Abstract
We have characterized a pathway for nuclear export of the glucocorticoid receptor (GR) in mammalian cells. This pathway involves the Ca2+ -binding protein calreticulin (CRT), which directly contacts the DNA binding domain (DBD) of GR and facilitates its delivery from the nucleus to the cytoplasm. In the present study, we investigated the role of Ca2+ in CRT-dependent export of GR. We found that removal of Ca2+ from CRT inhibits its capacity to stimulate the nuclear export of GR in digitonin-permeabilized cells and that the inhibition is due to the failure of Ca2+-free CRT to bind the DBD. These effects are reversible, since DBD binding and nuclear export can be restored by Ca2+ addition. Depletion of intracellular Ca2+ inhibits GR export in intact cells under conditions that do not inhibit other nuclear transport pathways, suggesting that there is a Ca2+ requirement for GR export in vivo. We also found that the Ran GTPase is not required for GR export. These data show that the nuclear export pathway used by steroid hormone receptors such as GR is distinct from the Crm1 pathway. We suggest that signaling events that increase Ca2+ could positively regulate CRT and inhibit GR function through nuclear export.
The nuclear transport machinery integrates a variety of nuclear and cytoplasmic activities by mediating the translocation of housekeeping and regulatory proteins and RNAs. Translocation of these macromolecules generally requires a cis-acting transport signal, recognition of the signal by a receptor, and movement of the signal-receptor complex through the nuclear pore complex (NPC) (40, 45). In the case of nuclear export, most proteins rely on a hydrophobic nuclear export signal (NES) and its recognition by the receptor Crm1 (1, 10, 11, 39, 44). NES binding to Crm1 is stabilized by the presence of RanGTP, and the resulting trimeric complex of Crm1, NES, and RanGTP undergoes translocation through the nuclear pore (14, 28). Other proteins are important cofactors for this export pathway, including RanGAP, RanBP1, NXT1, and RanBP3 (1, 4, 22, 24).
A number of proteins that lack a hydrophobic NES are known to undergo nuclear export, and current evidence indicates that three distinct mechanisms can account for nuclear export of these proteins. First, an NES-containing adapter could be used to bridge the interaction between the protein and Crm1 (21). Second, the protein could use a different signal for nuclear export and undergo Crm1-independent export (3). Third, the protein could interact directly with nucleoporins in the NPC and undergo receptor-independent nuclear export (46). An advantage of these mechanisms is that they provide the potential for additional levels of regulation for protein sorting between the nucleus and cytoplasm.
The glucocorticoid receptor (GR) is an example of a protein that undergoes export from the nucleus even though it lacks a hydrophobic NES. Moreover, GR export is insensitive to the Crm1 inhibitor leptomycin B, which seems to rule out the use of NES adapters and Crm1 as the major receptor for this pathway (18, 25). The signal that specifies nuclear export of GR maps to the 67-amino-acid DNA binding domain (DBD), which is both sufficient to mediate the export of green fluorescent protein (GFP) reporter proteins and necessary for export of GR (3). Mutations that disrupt DBD structure, whether in the context of an isolated DBD or in full-length GR, also reduce its export activity. The most severe mutations that reduce DBD-dependent nuclear export are two phenylalanine-to-alanine mutations in the DNA recognition helix (3). The structural conservation of the DBD in the nuclear receptor superfamily suggests that the DBD could be widely used as an export signal. Support for this hypothesis was obtained by showing that the DBDs from steroid, nonsteroid, and orphan nuclear receptors can function as export signals when fused to a GFP reporter protein (3).
Nuclear export mediated by the DBD of GR involves a Ca2+ binding protein named calreticulin (CRT), which was first described as a protein in the lumen of the endoplasmic reticulim (ER) (29). The original link between these proteins came with the finding that a peptide sequence, KLGFFKR, which is recognized by CRT, is related to the peptide sequence KVFFKR, which is found in the DNA recognition helix of GR (33). CRT binds to GR and blocks its interaction with DNA in gel shift experiments, and overexpression of CRT inhibits GR-dependent transcription in cells (6). Similar results were obtained when the interactions between the androgen, retinoic acid, and vitamin D receptors and CRT were examined (8). The initial interpretation of these results was that a pool of CRT outside of the lumen of the ER acts as a negative regulator of transcription. The technical difficulty of showing that CRT is outside the ER, however, gave way to the view that CRT is restricted to the ER lumen and that the effects of CRT on gene expression are indirect (23).
Our laboratory purified CRT from HeLa cell cytosol in a search for novel export factors (18). We used a permeabilized cell assay that measures the nuclear export of protein kinase inhibitor (PKI), a protein that contains a hydrophobic NES (19). CRT can bind directly to the NES in PKI, which contains the peptide sequence LALKLAGLDIN. In binding experiments the interaction of CRT with PKI is stabilized in the presence of RanGTP, and in permeabilized cells CRT-dependent export of PKI is enhanced by RanGTP (18). The ability of CRT to act as an export factor for PKI and its prior link with steroid receptor function led us to test whether CRT can function as an export factor for GR. We found that recombinant CRT can stimulate GR export and that the GR export deficiency in crt−/− cells can be rescued with CRT (18). These observations support the view that CRT functions as an export factor for GR.
In the present study we characterized the CRT-dependent nuclear export of GR, with particular emphasis on how this pathway could be regulated. Because CRT is a Ca2+ binding protein, we have examined how removing Ca2+, either by chelation or by deletion of Ca2+ binding domains, affects CRT binding to the GR DBD and CRT-dependent nuclear export of GR. We show that Ca2+ binding to CRT is necessary for direct binding to the DBD and for nuclear export of GR in permeabilized cells. This Ca2+ requirement involves the C-terminal domain of CRT, which contains multiple low-affinity, high-capacity binding sites for Ca2+ (2). Removal of the C-terminal domain renders CRT insensitive to Ca2+ chelation, suggesting that this domain performs a regulatory function that is linked to Ca2+ binding. While the Ca2+-loaded CRT is active for GR export and Ca2+-free CRT is inactive for GR export, the opposite is the case for NES export in permeabilized cells. Thus, Ca2+-loaded CRT is inactive for Rev export and Ca2+-free CRT is active for Rev export. The Ca2+-loaded and Ca2+-free forms of CRT were shown to have different sensitivities to proteases (7), indicating that CRT adopts different protein conformations. Our data show that these Ca2+-dependent conformations of CRT are correlated with the ability to recognize different protein substrates. Ca2+ depletion in vivo results in the inhibition of GR export, consistent with a Ca2+ requirement for the translocation of GR from the nucleus to the cytoplasm. Our finding that CRT requires Ca2+ for binding and nuclear export of GR suggests a potential mechanism for regulating this pathway.
MATERIALS AND METHODS
Plasmids and recombinant proteins.
Standard methods were used for the expression and purification of glutathione S-transferase (GST)- and His-tagged proteins, all of which were stored at −80°C as single-use aliquots. The plasmid encoding the GST fusion with CRT was constructed in pGEX4T3, using the open reading frame of mouse CRT lacking the N-terminal 17-amino-acid signal sequence. GST-CRT was expressed in DH5α bacteria as described previously (18). Full-length and deletion mutants of mouse CRT were cloned into pQE30, and the His-tagged proteins were expressed in TG1 bacteria (3). The wild-type (WT) and NES mutant forms of PKI (L41A, L44A) (44) were expressed in BL21(DE3) bacteria and purified without fusion tags as described previously (19). The plasmid encoding a hydrophobic NES was generated by cloning the DNA sequence that encodes residues 35 to 49 of human PKI into pGEX4T3. Likewise, the plasmid encoding a nuclear receptor DBD was generated by cloning the DNA sequence that encodes residues 413 to 509 of human GR (3). The GST-NES and GST-DBD proteins immobilized on glutathione beads were either used directly for binding experiments or eluted and used in microtiter plate binding assays. For certain competition experiments, WT and mutant (MUT) forms of the GR DBD (FF to AA) were expressed as GST fusion proteins, cleaved from GST using thrombin, and further purified by ion-exchange chromatography (3). Plasmids encoding His-tagged Ran (WT, Q69L, and T24N; a gift of D. Görlich) and His-tagged Crm1 (a gift of L. Gerace) were used to express proteins in TG1 bacteria, which were purified on Talon resin (Clontech).
Binding assays.
The three formats used for the solid-phase binding assays were microtiter wells, biosensor cuvettes, and Sepharose beads. The microtiter well assay was performed as described previously (4). Briefly, purified target proteins were immobilized in high-binding 96-well plates (Costar no. 3590) overnight at 4°C in 1× transport buffer (30). Unbound protein was removed, and the plates were blocked overnight with bovine serum albumin (30 mg/ml). Binding assays (with 100-μl mixtures) were performed in triplicate, using radiolabeled Ran and CRT or Crm1, and the level of binding was measured by scintillation counting as described previously (4). The biosensor assay was performed as described previously (18). Briefly, biotinylated NES peptide was immobilized in streptavidin-coated cuvettes (Fisons) for 15 min at room temperature. The cuvettes were washed with PBS and used for binding assays with the proteins indicated in the legend to Fig. 3. A detailed description of the Fisons biosensor, which measures the change in refractive index that occurs on protein-ligand binding and dissociation, has been published (34). The Sepharose bead binding assays, using either glutathione beads and GST proteins or Talon beads and His-tagged proteins, were carried out by standard methods. Briefly, proteins were immobilized on the beads and blocked overnight with bovine serum albumin (30 mg/ml), and the assays (with 100-μl mixtures) were performed using the proteins indicated in the legends. The bound fractions were examined by immunoblotting using antibodies to CRT or Crm1 and enhanced chemiluminescence.
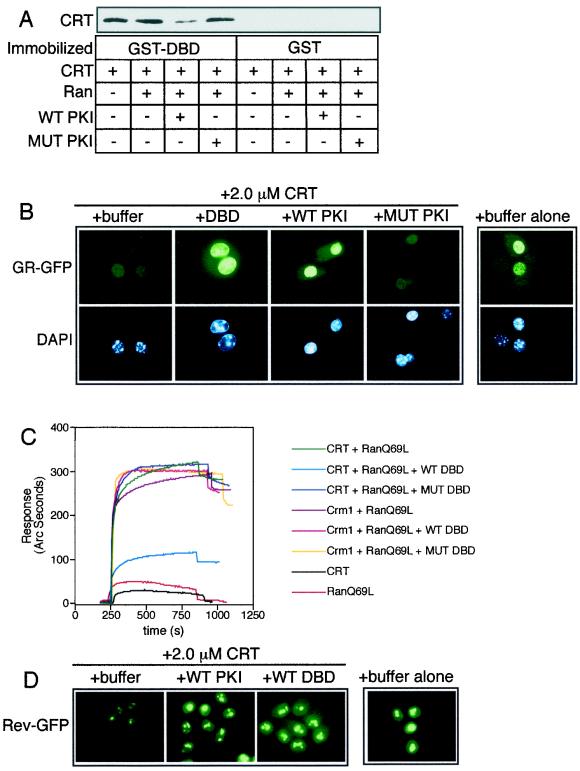
FIG. 3.
The DBD and hydrophobic NES use a common or overlapping binding site on CRT. (A) Binding assay with GST-DBD or GST (2 μg each) immobilized on glutathione beads and CRT (500 ng), RanGTP (2 μg), and WT or MUT PKI (2 μg) added in solution. The bound fractions were analyzed by immunoblotting for CRT. Including WT PKI in the reaction reduced the level of CRT bound to the DBD, indicating that these proteins bind to similar sites on CRT. This competition was not observed when RanGTP was omitted from the assay (data not shown), consistent with Ran acting as a cofactor for NES binding but not for DBD binding. (B) Nuclear export of GR-GFP was assayed in permeabilized cells using CRT (2.0 μM) in the presence of buffer, excess DBD (4.5 μM), or PKI (12 μM WT or MUT). (C) Competitive binding interactions between NES, DBD, and CRT measured in a biosensor assay. NES peptide was immobilized on the cuvette surface through a biotin-neutravidin linkage and was used to measure the Ran-dependent binding of CRT in the absence and presence of DBD in the solution. The proteins used in the assay were CRT (1.1 μM), Crm1 (1.1 μM), RanQ69L (1.9 μM), and WT and MUT DBD from GR (9.1μM each). CRT binds efficiently to NES peptide in the presence of Ran (green tracing), and this can be competed with the WT DBD (light blue tracing) but not with the transport-defective MUT DBD that contains the FF-to-AA mutations (dark blue tracing). Crm1 binding to the NES is unaffected by the presence of excess DBD (fuchsia tracing). (D) The DBD competes with NES in the CRT-dependent export pathway. The cell line expressing Rev-GFP was used to assay export in the presence of CRT (1.1 μM), WT PKI (12 μM), and WT DBD (9.1 μM) as indicated.
Ca2+ removal from CRT.
Ca2+ was removed from CRT by a published procedure that involves treatment with EGTA (43). Recombinant CRT (0.5 mg/ml in PBS) was incubated for 10 min at 30°C in the presence of 10 mM EGTA, and the sample was then transferred to ice. Ca2+ was rebound to CRT by supplementing half of the EGTA-treated sample with excess CaCl2 (final Ca2+ concentration, 20 mM). The EGTA- and Ca2+-treated samples were used at a dilution of at least 1:10 in nuclear export and binding assays, such that the maximum concentration of EGTA in the assays was ∼1 mM.
GR export assays.
Nuclear export of GR in permeabilized cells was performed essentially as described previously (18), except that a stable cell line expressing GR-GFP (a gift of G. Hager) was used instead of transiently transfected cells. The cell line (3676 cells) (27) expresses GR-GFP under the control of a tetracycline-regulated promoter. The cells were grown on glass coverslips for 16 h in the absence of tetracycline to allow GR-GFP expression, and nuclear import of GR-GFP was induced in vivo by dexamethasone (Dex; 1μM) addition to the media. The cells were permeabilized with digitonin (0.005%) for 5 min and used for export assays in vitro with the combinations of transport factors indicated in the legends. At the end of the 20-min export reaction, the samples were washed, fixed, stained with 4′,6-diamidino-2-phenylindole (DAPI), and mounted on glass slides. Using a Nikon Microphot SA microscope (60× objective, numerical aperture N.A. = 1.40) and a Hamamatsu C-4742-95 charge-coupled device camera, ∼50 nuclei from each coverslip were selected using the DAPI channel and DAPI and GFP images were captured. The digital images were acquired using Openlab 2.06 on a Macintosh G3 computer (OS 9.0), and figures were assembled using Adobe Photoshop 5.5 and Freehand 9.0. Mixtures for reactions that measured export and import in the same nuclei contained, in addition to CRT or Crm1, the fluorescent protein allophycocyanin coupled with NLS peptide (APC-NLS) and recombinant import factors (importins and NTF2).
Hydrophobic NES export assays.
The assay for nuclear export of Rev in permeabilized cells has been described previously (RGG2.2 cells) (18, 26). The fluorescent reporter in this cell line (denoted Rev-GFP) also contains the ligand binding domain of GR, which confers Dex-inducible import in vivo. Digitonin permeabilization, nuclear export, and analysis using fluorescence microscopy were performed as described above for GR-GFP. All export assay mixtures contained Ran, NXT1, and RanBP1. In experiments designed to test the role of Ran in CRT-dependent export, the T24N and Q69L mutant forms of Ran were preloaded with unlabeled GDP and GTP, respectively, and unincorporated nucleotide was removed on a desalting column.
Ca2+ depletion in living cells.
Nuclear export of GR was assayed under conditions of Ca2+ depletion in the 3676 cells by measuring the net redistribution from the nucleus to the cytoplasm. Cells expressing GR-GFP were treated with Dex for 1 h to induce nuclear import and subsequently transferred to phenol red-free media containing ionomycin (Ion; 1 μM) or thapsigargin (TG; 1 μM) in the presence of 1,2-bis(O-aminophenoxy)ethane-N,N,N′, N′-tetraacetic acid-acetoxymethyl ester (BAPTA-AM; 10 μM). Cells were also treated with dimethylsulfoxide (DMSO; 0.1%) as a control since the Ion and TG were prepared as 1,000× stocks in DMSO. At 0-, 3-, and 6-h time points, the coverslips were fixed in formaldehyde (3.7%) and mounted using Vectashield. The ratio of nuclear to cytoplasmic GR-GFP fluorescence was measured using Openlab software in at least 50 cells per condition. Similar methods were used to assay the effect of Ca2+ depletion on Rev-GFP export using the RGG2.2 cells.
RESULTS
Assembly of CRT complexes in vitro.
In digitonin-permeabilized cells, CRT can stimulate the nuclear export of proteins such as PKI that contain a hydrophobic NES, in a reaction that is dependent on Ran (18). In addition, CRT can stimulate the nuclear export of steroid hormone receptors that lack a hydrophobic NES. The export signal for CRT-dependent export of steroid hormone receptors is contained within the DBD of these proteins (3). The lack of apparent structural similarity between the hydrophobic NES and the DBD led us to characterize how CRT can recognize distinct export signals.
Because Ran is a stoichiometric component of export complexes that contain Crm1 and NES proteins, we first examined whether Ran might also function as a component of export complexes that contain CRT. Previously, we have shown that Ran assembles into a complex containing NES and CRT (18). In the present study, we tested whether Ran can assemble into a complex containing the DBD of GR (amino acids 413 to 509) and CRT. WT PKI, NES MUT PKI, and GST-DBD were immobilized in microtiter wells and incubated with recombinant CRT or Crm1 (5 μg each) in the presence of radiolabeled RanGTP (2 × 104 cpm). RanGTP assembled into a complex with CRT or Crm1 in the presence of WT PKI. The reaction is specific since a functional NES in PKI is required for complex assembly. In contrast to these results obtained with the NES-containing protein PKI, RanGTP did not coassemble into a complex with CRT or Crm1 in the presence of the DBD from GR (Fig. 1A). Thus, CRT binding to the DBD is qualitatively different from CRT binding to the hydrophobic NES, since only the latter involves RanGTP as a stoichiometric component.
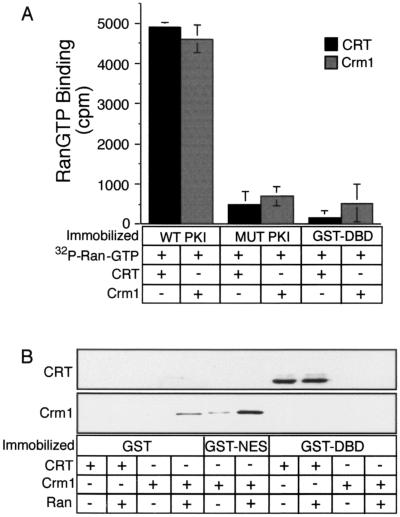
FIG. 1.
Formation of export complexes involving CRT and Crm1. (A) The incorporation of RanGTP into complexes containing CRT and Crm1 was assayed using Ran preloaded with [γ-32P] GTP. Target proteins were immobilized in microtiter wells (500 ng/well), and CRT or Crm1 (5 μg each) was added to each well together with 2 × 104 cpm of radiolabeled RanGTP. Following incubation for 1 h at room temperature, the wells were washed four times and the bound fractions were released and assayed by scintillation counting. (B) RanGTP is not a cofactor for CRT binding to the DBD. Target proteins (1 μg each) were immobilized on glutathione beads, and CRT or Crm1 (500 ng each) was added to each sample in the absence or presence of Ran (1 μg) preloaded with cold GTP. The samples were mixed end over end for 2 h at room temperature, washed three times, eluted, and analyzed by immunoblotting with antibodies to CRT and Crm1. These data show that, like Crm1, NES recognition by CRT involves RanGTP. In contrast, DBD recognition by CRT does not involve RanGTP.
We carried out binding reactions with the DBD immobilized on glutathione beads and confirmed that CRT binds directly to the DBD and that binding is neither enhanced nor prevented by RanGTP (Fig. 1B). Under the reaction conditions, Crm1 binding to the hydrophobic NES was stimulated by the presence of RanGTP (lane 6). We did not observe Crm1 binding to the DBD in the absence or presence of RanGTP, consistent with our data that Crm1 is not the receptor for proteins that use the DBD as an export signal (18). Our data demonstrate that CRT can assemble into two types of export complexes in vitro: a CRT-NES-RanGTP complex and a CRT-DBD complex.
Ran-dependent and Ran-independent export by CRT.
The results of our binding assays suggested that CRT-mediated export might occur by both Ran-independent and Ran-dependent mechanisms. That is, the data suggested that nuclear export of hydrophobic NES-containing proteins could be Ran dependent and nuclear export of DBD-containing proteins could be Ran independent. We tested this hypothesis by manipulating the composition and concentration of recombinant export factors in permeabilized cell assays, using GFP fusions of Rev and GR as the export substrates to assay NES- and DBD-dependent export, respectively. Both GFP export substrates contain the hormone binding domain of GR, which directs efficient Dex-dependent nuclear import in vivo (31). Following a digitonin permeabilization step, nuclear export of the GFP reporters can be stimulated by the addition of soluble transport factors (26).
We tested for the role of Ran in CRT-dependent NES export by using Rev, which contains a hydrophobic NES (LPPLERLTL) (10). We used a concentration of CRT that was determined to be subsaturating with respect to Rev-GFP export. In the presence of 0.2 μM CRT, WT Ran preloaded with GTP stimulated Rev-GFP export (Fig. 2A). In contrast, the Ran mutant T24N, which mimics the GDP-bound form of Ran, was inactive for CRT-dependent export. The Ran mutant Q69L, which mimics the GTP-bound form of Ran, showed little effect on export mediated by CRT, which is different from previous results obtained using another RanGTP mutant (G19V) and a fluorescent conjugate of PKI as the export substrate (18). The reason for this discrepancy is unclear, but it could relate to subtle differences in the structure and activity of these mutant proteins or to the fact that different NES proteins were used in the two assays, or both. Crm1 in these assays (Fig. 2B) supports robust export of Rev-GFP in the presence of WT Ran preloaded with GTP. In the presence of the Ran mutant Q69L, a moderate level of export was observed, while the Ran mutant T24N failed to support Crm1-dependent export. Thus, Ran stimulates both CRT- and Crm1-dependent export in this system, and maximal export activity of either protein is observed only in the presence of WT Ran.
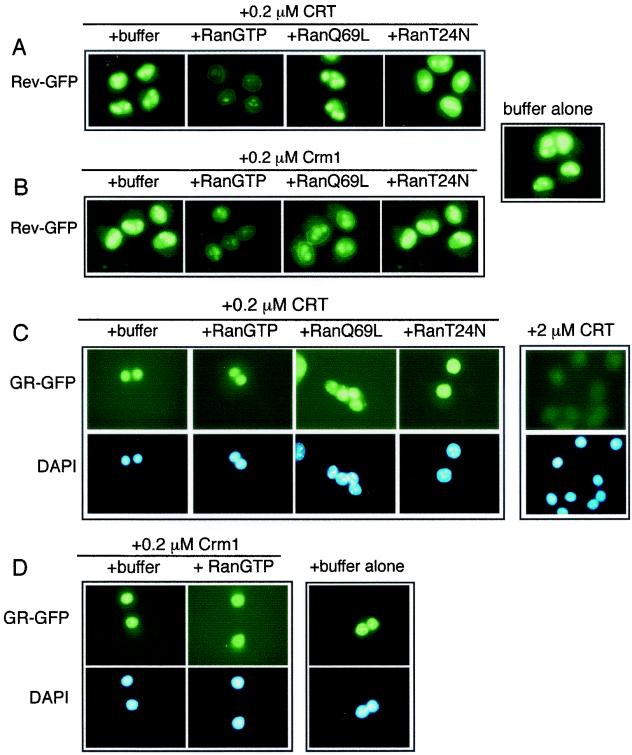
FIG. 2.
Ran is a cofactor for NES-dependent export but not for DBD-dependent export. Nuclear export was assayed by supplementing digitonin-permeabilized cell assay mixtures with CRT (0.2 or 2.0 μM), Crm1 (0.2 μM), and different forms of Ran (1.9 μM each). NES-dependent export was assayed using a cell line that expresses a GFP fusion with Rev (RGG2.2) (26), and DBD-dependent export was assayed using a cell line that expresses a GFP fusion with GR (3676) (27). The Rev-GFP fusion also contains the ligand binding domain of GR, which imparts ligand-dependent nuclear import of the reporter protein. Prior to digitonin permeabilization, both cell lines were treated with Dex (1 μM) to induce nuclear import of the GFP reporters. (A and B) RanGTP stimulates Rev-GFP export in the presence of CRT or Crm1. (C and D) In contrast, neither WT nor MUT forms of Ran stimulate GR-GFP export in the presence of CRT or Crm1.
GR-GFP export was assayed at the same concentration of CRT (0.2 μM) that, in the presence of RanGTP, promotes efficient export of Rev GFP. We determined that the addition of subsaturating CRT together with Ran does not support nuclear export of GR. We also found that the addition of Crm1 and RanGTP at concentrations (0.2 and 1.9 μM, respectively) that promote efficient Rev export fails to support GR export. These transport data correlate with our in vitro binding data, since CRT-dependent binding and export of an NES-containing protein is dependent on RanGTP. In contrast, CRT-dependent binding and export of the DBD appears to be independent of Ran. In the context of permeabilized cell assays, CRT can substitute for Crm1 in the NES pathway; however, Crm1 cannot substitute for CRT in the DBD pathway.
Overlapping export substrate binding sites on CRT.
We considered two models to account for the ability of CRT to bind and mediate the nuclear export of two substrates with apparently unrelated export signals. In the first model, CRT could contain two distinct substrate binding sites, with only one of the two sites regulated by RanGTP. In the second model, CRT could contain a single substrate- binding site that accommodates both types of substrate and could use RanGTP to regulate the binding of only one of the two types of substrate. We reasoned that if CRT uses a single substrate binding site (or a single type of binding site), then CRT binding to the DBD should be inhibited in the presence of excess NES. To address this issue, we assayed CRT binding to an immobilized DBD in the absence and presence of WT and NES mutant forms of PKI. CRT binding to the DBD was reduced by the presence of WT PKI but not by the NES mutant form of PKI (Fig. 3A). Our data are consistent with a single type of substrate binding site on CRT, although we cannot rule out the possibility that CRT contains a second substrate binding site that is inhibited by an allosteric mechanism.
We also tested whether CRT contains a single type of substrate binding site by assaying nuclear export in permeabilized cells. Nuclear export of GR-GFP was tested in the absence and presence of WT and NES mutant forms of PKI. Inclusion of excess WT PKI (12 μM) in the reaction mixture inhibited the nuclear export of GR, whereas inclusion of the NES mutant form of PKI at the same concentration had no effect (Fig. 3B). GR export mediated by CRT was blocked by excess GR DBD (4.5 μM), consistent with previous data showing that the DBD functions as the signal for nuclear export of GR (3, 18).
The assays described above (Fig. 3A and B) were designed to measure the effect of excess NES on CRT binding to, and export of, the DBD. We performed analogous protein binding and nuclear export experiments that, instead, measured the effect of excess DBD on the interaction between CRT and the NES (Fig. 3C). In the biosensor assay, CRT binding to the NES (green tracing) or Crm1 binding to the NES (purple tracing) were both observed, but only in the presence of Ran GTP. CRT binding to the NES was reduced markedly by the presence of a WT DBD (light blue tracing), but this interaction was unaffected by the presence of a MUT DBD (dark blue tracing). The ability of the DBD to compete with NES for binding to CRT provides evidence that a single type of binding site is used for both substrates.
The effect of excess DBD on CRT-dependent export of NES substrate was tested in permeabilized cells by using Rev-GFP as a reporter. CRT-dependent export of Rev-GFP was inhibited by excess DBD and, as expected, by excess WT PKI (Fig. 3D). Addition of excess WT PKI resulted in inhibition of Crm1-dependent export of Rev-GFP, while addition of excess DBD had no effect on Rev-GFP export (data not shown). In summary, our results demonstrate that (i) CRT can bind to and mediate the nuclear export of a protein that contains either a hydrophobic NES or a steroid hormone receptor DBD, (ii) CRT-dependent binding and export of a protein containing the hydrophobic NES is dependent on Ran, (iii) CRT-dependent binding and export of a protein containing an appropriate DBD is not dependent on Ran, and (iv) CRT uses a similar substrate binding site for proteins that contain either a hydrophobic NES or an appropriate DBD.
Ca2+ is critical for CRT export activity.
CRT was originally discovered as a Ca2+ binding protein (29), and many of its functions in the ER have been proposed to be linked to its ability to bind Ca2+ (20). To determine if Ca2+ influences the nuclear export activity of CRT, we used an established method to release Ca2+ from recombinant CRT (43) and tested the protein in binding and transport assays. EGTA-induced Ca2+ release from CRT resulted in the loss of CRT binding to the DBD. Binding to the DBD was, however, restored by the addition of excess Ca2+ (Fig. 4A). Ca2+ removal from CRT caused a corresponding reduction in its capacity to promote nuclear export of GR in permeabilized cells, and the export activity was restored by the addition of excess Ca2+ (Fig. 4B). Unexpectedly, the capacity of CRT to mediate nuclear export of the NES substrate Rev-GFP was unaffected by EGTA, and the addition of excess Ca2+ to this assay actually inhibited Rev-GFP export (Fig. 4C). The effect of Ca2+ on Rev-GFP export appears to be linked to CRT since these treatments did not affect Crm1-dependent export of Rev-GFP (Fig. 4D). These data suggest that the Ca2+-bound state of CRT determines whether it binds to DBD- or NES-containing proteins and that these interactions are mutually exclusive under the conditions used in our assays.
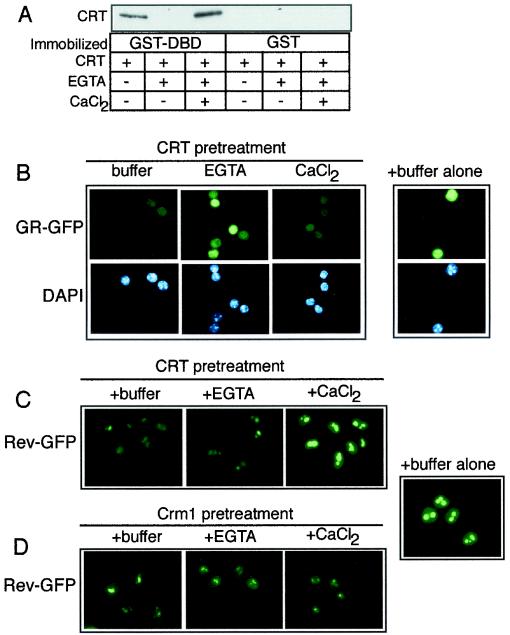
FIG. 4.
Ca2+ binding to CRT is necessary for nuclear export of GR. (A) Binding assay performed with GST-DBD or GST (2.5 μg each) immobilized on glutathione beads and CRT (500 ng). The amount of CRT bound in each reaction fraction was examined by immunoblotting for CRT. The CRT used in the binding assay was untreated, pretreated with 10 mM EGTA, or pretreated with 10 mM EGTA and 20 mM CaCl2. Ca2+ removal from CRT inhibits binding to the DBD, and this can be reversed by addition of Ca2+. (B and C) Ca2+ is required for CRT-dependent GR export; however, Ca2+ inhibits CRT-dependent NES export. (D) The presence of excess EGTA and Ca2+ in the permeabilized-cell assay mixture does not affect the export mediated by Crm1. CRT and Crm1 were used at a final concentration of 1.1 μM each in the export assays. The pretreatment of CRT and Crm1 with EGTA is described in Materials and Methods.
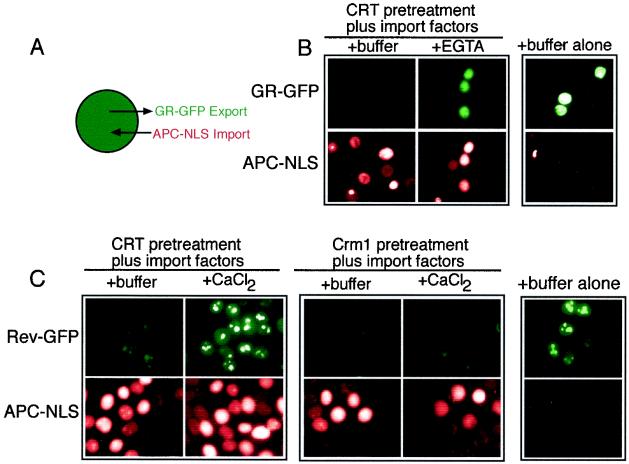
We considered it formally possible that addition of EGTA to digitonin-permeabilized cells could block GR export by inhibiting NPC function. Ca2+ depletion in cells has been reported to inhibit the nuclear import of an NLS reporter protein (15), and there is structural evidence that depletion of Ca2+ from the lumen of the nuclear envelope can alter NPC structure (41). It was also possible that Ca2+-free CRT might accumulate at the NPC and block transit through the nuclear pore. We addressed this issue in a permeabilized cell assay that reconstitutes both nuclear import and export (Fig. 5A), reasoning that nuclear import would provide a relevant readout of NPC activity that is independent of nuclear export. Import in these assays was reconstituted by the addition of α- and β-importin, Ran, and NTF2 and was monitored by the nuclear accumulation of allophycocyanin conjugated with the simian virus 40 large T-antigen NLS (APC-NLS). EGTA effectively blocked GR export without affecting APC-NLS import (Fig. 5B). Moreover, excess Ca2+ blocked CRT-dependent export of Rev-GFP without affecting APC-NLS import. This effect was specific to CRT since Crm1-dependent export of Rev-GFP was unaffected by excess Ca2+ (Fig. 5C). Our data are consistent with Ca2+-mediated regulation of the activity of CRT and not with the inactivation of NPC function. We note that our standard assay buffer contains 1 mM EGTA. Because this condition is permissive for CRT-dependent export, removal of Ca2+ from CRT may require the higher concentration of EGTA (10 mM) or pretreatment of purified CRT with EGTA at 30°C, or both.
FIG. 5.
Ca2+ chelation with EGTA inhibits CRT-dependent export without affecting nuclear import in permeabilized cells. (A) Diagram illustrating the assay for nuclear import (APC-NLS) and export (GR-GFP) in the same cells. (B) EGTA blocks CRT-dependent export of GR-GFP without affecting the nuclear import of APC-NLS in the presence of β-importin, Rch1, Ran, NTF2, NXT1, and RanBP1 (50μg/ml each). (C) Excess Ca2+ blocks CRT-dependent export of Rev-GFP without affecting the nuclear import of APC-NLS.
The P-domain and C-terminal domain of CRT Impart Ca2+ regulation.
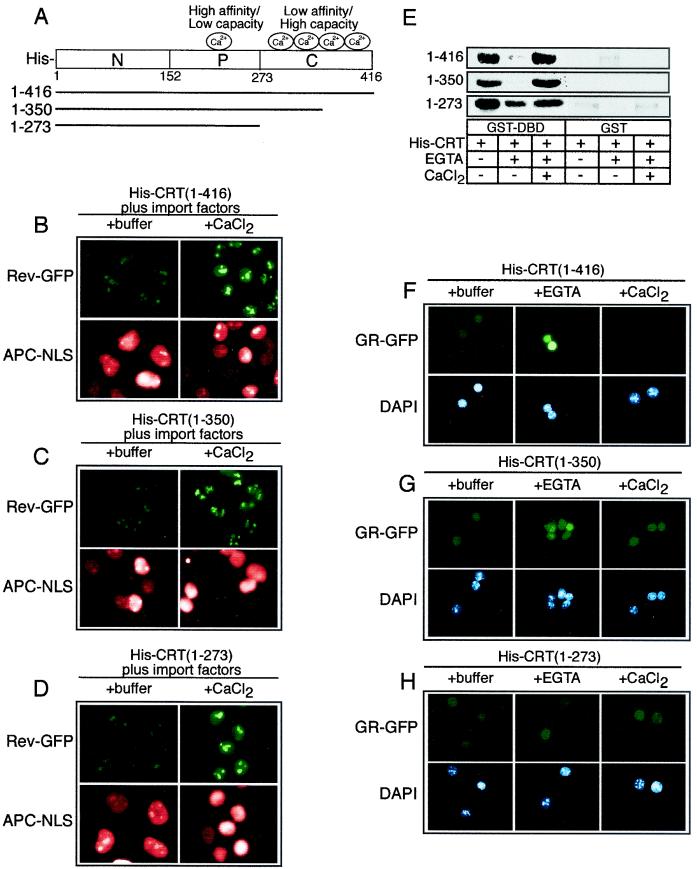
As diagrammed in Fig. 6A, the domain structure of CRT includes an acidic C-terminal domain that contains multiple low-affinity, high-capacity Ca2+ binding sites (KD ≈ 2 mM; >25 mol/mol) and a proline-rich P-domain that contains high-affinity, low-capacity Ca2+ binding sites (KD ≈ 1 μM) (2). In light of our data showing that Ca2+ can regulate CRT-dependent export of GR, we examined whether the C-terminal domain is required for CRT activity in our assays. CRT mutants lacking a portion of the C-terminal domain (retaining residues 1 to 350) or the entire C-terminal domain (retaining residues 1 to 273) were still functional for nuclear export of Rev-GFP, and the activities of these mutants was blocked by excess Ca2+ (Fig. 6B to D). From these data we infer that the Ca2+-dependent inhibition of hydrophobic NES export by CRT can be ascribed to the high-affinity low-capacity Ca2+ binding site in the P-domain.
FIG. 6.
The low-affinity Ca2+ binding sites in the C-terminal domain of CRT are not essential for nuclear export activity. (A) Diagram of the CRT structure and sites of Ca2+ binding. (B to D) Export assays performed with CRT proteins containing deletions in the low-affinity Ca2+ binding C-terminal domain. CRT lacking the entire C-terminal domain retains its Ca2+-dependent inhibition of NES export, indicating that this is probably due to the high-affinity, low-capacity Ca2+ binding site. (E) EGTA-sensitive binding of CRT to the DBD is partially lost on removal of the C-terminal domain (residues 1 to 273). (F to H) Removal of the entire C-terminal domain (residues 1 to 273) from CRT abrogates the EGTA-sensitive export of GR. Although the C-terminal domain of CRT is not required for export, it is necessary for Ca2+ regulation of GR export.
The deletion mutants were also examined for the Ca2+ dependence of DBD binding and GR export. We found that the deletion mutants still bound to the DBD in the presence of Ca2+ and that the mutant (residues 1 to 273) lacking the entire C-terminal domain was able to bind to the DBD in the presence of EGTA, albeit to a lesser extent than was the full-length CRT (Fig. 6E). This suggests that in the absence of Ca2+, the C-terminal domain normally exerts a negative regulation on the substrate binding activity of CRT. Removal of the entire C-terminal domain (residues 1 to 273) also resulted in loss of the EGTA-dependent inhibition of GR export that is observed with both the full-length and partial C-terminal domain deletion mutant (Fig. 6F to H). These data indicate that the low-affinity, high-capacity Ca2+ binding sites in the C-terminal domain of CRT are not required for nuclear export of hydrophobic NES or DBD-containing proteins but that they do contribute a regulatory function. The Ca2+ binding sites in the C-terminal domain are important for the EGTA-sensitive GR export, and the Ca2+ binding site in the P-domain appears to be sufficient to confer Ca2+-induced inhibition of NES export.
GR-GFP Export is Sensitive to Ca2+ Depletion.
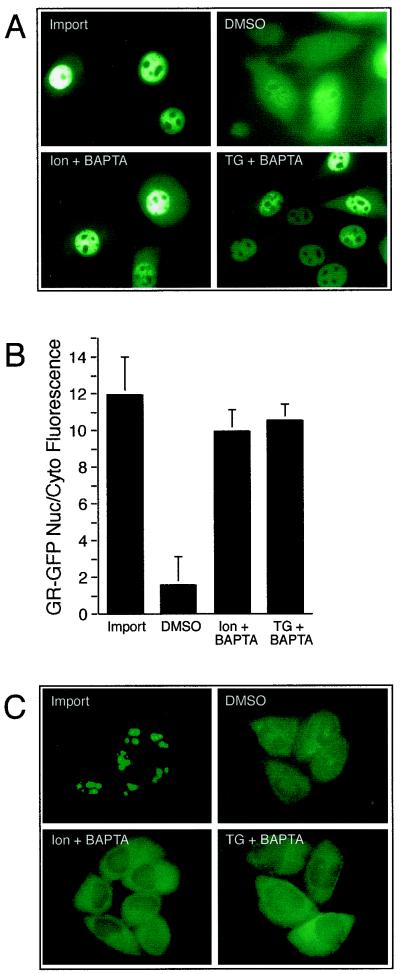
Our results showing that GR undergoes CRT- and Ca2+-sensitive export in permeabilized cells prompted us to examine whether this pathway is operational in living cells. The 3676 cell line (27), which stably expresses GR-GFP, was treated with Dex to induce import and then transferred to Dex-free medium that contained reagents known to deplete luminal and cytosolic Ca2+. The effect of Ca2+ depletion on nuclear export was examined by fluorescence microscopy after 6 h and quantitated by measuring the nuclear/cytoplasmic fluorescence ratio of GR-GFP. The conditions included treatment with the Ca2+ ionophore Ion (1 μM) or the ER Ca2+ pump inhibitor TG (1 μM). The membrane-permeable form of BAPTA-AM (10 μM) was included with Ion and TG to chelate Ca2+ that was released. We found that Ion or TG administered in the presence of BAPTA-AM were both effective at blocking the nuclear export of GR-GFP (Fig. 7A). During the export phase of the experiment, the nuclear/cytoplasmic fluorescence in the control cells showed a significant reduction from 11.9 ± 2.1 to 1.5 ± 1.5 (Fig. 7B). In contrast, the nuclear/cytoplasmic fluorescence in the Ion- and TG-treated cells showed only a slight reduction, to 9.9 ± 1.2 and 10.5 ± 0.9, respectively.
FIG. 7.
Ca2+ depletion in vivo inhibits the nuclear export of GR. (A) Representative fields of cells expressing GR-GFP after induction of nuclear import with Dex (Import). Following Dex removal, the cells were treated for 5 h with vehicle (DMSO), Ion and BAPTA-AM, or TG and BAPTA-AM, and the GR-GFP distribution was recorded in living cells. (B) Measurements of the nuclear/cytoplasmic (Nuc/Cyto) ratios of GR-GFP fluorescence in cells incubated under conditions that deplete Ca2+. Depletion of luminal stores of Ca2+ with Ion andTG and chelation with BAPTA-AM results in significant reduction of GR export to the cytoplasm. (C) Depletion of luminal Ca2+ stores does not inhibit NES export. The RGG 2.2 cell line expressing Rev-GFP was treated with Dex to induce importing of the reporter, which concentrates in the nucleoli. The cells were then maintained for 6 h under conditions that deplete Ca2+. During the last 1 h, Dex was removed to allow Rev-GFP export to the cytoplasm. Rev-GFP export was observed whether or not Ca2+ was depleted.
It is unlikely that Ion, TG, and BAPTA-AM cause a global disruption of NPC structure because NES-dependent export of Rev-GFP continues under Ca2+ depletion conditions in vivo (Fig. 7C) as well as in vitro (26). The transport of other cargos is unaffected by Ca2+ depletion in vivo, including Crm1-dependent nuclear export of mitogen-activated protein kinase-activated protein kinase 2 (42) and importin/karyopherin-dependent nuclear import of Rev-GFP (B. Black, unpublished observations). Our finding that nuclear export of GR in permeabilized cells is sensitive to the Ca2+-bound state of the CRT used in the assays suggests that depletion of Ca2+ in vivo blocks the CRT-dependent export of GR in the cell.
DISCUSSION
Our study has revealed that Ca2+ and RanGTP can regulate the nuclear export activity of CRT. Ca2+ binding to CRT is necessary for its interaction with the DBD of GR in solid-phase binding assays and for nuclear export of GR in digitonin-permeabilized cell assays. These interactions appear to be independent of Ran, since Ran is not required for CRT binding to the DBD. Ran does not stimulate GR export when CRT is rate-limiting in the assay, and GTPase-deficient mutants of Ran do not block GR export. Under conditions where the low-affinity Ca2+ binding sites of CRT are saturated with Ca2+, CRT does not support the nuclear export of a hydrophobic NES substrate. The export capacity for a hydrophobic NES substrate can be restored by EGTA-mediated removal of Ca2+ from CRT. Under these conditions of low Ca2+, RanGTP acts as a stimulatory factor of CRT in a mechanism that involves the assembly of CRT, NES, and RanGTP into a complex (18). Collectively, our data suggest that Ca2+ binding determines whether CRT interacts with DBD containing cargo or with NES-containing cargo, and only the latter involves Ran.
The characterization of CRT over the past several years has revealed that it can associate with structurally distinct substrates (20). Studies of the chaperone-like functions of CRT proposed to occur in the ER lumen have addressed the in vitro interaction of CRT with oligosaccharides, both free in solution and as a structural component of glycoproteins. CRT is capable of binding directly to high-mannose-containing oligosaccharides, and CRT can partially suppress the aggregation of certain glycosylated proteins exposed to elevated temperatures (35). It can also suppress the thermal aggregation of nonglycosylated proteins (35). These data have been taken as evidence that in the ER, CRT may use both its lectin and peptide binding properties to stabilize protein-folding intermediates. The view that CRT participates in the folding of glycoproteins in the ER is logical, given the sequence relatedness (34% identity; expectation value, <10−50) and functional similarities to the ER chaperone calnexin. Calnexin is a key component of a quality control pathway that monitors the folding state of glycoproteins in the ER. The current view is that calnexin and CRT interact transiently with protein-folding intermediates, effectively retaining them in the ER until a native conformation is attained (17).
Studies of the functions of CRT that occur outside the ER have yielded an even more diverse collection of substrates (20). These include the cytoplasmic domain of α-integrins (33), steroid hormone receptors (6), NES-containing proteins (18), and a stem-loop structure from rubella virus RNA (38). Some of these substrates contain a binding site for CRT that can be recognized at the sequence level, while other substrates seem structurally distinct. The site within α-integrin recognized by CRT is the sequence KXGFFKR, which is similar to the sequence KVFFKR within the DNA recognition helix of GR and other nuclear receptors. The NESs in PKI (LALKLAGLDIN) and Rev (LPPLERLTL) are similar to each other, but they show little resemblance to the CRT binding sites in α-integrin and the DNA recognition helix. One feature that is shared by most of these signals is the presence of hydrophobic amino acids that, when mutated, abrogates binding to CRT. In the case of the hydrophobic NES, the critical leucines are probably part of an amphipathic helix and the side chains are predicted to physically contact the receptors Crm1 and CRT. This is not the case for the two phenylalanines within the DNA recognition helix that are necessary for binding to CRT (3). The crystal structures of different nuclear receptors reveal that the phenylalanine side chains pack against the core of the DBD, at least when bound to DNA (32). Thus, the FF-to-AA mutations in the DNA recognition helix of GR that inhibit nuclear export probably affect the folding of the DBD, resulting in a structure that is no longer recognized by CRT (3).
How does CRT recognize its substrates? Our present study and previous work from other laboratories suggest that the N-terminal domain mediates substrate recognition and that regulation of binding is imparted by the Ca2+-dependent conformational changes in the P-domain and C-terminal domain of CRT. Multiple low-affinity Ca2+ binding sites are contained within the C-terminal domain of CRT, and at least one high-affinity Ca2+ binding site is contained in the middle domain (P-domain) (2). Ca2+ is a cofactor for CRT binding to oligosaccharides, glycoproteins, and certain nonglycosylated proteins in vitro (43). Moreover, in our assays, Ca2+ is necessary for the physical interaction between CRT and the DBD as measured by direct binding and as assayed by nuclear export of GR in permeabilized cells.
Ca2+ binding induces structural changes in CRT that dramatically alter its fragmentation pattern that results when it is treated with several different proteases (7). Of particular relevance to our study is the N-terminal 27 kDa of CRT, which is protected from tryptic digestion in the presence of Ca2+ but is highly susceptible in the absence of Ca2+ (7). This protease-resistant core of CRT retains at least one high-affinity Ca2+ binding site from the P-domain, while all of the low-affinity Ca2+ binding sites in the C-terminal domain are removed by digestion. We found that a CRT deletion mutant (residues 1 to 273) that contains the protease-resistant core is still functional for nuclear export of GR in permeabilized cells, indicating that the low-affinity Ca2+ binding sites are dispensable for our assays that score a positive interaction between CRT and GR. It is interesting that this CRT deletion mutant (residues 1 to 273) shows some Ca2+-independent binding to the DBD, although the high-affinity Ca2+ binding site is still present in this mutant. The fact that the C-terminal Ca2+ binding sites are necessary for EGTA-induced inhibition of CRT binding to the DBD strongly suggests that the C-terminal domain functions in negative regulation of substrate binding. We have also observed that the N-terminal domain of CRT (residues 1 to 150) expressed as a GST fusion protein is sufficient to mediate nuclear export of GR in permeabilized cells (unpublished observations). This is consistent with previous data showing that the N-terminal domain of CRT can block GR binding to the GRE in vitro (6). All available information indicates, therefore, that the N-terminal domain of CRT is the substrate binding domain that physically contacts the DBD of GR. We infer that the N-terminal domain also binds hydrophobic NES substrates, since the DBD and NES display competitive interactions with CRT in our binding assays.
Emerging structural information provides a context for interpreting how Ca2+ might regulate CRT activity. Although the atomic structure of CRT has not yet been solved, the structure of the luminal domain of calnexin, which is 35% identical to CRT, has been solved at 2.9 Å resolution (37). The structure of calnexin reveals that it contains a compact globular domain (residues 61 to 262 and 415 to 458) and a large hairpin that forms a highly extended arm (residues 277 to 410). Tandem repeats in the P-domain comprise the arm, which projects 140 Å away from globular domain. The hairpin structure brings the N- and C-terminal domains together to form the globular domain (37). Thus, the close proximity of these domains may help explain how the occupancy of Ca2+ binding sites in the C-terminal domain can influence substrate recognition by the N-terminal domain in both calnexin and CRT. The nuclear magnetic resonance spectroscopy structure of the P-domain of CRT (residues 189 to 288) was recently solved, and, like calnexin, this domain forms a highly extended arm (9). The crystal structure of calnexin and the nuclear magnetic resonance spectroscopy structure of the P-domain of CRT were both solved in the presence of Ca2+, and so the basis of Ca2+-dependent changes in structure awaits further studies. It has been suggested that the P-domain or arm is a Ca2+-sensitive protein interaction site for CRT (9).
Does Ca2+ regulate the engagement of CRT with nuclear export substrates? Both cytoplasmic and nuclear Ca2+ levels can respond to signaling events, and in some cases the modulation of nuclear Ca2+ occurs independently of cytoplasmic Ca2+ (36). Depending on the system and method of measurement, Ca2+ levels in the cell are thought to increase to approximately micromolar concentrations on stimulation (5). This concentration would be predicted to fill the high-affinity Ca2+ binding sites on CRT but not the low-affinity sites. It should be noted, however, that the affinity of CRT for Ca2+ was measured in vitro. While it is clear that CRT contains at least two classes of binding sites with different affinities for Ca2+, demonstrating the Ca2+ occupancy of particular sites on CRT in the cell may require the development of fluorescence-based assays that can register Ca2+ binding. This approach could be used to determine if Ca2+ is constitutively bound to CRT or if Ca2+ binds reversibly, thereby acting as a regulator of CRT function. We speculate that transient increases in Ca2+ concentration, theoretically, could promote the assembly of CRT-DBD complexes in the nucleus. The complex could then undergo export to the cytoplasm, where the complex is disassembled, which would be favored by low free Ca2+ concentrations.
Although the origin of nuclear Ca2+ remains an issue of debate, it is clear that processes including transcription are regulated by nuclear Ca2+ (16). Several observations are actually consistent with the notion that Ca2+ gradients between the nucleus and cytoplasm might be used to promote the assembly and disassembly of CRT complexes. First, the presence of Ca2+ ATPases in the nuclear envelope and the lack of Ca2+ buffering and sequestration proteins in the nucleus should prolong the availability of free Ca2+ (12). Second, the inner membrane of the nuclear envelope contains both major Ca2+ release channels, which may be activated independently of channels in the ER membrane that face the cytoplasm (36). Third, morphological data show that the ER forms invaginations that reach deep into the nucleus (13), which could place Ca2+ release channels close to sites of CRT-DBD assembly in the nucleoplasm. While it is clear that Ca2+ levels in the nucleus do change in response to signaling events, it remains to be established whether Ca2+ regulation of CRT is a dynamic process that involves reversible binding or whether Ca2+ is constitutively bound and simply maintains the tertiary structure of CRT.
CRT is thought to function in multiple pathways in the cell (23). In the ER, CRT may function as (i) a chaperone that mediates folding by transiently binding to oligosaccharide side chains and peptide domains, (ii) a Ca2+ storage protein involved in Ca2+ homeostasis, and (iii) a factor that directly regulates the activity of the Ca2+ pumps. At the plasma membrane, CRT may modulate (i) cell migration by interacting with adhesion plaque proteins and (ii) cell-cell interactions by interacting with cell surface proteins. In the nucleocytoplasmic compartment, CRT can regulate (i) the activity of steroid receptors through direct binding to the DBD of these proteins and (ii) the redistribution of steroid receptors to the cytoplasm. Because most of these functions mentioned above have been linked to the Ca2+ binding activity of CRT, conditions that influence the Ca2+ levels are predicted to regulate the activity of CRT. In this regard, our findings that Ca2+ is necessary for the nuclear export function of CRT in vitro and that Ca2+ depletion results in GR export defects in vivo suggest that this pathway is used to regulate the nucleocytoplasmic distribution of GR and possibly other steroid hormone receptors. This link between Ca2+ and GR export could negatively regulate transcription through a nuclear transport pathway, perhaps using Ca2+ as a second messenger.
Acknowledgments
We thank Gordon Hager, Dona Love, and John Hanover for cell lines and Ian Macara, Dirk Görlich, and Larry Gerace for plasmids.
Financial support was provided by the NIH (GM58639-01 to B.M.P.) and the DOD (DAMD 17-00-1-0048 to F.R.).
REFERENCES
- 1.Askjaer, P., T. H. Jensen, J. Nilsson, L. Englmeier, and J. Kjems. 1998. The specificity of the CRM1-Rev nuclear export signal interaction is mediated by RanGTP. J. Biol. Chem. 273:33414-33422. [DOI] [PubMed] [Google Scholar]
- 2.Baksh, S., and M. Michalak. 1991. Expression of calreticulin in Escherichia coli and identification of its Ca2+ binding domains. J. Biol. Chem. 266:21458-21465. [PubMed] [Google Scholar]
- 3.Black, B. E., J. M. Holaska, F. Rastinejad, and B. M. Paschal. 2001. DNA binding domains in diverse nuclear receptors function as nuclear export signals. Curr. Biol. 11:1749-1758. [DOI] [PubMed] [Google Scholar]
- 4.Black, B. E., L. Lévesque, J. M. Holaska, T. C. Wood, and B. M. Paschal. 1999. Identification of an NTF2-related factor that binds Ran-GTP and regulates nuclear protein export. Mol. Cell. Biol. 19:8616-8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brini, M., and E. Carafoli. 2000. Calcium signalling: a historical account, recent developments and future perspectives. Cell Mol. Life Sci. 57:354-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns, K., B. Duggan, E. A. Atkinson, K. S. Famulski, M. Nemer, R. C. Bleackley, and M. Michalak. 1994. Modulation of gene expression by calreticulin binding to the glucocorticoid receptor. Nature 367:476-480. [DOI] [PubMed] [Google Scholar]
- 7.Corbett, E. F., K. M. Michalak, K. Oikawa, S. Johnson, L. D. Campbell, P. Eggleton, C. Kay, and M. Michalak. 2000. The conformation of calreticulin is influenced by the endoplasmic reticulum lumenal environment. J. Biol. Chem. 275:27177-27185. [DOI] [PubMed] [Google Scholar]
- 8.Dedhar, S., P. S. Rennie, M. Shago, C. Y. Hagesteijn, H. Yang, J. Filmus, R. G. Hawley, N. Bruchovsky, H. Cheng, R. J. Matusik, and V. Giguere. 1994. Inhibition of nuclear hormone receptor activity by calreticulin. Nature 367:480-483. [DOI] [PubMed] [Google Scholar]
- 9.Ellgaard, L., R. Riek, T. Herrmann, P. Peter Güntert, D. Braun, A. Helenius, and K. Wüthrich. 2001. NMR structure of the calreticulin P-domain. Proc. Natl. Acad. Sci. USA 98:3133-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer, U., J. Huber, W. C. Boelens, I. W. Mattaj, and R. Luhrmann. 1995. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell 82:475-483. [DOI] [PubMed] [Google Scholar]
- 11.Fornerod, M., M. Ohno, M. Yoshida, and I. W. Mattaj. 1997. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90:1051-1060. [DOI] [PubMed] [Google Scholar]
- 12.Fox, J. L., A. D. Burgstahler, and M. H. Nathanson. 1997. Mechanism of long-range calcium signalling in the nucleus of isolated rat hepatocytes. Biochem. J. 326:491-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fricker, M., M. Hollinshead, N. White, and D. Vaux. 1997. Interphase nuclei of many mammalian cell types contain deep, dynamic, tubular membrane-bound invaginations of the nuclear envelope. J. Cell Biol. 136:531-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorlich, D., and U. Kutay. 1999. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 15:607-660. [DOI] [PubMed] [Google Scholar]
- 15.Greber, U. F., and L. Gerace. 1995. Depletion of calcium from the lumen of endoplasmic reticulum reversibly inhibits passive diffusion and signal-mediated transport into the nucleus. J. Cell Biol. 128:5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardingham, G. E., S. Chawla, C. M. Johnson, and H. Bading. 1997. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature 385:260-265. [DOI] [PubMed] [Google Scholar]
- 17.Helenius, A., T. S. Trombetta, D. N. Hebert, and J. F. Simons. 1997. Calnexin, calreticulin, and the folding of glycoproteins. Trends Cell Biol. 7:193-200. [DOI] [PubMed] [Google Scholar]
- 18.Holaska, J. M., B. E. Black, D. C. Love, J. A. Hanover, J. Leszyk, and B. M. Paschal. 2001. Calreticulin is a receptor for nuclear export. J. Cell Biol. 152:127-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holaska, J. M., and B. M. Paschal. 1998. A cytosolic activity distinct from Crm1 mediates nuclear export of protein kinase inhibitor in permeabilized cells. Proc. Natl. Acad. Sci. USA 95:14739-14744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, S., M. Michalak, M. Opas, and P. Eggleton. 2001. The ins and outs of calreticulin: from the ER lumen to the extracellular space. Trends Cell Biol. 11:122-129. [DOI] [PubMed] [Google Scholar]
- 21.Katagiri, Y., K. Takeda, Z. X. Yu, V. J. Ferrans, K. Ozato, and G. Guroff. 2000. Modulation of retinoid signalling through NGF-induced nuclear export of NGFI-B. Nat. Cell Biol. 2:435-440. [DOI] [PubMed] [Google Scholar]
- 22.Kehlenbach, R. H., A. Dickmanns, A. Kehlenbach, T. Guan, and L. Gerace. 1999. A role for RanBP1 in the release of CRM1 from the nuclear pore complex in a terminal step of nuclear export. J. Cell Biol. 145:645-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krause, K. H., and M. Michalek. 1997. Calreticulin. Cell 88:439-443. [DOI] [PubMed] [Google Scholar]
- 24.Lindsay, M. E., J. M. Holaska, K. Welch, B. M. Paschal, and I. G. Macara. 2001. Ran-binding protein 3 is a cofactor for Crm1-mediated nuclear protein export. J. Cell Biol. 153:1391-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, J., and D. B. DeFranco. 2000. Protracted nuclear export of glucocorticoid receptor limits its turnover and does not require the exportin 1/CRM1-directed nuclear export pathway. Mol. Endocrinol. 14:40-51. [DOI] [PubMed] [Google Scholar]
- 26.Love, D. C., T. D. Sweitzer, and J. A. Hanover. 1998. Reconstitution Of HIV-1 Rev nuclear export-independent requirements for nuclear import and export. Proc. Natl. Acad. Sci. USA 95:10608-10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNally, J. G., W. G. Muller, D. Walker, R. Wolford, and G. L. Hager. 2000. The glucocorticoid receptor: rapid exchange with regulatory sites in living cells. Science 287:1262-1265. [DOI] [PubMed] [Google Scholar]
- 28.Nakielny, S., and G. Dreyfuss. 1999. Transport of proteins and RNAs in and out of the nucleus. Cell 99:677-690. [DOI] [PubMed] [Google Scholar]
- 29.Ostwald, T. J., and D. H. MacLennan. 1974. Isolation of a high affinity calcium-binding protein from sarcoplasmic reticulum. J. Biol. Chem. 249:974-979. [PubMed] [Google Scholar]
- 30.Paschal, B. M., and L. Gerace. 1995. Identification of NTF2, a cytosolic factor for nuclear import that interacts with nuclear pore complex protein p62. J. Cell Biol. 129:925-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Picard, D., and K. R. Yamamoto. 1987. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 6:3333-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rastinejad, F. 2001. Retinoid X receptor and its partners in the nuclear receptor family. Curr. Opin. Struct. Biol. 11:33-38. [DOI] [PubMed] [Google Scholar]
- 33.Rojiani, M. V., B. B. Finlay, V. Gray, and S. Dedhar. 1991. In vitro interaction of a polypeptide homologous to human Ro/SS-A antigen (calreticulin) with a highly conserved amino acid sequence in the cytoplasmic domain of integrin alpha subunits. Biochemistry 30:9859-9866. [DOI] [PubMed] [Google Scholar]
- 34.Rubio, I., P. Buckle, H. Trutnau, and R. Wetzker. 1997. Real-time assay of the interaction of a GST fusion protein with a protein ligate using resonant mirror technique. Bio/technology 22:269-271. [DOI] [PubMed] [Google Scholar]
- 35.Saito, Y., Y. Ihara, M. R. Leach, M. F. Cohen-Doyle, and D. B. Williams. 1999. Calreticulin functions in vitro as a molecular chaperone for both glycosylated and non-glycosylated proteins. EMBO J. 18:6718-6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santella, L., and K. Kyozuka. 1997. Effects of 1-methyladenine on nuclear calcium transients and meiosis resumption in starfish oocytes are mimicked by the nuclear injection of inositol 1,4,5-triphosphate and cADP-ribose. Cell Calcium 22:11-20. [DOI] [PubMed] [Google Scholar]
- 37.Schrag, J. D., J. J. Bergeron, Y. Li, S. Borisova, M. Hahn, D. Y. Thomas, and M. Cygler. 2001. The structure of calnexin, an ER chaperone involved in quality control of protein folding. Mol. Cell 8:633-644. [DOI] [PubMed] [Google Scholar]
- 38.Singh, N. K., C. D. Atreya, and H. L. Nakhasi. 1994. Identification of calreticulin as a rubella virus RNA binding protein. Proc. Natl. Acad. Sci. USA 91:12770-12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stade, K., C. S. Ford, C. Guthrie, and K. Weis. 1997. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell 90:1041-1050. [DOI] [PubMed] [Google Scholar]
- 40.Steggerda, S. M., and B. M. Paschal. 2002. Regulation of nuclear import and export by the GTPase Ran. Int. Rev. Cytol. 217:41-91. [DOI] [PubMed] [Google Scholar]
- 41.Stoffler, D., K. N. Goldie, B. Feja, and U. Aebi. 1999. Calcium-mediated structural changes of native nuclear pore complexes monitored by time-lapse atomic force microscopy. J. Mol. Biol. 287:741-752. [DOI] [PubMed] [Google Scholar]
- 42.Strubing, C., and D. E. Clapham. 1999. Active nuclear import and export is independent of lumenal Ca2+ stores in intact mammalian cells. J. Gen. Physiol. 113:239-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vassilakos, A., M. Michalak, M. A. Lehrman, and D. B. Williams. 1998. Oligosaccharide binding characteristics of the molecular chaperones calnexin and calreticulin. Biochemistry 37:3480-3490. [DOI] [PubMed] [Google Scholar]
- 44.Wen, W., J. L. Meinkoth, R. Y. Tsien, and S. S. Taylor. 1995. Identification of a signal for rapid export of proteins from the nucleus. Cell 82:463-473. [DOI] [PubMed] [Google Scholar]
- 45.Wente, S. R. 2000. Gatekeepers of the nucleus. Science 288:1374-1377. [DOI] [PubMed] [Google Scholar]
- 46.Wiechens, N., and F. Fagotto. 2001. CRM1- and Ran-independent nuclear export of beta-catenin. Curr. Biol. 11:18-27. [DOI] [PubMed] [Google Scholar]