Abstract
The Akt (or protein kinase B) and Cot (or Tpl-2) serine/threonine kinases are associated with cellular transformation. These kinases have also been implicated in the induction of NF-κB-dependent transcription. As a member of the mitogen-activated protein kinase kinase kinase (MAP3K) family, Cot can also activate MAP kinase signaling pathways that target AP-1 and NFAT family transcription factors. Here we show that Akt and Cot physically associate and functionally cooperate. Akt appears to function upstream of Cot, as Akt can enhance Cot induction of NF-κB-dependent transcription, and dominant-negative Cot blocks the activation of this element by Akt. Furthermore, deletion analysis shows that binding to Akt is critical for Cot function. The regulation of NF-κB-dependent transcription by Cot requires Akt-dependent phosphorylation of serine 400 (S400), near the carboxy terminus of Cot. However, phosphorylation at this site is not required for Cot kinase activity or AP-1 induction, suggesting it specifically regulates Cot effector function at the level of the NF-κB pathway. Mutation of S400 in Cot does indeed abolish its ability to activate IκB-kinase (IKK) complexes, but paradoxically it allows for increased Cot association with the IKK complex. This mutated form of Cot also acts as a dominant negative for T-cell antigen receptor/CD28- or Akt/phorbol myristate acetate-induced NF-κB induction, while having relatively little effect on tumor necrosis factor induction of NF-κB. These findings suggest that the activation of different signaling pathways by MAP3Ks may be regulated separately and may provide evidence for how such discrimination by one member of this kinase family occurs.
Cot (cancer osaka thyroid) was initially identified in a screen for transforming genes expressed by a human thyroid carcinoma cell line (28). Although the relationship of Cot to the original tumor is unclear, a truncated form of the protein could transform cell lines (28). The rat homologue, Tpl-2 (tumor progression locus 2), was subsequently isolated in a screen for genes which influence the progression of Moloney leukemia virus-induced rat thymomas (33). In addition, disregulation of murine Tpl-2 has been associated with mouse mammary tumor virus-induced transformation (10). Transformation by Cot/Tpl-2 is due in part to truncation of the protein after the seventh, or penultimate, exon. However, overexpression also appears to play a role in transformation, since relatively high levels of expression are required for a truncated form of Tpl-2 to cause thymomas in transgenic mice (7). The proto-oncogenic form of Cot is expressed primarily in hematopoietic tissue, although some message is also seen in lung tissue (26, 30). In addition, message levels are upregulated after stimulation of splenocytes with ConA (33). Thus, the primary focus of Cot function would appear to reside within the immune system.
The endogenous Cot/Tpl-2 gene encodes a serine/threonine kinase of the mitogen-activated protein kinase kinase kinase (MAP3K) family. This family consists of over 10 members, each of which contains a serine/threonine kinase domain that is conserved at the level of 25 to 30% identity or 50% homology between family members. Some family members contain additional domains, such as pleckstrin homology (PH), GTPase-interacting, or proline-rich domains (38). Many of these kinases fit into prototypical signaling cascades where they relay signals from small G proteins to MAP kinase kinases and MAP kinases, leading to the activation of inducible transcription factors such as Elk and c-Jun. Cot can activate both the ERK and c-Jun N-terminal kinase signaling pathways, acting through MEK-1 and SEK-1, respectively (36). This results in induction of AP-1 and NFAT-dependent transcription. In addition, several MAP3Ks, including Cot, have been implicated in NF-κB induction (38). Thus, Cot was shown to activate the IκB-kinase (IKK) complex, possibly acting through NF-κ B-inducing kinase (NIK) (22). Another mechanism postulated for NF-κB induction by Cot is the inducible degradation of the inhibitory p105 protein (5), which may itself be phosphorylated by the IKK complex (37).
It has been reported that the Akt serine/threonine kinase, which is also capable of inducing transformation (2, 6), can participate in NF-κB induction (13, 16, 24, 25, 32, 35). Our studies suggested that Akt can contribute to T-cell NF-κB induction in a manner similar to that of the CD28 coreceptor (14), which synergizes with signals from the T-cell receptor for antigen (TCR)/CD3 complex. Thus, overexpression of Akt alone resulted in no significant effects on NF-κB-dependent transcription (16). However, Akt could synergize with a suboptimal concentration of phorbol myristate acetate (PMA) or a TCR signal to activate transcription of an NF-κB-dependent reporter or the proximal interleukin-2 (IL-2) promoter (14, 16).
Since previous studies had shown that Cot can replace TCR and CD28 signals for NF-κB induction or IL-2 production (3, 22, 43) and that Cot mRNA is most highly expressed in thymus and spleen (30), we sought to determine whether there might be a functional connection between these two kinases in NF-κB induction in T cells. Our data demonstrate that Akt and Cot can physically interact, through the amino terminus of Cot, in an interaction that is apparently required for Cot activation of NF-κB. The interaction between Akt and Cot also results in the phosphorylation of Cot at two sites near its carboxyl terminus, one of which is required for Cot induction of NF-κB. Intriguingly, neither interaction with nor phosphorylation by Akt is required for activation of other inducible transcription factors by Cot. Thus, our data show that activation of different downstream pathways by a MAP3K can be regulated at the level of phosphorylation.
MATERIALS AND METHODS
Plasmids and antibodies.
Anti-Glu tag antibody conjugated to Protein G beads was obtained from D. Stokoe. Anti-Akt PH domain antibody conjugated to Protein G beads was obtained from Upstate Biotechnology Incorporated. Anti-myc (9E10) and anti-hemagglutinin (HA) (12CA5) antibodies were generated in a Miniperm apparatus (Heraeus). Anti-Akt monoclonal antibody was obtained from BD/Transduction Laboratories. 9E10-Cy3 was obtained from Sigma. Rabbit-anti-p65/RelA was from Santa Cruz Biotechnology, and anti-rabbit-fluorescein isothiocyanate was from Jackson Immunochemicals.
Site-directed mutagenesis.
Serines 400 and 413 of Cot were mutated to alanines by using oligonucleotide primers and the Quickchange Mutagenesis kit from Stratagene, according to the manufacturer's instructions. Mutant constructs were verified by sequencing.
Transfections and luciferase assays.
Jurkat T cells were transfected and luciferase assays were performed as described previously (16). All luciferase reporters were transfected at 20 μg/cuvette. 293T human embryonic kidney fibroblasts were maintained in DMEM with 10% fetal calf serum and were transfected by the calcium phosphate method. The day before transfection, cells were split into 6-well plates to achieve 50 to 70% confluence. One hour before transfection cells were fed with 4.5 ml of complete Dulbecco's modified Eagle medium. DNA was thoroughly mixed with 218 μl of double-distilled water and 32 μl of 1 M CaCl2. Hanks balanced salt solution (250 μl) was then added dropwise while the mixture was being shaken. The mixture (500 μl total) was added dropwise to a well of 293 cells. The cells were washed with phosphate-buffered saline (PBS) the next day and immediately lysed with 1% NP-40 lysis buffer and protease and phosphatase inhibitors.
Immunoprecipitation and Western blots.
Jurkat cells were lysed at 5 × 107 cells/ml in 1% NP-40 lysis buffer and protease and phosphatase inhibitors. Postnuclear supernatants equivalent to 106 cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were blotted to polyvinylidene difluoride (PVDF) (NEN). For immunoprecipitations, 20 μl of protein A bead slurry was incubated with lysates for 2 h, with tumbling at 4°C. Beads were washed three times with lysis buffer and then were resuspended in 40 μl of 2× SDS sample buffer, boiled for 5 min, and separated by SDS-PAGE. Blots were blocked with 5% milk in Tris-buffered saline with 0.05% Tween-20 (TBS-T). Primary and secondary (horseradish peroxidase-conjugated) antibody incubations were carried out at room temperature in TBS-T. Washes after primary and secondary incubations were performed with TBS-T three times for 10 min each time. Blots were visualized with ECL reagents from Amersham.
Phosphorylation of Cot and kinase assays.
For in vitro phosphorylation, immunoprecipitations were carried out as described above. Akt kinase buffer was then added (40 μl), containing 10 mM MgCl2, 1 mM dithiothreitol, 50 mM Tris (pH 7.5), 500 nM protein kinase A inhibitor peptide (Calbiochem), 20 μM ATP, and 7 μCi of [γ-32P]ATP. Samples were incubated at 30°C for 30 min, at which point 8 μl of 6× SDS sample buffer was added.
For phosphorylation in intact cells, 293T cells (3 wells/point) were washed twice with phosphate-free RPMI the day after transfection. Cells were incubated with 4 ml of phosphate-free RPMI containing orthophosphoric acid (0.5 mCi/ml; ICN) for 2 h at 37°C. Cells were washed twice with ice-cold PBS and then were lysed in 1% NP-40 lysis buffer plus protease and phosphatase inhibitors. Immunoprecipitation, SDS-PAGE, and Western blotting were carried out as described above.
For Cot kinase assays, Myc-tagged Cot constructs were immunoprecipitated from transfected cells after lysis as described above. Beads were washed three times in lysis buffer, washed once with kinase buffer (20 mM HEPES [pH 7.5], 10 mM MgCl2, 5 mM MnCl2, 1 mM dithiothreitol), and then resuspended in 40 μl of kinase buffer containing 10 μM ATP, 0.5 μg of histone H2B, and 10 μCi of [γ-32P]ATP. Kinase assays were incubated at 30°C for 30 min and were terminated by the addition of sample buffer. Immunocomplex kinase assays for IKK activity were carried out as described previously (16).
Phosphopeptide mapping.
Six wells per point of 293T cells transfected with kinase-inactive Cot and myristylated Akt were pooled. Cells were lysed and Akt immunoprecipitations and in vitro labeling reactions were carried out as described above. After SDS-PAGE the gel was wrapped in plastic film and exposed directly to X-ray film. The Cot-containing gel slice was cut into small pieces and was washed twice with 20 mM ammonium bicarbonate-acetonitrile (50:50). The gel pieces were then dried under a stream of nitrogen. For digestion, 1 μg of trypsin (Promega, Madison, Wis.) in 50 μl of 20 mM ammonium bicarbonate was added and incubated overnight at 37°C. Peptides were extracted twice with 0.1% trifluoroacetic acid (TFA)-acetonitrile (40:60) for 45 min at 37°C. Aliquots of the resultant peptide mixtures were then either subjected to a Vydac C18 reverse-phase high-pressure liquid chromatography (RP-HPLC) column (1 mm by 25 cm; The Separations Group) or spotted onto a cellulose plate (20 by 20 cm; Baker, Phillipsburg, N.J.). For RP-HPLC the peptides were eluted with a linear gradient of acetonitrile in 0.1% TFA over 1 h at a flow rate of 100 μl/min. Peptide fractions were collected at 1 min, and 10% of each fraction was subjected to scintillation counting (Beckman, Fullerton, Calif.). For thin layer electrophoresis the cellulose plate was run for 2 h at 900 V in pyridine-acetic acid-acetone-water (1:2:8:40) by using a high-voltage electrophoresis chamber (Savant Instruments, Holbrook, N.Y.). After being dried overnight the plate was subjected to thin-layer chromatography and developed in pyridine-n-butanol-acetic acid-water (50:75:15:60). After being dried the cellulose plate was exposed to X-ray film at −80°C.
Radiolabeled phosphopeptides from RP-HPLC were concentrated to a volume of 5 μl and spotted onto a Sequelon AA membrane. Peptides were coupled to the membrane according to the manufacturer's instructions (PE Biosystems). The membranes were then placed into the cartridge of a Procise Protein Sequencer, Model 492 (PE Biosystems) and subjected to automated Edman degradation. The released anilinothiazolinone-amino acids were collected, and scintillation was counted. Results were obtained on three separate preparations of phosphorylated Cot.
HeLa transfections and immunofluorescence microscopy.
One day before transfection, HeLa cells were seeded onto glass coverslips in 6-well plates. Two hours before transfection, cells were fed with 2 ml of complete Dulbecco's modified Eagle medium. Cells were transfected by using 6 μl of the Fugene reagent (Roche)/sample plus 2 μg of the indicated plasmid. Sixteen hours after transfection cells were washed with PBS and fixed and stained as previously described (12). Jurkat T cells were transfected as described above and settled onto poly-l-lysine-coated slides for fixation and staining. Images were acquired with a Marianas Turn-Key system from Intelligent Imaging and were analyzed with SlideBook software. Single-plane images were exported as TIFF files and further processed by using Photoshop 4.0 (Adobe) and Canvas 7 (Deneba).
RESULTS
Akt/Cot functional interaction.
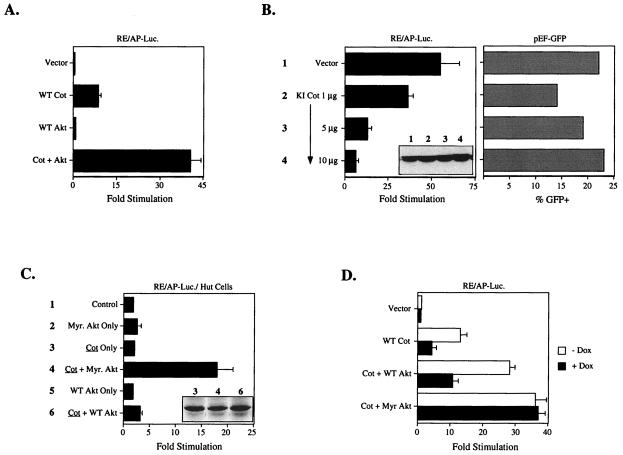
Overexpression of Cot in T-cell lines leads to the production of IL-2 and tumor necrosis factor alpha (TNF-α) in a manner similar to stimulation through the TCR/CD3 complex and CD28 (3, 4, 42, 43). Lin et al. recently demonstrated that transcription from the NF-κB-like RE/AP element in the IL-2 promoter can be upregulated by Cot overexpression in Jurkat T cells (22). As shown in Fig. 1A, this effect can be augmented five- to sixfold by coexpression of wild-type Akt. Akt can also induce transcription from the RE/AP element, an effect that requires an additional signal, which can be provided by a low concentration of PMA (16). As seen in Fig. 1B (left panel), RE/AP activation by Akt and PMA is inhibited by kinase-inactive (KI) Cot in a dose-dependent manner, while expression of green fluorescent protein (GFP) driven by a constitutive promoter is not inhibited by the KI Cot construct (Fig. 1B, right panel). Similar results were obtained when a classical NF-κB reporter was used (16 and data not shown).
FIG. 1.
Functional interaction between Akt and Cot. (A) Jurkat T cells were transfected with the RE/AP luciferase (RE/AP-Luc.) reporter and 3 μg of wild-type (WT) Cot and/or 10 μg of wild-type Akt. The next day luciferase activity was determined as described in Materials and Methods. (B) Jurkat T cells were transfected with 10 μg of wild-type Akt, the indicated amounts of kinase-inactive (KI) Cot, and either RE/AP-luciferase (left panel) or pEF-GFP (right panel). The next day cells were stimulated with 10 ng of PMA/ml or were analyzed for GFP expression by flow cytometry. The inset in the left panel shows expression of Akt in the samples with RE/AP luciferase. (C) Hut78 cells were transfected with 10 μg of the indicated constructs and the RE/AP luciferase reporter. The inset shows constant levels of Cot expression in the relevant samples. (D) Jurkat cells stably transfected with a tetracycline-regulated PTEN allele were transfected with the RE/AP-luciferase reporter and 5 μg of the indicated constructs. Samples were split into wells without or with 1 μg of doxycycline (Dox)/ml. Twenty-four hours after transfection luciferase activity was determined. Myr. Akt, myristylated Akt.
The Jurkat T-cell line lacks a negative regulator of phosphatidylinositol 3-kinase (PI3K) effectors, a PIP3 phosphatase known as PTEN/MMAC (39). This is consistent with the fact that the PH-domain-containing kinase Akt has higher basal activity in these cells (39), and that wild-type Akt is capable of activating RE/AP-dependent transcription with nearly the same efficiency as myristylated Akt (16). By contrast, the transformed human T-cell line Hut78 appears to express normal levels of PTEN; in this cell line overexpression of wild-type Akt does not activate RE/AP-dependent transcription in conjunction with PMA, although the myristylated form of the kinase can still do so (unpublished data). As shown in Fig. 1C, overexpression of Cot was insufficient to induce RE/AP in Hut78 cells, even when wild-type Akt was cotransfected. However, when myristylated Akt was cotransfected with Cot, RE/AP-dependent transcription was activated efficiently. PMA addition is not required to see these effects, most likely due to the fact that Cot overexpression bypasses this requirement. The level of RE/AP induction noted when Cot is overexpressed by itself in Jurkat T cells is therefore probably due in part to the high basal activity of Akt in these cells (39). This was examined another way, i.e., with Jurkat cells engineered to inducibly express PTEN at physiologic levels in response to the tetracycline analogue doxycycline (Z. Xu and A. Weiss, unpublished data). Thus, as shown in Fig. 1D, induction of PTEN expression with doxycycline (dox) inhibited the ability of Cot to induce RE/AP, either by itself or with wild-type Akt overexpression. However, the effect of myristylated Akt on Cot induction of RE/AP was completely unaffected by induction of PTEN. Similar results were seen with a classical NF-κB reporter (data not shown). Taken together, the results shown in Fig. 1 demonstrate a functional interaction between Akt and Cot that depends upon regulation of Akt by PI3K products. Moreover, the inhibitory effects of kinase-inactive Cot on Akt-induced NF-κB-dependent transcription suggest that Cot functions downstream of Akt.
Akt and Cot/Tpl-2 physical association.
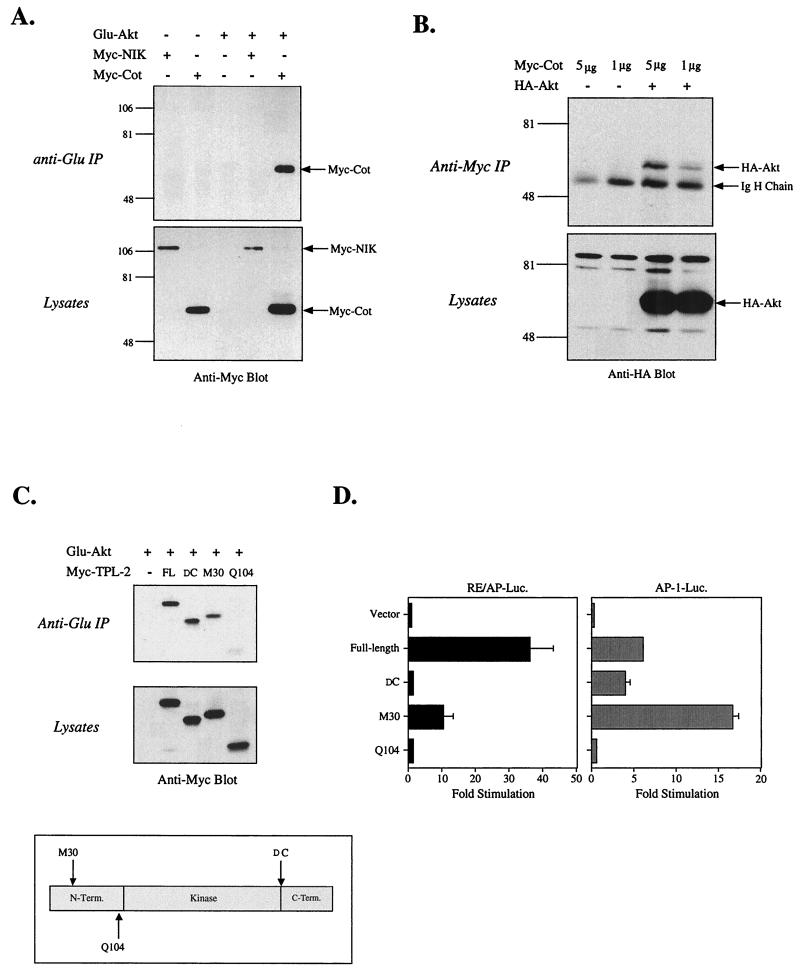
Because the results shown in Fig. 1 demonstrated a functional interaction, we investigated whether a physical interaction between Akt and Cot can occur. Figure 2A shows that Cot can be coimmunoprecipitated with epitope-tagged Akt when the two proteins are coexpressed in 293T cells. Likewise, precipitation of tagged Cot reveals the presence of Akt (Fig. 2B). The interaction of Akt with Cot appears to be relatively specific, as we were unable to detect Akt association with coexpressed NIK, a kinase that is closely related to Cot (23, 27) (Fig. 2A). Even when NIK was expressed at much higher levels, no interaction with Akt was detected (data not shown). Thus, Akt and Cot physically interact, although it is unclear at this point if the interaction is direct.
FIG. 2.
Physical interaction between Akt and Cot. 293T cells were transfected with 5 μg or the stated amount of the indicated plasmids. The next day cells were lysed and a small sample was removed to assess protein expression levels (lysates). The remainder was subjected to immunoprecipitation with Protein G beads covalently coupled to a monoclonal antibody against the Glu epitope tag (anti-Glu IP) of the transfected Akt construct (A) or the 9E10 monoclonal antibody against the myc epitope tag (anti-Myc IP) of the transfected Cot construct (B). Western blots of immunoprecipitated proteins and lysates were probed with anti-myc (A) or anti-HA antibody (B). (C) Tpl-2 constructs (2 to 10 μg; used in order to normalize expression levels) were analyzed for binding to Akt, as described for panel A. (D) Function of the Tpl-2 constructs was analyzed by cotransfection into Jurkat T cells with RE/AP or AP-1 luciferase (Luc.) reporters. Equal expression levels of the Tpl-2 constructs were confirmed by Western blotting (data not shown). IgH, immunoglobulin H; FL, full length; DC, deletion of C terminus.
The rat homologue of Cot, known as Tpl-2, also induces lymphoma formation when truncated (26, 33). We tested the ability of Akt to interact with Tpl-2 in 293T cells to determine the degree to which the interaction is conserved evolutionarily. In addition, various Tpl-2 truncation mutants have already been described (5) which allowed us to map the location of the interaction with Akt. As shown in Fig. 2C, full-length Tpl-2 can be coprecipitated with Akt. The truncation of 70 residues from the carboxy terminus (DC) leaves the interaction with Akt intact. However, as truncations are made at the amino terminus the binding to Akt is lost. Thus, truncation of the amino-terminal residues before M30 partially reduces binding to Akt, while a more severe truncation, deleting the amino-terminal 103 residues (Q104), completely ablates binding. When function of these constructs was assessed in transcriptional reporter assays, it was noted that partial truncation of the amino terminus (M30) had a negative impact on induction of an RE/AP element (or NF-κB; data not shown), with further truncation (Q104) resulting in a complete loss of function (Fig. 2D). Truncation of the 70 carboxy-terminal residues also ablated activation of RE/AP, even though interaction with Akt still occurred. By contrast, the carboxy-terminal truncation of Tpl-2 had little to no effect on AP-1 (or NFAT; data not shown) activation, and the M30 amino-terminal truncation had increased activity in this assay, although further truncation of the amino terminus (Q104) did eventually eliminate activation of AP-1. These results indicate that both the amino- and carboxy-terminal domains of Cot/Tpl-2 are important for RE/AP or NF-κB activation and that the former may be important because it regulates association with Akt. The interaction appears to require the kinase and/or carboxy-terminal domains of Akt, as an Akt mutant lacking the amino-terminal PH domain can still interact with Cot (data not shown). We have been unable to generate a carboxy-terminal truncation mutant of Akt that is expressed at high enough levels to perform further analyses (data not shown).
Phosphorylation of Cot by Akt.
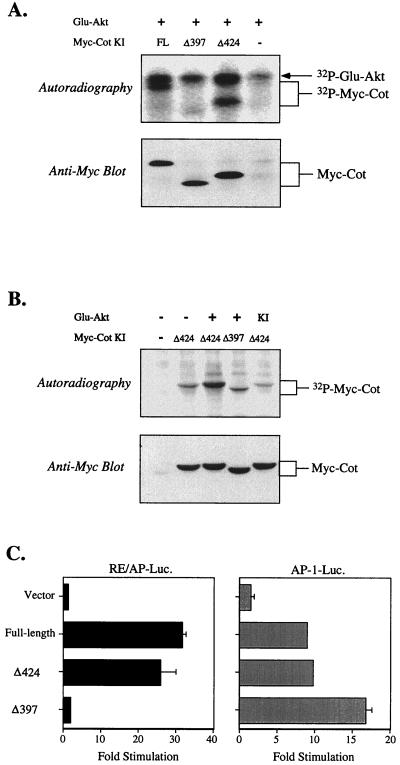
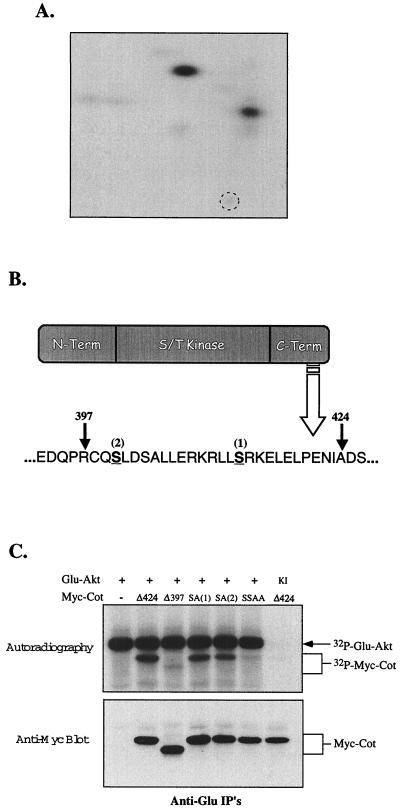
Because Akt and Cot interact, and because the experiments shown in Fig. 1 suggested that Cot functions downstream of Akt, we hypothesized that Cot may be a substrate for Akt. Previous studies showed that Cot is negatively regulated by its carboxy-terminal tail (7, 28, 33), and it has been postulated that phosphorylation of this region of Cot may relieve a conformational inhibition (7). As shown in Fig. 3A, coprecipitated kinase-inactive Cot was phosphorylated in vitro by Akt. A deletion mutant of Cot lacking the 44 amino acids following residue 424 (Δ424) was also phosphorylated by Akt in vitro, apparently with the same efficiency. When an additional 27 residues were removed (Δ397; approximately the same size as the Tpl-2 carboxy-terminal truncation mutant used above), Cot was no longer phosphorylated by Akt in vitro, although both truncated Cot constructs were still coprecipitated with Akt (Fig. 3A, bottom gel). The same pattern was noted when phosphorylation of Cot was investigated in intact cells. In this experiment, shown in Fig. 3B, only the truncated forms of Cot were employed, as they are more easily separated from the Akt band. Basal levels of phosphorylation of kinase-inactive Cot immunoprecipitated from cells labeled with 32P-orthophosphate were consistently higher than in the in vitro experiments (data not shown). Nonetheless, Cot Δ424 isolated from cells coexpressing myristylated Akt contained more 32P (Fig. 3B). Similar to the results seen in the in vitro experiments, however, the shorter form of Cot Δ397 contained no more than basal levels of phosphate (Fig. 3B, compare the second and fourth lanes, and data not shown). These results suggest that the site(s) of Akt-induced phosphorylation lies between amino acids 398 and 424 in Cot.
FIG. 3.
Phosphorylation of Cot by Akt. (A) 293T cells were transfected with 5 μg of the indicated constructs. Glu epitope tag immunoprecipitates were incubated with kinase buffer and [32P]ATP to allow phosphorylation of coprecipitated proteins. After SDS-PAGE, gels were transferred to PVDF and exposed to X-ray film (top gel), followed by rehydration and Western blotting with anti-myc antibody (bottom gel) to confirm the presence of coprecipitated kinase-inactive (KI) Cot proteins. (B) 293T cells were transfected with 5 μg of the indicated constructs, and the next day they were incubated with medium containing 32P-orthophophoric acid. Transfected kinase-inactive Cot proteins were immunoprecipitated with anti-myc antibody, separated by SDS-PAGE, and transferred to PVDF. After exposure to X-ray film, membranes were probed with anti-myc antibody to confirm the presence of transfected Cot proteins. (C) The indicated constructs (5 μg) were transfected into Jurkat T cells with either the RE/AP or AP-1 luciferase reporter; luciferase activity was determined the next day as described in the text. FL, full length.
Next, the two truncated forms of Cot were compared with the full-length kinase for their relative abilities to induce transcriptional reporters. As with the carboxy-terminal truncation of Tpl-2 shown in Fig. 2D, Cot Δ397 lost the ability to activate RE/AP (or NF-κB; data not shown) in Jurkat T cells. Nonetheless, as reported previously (11, 34, 36), this form of Cot could still efficiently activate AP-1 (or NFAT)-dependent transcription (Fig. 3C), consistent with the fact that this mutant possesses as much catalytic activity as the full-length kinase (Fig. 5 and reference 7). Thus, phosphorylation of the carboxy-terminal domain of Cot correlates with RE/AP (and NF-κB), but not AP-1 (or NFAT), activation by this kinase.
FIG. 5.
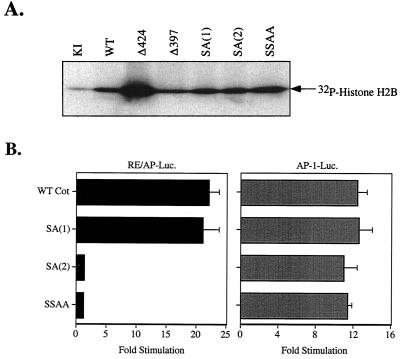
Effects of mutated Cot constructs on kinase activity and transcriptional reporters. (A) Kinase activity of mutated kinase-inactive (KI) Cot constructs. 293T cells were transfected with 5 μg of the indicated myc-tagged Cot constructs. The next day anti-myc immunoprecipitates were washed and kinase activity was determined as described in Materials and Methods. Δ424 and Δ397 are the truncations referred to in the text. Equivalent levels of transfected Cot constructs were confirmed by Western blotting (data not shown). (B) Functional effects on inducible transcription of the Cot serine mutations were determined by cotransfection with RE/AP or AP-1 luciferase (Luc.) reporters in Jurkat T cells. WT, wild type.
The results shown in Fig. 3 suggest that Akt can phosphorylate one or more sites in the carboxy-terminal tail of Cot, somewhere between residues 398 and 424. This was confirmed by labeling of Cot to high stoichiometry in vitro with activated Akt. SDS-PAGE and two-dimensional phosphopeptide mapping were then performed (Fig. 4A), followed by Edman degradation. The results of these experiments showed that Akt could phosphorylate two sites in the carboxy-terminal portion of Cot, at serines 400 and 413 (Fig. 4A and B). The latter site was not unexpected, since it conforms reasonably well to a consensus Akt phosphorylation site (RxRxxS/Tφ, where φ is any hydrophobic amino acid) (1, 29). However, the sequence around the serine at residue 400 is rather unlike this consensus, except for the arginine at position −3. Both serines are contained within sequences that are highly conserved among human, rat, and murine forms of Cot/Tpl-2 (data not shown), suggesting their likely importance. The two serines in the carboxy-terminal tail of Cot identified as Akt phosphorylation sites were replaced with alanines, individually or in combination, by site-directed mutagenesis. The ability of Akt to phosphorylate these constructs in vitro was then tested. As shown in Fig. 4C (top panel), mutation of the Akt consensus site (S413) to alanine [construct SA(1)] had a moderate effect on Cot phosphorylation by Akt, as did mutation of the nonconsensus (S400) site [construct SA(2)]. However, mutation of both serines (SSAA) reduced the level of phosphorylation to that seen with the Δ397 truncation of Cot. Coprecipitation of Cot with KINASE-INACTIVEAkt led to no phosphorylation of Cot in vitro, implying a lack of contaminating kinases in the Akt immunoprecipitations. All of these constructs still interacted with Akt (Fig. 4C, bottom panel).
FIG. 4.
Mapping Akt-induced Cot phosphorylation sites. (A) Two-dimensional phosphopeptide analysis of Cot phosphorylated in vitro by Akt. The broken circle indicates the origin. A similar pattern of Cot phosphorylation was noted with samples phosphorylated in intact cells, although two additional spots appeared, consistent with the higher basal phosphorylation of Cot in intact cells (Fig. 3B and data not shown). (B) Schematic diagram of the Cot domain structure and primary amino acid sequence. The two Akt-induced phosphorylation sites, determined as described in Materials and Methods, are underlined, and the sites of truncation described in the legend to Fig. 3 are indicated by arrows. (C) Phosphorylation of Cot constructs by Akt. 293T cells were transfected with 5 μg of the indicated constructs, and in vitro phosphorylation was assessed as described for panel A. IP's, immunoprecipitates.
Function of Akt-dependent phosphorylation sites in Cot.
Having shown that the Akt-dependent phosphorylation of Cot occurs exclusively on serines 400 and 413, the functional consequences of mutating one or both of these residues were examined. None of the serine mutations affected kinase activity, as measured by in vitro kinase assays with an exogenous substrate, histone H2B (Fig. 5A). The effects of these serine mutations were further tested in Jurkat T cells with transcriptional reporter assays. As shown in Fig. 5B, mutation of the Akt consensus site [SA(1)] had no effect on either RE/AP or AP-1 activation by Cot. However, both the SA(2) and SSAA forms, while still capable of activating AP-1 (right panel), lost the ability to induce RE/AP (or NF-κB; data not shown) activity (left panel), the same phenotype seen with Cot truncated at residue 397 (Fig. 3C, Δ397).
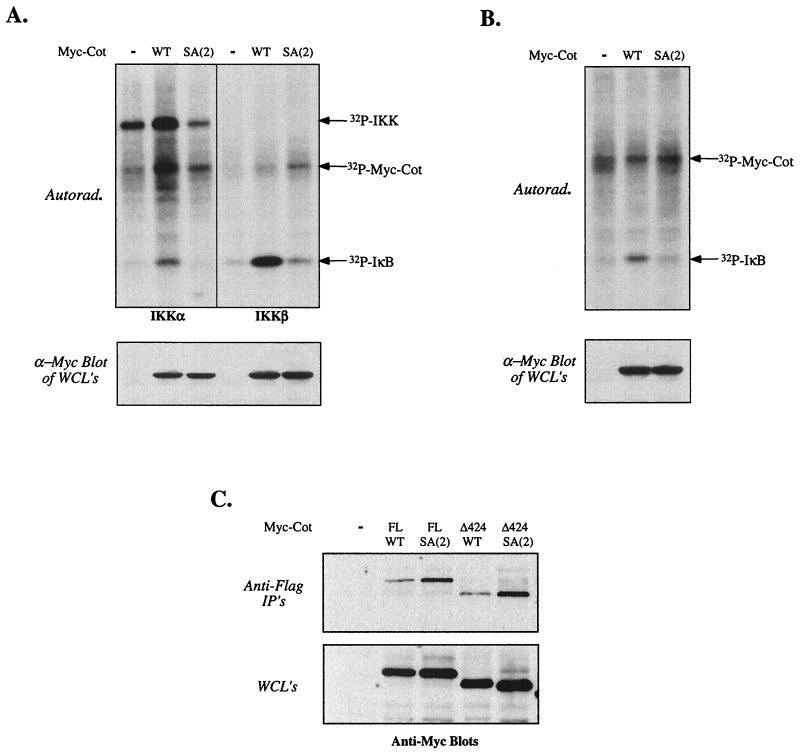
Since we previously mapped the effects of Akt upstream of activation of the IKKs, we examined whether mutation of S400 affected the ability of Cot to activate the IKK complex. Cotransfection of wild-type Cot with Flag-tagged IKKα (left panel) or IKKβ (right panel) resulted in activation of the kinases, as measured by phosphorylation of glutathione S-transferase-IκB in vitro (Fig. 6A). However, Cot SA(2), mutated at S400, was incapable of activating complexes containing either tagged IKKα or IKKβ. The ability of Cot to activate endogenous IKK complexes was also assessed. Wild-type Cot or Cot SA(2) was transfected into 293T cells, followed by immunoprecipitation of endogenous IKK complexes with an anti-IKKγ antibody. As shown in Fig. 6B, wild-type Cot, but not the SA(2) form, efficiently increased endogenous IKK activity. These results suggest that the inability of Cot lacking serine 400 to activate NF-κB-dependent transcription is due to failure to activate the IKK complex, perhaps due to an inability to interact with the complex. In an attempt to understand the lack of IKK activation by Cot SA(2), we examined the ability of wild-type or mutated Cot to interact with the IKK complex when cotransfected with Flag-tagged IKKα, as shown in Fig. 6C. As expected, either full-length or Cot Δ424 could be coprecipitated with IKKα (Fig. 6C, top gel, third and fourth lanes). However, we consistently noted a modest but reproducible increase in the amount of Cot coprecipitated with IKKα when the Cot construct (full length or truncated) was mutated at S400. Similar results were obtained when endogenous IKKγ was precipitated from cells transfected with the same Cot constructs (data not shown).
FIG. 6.
Effects of Cot S400 mutation on IKK complex activation and association. (A and B) Activation of cotransfected (A) or endogenous (B) IKK by wild-type (WT) or SA(2) Cot. 293T cells were transfected with 5 μg of empty vector or the indicated Cot constructs; samples shown in panel A were also transfected with 5 μg of Flag-tagged IKKα or 0.1 μg of IKKβ. Twenty hours after transfection cells were harvested, lysed, and subjected to immunoprecipitation with either anti-Flag antibody M2 (A) or anti-IKKγ (B). IKK kinase assays were then carried out as described in Materials and Methods, with recombinant IκBα as an exogenous substrate. A small aliquot of each lysate was assayed by Western blot for expression of the Cot constructs (lower gels). Equal expression and immunoprecipitation of IKKα were confirmed by reprobing the kinase assay blot with anti-Flag antibody (data not shown). As IKKβ displays higher levels of kinase activity than IKKα, much lower quantities of the former were transfected, precluding detection of the autophosphorylated kinase (A, upper right gel). (C) 293T cells were cotransfected with 5 μg of Flag-tagged IKKα and the indicated Cot constructs. Twenty hours after transfection cells were harvested, lysed, and subjected to immunoprecipitation with anti-Flag antibody. After SDS-PAGE and Western blotting, membranes were probed with anti-Myc antibody 9E10 to reveal coprecipitated Cot. IP's, immunoprecipitates; FL, full length; Autorad., autoradiography; WCL, whole-cell lysates.
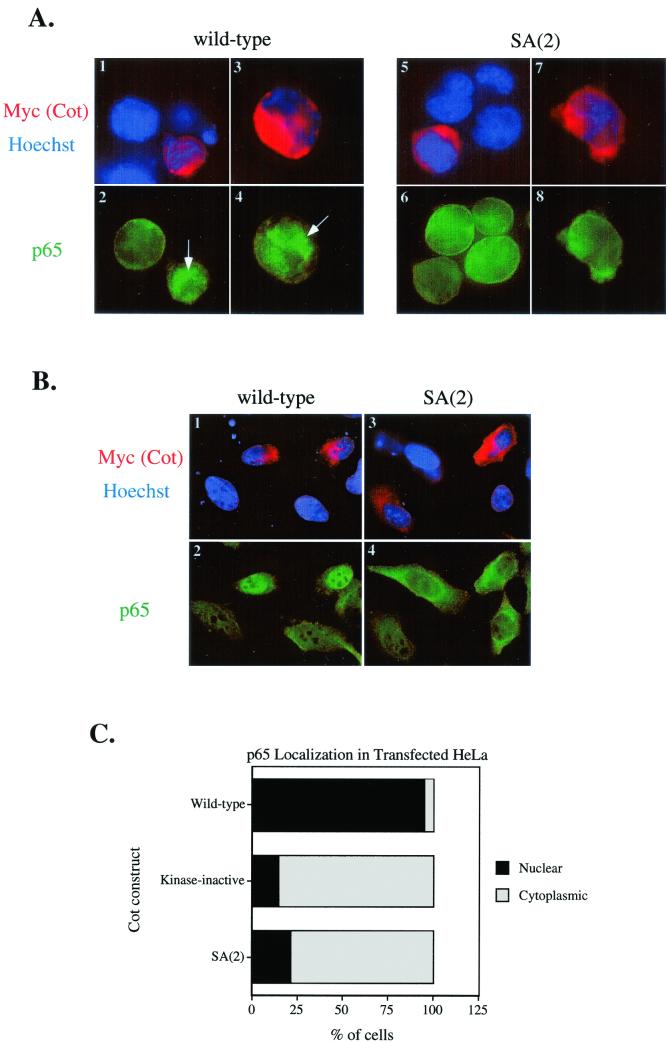
In order to further define the defect in NF-κB induction of the SA(2) form of Cot, we investigated whether this construct could induce nuclear entry of NF-κB. We therefore transfected wild-type or SA(2) Cot into Jurkat (Fig. 7A) or HeLa (Fig. 7B) cells and followed the localization of the NF-κB protein p65/RelA by immunofluorescence microscopy. Figure 7A and B show representative images from such experiments, where transfected Cot is revealed by anti-Myc staining (red), nuclei are revealed by Hoechst 33342 staining of DNA (blue), and p65 is revealed by indirect staining with a rabbit antiserum (green). Thus, cells expressing wild-type Cot efficiently transport p65 into the nucleus (Fig. 7A, images 1 to 4, and B, images 1 and 2), consistent with the activation of IKK shown in Fig. 6. This is more obvious in the HeLa cells (Fig. 7B, image 2), although it still occurs in Jurkat T cells (Fig. 7A, images 2 and 4). Conversely, SA(2) Cot (and kinase-inactive Cot; data not shown) is unable to induce significant nuclear entry of p65, also consistent with IKK and NF-κB reporter data. Figure 7C shows quantitation of nuclear versus cytoplasmic p65 staining for the HeLa (Fig. 7D) experiment represented in the immunofluorescence images of Fig. 7B. Although images of cells transfected with kinase-inactive Cot are not shown, quantitation of nuclear versus cytoplasmic localization of p65 is shown for comparison with SA(2) Cot.
FIG. 7.
Cot induction of p65/RelA nuclear entry. Jurkat (A) or HeLa (B) cells were transfected with the indicated Cot constructs and were stained with 9E10-Cy3 (to detect myc-tagged Cot) and Hoechst (to reveal nuclei) (images 1, 3, 5, and 7 in panel A and images 1 and 3 in panel B) and anti-p65 plus goat anti-rabbit-fluorescein isothiocyanate (images 2, 4, 6, and 8 in panel A and images 2 and 4 in panel B). (C) Quantitation of nuclear versus cytoplasmic localization of p65 in Cot-transfected HeLa cells. At least 40 Cot-transfected (red) cells of each type were scored for localization of p65 staining. Nuclear staining was scored when p65 intensity in the nucleus was greater than the overall staining intensity in the cytoplasm. Results in each part are representative of three independent experiments.
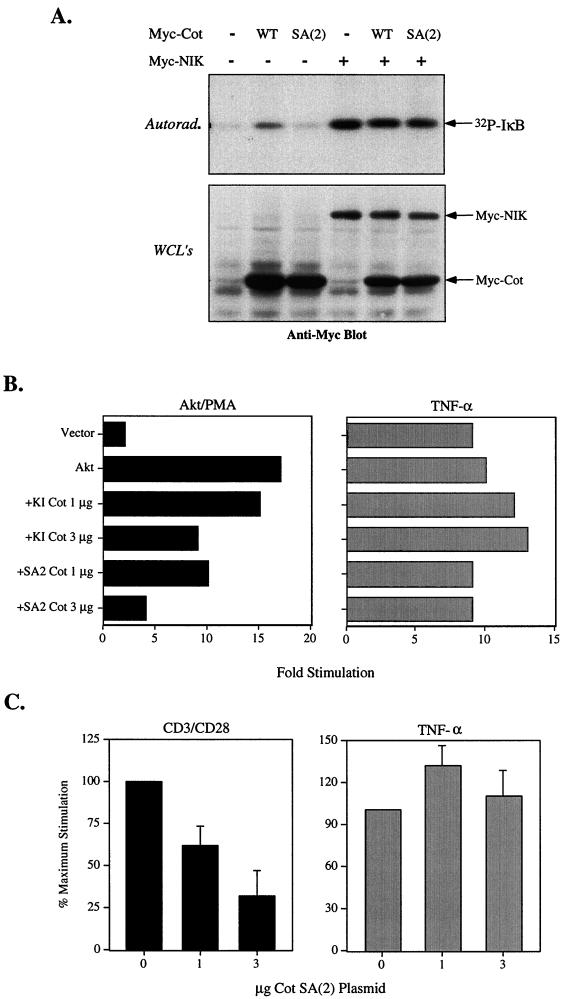
Previous studies are consistent with the hypothesis that another kinase, such as NIK, might mediate IKK activation by Akt and Cot. Our own work had shown that NF-κB induction by Akt and PMA could be potently inhibited by a dominant-negative form of NIK (16). Lin et al. showed that KI Cot could not inhibit NIK-induced IKK activation (22). We therefore investigated whether Cot mutated at S400 could inhibit IKK activation by NIK. As shown in Fig. 8A, wild-type Cot but not SA(2) could activate IKK, consistent with data shown above. NIK could also activate cotransfected IKKα, which was unaffected by cotransfection with Cot SA(2), even though the latter was expressed at higher levels (Fig. 8A, lower gel).
FIG. 8.
Specificity of NF-κB inhibition by Cot mutated at S400. (A) 293T cells were transfected with Flag-tagged IKKα and the indicated myc-tagged Cot or NIK constructs. Twenty hours after transfection cells were harvested, lysed, and subjected to immunoprecipitation with anti-Flag antibody M2. IKK kinase assays were conducted as described in Materials and Methods (upper gel). Aliquots of the lysates were assayed for expression of the myc-tagged proteins (lower gel). Equivalent levels of IKKα were confirmed by Western blotting (data not shown). (B and C) Dominant-negative activity of Cot SA(2). Jurkat T cells were transfected with an NF-κB-luciferase reporter and the indicated constructs. Cells were then stimulated for 6 h with 20 ng of PMA/ml (B), anti-CD3/CD28 (1 μg of anti-CD3/ml and 2 μg of anti-CD28/ml) (C), or 50 ng of TNF-α/ml (B and C), after which luciferase activity was determined. The results shown in panel C are shown as the percent maximal stimulation (versus vector control) and are the averages ± standard deviations of three experiments. WT, wild type; Autorad., autoradiography; WCL's, whole-cell lysates.
Both Akt and Cot have been implicated in T-cell activation downstream of the TCR/CD3 complex and the costimulatory receptor CD28 (14, 22). Since phosphorylation of serine 400 is required for Cot activation of NF-κB-dependent transcriptions, we hypothesized that this form of Cot [Cot SA(2)] may act like a dominant negative to block activation of NF-κB and RE/AP, as seen with kinase-inactive Cot (Fig. 1). As shown in Fig. 8B, Cot SA(2) could indeed inhibit Akt- or PMA-induced NF-κB even more efficiently than kinase-inactive Cot. Consistent with previous results obtained with kinase-inactive Cot (22), TNF-induced induction of NF-κB was not sensitive to the effects of low levels of Cot SA(2) (Fig. 8B). We also noted that this form of Cot could inhibit NF-κB induction through endogenous pathways. Thus, Cot SA(2) suppressed CD3/CD28-mediated NF-κB (Fig. 8C) or RE/AP (data not shown) induction in a dose-dependent manner, even though this construct still possesses kinase activity. Furthermore, Cot SA(2) could also inhibit Akt-dependent activation of the IKK complex (data not shown). These results provide further evidence that the MAP3K Cot plays a role in the activation of NF-κB by at least a subset of stimulatory pathways.
DISCUSSION
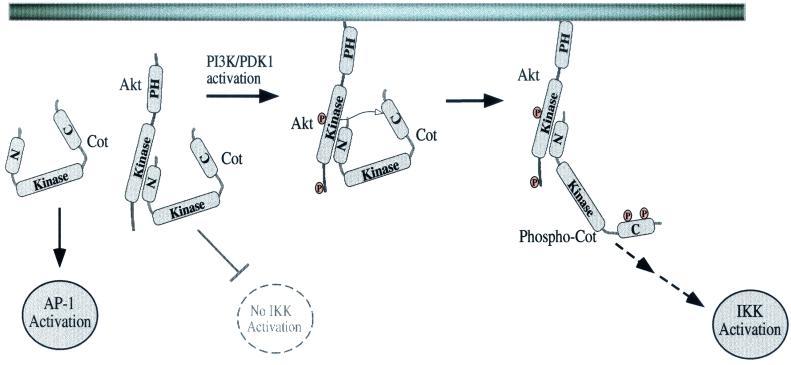
This report provides evidence for a novel regulatory mechanism of a MAP3K, i.e., Cot, which is modulated by its phosphorylation by the PIP3-regulated kinase Akt. Our studies with cells with or without the negative regulator PTEN indicate that the PI3K/Akt pathway is important for NF-κB induction by Cot. Furthermore, Akt and Cot can physically associate through the amino terminus of Cot. Akt can also mediate the phosphorylation of Cot, as demonstrated both in vitro and in intact cells. Although two sites of serine phosphorylation in Cot were mapped, only one of these sites appears to be critical for Cot induction of NF-κB-dependent transcription. However, neither site was required for Cot induction of AP-1 or NFAT, consistent with the dispensability of the carboxy terminus for activating these reporters. Our data provide evidence for a pool of Akt complexed with Cot, probably maintained in the cytoplasm, since Akt lacking a PH domain can still associate with Cot (Fig. 9 and data not shown). Upon PI3K activation this complex is likely recruited to the membrane through the Akt PH domain, which binds the phospholipid PIP3. Akt is then activated by the kinase PDK-1 and perhaps another intermediate kinase (data not shown). This in turn allows Akt to phosphorylate Cot at two sites in the carboxy-terminal domain, at least one of which may promote binding of substrates or coactivators to Cot, or alternatively may relieve binding of a negative regulator. Strikingly, this mechanism for regulation of Cot does not affect Cot kinase activity or induction of AP-1-dependent transcription by Cot. What it may regulate, however, is release of Cot from the IKK complex, allowing each Cot molecule to serially activate many such complexes (not depicted in the model shown in Fig. 9).
FIG. 9.
Model for regulation of Cot by Akt. See the text for details.
In total our data demonstrate that Akt and Cot can induce NF-κB-dependent transcription through activation of the IKK complex and subsequent nuclear translocation of NF-κB factors, such as p65/RelA (16) (Fig. 6 and 7). Recently it has been suggested that the major effect of Akt in this pathway occurs through phosphorylation-dependent increases in the transactivation potential of p65 (24, 25). We have investigated whether this may occur in T cells, but we have been unable to detect Akt-mediated increases in p65 transactivation, as measured with a p65/gal4 transcriptional reporter (L. P. Kane and A. Weiss, unpublished data). This discrepancy may be due to cell type differences in the coupling of upstream signaling pathways to NF-κB. In addition, we have noted that a rapidly inducible, estrogen-regulated Akt fusion protein is capable of upregulating NF-κB-dependent transcription in conjunction with PMA (L. P. Kane and A. Weiss, unpublished data). This is also consistent with a relatively direct effect on the NF-κB nuclear translocation pathway, as discussed above.
Two sites of apparent Akt-dependent serine phosphorylation in the C-terminal tail of Cot were identified in this study (Fig. 4). Phosphorylation of these sites is probably not carried out in a cooperative manner, as mutation of either one only partially decreased phosphorylation of Cot in vitro or in intact cells (Fig. 4C and data not shown). Only one of these sites (S413) conforms to the established consensus for phosphorylation by Akt (1, 29). The sequence around the S400 site (QPRCQSL) partially fulfills the Akt consensus phosphorylation site (RXRXXS/TΦ). It is possible but unlikely that the high concentrations of proteins used in the in vitro and intact cell experiments resulted in spurious phosphorylation of S400 by Akt. It also seems unlikely that an associated kinase was carrying out this phosphorylation, as use of a kinase-inactive Akt resulted in no increase in Cot phosphorylation (Fig. 3B and 4C). However, it is formally possible that Akt itself activates another kinase, which then carries out the phosphorylation of S400. Another possibility is that binding to Cot somehow alters the specificity of Akt, allowing it to directly phosphorylate this site. Regardless of the precise mechanism, either perturbation of the Akt pathway (Fig. 1) or mutation of S400 (Fig. 5) has profound consequences for Cot induction of NF-κB.
Regulatory phosphorylation of kinases is a common theme. Phosphorylation by Akt negatively regulates other kinases, such as Raf and GSK3 (8), by allowing complex formation with 14-3-3 proteins, which sequester the kinases. Src family tyrosine kinases are both positively and negatively regulated by phosphorylation, as is the ZAP-70 tyrosine kinase (15, 21). These tyrosine kinases appear to be regulated at the level of protein tertiary structure, which controls access to the catalytic domain. These models probably do not account for the importance of serine 400 in Cot, which is dispensable for catalytic activity and some downstream effects. Other molecules regulated by serine phosphorylation include the tyrosine kinase Btk (17) and the transcription factor STAT1 (20). In the former case, serine phosphorylation negatively regulates the kinase, possibly at the level of membrane recruitment (17). The case of STAT1 regulation is more similar to the effects noted here with Cot, in that only a subset of downstream target genes are affected by mutation of S727 in STAT1 (20). This specificity may be achieved through serine-dependent interactions with gene-specific coactivators (31, 49). Such a protein-protein interaction model is appealing for the function of S400 in Cot and will be tested.
Although Cot may affect NF-κB coactivators, it is clear that there is a role for S400 in the activation of the IKK complex and nuclear translocation of p65. The precise mechanism by which phosphorylation at S400 influences Cot activation of the IKK complex is not yet clear. We propose that efficient activation of the IKK complex by Cot requires serial interaction of Cot with multiple complexes. As Cot interacts with and activates each complex, phosphorylation at S400 by Akt may cause the release of Cot, followed by dephosphorylation and association with a different IKK complex. Cot mutated at S400 may therefore be unable to efficiently disengage from complexes with which it is associated. How would this type of mechanism operate even in a system where Cot is overexpressed, as it is in these experiments? While we noted no effect of the S400 mutation on total Cot kinase activity (Fig. 5A), it remains possible that local activation of Cot, perhaps by another protein associated with the IKK complex, is affected by this mutation. In this regard, our results are consistent with those of Lin et al. (22), who noted that Cot appears not to associate directly with the IKK complex, as demonstrated by the inability of Cot SA(2) to inhibit NIK-induced IKK activation (Fig. 8A). Furthermore, NF-κB induction by some inducers (CD3/CD28) is affected more than others (TNF) by this form of Cot. We have investigated whether the mutation in S400 affects the association of Cot with NIK but have not detected any difference in the efficiency of this association (data not shown). Thus, additional proteins may be required for the effects of Cot on the IKK complex.
Cot/Tpl-2 is capable of activating classical NF-κB transcription and the NF-κB-like RE/AP element in the IL-2 promoter, both of which are targets of TCR/CD28 signal integration. Our results with the S400 mutation in Cot also suggest that Cot participates in the TCR/CD28, but not the TNF, pathway leading to NF-κB-dependent transcription. However, there are discrepancies regarding the mechanism by which this occurs. Lin et al. provided evidence that the IKK complex is targeted by Cot, possibly through the intermediate kinase NIK (22). Consistent with this evidence is the fact that T cells from aly mice, which express a mutant NIK protein, have a cell-autonomous defect in IL-2 production (41, 47). Belich et al. demonstrated an interaction of Tpl-2 with p105, while these investigators failed to see an effect on IκB degradation (5). The requirement for phosphorylation of the cytoplasmic tail of Cot that we have uncovered seems unlikely to regulate interactions with p105, since Belich et al. were able to observe interactions between Tpl-2 and p105 even when the proteins were translated in vitro. It should be pointed out that although IKK activation is thought to lie upstream of RE/AP induction, this pathway might differ from classical NF-κB-dependent transcription (18, 22, 45). Previous studies are consistent with the RE/AP element being regulated by c-Rel rather than the classical RelA/p50 heterodimer (40). Clearly, the pathway lying between activation of Akt and Cot and inducible transcription regulated by the RE/AP element requires further investigation.
Recently the relevance of Cot for NF-κB induction has been called into question by the analysis of Cot-deficient mice (9). Although macrophages from these mice display decreased TNF-α production, no defect in NF-κB gel shift activity was seen, either in macrophages or in T cells. What could account for this discrepancy with the cell line experiments? It is likely that this is the result of redundancy with other MAP3Ks, which comprise a rather large family of homologous kinases (38). The kinase domain of Cot is highly related to those of other members of this family, especially MEKK1 and ASK1 (MEKK5). Indeed, recent studies indicate that Akt can complex with and phosphorylate ASK1 (19). At least two other members of the MAP3K family, MEKK1 and MLK3, have been implicated in NF-κB induction associated with T-cell activation (12, 44). Intriguingly, although MEKK1 to -3 are all capable of inducing NF-κB in HeLa cells, MLK3 is not (50), suggesting that there is cell type specificity in the pathways activated by various MAP3Ks. Finally, recent genetic data from mice and flies demonstrate that at least two members of the MAP3K family, MEKK3 and TAK1, are required for induction of NF-κB by certain stimulatory receptors (46, 48).
Regardless of the possible redundancy within this family, functions ascribed to overexpressed MAP3Ks may be biologically relevant for an additional reason. In the case of Cot, cellular transformation is associated with not only truncated forms of the protein that have increased kinase activity but also with overexpression (7, 10). In this regard it may be interesting to determine how the mutation of serine 400 in Cot affects the ability of the kinase to effect transformation. To date, no analysis of transformation by Cot has focused on the roles of NF-κB versus those of MAP kinases in this process.
Acknowledgments
We are grateful to the following individuals for providing reagents used in these studies: W. Greene, S. Ley, X. Lin, and D. Stokoe.
L.P.K. is the recipient of an Arthritis Foundation Investigator award. A.W. is an Investigator of the Howard Hughes Medical Institute. This work was also supported in part by the Rosalind Russell Medical Research Center for Arthritis.
REFERENCES
- 1.Alessi, D. R., F. B. Caudwell, M. Andjelkovic, B. A. Hemmings, and P. Cohen. 1996. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 399:333-338. [DOI] [PubMed] [Google Scholar]
- 2.Aoki, M., O. Batista, A. Bellacosa, P. Tsichlis, and P. K. Vogt. 1998. The Akt kinase: molecular determinants of oncogenicity. Proc. Natl. Acad. Sci. USA 95:14950-14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballester, A., R. Tobena, C. Lisbona, V. Calvo, and S. Alemany. 1997. Cot kinase regulation of IL-2 production in Jurkat T cells. J. Immunol. 159:1613-1618. [PubMed] [Google Scholar]
- 4.Ballester, A., A. Velasco, R. Tobena, and S. Alemany. 1998. Cot kinase activates tumor necrosis factor-alpha gene expression in a cyclosporin A-resistant manner. J. Biol. Chem. 273:14099-14106. [DOI] [PubMed] [Google Scholar]
- 5.Belich, M. P., A. Salmeron, L. H. Johnston, and S. C. Ley. 1999. TPL-2 kinase regulates the proteolysis of the NF-kappaB-inhibitory protein NF-kappaB1 p105. Nature 397:363-368. [DOI] [PubMed] [Google Scholar]
- 6.Bellacosa, A., J. R. Testa, S. P. Staal, and P. N. Tsichlis. 1991. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science 254:274-277. [DOI] [PubMed] [Google Scholar]
- 7.Ceci, J. D., C. P. Patriotis, C. Tsatsanis, A. M. Makris, R. Kovatch, D. A. Swing, N. A. Jenkins, P. N. Tsichlis, and N. G. Copeland. 1997. Tpl-2 is an oncogenic kinase that is activated by carboxy-terminal truncation. Genes Dev. 11:688-700. [DOI] [PubMed] [Google Scholar]
- 8.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 9.Dumitru, C. D., J. D. Ceci, C. Tsatsanis, D. Kontoyiannis, K. Stamatakis, J.-H. Lin, C. Patriotis, N. A. Jenkins, N. G. Copeland, G. Kollias, and P. N. Tsichlis. 2000. TNF-α induction by LPS is regulated posttranscriptionally via a Tpl-2/ERK-dependent pathway. Cell 103:1071-1083. [DOI] [PubMed] [Google Scholar]
- 10.Erny, K. M., J. Peli, J. F. Lambert, V. Muller, and H. Diggelmann. 1996. Involvement of the Tpl-2/cot oncogene in MMTV tumorigenesis. Oncogene 13:2015-2020. [PubMed] [Google Scholar]
- 11.Hagemann, D., J. Troppmair, and U. R. Rapp. 1999. Cot protooncoprotein activates the dual specificity kinases MEK-1 and SEK-1 and induces differentiation of PC12 cells. Oncogene 18:1391-1400. [DOI] [PubMed] [Google Scholar]
- 12.Hehner, S. P., T. G. Hofmann, A. Ushmorov, O. Dienz, I. Wing-Lan Leung, N. Lassam, C. Scheidereit, W. Droge, and M. L. Schmitz. 2000. Mixed-lineage kinase 3 delivers CD3/CD28-derived signals into the IκB kinase complex. Mol. Cell. Biol. 20:2556-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones, R. G., M. Parsons, M. Bonnard, V. S. Chan, W. C. Yeh, J. R. Woodgett, and P. S. Ohashi. 2000. Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X(L) levels in vivo. J. Exp. Med. 191:1721-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kane, L. P., P. G. Andres, K. C. Howland, A. K. Abbas, and A. Weiss. 2001. Akt provides the CD28 co-stimulatory signal for upregulation of IL-2 and IFN-γ but not Th2 cytokines. Nat. Immunol. 2:37-44. [DOI] [PubMed] [Google Scholar]
- 15.Kane, L. P., J. Lin, and A. Weiss. 2000. Signal transduction by the TCR for antigen. Curr. Opin. Immunol. 12:242-249. [DOI] [PubMed] [Google Scholar]
- 16.Kane, L. P., V. S. S. Shapiro, D. Stokoe, and A. Weiss. 1999. Induction of NF-κB by the Akt/PKB kinase. Curr. Biol. 9:601-604. [DOI] [PubMed] [Google Scholar]
- 17.Kang, S. W., M. I. Wahl, J. Chu, J. Kitaura, Y. Kawakami, R. M. Kato, R. Tabuchi, A. Tarakhovsky, T. Kawakami, C. W. Turck, O. N. Witte, and D. J. Rawlings. 2001. PKCbeta modulates antigen receptor signaling via regulation of Btk membrane localization. EMBO J. 20:5692-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khoshnan, A., S. J. Kempiak, B. L. Bennett, D. Bae, W. Xu, A. M. Manning, C. H. June, and A. E. Nel. 1999. Primary human CD4+ T cells contain heterogeneous I kappa B kinase complexes: role in activation of the IL-2 promoter. J. Immunol. 163:5444-5452. [PubMed] [Google Scholar]
- 19.Kim, A. H., G. Khursigara, X. Sun, T. F. Franke, and M. V. Chao. 2001. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol. Cell. Biol. 21:893-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovarik, P., M. Mangold, K. Ramsauer, H. Heidari, R. Steinborn, A. Zotter, D. E. Levy, M. Muller, and T. Decker. 2001. Specificity of signaling by STAT1 depends on SH2 and C-terminal domains that regulate Ser727 phosphorylation, differentially affecting specific target gene expression. EMBO J. 20:91-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, J., and A. Weiss. 2001. T cell receptor signalling. J. Cell Sci. 114:243-244. [DOI] [PubMed] [Google Scholar]
- 22.Lin, X., E. T. Cunningham, Y. Mu, R. Geleziunas, and W. C. Greene. 1999. The proto-oncogene Cot kinase participates in CD3/CD28 induction of NF-κB acting through the NF-κB-inducing kinase and IκB kinases. Immunity 10:271-280. [DOI] [PubMed] [Google Scholar]
- 23.Lin, X., Y. Mu, E. T. Cunningham, Jr., K. B. Marcu, R. Geleziunas, and W. C. Greene. 1998. Molecular determinants of NF-κB-inducing kinase action. Mol. Cell. Biol. 18:5899-5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madrid, L. V., M. W. Mayo, J. Y. Reuther, and A. S. Baldwin, Jr. 2001. Akt stimulates the transactivation potential of the RelA/p65 subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J. Biol. Chem. 276:18934-18940. [DOI] [PubMed] [Google Scholar]
- 25.Madrid, L. V., C. Y. Wang, D. C. Guttridge, A. J. Schottelius, A. S. Baldwin, Jr., and M. W. Mayo. 2000. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-κB. Mol. Cell. Biol. 20:1626-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makris, A., C. Patriotis, S. E. Bear, and P. N. Tsichlis. 1993. Genomic organization and expression of Tpl-2 in normal cells and Moloney murine leukemia virus-induced rat T-cell lymphomas: activation by provirus insertion. J. Virol. 67:4283-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malinin, N. L., M. P. Boldin, A. V. Kovalenko, and D. Wallach. 1997. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature 385:540-544. [DOI] [PubMed] [Google Scholar]
- 28.Miyoshi, J., T. Higashi, H. Mukai, T. Ohuchi, and T. Kakunaga. 1991. Structure and transforming potential of the human cot oncogene encoding a putative protein kinase. Mol. Cell. Biol. 11:4088-4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obata, T., M. B. Yaffe, G. G. Leparc, E. T. Piro, H. Maegawa, A. Kashiwagi, R. Kikkawa, and L. C. Cantley. 2000. Peptide and protein library screening defines optimal substrate motifs for AKT/PKB. J. Biol. Chem. 275:36108-36115. [DOI] [PubMed] [Google Scholar]
- 30.Ohara, R., J. Miyoshi, M. Aoki, and K. Toyoshima. 1993. The murine cot proto-oncogene: genome structure and tissue-specific expression. Jpn. J. Cancer Res. 84:518-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ouchi, T., S. W. Lee, M. Ouchi, S. A. Aaronson, and C. M. Horvath. 2000. Collaboration of signal transducer and activator of transcription 1 (STAT1) and BRCA1 in differential regulation of IFN-gamma target genes. Proc. Natl. Acad. Sci. USA 97:5208-5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozes, O. N., L. D. Mayo, J. A. Gustin, S. R. Pfeffer, L. M. Pfeffer, and D. B. Donner. 1999. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401:82-85. [DOI] [PubMed] [Google Scholar]
- 33.Patriotis, C., A. Makris, S. E. Bear, and P. N. Tsichlis. 1993. Tumor progression locus 2 (Tpl-2) encodes a protein kinase involved in the progression of rodent T-cell lymphomas and in T-cell activation. Proc. Natl. Acad. Sci. USA 90:2251-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patriotis, C., A. Makris, J. Chernoff, and P. N. Tsichlis. 1994. Tpl-2 acts in concert with Ras and Raf-1 to activate mitogen-activated protein kinase. Proc. Natl. Acad. Sci. USA 91:9755-9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romashkova, J. A., and S. S. Makarov. 1999. NF-kB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401:86-90. [DOI] [PubMed] [Google Scholar]
- 36.Salmeron, A., T. B. Ahmad, G. W. Carlile, D. Pappin, R. P. Narsimhan, and S. C. Ley. 1996. Activation of MEK-1 and SEK-1 by Tpl-2 proto-oncoprotein, a novel MAP kinase kinase kinase. EMBO J. 15:817-826. [PMC free article] [PubMed] [Google Scholar]
- 37.Salmeron, A., J. Janzen, Y. Soneji, N. Bump, J. Kamens, H. Allen, and S. C. Ley. 2001. Direct phosphorylation of NF-kappaB1 p105 by the IkappaB kinase complex on serine 927 is essential for signal-induced p105 proteolysis. J. Biol. Chem. 276:22215-22222. [DOI] [PubMed] [Google Scholar]
- 38.Schlesinger, T. K., G. R. Fanger, T. Yujiri, and G. L. Johnson. 1998. The Tao of MEKK. Front. Biosci. 3:1181-1186. [DOI] [PubMed] [Google Scholar]
- 39.Shan, X., M. J. Czar, S. C. Bunnell, P. Liu, Y. Liu, P. L. Schwartzberg, and R. L. Wange. 2000. Deficiency of PTEN in Jurkat T cells causes constitutive localization of itk to the plasma membrane and hyperresponsiveness to CD3 stimulation. Mol. Cell. Biol. 20:6945-6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shapiro, V. S., K. E. Truitt, J. B. Imboden, and A. Weiss. 1997. CD28 mediates transcriptional upregulation of the interleukin-2 (IL-2) promoter through a composite element containing the CD28RE and NF-IL-2B AP-1 sites. Mol. Cell. Biol. 17:4051-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shinkura, R., K. Kitada, F. Matsuda, K. Tashiro, K. Ikuta, M. Suzuki, K. Kogishi, T. Serikawa, and T. Honjo. 1999. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-kappa b-inducing kinase. Nat. Genet. 22:74-77. [DOI] [PubMed] [Google Scholar]
- 42.Tsatsanis, C., C. Patriotis, S. E. Bear, and P. N. Tsichlis. 1998. The Tpl-2 protooncoprotein activates the nuclear factor of activated T cells and induces interleukin 2 expression in T cell lines. Proc. Natl. Acad. Sci. USA 95:3827-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsatsanis, C., C. Patriotis, and P. N. Tsichlis. 1998. Tpl-2 induces IL-2 expression in T-cell lines by triggering multiple signaling pathways that activate NFAT and NF-kappaB. Oncogene 17:2609-2618. [DOI] [PubMed] [Google Scholar]
- 44.Tuosto, L., A. Costanzo, F. Guido, B. Marinari, S. Vossio, F. Moretti, M. Levrero, and E. Piccolella. 2000. Mitogen-activated kinase kinase kinase 1 regulates T cell receptor-and CD28-mediated signaling events which lead to NF-kB activation. Eur. J. Immunol. 30:2445-2454. [DOI] [PubMed] [Google Scholar]
- 45.Venkataraman, L., W. Wang, and R. Sen. 1996. Differential regulation of c-Rel translocation in activated B and T cells. J. Immunol. 157:1149-1155. [PubMed] [Google Scholar]
- 46.Vidal, S., R. S. Khush, F. Leulier, P. Tzou, M. Nakamura, and B. Lemaitre. 2001. Mutations in the Drosophila dTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-kappaB-dependent innate immune responses. Genes Dev. 15:1900-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamada, T., T. Mitani, K. Yorita, D. Uchida, A. Matsushima, K. Iwamasa, S. Fujita, and M. Matsumoto. 2000. Abnormal immune function of hemopoietic cells from alymphoplasia (aly) mice, a natural strain with mutant NF-kappa B-inducing kinase. J. Immunol. 165:804-812. [DOI] [PubMed] [Google Scholar]
- 48.Yang, J., Y. Lin, Z. Guo, J. Cheng, J. Huang, L. Deng, W. Liao, Z. Chen, Z. Liu, and B. Su. 2001. The essential role of MEKK3 in TNF-induced NF-kappaB activation. Nat. Immunol. 2:620-624. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, J. J., Y. Zhao, B. T. Chait, W. W. Lathem, M. Ritzi, R. Knippers, and J. E. Darnell, Jr. 1998. Ser727-dependent recruitment of MCM5 by Stat1alpha in IFN-gamma-induced transcriptional activation. EMBO J. 17:6963-6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao, Q., and F. S. Lee. 1999. Mitogen-activated protein kinase/ERK kinase kinases 2 and 3 activate nuclear factor-kappaB through IkappaB kinase-alpha and IkappaB kinase-beta. J. Biol. Chem. 274:8355-8358. [DOI] [PubMed] [Google Scholar]