Abstract
Using a system that expresses a constitutively kinase-active c-Abl protein [c-Abl(KA)], we identified the protein IκBα as a novel substrate of c-Abl. This kinase-substrate relationship is not only confirmed at the level of endogenous proteins but is also supported by a physical interaction between the two proteins. Interestingly, the association of c-Abl with IκBα, which is detectable in the form of nonphosphorylated proteins, is remarkably enhanced by an inducible binding of tyrosine-phosphorylated IκBα to the c-Abl SH2 domain. In contrast to the serine 32/34 phosphorylation that triggers ubiquitination and degradation of IκBα, c-Abl-mediated phosphorylation at tyrosine 305 is associated with an increase of the IκBα protein stability. Significantly, this activity is not shared by the oncogenic Bcr-Abl, because it is unique to the nuclear c-Abl. We also demonstrate that c-Abl targets the nuclear subpopulation of IκBα for phosphorylation and induces it to accumulate in the nucleus. As a consequence, NF-κB transcription activity is abolished, leading to an increased cellular sensitivity to the induction of apoptosis. The functional importance of c-Abl-mediated IκBα phosphorylation is highlighted by a loss of response of the IκBα(Y305F) protein to c-Abl-mediated regulation. Using cells expressing the c-Abl(KA) protein under the control of an inducible promoter, we demonstrate inactivation of the NF-κB-dependent cell survival pathway as one of the mechanisms for c-Abl-mediated apoptosis.
c-Abl is a ubiquitously expressed nonreceptor tyrosine kinase that distributes in both the cytoplasm and the nucleus. In contrast to the oncogenic v-Abl and Bcr-Abl kinases, whose activities are constitutively activated, the in vivo kinase activity of c-Abl is very low, which is attributable to tight regulation. The kinase activity of c-Abl is, however, induced in response to genotoxic stress, cell adhesion, and growth factor (8, 9, 13, 15, 16). The cellular response to c-Abl activation depends on the location of c-Abl within the cells, since nuclear c-Abl and cytoplasmic c-Abl have different effects. The oncogenic forms of Abl, including v-Abl and Bcr-Abl, are exclusively cytoplasmically localized. They induce cellular transformation by promoting proliferation and inhibiting apoptotic cell death (8, 9, 13, 15, 16). These activities contrast with nuclear c-Abl, which inhibits cell growth. In keeping with this notion, numerous studies have shown that c-Abl contributes to apoptotic cell death induced by a number of physiological and pharmacological agents (8, 9, 13, 15, 16). Among many proteins that are phosphorylated by the c-Abl kinase, c-Jun and p73 are the only known nuclear substrates. While c-Jun has been implicated in apoptosis, this activity of c-Jun is observed only in certain cell types (6), and it is not known whether c-Jun participates in c-Abl-mediated apoptosis. Whereas it has been clearly shown that p73 is a downstream effecter of the c-Abl-dependent apoptotic pathway (8, 9, 13, 15, 16), the fact that the p73 expression is tissue type and developmental-stage dependent suggests a possibility for the presence of one or more additional nuclear substrates of c-Abl that mediate its cytotoxic effect.
NF-κB is an inducible transcription factor that regulates the expression of a number of proteins involved in the regulation of cell survival and immune responses (7). The biological activity of NF-κB is tightly controlled by its inhibitor protein ΙκB, which binds to and sequesters NF-κB in the cytoplasm. Upon exposure to extracellular signals, a series of biochemical events target the inhibitor protein for degradation, resulting in release and subsequent translocation of NF-κB into the nucleus, where it transactivates the expression of its downstream target genes. IκBα, the best-characterized member of the ΙκB family, contains both nuclear localization sequence (NLS) and nuclear export signal (NES) that render to IκBα the ability to rapidly shuttle between the nucleus and the cytoplasm. Newly synthesized IκBα proteins can enter the nucleus to bind to and disassociate NF-κB from DNA. IκBα, through its NES, can mediate the nuclear export of IκBα/NF-κB complexes, a process called postinduction repression that is essential to restore the preinduction state of the complexes. Importantly, this activity of IκBα is not shared by other ΙκB members and is important for certain physiological and pathological situations in which NF-κB needs to be rapidly reactivated (4).
In an effort to gain a better understanding of the proapoptotic function of nuclear c-Abl, we recently generated vectors expressing constitutive kinase-active c-Abl [c-Abl(KA)] to search for its potential substrates. In this report, we present evidence to identify IκBα as another c-Abl substrate that plays an important role in mediating c-Abl-dependent cell death.
MATERIALS AND METHODS
Cell culture and transfection.
293T cells and U2OS cells (American Type Culture Collection) and c-Abl−/− MEF (Bruce Mayer, Harvard Medical School, Boston, Mass.) were maintained in minimal essential medium (MEM) supplemented with 10% fetal bovine serum. Cells were transfected by a calcium-phosphate method as described previously (3). Luciferase activity was measured 24 h posttransfection using a Lumat 9507 illuminometer (EG&G Berthold) as described previously (3).
Preparation of whole-cell extracts and immunoprecipitation analysis.
Cells were transfected in 60-mm-diameter plates with 5 μg of DNA and harvested at 24 h posttransfection. Cells were lysed in 200 μl of lysis buffer (10 mM Tris-HCl, pH 7.5, 1 mM EDTA, 1% Triton X-100, 150 mM NaCl, 1 mM dithiothreitol [DTT], 10% glycerol, 0.2 mM phenylmethylsulfonyl fluoride [PMSF], and protease inhibitors) by incubating on ice for 30 min, and the extracts were centrifuged at 30 × 3.75 g (Eppendorf) for 15 min to remove cell debris. Protein concentrations were determined using a Bio-Rad protein assay (Hercules, Calif.). After addition of 5× loading buffer, the samples were incubated at 95°C for 5 min and resolved through sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). For immunoprecipitation, cell lysates were prepared in 0.5% Triton X-100 lysis buffer and incubated with the indicated antibody for 4 h followed by incubation with protein A-protein G beads (Oncogene Science, Cambridge, Mass.) for an additional 4 h. Immune complexes and whole lysates were separated by SDS-PAGE. Proteins were transferred onto nitrocellulose membranes (Schleicher & Schuell) and probed with the indicated antibody. Proteins were visualized with an enhanced chemiluminescence detection system (NEN).
Subcellular distribution assay.
Cells were grown on chamber slides (Nunc, Naperville, Ill.) and transfected with the indicated vector. Cells were washed with cold phosphate-buffered saline (PBS) 24 h after transfection and fixed with 4% paraformaldehyde (Sigma) for 30 min at 4°C. After washing with PBS, the slides were incubated with DAPI (4′,6′-diamidino-2-phenylindole) (10 μg/ml; Sigma) for 1 h. Following PBS washing, the slides were mounted with Fluoromount-G (Southern Biotechnology Associates) containing 2.5 mg of n-propyl gallate/ml (Sigma). Specimens were examined under a fluorescence microscope (Zeiss).
Subcellular fractionation.
For fractionation experiments, cells were trypsinized, rinsed with PBS and pelleted. Cells were then suspended in 400 μl of hypotonic buffer (50 mM Tris [pH 7.5], 5 mM EDTA, 10 mM NaCl) containing 0.05% NP-40 and spun at 2,000 rpm for 2 min before the supernatant was collected. The remaining pellet was washed 5 times with 400 μl of hypotonic buffer containing 0.1% NP-40. The remaining pellet was resuspended in 500 μl of 1× RIPA buffer (20 mM Tris [pH 7.5], 5 mM EDTA, 150 mM NaCl, 1.0% Nonidet P-40, 1.0% deoxycholate, 0.025% SDS and 1 mM PMSF) and briefly sonicated. After centrifugation at 14,000 rpm for 15 min at 4°C, the nuclear or cytoplasmic fractions were assessed for purity using nucleus-specific (histone) and cytoplasm-specific (tubulin) marker proteins.
Electrophoretic mobility shift assay (EMSA).
A total of 2 × 106 cells were washed with cold PBS and suspended in 0.4 ml of hypotonic buffer. The cells were allowed to swell on ice for 15 min, after which 12.5 μl of 10% Nonidet P-40 was added. The tube was then vigorously vortexed for 10 s, and the homogenate was centrifuged for 30 s at 14,000 rpm. The nuclear pellet was resuspended in 25 μl of ice-cold nuclear extraction buffer (20 mM HEPES [pH 7.9], 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF plus protease inhibitor cocktails) and the tube was incubated on ice for 30 min with intermittent mixing. This nuclear extract was then centrifuged for 15 min at 14,000 rpm at 4°C, and the supernatant was either used immediately or stored at −70°C for later use. The protein content was measured by the method of Bradford. EMSAs were performed by incubating 4 to 5 μg of nuclear extract with 16 fmol of 32P-end- labeled probe (GGGGACTTTCCC; Santa Cruz Biotechonology) in the presence of 1 to 2 μg of poly(dI-dC) in a binding buffer (25 mM HEPES [pH 7.9], 0.5 mM EDTA, 0.5 mM DTT, 1% Nonidet P-40, 5% glycerol, and 50 mM NaCl) for 20 min at 37°C. The DNA-protein complex formed was separated from free oligonucleotide on 7.5% native polyacrylamide gel using buffer containing 50 mM Tris, 200 mM glycine, pH 8.5, and 1 mM EDTA and then the gel was dried and exposed to film.
Measurement of apoptosis by fluorescence-activated cell sorting analysis (FACS).
Cells were fixed in 70% ethanol and stained with 40 μg of propidium iodide (Sigma) per ml and 10 μg of RNase A (Sigma) per ml for 1 h before analysis on an Ortho 2150 Cytofluorograf (Cyonics/Uniphase).
RESULTS
IκBα is a novel substrate of c-Abl.
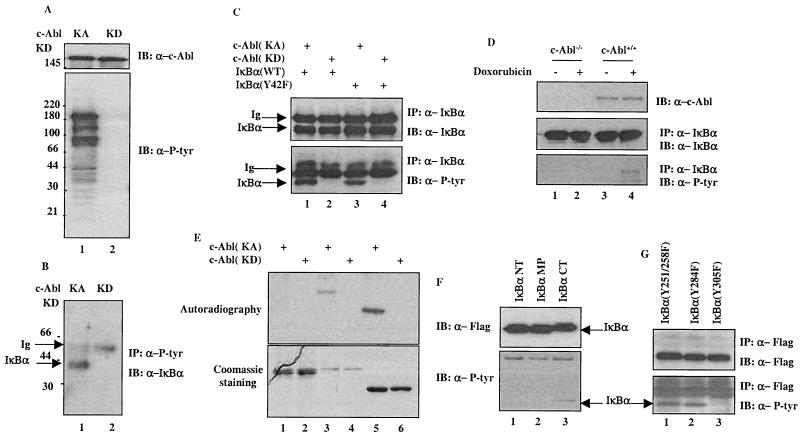
To search for additional substrates of the c-Abl tyrosine kinase, we generated a Flag-tagged vector that expresses the c-Abl proteins with a constitutive kinase activity. Characterization of this c-Abl(KA) has been described elsewhere (L. Nie, H. Kawai, and Z.-M. Yuan, submitted for publication). When transiently transfected into 293T cells, expression of the c-Abl(KA) was associated with an induction of tyrosine phosphorylation of numerous proteins, as revealed by anti-P-Tyr immunoblotting analysis (Fig. 1A, lower panel, lane 1). The induced tyrosine phosphorylation is attributed specifically to the kinase activity of c-Abl, as cells expressing the kinase-dead c-Abl [c-Abl(KD)] did not exhibit any apparent reactivity towards the anti-P-Tyr antibody (Fig. 1A, lower panel, lane 2). To identify the nature of the phosphorylated proteins, extracts from the c-Abl(KA)- or c-Abl(KD)-expressing cells were subjected to purification with an anti-P-Tyr affinity column. Mass spectrometry analysis of the eluted fractions suggested one of the phosphorylated proteins as IκBα (not shown), which was subsequently confirmed by immunoprecipitation (IP)-Western analysis. As shown in Fig. 1B, when probed with an antibody specific against IκBα, the anti-P-Tyr immunocomplexes displayed a positive band corresponding approximately to 40 kDa. This band was only detected in the c-Abl(KA)-, but not in the c-Abl(KD)-, expressing cells (Fig. 1B, lane 1 versus lane 2). The c-Abl-dependent tyrosine phosphorylation of IκBα was further demonstrated in a separate experiment where anti-IκBα immunocomplexes were analyzed with an anti-P-Tyr antibody (Fig. 1C, lower panel, lane 1 versus lane 2). Several published studies have reported that tyrosine 42 of IκBα is phosphorylated by nonreceptor tyrosine kinases (12); it was therefore of interest to determine whether the c-Abl tyrosine kinase also phosphorylated the same site. An IκBα mutant with the tyrosine 42 replaced by phenylalanine was prepared for testing this possibility. Anti-P-Tyr immunoblotting analysis revealed that the Y42F mutant of IκBα remained phosphorylated by c-Abl (Fig. 1C, lower panel, lane 3 versus lane 4), indicating that the tyrosine residues other than 42 were targeted by c-Abl for phosphorylation. To examine if this c-Abl-dependent IκBα phosphorylation could also be observed at the level of endogenous proteins, MEFs derived from wild type or c-Abl knockout mice were tested. As shown in Fig. 1D, doxorubicin, an activator of c-Abl (2), induced IκBα tyrosine phosphorylation in the wild-type, but not the c-Abl-deficient, cells, indicating that the posttranslational modification of IκBα is indeed dependent on c-Abl activation.
FIG. 1.
IκBα is a novel substrate of c-Abl. (A) A plasmid (2 μg) encoding c-Abl(KA) (lane 1) or c-Abl(KD) (lane 2) was transfected into 293T cells. The cells were harvested at 24 h posttransfection and subjected to Western analysis with anti-c-Abl (top panel) or anti-P-Tyr (bottom panel). (B) Cell lysates prepared from the transfectants were also analyzed by anti-P-tyr immunoprecipitation followed by anti-IκBα immunoblotting. (C) A vector expressing c-Abl(KA) (lanes 1 and 3) or c-Abl(KD) (lanes 2 and 4) was cotransfected with wild type (lanes 1 and 2) or Y42F (lanes 3 and 4) of IκBα into U2OS cells. The cells were subjected to anti-IκBα immunoprecipitation at 24 h posttransfection. The immunocomplexes were analyzed by immunoblotting using anti-IκBα (top panel) or anti-P-tyr (bottom panel). (D) c-Abl−/− or c-Abl+/+ MEFs were treated with doxorubicin (2 μM; lanes 2 and 4) or left untreated (lanes 1 and 3). The cells were harvested 12 h after the treatment and subjected to anti-IκBα immunoprecipitation. Proteasome inhibitor (MG132; 7.5 μM; Sigma) was added 6 h before harvesting. The IκBα immunocomplexes were analyzed with anti-IκBα (middle panel) or anti-P-tyr (bottom panel). (E) Purified recombinant c-Abl(KA) (lanes 1, 3, and 5) or c-Abl(KD) (lanes 2, 4, and 6) was incubated with GST-fusion proteins of p53DBD (lanes 1 and 2), IκBα (lanes 3 and 4) or Crk (lanes 5 and 6) in the presence of [32P]ATP for 15 min at 30°C. After washing, the GST-fusion proteins were resolved on SDS-PAGE and the gel was either stained with Coomassie dye (bottom panel) or dried followed by autoradiography (top panel). (F) Immunopurified Flag-tagged IκBα NT, middle portion (MP) or CT was incubated with purified recombinant c-Abl(KA) for 30 min at 30°C. The reaction products were analyzed by Western analysis using anti-IκBα (upper panel) or anti-P-tyr (lower panel). (G) A plasmid encoding IκBα(Y251/258F), (Y284F) or (Y305F) was cotransfected with c-Abl(KA). Lysates prepared 24 h posttransfection were immunoprecipitated with anti-Flag followed by Western analysis using anti-Flag (upper panel) or anti-P-tyr (lower panel).
The tyrosine phosphorylation of IκBα observed in the c-Abl(KA)-expressing cells could be the result of a direct phosphorylation by c-Abl or of an intermediate tyrosine kinase that was activated by c-Abl. An in vitro kinase assay was therefore performed to distinguish these possibilities. A GST-IκBα fusion protein was prepared to incubate with purified recombinant c-Abl in the presence of ATP. GST-p53 DNA-binding domain (DBD) and GST-Crk were used as negative and positive controls, respectively. Analysis of the reaction products demonstrated that IκBα (Fig. 1E, lanes 3 and 4) and Crk (lanes 5 and 6), but not p53DBD (lanes 1 and 2), were phosphorylated by the c-Abl(KA) (Fig. 1E, compare lane 1 with lanes 3 and 5), indicating that IκBα is a substrate of the c-Abl tyrosine kinase. Coomassie staining showed the amount of substrates used (lower panel) and the c-Abl(KD) was included to ensure specificity of the reaction (lanes 2, 4, and 6). To map the putative tyrosine residue that was phosphorylated by c-Abl, we divided IκBα into three portions and subjected them to an in vitro kinase assay. The result indicated that the C terminus, but not the N terminus and the middle part, was phosphorylated (Fig. 1F, lanes 1 and 2 versus lane 3). Inspection of the amino acid sequence revealed four tyrosine residues in the C terminus of IκBα, which were replaced by phenylalanine using site-directed mutagenesis to generate Y-F mutants. When coexpressed with the c-Abl(KA), IκBα(Y305F) exhibited no detectable reactivity to anti-P-Tyr antibody whereas IκBα(Y254/258F) and IκBα(Y284F) remained tyrosine phosphorylated (Fig. 1G, lanes 1 and 2 versus lane 3), indicating the Tyr-305 as the c-Abl phosphorylation site.
Upregulation of IκBα by c-Abl in a kinase-dependent manner.
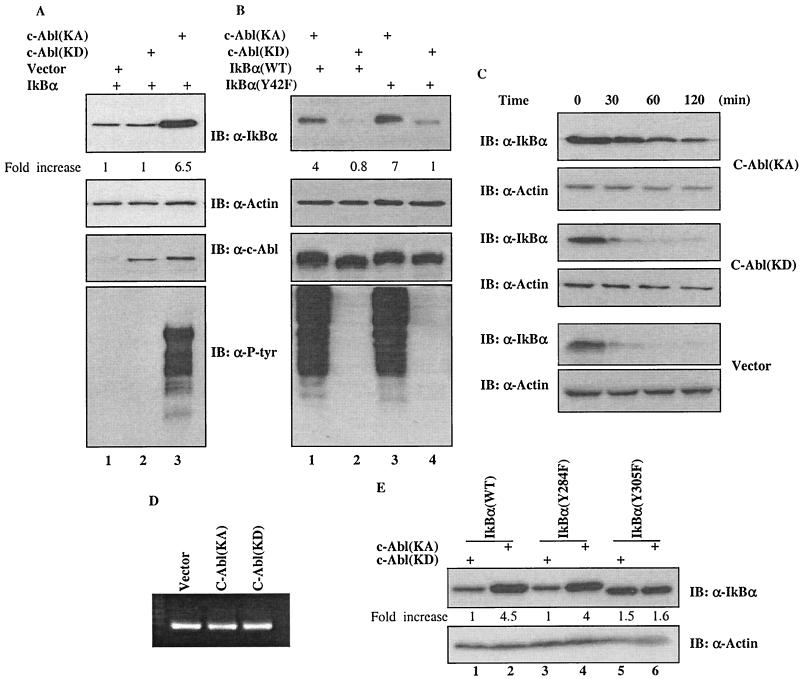
The finding that IKK-mediated phosphorylation of IκBα at Ser32 and 36 triggers ubiquitination and subsequent degradation of IκBα (1, 5, 7, 14) led us to ask whether c-Abl-mediated tyrosine phosphorylation could affect the cellular abundance of IκBα. Western analysis was performed to test this possibility. Interestingly, the IκBα protein levels from the c-Abl(KA)-expressing 293T cells were found significantly elevated (Fig. 2A, top panel, lane 3) when compared with that of the c-Abl(KD) (lane 2) or parental vector (lane 1) expressing cells. The cellular actin levels were measured to ensure equal loadings (Fig. 2A, second panel). The anti-c-Abl blot (panel 3) showed that comparable levels of c-Abl(KA) and c-Abl(KD) expression were achieved, and anti-P-Tyr analysis demonstrated that kinase activity was elicited only by the c-Abl(KA) (bottom panel). A similar increase of the IκBα protein levels by the c-Abl(KA) was also evident when U2OS cells were tested (Fig. 2B), indicating that the observation is not cell type dependent. Consistent with the phosphorylation result described in the legend to Fig. 1C, the abundance of IκBα(Y42F) mutant was also found to be markedly higher in the c-Abl(KA)- than the c-Abl(KD)-expressing cells (Fig. 2B, lanes 3 and 4).
FIG. 2.
Upregulation of IκBα by c-Abl in a kinase-dependent manner. (A) A vector expressing IκBα was cotransfected with a plasmid encoding control vector (lane 1), c-Abl(KD) (lane 2) or c-Abl(KA) (lane 3) into 293T cells. Cell lysates prepared at 24 h after transfection were subjected to Western analysis using the indicated antibody. The densitometric intensity of the IκBα band was scanned and numbers represent fold increase over that of vector control that was set as 1. (B) A plasmid containing cDNA of wild type (lanes 1 and 2) or Y42F (lanes 3 and 4) mutant of IκBα was coexpressed with c-Abl(KA) (lanes 1 and 3) or c-Abl(KD) (lanes 2 and 4) in U2OS cells and the cells were analyzed as described for panel A. (C) Half-life of IκBα in the vector, c-Abl(KD)- or c-Abl(KA)-expressing cells was determined by monitoring the disappearance of IκBα after addition of cycloheximide (20 μg/ml). (D) mRNA was isolated and analyzed by RT-PCR using a kit according to the manufacturer's protocol (Promega). (E) A plasmid encoding wild-type IκBα (lanes 1 and 2), IκBα(Y284F) (lanes 3 and 4) or IκBα(Y305F) (lanes 5 and 6) was cotransfected with c-Abl(KA) (lanes 2, 4 and 6) or c-Abl(KD) (lanes 1, 3 and 5). Cellular abundance of IκBα was analyzed 24 h after transfection and actin was used as a loading control.
In light of the fact that the IκBα abundance is, at least to a large extent, regulated by rapid protein degradation (1, 5, 7, 14), we asked whether the induced IκBα levels by c-Abl could be a consequence of increased protein stability by measuring the half-life of IκBα. Using cycloheximide to block new protein synthesis, we performed Western analysis to monitor the disappearance of IκBα upon addition of the protein synthesis inhibitor. As shown in Fig. 2C, the half-life of IκBα in the c-Abl(KA)-expressing cells was considerably prolonged over that in the vector or the c-Abl(KD)-expressing cells. Together with the result showing no detectable effect of c-Abl on the cellular IκBα mRNA levels (Fig. 2D), we draw the conclusion that the c-Abl tyrosine kinase increases the IκBα levels via a posttranscriptional mechanism.
With the finding that Tyr-305 of IκBα is the phosphorylation site, we tested IκBα(Y305F) for the possibility that this phosphorylation was responsible for the increased protein stability of IκBα. While the levels of IκBα(Y284F) were increased to a comparable extent to that of wild-type IκBα in a kinase-dependent fashion (Fig. 2F, lanes 1 to 4), there was no apparent difference of IκBα(Y305F) levels between the c-Abl(KA)- and c-Abl(KD)-expressing cells (Fig. 2F, lane 5 versus 6), supporting a critical role of phosphorylation of tyrosine 305 in the regulation of IκBα stability.
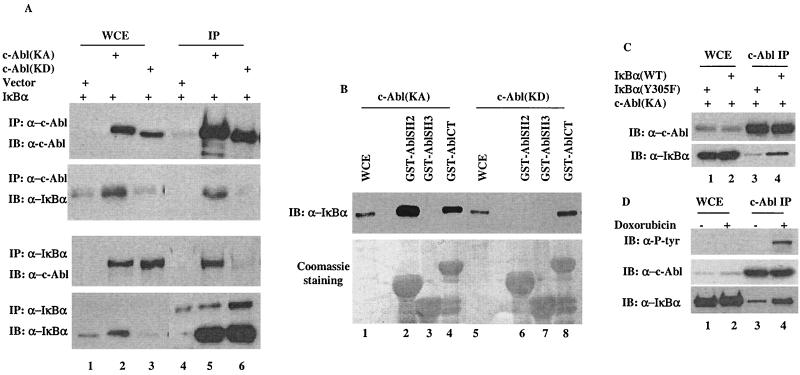
The c-Abl-mediated tyrosine phosphorylation of IκBα suggests a possible physical interaction between the two proteins. Coimmunoprecipitation was then performed to test this possibility. Anti-c-Abl immunocomplexes derived from cells coexpressing IκBα with a control vector, c-Abl(KA), or c-Abl(KD) were probed with an anti-IκBα antibody. The readily detectable IκBα signal in the c-Abl immunocomplexes, (Fig. 3A, panel 2, lanes 5 and 6) suggested an in vivo interaction between the two proteins. Interestingly, the interaction between c-Abl and IκBα in the c-Abl(KA)-expressing cells was substantially stronger than that in the c-Abl(KD)-expressing cells (lane 5 versus lane 6). This suggests a phosphorylation-dependent nature of the interaction, which was further demonstrated by the reciprocal coimmunoprecipitation experiment (Fig. 3A, panels 3 and 4). With the notion that the SH2 domain tends to bind to phosphorylated tyrosine residues, we examined whether the SH2 domain of c-Abl mediated the interaction by incubating a GST-fusion protein of the c-Abl SH2 domain with cell lysates isolated from cells expressing c-Abl(KA). GST-fusion proteins of the Abl SH3 or C terminus (CT) were included as controls. In contrast to the c-Abl SH3 domain that did not show any detectable association (Fig. 3B, lane 3), both the SH2 and CT of c-Abl were found to bind to IκBα and the affinity of SH2 appeared higher than that of the CT (Fig. 3B, lane 2 versus lane 4). Significantly, this SH2-mediated binding was not detectable when lysates prepared from the c-Abl(KD)-expressing cells were tested (Fig. 3B, lane 6). Coomassie staining showed that the difference in binding was not due to the different amounts of the GST proteins used (lower panel). The SH2 domain-mediated binding is consistent with the phosphorylation-enhanced interaction between c-Abl and IκBα observed in cells. To investigate this phosphorylation-enhanced interaction further, the IκBα(Y305F) mutant was tested for its association with c-Abl. When compared with wild-type IκBα, the Tyr-305 mutant exhibited a markedly reduced binding to c-Abl (Fig. 3C, lane 3 versus lane 4), consistent with a tyrosine-phosphorylation-mediated interaction. To exclude the possibility that this observed interaction was caused by overexpression, we performed IP-Western to analyze endogenous c-Abl and IκBα for complex formation. The readily detectable IκBα protein in c-Abl immunocomplexes confirmed the results derived from the transfection experiments (Fig. 3D). Significantly, treatment of doxorubicin resulted in a considerable increase of the IκBα proteins that were associated with c-Abl (Fig. 3D, bottom panel, lane 3 versus lane 4). When probed with anti-P-Tyr antibody, the c-Abl proteins isolated from the doxorubicin-treated cells exhibited strong positive reactivity (Fig. 3D, top panel, lane 4), indicative of c-Abl activation. Taken together, it appears that c-Abl physically associates with IκBα and this interaction is substantially augmented by an inducible binding of tyrosine phosphorylated IκBα to the c-Abl SH2 domain.
FIG. 3.
A phosphorylation-facilitated interaction between IκBα and c-Abl. (A) A vector expressing IκBα was coexpressed with a control vector, c-Abl(KA) or c-Abl(KD). Cell lysates prepared 24 h after transfection were subjected to immunoprecipitation with anti-c-Abl (top two panels) or anti-IκBα (lower two panels). Whole-cell extracts (lanes 1 to 3) or immunocomplexes (lanes 4 to 6) were analyzed by Western blotting using the indicated antibodies. (B) Cell lysates prepared from c-Abl(KA)- (lanes 1 to 4) or c-Abl(KD)- (lanes 5 to 8) expressing cells were incubated with the indicated GST-fusion proteins. The adsorbates were extensively washed, resolved on SDS-PAGE and either immunoblotted with anti-IκBα (top panel) or stained with Coomassie dye (bottom panel). Whole-cell extracts (WCE, lanes 1 and 5) were included as blotting controls. (C) A vector expressing wild-type IκBα (lanes 2 and 4) or IκBα (Y305F) mutant (lanes 1 and 3) was cotransfected with c-Abl(KA). Cell lysates prepared 24 h after transfection were subjected to immunoprecipitation with anti-c-Abl. Whole-cell extract (lanes 1 and 2) and c-Abl immunocomplexes (lanes 3 and 4) were analyzed by Western blotting using anti-c-Abl (top panel) or anti-P-Tyr (bottom panel). (D) U2OS cells treated with or without doxorubicin (2 μM for 12 h) were analyzed by anti-c-Abl immunoprecipitation followed by Western analysis using the indicated antibodies.
The ability of c-Abl to stabilize IκBα is not shared by the oncogenic Abl.
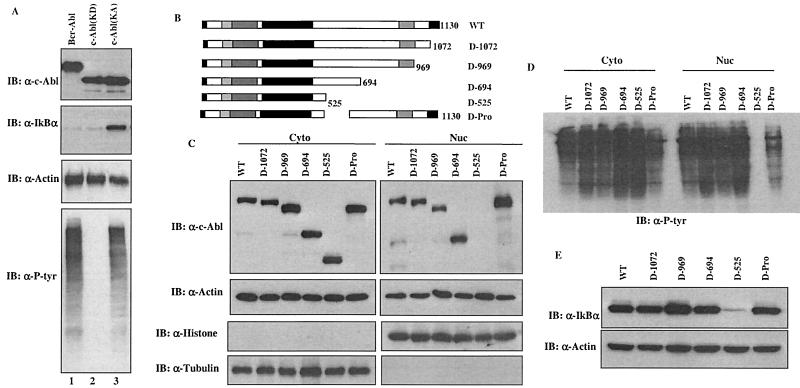
IκBα is primarily localized in the cytoplasm where it binds to and prevents NF-κB from nuclear import (1, 5, 7, 14). While the c-Abl protein distributes in both cytoplasmic and nuclear compartments, the oncogenic Abl proteins, including v-Abl and Bcr-Abl, are exclusively cytoplasmically localized (8, 9, 13, 15, 16). It is, therefore, of importance to determine whether the oncogenic Abl proteins also share the ability to regulate the stability of IκBα. To accomplish this, a plasmid encoding Bcr-Abl was coexpressed with IκBα and the protein levels were determined 24 h posttransfection. Remarkably, despite the finding that the Bcr-Abl proteins expressed were highly kinase active, as demonstrated by anti-P-Tyr immunoblotting analysis (Fig. 4A, bottom panel, lane 1), no apparent increase of the IκBα protein levels was detected (Fig. 4A, panel 2, lane 1).
FIG. 4.
The ability of c-Abl to stabilize IκBα is not shared by the oncogenic Abl. (A) A vector expressing IκBα was cotransfected with a plasmid encoding Bcr-Abl (lane 1), c-Abl(KD) (lane 2) or c-Abl(KA) (lane 3). The cells were analyzed as described for Fig. 2. (B) A series of deletion mutants of c-Abl were prepared. (C) cDNAs of the indicated c-Abl deletion mutants were subcloned into Flag-tagged pCDNA3 vector and were transfected into 293T cells. The cells were then fractionated into cytoplasmic and nuclear fractions, which then were subjected to anti-Flag (top panels) or anti-actin (second panels) immunoblotting analysis. Anti-histone (nuclear marker) and anti-tubulin (cytoplasmic marker) immunoblotting were included to assess the purity of each fraction. (D) The cytoplasmic and nuclear fractions were also immunoblotted with anti-P-tyr. (E) A plasmid encoding IκBα was cotransfected with the indicated mutants of c-Abl and the IκBα levels were analyzed as described for Fig. 2.
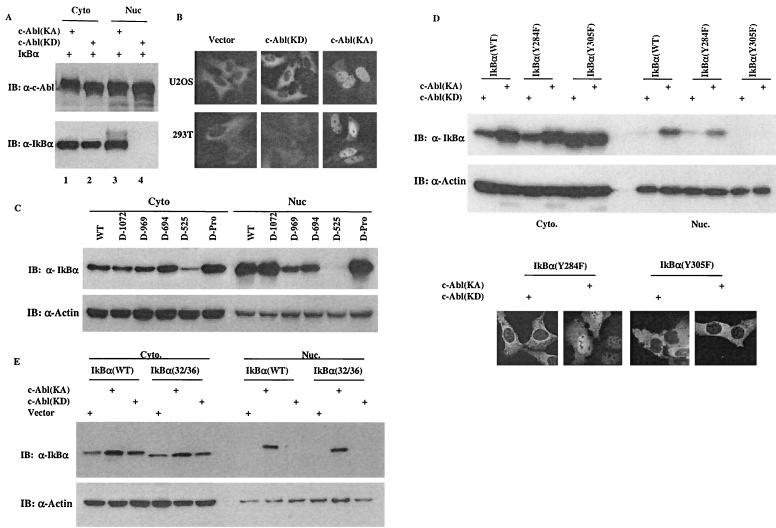
The inability of the kinase-active but cytoplasmically localized Bcr-Abl to affect IκBα levels suggested a possibility that stabilization of IκBα was a function of the nuclear c-Abl. A series of c-Abl deletion mutants as shown in Fig. 4B were generated to address this possibility. It has been reported that the c-Abl protein contains three NLSs at its C terminus. Each NLS can function independently and is sufficient to mediate the nuclear import of c-Abl (15). Results obtained from our c-Abl deletion mutants are consistent with the published data. As shown in Fig. 4C, the c-Abl(1-694) mutant that contains only one NLS was still found to have a similar distribution pattern to that of wild-type c-Abl, localized in both the cytoplasm and the nucleus. In contrast, the c-Abl(1-525) mutant that lacks NLS was detected only in the cytoplasm. Removal of the C terminus or the prolin rich domain of c-Abl did not have an apparent effect, indicating that the exclusive cytoplasmic localization of the c-Abl(1-525) mutant is attributable to its lack of an NLS. Anti-histone or anti-tubulin blotting was used as a marker of the nucleus or the cytoplasm, respectively. In keeping with the intact kinase domain retained in the deletion mutants, anti-P-Tyr immunoblotting analysis demonstrated that all the c-Abl deletion mutants remained highly kinase active (Fig. 4D). Having verified the distribution patterns of the c-Abl deletion mutants, we went on assessing their ability to regulate IκBα levels. Significantly, a perfect temporal correlation between the c-Abl nuclear distribution and the ability to upregulate IκBα levels was observed. In contrast to the wild-type c-Abl and those mutants that exhibited a cytoplasmic and nuclear distribution pattern, the c-Abl(1-525) mutant, which was exclusively cytoplasmically localized, lost its ability to upregulate IκBα levels (Fig. 4E). Together with the observation of the inability of cytoplasmically localized Bcr-Abl to stabilize IκBα, these results demonstrate that the stabilization of IκBα is a function of the nuclear c-Abl.
The c-Abl tyrosine kinase induces nuclear accumulation of IκBα.
The finding that the nuclear c-Abl is responsible for increasing IκBα levels raised a question as to how the nuclear c-Abl could regulate the stability of IκBα protein that is mainly cytoplasmically localized. Recent studies have shown that although IκBα is a predominantly cytoplasmically distributed protein, it undergoes active nuclear-cytoplasmic shuttling (11). Thus, we tested the possibility that c-Abl interacted with the nuclear population of IκBα. The cytoplasmic and nuclear fractions were then isolated from cells expressing c-Abl(KA) or c-Abl(KD) for Western analysis. The result revealed a predominantly cytoplasmic distribution of IκBα in cells expressing c-Abl(KD) (Fig. 5A, lanes 2 and 4), a distribution pattern of IκBα consistent with that in normal cells. In striking contrast, however, the c-Abl(KA)-expressing cells exhibited a very abundant nuclear distribution of IκBα in addition to the cytoplasmic pool (Fig. 5A, lanes 1 and 3). Notably, a slower migration form of IκBα, which most likely resulted from phosphorylation, was associated with only the nuclear, but not cytoplasmic, IκBα. To further substantiate this result, a GFP-IκBα-expressing vector was prepared for an immunostaining analysis. When transfected into U2OS cells, c-Abl indeed induced the very pronounced nuclear accumulation of IκBα in a kinase-dependent fashion (Fig. 5B, top panel). An almost identical result was obtained when 293T cells were examined (Fig. 5B, bottom panel).
FIG. 5.
The c-Abl-induced IκBα nuclear accumulation contributes to its ability to upregulate the IκBα protein levels. (A) A vector containing cDNA of IκBα was cotransfected with a plasmid encoding c-Abl(KA) or c-Abl(KD). Cytoplasmic and nuclear fractions were prepared at 24 h posttransfection and subjected to Western analysis using anti-c-Abl (top panel) or anti-IκBα (bottom panel). (B) A plasmid encoding GFP-IκBα was cotransfected with a control vector, c-Abl(KD) or c-Abl(KA) into U2OS (top panels) or 293T cells (bottom panels). The cells were processed as described in Materials and Methods and examined under a fluorescence microscope. (C) An IκBα-expressing vector was cotransfected with plasmids encoding the indicated c-Abl deletion mutants. Cell fractions were prepared at 24 h posttransfection and analyzed by Western blotting using anti-IκBα (top panel) and anti-actin (bottom panel). (D) A plasmid encoding IκBα(wild type), (Y284F) or (Y305F) was cotransfected with c-Abl(KA) or c-Abl(KD). Cell fractions were prepared at 24 h after transfection and Western blotted with anti-IκBα or anti-actin. U2OS cells were transfected with the indicated vectors and analyzed as described for panel B. (E) Cell fractions were prepared from cells transfected with the indicated vectors and analyzed as described for panel C.
The IκBα protein is ubiquitinated and degraded largely, if at all, in the cytoplasm. Interference with its nuclear export would likely result in nuclear accumulation and thereby inhibition of IκBα degradation. We asked whether this c-Abl-induced IκBα nuclear accumulation contributed to its ability to increase IκBα levels. With the finding that stabilization of IκBα is mediated by the nuclear c-Abl, we tested the ability of the c-Abl deletion mutants described above to regulate the subcellular distribution of IκBα. Measurement of the cytoplasmic and nuclear IκBα levels revealed that the c-Abl mutants that retained the ability to stabilize IκBα were all capable of inducing IκBα nuclear accumulation, albeit to slightly different extents (Fig. 5C), correlating the IκBα nuclear accumulation to the increased protein stability. In support of this correlation, the c-Abl(1-525) mutant that is unable to increase IκBα levels failed to induce any apparent IκBα nuclear accumulation (Fig. 5C). Taken together, it appears that the nuclear retention of IκBα contributes to the ability of c-Abl to increase IκBα levels.
While the inability of c-Abl(KD) to induce IκBα nuclear retention indicated a requirement of the c-Abl kinase activity, it is unclear whether this induced nuclear distribution was attributed either to phosphorylation of IκBα or to an impaired function of the nuclear exporter proteins. The phosphorylation-resistant mutant IκBα(Y305F) was examined to address this issue. Similar to the wild-type IκBα, the IκBα(Y284F) mutant exhibited a significant nuclear distribution in the c-Abl(KA)-expressing cells (Fig. 5D, top). In contrast, substitution of tyrosine 305 almost completely abolished the c-Abl-induced IκBα nuclear retention. The data obtained from immunostaining further confirmed this tyrosine-305 phosphorylation-dependent nuclear retention of IκBα (Fig. 5D, bottom).
IKK-mediated phosphorylation of IκBα at Ser32/36 triggers ubiquitination and subsequent degradation of IκBα (1, 5, 7, 14). To assess if the IKK-mediated pathway was affected, an IκBα 32/36 Ser to Ala substitution mutant was prepared. When compared with the wild-type IκBα, the Ser to Ala mutant of IκBα was similarly retained in the nucleus by c-Abl in a kinase-dependent manner (Fig. 5E), excluding the involvement of the IKK-dependent phosphorylation.
Functional regulation of NF-κB by the c-Abl tyrosine kinase.
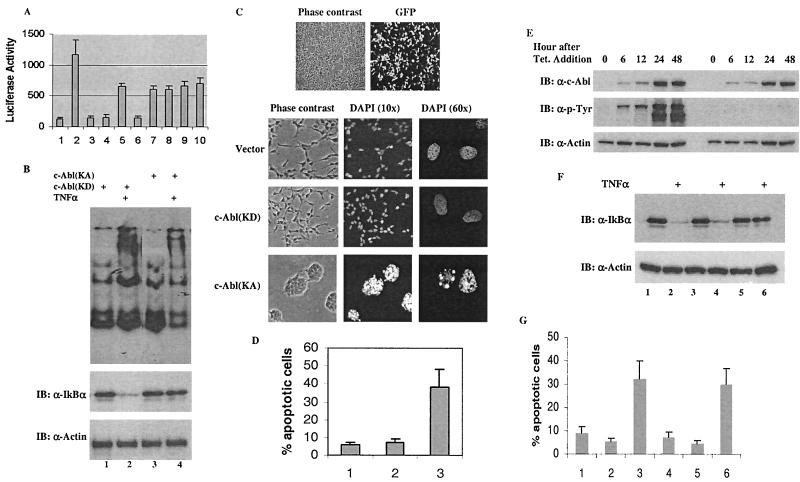
In light of the fact that the nuclear-distributed IκBα binds to NF-κB and disables its function as a transcription factor (11), we were interested in knowing whether the nuclear accumulation of IκBα induced by the c-Abl tyrosine kinase could be translated into a functional inhibition of NF-κB activity. First, we examined the effect of c-Abl on NF-κB transcriptional activity by performing a luciferase assay. A κB-luciferase reporter plasmid (Promega) was cotransfected together with an expression vector to normalize the difference in transfection efficiencies. As expected, expression of NF-κB (Fig. 6A, bar 2), but not c-Abl (bars 3 and 4), resulted in a significant increase of the luciferase activity and this NF-κB-mediated activity was inhibited by IκBα (bar 5). In support of the kinase-dependent stabilization of IκBα, introduction of c-Abl(KA), but not c-Abl(KD) (bar 7), was associated with a significant augmentation of IκBα-mediated inhibitory potency (bar 6). While it remained to be a potent inhibitor of NF-κB (bar 8), the inhibitory potential of IκBα(Y305F) was not further enhanced by c-Abl (bars 9 and 10), consistent with the observation that this IκBα mutant was not nuclear accumulated and stabilized by c-Abl.
FIG. 6.
Functional inhibition of NF-κB contributes to the proapoptotic function of c-Abl. (A) A luciferase-expressing vector containing a κB-responsive element was cotransfected with control vectors (bar 1), NF-κB/control vector (bar 2), c-Abl(KA) (bar 3) or c-Abl(KD) (bar 4), NF-κB/IκBα (bar 5), NF-κB/IκBα/c-Abl(KA) (bar 6), NF-κB/IκBα/c-Abl(KD) (bar 7), NF-κB/IκBα(Y305F) (bar 8), NF-κB/IκBα(Y305F)/c-Abl(KA) (bar 9), or NF-κB/IκBα(Y305F)/c-Abl(KD) (bar 10). pRL-TK vector (Promega) was included to control the low level of Renilla luciferase expression and serve as a transfection efficiency control. Luciferase assays were performed 24 h posttransfection using the Promega Rapid Detection system. Values are averages ± SE of the mean derived from two separate experiments performed in triplicate. (B) Cells expressing c-Abl(KD) (lanes 1 and 2) or c-Abl(KA) (lanes 3 and 4) were treated with TNF-α (10 ng/ml) for 15 min (lanes 2 and 4) or left untreated (lanes 1 and 3). Nuclear extracts were prepared for EMSA using a labeled consensus NF-κB-binding oligonucleotide (top panel) and whole-cell extracts were analyzed by Western blotting using anti-IκBα and anti-actin (lower panels). (C) 293T cells were transfected with 2 μg of GFP-c-Abl(KD) and the cells were visualized via phase-contrast optics (left) and fluorescent filter (right) to show transfection efficiency. Cells expressing a control vector (2 μg; top panels), c-Abl(KD) (2 μg; middle panels) or c-Abl(KA) (2 μg; bottom panels) were treated with TNF-α (40 ng/ml) at 16 h posttransfection. After incubation at 37°C for an additional 8 h, the cells were fixed and stained with DAPI. The whole cells were visualized by phase-contrast optics and the nuclei were visualized by UV optics (two different amplifications, 10× or 60×). (D) Cells expressing a control vector (bar 1), c-Abl(KD) (bar 2) or c-Abl(KA) (bar 3) were processed as described for panel C. The cells were then fixed in 70% methanol, stained with PI and subjected to FACS analysis. Sub-G1 populations were recorded as the apoptotic cells. Values are averages ± SE of the mean derived from two separate experiments performed in triplicate. (E) The pooled cell populations that stably expressed c-Abl(KA) or c-Abl(KD) were treated with tetracycline (1 μg/ml) and harvested at the indicated times for Western analysis with anti-c-Abl (top), anti-p-Tyr (middle) or anti-actin (bottom). (F) At 24 h postaddition of tetracycline, cells expressing the control vector (lanes 1 and 2), c-Abl(KD) (lanes 3 and 4) and c-Abl(KA) (lanes 5 and 6) were treated with TNF-α (10 ng/ml) for 15 min and the cell lysates were subjected to Western analysis. (G) After induction with tetracycline for 12 h, cells that expressed the control vector (bars 1 and 4), c-Abl(KD) (bars 2 and 5) or c-Abl(KA) (bars 3 and 6) were treated with TNF-α (40 ng/ml) (bars 1 to 3) or UV (20 J/m2) (bars 4 to 6). The cells were harvested (8 h after addition of TNF-α or 24 h after UV treatment) for FACS analysis as described for panel D.
Recent studies have shown that cytoplasmic localization of IκBα/NF-κB complexes is important for efficient cytokine-dependent phosphorylation-ubiquitination and subsequent degradation of IκBα proteins, which results in the release of NF-κB to the nucleus to induce gene expression (4). We asked whether c-Abl-induced IκBα nuclear accumulation could interfere with cytokine-induced NF-κB activation by examining TNF-α-induced activation of NF-κB. An EMSA was performed to assess TNF-α-induced binding of NF-κB to its responsive sequence. Cells were transfected with the indicated vectors and treated with TNF-α for 20 min at 24 h posttransfection. Cell nuclear extracts were then isolated for incubation with a radiolabeled probe containing the NF-κB binding sequence. The products were then resolved on nondenaturating polyacrylamide gel and analyzed by autoradiography. As anticipated, TNF-α induced a very pronounced binding of NF-κB to the κB sequence in the c-Abl(KD)-expressing cells, as evidenced by the band shift (Fig. 6B, top). Under the same condition, this TNF-α-induced NF-κB binding was significantly diminished in the c-Abl(KA)-expressing cells (Fig. 6B, top panel). In a parallel experiment, Western blotting was performed to monitor the TNF-α-induced IκBα degradation. Exposure of cells expressing c-Abl(KD) to TNF-α was associated with a rapid disappearance of cellular IκBα (Fig. 6B, lower panel, lanes 1 and 2). In contrast, the IκBα levels in cells expressing c-Abl(KA) were resistant to degradation in response to TNF-α (Fig. 6B, lower panels, lanes 3 and 4), supporting the result obtained by EMSA. Taken together, it appears that the c-Abl-induced IκBα nuclear accumulation results in the inhibition of NF-κB-mediated transcription activation in response to TNF-α.
In addition to the activation of the NF-κB-dependent survival, TNF-α is also a potent inducer of the caspase-mediated apoptosis. The impaired activation of NF-κB by TNF-α in the c-Abl(KA)-expressing cells would predict a higher sensitivity of the cells to TNF-α-induced apoptosis. We first set up a condition where approximately 90% transfection efficiency was obtained for assessing the role of c-Abl in the regulation of TNF-α-induced apoptosis (Fig. 6C, top). As shown in Fig. 6C, under the condition where NF-κB was activated by TNF-α no significant apoptosis was evident in the vector or the c-Abl(KD)-expressing cells. Treatment of the c-Abl(KA)-expressing cells with TNF-α, however, resulted in the typical apoptotic phenotypes, cells roundup with highly condensed nuclei. Measurement of the sub-G1 population by FACS analysis demonstrated a significant induction of apoptosis in the c-Abl(KA)-expressing cells in response to TNF-α (Fig. 6D, bar 3). In contrast, the control vector or the c-Abl(KD)-expressing cells did not show any significantly increased sensitivity towards TNF-α-induced apoptosis (bars 1 and 2). Together, these results indicate a significantly increased sensitivity to TNF-α-induced apoptosis in the c-Abl(KA)-expressing cells.
Finally, to demonstrate the c-Abl-mediated functional inhibition of NF-κB in a more biologically relevant context, we established stable cell lines that expressed c-Abl(KA) or c-Abl(KD) under the control of a tetracycline-responsive promoter. To eliminate the possibility of a nonspecific effect derived from the clonal selection, the drug-resistant clones were pooled for functional analysis. Upon addition of tetracycline the c-Abl expressions were modestly induced to comparable levels (approximate 5-fold over the levels of endogenous c-Abl; data not shown) in the c-Abl(KA)- and the c-Abl(KD)-expressing cells (Fig. 6E, top panel). Anti-P-Tyr analysis demonstrated a kinase activity elicited only in the c-Abl(KA)-expressing cells (Fig. 6E, middle panel). Characterization of the cell lines has been described elsewhere (H. Kawai, L. Nie, and Z. M. Yuan, unpublished data). We first examined the activation of NF-κB in response to TNF-α. Analogous to the results derived from the transient-transfection experiments, TNF-α-induced degradation of IκBα was severely compromised in the c-Abl(KA)-expressing cells (Fig. 6F, lane 5 versus lane 6) compared with that in the vector (lanes 1 and 2) or the c-Abl(KD)-expressing cells (lanes 3 and 4). We then analyzed the cells for their sensitivity to TNF-α- or UV-induced apoptosis. The result supported a kinase-dependent proapoptotic function of c-Abl, showing that the c-Abl(KA)-expressing cells (Fig. 6G, bars 3 and 6) exhibited a substantially increased sensitivity to TNF-α (bars 1 to 3) and UV (bars 4 to 6) compared with the cells expressing the control vector (bars 1 and 4) or c-Abl(KD) (bars 2 and 5).
DISCUSSION
NF-κB is an inducible transcription factor that plays an important role in the expression of a variety of genes involved in cell survival. The biological activity of NF-κB is tightly controlled by its inhibitor protein, which binds to and sequesters the transcription factor in the cytoplasm (1, 5, 7, 14). IκBα, the best-characterized member of the IκB family, is an unstable protein whose stability is largely regulated through a mode of posttranslational modification. IKK-mediated phosphorylation of 32/36 serine residues triggers IκBα ubiquitination and subsequent degradation via the proteasome-dependent proteolysis, thereby releasing NF-κB to the nucleus to function as a transcription activator (1, 5, 7, 14). IκBα is also phosphorylated at tyrosine 42 by a number of cytoplasmic nonreceptor tyrosine kinases and this phosphorylation has been reported to contribute to the activation of NF-κB transcription activity (12). We identified tyrosine 305 of IκBα as a novel phosphorylation site for the nuclear c-Abl both in vitro and in cells. What is unique to Tyr-305 phosphorylation is its association with a profound increase of IκBα protein stability. The temporal correlation of the increased IκBα stability and its nuclear retention suggests that c-Abl inhibits IκBα degradation by inducing its accumulation in the nucleus as the proteasome-mediated IκBα protein proteolysis occurs, at least primarily, in the cytoplasm (1, 5, 7, 14). It is presently unclear how c-Abl-mediated phosphorylation results in a nuclear retention of the IκBα proteins. The remarkably enhanced interaction between c-Abl and IκBα via the phosphorylated Tyr-305 might lead to blocking the nuclear export sequence or preventing the access of proteases. Further study is required to address these possibilities.
c-Abl is a protein that actively shuttles between the cytoplasm and the nucleus and the cytotoxic activity of c-Abl is a function of the nuclear c-Abl (9, 13, 15, 16; Nie et al., submitted). We demonstrate here that it is the nuclear c-Abl that targets IκBα for phosphorylation. Interestingly, IκBα also actively undergoes nucleus-cytoplasm shuttling (11). IκBα enters the nucleus to complex with NF-κB and to disable its transcriptional activity by promoting nuclear export. This activity of IκBα has been shown to be essential to restore the cellular response postinduction as blockage of IκBα nuclear export prevented NF-κB from reactivation (4). The observation that the c-Abl(KA)-expressing cells exhibited an impaired activation of NF-κB in response to TNF-α is consistent with this finding. In addition to the activation of NF-κB, TNF-α is also a potent activator of the apoptotic pathway. The propensity of a cell to survive or die is set by the balance between proapoptotic and prosurvival signals. The compromised NF-κB-dependent cell survival renders the c-Abl(KA)-expressing cell with a markedly increased sensitivity to TNF-α and UV-induced cell death. The functional inhibition of NF-κB therefore serves as one of the mechanisms for the proapoptotic function of c-Abl.
The finding that the inhibition of NF-κB by c-Abl requires its nuclear localization is of great significance because the oncogenic Bcr-Abl, which is highly kinase active but exclusively cytoplasmically localized, is unable to share the same activity. On the contrary, Bcr-Abl can in fact potently activate NF-κB, which has been demonstrated to be essential for Bcr-Abl to induce transformation (10). This completely opposite effect on the NF-κB-mediated cell survival pathway seems to represent a key point that differentiates the oncogenic Bcr-Abl from the growth-suppressive normal c-Abl.
Acknowledgments
We thank Colleen Dionne for critical reading of the manuscript.
The research was supported by NCI grant CA76275-01A1.
REFERENCES
- 1.Aradhya, S., and D. L. Nelson. 2000. NF-κB signaling and human disease. Curr. Opin. Genet. Dev. 11:300-306. [DOI] [PubMed] [Google Scholar]
- 2.Costanzo, A., P. Merlo, N. Pediconi, M. Fulco, V. Sartorelli, P. A. Cole, G. Fontemaggi, M. Fanciulli, L. Schiltz, G. Blandino, C. Balsano, and M. Levrero. 2002. DNA damage-dependent acetylation of p73 dictates the selective activation of apoptotic target genes. Mol. Cell 9:175-186. [DOI] [PubMed] [Google Scholar]
- 3.Gu, J., D. Chen, J. Rosenblum, R. M. Rubin, and Z. M. Yuan. 2000. Identification of a sequence element from p53 that signals for Mdm2-targeted degradation. Mol. Cell. Biol. 20:1243-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang, T. T., and S. Miyamoto. 2001. Postrepression activation of NF-κB requires the amino-terminal nuclear export signal specific to IκBα. Mol. Cell. Biol. 21:4737-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huxford, T., S. Malek, and G. Ghosh. 1999. Structure and mechanism in NF-kappa B/I kappa B signaling. Cold Spring Harbor Symp. Quant. Biol. 64: 533-540. [DOI] [PubMed] [Google Scholar]
- 6.Karin, M., Z. Liu, and E. Zandi. 1997. AP-1 function and regulation. Curr. Opin. Cell Biol. 9:240-246. [DOI] [PubMed] [Google Scholar]
- 7.Karin, M., and A. Lin. 2001. NF-κB at the crossroads of life and death. Nat. Immunol. 3:221-227. [DOI] [PubMed] [Google Scholar]
- 8.Kharbanda, S., Z. M. Yuan, R. Weichselbaum, and D. Kufe. 1998. Determination of cell fate by c-Abl activation in the response to DNA damage. Oncogene 17:3309-3318. [DOI] [PubMed] [Google Scholar]
- 9.Raitano, A. B., Y. E. Whang, and C. L. Sawyers. 1997. Signal transduction by wild-type and leukemogenic Abl proteins. Biochim. Biophys. Acta 1333:F201-F216. [DOI] [PubMed] [Google Scholar]
- 10.Reuther, J. Y., G. W. Reuther, D. Cortez, A. M. Pendergast, and A. S. Baldwin, Jr. 1998. A requirement for NF-κB activation in Bcr-Abl-mediated transformation. Genes Dev. 12:968-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sachdev, S., S. Bagchi, D. D. Zhang, A. C. Mings, and M. Hannink. 2000. Nuclear import of IκBα is accomplished by a Ran-independent transport pathway. Mol. Cell. Biol. 20:1571-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoonbroodt, S., and J. Piette. 2000. Oxidative stress interference with the nuclear factor-kappa B activation pathways. Biochem. Pharmacol. 60:1075-1083. [DOI] [PubMed] [Google Scholar]
- 13.Shaul, Y. 2000. c-Abl: activation and nuclear targets. Cell Death Differ. 7:10-16. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka, K., T. Kawakami, K. Tateishi, H. Yashiroda, and T. Chiba. 2001. Control of IκB alpha proteolysis by the ubiquitin-proteasome pathway. Biochimie 83:351-382. [DOI] [PubMed] [Google Scholar]
- 15.Van Etten, R. A. 1999. Cycling, stressed-out and nervous: cellular functions of c-Abl. Trends Cell Biol. 9:179-186. [DOI] [PubMed] [Google Scholar]
- 16.Wang, J. Y. 2000. Regulation of cell death by the Abl tyrosine kinase. Oncogene 19:5643-5650. [DOI] [PubMed] [Google Scholar]