Abstract
c-Myc promotes apoptosis by destabilizing mitochondrial integrity, leading to the release of proapoptotic effectors including holocytochrome c. Candidate mediators of c-Myc in this process are the proapoptotic members of the Bcl-2 family. We show here that fibroblasts lacking Bak remain susceptible to c-Myc-induced apoptosis whereas bax-deficient fibroblasts are resistant. However, despite this requirement for Bax, c-Myc activation exerts no detectable effects on Bax expression, localization, or conformation. Moreover, susceptibility to c-Myc-induced apoptosis can be restored in bax-deficient cells by ectopic expression of Bax or by microinjection of a peptide comprising a minimal BH3 domain. Microinjection of BH3 peptide also restores sensitivity to c-Myc-induced apoptosis in p53-deficient primary fibroblasts that are otherwise resistant. By contrast, there is no synergy between BH3 peptide and c-Myc in fibroblasts deficient in both Bax and Bak. We conclude that c-Myc triggers a proapoptotic mitochondrial destabilizing activity that cooperates with proapoptotic members of the Bcl-2 family.
Expression of c-Myc sensitizes cells to many mechanistically diverse apoptotic stimuli that a tumor cell might encounter, including hypoxia, genotoxic stress, nutrient deprivation, and death receptor signaling. Substantial evidence supports the notion that such sensitization to apoptosis acts as a significant restraint to the oncogenic potential of c-Myc (10, 39, 44). Two proapoptotic effector pathways have been identified downstream of c-Myc, both of which converge on the mitochondrion. One involves up-regulation of p53 through the ARF/Mdm-2 pathway (56), whereupon p53 downstream proapoptotic effectors, such as the proapoptotic Bcl-2 family members Bax (17, 34), PUMA (35, 55), and Noxa (38), promote release of holocytochrome c from the mitochondria. A separate pathway involves the direct, p53-independent destabilization of mitochondrial integrity through an undefined mechanism, also with consequent release of holocytochrome c (24). Both of these apoptotic pathways share Apaf-1 and caspase 9 as final apoptotic effectors downstream of the mitochondrion. Inhibition of this mitochondrial pathway, either by suppression of holocytochrome c release by survival signals (19) or Bcl-2/Bcl-xL proteins (2, 13, 49, 51) or by incapacity of the downstream mitochondrial apoptotic effector pathway through genetic loss of Apaf-1 or caspase 9 (47), inhibits c-Myc-induced apoptosis and promotes c-Myc oncogenicity.
The best-characterized modulators of mitochondrial apoptotic function are the members of the Bcl-2 family. Both the Bcl-2/Bcl-xL apoptosis suppressors and the Bax/Bak/Bok apoptosis inducers modulate mitochondrial outer membrane integrity, possibly through their interactions with structural components of the mitochondria such as the mitochondrial porin (46) and the adenine nucleotide translocator (30). BH3-only proteins are more distant members of the Bcl-2 family that heterodimerize with, and antagonize, the protective Bcl-2/Bcl-xL proteins, most likely through interaction between their eponymous Bcl-2 homology (BH) 3 domain and the hydrophobic cleft formed by the conserved BH1, BH2, and BH3 domains in Bcl-2/Bcl-xL proteins (42). Unlike the multidomain conserved proapoptotic family members Bax or Bak, BH3-only proteins lack innate cytotoxic activity, and the presence of either Bax or Bak is essential for induction of apoptosis by these proteins (52, 57). This indicates that BH3-only proteins promote apoptosis by quelling the protective action of Bcl-2/Bcl-xL and/or promoting the proapoptotic assembly and function of the Bax/Bak killers (3).
In general terms, the relative abundance of active proapoptotic versus antiapoptotic Bcl-2 proteins appears to establish the innate susceptibility of the mitochondrial death pathway to be triggered. Superimposed on this, however, are multiple mechanisms that regulate the activities of individual Bcl-2 family members. For example, Bax proapoptotic action is in great part modulated by factors affecting its localization to mitochondria (53), whereas most BH3-only proteins serve as terminal apoptotic effectors of specific proapoptotic or antiapoptotic signaling pathways (23). Thus, ligation of death receptors activates caspase 8, which cleaves and activates the proapoptotic activity of Bid, triggering a feed-forward amplifying loop that recruits the mitochondrial apoptotic pathway into death receptor signaling. The activity of Bad is modulated by signaling through the Akt/PKB survival signaling pathway that phosphorylates Bad and triggers its inactivation by sequestration (5). Similarly, integrin-mediated survival pathways regulate the exposure of the BH3 domain of Bax (14), while Bim is bound to the LC8 cytoplasmic dynein light chain that sequesters it to the microtubule-associated dynein motor complex until it is released in response to multiple apoptotic stimuli (41).
Given the dramatic effects of c-Myc activation on mitochondrial integrity, the proapoptotic Bax/Bak/BH3 proteins are plausible candidate Myc proapoptotic effectors. Indeed, Bax has been shown to play an important part in the p53-dependent apoptotic pathway induced via ARF/MDM2 that suppresses oncogenic transformation by both c-Myc (56) and E1A (7, 32). Bax is a direct transcriptional target of p53 in humans (17, 34) (but not in mice [43]), is required for effective p53-induced apoptosis in response to chemotherapy (32), and is directly implicated in mitochondrial damage induced by enforced expression of p53 (45). However, up-regulation of Bax is not the sole determinant of p53-induced apoptosis, and other p53-induced proteins, such as PERP and the BH3 proteins Noxa (38) and PUMA (35, 55), often correlate more closely than Bax with p53-induced apoptosis (1, 38). Moreover, Bax is also induced in many situations where p53 activation triggers growth arrest without cell death (1). More recently, Bax has also been implicated as a direct proapoptotic effector in the c-Myc-induced mitochondrial pathway. The Bax promoter possesses two consensus Myc box binding sites, and bax-deficient mouse embryo fibroblasts (MEFs) demonstrate significant resistance to c-Myc-induced apoptosis in low-serum conditions (33).
In the present study, we explore the relationship between c-Myc-induced apoptosis and proapoptotic Bcl-2 family members. We confirm that bax-deficient MEFs are resistant to c-Myc-induced apoptosis but show that activation of c-Myc has no effect on Bax levels, conformation, or localization. Moreover, we demonstrate that susceptibility to c-Myc-induced apoptosis can be restored in bax-deficient cells by ectopic expression of Bax or Bak driven by a heterotypic promoter or by introduction of a minimal generic BH3 peptide that acts to antagonize the antiapoptotic Bcl-2 family members. This minimal BH3 peptide also restores c-Myc-induced apoptosis to p53-deficient cells that are otherwise resistant. Our data favor a model in which Myc and the BH3 peptide provide cooperative functions that promote cell death through a mechanism dependent on the presence of either Bax or Bak.
MATERIALS AND METHODS
Reagents and antibodies.
The antibodies used in this study were as follows: monoclonal anti-cytochrome antibody 6H2B4 (PharMingen, San Diego, Calif.), polyclonal anti-Bax antibody TL41 (raised against the BH3 domain of Bax, residues 57 to 72), polyclonal anti-Bid antibody (raised against the BH3 domain of Bid, residues 84 to 99), anti-Bak antibody (Becton Dickinson Transduction Labs), polyclonal anti-Bax-NT N-terminal-specific antibody (Upstate Biotechnology). Horseradish peroxidase-conjugated antibodies and enhanced chemiluminescence reagents were obtained from Amersham, Little Chalfont, United Kingdom. Alexa488- and Alexa568-conjugated secondary antibodies were obtained from Molecular Probes. Our use of 4-hydroxytamoxifen (OHT) to activate the OHT conditional allele of c-Myc (c-MycERTAM) and of zVADfmk to inhibit caspases has been described previously (24). Unless otherwise indicated, all other reagents used in this study were obtained from Sigma.
Cells and cell culture techniques.
Rat-1 fibroblasts expressing c-MycERTAM (Rat-1/c-MycERTAM) were as described previously (29). MEFs derived from bid−/− (54), bax−/− (32), p53−/−/bax−/− (32), and bax−/−/bak−/− (28) mice were as previously described. The bak−/− mice were kindly provided by Craig Thompson, University of Pennsylvania. Mice with a targeted disruption in the p53 gene were a kind gift of Larry Donehower. MEFs were prepared as described previously (25) and were cultured in Dulbecco's modified Eagle medium supplemented with 20% heat-inactivated fetal calf serum (FCS), 2 mM glutamine, and 0.1 mM nonessential amino acids. Presenescent MEFs maintained on a 3T9 protocol (56) were used throughout this study.
Retrovirus infection was performed as described previously (7) by using high-titer ecotropic retrovirus supernatants generated by transient transfection with the Phoenix retrovirus packaging system (G. Nolan, Stanford University, Stanford, Calif.). The retrovirus vectors used were as follows: pBabepuro (pBp), a control vector expressing puromycin phosphotranferase; pBp(c-MycERTAM), a c-Myc-ERTAM cDNA in pBp (29); and pBp(BAX), full-length Baxα cDNA in pBp. Homogeneous populations of cells expressing each transgene were isolated by selection for 2 days in 2 μg of puromycin/ml. Expression of transgene-encoded protein products was confirmed by immunoblotting and immunocytochemical staining.
Peptides and recombinant proteins.
The following peptides purified by high-performance liquid chromatography were used in this study: Bax-BH3, KKLSECLKRIGDELDS; Bax-BH3L63A, KKLSECAKRIGDELDS; Bak-BH3, GQVGRQLAIIGDDINR; and Bid-BH3, RNIARHLAQVGDSMDR.
Bcl-xL(ΔTM) cDNA (encoding Bcl-xL with a C-terminal deletion comprising amino acids 211 to 232) was subcloned into the pGEX-2T bacterial expression vector, and expression of recombinant glutathione S-transferase (GST)-Bcl-xL(ΔTM) or of GST alone was induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside to bacterial cultures. Cells were harvested 1 h later and lysed by sonication in 50 mM Tris (pH 8.0), 200 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 10 μM E64, and 10 μg of aprotinin/ml. Igepal (Sigma) was added to the lysates to a final concentration of 0.1% (vol/vol). GST and GST-Bcl-xL(ΔTM) were isolated by binding to glutathione Sepharose (Amersham) and were eluted from the matrix in detergent-free lysis buffer containing 10 mM glutathione. Recombinant proteins were dialyzed against phosphate-buffered saline (PBS) and stored at −70°C.
Biochemical techniques.
For GST pull-down assays, Rat-1/c-MycERTAM cells were scraped into ice-cold PBS, pelleted by centrifugation, and lysed in Triton lysis buffer (1% [vol/vol] Triton X-100, 20 mM HEPES [pH 7.4], 200 mM NaCl, 2 mM EGTA, 10% [vol/vol] glycerol, 1 mM Na3VO4, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 10 μM E64, 10 μg of aprotinin/ml). GST or GST-Bcl-xL(ΔTM) was prebound to glutathione Sepharose beads by incubation in lysis buffer for 1 h at 4°C, washed three times in lysis buffer, and then incubated with cell lysates for 3 h at 4°C. Protein complexes were then centrifuged at 13,000 × g for 5 min at 4°C, washed three times in lysis buffer, eluted with sodium dodecyl sulfate (SDS) sample buffer, fractionated by SDS-polyacrylamide gel electrophoresis (PAGE), and analyzed by immunoblotting. Subcellular fractions were generated as described previously (24).
Immunocytochemistry.
Immunocytochemical staining of cells was performed as described previously (15). The primary monoclonal anti-cytochrome antibody, 6H2B4, and polyclonal anti-Bax antibody TL41 were used at dilutions of 1:200 and 1:1,000, respectively. Both anti-rabbit and anti-mouse secondary antibodies were used at a 1:200 dilution. Images were acquired as described below.
Microinjection experiments.
For both nuclear and cytoplasmic injections, cells were seeded on glass-bottomed coverslip dishes (Matek Corp., Ashland, Oreg.) and microinjected by using sterile microcapillaries (Femtotips II; Eppendorf) mounted on an automated microinjection system as described previously (24). Identical, standardized conditions of pressure (100 hPa for cytoplasmic injection and 150 hPa for nuclear injection) and time (0.1 s) were used in all experiments. For cytoplasmic microinjections, peptides and/or recombinant proteins were dissolved in PBS together with dextran (10 kDa)-conjugated lysine-fixable Oregon Green (0.5% final concentration; Molecular Probes) as a coinjection marker. Typically, 100 cells were microinjected for each condition in each experiment. Calibration assays indicated that a 2- to 5-pl volume was delivered to each cell (unpublished results). The percentage of positive (i.e., fluorescent) cells exhibiting morphological features of apoptosis was evaluated by fluorescence microscopy as described previously (24). For nuclear microinjection studies, plasmid DNA was purified on Qiagen columns according to the manufacturer's instructions. DNA comprising empty expression vector pcDNA3, pcDNA3(c-Myc) (kindly provided by E. Verschuren), or pcDNA3(Bak) (a kind gift of Pierre François Cartron) at a final concentration of 100 ng/μl was mixed with pEGFP-N1 (20 ng/μl) as a coinjection marker.
Fluorescence microscopy.
Unless otherwise indicated, the images presented in this study were recorded by using a ×32 Plan Achromatic objective on a Zeiss inverted fluorescence microscope (Axiovert 100TV) with a charge-coupled device camera (Hammamatsu Orca C-7525) controlled by a Macintosh G3 computer.
RESULTS
Requirement for Bax in c-Myc-induced apoptosis of fibroblasts.
A major proapoptotic effector pathway triggered by c-Myc involves the release of holocytochrome c from mitochondria. This release, and the apoptosis that ensues, are potently inhibited by survival factors and antiapoptotic members of the Bcl-2 family, both of which inhibit Myc-induced apoptosis and dramatically enhance c-Myc oncogenic potential. Proapoptotic relatives of Bcl-2 are prominently implicated in the release of mitochondrial holocytochrome c and are hence candidate effectors of Myc-induced apoptosis. The recent identification of canonical Myc/Max binding sites in the bax promoter and the reported resistance to Myc-induced apoptosis in bax-deficient MEFs together suggest a specific role for Bax as a key apoptotic effector of c-Myc (33).
To assess whether known proapoptotic Bcl-2 family members play any role in Myc-induced apoptosis, we began by determining whether c-Myc-induced apoptosis is compromised in serum-deprived embryo fibroblasts derived from mice in which Bax or two other well-characterized proapoptotic Bcl-2 family members, Bak and Bid, had been genetically eliminated. Nuclei of such MEFs were microinjected with calibrated quantities of a plasmid encoding c-Myc (pcDNA3c-Myc) together with a plasmid directing expression of green fluorescent protein (GFP) to mark successfully microinjected cells. Insignificant apoptosis was observed in GFP-positive MEFs injected with either empty vector or pcDNA3c-Myc and maintained in high-serum conditions (data not shown), conditions in which c-Myc by itself is insufficient to induce either cytochrome c release or apoptosis. Immunocytochemical analysis (data not shown) demonstrated that the pcDNA3c-Myc plasmid induced significant levels of c-Myc expression in all microinjected fibroblasts. Following microinjection, MEFs were then deprived of serum, and cell viability was assayed after 24 h. As shown in Fig. 1A, serum deprivation of cells microinjected with c-Myc plasmid led to significant loss of viability in wild-type MEFs, whereas microinjection with the vector alone caused negligible cell death. In bid−/− MEFs, the extent and rate of induction of c-Myc-induced apoptosis was essentially identical to that in wild-type MEFs, suggesting that Bid plays no obligate role in c-Myc induction of apoptosis (Fig. 1A). This is of interest given the established role of Bid in coupling Fas signaling to mitochondria and the published requirement of Fas signaling in c-Myc-induced apoptosis of rodent fibroblasts (22). Similarly, the absence of Bak had no discernible inhibitory effect on Myc-induced apoptosis. By contrast, the absence of the close Bak homologue Bax conferred dramatic resistance to the induction of cell death following nuclear microinjection of pcDNA3c-Myc (Fig. 1A). An analogous apparent dependency of c-Myc killing on Bax was also observed when a retrovirus vector was used to drive expression of OHT-dependent c-MycERTAM in bax−/− MEFs (data not shown). Thus, efficient induction of apoptosis by c-Myc in serum-deprived MEFs requires expression of Bax, supporting the published notion that Bax is a critical proapoptotic Myc effector. However, infection of bax−/− MEFs with a recombinant retrovirus vector directing constitutive expression of Bax, though having little discernible effect on cell viability on its own, effectively restored apoptosis following c-Myc activation (Fig. 1B). This implies that Bax expression is required for c-Myc-induced apoptosis even when bax is not a transcriptional target of c-Myc.
FIG. 1.
c-Myc-induced apoptosis in fibroblasts requires Bax but not Bid or Bak. (A) MEFs of the indicated genotypes were grown in 20% heat-inactivated FCS and were microinjected with either empty pcDNA3 or pcDNA3(Myc) (100 ng/ml) mixed in RNase-free water with pEGFP-N1 (20 ng/ml) indicator as described in Materials and Methods. Eighteen hours after microinjection, the number of GFP-expressing cells was evaluated by fluorescence microscopy; cells were then washed twice in PBS and cultured in the absence of FCS for an additional 24 h at 37°. The number of GFP-expressing cells still viable was then determined. Cell loss or cell gain was expressed as a percentage of the initial number of GFP-positive cells prior to serum deprivation. Data presented are mean values ± standard errors of the means (SEM) from the indicated number of independent experiments. wt, wild type. Insert: MEFs with genes knocked out were validated for loss of relevant BH3 protein by immunoblot analysis. Lysates of wild-type MEFs and bid−/−, bax−/−, or bak−/− MEFs were fractionated by SDS-PAGE (105 cells per lane) and immunoblotted with anti-Bid, anti-Bax, or anti-Bak antibodies as indicated. (B) bax−/− MEFs were infected with either pBp(Bax) or retrovirus vector alone, and their sensitivities to c-Myc-induced apoptosis were assayed by microinjection with either empty pcDNA3 or pcDNA3(Myc) mixed with pEGFP-N1 indicator as described above. Data presented are mean values ± SEM from the indicated number of independent experiments. For each type of infected cell, each experiment comprised two paired observations, with either pcDNA3 or pcDNA3(Myc) plasmid DNA being injected within distinct areas of the same cell population seeded on glass coverslips the previous day. For each type of infected cell and each type of microinjected plasmid, the average number of GFP-positive cells prior to serum deprivation is indicated in brackets.
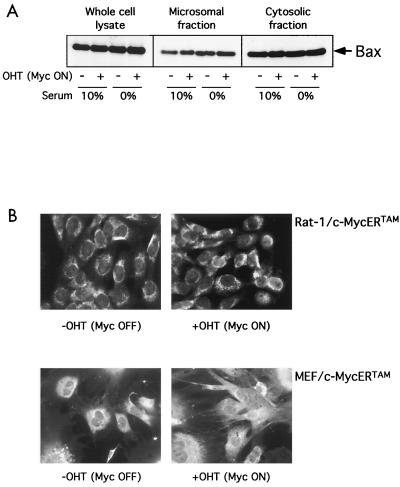
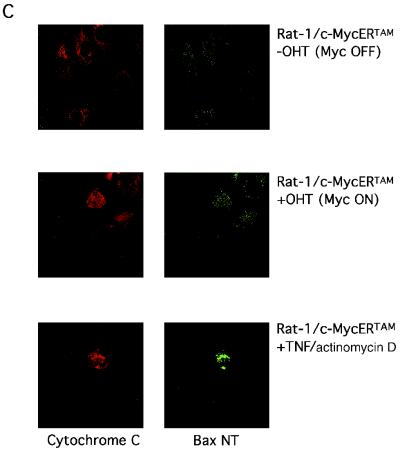
To further explore the Myc proapoptotic effector role for Bax, we examined the effect of c-Myc activation on Bax protein levels, localization, and conformation under conditions where c-Myc activation induces apoptosis. Rat-1/c-MycERTAM were either serum-deprived or maintained in 10% serum for 24 h as a control, after which c-Myc was activated by the addition of 100 nM OHT to the cell culture medium for 6 h. Bax levels were then determined by immunoblotting in whole-cell extracts as well as in a subcellular heavy membrane fraction containing mitochondria and its corresponding postmitochondrial cytosolic supernatant fraction. We observed no detectable change in Bax levels in whole cells or in either subcellular fraction (Fig. 2A) following c-Myc activation, even though significant loss of mitochondrial holocytochrome c (an early event of c-Myc-induced apoptosis) had occurred in serum-deprived cells by this time (24). This confirms our earlier conclusion that c-Myc does not need to transcriptionally induce Bax to induce apoptosis. Next, using immunocytochemical analysis, we assayed Bax levels and subcellular localization in serum-deprived Rat-1/c-MycERTAM and MEFs expressing c-MycERTAM. After 8 h of c-Myc activation in low-serum conditions, we could detect no change either in overall Bax immunofluorescence or in its subcellular distribution (Fig. 2B). It has recently been reported that in response to some apoptotic stimuli, Bax undergoes a conformational change upon its activation to a proapoptotic effector that can be detected with an N-terminal antibody (40). To assess whether such a change in Bax conformation is required for c-Myc to initiate apoptosis, we incubated appropriate permeabilized fibroblasts with the conformation-specific antibody as well as with a cytochrome c-specific antibody to assay for cytochrome c localization in the same cells. We observed no significant change in antibody binding to Bax following 24 h of OHT treatment in Rat-1/c-MycERTAM cultured under low-serum conditions, even in cells that exhibited significant cytochrome c release and in which apoptosis had been initiated (Fig. 2C). Similarly, we observed no change in antibody binding to Bax in permeabilized MEFs expressing c-MycERTAM and treated with OHT (data not shown). In sharp contrast, treatment of either Rat-1/c-MycERTAM cells (Fig. 2C) or MEFs expressing c-MycERTAM (data not shown) with a combination of tumor necrosis factor (TNF) and actinomycin D induced a dramatic increase in binding by the conformation-specific Bax antibody.
FIG. 2.
Activation of c-Myc induces apoptosis but has no effect on Bax expression, localization, or conformation. (A) Rat-1/c-MycERTAM cells were either serum deprived for 24 h or maintained in 10% FCS prior to stimulation with either 100 nM OHT or ethanol vehicle control for 6 h. Cells were then harvested and either lysed directly or biochemically fractionated into cytosolic and heavy membrane fractions prior to solubilization in SDS sample buffer, as described in Materials and Methods. Forty micrograms of heavy membrane fraction, 20 μg of cytosolic fraction, or 20 μg of whole-cell lysate was thenfractionated by SDS-PAGE and immunoblotted with anti-Bax antibody (see Materials and Methods). (B) Rat-1/c-MycERTAM cells (upper panels) serum deprived for 48 h or MEFs infected with pBp(c-MycERTAM) (lower panels) and serum deprived for 24 h were either left untreated (left panels) or treated with OHT for 8 h (right panels) prior to fixation and immunostaining with polyclonal Bax (57-72) antibody as described in Materials and Methods. Bar = 20 μM. (C) Rat-1/c-MycERTAM cells were serum deprived for 48 h prior to treatment with OHT for an additional 24 h. Cells were then fixed and immunostained by using polyclonal Bax NT antibody (green) and monoclonal anti-cytochrome c antibody (red). As a positive control for altered Bax conformation during apoptosis, Rat-1/c-MycERTAM cells grown in 10% FCS in the absence of OHT were either treated with human recombinant TNF alpha (5 ng/ml) and actinomycin D (2 μg/ml) or left untreated for 20 h prior to fixation and immunostaining with polyclonal Bax NT antibody and monoclonal anti-cytochrome c antibody. Images were collected on a Leica TCS NT confocal microscope with a 63 × 1.4 PL Apo objective (Leica, Rueil-Malmaison, France).
In summary, although both the reported presence of Myc binding sites in the bax promoter and the clear requirement for functional Bax in Myc-induced apoptosis suggest that Bax may be a transcriptional proapoptotic effector target of Myc that acts as its critical proapoptotic effector, we found no evidence for any change in Bax expression, localization, or conformation under circumstances where c-Myc potently induces both cytochrome c release from mitochondria and apoptosis.
One possible explanation for these ostensibly paradoxical results is that Bax is not a c-Myc transcriptional target effector; instead, c-Myc merely requires a minimal level of mitochondrial Bax activity to promote efficient release of holocytochrome c. This idea is the most consistent with our earlier observation that ectopic expression of Bax restores the sensitivity of bax-deficient cells to c-Myc-induced apoptosis even when Bax expression is driven by a heterologous promoter (see above). Thus, c-Myc might promote the proapoptotic action of Bax or, alternatively, might negate the action of the antiapoptotic Bcl-2 family members that normally serve to restrain the lethal actions of Bax.
To further examine these possibilities, we next asked whether c-Myc activation can still potentiate apoptosis in cells in which the protective members of the Bcl-2 family had been incapacitated. To do this, we made use of a minimal BH3 domain synthetic peptide (Bax-BH3), which comprised the 16 residues of the defined Bax BH3 domain (Bax-BH3 [57-72]). Such BH3 peptides have been shown to induce cytochrome c release and activation of caspases in a Xenopus laevis cell-free system (4). As a control, we used a defective point variant of the Bax-BH3 peptide (Bax-BH3L63A) in which an alanine had been substituted for the critical leucine at position 63. This peptide possesses no proapoptotic activity (20). In addition, all analyses with this Bax peptide were reproduced with two other analogous BH3 peptides corresponding to the minimal BH3 domains of either of the two proapoptotic Bcl-2 family members, Bak (residues 72 to 87) or Bid (residues 84 to 99).
Bax-BH3 peptide microinjection inhibits Bcl-xL function.
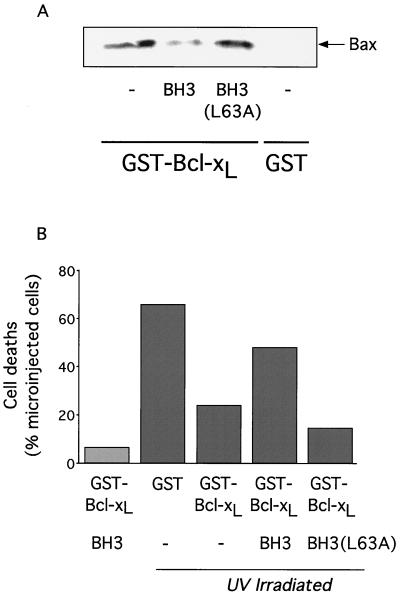
We first assessed the effect of the BH3 peptide on the interaction between the death suppressor Bcl-xL and the death promoter Bax by assaying the ability of a recombinant GST-Bcl-xL fusion protein to bind endogenous Bax in the presence or absence of peptide. As shown in Fig. 3A, the addition of 200 μM BH3 peptide resulted in a marked decrease in the amount of Bax associated with GST-Bcl-xL. In contrast, the addition of the same concentration of the BH3L63A variant had no effect on the interaction between GST-Bcl-xL and Bax. In no case did we observe any interaction between GST alone and Bax.
FIG. 3.
Inhibition of Bcl-xL antiapoptotic function by Bax-BH3 peptide. (A) Bax-BH3 peptide inhibits interaction between GST-Bcl-xL and Bax in vitro. Rat-1/c-MycERTAM fibroblasts grown in 10% FCS in the absence of OHT were lysed as described in Materials and Methods. Cell lysates were incubated with glutathione Sepharose beads prebound with either GST or GST-Bcl-xL(ΔTM) in the presence or absence of 200 μM Bax-BH3 peptide or Bax-BH3L63A mutant peptide as indicated. Glutathione Sepharose beads were then washed, bound polypeptides were eluted with SDS sample buffer and fractionated by SDS-PAGE, and the presence of endogenous Bax was assessed by immunoblotting with anti-Bax antibody. (B) Bax-BH3 peptide inhibits the antiapoptotic action of Bcl-xL in intact cells. Either GST (0.2 mg/ml) or GST-Bcl-xL(ΔTM) (0.2 mg/ml) in PBS were mixed with the fluorescent microinjection marker Oregon Green dextran (0.5%, wt/vol) in the presence or absence of either Bax-BH3 peptide (0.2 mg/ml) or Bax-BH3L63A mutant peptide (0.2 mg/ml) and then microinjected into Rat-1/c-MycERTAM fibroblasts grown in 10% FCS in the absence of OHT. Thirty minutes following microinjection, cells were washed once in PBS and subjected to 25 mJ of UV irradiation (Stratalinker UV cross-linker; Stratagene, La Jolla, Calif.) in the absence of medium, followed by incubation at 37°C for 6 h in normal growth medium. The viability of microinjected cells was then assessed by fluorescence microscopy as described in Materials and Methods. The data presented are from one experiment that is representative of four independent experiments. Each experiment comprised five observations; GST, GST-Bcl-xL, GST-Bcl-xL/Bax-BH3, and GST-Bcl-xL/Bax-BH3L63A solutions were injected within distinct areas of the same cell population seeded on glass coverslips 24 h prior to UV treatment. The same microcapillary loaded with GST-Bcl-xL/Bax-BH3 was used to microinject the cell population that was not treated with UV.
We next used microinjection to directly assess the ability of the BH3 peptide to antagonize the antiapoptotic function of Bcl-xL in intact cells. GST-Bcl-xL was mixed with the inert fluorescent marker Oregon Green dextran and microinjected into Rat-1 fibroblasts in the presence or absence of either the BH3 or BH3L63A peptide. Cells were then subjected to 25 mJ of UV irradiation, and subsequent cell deaths in the microinjected populations were monitored by fluorescence microscopy. Microinjection of GST-Bcl-xL (0.2 mg/ml) significantly enhanced the survival of UV-treated cells compared with cells microinjected with GST alone (Fig. 3B). Such enhanced survival was reversed following microinjection of BH3 peptide (0.2 mg/ml) but not in cells microinjected with the same amount of BH3L63A variant peptide. In the absence of UV irradiation, coinjection of GST-Bcl-xL and the BH3 peptide induced negligible apoptosis (Fig. 3B), indicating that at this concentration the only apparent action of the BH3 peptide is to counteract the protective effect of Bcl-xL against the UV insult. Taken together, these data demonstrate that the BH3 peptide acts as a functional antagonist of Bcl-xL in intact cells.
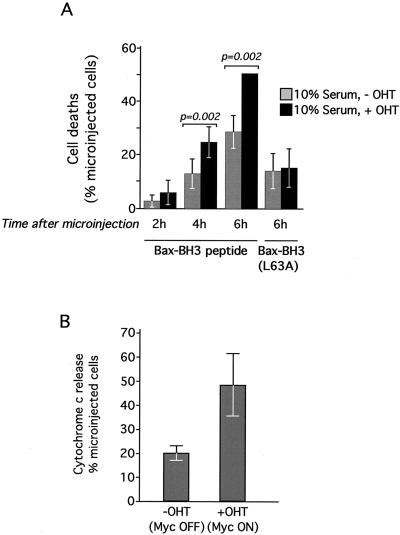
We next assessed the innate proapoptotic activity of the BH3 peptide in Rat-1 fibroblasts. Microinjection of BH3 peptide induced apoptosis in a dose-dependent manner: 10 μg of peptide/ml triggered more widespread apoptosis over a given time than did 2 μg of peptide/ml. However, doses of BH3 peptide above 5 μg/ml led to no further exacerbation of cell death (Fig. 4A). We estimated the volume delivered per cell by the calibrated microinjection capillaries to be approximately 5 pl (data not shown). This indicates that the proapoptotic function of the BH3 peptide saturates at around 8 × 106 molecules of peptide per cell. zVADfmk at 100 μM had no significant effect on the onset of apoptosis induced by Bax-BH3 peptide as assessed by membrane blebbing (Fig. 4A). This is consistent with previously presented data indicating that proapoptotic Bcl-2 family members can trigger zVAD-independent cytotoxicity (31). Interestingly, even cells microinjected at the same time with the same amount of peptide exhibited asynchronous cytochrome c release (Fig. 4B) and apoptosis, suggesting the existence of additional variables and factors that determine the kinetics of BH3 killing.
FIG. 4.
Induction of cell death by Bax-BH3 peptide in intact cells. (A) Induction of cell death by Bax-BH3 peptide is titratable. Bax-BH3 peptide at the indicated concentrations was mixed with 0.5% (wt/vol) Oregon Green dextran in PBS and microinjected into Rat-1/c-MycERTAM fibroblasts, which were grown in 10% FCS in the absence of OHT. Cells were then incubated at 37°C, and the deaths of microinjected cells were assessed morphologically by fluorescence microscopy at the indicated times. Where indicated, zVADfmk was added to a final concentration of 100 μM 2 h prior to microinjection. (B) Bax-BH3 peptide triggers asynchronous release of cytochrome c from mitochondria. Bax-BH3 peptide (1 mg/ml) mixed with 0.5% (wt/vol) Oregon Green dextran in PBS was microinjected into Rat-1/c-MycERTAM fibroblasts cultured in the absence of OHT. Cells were then incubated for 5 h at 37°C prior to fixation and immunostaining with anti-cytochrome c antibody. Cells were analyzed by fluorescence microscopy. Left panel, an overlay of phase contrast and green fluorescence allows identification of microinjected cells; right panel, cyto-chrome c immunostaining of the same cells. Bar = 20 μM. This frame is representative of at least four experiments. (C) Dependence of Bax-BH3 peptide-induced cell death on endogenous Bax/Bak. Wild-type (wt) and knockout MEFs of the indicated genotypes were microinjected with either Bax-BH3 (2 mg/ml) or Bax-BH3L63A (2 mg/ml) as indicated, and cell viability was assessed as described above.
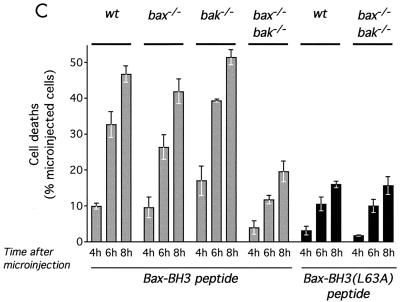
Induction of apoptosis by BH3-only proteins requires the presence of either Bax or Bak (52, 57). In order to test whether this were also true of BH3 peptide-induced apoptosis, we investigated whether BH3 peptide-induced apoptosis is compromised in embryo fibroblasts derived from mice in which Bax, Bak, or both had been genetically eliminated. As shown in Fig. 4C, wild-type, bax−/−, and bak−/− MEFs exhibited similar sensitivities to BH3 peptide-induced apoptosis. In sharp contrast, BH3 peptide microinjection induced only background apoptosis in bax−/−/bak−/− double-knockout MEFs that was similar to that induced upon microinjection of the inactive Bax-BH3L63A variant peptide (Fig. 4C). Taken together, these studies indicate that the specific proapoptotic function of the BH3 peptide, like that of intact BH3-only proteins, requires the presence of one or the other of the multidomain proapoptotic Bcl-2 family members, Bax and Bak.
c-Myc synergizes with BH3 peptide to induce apoptosis in fibroblasts.
Having validated the minimal BH3 peptide as an inhibitor of antiapoptotic Bcl-2 family member (protector) function, we next sought to investigate the relationship between c-Myc and BH3 in the induction of apoptosis. We reasoned that if the sole apoptosis-promoting effect of c-Myc is to incapacitate protectors, then activation of c-Myc should have no effect on cells loaded with saturating levels of BH3 peptide in which those protectors had already been quenched. However, should c-Myc and BH3 contribute discrete functions to the proapoptotic induction of holocytochrome c release, then we would expect to see synergy between c-Myc and the BH3 peptide, even at saturating levels of BH3 peptide. To investigate these possibilities, Rat1/c-MycERTAM fibroblasts were microinjected with saturating proapoptotic levels of BH3 peptide and were then examined to see whether c-Myc activation further exacerbated apoptosis. Importantly, these experiments were performed in high-serum conditions, conditions under which c-Myc by itself induces neither cytochrome c release (24) nor apoptosis (11, 18).
We observed that activation of c-Myc dramatically increased both the rate and extent of the apoptosis induced by microinjection of saturating amounts of Bax-BH3 peptide (1 mg/ml) (Fig. 5A). In contrast, the coactivation of c-Myc induced no further death in cells microinjected with the defective BH3L63A variant peptide (Fig. 5A). Potentiation of BH3 peptide-induced apoptosis by c-Myc could be detected within 6 h of c-Myc activation; immunocytochemical analysis indicated that the effect of c-Myc coactivation was to increase significantly the proportion of microinjected cells at any time that had released holocytochrome into the cytosol (Fig. 5B). Thus, c-Myc cooperates with the saturating amounts of BH3 peptide to potentiate cytochrome c release. The synergy observed between c-Myc and the BH3 peptide contrasts sharply with the lack of synergy between c-Myc and microinjected holocytochrome c (24). Taken together, these data indicate that c-Myc and the BH3 peptide each provide different functions that converge on release of their common cytochrome c effector.
FIG. 5.
c-Myc and Bax-BH3 peptide cooperate to induce cell death. (A) c-Myc sensitizes cells to Bax-BH3 peptide-induced apoptosis. Rat-1/c-MycERTAM fibroblasts grown in 10% FCS were treated with either ethanol vehicle control or 100 nM OHT to activate c-Myc 2 h prior to microinjection with Bax-BH3 peptide (1 mg/ml) as described in the legend to Fig. 4A. Cells were then incubated at 37°C for the indicated times and analyzed by fluorescence microscopy for cell death. The data are mean values ± SEM from at least five experiments. Each experiment comprised two paired observations, and the same microcapillary was used to microinject cells, which were treated with either OHT or ethanol, with the same peptide solution on the same day. (B) Effect of c-Myc on Bax-BH3 peptide-induced holocytochrome c release. Rat-1/c-MycERTAM fibroblasts grown in 10% FCS were treated with either ethanol vehicle control or 100 nM OHT to activate c-Myc 2 h prior to microinjection with Bax-BH3 peptide (1 mg/ml) as described above. Cells were then incubated for 5 h at 37°C, fixed, and immunostained with anti-cytochrome c antibody as described in the legend to Fig. 4B. The percentage of microinjected cells exhibiting cytosolic cytochrome c was then determined. The data are mean values ± SEM from four independent experiments performed as described above.
The proapoptotic effect of c-Myc is independent of Bax.
Our data indicate that c-Myc and BH3 peptide exert discrete effects on mitochondria that cooperate to induce holocytochrome c release and consequent apoptosis. This cooperation is especially evident in situations such as high-serum conditions where, as described above, c-Myc alone does not trigger cytochrome c release unless sufficient BH3 peptide is also present.
The observation that bax-deficient MEFs are resistant to c-Myc-induced apoptosis, even in low-serum conditions, has been interpreted as indicating that Bax is the key proapoptotic effector of c-Myc. One problem with this idea, however, is that we found no evidence that c-Myc regulates any aspect of Bax expression, localization, or conformation. An alternative explanation for the resistance of bax-deficient MEFs to c-Myc-induced apoptosis is that the absence of Bax depletes the total amount of proapoptotic Bax/Bak proteins, thus shifting the net complement of Bcl-2 family members towards the apoptosis suppressors. The two models make very different predictions. If Bax were the critical proapoptotic effector of c-Myc, then we would expect bax-deficient cells to remain resistant to c-Myc-induced apoptosis even in the presence of the BH3 peptide. In contrast, if Bax depletion merely shifts the balance of Bcl-2 family members towards the apoptosis suppressors, then the addition of BH3 peptide should counteract this shift and reinstate sensitivity to c-Myc.
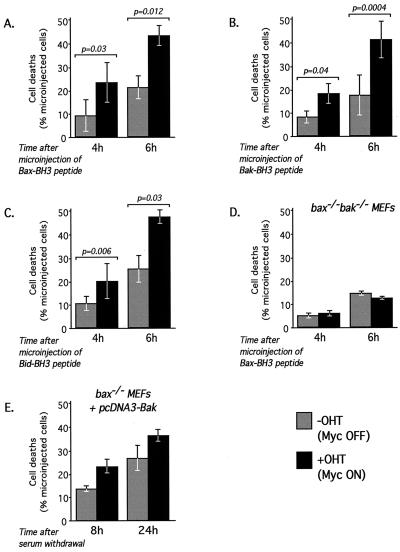
To explore these possibilities, bax−/− MEFs expressing c-MycERTAM were microinjected with the BH3 peptide either in the presence or absence of OHT to activate c-MycERTAM. As already described and documented, activation of c-Myc alone has no apparent deleterious effect on the viability of bax−/− MEFs and microinjection of BH3 peptide alone exerted only a modest proapoptotic effect. However, microinjection of BH3 peptide into bax−/− MEFs restored the ability of c-Myc to induce apoptosis (Fig. 6A). Moreover, equivalent BH3 mimetic peptides derived from the sequences of human Bak (Bak-BH3) and human Bid (Bid-BH3) also synergized with c-Myc to induce apoptosis in Rat-1 fibroblasts (data not shown) and could reconstitute c-Myc-induced apoptosis in Bax-knockout MEFs (Fig. 6B and C). These data unambiguously confirm that there is no special relationship between Myc and Bax (or the Bax-BH3 domain) and further intimate that any active BH3 protein would cooperate with c-Myc to induce apoptosis, independent of Bax status.
FIG. 6.
Multiple BH3 peptides cooperate with c-Myc to induce cell death in Bax-deficient cells. (A to C) bax−/− MEFs infected with pBp(c-MycERTAM) were treated with either ethanol vehicle control or 100 nM OHT to activate c-Myc 2 h prior to microinjection with 2 mg of Bax-BH3 (A), Bak-BH3 (B), or Bid-BH3 (C) peptide per ml. Cell death was assessed as described in the legend to Fig. 4A. Data are the mean values ± SEM from at least three independent experiments. Each experiment comprised two paired observations as described in the legend to Fig. 5A. (D) Additional experiments were performed as described for panels A to C by using bax−/−/bak−/− MEFs infected with pBp(c-MycERTAM). (E) bax−/− MEFs infected with pBp(c-MycERTAM) were microinjected with pcDNA3(Bak) (100 ng/ml) and pEGFP-N1 (20 ng/ml). The number of GFP-expressing cells was evaluated by fluorescence microscopy 18 h after microinjection. Cells were then washed twice in PBS and treated with 100 nM OHT or ethanol vehicle control in the absence of FCS. Cell loss was evaluated at the times indicated, as described in the legend to Fig. 1A. The data presented are mean values ± SEM from three independent experiments.
As demonstrated above, the specific proapoptotic function of BH3 peptides requires expression of either Bax or Bak or both. Thus, if c-Myc and Bax BH3 still cooperate in Bax-deficient MEFs, then it is presumably due to the presence of Bak (or some other, uncharacterized equivalent). To explore this idea, we infected bax−/−/bak−/− double-knockout MEFs with recombinant pBp retrovirus directing expression of c-MycERTAM and then microinjected the cells with the Bax-derived BH3 peptide either in the presence or absence of OHT to activate c-MycERTAM. As shown in Fig. 6D, the combination of c-Myc and the BH3 peptide was unable to induce apoptosis in bax−/−/bak−/− double-knockout MEFs.
This result implies that Bak can indeed be a substitute for Bax in sensitizing fibroblasts to the proapoptotic effects of c-Myc but only in cells in which the antiapoptotic action of the Bcl-2 proteins have been in effect neutralized by BH3 peptide. It therefore seems likely that the inability of endogenous Bak to mediate c-Myc-induced apoptosis in bax-deficient fibroblasts is not because Bax and Bak have nonoverlapping functions but because Bak is insufficiently active. This could be because it is expressed at lower levels than Bax or because it is more susceptible to inhibition by Bcl-2/xL. To investigate these possibilities, we asked whether the increasing expression of Bak would restore the sensitivity of bax-deficient fibroblasts to c-Myc proapoptotic function. The nuclei of bax−/− MEFs expressing c-MycER were microinjected with a plasmid encoding Bak (pcDNA3Bak) and a plasmid encoding GFP. Cells were then deprived of serum in the presence or absence of OHT, and cell viability was assayed after 8 and 24 h. As shown in Fig. 6E, ectopic expression of Bak effectively restored sensitivity to c-Myc-induced apoptosis in bax-deficient cells.
Reduced Bax expression contributes only a part of the resistance of p53−/− MEFs to c-Myc-induced apoptosis.
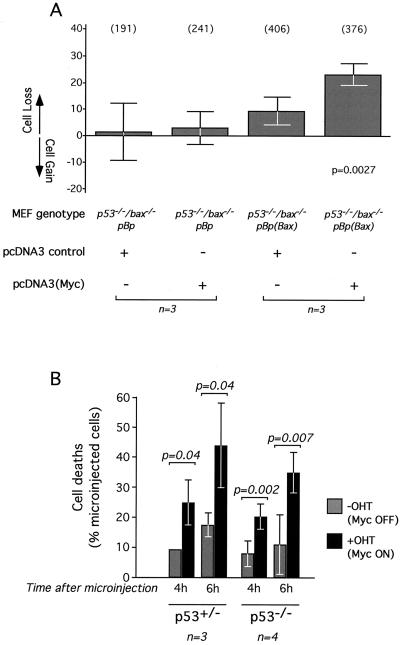
p53-knockout MEFs are refractory to c-Myc-induced cell death, suggesting an important role for p53 in oncogene-induced apoptosis of these cells (19, 50). Recently, c-Myc has been shown to induce expression of p53 through the ARF/MDM-2 pathway in primary fibroblasts (56), thereby indirectly inducing activation of p53 proapoptotic target genes such as bax. Indeed, expression of both bax mRNA and protein are constitutively low in p53-negative MEFs (27, 32), so the immunity of p53-negative cells to c-Myc-induced apoptosis might be a consequence of such reduced Bax expression. To investigate this possibility, we used a retrovirus vector to ectopically express Bax in p53−/−/bax−/− double-knockout MEFs and then assessed any effect on c-Myc-induced apoptosis. Western blot analysis indicated that the levels of Bax obtained after retrovirus infection were comparable to those obtained in analogously infected bax−/− MEFs and similar to endogenous Bax levels in wild-type MEFs (data not shown). We found that p53−/−/bax−/− MEFs expressing Bax exhibit a significant loss of viability upon expression of c-Myc in low-serum conditions (Fig. 7A). This indicates that Bax and c-Myc can functionally cooperate in the absence of p53 and confirms that c-Myc exerts a p53-independent effect on mitochondrial integrity, as described in a previous study (24). The resistance of p53−/− MEFs to c-Myc-induced apoptosis may therefore be attributed, at least in part, to the reduced Bax levels of these cells. However, it should be noted that the extent of cell death observed was modest when compared to that observed in p53-positive bax−/− MEFs expressing ectopic Bax (Fig. 1B). Thus, in addition to promoting basal Bax expression, p53 also seems to provide additional functions that potentiate pro-cytochrome c-releasing apoptotic synergy between Bax and c-Myc. This is consistent with published data showing that Bax is less effective in promoting fibroblast apoptosis in a p53-deficient background (32).
FIG. 7.
Role of p53 in c-Myc-induced sensitization to Bax-BH3 peptide and Bax. (A) c-Myc induces apoptosis of p53−/−/bax−/− MEFs ectopically expressing Bax. Experiments were performed as described in the legend to Fig. 1B with p53−/−/bax−/− MEFs. (B) Cooperation between c-Myc and the Bax-BH3 peptide in p53-null cells. Experiments were performed as described in the legend to Fig. 6 with p53+/− and p53−/− MEFs infected with pBp(c-MycERTAM). Microinjection was performed with Bax-BH3 peptide (2 mg/ml). The data are mean values ± SEM from the indicated number of experiments.
As shown in Fig. 7B, microinjection of BH3 peptide induced apoptosis as efficiently in p53-deficient cells as in cells with a functional p53. Moreover, BH3 microinjection restored the apoptotic response to c-Myc in p53-deficient MEFs. This means that although p53-deficient MEFs are resistant to induction of apoptosis by c-Myc, c-Myc nonetheless exerts its proapoptotic action in such cells, an action that is revealed upon introduction of a sufficient level of the BH3 peptide.
DISCUSSION
c-Myc sensitizes cells to a wide range of mechanistically diverse proapoptotic insults, including DNA damage, death receptor signaling, hypoxia, interferon activity, and nutrient deprivation. At least two discrete proapoptotic effector pathways mediate this sensitization. One of these involves stabilization of p53 through the ARF/Mdm-2 pathway, which serves as a sentinel for genotoxic damage. The second promotes release of holocytochrome c from mitochondria by a mechanism that is independent of both the Fas and the DNA damage proapoptotic pathways (24). Since the release of holocytochrome c is the principal target for suppression by survival factors (24), this pathway acts as a trophic sentinel (12), triggering Myc-induced apoptosis should the affected cell exhaust or elude its local supply of survival signals. c-Myc-induced release of holocytochrome c is also suppressed by Bcl-2/Bcl-xL, which, like survival factors, potently exacerbates c-Myc oncogenicity. Curiously, cells expressing c-Myc are sensitized to many mechanistically diverse apoptotic triggers, even under conditions where c-Myc by itself is not sufficient to trigger holocytochrome c release, such as in high-serum conditions. Thus, c-Myc must act in some way to potentiate the ability of other factors to induce holocytochrome c release. Since all proapoptotic actions of c-Myc require its integrity as a transcription factor, it seems most plausible that c-Myc modulates target genes that encode proteins that predispose the mitochondrion to permeabilization.
The proapoptotic members of the Bcl-2 family antagonize the protective actions of Bcl-2 and Bcl-xL on mitochondria and promote the release of holocytochrome c. These members include Bax, Bak, and Bok, bona fide homologs of Bcl-2/Bcl-xL that share the conserved Bcl-2 homology domains BH1, BH2, and BH3, and the BH3-only proteins, most of which act as downstream effectors of distinct proapoptotic signaling pathways. The propensity for proapoptotic Bcl-2 family members to induce holocytochrome c release and consequent apoptosis makes them good candidate effectors for the mitochondrial apoptotic pathway that c-Myc elicits. Indeed, recent studies have implicated a specific role for Bax in c-Myc-induced apoptosis. First, inspection of the bax promoter revealed two consensus Myc/Max binding sites. Second, bax-deficient MEFs exhibit marked resistance to c-Myc-induced apoptosis in low-serum conditions (33). Taken together, these data have implicated bax as a key transcriptional target and proapoptotic effector of c-Myc.
We began by examining the susceptibility of MEFs deficient in members of the Bax/BH3 families to c-Myc-induced apoptosis. Like other researchers, we found Bax-deficient MEFs to be markedly resistant to c-Myc-induced apoptosis. Immunocytochemical analysis revealed that c-Myc is unable to induce cytochrome c release in bax-deficient MEFs, indicating that the defect lies upstream of the mitochondrion (data not shown). In contrast, MEFs deficient in either Bak or Bid exhibited normal sensitivity to c-Myc-induced apoptosis. The inconsequence of Bak is intriguing since it is a close structural and functional homolog of Bax that can be a substitute for Bax in some of its functions during mouse development (28). The lack of a requirement for Bid in c-Myc killing is consistent with previously reported observations indicating that the observed synergy between c-Myc and Fas/CD95 results from the two proapoptotic pathways converging on a common death machinery and not from c-Myc triggering of the Fas/CD95 pathway or vice versa (22, 24).
We next asked whether ectopic expression of Bax in bax-deficient MEFs restores sensitivity to the induction of apoptosis by c-Myc. Ectopic Bax expression by itself had little effect on cell viability, as reported previously (32), but did restore the ability of c-Myc to induce apoptosis. Since, in this instance, expression of Bax is driven by an exogenous and c-Myc-independent promoter, the dependence of c-Myc-induced apoptosis on Bax does not seem to be because bax is a transcriptional target for c-Myc. Consistent with this was our observation that c-Myc had no detectable effect on Bax levels in Rat-1 fibroblasts under conditions where c-Myc promotes apoptosis, either in whole-cell lysates or in separated mitochondrial and cytosolic cell fractions. A similar lack of effect of c-Myc on Bax expression was previously reported in MEFs (56). This indicates that an increase in Bax levels is not necessary for c-Myc to induce apoptosis. It is of note that our ability to restore c-Myc-induced apoptosis in bax-deficient MEFs by ectopic Bax expression implies that the resistance of bax-deficient cells to c-Myc induction of apoptosis is a bona fide consequence of the absence of Bax rather than some indirect consequence of the absence of bax expression during murine development.
If c-Myc does not need to induce Bax expression in order to exert its apoptotic effect, then it is possible that it triggers activation of existing Bax. Nascent Bax in healthy cells is predominantly cytosolic. Upon receipt of certain proapoptotic triggers, Bax migrates to the mitochondria, dimerizes (9, 16, 37), and undergoes a conformational change that can be detected by certain N-terminal antibodies (21). Consistent with this scenario, subcellular fractionation indicated that most endogenous Bax in Rat-1 fibroblasts is indeed nonmitochondrial. However, activation of c-Myc had no detectable effect on Bax localization, even under low-serum conditions, where c-Myc activation efficiently induces apoptosis. This observation is consistent with the findings of Soucie et al. (48) and indicates that c-Myc exerts no direct effect on Bax localization. Immunocytochemical studies using a conformation-specific Bax antibody that readily detects a change in Bax conformation following TNF alpha and actinomycin D treatment revealed no detectable change in Bax conformation in response to c-Myc activation. Thus, a change in Bax conformation is not required for c-Myc to induce cytochrome c release and initiate apoptosis in serum-deprived fibroblasts. This observation is also consistent with the results previously presented by Soucie et al., who found no direct effect of Myc ectopic expression on Bax conformation but, interestingly, did observe that the ability of certain DNA-damaging agents to induce a change in Bax conformation is compromised in the absence of Myc. Taken together, our data indicate that while Bax activity is required for efficient c-Myc-induced apoptosis, Bax is not a direct effector of c-Myc. Rather, Bax provides some requisite basal proapoptotic function that is insufficient to induce apoptosis by itself but with which c-Myc cooperates to induce efficient cell death.
The potential proapoptotic actions of c-Myc could be to potentiate the proapoptotic activity of Bax or, alternatively, to antagonize antiapoptotic Bcl-2 family members. Indeed, a recent study has demonstrated that c-Myc activation does suppress Bcl-xL and Bcl-2 mRNA and protein levels in lymphocytic cells, an effect that is eroded during Myc-induced lymphomagenesis (8). To explore these two possibilities, we sought to establish whether the proapoptotic function of Myc is still retained in cells where the function of the Bcl-2/xL protectors had been eliminated. To do this, we made use of a minimal 16-amino-acid Bax-BH3 peptide. Such BH3 peptides have been shown to bind Bcl-xL and promote mitochondrial dysfunction in vitro (4, 36). We established that the Bax-BH3 16-mer peptide acts as a functional antagonist of Bcl-xL-Bax interactions in vitro and suppresses Bcl-xL-mediated protection from UV-induced apoptosis in intact cells. In contrast, a mutant peptide control, differing by one conservative amino acid change and shown to be unable to interact with Bcl-xL, did neither.
Upon microinjection into cells, the Bax-BH3 peptide exhibited dose-dependent proapoptotic activity and triggered caspase-independent release of holocytochrome c. However, the lethal action of the peptide saturated at around 8 million molecules of peptide per cell, at a level where presumably all protective Bcl-2 family members have been antagonized. However, even at levels well above such saturating levels of BH3 peptide and in high-serum conditions, activation of c-Myc triggered a dramatic increase in apoptosis, which is evident in both an increased probability of cell death and an increased proportion of microinjected cells exhibiting cytosolic holocytochrome c. Thus, the contribution of c-Myc cannot be solely to incapacitate the protectors. Rather, the cooperation between c-Myc and the BH3 peptide seems to reflect a general sensitization induced by c-Myc to BH3 function, which extends to other BH3-bearing molecules, such as microinjected Bak-BH3 and Bid-BH3 peptides, and to overexpressed Bad and Bid (unpublished data). Mutational analysis of c-Myc indicates that its ability to potentiate BH3 peptide-induced killing requires its integrity as a bHLHZip transcription factor (data not shown), supporting the generally held assumption that c-Myc proapoptotic activity is mediated through modulation of target genes. Interestingly the proapoptotic synergy between c-Myc and BH3 is also evident in bax-deficient cells, in which c-Myc activation alone is unable to induce apoptosis. This indicates that the immediate proapoptotic activity of c-Myc is independent of Bax. We suspect that the inability of c-Myc to induce apoptosis in bax-deficient cells is not because of any dedicated affector-effector relationship between Myc and Bax. Rather, it arises because the absence of Bax skews the overall balance of proapoptotic and antiapoptotic Bcl-2 family members towards the apoptotic suppressors. Subsequent negation of these apoptosis suppressors by BH3 peptides shifts the balance back to a state where the proapoptotic action of c-Myc can once again be manifest. Interestingly, MEFs lacking both Bax and Bak are completely resistant to induction of apoptosis by c-Myc and the BH3 peptide, which is consistent with the previously reported results showing that apoptosis induced by BH3-only proteins requires the presence of at least one or the other of the two multidomain proapoptotic Bcl-2 family proteins.
Taken together, our data offer a new interpretation of the apparent dependence of c-Myc-induced apoptosis on Bax in fibroblasts. Rather than c-Myc acting on mitochondria through Bax, c-Myc and Bax both contribute independent and cooperative functions that together trigger the release of holocytochrome c. Our data suggest that c-Myc activation results in the enhanced efficiency of both active forms of Bax and Bak. In low-serum conditions or in conditions where there are sufficient levels of BH3 to counter the antiapoptotic Bcl-2 family proteins, this is sufficient to destabilize mitochondrial integrity and induce apoptosis. In the absence of Bax, c-Myc activation by itself is insufficient to induce apoptosis. However, increased Bak expression restores the sensitivity of bax-deficient fibroblasts to c-Myc. Furthermore, microinjection of the BH3 peptide also restores such sensitivity by a mechanism that requires the presence of Bak. It therefore seems likely that in bax-deficient fibroblasts there is not enough active Bak to facilitate c-Myc killing; this may be because of low Bak expression and/or because it is overwhelmed by Bcl-2/xL protectors. In such cells, Myc-induced apoptosis can therefore be recovered either by increasing Bak expression or by antagonizing the Bcl-2/xL protectors with BH3 peptides. Our data are consistent with the previously reported stimulation of Bax mitochondrial activity by c-Myc (48) and indicate that such stimulation extends to Bak activity. Moreover, the notion that c-Myc cooperates with active forms of Bax and Bak is consistent with the observation that c-Myc sensitizes cells to apoptosis induced by microinjected tBid (P. Juin, A. Hunt, O. von Ahsen, D. Green, and G. Evan, unpublished data), which has been shown to activate both proteins.
The identification of Bax as a cofactor, rather than an effector, for c-Myc-induced apoptosis sheds some light on the possible roles of p53 in this process. p53-deficient MEFs are profoundly resistant to c-Myc-induced apoptosis (19, 50). However, we find that p53 is not required for the susceptibility of MEFs to BH3 peptide-induced apoptosis or for the apoptotic synergy between c-Myc and BH3 peptide, indicating that the immediate proapoptotic action exerted by c-Myc in these cells is independent of p53. One possibility is that the observed resistance of p53-deficient cells to c-Myc-induced apoptosis arises because p53-deficient MEFs express constitutively lower levels of Bax than those expressed by wild-type MEFs (27, 32). Indeed, ectopic reexpression of Bax in p53−/−/bax−/− double-knockout fibroblasts does restore c-Myc-induced apoptosis to some degree. However, it is modest compared with that observed in bax−/− single-knockout MEFs in which Bax has been reintroduced, implying that p53 must provide additional functions necessary for Bax to cooperate effectively with c-Myc to induce apoptosis. We presume that p53 also influences other factors that modulate Bax activation, such as Peg3, Noxa, or PUMA (6, 35, 38, 55). These analyses clearly indicate that variation in constitutive Bax levels can affect the efficacy with which Myc induces apoptosis. Thus, even in mouse fibroblasts, where Myc itself has little or no effect on Bax expression, other factors that determine Bax expression will exert significant influence on the ability of Myc to trigger cell death.
In summary, our study has uncovered a novel synergistic relationship between c-Myc and the BH3-only proteins and show that they conspire to promote the release of mitochondrial holocytochrome c through a mechanism dependent upon the presence of the multidomain proapoptotic Bax/Bak proteins. BH3-only proteins are a heterogeneous family whose individual members are activated by a diverse variety of signals and insults (26). For this reason, the precise BH3 activity synergizing with c-Myc in any instance is likely to be both cell type and insult dependent. Since cancer cells with activated Myc are under strong selective pressure to evade apoptosis, the loss or inactivation of differing BH3 proteins is likely to be an important, if highly variable, feature of carcinogenesis. Nonetheless, our studies show that reinstatement of lost BH3 functionality can recover effective oncogene-induced apoptosis even in cells that have lost Bax, Bak, or p53, thus offering a novel therapeutic strategy for the treatment of malignancies.
Acknowledgments
We thank Craig Thompson for the gift of Bak-deficient mice and the bax−/−/bak−/− MEFs, Larry Donehower for the gift of p53-deficient mice, and Emmy Verschuren for the pcDNA3c-Myc plasmid. We thank Scott Lowe for the gift of p53−/−/bax−/− MEFs and for fruitful discussion throughout this work and Oliver von Ahsen, Ingram Iaccarino, Douglas Green, and François Vallette for invaluable comments. We also thank all members of the Evan lab for their constant support. We are particularly indebted to Dave Hancock for his help and criticism throughout this study.
REFERENCES
- 1.Attardi, L. D., E. E. Reczek, C. Cosmas, E. G. Demicco, M. E. McCurrach, S. W. Lowe, and T. Jacks. 2000. PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev. 14:704-718. [PMC free article] [PubMed] [Google Scholar]
- 2.Bissonnette, R., F. Echeverri, A. Mahboubi, and D. Green. 1992. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature 359:552-554. [DOI] [PubMed] [Google Scholar]
- 3.Cheng, E. H., M. C. Wei, S. Weiler, R. A. Flavell, T. W. Mak, T. Lindsten, and S. J. Korsmeyer. 2001. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell 8:705-711. [DOI] [PubMed] [Google Scholar]
- 4.Cosulich, S. C., V. Worrall, P. J. Hedge, S. Green, and P. R. Clarke. 1997. Regulation of apoptosis by BH3 domains in a cell-free system. Curr. Biol. 7:913-920. [DOI] [PubMed] [Google Scholar]
- 5.Datta, S. R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231-241. [DOI] [PubMed] [Google Scholar]
- 6.Deng, Y., and X. Wu. 2000. Peg3/Pw1 promotes p53-mediated apoptosis by inducing Bax translocation from cytosol to mitochondria. Proc. Natl. Acad. Sci. USA 97:12050-12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Stanchina, E., M. E. McCurrach, F. Zindy, S. Y. Shieh, G. Ferbeyre, A. V. Samuelson, C. Prives, M. F. Roussel, C. J. Sherr, and S. W. Lowe. 1998. E1A signaling to p53 involves the p19(ARF) tumor suppressor. Genes Dev. 12:2434-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eischen, C. M., D. Woo, M. F. Roussel, and J. L. Cleveland. 2001. Apoptosis triggered by Myc-induced suppression of Bcl-XL or Bcl-2 is bypassed during lymphomagenesis. Mol. Cell. Biol. 21:5063-5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eskes, R., S. Desagher, B. Antonsson, and J. C. Martinou. 2000. Bid induces the oligomerization and insertion of bax into the outer mitochondrial membrane. Mol. Cell. Biol. 20:929-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evan, G., and T. Littlewood. 1998. A matter of life and cell death. Science 281:1317-1322. [DOI] [PubMed] [Google Scholar]
- 11.Evan, G., A. Wyllie, C. Gilbert, T. Littlewood, H. Land, M. Brooks, C. Waters, L. Penn, and D. Hancock. 1992. Induction of apoptosis in fibroblasts by c-myc protein. Cell 63:119-125. [DOI] [PubMed] [Google Scholar]
- 12.Evan, G. I., and K. H. Vousden. 2001. Proliferation, cell cycle and apoptosis in cancer. Nature 411:342-348. [DOI] [PubMed] [Google Scholar]
- 13.Fanidi, A., E. Harrington, and G. Evan. 1992. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature 359:554-556. [DOI] [PubMed] [Google Scholar]
- 14.Gilmore, A. P., A. D. Metcalfe, L. H. Romer, and C. H. Streuli. 2000. Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J. Cell Biol. 149:431-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein, J., N. Waterhouse, P. Juin, G. Evan, and D. Green. 2000. The co-ordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat. Cell Biol. 2:156-162. [DOI] [PubMed] [Google Scholar]
- 16.Gross, A., J. Jockel, M. C. Wei, and S. J. Korsmeyer. 1998. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 17:3878-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han, J., P. Sabbatini, D. Perez, L. Rao, D. Modha, and E. White. 1996. The E1B 19K protein blocks apoptosis by interacting with and inhibiting the p53-inducible and death-promoting Bax protein. Genes Dev. 10:461-477. [DOI] [PubMed] [Google Scholar]
- 18.Harrington, E. A., M. R. Bennett, A. Fanidi, and G. I. Evan. 1994. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 13:3286-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermeking, H., and D. Eick. 1994. Mediation of c-Myc-induced apoptosis by p53. Science 265:2091-2093. [DOI] [PubMed] [Google Scholar]
- 20.Holinger, E. P., T. Chittenden, and R. J. Lutz. 1999. Bak BH3 peptides antagonize Bcl-xL function and induce apoptosis through cytochrome c-independent activation of caspases. J. Biol. Chem. 274:13298-13304. [DOI] [PubMed] [Google Scholar]
- 21.Hsu, Y. T., and R. J. Youle. 1998. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J. Biol. Chem. 273:10777-10783. [DOI] [PubMed] [Google Scholar]
- 22.Hueber, A. O., M. Zornig, D. Lyon, T. Suda, S. Nagata, and G. I. Evan. 1997. Requirement for the CD95 receptor-ligand pathway in c-Myc-induced apoptosis. Science 278:1305-1309. [DOI] [PubMed] [Google Scholar]
- 23.Hunt, A., and G. Evan. 2001. Apoptosis. Till death us do part. Science 293:1784-1785. [DOI] [PubMed] [Google Scholar]
- 24.Juin, P., A. O. Hueber, T. Littlewood, and G. Evan. 1999. c-Myc-induced sensitization to apoptosis is mediated through cytochrome c release. Genes Dev. 13:1367-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamijo, T., F. Zindy, M. F. Roussel, D. E. Quelle, J. R. Downing, R. A. Ashmun, G. Grosveld, and C. J. Sherr. 1997. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91:649-659. [DOI] [PubMed] [Google Scholar]
- 26.Kelekar, A., and C. Thompson. 1998. Bcl-2 family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 8:324-330. [DOI] [PubMed] [Google Scholar]
- 27.Koumenis, C., R. Alarcon, E. Hammond, P. Sutphin, W. Hoffman, M. Murphy, J. Derr, Y. Taya, S. W. Lowe, M. Kastan, and A. Giaccia. 2001. Regulation of p53 by hypoxia: dissociation of transcriptional repression and apoptosis from p53-dependent transactivation. Mol. Cell. Biol. 21:1297-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindsten, T., A. J. Ross, A. King, W. X. Zong, J. C. Rathmell, H. A. Shiels, E. Ulrich, K. G. Waymire, P. Mahar, K. Frauwirth, Y. Chen, M. Wei, V. M. Eng, D. M. Adelman, M. C. Simon, A. Ma, J. A. Golden, G. Evan, S. J. Korsmeyer, G. R. MacGregor, and C. B. Thompson. 2000. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell 6:1389-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Littlewood, T., D. Hancock, P. Danielian, M. Parker, and G. Evan. 1995. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res. 23:1686-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marzo, I., C. Brenner, N. Zamzami, J. M. Jurgensmeier, S. A. Susin, H. L. Vieira, M. C. Prevost, Z. Xie, S. Matsuyama, J. C. Reed, and G. Kroemer. 1998. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science 281:2027-2031. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy, N., M. Whyte, C. Gilbert, and G. Evan. 1997. Inhibition of Ced-3/ICE-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak. J. Cell Biol. 136:215-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCurrach, M. E., T. M. Connor, C. M. Knudson, S. J. Korsmeyer, and S. W. Lowe. 1997. bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc. Natl. Acad. Sci. USA 94:2345-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell, K. O., M. S. Ricci, T. Miyashita, D. T. Dicker, Z. Jin, J. C. Reed, and W. S. El-Deiry. 2000. Bax is a transcriptional target and mediator of c-myc-induced apoptosis. Cancer Res. 60:6318-6325. [PubMed] [Google Scholar]
- 34.Miyashita, T., and J. C. Reed. 1995. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80:293-299. [DOI] [PubMed] [Google Scholar]
- 35.Nakano, K., and K. H. Vousden. 2001. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 7:683-694. [DOI] [PubMed] [Google Scholar]
- 36.Narita, M., S. Shimizu, T. Ito, T. Chittenden, R. J. Lutz, H. Matsuda, and Y. Tsujimoto. 1998. Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc. Natl. Acad. Sci. USA 95:14681-14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nomura, M., S. Shimizu, T. Ito, M. Narita, H. Matsuda, and Y. Tsujimoto. 1999. Apoptotic cytosol facilitates Bax translocation to mitochondria that involves cytosolic factor regulated by Bcl-2. Cancer Res. 59:5542-5548. [PubMed] [Google Scholar]
- 38.Oda, E., R. Ohki, H. Murasawa, J. Nemoto, T. Shibue, T. Yamashita, T. Tokino, T. Taniguchi, and N. Tanaka. 2000. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288:1053-1058. [DOI] [PubMed] [Google Scholar]
- 39.Pelengaris, S., M. Khan, and G. I. Evan. 2002. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell 109:321-334. [DOI] [PubMed] [Google Scholar]
- 40.Perez, D., and E. White. 2000. TNF-alpha signals apoptosis through a bid-dependent conformational change in Bax that is inhibited by E1B 19K. Mol. Cell 6:53-63. [PubMed] [Google Scholar]
- 41.Puthalakath, H., D. C. Huang, L. A. O'Reilly, S. M. King, and A. Strasser. 1999. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell 3:287-296. [DOI] [PubMed] [Google Scholar]
- 42.Sattler, M., H. Liang, D. Nettesheim, R. P. Meadows, J. E. Harlan, M. Eberstadt, H. S. Yoon, S. B. Shuker, B. S. Chang, A. J. Minn, C. B. Thompson, and S. W. Fesik. 1997. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 275:983-986. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt, T., K. Korner, H. Karsunky, S. Korsmeyer, R. Muller, and T. Moroy. 1999. The activity of the murine Bax promoter is regulated by Sp1/3 and E-box binding proteins but not by p53. Cell Death Differ. 6:873-882. [DOI] [PubMed] [Google Scholar]
- 44.Schmitt, C. A., J. S. Fridman, M. Yang, S. Lee, E. Baranov, R. M. Hoffman, and S. W. Lowe. 2002. A senescence program controlled by p53 and p16 INK4a contributes to the outcome of cancer therapy. Cell 109:335-346. [DOI] [PubMed] [Google Scholar]
- 45.Schuler, M., E. Bossy-Wetzel, J. C. Goldstein, P. Fitzgerald, and D. R. Green. 2000. p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J. Biol. Chem. 275:7337-7342. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu, S., M. Narita, and Y. Tsujimoto. 1999. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399:483-487. [DOI] [PubMed] [Google Scholar]
- 47.Soengas, M. S., R. M. Alarcon, H. Yoshida, A. J. Giaccia, R. Hakem, T. W. Mak, and S. W. Lowe. 1999. Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science 284:156-159. [DOI] [PubMed] [Google Scholar]
- 48.Soucie, E. L., M. G. Annis, J. Sedivy, J. Filmus, B. Leber, D. W. Andrews, and L. Z. Penn. 2001. Myc potentiates apoptosis by stimulating Bax activity at the mitochondria. Mol. Cell. Biol. 21:4725-4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strasser, A., A. W. Harris, D. L. Vaux, E. Webb, M. L. Bath, J. M. Adams, and S. Cory. 1990. Abnormalities of the immune system induced by dysregulated bcl-2 expression in transgenic mice. Curr. Top. Microbiol. Immunol. 166:175-181. [DOI] [PubMed] [Google Scholar]
- 50.Wagner, A. J., J. M. Kokontis, and N. Hay. 1994. Myc-mediated apoptosis requires wild-type p53 in a manner independent of cell cycle arrest and the ability of p53 to induce p21waf1/cip1. Genes Dev. 8:2817-2830. [DOI] [PubMed] [Google Scholar]
- 51.Wagner, A. J., M. B. Small, and N. Hay. 1993. Myc-mediated apoptosis is blocked by ectopic expression of bcl-2. Mol. Cell. Biol. 13:2432-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei, M. C., W. X. Zong, E. H. Cheng, T. Lindsten, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, C. B. Thompson, and S. J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolter, K. G., Y. T. Hsu, C. L. Smith, A. Nechushtan, X. G. Xi, and R. J. Youle. 1997. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 139:1281-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin, X. M., K. Wang, A. Gross, Y. Zhao, S. Zinkel, B. Klocke, K. A. Roth, and S. J. Korsmeyer. 1999. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400:886-891. [DOI] [PubMed] [Google Scholar]
- 55.Yu, J., L. Zhang, P. Hwang, K. Kinzler, and B. Vogelstein. 2001. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell 7:673-682. [DOI] [PubMed] [Google Scholar]
- 56.Zindy, F., C. M. Eischen, D. H. Randle, T. Kamijo, J. L. Cleveland, C. J. Sherr, and M. F. Roussel. 1998. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 12:2424-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zong, W. X., T. Lindsten, A. J. Ross, G. R. MacGregor, and C. B. Thompson. 2001. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 15:1481-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]