Abstract
DNA double-strand breaks (DSBs) arise spontaneously after the conversion of DNA adducts or single-strand breaks by DNA repair or replication and can be introduced experimentally by expression of specific endonucleases. Correct repair of DSBs is central to the maintenance of genomic integrity in mammalian cells, since errors give rise to translocations, deletions, duplications, and expansions, which accelerate the multistep process of tumor progression. For p53 direct regulatory roles in homologous recombination (HR) and in non-homologous end joining (NHEJ) were postulated. To systematically analyze the involvement of p53 in DSB repair, we generated a fluorescence-based assay system with a series of episomal and chromosomally integrated substrates for I-SceI meganuclease-triggered repair. Our data indicate that human wild-type p53, produced either stably or transiently in a p53-negative background, inhibits HR between substrates for conservative HR (cHR) and for gene deletions. NHEJ via microhomologies flanking the I-SceI cleavage site was also downregulated after p53 expression. Interestingly, the p53-dependent downregulation of homology-directed repair was maximal during cHR between sequences with short homologies. Inhibition was minimal during recombination between substrates that support reporter gene reconstitution by HR and NHEJ. p53 with a hotspot mutation at codon 281, 273, 248, 175, or 143 was severely defective in regulating DSB repair (frequencies elevated up to 26-fold). For the transcriptional transactivation-inactive variant p53(138V) a defect became apparent with short homologies only. These results suggest that p53 plays a role in restraining DNA exchange between imperfectly homologous sequences and thereby in suppressing tumorigenic genome rearrangements.
In response to DNA damage the tumor suppressor p53 induces a transient cell cycle arrest by transcriptional transactivation of target genes or triggers apoptotic cell death by transcriptional transactivation-dependent and -independent pathways (25). The generation of mice nullizygous for p53 made clear that, in addition, p53 counteracts aneuploidies, allelic losses, sister chromatid exchanges, and gene amplifications (17, 28). With respect to the underlying mechanism, a direct participation of p53 in DNA repair was proposed. This was due to biochemical observations that revealed activities of p53 in the recognition of DNA damage, in DNA reannealing, and in exonucleolytic DNA degradation (1). p53 also binds to a plethora of repair-related proteins. The meaning of most of these interactions is not yet clear. Thus, uncertainties exist, whether p53 participates in nucleotide excision repair by modulating TFIIH activities (12, 32, 48) or rather counteracts sister chromatic exchanges after UV irradiation (7, 17).
More convincingly, several groups unanimously reported on 5- to >100-fold rate increases of spontaneous inter- and intrachromosomal homologous recombination (HR), when wild-type p53 (wtp53) was inactivated genetically or by interactions with viral tumor antigens (2, 9, 10, 31, 42, 50, 51). Conversely, with respect to a possible involvement of p53 in non-homologous end joining (NHEJ) contradictory results have been obtained (3, 24, 46, 54). Physical and genetic links were established between p53 and factors involved in HR, namely the recombinase Rad51, the Rad51 complex partner BRCA1 and BRCA2, and the helicase BLM (1, 30, 49). Further clues to the involvement of p53 in HR came from investigations on its DNA binding activities. Applying gel retardation, protection assays, electron microscopy, and enzyme analysis, wtp53 was demonstrated to specifically interact with and preferentially degrade recombination intermediates in a sequence-nonspecific and Rad51-stimulated fashion (9, 18, 23, 45). Certain mismatched bases, when arising within recombination intermediates, caused an enhancement of DNA binding, of the exonucleolytic attack, and of recombination regulation by p53 (9, 45). This has led to a model suggesting that p53 monitors the fidelity of strand exchange events in close contact to the recombinase Rad51 and in a manner complementary to the mismatch repair factor MSH2.
In mammalian cells the relative contribution of homology-directed repair of chromosomal double-strand breaks (DSBs) seems to account for 30 to 50%, and this activity increases further in replicating cells, when sister chromatids as the preferred homologous templates are available (20). HR mechanisms initiated by strand invasion, i.e., gene conversion, crossover, and break-induced replication, rely on the strand transferase Rad51 and on other members of the Rad52 epistasis group (22). Single-stranded annealing, a nonconservative mechanism of homology-directed DSB repair, and NHEJ, which implies simple religation or the alignment of DNA ends at microhomologies of a few base pairs, proceed independently of Rad51 (14, 22). A recent study by Richardson and Jasin (37) provided convincing evidence that homologous repair and NHEJ are not completely separable. Instead, compound events initiated by gene conversion and completed by NHEJ are frequent and protect cells against deleterious chromosome rearrangements.
Several groups have begun to elucidate the role of p53 in HR (9, 13, 42). In these studies, systems for probing recombination were based either on procedures selecting for survival phenotypes or on the rescue of simian virus 40 in a plaque assay. Here, we developed and applied a fast-readout assay system for DSB repair, which is based on the enhanced green fluorescent protein (EGFP) and the I-SceI meganuclease, similar to the gene conversion assay generated by Pierce et al. (34). The assay was used to systematically analyze the activities of wtp53 in comparison to p53 mutants and to examine the substrate requirements for recombination surveillance by p53, thereby providing critical information on the involvement in different DSB repair pathways.
MATERIALS AND METHODS
Plasmids and DNA manipulations.
Plasmids for HR measurements were constructed by insertion of the puromycin resistance gene, of one acceptor gene cassette, of one spacer cassette, and of one donor gene in series into the multiple cloning site of the retroviral vector p5NM (21) (Fig. 1 and 2). Donor-free vector with EJ-EGFP at the acceptor position was prepared in order to test NHEJ. Other vectors, lacking either the donor or the acceptor genes, were produced as negative controls. Plasmids, carrying a wt EGFP gene at the acceptor position, were generated for the determination of transfection efficiencies.
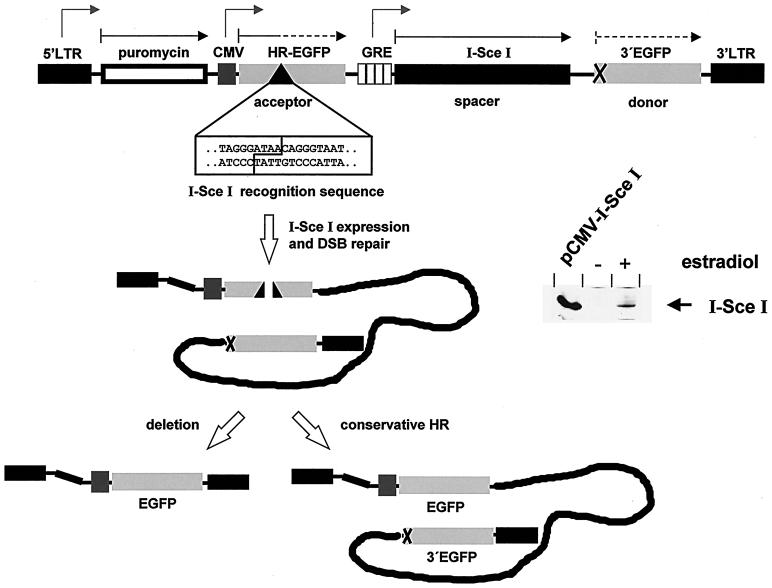
FIG. 1.
Structure of the test system based on the reconstitution of an EGFP gene. The vector design for the concomitant analysis of deletion events and conservative HR is drawn schematically for one representative variant. The 5′ long terminal repeat (LTR) and the 3′ LTR from the retroviral vector p5NM (21) are flanking the construct, and the puromycin acetyltransferase gene follows the 5′ LTR. Mutated EGFP genes, namely the CMV-driven acceptor gene HR-EGFP, which comprises an I-SceI recognition sequence, and the donor gene 3′EGFP, serve as HR substrates and are arranged in series. DSB repair is initiated by I-SceI meganuclease expression, which is driven by the estradiol-responsive GRE promoter within the spacer region. Expression of the HA-I-SceI fusion protein after estradiol induction (200 nM) for 24 h was verified by immunoblotting with the anti-HA antibody 12CA5 (right panel). pCMV-I-SceI-electroporated cells served as positive control. Grey, kinked arrows indicate the direction of transcription, black arrows indicate the orientation of the reading frame, and dashed arrows indicate the disrupted EGFP frames.
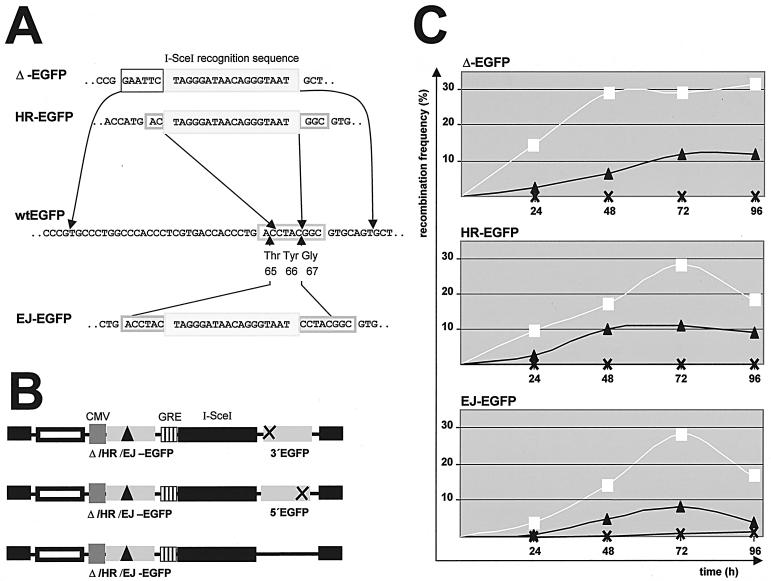
FIG. 2.
Time course measurements with different recombination substrates. (A) Acceptor gene design. Acceptor gene substrates were designed such that the I-SceI recognition sequence (light grey box) replaces either 46 bp (Δ-EGFP) or 4 bp (HR-EGFP) within the wt EGFP sequence. For in-frame wt EGFP reconstitution via NHEJ, microhomologies of 5 bp (dark grey boxes) were generated 5′and 3′ to the I-SceI recognition sequence in EJ-EGFP. The grey frames mark the codons for the chromophore residues 65 to 67 within wt EGFP. (B) Acceptor and donor gene combinations. Wild-type EGFP reconstitution was analyzed with constructs comprising either one of the three different acceptor genes (Δ-EGFP, HR-EGFP, EJ-EGFP) together with the 5′-truncated donor gene 3′EGFP, with the 3′-truncated donor gene 5′EGFP, or without a donor gene. The extent of homologies between acceptor and donor genes 5′ and 3′ to the I-SceI site was 164 and 519 bp for Δ-EGFP/3′EGFP, 191 and 528 bp for HR-EGFP/3′EGFP, 199 and 533 bp for EJ-EGFP/3′EGFP, 168 and 233 bp for Δ-EGFP/5′EGFP, 195 and 242 bp for HR-EGFP/5′EGFP, and 203 and 247 bp for EJ-EGFP/5′EGFP. (C) Recombination measurements. Twenty micrograms of each recombination plasmid was electroporated into KMV cells. To induce I-SceI-mediated cleavage, estradiol was included in the growth medium. At different times after electroporation, the numbers of green fluorescing cells were determined by flow cytometry. Positive controls with wt EGFP expression plasmid unveiled stable transfection efficiencies of 27% ± 2% for each construct and time point. Recombination frequency was calculated as the number of fluorescing cells divided by the total number of successfully transfected cells. The mean values of three to eight independent recombination measurements are shown graphically. A total of 107 cells were electroporated in each sample, with an estimated 50% survival. White squares, values for recombination plasmids with 3′EGFP at the donor position; black triangles, values for 5′EGFP plasmids; black crosses, values for donor-free constructs.
The acceptor genes Δ-EGFP, HR-EGFP, and EJ-EGFP were constructed by PCR mutagenesis (Fig. 2). In Δ-EGFP, we replaced 46 bp encompassing the chromophore codons 65 to 67 by EcoRI and I-SceI recognition sites. In HR-EGFP, we replaced 4 bp out of the chromophore codons 65 to 66 by the I-SceI site. The resulting HR-EGFP gene additionally carried a single nucleotide mutation in codon 64 (CTG→ATG). In EJ-EGFP, the I-SceI site was inserted between a 5-bp duplication. Δ-EGFP, HR-EGFP, and EJ-EGFP were each transferred into the BamHI site of p5NM together with the cytomegalovirus (CMV) promoter from pEGFP-N1 and the puromycin resistance gene from pRetroOn (Clontech).
For the analysis of cHR we constructed the donor gene 5′EGFP, and for cHR and deletion events we constructed 3′EGFP. 5′EGFP was engineered by AgeI and GsuI digestion of the vector pEGFP-N1, resulting in 3′ truncation of the wt EGFP coding sequence by 276 bp. 3′EGFP was PCR amplified by use of the mutagenizing oligonucleotide 5′-AGGAGGCTCGAGTAGTGAGTG AGCAAGGGCGAGGAGCTGT-3′ (mutating bases are underlined), thereby replacing the start ATG from wt EGFP by the stop codons TAG and TGA. 5′EGFP and 3′EGFP were ligated into the XhoI restriction site of p5NM.
As a spacer, an I-SceI expression cassette, consisting of the GRE promoter from pGC (4) and the sequence coding for N-terminally hemagglutinin (HA) epitope-tagged, mammalianized I-SceI (40), was inserted into the SalI site of p5NM. Alternatively, the spacer consisted of the hygromycin cDNA from pIREShyg (Clontech) and the simian virus 40 promoter from pEGFP-N1.
To generate plasmid pGC-wtp53 for Gal4ERVP-dependent wtp53 expression, we inserted the p53 cDNA into the EcoRV site of pGC (4). The pSV53her derivative pSVp53(138V)her was engineered by in vitro mutagenesis with oligonucleotide 5′-ACAGGGCAGGTCTTGACCAGTTGGCAAAACATC-3′ (the mutating base is underlined) and the U.S.E. mutagenesis kit from Pharmacia. pSVp53(174Y-r)her was created by PCR amplification with pKEX-p53(174Y) template (36) and primers 5′-AAGAATTCATGGAGGATTCACAG-3′ and 5′-TTGGATCCAGGTCTGAGTCAGGCC-3′, EcoRI/BamHI digestion, and ligation with pSV53her. Sequences encoding wtp53, mutant p53, and I-SceI within expression constructs were verified by use of the ABi PRISM Big Dye ready reaction cycle sequencing kit and an ABi 377 sequencer. Recombination plasmids were sequenced as well.
Generation of transgenic clones and genomic PCR analysis.
The human myeloid leukemia cell line K562 and the human B-lymphoblastoid cell lines TK6 and WTK1 (26) were grown in RPMI medium supplemented with 12% fetal calf serum. KMV cells, expressing the transcription factor Gal4ERVP for estradiol-inducible expression, were established by electroporation of K562 cells with pMVGalERVP (4) and soft agar cloning in phenol red-free RPMI with 12% charcoal-stripped fetal calf serum (9) and 1.2 mg of G418 per ml. To generate stable integrants for recombination assays, KMV cells were electroporated with recombination plasmids carrying either the Δ-EGFP, the HR-EGFP, or the EJ-EGFP acceptor, the 3′EGFP donor, and a hygromycin spacer. For the analysis of DSB repair in the presence of wtp53, we stably coelectroporated KMV cells with one of the recombination plasmids and with pSV53her or we stably introduced pGC-wtp53 first and the recombination plasmid next. Subsequently, puromycin-resistant (0.25 μg/ml) colonies were screened by genomic PCR for the integrity of the chromosomally integrated recombination plasmids and by Western analysis for p53 expression. For recombination measurements we utilized clones, which showed I-SceI-inducible cellular fluorescence. To verify the DNA exchange products, we isolated 4,000 to 9,000 cells after transfection with pCMV-I-SceI on the basis of green fluorescence on a fluorescence-activated cell sorter (FACS), M.Fl. cell sorter (Cytomation BioInstruments, Ft. Collins, Colo.). Additionally, we isolated green fluorescent cells by soft agar cloning. Subsequent genomic PCR analyses relied on the oligonucleotides PCR-1-1 (5′-CCCGCAACCTCCCCTTCTAC-3′), PCR-1-2 (5′-GAACCCGCTCGTCTGGCTAAG-3′), PCR-2-1 (5′-TACACAAATCGCCC GCAGAAGC-3′), and PCR-2-2 (5′-CTGTCTTTAACAAATTGGACTAATCG-3′). The PCR conditions used were 5 min at 94°C, then 35 cycles at 92°C for 60 s, 60°C for 60 s, and 72°C for 120 s, followed by a final extension step of 72°C for 7 min. At least three PCRs were performed for each construct from genomic DNA prepared from fluorescing and control cells. Reaction products were purified by QiAquick PCR purification kit (Qiagen) and subjected to restriction analysis. Primers were synthesized by MWG Biotech. I-SceI meganuclease was purchased from Roche.
Extraction of mammalian cells and Western blot analysis.
Total cellular homogenates were obtained as before (50). Proteins, separated on a sodium dodecyl sulfate-polyacrylamide gel (10 to 15% acrylamide) and transferred to Immobilon-P membranes (Millipore), were immunodetected by the affinity-purified, monoclonal anti-p53 antibodies DO1 or PAb421 (Oncogene Science) or the anti-HA antibody 12CA5, coupled to recognition by the affinity-purified and peroxidase-conjugated secondary serum, anti-mouse immunoglobulin G from goat (Rockland). Visualization of the immunocomplexed bands was achieved by Super-Signal ULTRA chemiluminescence enhancement (Pierce). Autoradiographies from titration analysis were subjected to phosphorimaging in order to quantify protein levels.
Transfections and recombination assays.
To assay EGFP expression for the determination of recombination frequencies, 107 cells were electroporated at 240 V and 1,050 mF with a total amount of 20 μg of plasmid DNA and subsequently split into two aliquots of 5-ml tissue culture medium with or without 200 nM β-estradiol. Cells were cultivated for 1 to 96 h at 37°C, except for assays with pCMV-p53(143A) at 39°C. Subsequently, cells were subjected to FACS cytometry by use of a Coulter Counter EPICS XL-MCL FACScan using the 488-nm laser line for excitation in combination with the filters used for green (Fl1) and orange (Fl2) fluorescence detection. EGFP-positive and -negative cells were distinguished by the published diagonal gating procedure in the Fl1/Fl2 dot plot (34). In parallel to each recombination measurement, we performed controls with plasmid devoid of the acceptor gene cassette and plasmid devoid of the donor gene to ascertain that EGFP signals were caused by homologous exchange. To determine the individual expression levels of EGFP in the presence or absence of I-SceI and of p53, each experiment included a cotransfection with a control plasmid, which carried wt EGFP at the acceptor position. The fraction of EGFP-positive cells was individually determined and was used to normalize each single recombination frequency, to exclude rate deviations related to growth regulatory effects. At least three independent measurements from at least two independent cell clones or cell batches were performed each, to calculate mean values and standard errors. Dose-response curves for p53-dependent recombination regulation were generated after transient electroporation experiments with 1 ng to 10 μg of pCMV-wtp53 and of pCMV-p53(273H) plasmid, respectively.
RESULTS
Fluorescence-based assessment of DSB repair pathways in human cells.
For the analysis of DSB repair in human cells we created a short-time test system based on the exchange between differently inactivated EGFP genes (Fig. 1). To initiate DSB repair precisely within one of the recombination substrate genes (acceptor gene), we inserted the 18-bp recognition sequence for I-SceI meganuclease into the EGFP variant under control of a CMV promoter. Homologous exchange between the cleaved acceptor gene and a 3′-positioned, equally oriented, and promoter-free EGFP variant (donor gene) was expected to restore the wt EGFP sequence at the acceptor position. The frequency of reconstituting recombination events was measured by FACS analysis as the fraction of green fluorescing cells within the transfected cell population. The mutations within the acceptor genes Δ-EGFP and HR-EGFP were chosen such that the I-SceI site substituted either 46 or 4 bp in the region encoding the EGFP chromophore, thereby varying the extent of homology with the donor sequence (Fig. 2A). To measure NHEJ via microhomologies, we incorporated the I-SceI site between a 5-bp duplication within the EJ-EGFP acceptor gene. To record NHEJ and HR concomitantly, we constructed plasmids with the EJ-EGFP acceptor and a donor gene (Fig. 2B). To record conservative HR (cHR) (crossing over, gene conversion) exclusively, we designed the 3′-truncated 5′EGFP donor gene. To detect both cHR and the deletion of intervening sequences (single-stranded annealing, replication slippage), we created 5′-truncated 3′EGFP (14, 20, 22). To transiently express the meganuclease, we introduced I-SceI cDNA (40) under the control of Gal4 responsive elements (GRE) as a spacer between the acceptor and the donor genes. Gal4ERVP (4)-producing KMV clones derived from the human leukemia cell line K562 were established, so that GRE-dependent expression relied on the estradiol responsiveness of this transcription factor (Fig. 1).
KMV cells were electroporated with each one of the six recombination plasmids, which resulted from the incorporation of one of the three acceptor genes and one of the two donor genes in different combinations and with the donor-free and acceptor-free plasmids (Fig. 2B and C). For I-SceI-mediated cleavage, estradiol was included into the growth medium. Twenty-four hours later we detected up to 15% green fluorescent cells within the population transfected with plasmids comprising acceptor and donor genes, and the percentage reached a maximum of up to 30% 48 to 72 h after electroporation. This indicated wt EGFP reconstitution by HR, since the signals for control plasmids stayed below the detection limit (10−4). With the donor-free EJ-EGFP plasmid a NHEJ frequency of 1% was determined. Similar HR and NHEJ frequencies were obtained in coelectroporation experiments with recombination plasmids, which contained a hygromycin spacer, and with pCMV-I-SceI for meganuclease expression.
With respect to the three differently mutated acceptor genes, similar HR frequencies were recorded. With respect to the two donor genes, we noticed an increase when we applied constructs with 3′EGFP compared to 5′EGFP. After 72 h this increase was 2.8-fold for HR-EGFP constructs in KMV cells (25.2 × 10−2 ± 1.5 × 10−2 for HR-EGFP/3′EGFP and 8.9 × 10−2 ± 2.5 × 10−2 for HR-EGFP/5′EGFP; n = 3). The 5′EGFP and 3′EGFP donor genes contained 233 to 247 bp versus 519- to 533-bp fragments that were homologous with the acceptor gene 3′ to the I-SceI site. Another plasmid, 5′EGFP/HR-EGFP, which like HR-EGFP/3′EGFP was designed to measure cHR and deletion events, displayed a homology length of 233 to 247 bp. After 72 h HR occurred at a 1.3-fold-lower frequency with 5′EGFP/HR-EGFP (20.0 × 10−2 ± 2.2 × 10−2) than with HR-EGFP/3′EGFP, which reflected the effect of the homology length difference. Therefore, we interpreted the 2.8-fold difference between the frequencies for HR-EGFP/5′EGFP and HR-EGFP/3′EGFP (Fig. 2C) by an additional contribution of deletion events. These data demonstrated that our fluorescence-based assay allowed to record DSB repair by the major pathways, namely cHR, deletions, and NHEJ in human cells.
Effect of p53 on NHEJ.
To assess a possible role of p53 in NHEJ, we tested whether the in-frame reconstitution of wt EGFP within a unique EJ-EGFP sequence was affected by p53. The human myeloid leukemia cell line K562 was chosen because it does not express p53 and does not undergo apoptosis when wtp53 is expressed (29). To further exclude rate deviations related to growth-regulatory effects, the fraction of EGFP-positive cells in the presence or absence of p53 was determined in parallel to each recombination experiment and was used to normalize the recombination frequencies. In electroporation experiments with donor-free EJ-EGFP plasmids, we obtained NHEJ activities of 1% ± 0% after estradiol-induced I-SceI production (n = 3). After wtp53 expression by pCMV-wtp53 coelectroporation, green fluorescent cells were undetectable, which indicated >10-fold-reduced NHEJ frequencies. This suggested an inhibitory involvement of p53 in NHEJ within I-SceI-linearized plasmids.
p53 inhibits DSB-induced homologous recombination.
We and others have previously shown that inactivation of wtp53 enhances spontaneous HR (2, 31, 50). Since p53 accumulation is triggered most rapidly by DNA strand breaks (25), we analyzed the role of p53 in DSB-induced HR by applying our fluorescence and I-Sce I-based test system.
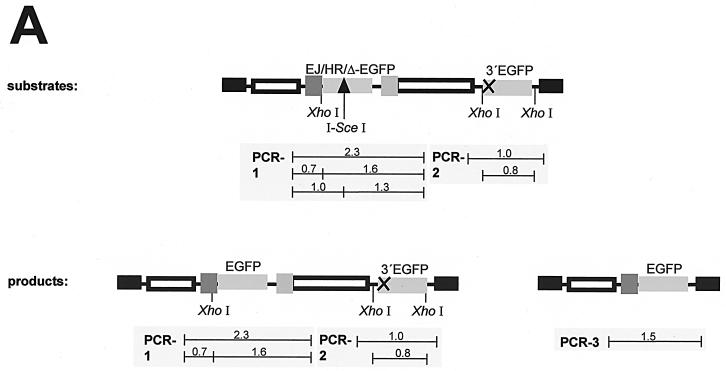
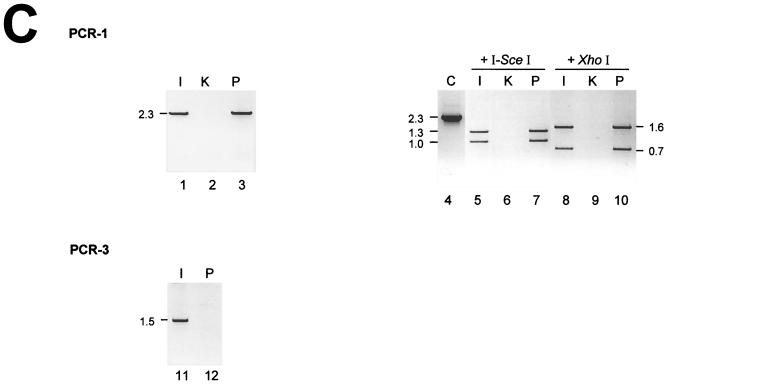
For the analysis, we stably transfected KMV cells with recombination plasmids that carried the Δ-EGFP, HR-EGFP, or EJ-EGFP acceptor and the 3′EGFP donor genes (Fig. 2). To understand the DSB repair events which underlie the reconstitution of wt EGFP genes within chromosomally integrated plasmids, we sorted green fluorescent cells flow cytometrically after the expression of I-SceI meganuclease and performed genomic PCR analysis (Fig. 3). cHR within one of the integrated recombination plasmids was indicated when the 2.3-kb PCR-1 fragment, which covers the acceptor and overlaps with the spacer region (Fig. 3A), showed resistance to total I-SceI cleavage. In combination with the absence of an I-SceI-cleavable PCR-2 fragment, which covers the donor region, cHR was interpreted as the result of gene conversion rather than double crossover. Gene conversion-specific products were detected in reactions from all types of sorted cells (Fig. 3B, lanes 8, 13, 23, and 28). Reaction PCR-3 was designed to detect a 1.5-kb product, which was expected to appear after a deletion event or, more rarely, after gene conversion with a tract length of at least 612 bp 3′ to the DSB (11). The predicted band was amplified from cells with HR-EGFP/3′EGFP plasmid (Fig. 3B, lane 32), whereas it was below the detection limit in reactions from EJ-EGFP/3′EGFP cells (Fig. 3B, lane 34). Altogether, we monitored both gene conversion and deletions in sorted cells with HR-EGFP/3′EGFP (Fig. 3B) and with Δ-EGFP/3′EGFP plasmid (not shown). In cells with EJ-EGFP/3′EGFP, gene conversion was the predominant DSB repair pathway. Finally, not only gene conversion but also deletion was detected as an independent event, as shown in Fig. 3C, lanes 5 and 11, displaying data from the analysis of an EGFP-positive single cell clone derived from Δ-EGFP/3′EGFP cells (Fig. 3C, lanes 5 and 11).
FIG. 3.
PCR analysis of green fluorescent cells. (A) Scheme of the recombination substrates and products, showing the positions of PCR fragments from the three reactions (PCR-1, -2, and -3). The expected sizes of these fragments and of restriction enzyme cleavage products are given in kilobases. (B) Genomic PCR of sorted cells. Clones with stably integrated HR-EGFP and EJ-EGFP recombination substrates were electroporated with vector pCMV-I-SceI. Green fluorescent cells were sorted (S; lanes 2, 5, 8, 10, 13, 15, 17, 20, 23, 25, 28, 30, 32, and 34) and analyzed by the genomic PCRs (PCR-1, -2, and -3) in parallel to reactions with DNA from untransfected (U; lanes 1, 4, 7, 9, 12, 14, 16, 19, 22, 24, 27, 29, 31, and 33) cells. The PCR products were cleaved by the meganuclease I-SceI and the control enzyme XhoI and subsequently analyzed by agarose gel electrophoresis. Genomic DNA from the parental KMV cells (K; lanes 3 and 18) did not support the amplification of products specific for the recombination plasmids. PCR products from bacterial recombination plasmid (C; lanes 21 and 26) were applied as size markers. (C) Genomic PCR of a fluorescent clone. Green fluorescent cells were selected by soft agar cloning after successful recombination. The isolated cell line (I; lanes 1, 5, 8, and 11), the parental line with the stably integrated Δ-EGFP plasmid (P; lanes 3, 7, 10, and 12), and the original KMV cells (K; lanes 2, 6, and 9) were analyzed by the genomic PCRs (PCR-1 and -3) and subsequently by I-SceI and XhoI cleavage as in panel B. C, PCR product from the bacterial recombination plasmid (lane 4).
To examine the effect of p53, we stably transfected KMV cells with both the HR-EGFP/3′EGFP recombination plasmid and pSV53her, the latter for stable expression of human wtp53, C-terminally fused to the human estrogen receptor hormone-binding domain (p53her). This construct allows to express p53 at physiological levels and to stimulate wtp53 functions after estradiol application, while minimizing growth retardation during cell culture (9, 38). Several puromycin-resistant clones were isolated and screened by genomic PCR for the integrity of the recombination substrate and by Western blotting for the expression of the p53her protein (Fig. 4). The newly generated cells were electroporated with pCMV-I-SceI, and flow cytometric analysis was performed after 48, 72, and 96 h of cultivation in the presence of estradiol (Fig. 4). In p53-negative clones I-SceI expression caused frequent wt EGFP reconstitution, with fluorescent signals increasing until 96 h after electroporation (36 × 10−4). In contrast, after electroporation with pBS only a small number of EGFP-positive cells were detected (2 × 10−4), indicating an 18-fold induction of DSB repair by specific I-SceI cleavage. The corresponding values for p53her-expressing cells were 10−4 with pCMV-I-SceI and <10−4 with pBS. Thus, with chromosomally integrated HR-EGFP/3′EGFP substrate we recorded a 36-fold inhibition by p53 after I-SceI-mediated cleavage and without it we recorded a >4-fold HR inhibition.
FIG. 4.
Homologous DSB repair in the presence of stably expressed wtp53. Homologous exchange was analyzed between HR-EGFP and 3′EGFP within plasmids (upper panel map), which were stably integrated into the cellular genome of KMV cells alone (−p53her) or together with p53her expression plasmid (+ p53her). After electroporation with 20 μg of pCMV-I-SceI (+ I-SceI) or pBS (− I-SceI), cells were cultivated for 48, 72, or 96 h and recombination frequencies were determined as for Fig. 2. Mean values and standard errors are diagrammatically shown for two clones and three to six independent measurements each. The absence of p53 in KMV cells and protein expression of p53her with an apparent molecular mass of 90 kDa in clones 12 and 13 were visualized by Western blotting (bottom panel). The primate cell line LMV with endogenous mutant p53 served as a control (10).
Effect of wtp53 on DSB repair involving HR and NHEJ.
It was recently shown that in the majority of DSB repair events the genomic integrity is preserved by a coupling of short-tract gene conversion and NHEJ (37). Here, we examined the effect of p53 on the chromosomally integrated EJ-EGFP/3′EGFP plasmid, which allows wt EGFP reconstitution by both homologous DNA exchange between EJ-EGFP and 3′EGFP and by NHEJ within EJ-EGFP (Fig. 5A). Our results from PCR analyses (Fig. 3B) were compatible with the idea that this substrate promoted the coupled DSB pathway. To determine DSB repair in the presence and absence of p53, we cotransfected the cells with pCMV-I-SceI and the pCMV-wtp53 expression vector or with pCMV-I-SceI and pBS (Fig. 5A). Transient wtp53 expression resulted in a fourfold reduction of the DNA exchange frequency for EJ-EGFP/3′EGFP. For comparison, with chromosomally integrated substrates designed for HR analysis, wtp53 led to a 27-fold decrease in cells with HR-EGFP/3′EGFP and a 43-fold decrease in cells with Δ-EGFP/3′EGFP plasmid. Due to this differential effect, it was unlikely that overexpressed p53 simply caused recombination inhibition by exonucleolytic degradation of I-SceI-linearized DNA substrates. However, to study the effect of wtp53 at low expression levels as well, we utilized the pGC vector, which induces protein amounts approximately 100-fold lower than those induced by the pCMV vector. In cells which were stably transfected with pGC-wtp53, we still noticed a p53-dependent recombination suppression by 50 to 60% for the chromosomally integrated Δ-EGFP/3′EGFP substrate (Fig. 5B). For EJ-EGFP/3′EGFP the reduction was only 10 to 20%, i.e., again less pronounced.
FIG. 5.
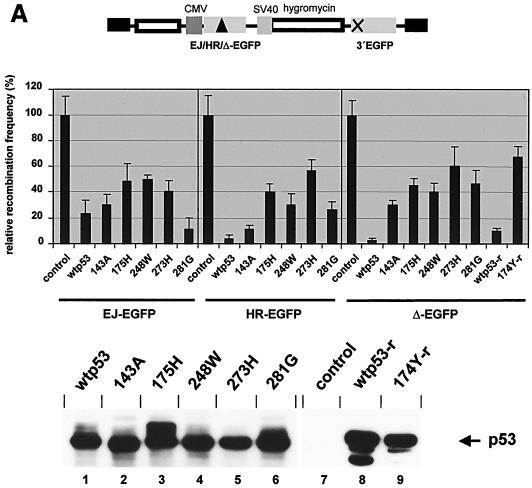
Effects on homology-directed DSB repair by wtp53 and tumor-derived mutants. Recombination constructs (a map is shown in the upper part of each panel) with different acceptor genes were stably introduced into the cellular genome of KMV cells. The resulting clones were subjected to pCMV-I-SceI electroporation. After further cultivation for 72 h the percentage of green fluorescing cells was determined. Recombination frequencies in cells without p53 were taken as 100% (14 × 10−4 for EJ-EGFP, 27 × 10−4 for HR-EGFP, 22 × 10−4 for Δ-EGFP), and the relative recombination frequencies were calculated. Values are the means ± standard errors from two to four clones per acceptor type, which were analyzed by 6 to 12 independent measurements each. (A) Homologous DSB repair after expression of wtp53 via pCMV constructs. For transient expression of p53 variants KMV-derived clones were coelectroporated with pCMV-I-SceI and pCMV-wtp53, pCMV-p53(143A), pCMV-p53(175H), pCMV-p53(248W), pCMV-p53(273H), pCMV-p53(281G), or pBS (control). For the analysis of wtp53 (wtp53-r) and mutant p53(174Y) (174Y-r) from rat, the expression vector pKEX-p53 or pKEX-p53(174Y) was used. The expressions of the different p53 proteins were compared by immunoblotting (bottom panel), utilizing antibody DO1 (lanes 1 to 6) or PAb421 (lanes 7 to 9). (B) Homologous DSB repair after expression of wtp53 via pGC-wtp53. An analysis was made of the homologous exchange between EJ-EGFP and 3′EGFP and Δ-EGFP and 3′EGFP within plasmids, which were integrated into the genome of KMV cells (−p53) or of KMV cells stably transfected with pGC-wtp53 (+ p53). Relative recombination frequencies were determined in the presence (+ I-SceI) or absence (− I-SceI) of I-SceI meganuclease. The expression of p53 in stably transfected cells after estradiol induction (200 nM) was verified by immunoblotting (bottom panel). TK6 cells served as positive control.
Regulation of homology-directed DSB repair by cancer-related p53 mutants.
To investigate the modulatory efficiency of tumor-derived p53 mutants during I-SceI-triggered recombination, we transiently expressed five p53 proteins carrying alterations at amino acids, which are frequently mutated in colorectal cancer (15). To record differences between the residual activities of the hotspot mutants, high-level expression conditions with 10 μg of the corresponding CMV promoter plasmid were chosen. With p53(273H), a mutant devoid of a DNA contacting residue and with wtp53 conformation (6), recombination frequencies were 40 to 60% of the frequencies without p53 (Fig. 5A). It should be noted that with lower amounts of the expression plasmid the weak downregulatory effect by p53(273H) disappeared, whereas a wtp53-dependent inhibition by 10% was noticeable even with 1 ng of plasmid DNA. With another DNA contact mutant, p53(248W), and with the conformationally altered mutant p53(175H), 30 to 50% of the control values were measured. In experiments with p53(281G) and with the temperature-sensitive mutant p53(143A) (55), we observed recombination frequencies that were in between the values for wtp53 and p53(273H) or even similar to the values for wtp53. The oncogenic mutant p53(174Y) from rat caused a reduction down to 68% of the control value (Δ-EGFP), while a reduction to 10% was measured with wtp53 from rat (36). In summary, HR suppression by the mutants with amino acid changes at position 273, 248, or 175 compared to wtp53 was 18- to 26-fold less pronounced with Δ-EGFP/3′EGFP substrate, 8- to 16-fold with HR-EGFP/3′EGFP, and 2-fold with EJ-EGFP/3′EGFP plasmid.
Activities of specifically inactivated p53 mutants in regulating DSB repair.
To further clarify the importance of DNA substrates in the p53-dependent regulation of DSB repair, we examined the activities of specifically mutated p53 variants (Fig. 6). Human p53(138V) is inactive as a transcriptional transactivator (47). The C-terminally truncated form p53(1-363) lacks the negative regulatory region and p53(1-333) lacks the oligomerization domain. To detect subtle recombination frequency changes, the proteins were transiently produced at 100-fold-reduced levels by use of pSV53her vector derivatives. Under these expression conditions, severely defective mutants like p53(174Y-r) did not interfere with recombination at all (Fig. 5A and 6). Repair within chromosomally integrated recombination plasmids (Fig. 6) was triggered by pCMV-I-SceI coelectroporation. For EJ-EGFP/3′EGFP the same threefold recombination downregulation was seen with wtp53her and with the mutant proteins. For HR-EGFP/3′EGFP the decrease was three- to fourfold. For Δ-EGFP/3′EGFP the inhibition was six- and fourfold after wtp53her and p53(1-363)her expression, respectively. Expression of the mutant proteins p53(138V)her and (1-333)her led to a twofold reduction. Thus, unlike what was observed with wtp53, we measured intermediate suppression of DSB repair by the mutants p53(138V) and p53 (1-333) with Δ-EGFP/3′EGFP plasmid.
FIG. 6.
Regulatory activities by specifically inactivated p53 mutants. One representative clone for each chromosomally integrated recombination construct was coelectroporated with the vectors pCMV-I-SceI and with either pSVp53her (wtp53), pSVp53(138V)her, pSVp53(1-333)her, pSVp53(1-363)her, pSVp53(174Y-r)her, or pBS (control) for the expression of the I-SceI meganuclease and different p53her variants. Recombination frequencies with pBS-transfected cells (control) were taken as 100% (31 × 10−4 for EJ-EGFP, 49 × 10−4 for HR-EGFP, and 44 × 10−4 for Δ-EGFP), and relative recombination frequencies were calculated. Immunoblotting was performed with DO1 antibody.
Effect of p53 on cHR between sequences with limiting homologies.
Rubnitz and Subramani (41) noticed that the sharpest drop in recombination frequency occurred when the homology was reduced from 197 to 163 bp. Within the recombination plasmids generated in this study, uninterrupted homologies of <200 bp were present 5′ to the I-SceI recognition sequence. Within HR-EGFP, 191 to 195 bp were homologous between the acceptor and the donor gene, compared to 164 to 168 bp within Δ-EGFP (Fig. 2B). To study the influence of sequence homology length in HR regulation by p53, we determined DSB repair frequencies in electroporation assays with the HR-EGFP/3′EGFP, Δ-EGFP/3′EGFP, HR-EGFP/5′EGFP, and HR-EGFP/5′EGFP plasmids. I-SceI meganuclease production relied on the GRE expression cassette located on the recombination plasmid. We varied the p53 status by coelectroporating KMV cells with either pCMV-wtp53 or with pBS. After wtp53 expression in assays with HR-EGFP/3′EGFP the frequency was reduced fourfold, and in assays with Δ-EGFP/3′EGFP it was reduced fivefold (Fig. 7A). With HR-EGFP/5′EGFP we measured a 7-fold p53-specific reduction and with Δ-EGFP/5′EGFP a 17-fold p53-specific reduction. To examine the effect of endogenously expressed wtp53, we determined wt EGFP reconstitution in the human B-lymphoblastoid cell lines TK6 and WTK1, which are derived from the same donor and express wtp53 and mutant p53(237I), respectively (26). In TK6 versus WTK1 cells, recombination was 4-fold less frequent both with HR-EGFP/3′EGFP and Δ-EGFP/3′EGFP plasmids, 7-fold with HR-EGFP/5′EGFP plasmid, and 50-fold with Δ-EGFP/5′EGFP plasmid (Fig. 7B). In conclusion, recombination suppression by both ectopically and endogenously expressed wtp53 was particularly evident in assays with Δ-EGFP/5′EGFP plasmids, i.e., during cHR processes under conditions of limiting sequence homologies.
FIG. 7.
Regulatory activities of wtp53 with respect to different substrates for HR. (A) Effect of transiently expressed p53. Recombination assays were performed by coelectroporating KMV cells with 10 μg of pCMV-wtp53 (+ p53) or pBS (− p53) and with either one of the two constructs for assaying both cHR and deletion (HR-EGFP/3′EGFP, Δ-EGFP/3′EGFP) or with either one of the two constructs for assaying cHR exclusively (HR-EGFP/5′EGFP, Δ-EGFP/5′EGFP). For the expression of I-SceI, estradiol was added to the growth medium. Flow cytometric analysis was performed 72 h later. For the determination of relative recombination frequencies after wtp53 expression, the frequencies with pBS were taken as 100% (22 × 10−2 for HR-EGFP/3′EGFP, 28 × 10−2 for Δ-EGFP/3′EGFP, 13 × 10−2 for HR-EGFP/5′EGFP, 7 × 10−2 for Δ-EGFP/5′EGFP). Mean values and standard errors were calculated from three to six measurements each. (B) Effects of endogenously expressed p53. Isogenic cells with wtp53 (TK6) and mutant p53 (WTK1) were electroporated with the same plasmid substrates as for panel A. Mean values and standard errors were calculated from four measurements. For the determination of relative recombination frequencies, the frequencies with WTK1 cells were taken as 100% (3 × 10−2 for HR-EGFP/3′EGFP and Δ-EGFP/3′EGFP and 1 × 10−2 for HR-EGFP/5′EGFP and Δ-EGFP/5′EGFP).
DISCUSSION
In the present work, we investigated the role of p53 in DSB repair by use of the rare-cutting, site-specific I-SceI nuclease. Due to this strategy, it could be excluded that the p53-dependent effects on recombination were indirectly caused by other repair activities of p53, such as during nucleotide or base excision repair. Utilizing an EGFP-based system that detects reactivating repair events without applying selective pressure and with a short delay, we found that wtp53 downregulates homology-directed DSB repair when expressed either endogenously or exogenously. Our observations are consistent with several studies which indicated suppression of spontaneous HR by p53 (2, 31, 50). Conversely, Willers and colleagues (52) did not see this effect with episomal recombination substrates, which were amplified by a polyomavirus-based replicon. Here, we have demonstrated recombination downregulation by p53 with extrachromosomal as well as with chromosomally integrated substrates. Recombination downregulation was still observed at low protein levels, but the degree of inhibition was dose dependent. Furthermore, suppression by p53 at fixed levels was 10-fold less pronounced when the recombination plasmids were introduced at high copy numbers by electroporation (Fig. 7A) than with chromosomal integrates (Fig. 5A). From these observations it appears that the extent of recombination regulation is influenced by the p53/DNA substrate ratio, which might explain the failure to detect recombination regulation with certain episomes.
Two hundred base pairs seems to represent a critical limit for HR in mammalian cells according to the analysis of the minimum amount of homology required (41) and according to the length distribution of gene conversion tracts (11). Our analysis of the p53-dependent regulation of homology-directed DSB repair with different substrate types suggested that downregulation is particularly evident during gene conversion events (5′EGFP donor), when sequence homologies were significantly shorter than 200 bp (Δ-EGFP acceptor). It should be noted that the decrease of homologies with Δ-EGFP versus HR-EGFP substrate did not significantly alter the HR frequencies in K562 and WTK1 cells devoid of functional p53. These results provide further evidence for a possible role of p53 in creating a threshold for recombination between short and long homologies, as was postulated from studies on the stability of repetitive sequences (13). Thus, our data support the idea that p53 plays an important role in the fidelity control during HR (9, 45), thereby possibly preventing error-prone repair and detrimental rearrangements by misalignment of repetitive DNA.
An inhibitory effect by wtp53 also became apparent with the donor-free plasmid, which we utilized for probing NHEJ. This is in agreement with data from reporter gene reconstitution and integration assays (3, 24) and with experimental results from Comet assays, showing that after the exposure to ionizing radiation DSB rejoining increases with loss of wtp53 function (5). Conversely, others observed a stimulatory role of p53 in NHEJ with plasmid substrates, which promote rejoining of broken DNA ends via short homologies (46, 54). However, unlike what was seen with the linearized plasmids used in the latter studies, here wt EGFP reconstitution within the EJ-EGFP gene after I-SceI cleavage required the removal of out-of-frame nonhomologous DNA overhangs. Therefore, in view of a fidelity control function of p53 in HR, p53 might play a similar role also in NHEJ, either by mere recognition of heterologies and inhibition of the process or by exonucleolytic proofreading. This would explain why in earlier studies error-free NHEJ was not inhibited by p53, whereas here we show that a NHEJ process, which creates nonhomologous overhangs, is downregulated. Interestingly, physical interactions of p53 with polymerase β have been reported (56). Polymerase β may participate in repair synthesis during meiotic, i.e., homologous, recombination as well as in gap filling during NHEJ together with an unidentified nuclease that removes terminal mismatches (35, 53). Thus, p53 is a conceivable candidate to provide a proofreading activity for polymerase β during recombination, since wtp53 is able to exonucleolytically process DNA ends in a 3′→5′ orientation with mismatch excision activity in a polymerase-based assay and with a preference for recombination intermediates in vitro (16, 33, 44, 45).
Recently, convincing evidence that homologous repair and NHEJ are not completely separable and that coupling of invasion of one chromosome end into homologous sequences and subsequent NHEJ of the originally broken chromosome effectively prevents rearrangements between different chromosomes was presented (37). When we studied DSB repair with a construct in which EJ-EGFP served both as the acceptor gene during homologous exchange and as a substrate for NHEJ via microhomologies, the inhibitory effect of wtp53 compared to cancer-related p53 mutants was minimal. Our PCR analysis of recombination products was compatible with a coupled pathway, since the fraction of deletions and long-tract gene conversion events was low for EJ-EGFP (37). Thus, it is tempting to speculate that the tumor suppressor p53 is less inhibitory to recombination when the coupled pathway reduces gene conversion tracts, which are subject to p53-dependent surveillance. These data are consistent with p53 being a central player in restraining chromosomal rearrangements, since the coupled pathway combines high-fidelity homologous exchange with intramolecular repair.
In previous studies, an increase in HR was found to correlate with the expression of structurally altered p53 mutants, while opposing results were obtained with respect to the DNA contact mutant p53(273H) (10, 42). In an attempt to resolve this discrepancy, we utilized the p53-negative cell line K562 to avoid complementation or dominant negative effects between endogenous and exogenous p53 forms. Under these conditions the structural mutant p53(175H), as well as the DNA contact mutants p53(248W) and p53(273H), was severely impaired in downregulating homologous DNA exchange (6). This suggested that dominant effects by endogenous p53 variants might have caused deviating results in earlier reports. From the fact that p53(273H) turned out to be the mutant with the lowest activities in recombination downregulation while transcriptional transactivation and growth regulatory functions are partially retained by p53(273H) (10), a central role of arginine 273 in the biochemistry underlying recombination control must be assumed. Intriguingly, arginine 273 is involved in sequence-independent DNA interactions according to the crystal structure and is essential for binding and exonucleolytically attacking recombination intermediates (6, 45). In comparison to p53(273H), p53(143A) and p53(281G) are detected less frequently in cancer patients and, accordingly, were significantly less defective in downregulating HR. This observation further supports the idea that the downregulation of HR contributes to tumor suppression. Importantly, with all cancer-related p53 mutants, we noticed a failure to fully counteract HR particularly with limiting homologies (Δ-EGFP), so that erroneous DNA exchange processes are expected to follow p53 alterations during tumorigenesis. Mutants p53(175H) and p53(174Y), which positively contribute to cancer progression, did not stimulate recombination beyond the p53-negative frequencies, which indicates a recombination-unrelated mechanism underlying their gain of function (36, 39).
Since p53 performs multiple functions in apoptosis, transcriptional transactivation, growth control, and recombination, it is important to understand possible interdependencies. Here, we excluded apoptosis-related effects on p53-dependent recombination regulation by choosing K562 cells (29). Earlier on, p53 mutations which allowed to distinguish functions in inhibiting HR and in inducing a G1/S arrest were identified (10, 42, 51). p53(138V) represents the human counterpart of the murine separation of function mutant p53(135V), which is devoid of transcriptional transactivation functions but is active in HR inhibition (51). Our analysis with p53(138V) confirmed that p53 regulates HR at least partially via a transactivation-independent mechanism, because p53(138V) downregulated HR like wtp53 with substrates that carried either the HR-EGFP or the EJ-EGFP acceptor gene. With Δ-EGFP acceptor substrates, i.e., for sequence homologies significantly below 200 bp, an intermediate effect was observed with p53(138V). From this, it is conceivable that transactivation-dependent pathways contribute to p53-dependent recombination control. p53 was reported to transcriptionally transactivate the gene encoding the recombination surveillance factor MSH2 (43). However, in response to DNA damage p53 accumulates, while MSH2 levels remain stable, which argues against a p53-dependent upregulation of MSH2 (57). Moreover, loss of p53 and MSH2 causes a synergistic increase in cancer susceptibilities and in the rate of frameshift mutations within CA repeat tracts after genotoxic treatment (8, 27). This argues against epistatic effects of p53 and MSH2 in genomic stabilization. At this point, it seems important to note that p53(135V) is an exonuclease-defective mutant and that oligomerization-inactive mutants, like p53(1-333), exonucleolytically attack single-stranded DNA but display reduced activities in binding and exonucleolytically processing heteroduplex junction DNAs (10, 18, 33). Therefore, instead of a failure to transcriptionally transactivate target genes, a loss of exonucleolytic proofreading might underlie the partial defects seen with p53(138V) and p53(1-333) during HR involving the Δ-EGFP acceptor. Transactivation-independent tumor suppressor activities by p53 were indicated by four- to fivefold-lower tumor incidences in mice transgenic for transactivation-deficient p53 than in p53 knockout mice (19).
In this work we show that p53 mutants seem to be particularly impaired in monitoring the exchange between sequences with short similarities, which suggests that HR inhibition by p53 increases with the error-proneness of the repair process. Moreover, it is now clear that apoptosis and transcriptional transactivation-independent activities of p53 are involved in recombination surveillance. Therefore, we hypothesize that p53 functions in tumor suppression as a gatekeeper of growth and as a caretaker by ensuring the fidelity of genome rearrangements.
Acknowledgments
We are especially indebted to Maria Jasin, Memorial Sloan Kettering Cancer Center, New York, N.Y., who generously provided the pCMV-I-SceI vector. We thank Carol Stocking, Heinrich-Pette-Institut Hamburg, for the retroviral vector p5NM and for expert advice.
This work was supported by the Deutsche Forschungsgemeinschaft grants Wi 1376/1-4 & -5 and grant 10-1281-Wi I from the Dr. Mildred Scheel Stiftung (Deutsche Krebshilfe).
REFERENCES
- 1.Albrechtsen, N., I. Dornreiter, F. Grosse, E. Kim, L. Wiesmüller, and W. Deppert. 1999. Maintenance of genomic integrity by p53: complementary roles for activated and non-activated p53. Oncogene 18:7706-7717. [DOI] [PubMed] [Google Scholar]
- 2.Bertrand, P., D. Rouillard, A. Boulet, C. Levalois, T. Soussi, and B. S. Lopez. 1997. Increase of spontaneous intrachromosomal homologous recombination in mammalian cells expressing a mutant p53 protein. Oncogene 14:1117-1122. [DOI] [PubMed] [Google Scholar]
- 3.Bill, C. A., Y. Yu, N. R. Miselis, J. B. Little, J. A. Nickoloff. 1997. A role for p53 in DNA end rejoining by human cell extracts. Mutat. Res. 385:21-29. [DOI] [PubMed] [Google Scholar]
- 4.Braselmann, S., P. Graninger, and M. Busslinger. 1993. A selective transcriptional induction system for mammalian cells based on Gal4-estrogen receptor fusion proteins. Proc. Natl. Acad. Sci. USA 90:1657-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bristow, R. G., Q. Hu, A. Jang, S. Chung, J. Peacock, S. Benchimol, and R. Hill. 1998. Radioresistant MTp53-expressing rat embryo transformants exhibit increased DNA-dsb rejoining during exposure to ionizing radiation. Oncogene 16:1789-1802. [DOI] [PubMed] [Google Scholar]
- 6.Cho, Y., S. Gorina, P. D. Jeffrey, and N. P. Pavletich. 1994. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science 265:346-355. [DOI] [PubMed] [Google Scholar]
- 7.Cleaver, E., V. Afzal, L. Feeney, M. McDowell, W. Sadinski, J. P. Volpe, D. B. Busch, D. M. Coleman, D. W. Ziffer, Y. Yu, H. Nagasawa, and J. B. Little. 1999. Increased ultraviolet sensitivity and chromosomal instability related to p53 function in the xeroderma pigmentosum variant. Cancer Res. 59:1102-1108. [PubMed] [Google Scholar]
- 8.Cranston, A., T. Bockerm, A. Reitmair, J. Palazzo, T. Wilson, T. Mak, and R. Fishel. 1997. Female embryonic lethality in mice nullizygous for both Msh2 and p53. Nat. Genet. 17:114-118. [DOI] [PubMed] [Google Scholar]
- 9.Dudenhöffer, C., G. Rohaly, K. Will, W. Deppert, and L. Wiesmüller. 1998. Specific mismatch recognition in heteroduplex intermediates by p53 suggests a role in fidelity control of homologous recombination. Mol. Cell. Biol. 18:5332-5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudenhöffer, C., M. Kurth, F. Janus, W. Deppert, and L. Wiesmüller. 1999. Dissociation of the recombination control and the sequence-specific transactivation function of P53. Oncogene 18:5773-5784. [DOI] [PubMed] [Google Scholar]
- 11.Elliott, B., C. Richardson, J. Winderbaum, J. A. Nickoloff, and M. Jasin. 1998. Gene conversion tracts from double-strand break repair in mammalian cells. Mol. Cell. Biol. 18:93-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford, J. M., and P. C. Hanawalt. 1997. Expression of wild-type p53 is required for efficient global genomic nucleotide excision repair in UV-irradiated human fibroblasts. J. Biol. Chem. 272:28073-28080. [DOI] [PubMed] [Google Scholar]
- 13.Gebow, D., N. Miselis, and H. L. Liber. 2000. Homologous and non-homologous recombination resulting in deletion: effects of p53 status, microhomology, and repetitive DNA length and orientation. Mol. Cell. Biol. 20:4028-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haber, J. E. 2000. Partners and pathways repairing a double-strand break. Trends Genet. 16:259-264. [DOI] [PubMed] [Google Scholar]
- 15.Hinds, P. W., C. A. Finlay, R. S. Quartin, S. J. Baker, E. R. Fearon, B. Vogelstein, and A. J. Levine. 1990. Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: a comparison of the “hot spot” mutant phenotypes. Cell Growth Differ. 1:571-580. [PubMed] [Google Scholar]
- 16.Huang, P. 1998. Excision of mismatched nucleotides from DNA: a potential mechanism for enhancing DNA replication fidelity by the wild-type p53 protein. Oncogene 17:261-270. [DOI] [PubMed] [Google Scholar]
- 17.Ishizaki, K., Y. Ejima, T. Matsunaga, R. Hara, A. Sakamoto, M. Ikenaga, Y. Ikawa, and S. Aizawa. 1994. Increased UV-induced SCEs but normal repair of DNA damage in p53-deficient mouse cells. Int. J. Cancer 58:254-257. [DOI] [PubMed] [Google Scholar]
- 18.Janz, C., S. Süsse, and L. Wiesmüller. 2002. p53 and recombination intermediates: role of tetramerization at DNA junctions in complex formation and exonucleolytic degradation. Oncogene 21:2130-2140. [DOI] [PubMed] [Google Scholar]
- 19.Jimenez, G. S., M. Nister, J. M. Stommel, M. Beeche, E. A. Barcarse, X. Q. Zhang, S. O'Gorman, and G. M. Wahl. 2000. A transactivation-deficient mouse model provides insights into Trp53 regulation and function. Nat. Genet. 26:37-43. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, R. D., and M. Jasin. 2000. Sister chromatid gene conversion is a prominent double-strand break repair pathway in mammalian cells. EMBO J. 19:3398-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laker, C., J. Meyer, A. Schopen, J. Friel, C. Heberlein, W. Ostertag, and C. Stocking. 1998. Host cis-mediated extinction of a retrovirus permissive for expression in embryonal stem cells during differentiation. J. Virol. 72:339-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert, S., Y. Saintigny, F. Delacote, F. Amiot, B. Chaput, M. Lecomte, S. Huck, P. Bertrand, and B. S. Lopez. 1999. Analysis of intrachromosomal homologous recombination in mammalian cell, using tandem repeat sequences. Mutat. Res. 433:159-168. [DOI] [PubMed] [Google Scholar]
- 23.Lee, S. M., L. Cavallo, and J. Griffith. 1997. Human p53 binds Holliday junctions strongly and facilitates their cleavage. J. Biol. Chem. 272:7532-7539. [DOI] [PubMed] [Google Scholar]
- 24.Lee, H., D. Sun, J. M. Larner, and F. S. Wu. 1999. The tumor suppressor p53 can reduce stable transfection in the presence of irradiation. J. Biomed. Sci. 6:285-292. [DOI] [PubMed] [Google Scholar]
- 25.Levine, A. 1997. p53, the cellular gatekeeper for growth and division. Cell 88:323-331. [DOI] [PubMed] [Google Scholar]
- 26.Levy, J. A., M. Virolainen, and V. Defendi. 1968. Human lymphoblastoid lines from lymph node and spleen. Cancer (Philadelphia) 22:517-524. [DOI] [PubMed] [Google Scholar]
- 27.Lin, X., K. Ramamurthi, M. Mishima, A. Kondo, and S. B. Howell. 2000. p53 interacts with the DNA mismatch repair system to modulate the cytotoxicity and mutagenicity of hydrogen peroxide. Mol. Pharmacol. 58:1222-1229. [DOI] [PubMed] [Google Scholar]
- 28.Livingstone, L. R., A. White, J. Sprouse, E. Livanos, T. Jacks, and T. D. Tlsty. 1992. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell 70:923-935. [DOI] [PubMed] [Google Scholar]
- 29.Mahdi, T., D. Alcalay, C. Cognard, J. Tanzer, and A. Kitzis. 1998. Rescue of K562 cells from MDM2-modulated p53-dependent apoptosis by growth factor-induced differentiation. Biol. Chem. 90:15-27. [PubMed] [Google Scholar]
- 30.Marmorstein, L. Y., T. Ouchi, and S. A. Aaronson. 1998. The BRCA2 gene product functionally interacts with p53 and RAD51. Proc. Natl. Acad. Sci. USA 95:13869-13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mekeel, K. L., W. Tang, L. A. Kachnic, C.-M. Luo, J. S. DeFrank, and S. N. Powell. 1997. Inactivation of p53 results in high rates of homologous recombination. Oncogene 14:1847-1857. [DOI] [PubMed] [Google Scholar]
- 32.Mirzayans, R., L. Enns, K. Dietrich, R. D. C. Barley, and M. C. Paterson. 1996. Faulty DNA polymerase delta/epsilon-mediated excision repair in response to gamma radiation or ultraviolet light in p53-deficient fibroblast strains from affected members of a cancer-prone family with Li-Fraumeni syndrome. Carcinogenesis 17:691-698. [DOI] [PubMed] [Google Scholar]
- 33.Mummenbrauer, T., F. Janus, B. Müller, L. Wiesmüller, W. Deppert, and F. Grosse. 1996. p53 protein exhibits 3′-to-5′ exonuclease activity. Cell 85:1089-1099. [DOI] [PubMed] [Google Scholar]
- 34.Pierce, A. J., R. D. Johnson, L. H. Thompson, and M. Jasin. 1999. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 13:2633-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plug, A. W., C. A. Clairmont, E. Sapi, T. Ashley, and J. B. Sweasy. 1997. Evidence for a role for DNA polymerase β in mammalian meiosis. Proc. Natl. Acad. Sci. USA 94:1327-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Preuss, U., R. Kreutzfeld, and K.-H. Scheidtmann. 2000. Tumor-derived p53 mutant C174Y is a gain-of-function mutant which activates the fos promoter and enhances colony formation. Int. J. Cancer 88:162-171. [DOI] [PubMed] [Google Scholar]
- 37.Richardson, C., and M. Jasin. 2000. Coupled homologous and non-homologous repair of a double-strand break preserves genomic integrity in mammalian cells. Mol. Cell. Biol. 20:9068-9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roemer, K., and T. Friedmann. 1993. Modulation of cell proliferation and gene expression by a p53-estrogen receptor hybrid protein. Proc. Natl. Acad. Sci. USA 90:9252-9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roemer, K. 1999. Mutant p53: gain-of-function oncoproteins and wild-type p53 inactivators. Biol. Chem. 380:879-887. [DOI] [PubMed] [Google Scholar]
- 40.Rouet, P., F. Smih, and M. Jasin. 1994. Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol. Cell. Biol. 14:8096-8106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubnitz, J., and S. Subramani. 1984. The minimum amount of homology required for homologous recombination in mammalian cells. Mol. Cell. Biol. 4:2253-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saintigny,Y., D. Rouillard, B. Chaput, T. Soussi, and B. S. Lopez. 1999. Mutant p53 proteins stimulate spontaneous and radiation-induced intrachromosomal homologous recombination independently of the alteration of the transactivation activity and of the G1 checkpoint. Oncogene 18:3553-3565. [DOI] [PubMed] [Google Scholar]
- 43.Scherer, S. J., S. M. Maier, M. Seifert, R. G. Hanselmann, K. D. Zang, H. K. Müller-Hermelink, P. Angel, C. Welter, and M. Schartl. 2000. p53 and c-Jun functionally synergize in the regulation of the DNA repair gene hMSH2 in response to UV. J. Biol. Chem. 275:37469-37473. [DOI] [PubMed] [Google Scholar]
- 44.Skalski, V., Z.-Y. Lin, B. Y. Choi, and K. R. Brown. 2000. Substrate specificity of the p53-associated 3′-5′ exonuclease. Oncogene 19:3321-3329. [DOI] [PubMed] [Google Scholar]
- 45.Süsse, S., C. Janz, F. Janus, W. Deppert, and L. Wiesmüller. 2000. Role of heteroduplex joints in the functional interactions between human Rad51 and wild-type p53. Oncogene 19:4500-4512. [DOI] [PubMed] [Google Scholar]
- 46.Tang, W., H. Willers, and S. N. Powell. 1999. p53 directly enhances rejoining of DNA double-strand breaks with cohesive ends in γ-irradiated mouse fibroblasts. Cancer Res. 59:2562-2565. [PubMed] [Google Scholar]
- 47.Wang, F. J. 1998. Analysis of downstream effectors of p53 on cell growth arrest and apoptosis induced by a temperature-sensitive Val138 mutant. J. Med. Dent. Sci. 45:141-149. [PubMed] [Google Scholar]
- 48.Wang, X. W., H. Yeh, L. Schaeffer, R. Roy, V. Moncollin, J.-M. Egly, Z. Wang, E. C. Friedberg, M. K. Evans, B. G. Taffe, V. A. Bohr, G. Weeda, J. H. J. Hoeijmakers, K. Forrester, and C. C. Harris. 1995. p53 modulation of TFIIH-associated nucleotide excision repair activity. Nat. Genet. 10:188-195. [DOI] [PubMed] [Google Scholar]
- 49.Wang, X. W., A. Tseng, N. A. Ellis, E. A. Spillare, S. P. Linke, A. I. Robles, H. Seker, Q. Yang, P. Hu, S. Beresten, N. A. Bemmels, S. Garfield, and C. C. Harris. 2001. Functional interaction of p53 and BLM DNA helicase in apoptosis. J. Biol. Chem. 276:32948-32955. [DOI] [PubMed] [Google Scholar]
- 50.Wiesmüller, L., J. Cammenga, and W. W. Deppert. 1996. In vivo assay of p53 function in homologous recombination between simian virus 40 chromosomes. J. Virol. 70:737-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willers, H., E. E. McCarthy, B. Wu, H. Wunsch, W. Tang, D. G. Taghian, F. Xia, and S. N. Powell. 2000. Dissociation of p53-mediated suppression of homologous recombination from G1/S cell cycle checkpoint control. Oncogene 19:632-639. [DOI] [PubMed] [Google Scholar]
- 52.Willers, H., E. E. McCarthy, P. Hubbe, J. Dahm-Daphi, and S. N. Powell. 2001. Homologous recombination in extrachromosomal plasmid substrates is not suppressed by p53. Carcinogenesis 22:1757-1763. [DOI] [PubMed] [Google Scholar]
- 53.Wilson, T. E., and M. R. Lieber. 1999. Efficient processing of DNA ends during yeast non-homologous end joining. J. Biol. Chem. 274:23599-23609. [DOI] [PubMed] [Google Scholar]
- 54.Yang, T., H. Namba, T. Hara, N. Takmura, Y. Nagayama, S. Fukata, N. Ishikawa, K. Kuma, K. Ito, and S. Yamashita. 1997. p53 induced by ionizing radiation mediates DNA end-joining activity, but not apoptosis of thyroid cells. Oncogene 14:1511-1519. [DOI] [PubMed] [Google Scholar]
- 55.Zhang, W., X. Y. Guo, G. Y. Hu, W. B. Liu, J. W. Shay, and A. B. Deisseroth. 1994. A temperature-sensitive mutant of human p53. EMBO J. 13:2535-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou, J., J. Ahn, S. H. Wilson, and C. Prives. 2001. A role for p53 in base excision repair. EMBO J. 20:914-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zink, D., C. Mayr, C. Janz, and L. Wiesmüller. Association of p53 and MSH2 with recombinative repair complexes during S-phase. Oncogene, in press. [DOI] [PubMed]