Abstract
Utilizing mutants of extracellular signal-regulated kinase 2 (ERK2) that are defective for intrinsic mitogen-activated protein kinase or ERK kinase (MEK) binding, we have identified a convergent signaling pathway that facilitates regulated MEK-ERK association and ERK activation. ERK2-Δ19-25 mutants defective in MEK binding could be phosphorylated in response to mitogens; however, signaling from the Raf-MEK pathway alone was insufficient to stimulate their phosphorylation in COS-1 cells. Phosphorylation of ERK2-Δ19-25 but not of wild-type ERK2 in response to Ras V12 was greatly inhibited by dominant-negative Rac. Activated forms of Rac and Cdc42 could enhance the association of wild-type ERK2 with MEK1 but not with MEK2 in serum-starved adherent cells. This effect was p21-activated kinase (PAK) dependent and required the putative PAK phosphorylation sites T292 and S298 of MEK1. In detached cells placed in suspension, ERK2 was complexed with MEK2 but not with MEK1. However, upon replating of cells onto a fibronectin matrix, there was a substantial induction of MEK1-ERK2 association and ERK activation, both of which could be inhibited by dominant-negative PAK1. These data show that Rac facilitates the assembly of a mitogen-activated protein kinase signaling complex required for ERK activation and that this facilitative signaling pathway is active during adhesion to the extracellular matrix. These findings reveal a novel mechanism by which adhesion and growth factor signals are integrated during ERK activation.
The extracellular signal-regulated protein kinases (ERKs) are ubiquitous protein kinases that function downstream of the ras oncogene and are involved in cell proliferation, movement, and differentiation (35). Ras is activated in response to agonist stimulation and recruits Raf protein kinases to the plasma membrane (28, 34, 45), where Raf undergoes activation by a mechanism that is incompletely understood. Raf proteins phosphorylate and activate mitogen-activated protein kinase or ERK kinases (MEKs), which phosphorylate the two ERK proteins, ERK1 and ERK2, on a TEY sequence in the catalytic domain, resulting in ERK activation (24, 37). MEKs can serve as cytoplasmic anchors for the ERKs through a direct binding interaction, holding the ERKs in the cytoplasm at times when the signaling pathway is inactive (21). Stimulation of the pathway results in the phosphorylation of the ERKs and their dimerization (26) and translocation to the nucleus and other sites of action (10, 23, 29), where they phosphorylate the various substrates required for biological responses. Sustained ERK activation and nuclear translocation are required for S-phase entry and proliferation of fibroblasts (6, 36), and both aspects are dependent upon the integration of adhesion and growth factor signals (2, 40, 47). However, the mechanisms by which adhesion and growth factor signals are integrated to modify ERK function are presently unclear.
MEK proteins bind directly to the ERKs through a region in the N terminus of MEK (3, 4,21). However, other sequences within MEK appear to be important for functional coupling between MEK and ERK. Specifically, deletion of the MEK proline-rich sequence inhibits in vivo coupling between MEK1 and ERK even though catalytic activity of MEK is retained in vitro (9, 14). Furthermore, the regions of the ERKs that participate in association with MEK are unclear, as there appear to be multiple regions of ERK2 that are required for phosphorylation by the MEKs (48). Recent reports have described acidic (41, 46) and hydrophobic (49) residues in the C terminus of ERK2, as well as point mutations in the kinase domain (39), as being necessary for association with MEK. Experiments using chimeras of ERK2 and p38 have identified regions in both the N terminus through kinase subdomain II (50) as well as kinase subdomains III and IV (5) as being required for efficient phosphorylation by MEKs. We have recently identified residues 19 to 25 of ERK2 as being required for MEK2 binding (16). Thus, it appears that MEK may utilize several regions of ERK either to bind and/or recognize ERK as a substrate.
Signaling through the Ras-ERK pathway can be influenced by p21-activated kinase (PAK), an effector of the Rho family GTPases Rac and Cdc42. Expression of constitutively active Rac or Cdc42 activates the Jun N-terminal kinases (JNKs) and p38 but not the ERKs (12, 32). However, Rac and Cdc42 can synergize with Raf to promote activation of the ERKs through mechanisms involving PAK1 phosphorylation of the MEK1 proline-rich sequence (18) and PAK3 phosphorylation of Raf-1 (27). PAK1 can enhance the phosphorylation of T292 and S298 of MEK1 in vivo (11, 18), and mutation of these sites to alanine inhibits the association of MEK1 with Raf-1 (18). PAK3 can phosphorylate Raf-1 on S338, enhancing Raf-1 activation (27).
We have identified N-terminal mutants of ERK2 that are defective in their intrinsic ability to associate with MEK and have used them to study the involvement of other signaling pathways that facilitate MEK-ERK complex formation and ERK activation. In particular, we addressed the role of the direct MEK-ERK interaction in ERK phosphorylation in response to growth factors, cellular adhesion, and mutational activation of components of the Ras-to-ERK pathway. We have found that ERK2-Δ19-25 proteins, which do not detectably associate with MEK1 or MEK2, can be phosphorylated on their activating sites in a MEK-dependent manner in response to mitogens; however, activation of the Raf-1-MEK pathway alone is insufficient to stimulate significant phosphorylation of these mutants, in contrast to wild-type ERK2 (WT-ERK2). We show that Rac signaling is required downstream of active Ras for ERK2-Δ19-25 phosphorylation in the absence of an intrinsic interaction with MEK and furthermore that Rac signaling can increase the association of ERK2 and MEK1 above the basal association seen in serum-starved adherent cultures. These data are consistent with there being two components to the MEK1-ERK2 interaction in adherent cells: an intrinsic interaction requiring the ERK2 N terminus and a Rac-inducible component independent of the ERK2 N terminus. We further demonstrate that Rac signaling regulates the association of MEK1 and ERK2 as well as the activation of ERK2 during cellular adhesion: both processes are stimulated in a PAK-dependent manner during attachment of cells to fibronectin, conditions under which endogenous Rac and PAK have been shown to be activated (15). These findings suggest that Rac-PAK signaling provides a mechanism to specifically regulate MEK1-ERK complexes for signaling and adhesion functions and reveal a novel mechanism for integration of matrix and growth factor signals whereby growth factor signaling predominantly regulates Raf and MEK activation and integrin signaling regulates the binding of MEK to ERK.
MATERIALS AND METHODS
Cell culture and plasmids.
COS-1, 10T1/2, and REF-52 cells were grown in 10% fetal bovine serum in Dulbecco's modified Eagle medium (DMEM) (Life Technologies, Rockville, Md.) at 37°C with 5% CO2. HA-MEK and FLAG-ERK2 constructs and the mutants thereof have been described previously (9, 16). Hemagglutinin (HA)-tagged H-Ras V12 was provided by Channing Der. Raf-1 CAAX and Raf-1 SAAX cDNAs were obtained from Deborah Morrison and subcloned into the pCDNA3 FLAG vector. Rac1 L61, Rac1 N17, PAK1 K299R, Cdc42 L61, and Rho L63 constructs were obtained from Scott Weed and Tom Parsons.
Immunoprecipitations and kinase reactions.
COS-1 cells were transfected using Lipofectamine (Life Technologies), and REF-52 cells were transfected with Superfect (Qiagen). To assess ERK2 phosphorylation, COS-1 cells were transfected with either 2 μg of pCDNA3 vector; 0.25 μg of FLAG-WT-ERK2; 2 μg of ERK2-Δ19-25; 2 μg of ERK2-Δ19-25-7A; or 2 μg of ERK2-Δ19-25-GP to obtain equal protein expression. All transfections were brought up to 2.0 μg of total DNA with pCDNA3 vector. The cells were placed in fresh serum-free media after the transfection and were either harvested or stimulated with 10 ng of epidermal growth factor (EGF) (Upstate Biotechnology, Lake Placid, N.Y.)/ml at 24 h posttransfection. For cotransfections with active or dominant-negative Ras, Raf, or MEK, 0.3 μg of each plasmid was included in the transfection. For titrations with MEK1 Δ32-51 S218/222D, 25, 50, 100, and 300 ng of plasmid were transfected. For kinase reactions with purified components, immunoprecipitated ERKs were incubated for 30 min at 30°C with 100 μM ATP and either 5 μg of recombinant MEK1 S218/222D or MEK1 that had been activated with purified B-Raf (8).
Coimmunoprecipitations with MEK1 and ERK2 in adherent cells have been described previously (8, 16). Coimmunoprecipitation experiments stimulated with Rac1 L61, Cdc42 L63, or Rho L63 included 250 ng of each plasmid along with 0.8 μg of ERK2 and 3.2 μg of MEK plasmid. The experiment with PAK1 K299R included 1 μg of this plasmid. In all coimmunoprecipitation experiments, the cells were placed in 10% serum-containing media after the transfection. The following day the cells were washed twice and serum starved for 4 h before harvesting in hypotonic buffer (50 mM HEPES, pH 7.4, 2 mM MgCl2, 2 mM EGTA, and 5 μg of aprotinin/ml). The cells were lysed by centrifugation for 20 min at 13,000 × g at 4°C. MEK-ERK complexes were immunoprecipitated for 1 h with either anti-FLAG or anti-HA antibodies and were subsequently eluted by overnight incubation at 4°C with 20 μg of the corresponding FLAG or HA peptide. For coimmunoprecipitation experiments on suspended and replated cells, the day following transfection the cells were trypsinized using trypsin-EDTA solution (Life Technologies), washed with 1 mg of soybean trypsin inhibitor (Sigma)/ml, and suspended in serum-free DMEM for 90 min at 37°C and 5% CO2. Cells were either left in suspension or allowed to adhere to bovine fibronectin-coated dishes (10 μg/ml in phosphate-buffered saline, overnight at 4°C; Sigma) for the indicated times at 37°C and 5% CO2 prior to lysis in hypotonic buffer. Immunoprecipitations were performed as described above. Other immunoprecipitations and blots were carried out in FLAG-lysis buffer as described previously (16).
REF-52 cells (1.5 × 106/15-cm-diameter plate) were transfected with 0.5 μg of FLAG-ERK2 construct, 3.5 μg of empty pCDNA3 vector, and 16 μg of either pPRK5 Myc-PAK1 K299R or the corresponding empty vector using Superfect (Qiagen). After 48 h, cultures were harvested by trypsinization, suspended in serum-free media, and allowed to adhere to fibronectin-coated 10-cm-diameter dishes as described above. Extracts were prepared in ice-cold FLAG buffer (16) supplemented with sodium fluoride (50 mM), sodium pyrophosphate (5 mM), sodium orthovanadate (0.1 mM), microcystin (0.1 μg/ml), and phenylmethylsulfonyl fluoride (1 mM). FLAG immunoprecipitates were prepared as described previously, and bound proteins were eluted by boiling in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer.
Inorganic phosphate labeling.
10T1/2 cells were transfected using Superfect with 2.8 μg of HA-MEK1 and either 2.8 μg of Rac1 L61 or empty vector. The cells were serum starved for 5 h in phosphate-free DMEM with 0.2% fetal calf serum and were then labeled with 2 mCi of [32P]orthophosphate/ml for the last 4 h. The cells were then lysed and HA-MEK1 immunoprecipitated. Tryptic phosphopeptide maps were then performed on the immunoprecipitate and compared to previous MEK1 maps (9).
RESULTS
ERK2-Δ19-25 mutants are defective in MEK1 binding.
We previously performed mutational analysis on the N-terminal domain of ERK2 to determine potential roles of this region in regulating ERK activation and function. We determined that ERK2 molecules with mutations in residues 19 to 25 were unable to associate with MEK2 in coimmunoprecipitation experiments (16). In addition, MEK2 could not act as a cytoplasmic anchor for ERK2 molecules with mutations in this region (16). MEK1 differs from MEK2 in its interaction with a putative “scaffold” and regulated phosphorylation (9, 18, 42) that may target ERK signaling within the cell (Slack et al., submitted for publication). It thus was important to test whether MEK1 also was defective for binding to the ERK2 N-terminal mutants. Plasmids encoding WT-ERK2 and ERK2-Δ19-25 mutants (Fig. 1A) were cotransfected into COS-1 cells with HA-tagged MEK1, and binding was determined by coimmunoprecipitation from serum-starved cells. ERK2 mutants with deletions or substitutions in amino acid residues 19 to 25 (ERK2-Δ19-25, ERK2-Δ19-25-7A, and ERK2-Δ19-25-GP) were not present in MEK1 immunoprecipitates (Fig. 1B). Thus, ERK2 mutants that contained a mutation in residues 19 to 25 could bind neither to MEK1 nor to MEK2.
FIG. 1.
ERK2-Δ19-25 mutants do not bind to MEK1. (A) Murine ERK2 was subcloned into the pCDNA3 expression vector in frame with a sequence coding for an N-terminal FLAG tag (16). The sequence of the N terminus from residues 16 to 30 is shown up to the first glycine in the kinase domain using the single-letter code for amino acids. Residues that were deleted are underlined, while substitution mutations to alanine are boxed. (B) COS-1 cells were cotransfected with HA-MEK1 and a FLAG-ERK2 mutant. The following day HA-MEK1 immunoprecipitates (IP) were prepared as described in Materials and Methods and were immunoblotted with anti-HA and anti-FLAG antibodies.
ERK mutants defective in MEK binding can be phosphorylated in response to growth factors but not in response to mutationally activated MEK.
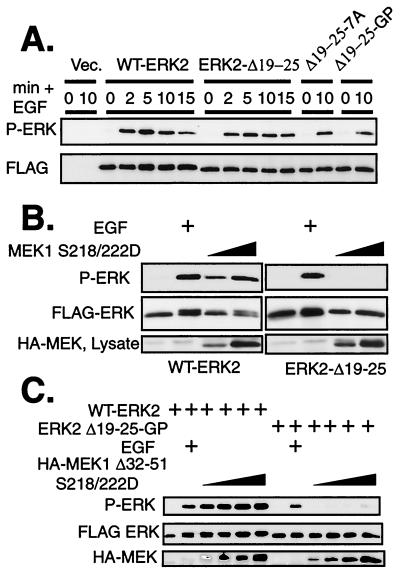
These ERK mutants that are unable to associate with MEK provide a tool for identifying additional signals within the cell that may be involved in regulation of ERK2 phosphorylation, both in response to extracellular signals and in response to mutational activation of the Ras to ERK pathway. ERK2 requires phosphorylation on both a threonine residue and tyrosine residue in the activation loop in order to be fully active as a kinase (24, 37). It was previously determined that 10 min of stimulation of COS-1 cells with EGF or serum induced phosphorylation of ERK2-Δ19-25 mutants and that the phosphorylation was MEK dependent (16). To determine if the kinetics of phosphorylation were altered by the mutations, we performed a time course of ERK phosphorylation in response to 10 ng of EGF/ml. WT-ERK2 was phosphorylated and activated within 2 min of EGF addition (Fig. 2A), as detected by immunoblotting with an antibody that specifically recognizes the dually phosphorylated form of the ERKs (51). Maximal phosphorylation was present at 5 min and continued throughout the 15-min time course. ERK2-Δ19-25 mutants were phosphorylated with kinetics to an extent similar to that for WT-ERK2, even with EGF concentrations as low as 1.0 pg/ml (Fig. 2A and data not shown). These results demonstrate that mutagenesis of the ERK2 N terminus does not change the kinetics or extent of ERK2 phosphorylation in response to EGF treatment.
FIG. 2.
Phosphorylation of ERK2-Δ19-25 mutants is induced by EGF but not by mutationally activated MEKs in vivo. (A) COS-1 cells were transfected with FLAG-ERK constructs and incubated in serum-free media overnight. Cells were stimulated with 10 ng of EGF/ml for the indicated times (min). FLAG-ERKs were immunoprecipitated and immunoblotted with antibodies to FLAG and phospho-ERK. Vec, vector. (B) COS-1 cells were cotransfected with FLAG-ERKs and mutationally active HA-MEK1 S218/222D. The cells were serum starved after the transfection, and FLAG-ERKs were immunoprecipitated 24 h after transfection. Immunoprecipitates were immunoblotted with antibodies to FLAG and phospho-ERK. (C) WT-ERK2 or ERK2-Δ19-25-GP was cotransfected with increasing amounts of MEK1 Δ32-51 S218/222D. Cultures were serum starved after transfection and harvested at 24 h. Stimulated cultures received 10 ng of EGF/ml for 10 min. FLAG-ERKs were immunoprecipitated with anti-FLAG antibodies and then immunoblotted with antibodies to FLAG and phospho-ERK.
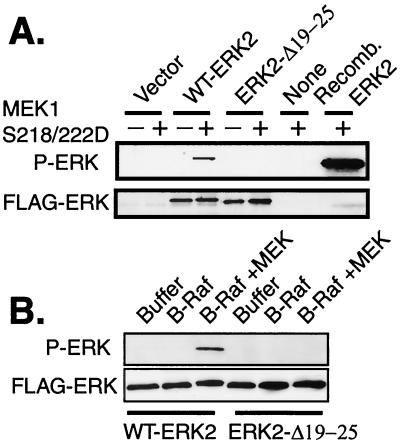
We next determined if MEK activation alone was sufficient for ERK phosphorylation in vivo in the absence of a direct MEK-ERK interaction. To bypass the stimulation of additional signaling pathways upstream of MEK that are activated by growth factors, we cotransfected COS-1 cells with a constitutively active form of MEK1 (MEK1 S218/222D) along with WT-ERK2 or mutant ERK2. Immunoblotting FLAG immunoprecipitates of these cultures revealed that, while WT-ERK2 was phosphorylated in response to cotransfection of MEK1 S218/222D, phosphorylation of ERK2-Δ19-25 could not be detected under these conditions (Fig. 2B and data not shown). Similar results were obtained using mutationally activated MEK2 and any of the ERK2 mutants containing substitutions or deletions at residues 19 to 25 (data not shown). Cotransfection of increasing amounts of MEK1 Δ32-51 S218/222D, a mutationally activated MEK1 that contains a deletion of the nuclear export sequence (20) and has fourfold-higher specific kinase activity than MEK1 S218/222D (31), was also unable to phosphorylate ERK2-Δ19-25-GP to levels comparable to those for WT-ERK2 (Fig. 2C). These data suggest that a quantitative difference in absolute MEK activity was not responsible for the different sensitivities of ERK2 and ERK2-Δ19-25-GP to phosphorylation by mutationally activated MEK and EGF-stimulated MEK. Specifically, the lowest concentration of mutationally activated MEK was sufficient to phosphorylate WT-ERK2 to levels in excess of those seen with EGF treatment, yet transfection with 12-fold-higher concentrations of activated MEK failed to stimulate phosphorylation of ERK2-Δ19-25-GP to levels approaching that seen with EGF treatment. Thus, WT-ERKs and mutant ERKs could be phosphorylated to similar extents by EGF stimulation, but even by conservative estimates ERK2-Δ19-25-GP was at least 20-fold less sensitive to phosphorylation by mutationally activated MEK. These data demonstrate that there was a qualitative difference between the abilities of endogenous MEK stimulated by EGF and of cotransfected mutationally activated MEK to phosphorylate ERK2 mutants that cannot bind directly to MEK. This distinction could result from conformational differences between the two MEK enzymes, or alternatively, other signaling pathways and/or proteins activated by growth factor stimulation upstream of or parallel to MEK might act to enhance the MEK-ERK interaction.
To test the first of these two possibilities, we determined the ability of recombinant WT-MEK1 activated by phosphorylation or MEK1 S218/222D to phosphorylate WT-ERK2 and ERK2 mutants in vitro. Inactive FLAG-ERK2 proteins were immunoprecipitated from transfected, serum-starved cells. These immunoprecipitates were mixed with high concentrations of recombinant MEK1 S218/222D and ATP, and the ability of the ERKs to be phosphorylated was assessed. Recombinant MEK1 S218/222D was able to phosphorylate WT-ERK2 but not an equal amount of ERK2-Δ19-25 (Fig. 3A). Similarly, recombinant WT-MEK1 protein activated in vitro with recombinant B-Raf was able to phosphorylate WT-ERK2 but was unable to phosphorylate ERK2-Δ19-25 (Fig. 3B). Therefore, direct signaling from MEK in vivo or from MEK and Raf in vitro was insufficient to stimulate detectable phosphorylation of an ERK mutant unable to bind directly to MEK. These data suggest that the ability of extracellular mitogens to stimulate phosphorylation of these ERK2 mutants was likely due to a difference in cell signaling upstream of, or parallel to, MEK.
FIG. 3.
ERK2-Δ19-25 mutants are not phosphorylated by active MEK1 in vitro. (A) COS-1 cells were transfected with FLAG-ERK constructs, and FLAG immunoprecipitates from serum-starved cells were each mixed with 5 μg of recombinant MEK1 S218/222D and ATP for 30 min. Purified, recombinant ERK2 protein was included as a positive control for MEK phosphorylation of ERK. Reactions were immunoblotted as indicated. (B) The reactions were identical to those for panel A, except that recombinant WT-MEK1 activated by recombinant B-Raf in vitro was used to phosphorylate the immunoprecipitated ERKs.
Ras is necessary and sufficient for phosphorylation of ERK2-Δ19-25.
Stimulation of the EGF receptor causes activation of Ras, which in turn causes activation of a kinase cascade leading to ERK activation. We therefore asked whether the second signal required for ERK2-Δ19-25 phosphorylation came from the EGF receptor itself and was independent of Ras or whether it was dependent on Ras signaling. Blocking Ras signaling with Ras N17 in the presence of EGF and mutationally activated MEK allowed us to determine if an additional signaling pathway stimulated by the EGF receptor could allow ERK2-Δ19-25 phosphorylation by mutationally activated MEK. Failure of ERK2-Δ19-25 to be phosphorylated would indicate that the second signal for phosphorylation depended on Ras activity. WT-ERK2 or ERK2-Δ19-25 along with Ras N17 and MEK1 S218/222D was transfected into cells, and the cultures were serum starved and then stimulated with EGF. As expected, WT-ERK2 could be phosphorylated by MEK1 S218/222D even when Ras N17 was present and when EGF was absent (Fig. 4A), indicating that activation of MEK1 alone is sufficient for phosphorylation of WT-ERK2. However, ERK2-Δ19-25, which was phosphorylated in response to EGF, was not phosphorylated in the presence of Ras N17, even when activated MEK1 and EGF were present (Fig. 4A). This suggests that the second signal required for ERK2-Δ19-25 phosphorylation was Ras dependent.
FIG. 4.
Ras activity is required for ERK2-Δ19-25 phosphorylation in response to EGF even in the presence of active MEK. (A) COS-1 cells were transfected with WT-ERK2 or ERK2-Δ19-25 and Ras N17, MEK1 S218/222D, or empty vector (Vec). The following day cells were serum starved for 5 h before stimulation with EGF for 10 min. FLAG-ERK immunoprecipitates were immunoblotted with anti-FLAG and anti-phospho-ERK antibodies. SF, serum free (B) Ras V12 requires Rac activity to stimulate ERK2-Δ19-25 phosphorylation. COS-1 cells were cotransfected with the indicated constructs and serum starved after the transfection. Cells were harvested at 24 h after transfection, and anti-FLAG and anti-phospho-ERK immunoblots were performed on anti-FLAG immunoprecipitates.
We next examined whether Ras activation, independent of growth factor treatment, is sufficient to stimulate phosphorylation of ERK2-Δ19-25 in vivo. Mutationally activated Ras V12 was cotransfected with either WT-ERK2 or ERK2-Δ19-25, and phosphorylation of the ERKs under serum-free conditions was determined by Western blotting. Unlike mutationally activated MEK, Ras V12 stimulated phosphorylation of both WT-ERK2 and ERK2-Δ19-25 (Fig. 4B), demonstrating that signaling pathways downstream of Ras were sufficient to induce ERK2-Δ19-25 phosphorylation. Together, these data indicate that Ras was necessary and sufficient to stimulate phosphorylation of an ERK2 mutant that does not intrinsically bind to MEK.
Rac-PAK signaling is required for activation of ERK mutants that are defective in MEK binding.
Ras is known to activate a number of downstream signaling pathways, including phosphatidylinositol 3-kinase, Ral GDS, Rap, and Rho family proteins (7). Previous reports have demonstrated that the Rho family proteins Rac and Cdc42, but not Rho, can synergize with Raf to activate the ERKs (18, 19). We examined the requirement for Rac activity downstream of Ras V12 for ERK2-Δ19-25 phosphorylation. While Rac1 N17 only slightly inhibited the phosphorylation of WT-ERK2 in response to Ras V12, there was a marked inhibition of phosphorylation of ERK2-Δ19-25-GP (Fig. 4B). These data indicate that Rac signaling is absolutely required for efficient phosphorylation of ERK2-Δ19-25-GP downstream of activated Ras. In fact, the amount of phosphorylation of ERK2-Δ19-25-GP in the presence of Ras V12 and Rac1 N17 was comparable to the small amount of ERK2-Δ19-25-GP phosphorylation stimulated by Raf-1 CAAX, a constitutively active membrane-targeted Raf-1 protein (45). Raf-1 SAAX, a mutant that is not targeted to the membrane, stimulated little phosphorylation of ERK2 or ERK2-Δ19-25-GP. These data suggest that signaling from active Ras to ERK2-Δ19-25-GP consists of a Raf component and a Rac component.
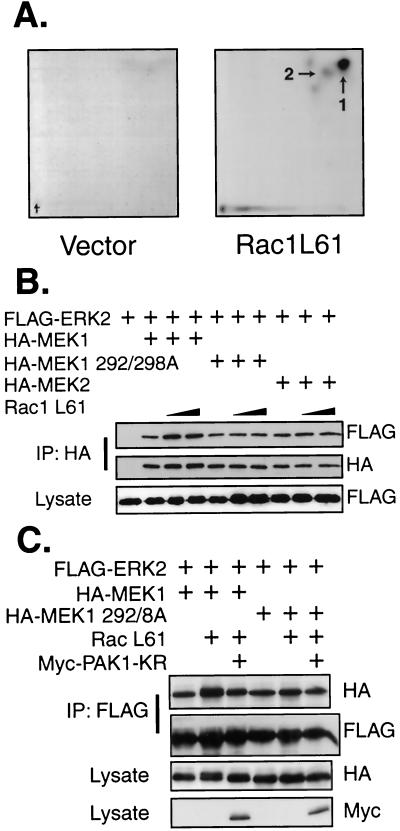
We hypothesized that Rac acts to promote functional interactions between MEK and ERK, thereby facilitating phosphorylation of ERK2-Δ19-25 mutants in response to active Raf. One possibility is that Rac signaling can promote the association of MEK and ERK2. Therefore, we performed coimmunoprecipitations of MEK1 and WT-ERK2 from serum-starved cells in the presence or absence of activated Rac (Fig. 5A). Importantly, Rac1 L61 increased the amount of MEK1 that could be coimmunoprecipitated with ERK2 from serum-starved cells, demonstrating that Rac signaling normally acts to increase the association between MEK1 and ERK2. Performing the reverse immunoprecipitation also showed this increase in MEK1-ERK2 binding in the presence of Rac1 L61 (Fig. 5B). To test the specificity of this enhancement, the experiment was repeated with activated Rac, Cdc42, or Rho (Fig. 5C). The results demonstrate that both activated Rac and Cdc42, but not activated Rho, were able to stimulate the association of MEK1 and ERK2 in serum-starved cells. Similar but slightly less consistent results were seen with ERK1 in this assay (data not shown). We have been unable to coimmunoprecipitate ERK2-Δ19-25 with MEK in the presence of Rac1 L61 (data not shown), most likely due to the fact that the interaction between MEK and even WT-ERK2 is very labile and thus difficult to assay upon removal from the cell. The inability of ERK2-Δ19-25 to intrinsically bind MEK may make this interaction more difficult to observe. Nevertheless, the results clearly demonstrate that both Rac and Cdc42 signaling can stimulate the association of MEK1 and WT-ERK2.
FIG. 5.
Rac signaling increases the association between ERK2 and MEK1. (A and B) COS-1 cells were transfected with FLAG-ERK2, HA-MEK1, and Rac1 L61 as indicated. The cells were serum starved the following day for 4 h before harvest in hypotonic buffer and lysedby centrifugation at 13,000 × g for 20 min. Anti-FLAG (A) or anti-HA (B) immunoprecipitations (IP) were performed from hypotonic extracts. Immunoprecipitated proteins were eluted with FLAG (A) or HA (B) peptides, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunoblotted with anti-FLAG and anti-HA antibodies. (C) Cells were transfected and processed as for panel A, with the substitution of either Cdc42 L61 or Rho L63 for Rac1 L61 as indicated. (D) COS-1 cells were transfected as shown in panel A with plasmids for FLAG-ERK2 and either HA-MEK1, HA-MEK1 S218A, which cannot be activated by Raf, or the activated mutant MEK1 S218/222D. The cells were serum starved and immunoprecipitations were performed as for panel A.
Rac-inducible MEK1-ERK association is regulated independently of MEK1 activity.
Rac and Raf signaling can synergize to promote activation of MEK and ERK. Under the conditions of our assay, MEK1 and ERK are in the cytoplasm in an inactive complex. To rule out a role for Raf or MEK activity in the increase in the MEK1-ERK2 interaction, we tested the ability of a nonactivatable MEK1 mutant and an activated MEK1 mutant to associate with ERK2 in the presence or absence of active Rac. COS-1 cells were transfected with ERK2 and either MEK1 or MEK1 S218A, which cannot be activated by Raf (13, 31), or the activated mutant MEK1 S218/222D. Similar amounts of all three MEK1 proteins were immunoprecipitated with ERK2 from serum-starved cells (Fig. 5D). Cotransfection of activated Rac enhanced the ability of ERK2 to immunoprecipitate each of the MEK1 proteins. These data demonstrate that the enhancement of the MEK1-ERK interaction by Rac was independent of Raf phosphorylation of MEK1 and MEK1 activity.
MEK1 phosphorylation regulates inducible but not basal MEK1-ERK binding.
It has been reported that Rac and Cdc42 act through their downstream effector PAK1 to induce phosphorylation of MEK1, but not MEK2, on S298 and perhaps on T292 (11, 18). We performed metabolic labeling with inorganic phosphate to determine if activated Rac would stimulate the phosphorylation of these sites on MEK1 in cells. HA-MEK1 isolated from transfected, serum-starved 10T1/2 cells was phosphorylated at low levels on both T292 and S298 (Fig. 6A), as determined by phosphotryptic mapping. Cotransfection of Rac1 L61 greatly increased the phosphorylation of transfected HA-MEK1 on T292 and S298 in serum-starved cells, demonstrating that phosphorylation of these sites was induced by Rac. We therefore investigated whether these phosphorylation sites are required for either the constitutive or Rac-inducible components of the MEK1-ERK2 association. We observed that the putative PAK1 phosphorylation sites on MEK1 are indeed required for the Rac-induced association of MEK1 with ERK2 but not for basal association in serum-starved cells (Fig. 6B and see below). Mutation of these residues in MEK1 to aspartic acid did not mimic the effect of phosphorylation, as this mutant did not show enhanced binding to WT-ERK2 (data not shown). Since MEK2 lacks T292 and is believed not to be a substrate for PAK1 in spite of conservation of S298 (18), our data predict that the MEK2-ERK2 interaction would lack a Rac-inducible component. Our data are consistent with this hypothesis: MEK2 forms a stable association with ERK2, but further association is not stimulated by cotransfection with activated Rac (Fig. 6B). Together these data indicate that the MEK-ERK interaction in adherent cells consists of a constitutive component and a Rac-regulated component and that the Rac-regulated component is MEK1 specific by virtue of PAK phosphorylation sites within this enzyme.
FIG. 6.
The increase in the MEK-ERK interaction is MEK1 specific and requires T292 and S298 of MEK1. (A) 10T1/2 cells were transfected with HA-MEK1 and either Rac1 L61 (right panel) or empty vector (left panel). The cells were serum starved for 5 h in phosphate-free DMEM with 0.2% fetal calf serum and were labeled with 2 mCi of [32P]orthophosphate/ml for the last 4 h. The cells were then lysed and HA-MEK1 immunoprecipitated (IP). Tryptic phosphopeptide maps were then performed on the immunoprecipitate and compared to previous MEK1 maps (9). Peptide 1, phospho-S298 peptide; peptide 2, doubly phosphorylated T292 and S298 peptide. (B) COS-1 cells were transfected with WT-ERK2, Rac1 L61, and either MEK1, MEK1 T292A/S298A, or MEK2 as indicated. The following day the cells were harvested in hypotonic buffer and lysed by centrifugation at 13,000 × g for 20 min. Anti-FLAG immunoprecipitates were prepared and eluted with FLAG peptides. Eluted proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with anti-HA and anti-FLAG antibodies. (C) Cells were transfected with plasmids encoding WT-ERK2, MEK1, Rac1 L61, and PAK1 K299R as indicated. The following day the cells were serum starved 4 h before harvest and anti-FLAG immunoprecipitates were immunoblotted as for panel A.
To confirm a role for PAK in the Rac-regulated, MEK-isozyme specific association with ERK2, we asked if a kinase-defective PAK1 could interfere with the complex-promoting activity of activated Rac. Kinase-defective PAK1 inhibited the enhanced MEK1-ERK2 interaction by activated Rac down to, but not below, the basal level of binding, while having no effect on the binding of ERK2 to the MEK1 T292A/S298A mutant (Fig. 6C). Thus, the specificity and Rac inducibility of the MEK1-ERK interaction can be explained in terms of MEK1 phosphorylation. Activated PAK1 phosphorylates MEK1 on S298 and possibly T292, enhancing the association between MEK1 and ERK, while the association between MEK2 and ERK2 is unaffected by active Rac since MEK2 lacks T292 and apparently is not a substrate for PAK1 (18). These experiments also distinguish the mechanism underlying basal MEK-ERK2 association from the Rac-inducible component. Basal association is not MEK-isozyme specific and does not require the Rac-PAK1-stimulated phosphorylation sites.
Rac-PAK signaling stimulates MEK1-ERK association upon cellular adhesion to fibronectin.
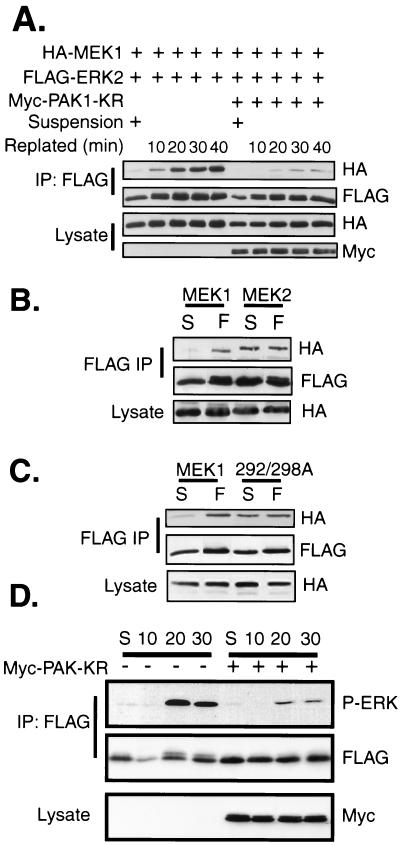
We next investigated the involvement of the Rac-PAK signaling pathway in physiological regulation of the MEK1-ERK2 interaction. The Rac-PAK and ERK signaling pathways are activated upon cellular adhesion to a fibronectin substratum (15, 38). We performed coimmunoprecipitations to determine the level of MEK1-ERK2 association in suspended cells compared to that in cells adhering to a fibronectin substratum. COS-1 cells transfected with MEK1 and ERK2 were placed in suspension for 90 min and were then allowed to adhere to dishes coated with a fibronectin matrix. There was little MEK1 in FLAG-ERK2 immunoprecipitates from suspended COS-1 cells (Fig. 7A). However, a substantial induction in a stable MEK1-ERK2 association was observed upon replating of suspended cells onto fibronectin-coated dishes for 10 min, and this increased over the 40-min time course of cellular adhesion. The increase of MEK1-ERK2 association upon adhesion was dependent on PAK1 activity, as cotransfection with kinase-defective PAK1 greatly inhibited the amount of MEK1 in FLAG-ERK2 immunoprecipitations.
FIG. 7.
(A) Adhesion to fibronectin stimulates the stable association of MEK1 and ERK2 and is PAK dependent. COS-1 cells were transfected with HA-MEK1 and FLAG-ERK2 plasmids. The following day the cells were trypsinized and suspended in serum-free media for 90 min at 37°C and 5% CO2. Cells were either left suspended (S) or allowed to adhere to a fibronectin-coated dish for the indicated time. The cells were harvested in hypotonic buffer and lysed by centrifugation at 13,000 × g for 20 min. Anti-FLAG immunoprecipitates (IP) were eluted off the antibody overnight at 4°C with FLAG peptide, run on a gel, and immunoblotted with anti-HA and anti-FLAG antibodies. An equal amount of cellular lysate from each sample was immunoblotted for HA-MEK and Myc-PAK. (B) COS-1 cells were transfected with plasmids for FLAG-ERK2 and either HA-MEK1 or HA-MEK2. The cells were placed in suspension (S) as for panel A or suspended and allowed to attach to fibronectin (F)-coated dishes for 20 min. ERK-MEK complexes were immunoprecipitated and immunoblottedas for panel A. (C) Same as for panel B, except the mutant MEK1 T292/S298A was transfected instead of MEK2. (D) PAK activity is required for fibronectin-stimulated ERK activation. REF cells were transfected with FLAG-ERK2 with or without Myc PAK1-K299R. After 48 h cells were suspended as described for panel A and were either left untreated in suspension (S) or allowed to adhere to fibronectin-coated dishes for the indicated times (given in minutes). Extracts were normalized with respect to protein, and anti-FLAG immunoprecipitates were prepared. Resolved immunoprecipitates were blotted sequentially with anti-FLAG and anti-phospho-ERK antisera. Equal amounts of lysate protein were blotted for the dominant-negative PAK1 protein with anti-Myc antisera.
Since the association between ERK2 and MEK2 was not regulated by Rac-PAK signaling, we determined whether there was an adhesion-dependent component of this interaction (Fig. 7B). Unlike MEK1, MEK2 could be coimmunoprecipitated with ERK2 from suspended COS-1 cells. Upon cellular adhesion, MEK2-ERK2 complexes remained at levels seen in suspended cells, showing no induction in complex formation. Interestingly, mutation of T292 and S298 of MEK1 to alanine enabled this mutant to behave like MEK2, associating with ERK2 in an adhesion-independent manner that could not be further induced upon cellular adhesion to fibronectin (Fig. 7C).
Finally, we assessed the requirement for PAK activity in the activation of ERK that occurs upon cellular adhesion. WT-ERK2 was transfected into REF-52 cells in the absence or presence of kinase-dead PAK1. The cells were later trypsinized and suspended in serum-free media for 90 min. The cells were then replated onto fibronectin-coated dishes, and the phosphorylation status of FLAG-ERK2 was analyzed by immunoprecipitation and immunoblotting (Fig. 7D). ERK2 was phosphorylated within 20 min after replating of the cells. Cotransfection of kinase-defective PAK substantially inhibited the activation of ERK2 upon cellular adhesion (Fig. 7D), demonstrating a requirement for PAK in the activation of ERK2 in newly adhering cells. Similar results were seen with ERK2-Δ19-25 mutants (data not shown). Together, these data demonstrate that physiological activation of PAK is required for both the MEK1-ERK2 association and ERK2 activation in response to cellular adhesion.
DISCUSSION
Regulation of MEK1-ERK complex formation.
Utilizing ERK2 mutants defective in their intrinsic ability to associate with MEK, we have been able to identify and analyze a second, regulated pathway that facilitates the ERK-MEK interaction. We demonstrate that ERK2-Δ19-25 mutants unable to bind to MEK in vivo were refractory to phosphorylation when cotransfected with plasmids encoding mutationally activated Raf or MEK. However, the mutant ERK became phosphorylated to the same level as that found in the WT when the cells were stimulated with extracellular agonists, such as serum, EGF, or cellular adhesion onto fibronectin (data not shown). These data imply that the extracellular agonists trigger an additional signal that was able to overcome the lack of intrinsic MEK binding in the ERK2-Δ19-25 mutant.
Our data are in contrast to those of Robinson et al. (39), who reported that point mutants of ERK2 that cannot associate with MEK show reduced activation by EGF in cells but can be phosphorylated by cotransfection with activated MEK1. In addition Tanoue et al. (46) reported that ERK2 C-terminal mutants deficient in MEK1 binding were activated in response to serum to only 60% of the level seen with WT-ERK2. Similarly, MEK1 mutants deficient for ERK binding were less efficient than WT-MEK1 at stimulating ERK activation in response to serum (46). Presumably the mutants used by Tanoue et al. (46) and Robinson et al. (39) can be only partially suppressed by the signals initiated by extracellular agonists.
We have considered the possibility that quantitative differences in enzyme activity between MEK activated in response to extracellular agonists versus mutationally activated MEK could be responsible for our results. For example, ERK activation has been demonstrated to behave like a “switch” mechanism, with only a 2.5-fold increase in MEK activation being sufficient for 100% of ERK activation (17). However, this mechanism does not explain the discrepancy in phosphorylation of ERK2-Δ19-25 mutants in response to EGF and mutationally activated MEKs, as the latter were able to phosphorylate WT-ERK2 to levels even higher than those induced by EGF stimulation. Our results suggest that the direct MEK-ERK interaction that requires the ERK2 N terminus is necessary for phosphorylation of ERK in response to simple activation of Raf and MEK but that additional mechanisms facilitate functional association in response to growth factors and active Ras.
Rac-PAK signaling regulates MEK1-ERK interactions.
Our data and previous reports (11, 18, 19, 27) suggest that Rac signaling plays a significant role in activation of ERK2 downstream of active Ras and is essential for phosphorylation of ERK2 mutants unable to directly bind to MEK. Rac modification of ERK activation has been proposed to occur through phosphorylation of MEK1 by PAK1 (11, 18) and by phosphorylation of Raf-1 by PAK3 (27). These phosphorylations were shown to increase the activation of MEK1 and Raf-1, respectively. In addition, Frost et al. (18) observed that mutation of MEK 1 at T292 and S298, phosphorylation sites that we and others (11, 18) have shown are induced by Rac-PAK signaling, inhibited basal association of MEK1 and Raf-1 and concluded that Rac-PAK signaling is required for full MEK1-Raf-1 association. However, they could not demonstrate an induction of MEK1-Raf-1 association in response to cotransfection with activated Rac2 (18).
In this report we demonstrate an alternative mechanism by which Rac signaling influences the functional coupling of MEK1 and ERK2. Rac-PAK signaling does not appear to be required for basal association of MEK1 and ERK2 in adherent cells, which occurs through an N-terminal site on MEK (3, 21), but rather regulates a distinct, inducible component of the MEK1-ERK interaction. We show that Rac-PAK1 signaling enhances the physical association between MEK1 and ERK2 in serum-starved cells and that this inducible association requires potential PAK phosphorylation sites (T292 and S298) on MEK1. These observations also account for the lack of inducible binding of ERK2 with MEK2, since MEK2 is not a good substrate for PAK (18) and lacks T292. Our data suggest that phosphorylation of these sites may either increase the intrinsic ability of MEK1 to bind to ERK or allow these proteins to interact with a scaffolding protein.
Integration of adhesion and growth factor signaling.
Stimulating cells in suspension with serum or growth factors leads to activation of Ras and Raf; however, activation of ERK requires cell adhesion to a substratum (1, 30, 33, 38, 44). Similarly, activation of PAK by Rac also requires cell adhesion (15). We observe little association of MEK1 with ERK2 in suspended cells, suggesting that both intrinsic and Rac-inducible components of the MEK-ERK association are adhesion dependent. Allowing suspended cells to attach to a fibronectin matrix robustly stimulated a stable association of MEK1 with ERK2 in a PAK1-dependent manner. Furthermore, PAK activity was required for ERK activation upon cellular adhesion. These data are consistent with the possibility that PAK signaling might in part underlie the anchorage dependence of ERK activation in response to growth factor stimulation by promoting functional coupling between MEK1 and ERK. This hypothesis is supported by our previous data showing that a subpopulation of MEK1 is constitutively phosphorylated on T292 and S298 in continuously adherent cells (9) and that T292 of MEK1 is necessary for ternary complex formation between Ras GTP, Raf-1, and MEK1 (25). Our observation that T292 and S298 of MEK1 are involved in regulation of the MEK1-ERK interaction suggests that PAK-mediated phosphorylation of MEK1 may enhance the formation of a MAP kinase signaling complex containing Ras-GTP, Raf-1, MEK1, and ERK. Note that in newly adherent cells, the majority of MEK1-ERK association can be inhibited with dominant-negative PAK. It is unclear when the Rac-PAK-independent component of the MEK1-ERK interaction is restored following adhesion. However, these data suggest that Rac-inducible MEK1-ERK association regulates ERK activation in newly adherent cells.
Our data also demonstrate functionally significant distinctions between MEK1 and MEK2. These isozymes are very closely related by sequence (80% identity at the amino acid level) and show considerable regulatory redundancy. However, in contrast to MEK1, the MEK2-ERK interaction was not stimulated by active Rac in adherent cells. Since MEK2 lacks T292 and is a poor substrate for PAK (18), these data are consistent with PAK phosphorylation regulating isozyme-specific, Rac-inducible MEK1-ERK association. Indeed, mutation of T292 and S298 to alanine eliminates Rac-inducible MEK1-ERK association in continuously adherent cells but does not inhibit the basal MEK1-ERK interaction.
A second difference between MEK1 and MEK2 is apparent in suspended and newly adherent cells. MEK1-ERK association was essentially undetectable in suspended cells but was rapidly and robustly inducible upon fibronectin stimulation. In contrast, both MEK2 and MEK1 T292A/S298A were able to associate with ERK in suspended cells, and these interactions were not further induced upon fibronectin stimulation. Thus, although phosphorylation of T292 and S298 correlated with inducible MEK1-ERK binding in continuously adherent cells, these sites play a more complex regulatory role in newly adherent cells. Their phosphorylation may control either the localization of MEK1 in suspended cells or MEK1's ability to dissociate from ERK when cells become detached from a substratum. One attractive possibility is that the two phosphorylation sites have distinct stimulatory and inhibitory roles with respect to ERK binding.
It has been reported that homozygous deletion of MEK1 is embryonically lethal for mice and that fibroblasts from these embryos are defective for migration on fibronectin (22). These observations demonstrate that MEK1 is essential for a subset of adhesion signaling events, and that, importantly, in spite of its continued expression in the MEK1-null fibroblasts, MEK2 does not substitute in these functions. Our data and those of Frost et al. (18) suggest that adhesion-stimulated Rac-PAK signaling likely influences the formation of MEK1-Raf and MEK1-ERK signaling complexes. MEK2 is not phosphorylated by PAK in a similar manner and hence cannot replace MEK1 in Rac-regulated ERK complex formation. Furthermore, since PAK activation is localized to focal contacts and lamellipodia (15, 43) in newly adherent cells, our data suggest that Rac signaling might stimulate the localized assembly of MEK1-Raf and MEK1-ERK complexes, leading to spatially restricted activation of ERK in structures important for cell motility. Thus, the migration defects of MEK1-null fibroblasts and embryonic lethality of MEK1-null mice might result from a loss of Rac and Ras signal integration at MEK1.
In summary, we have been able to identify a new mechanism that regulates MEK1-ERK interactions and is dependent on Rac and PAK. This provides a novel mechanism whereby adhesion-activated integrin signals can be integrated with growth factor signals to localize ERK activation or to confer anchorage dependence upon growth factor signaling. Importantly, subversion of this signal integration may contribute to anchorage-independent growth during malignancy.
Acknowledgments
We thank the members of the Weber lab and PWP for helpful discussions. We also thank Scott Weed and Tom Parsons for Rac plasmids, Channing Der for Ras V12, Debbie Morrison for Raf-1 CAAX and Raf-1 SAAX, Alexis Rahal for technical assistance and Cliff Martin for assistance in preparing the manuscript.
This work was supported by Public Health Service grants GM47332, CA40042, and CA39076 from the National Institutes of Health. S. T. Eblen was supported by National Research Service Award 5F32 GM18672-02. J. K. Slack was supported by NIH grants CA76465 and CA40042 to J. T. Parsons.
REFERENCES
- 1.Aplin, A. E., and R. L. Juliano. 1999. Integrin and cytoskeletal regulation of growth factor signaling to the MAP kinase pathway. J. Cell Sci. 112:695-706. [DOI] [PubMed] [Google Scholar]
- 2.Aplin, A. E., S. A. Stewart, R. K. Assoian, and R. L. Juliano. 2001. Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J. Cell Biol. 153:273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardwell, A. J., L. J. Flatauer, K. Matsukuma, J. Thorner, and L. Bardwell. 2001. A conserved docking site in MEKS mediates high-affinity binding to MAP kinases and cooperates with a scaffold protein to enhance signal transmission. J. Biol. Chem. 276:10374-10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardwell, L., J. G. Cook, E. C. Chang, B. R. Cairns, and J. Thorner. 1996. Signaling in the yeast pheromone response pathway: specific and high-affinity interaction of the mitogen-activated protein (MAP) kinases Kss1 and Fus3 with the upstream MAP kinase kinase Ste7. Mol. Cell. Biol. 16:3637-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunet, A., and J. Pouyssegur. 1996. Identification of MAP kinase domains by redirecting stress signals into growth factor responses. Science 272:1652-1655. [DOI] [PubMed] [Google Scholar]
- 6.Brunet, A., D. Roux, P. Lenormand, S. Dowd, S. Keyse, and J. Pouyssegur. 1999. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 18:664-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell, S. L., R. Khosravi-Far, K. L. Rossman, G. J. Clark, and C. J. Der. 1998. Increasing complexity of Ras signaling. Oncogene 17:1395-1413. [DOI] [PubMed] [Google Scholar]
- 8.Catling, A. D., S. T. Eblen, H. J. Schaeffer, and M. J. Weber. 2001. Scaffold protein regulation of mitogen-activated protein kinase cascade. Methods Enzymol. 332:368-387. [DOI] [PubMed] [Google Scholar]
- 9.Catling, A. D., H.-J. Schaeffer, C. W. M. Reuter, G. R. Reddy, and M. J. Weber. 1995. A proline-rich sequence unique to MEK1 and MEK2 is required for Raf binding and regulates MEK function. Mol. Cell. Biol. 15:5214-5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, R.-H., C. Sarnecki, and J. Blenis. 1992. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol. Cell. Biol. 12:915-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coles, L. C., and P. E. Shaw. 2002. PAK1 primes MEK1 for phosphorylation by Raf-1 kinase during cross-cascade activation of the ERK pathway. Oncogene 21:2236-2244. [DOI] [PubMed] [Google Scholar]
- 12.Coso, O. A., M. Chiariello, J. C. Yu, H. Teramoto, P. Crespo, N. Xu, T. Miki, and J. S. Gutkind. 1995. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell 81:1137-1146. [DOI] [PubMed] [Google Scholar]
- 13.Cowley, S., H. Paterson, P. Kemp, and C. J. Marshall. 1994. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell 77:841-852. [DOI] [PubMed] [Google Scholar]
- 14.Dang, A., J. A. Frost, and M. H. Cobb. 1998. The MEK1 proline-rich insert is required for efficient activation of the mitogen-activated protein kinases ERK1 and ERK2 in mammalian cells. J. Biol. Chem. 273:19909-19913. [DOI] [PubMed] [Google Scholar]
- 15.del Pozo, M. A., L. S. Price, N. B. Alderson, X. D. Ren, and M. A. Schwartz. 2000. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 19:2008-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eblen, S. T., A. D. Catling, M. C. Assanah, and M. J. Weber. 2001. Biochemical and biological functions of the N-terminal, noncatalytic domain of extracellular signal-regulated kinase 2. Mol. Cell. Biol. 21:249-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrell, J. E., Jr. 1996. Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends Biochem. Sci. 21:460-466. [DOI] [PubMed] [Google Scholar]
- 18.Frost, J. A., H. Steen, P. Shapiro, T. Lewis, N. Ahn, P. E. Shaw, and M. H. Cobb. 1997. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 16:6426-6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frost, J. A., S. Xu, M. R. Hutchison, S. Marcus, and M. H. Cobb. 1996. Actions of Rho family small G proteins and p21-activated protein kinases on mitogen-activated protein kinase family members. Mol. Cell. Biol. 16:3707-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuda, M., I. Gotoh, Y. Gotoh, and E. Nishida. 1996. Cytoplasmic localization of mitogen-activated protein kinase kinase directed by its NH2-terminal, leucine-rich short amino acid sequence, which acts as a nuclear export signal. J. Biol. Chem. 271:20024-20028. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda, M., Y. Gotoh, and E. Nishida. 1997. Interaction of MAP kinase with MAP kinase kinase: its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO J. 16:1901-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giroux, S., M. Tremblay, D. Bernard, J. F. Cardin-Girard, S. Aubry, L. Larouche, S. Rousseau, J. Huot, J. Landry, L. Jeannotte, and J. Charron. 1999. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr. Biol. 9:369-372. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez, F. A., A. Seth, D. L. Raden, D. S. Bowman, F. S. Fay, and R. J. Davis. 1993. Serum-induced translocation of mitogen-activated protein kinase to the cell surface ruffling membrane and the nucleus. J. Cell Biol. 122:1089-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Her, J. H., S. Lakhani, K. Zu, J. Vila, P. Dent, T. W. Sturgill, and M. J. Weber. 1993. Dual phosphorylation and autophosphorylation in mitogen-activated protein (MAP) kinase activation. Biochem. J. 296:25-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jelinek, T., A. D. Catling, C. W. Reuter, S. A. Moodie, A. Wolfman, and M. J. Weber. 1994. RAS and RAF-1 form a signalling complex with MEK-1 but not MEK-2. Mol. Cell. Biol. 14:8212-8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khokhlatchev, A. V., B. Canagarajah, J. Wilsbacher, M. Robinson, M. Atkinson, E. Goldsmith, and M. H. Cobb. 1998. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell 93:605-615. [DOI] [PubMed] [Google Scholar]
- 27.King, A. J., H. Sun, B. Diaz, D. Barnard, W. Miao, S. Bagrodia, and M. S. Marshall. 1998. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature 396:180-183. [DOI] [PubMed] [Google Scholar]
- 28.Leevers, S. J., H. F. Paterson, and C. J. Marshall. 1994. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature 369:411-414. [DOI] [PubMed] [Google Scholar]
- 29.Lenormand, P., C. Sardet, G. Pages, G. L'Allemain, A. Brunet, and J. Pouyssegur. 1993. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J. Cell Biol. 122:1079-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin, T. H., Q. Chen, A. Howe, and R. L. Juliano. 1997. Cell anchorage permits efficient signal transduction between ras and its downstream kinases. J. Biol. Chem. 272:8849-8852. [PubMed] [Google Scholar]
- 31.Mansour, S. J., W. T. Matten, A. S. Hermann, J. M. Candia, S. Rong, K. Fukasawa, G. F. Vande Woude, and N. G. Ahn. 1994. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science 265:966-970. [DOI] [PubMed] [Google Scholar]
- 32.Minden, A., A. Lin, F. X. Claret, A. Abo, and M. Karin. 1995. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell 81:1147-1157. [DOI] [PubMed] [Google Scholar]
- 33.Miyamoto, S., H. Teramoto, J. S. Gutkind, and K. M. Yamada. 1996. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J. Cell Biol. 135:1633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moodie, S. A., B. M. Willumsen, M. J. Weber, and A. Wolfman. 1993. Complexes of Ras GTP with Raf-1 and mitogen-activated protein kinase kinase. Science 260:1658-1661. [DOI] [PubMed] [Google Scholar]
- 35.Nishida, E., and Y. Gotoh. 1993. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem. Sci. 18:128-131. [DOI] [PubMed] [Google Scholar]
- 36.Pages, G., P. Lenormand, G. L'Allemain, J. C. Chambard, S. Meloche, and J. Pouyssegur. 1993. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc. Natl. Acad. Sci. U. S. A 90:8319-8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Payne, D. M., A. J. Rossomando, P. Martino, A. K. Erickson, J. H. Her, J. Shabanowitz, D. F. Hunt, M. J. Weber, and T. W. Sturgill. 1991. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 10:885-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renshaw, M. W., X. D. Ren, and M. A. Schwartz. 1997. Growth factor activation of MAP kinase requires cell adhesion. EMBO J. 16:5592-5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson, F. L., A. W. Whitehurst, M. Raman, and M. H. Cobb. 2002. Identification of novel point mutations in ERK2 that selectively disrupt binding to MEK1. J. Biol. Chem. 277:14844-14852. [DOI] [PubMed] [Google Scholar]
- 40.Roovers, K., G. Davey, X. Zhu, M. E. Bottazzi, and R. K. Assoian. 1999. Alpha5beta1 integrin controls cyclin D1 expression by sustaining mitogen-activated protein kinase activity in growth factor-treated cells. Mol. Biol. Cell 10:3197-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubinfeld, H., T. Hanoch, and R. Seger. 1999. Identification of a cytoplasmic-retention sequence in ERK2. J. Biol. Chem. 274:30349-30352. [DOI] [PubMed] [Google Scholar]
- 42.Schaeffer, H. J., A. D. Catling, S. T. Eblen, L. S. Collier, A. Krauss, and M. J. Weber. 1998. MP1: a MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science 281:1668-1671. [DOI] [PubMed] [Google Scholar]
- 43.Sells, M. A., J. T. Boyd, and J. Chernoff. 1999. p21-activated kinase 1 (PAK1) regulates cell motility in mammalian fibroblasts. J. Cell Biol. 145:837-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Short, S. M., J. L. Boyer, and R. L. Juliano. 2000. Integrins regulate the linkage between upstream and downstream events in G protein-coupled receptor signaling to mitogen-activated protein kinase. J. Biol. Chem. 275:12970-12977. [DOI] [PubMed] [Google Scholar]
- 45.Stokoe, D., S. G. Macdonald, K. Cadwallader, M. Symons, and J. F. Hancock. 1994. Activation of Raf as a result of recruitment to the plasma membrane. Science 264:1463-1467. [DOI] [PubMed] [Google Scholar]
- 46.Tanoue, T., M. Adachi, T. Moriguchi, and E. Nishida. 2000. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat. Cell Biol. 2:110-116. [DOI] [PubMed] [Google Scholar]
- 47.Welsh, C. F., K. Roovers, J. Villanueva, Y. Liu, M. A. Schwartz, and R. K. Assoian. 2001. Timing of cyclin D1 expression within G1 phase is controlled by Rho. Nat. Cell Biol. 3:950-957. [DOI] [PubMed] [Google Scholar]
- 48.Wilsbacher, J. L., E. J. Goldsmith, and M. H. Cobb. 1999. Phosphorylation of MAP kinases by MAPK/ERK kinase involves multiple regions of MAP kinases. J. Biol. Chem. 274:16988-16994. [DOI] [PubMed] [Google Scholar]
- 49.Xu, B., S. Stippec, F. L. Robinson, and M. H. Cobb. 2001. Hydrophobic as well as charged residues in both mek1 and erk2 are important for their proper docking. J. Biol. Chem. 276:26509-26515. [DOI] [PubMed] [Google Scholar]
- 50.Xu, B., J. L. Wilsbacher, T. Collisson, and M. H. Cobb. 1999. The N-terminal ERK-binding site of MEK1 is required for efficient feedback phosphorylation by ERK2 in vitro and ERK activation in vivo. J. Biol. Chem. 274:34029-34035. [DOI] [PubMed] [Google Scholar]
- 51.Zecevic, M., A. D. Catling, S. T. Eblen, L. Renzi, J. C. Hittle, T. J. Yen, G. J. Gorbsky, and M. J. Weber. 1998. Active MAP kinase in mitosis: localization at kinetochores and association with the motor protein CENP-E. J. Cell Biol. 142:1547-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]