Abstract
The Polycomb (Pc) group (Pc-G) of repressors is essential for transcriptional silencing of homeotic genes that determine the axial development of metazoan animals. It is generally believed that the multimeric complexes formed by these proteins nucleate certain chromatin structures to silence promoter activity upon binding to Pc-G response elements (PRE). Little is known, however, about the molecular mechanism involved in sequence-specific binding of these complexes. Here, we show that an immunoaffinity-purified Pc protein complex contains a DNA binding activity specific to the (GA)n motif in a PRE from the bithoraxoid region. We found that this activity can be attributed primarily to the large protein isoform encoded by pipsqueak (psq) instead of to the well-characterized GAGA factor. The functional relevance of psq to the silencing mechanism is strongly supported by its synergistic interactions with a subset of Pc-G that cause misexpression of homeotic genes.
The Hox genes in the Antennapedia complex (ANTP-C) and the bithorax complex (BX-C) of Drosophila melanogaster are essential for segmental specification along the anteroposterior body axis (32, 41). Aberrant expression of these genes often causes homeotic transformation, i.e., misspecification of body parts. Complex regulatory mechanisms exist to ensure silencing of these genes outside specific domains throughout development (42). The silenced state is initially established by segmentation genes, including hunchback and tailless, that are expressed transiently in early embryos (46). Subsequent maintenance requires the presence of large numbers of the Polycomb (Pc) group (Pc-G) repressors.
Studies of cis-acting sequences capable of maintaining the silenced state of homeotic genes have revealed that, although extended sequences are necessary for full function, short sequences of several hundreds of bases have been proven to be critical. These sequences are commonly referred to as Pc-G response elements (PRE) (53). PRE appear to silence a closely linked marker gene in an epigenetically transmittable manner during cell division (10).
Several salient features have been noted about PRE. For example, PRE can silence a distant marker gene (54). PRE can also exhibit a pairing-sensitive silencing effect, resulting in much stronger silencing on the marker gene when PRE is present on the homologous chromosome (31). A high incidence of PRE insertion occurs at sites that contain preexisting PRE or PRE-like sequences (19). In general, PRE insertion creates a new chromosomal binding site for many Pc-G proteins (17). Further, PRE can confer transcription repression on Ultrabithorax (Ubx) in a Pc-dependent manner in cultured cells (13). Thus, PRE appear to act as the core sequences upon which Pc-G proteins assemble into large functional silencing complexes. It has been speculated that PRE at different chromosomal sites, when spatially juxtaposed, might cooperate and become more effective (48).
How Pc-G can accomplish these tasks remains largely unclear. To date, less than half a dozen Pc-G have been thoroughly studied. Some Pc-G proteins contain domains that are capable of homophilic or heterophilic interaction (38, 45), potentially facilitating formation and/or interaction of multimeric protein complexes. Consistently, large protein complexes containing Pc-G proteins have been identified. For example, PC, Polyhomeotic (PH), and Posterior Sex Combs (PSC) are found in the Pc repression complex 1 of approximately 2 MDa (51). A smaller protein complex containing Enhancer of Zeste [E(Z)] and Extra Sex Combs (ESC) has also been reported (44, 59). Since some Pc-G proteins have not been shown to copurify with these complexes, additional complexes might be expected. Germ line clones of many Pc-G mutations display similar but distinct patterns of embryonic defects, suggesting partially overlapping functions (56). Chromatin immunoprecipitation has also revealed substantial variation in the composition of the Pc-G complexes at different sites (58). Surprisingly, some of these sites are found in actively expressed genes (58). Thus, multiple Pc-G complexes might function in different contexts during development.
A statistical estimation has suggested that there are about 40 Pc-G in the fly genome (29). Consistent with this view, many novel proteins have been found in Pc-G complexes (14, 44, 50, 51, 59). Recently, mutations of several new genes were found to enhance homeotic phenotypes when in combination with Pc-G mutations; however, they produced little effect by themselves (14, 33, 56). These types of genes might define functions distinct from those of previously characterized Pc-G. Indeed, the identification of MI-2 and histone deacetylase 1 (HDAC1) as crucial partners has revealed that nucleosome modification, via histone deacetylation, plays a crucial role in homeotic gene silencing (14, 33, 59).
A fundamental question yet to be addressed fully is how the Pc-G protein complexes recognize specific sequences in PRE. With the exception of pleiohomeotic (pho), which encodes the homologue of mammalian YY1 (8), no existing Pc-G has been shown to bind specific DNA sequences. The PHO binding site is a functional constituent of PRE (20); however, the inability of a LexA-PHO fusion protein to silence a linked reporter gene as other Pc-G fusion proteins suggests that PHO alone may not be sufficient to target functional Pc-G complexes (49). Recently, the (GA)n motif present in PRE has been suggested to be critical for homeotic gene silencing (24, 25). It has been further suggested that the GAGA factor (GAF), a well-characterized DNA binding protein for such a motif (55), is involved in the binding (24, 25). Contrary to the expected silencing effect, GAF has also been shown to act either as an antirepressor to alleviate the negative effects of histone H1 (36) or as a transactivator in vitro, in cultured cells, and in stress response (6, 21, 55). In addition, Trithorax-like (Trl), the GAF-encoding gene, has been formerly classified as a member of the trithorax group of genes (trx-G) that antagonize Pc-G (18). Therefore, the role of GAF remains unresolved.
An ∼440-bp DNA fragment from the bithoraxoid (bxd) region of Ubx that can recapitulate both positive and negative effects of trx and Pc, respectively, has previously been identified (13). In this study, we used immunoaffinity chromatography to purify tagged Pc-G complexes and then we assayed their DNA binding activity. We found that the (GA)n motif in this fragment is indeed a primary binding site for the Pc-G complexes. We also provided several lines of evidence to show that the DNA binding protein is encoded by the pipsqueak (psq) gene, previously only known for its role in oogenesis and eye development (27, 52, 61).
MATERIALS AND METHODS
Fly works.
Flies were raised at 25°C on standard food. To test genetic interactions, psq alleles were rebalanced with SM6-TM6B,Tb since the CyO balancer present in some of the original stocks appeared to show significant interaction with Pc4. Virgin females heterozygous for various psq alleles (0115, 2403, 8109, and Δ18 from C. Berg; F112, E34, and E39 from U. Weber) were crossed with males heterozygous for Pc4, Psc1, ScmD1, esc10, or E(z)63, and the number of sex comb teeth in the second and third legs of their male progeny were counted as described previously (14). To identify larvae heterozygous for both psq and Pc mutations, female flies carrying psq alleles balanced with SM6-TM6B,Tb were crossed with Pc4/TM6B,Tb male flies. Larvae without the dominant Tb phenotype carried by the TM6B balancer were selected for subsequent disk staining.
Plasmids and probes.
To generate the pMT/Pc-FH clone, the DNA sequence from the termination codon of Pc up to the BglII site of pAct/Pc-1 (13) was first replaced by the DNA sequence encoding a FLAG peptide and hexahistidine (TTGATATCAGATCTGATGGACTACAAGGACGATGACGATAAG AACGCGTCCCACCATCACCACCATCACTAGGATCT; details available upon request) to make pAct/Pc-FH. The SmaI/SalI fragment of pAct/Pc-FH was then cloned into the XhoI/SalI sites of pMt/Hy (37) after the repair of the XhoI site by Klenow enzyme. The bxd-a and bxd-b clones were generated from B-151 (13) by dividing the ∼440-bp bxd fragment at the internal BglI site and inserting it into the PstI/EcoRV sites of pBluescript after T4 polymerase treatment. bxd-1, -2, -3, and -4 were generated by PCR amplification and subsequent cloning into pBluescript (Stratagene). p(GA)n and p(Z)n were generated from B120/GAGA and B120/ZESTE plasmids (13, 39), respectively, by inserting into pBluescript the amplified DNA sequences corresponding to positions −321 to −12 of Ubx. For competition assays, DNA fragments were prepared following digestion by restriction enzymes: XbaI/XhoI for bxd-a and -b; SacII/XbaI for bxd-1, -2, -3, and -4 and p(GA)n and p(Z)n; PvuII for pBluescribe (Stratagene).
For the construction of pET/GAGA, a SmaI/KpnI fragment corresponding to the full-length cDNA of GAGA-519 was excised from pBluescript/GAGA (a gift from T. Tsukiyama and C. Wu) and first cloned into a modified pPac5c-PL vector (a gift from C. Thummel) that contains a DNA fragment encoding the FLAG peptide in the polylinker (details available upon request). The SnaBI/SacI fragment was then inserted at the NdeI/HindIII sites of the pET15b vector (Novagene), after repair of the NdeI site. For the construction of pQE/PSQ-N, the SpeI/MluI fragment corresponding to the C-terminal part of psq-1 was deleted from pHH14 (a gift from C. Berg) (27). For pQE/PSQ-C, the BamHI/SmaI fragment corresponding to the N-terminal part of psq-1 was deleted from pHH14.
Cell culture, protein, and antibody purification.
The plasmid pMT/Pc-FH was transfected into S2 cells and selected for with hygromycin (200 μg/ml) as described previously (37). Stable PC-FH cell lines were grown in spinner flasks and induced at a density of 2 × 106 to 4 × 106 cells/ml with 0.1 mM CuSO4 for 67 h. The amount of Pc proteins induced at this concentration was less than 10% of the amount induced by 0.7 mM CuSO4 (data not shown). As described earlier (14), the Pc protein complex was purified by immunoaffinity chromatography from the PC-FH cell line by making a 10 to 40% (NH4)2SO4 differential precipitation of the nuclear extract, followed by FLAG peptide elution from a FLAG antibody column (M2; Kodak).
For expression of recombinant proteins, pET/GAGA was transformed into BL21(DE3) cells and pQE/PSQ-N and PSQ-C were transformed into TG-1 cells. Soluble proteins were obtained under the following induction conditions: 25°C for 90 min for GAGA and 25°C for 5 h for both PSQ-N and PSQ-C. Proteins were purified from a nickel-nitrilotriacetic acid column according to the vendor's instructions (Qiagen). Purified PSQ-N and PSQ-C were coupled to Affi-Gel 10 resin (Bio-Rad) in acetate buffer (0.2 M, pH 5.0) (16) and Clark and Lubs buffer (pH 8.5) (16), respectively.
AS-2 serum (a gift from C. Berg) (27) was used for affinity purification of PSQ antibody against specific parts of Psq proteins. However, the antibody purified from the PSQ-N column appeared to have strong cross-reactivity against many proteins on the Western blot, presumably due to the BTB and glutamine-rich domains. Serum extensively preabsorbed with PSQ-N resin was then passed through a PSQ-C column to purify the corresponding antibody by standard acid elution (23).
Electrophoretic mobility shift assay (EMSA).
Binding reactions were carried out at 30°C for 20 min in a 10-μl solution containing binding buffer (20 mM Tris-HCl [pH 7.8], 60 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol, 0.1% Tween 20, 10% glycerol), 5 μg of bovine serum albumin, 0.5 to 1 ng of 32P-labeled probe, and poly(dI-dC) (0.1 μg for PC and 0.5 μg for GAF). Samples were run at 4°C for ∼3 h on a 3.5% polyacrylamide gel (37.5:1 acrylamide-to-bisacrylamide ratio; Bio-Rad) in 1× TAE (40 mM Tris-acetate [pH 8.0], 1 mM EDTA) after a preelectrophoresis for about half an hour. Competition experiments were carried out in the presence of ∼30 ng of purified DNA fragments. For antibody supershift experiments, the PC protein complex was incubated with affinity-purified PC (2.5 mg/ml) antibody or goat immunoglobulin G (IgG) (2.5 mg/ml) for 1 h on ice prior to the addition of other components. The amount of poly(dI-dC) was increased to 0.25 μg for these assays.
UV cross-linking.
The oligo probe used for UV cross-linking studies was made by incorporating radioactive dCTP and cross-linkable 5-[N-(p-azidodbenzoyl)-3-aminoallyl] dUTP (AB-dUTP) specifically at the binding site as described previously (2). The probe was made by first annealing a 47-mer (5′-CCTCCTCCTTCCTGGAGAGGGAGAGAGGCACGACTTAACGCATACAC-3′) to a 19-mer (5′-GTGTATGCGTTAAGTCGTG-3′). Approximately 50 ng of hybrid DNA was partially filled by exonuclease-free Klenow enzyme (New England Biolabs) in the presence of ∼0.67 μM [α-32P]dCTP (∼3,000 Ci/mmol; Amersham), 1 μM dCTP, 20 μM AB-dUTP (a gift from P. Geiduschek), and 5 μM dTTP, followed by the addition of 250 μM dNTP to fill up the remaining gap in the probe DNA. The probe was purified by phenol-chloroform extraction and repeated ethanol precipitation in the presence of 0.5 M ammonium acetate and glycogen (200 μg/ml) (Boehringer Mannheim).
DNA binding reactions with 10-μl reaction mixtures were carried out as described above, except that bovine serum albumin was omitted. Open vials were UV irradiated for 3 min at 0.4 W in a UVC-515 UV multilinker (UV LUM). MgCl2 and CaCl2 were then added to a final concentration of 8 mM. Following a 30-min incubation at 30°C with 10 to 30 U of DNase I (Sigma), the DNA-protein complexes were further digested by 0.5 U of micrococcal nuclease (Sigma) for 20 min. Samples were then analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis on a 9% gel according to standard procedure.
Immunostaining.
For disc staining, imaginal discs from wandering third-instar larvae were fixed and stained with UBX antibody (1:2 dilution of FP.3.38 [62]). Labeling was detected by the ABC detection method (Vector) and diaminobenzidine staining. For polytene chromosome staining, salivary glands were dissected, fixed, and squashed according to the standard protocol (65), except that the formaldehyde concentration was reduced to 2.5%. For double immunofluorescence staining, PSC monoclonal antibody 6E8 (1:10 purified antibody from Developmental Studies Hybridoma Bank) and affinity-purified rabbit PSQ antibody (1:500) were used. The secondary antibodies were conjugated with Cy5 and Rhodamine Red-X for the anti-mouse and anti-rabbit antibodies, respectively. Images were obtained with a Zeiss LSM310 confocal microscope.
RESULTS
(GA)n as a critical motif in PRE.
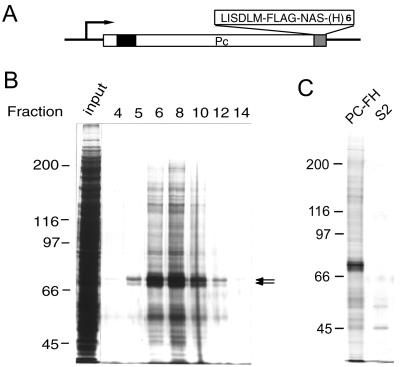
To facilitate the biochemical study of the Pc-G complex, we established a Drosophila S2 cell line PC-FH that can express Pc protein with both FLAG epitope and hexahistidine tags at its C terminus (Fig. 1A). These modifications do not appear to affect the activity of Pc, since similar constructs can repress the Ubx reporter gene in cultured cells and partially rescue Pc mutants in transgenic flies (data not shown). The tagged PC is under the control of a metallothionein promoter, which allows protein induction by the copper ion in a range up to 0.7 mM (9; data not shown). We chose a suboptimal concentration of CuSO4 (i.e., 0.1 mM) for induction, since it appears to provide sufficient amounts of tagged protein complexes for purification. Nuclear extracts prepared from induced cells were fractionated by 10 to 40% (NH4)2SO4 precipitation to enrich for large protein complexes. The extracts were then passed through a FLAG antibody column (i.e., M2 resin) and eluted with the FLAG peptide. We estimated that approximately 200-fold purification was obtained by affinity chromatography compared with the crude extracts (Fig. 1B). As shown in Fig. 1C, many proteins appeared to be specifically coeluted with the Pc protein. Although the region corresponding to the size of Pc proteins appears to be heavily stained, the relative abundance of Pc proteins has been exaggerated by the presence of several proteins of similar size that can be better separated in high-resolution gels (data not shown). An earlier study has shown that a specific subset of Pc-G proteins, including PSC, PH, and HDAC1 were copurified, indicating the presence of multimeric Pc-G complexes in these fractions (14). Since this is the first Drosophila Pc-G complex shown to contain both histone modification activity (14) and DNA binding activity (see below) and since homeotic genes are its best characterized targets, we shall refer to this complex as CHRASCH (chromatin-associated silencing complex for homeotics) to distinguish it from commonly referred Pc-G complexes or complexes characterized by other workers (44, 51, 59).
FIG. 1.
Purification of tagged Pc-G complex. (A) Structure of the epitope-tagged Pc construct. The chromodomain is indicated by a solid box. The C-terminal FLAG epitope and hexahistidine tags are labeled FLAG and (H)6, respectively. The extra amino acid residues introduced by the linker sequences are shown in single letter code. (B) Silver staining of eluted proteins. Aliquots (3.5 μl) of proteins from the column input and different fractions were resolved on an SDS-7% polyacrylamide gel. The fraction numbers are indicated at the top of each lane and are consistent with those in Fig. 2D and 3B. The position of PC is indicated by the arrows. Note that several discrete protein bands can be resolved in this region on high-resolution gels. (C) Specific association of Pc-G proteins. The eluted fractions prepared from PC-FH or S2 cells were resolved on an SDS-7% polyacrylamide gel. Only a small number of nonspecific proteins were eluted from S2 cell extracts.
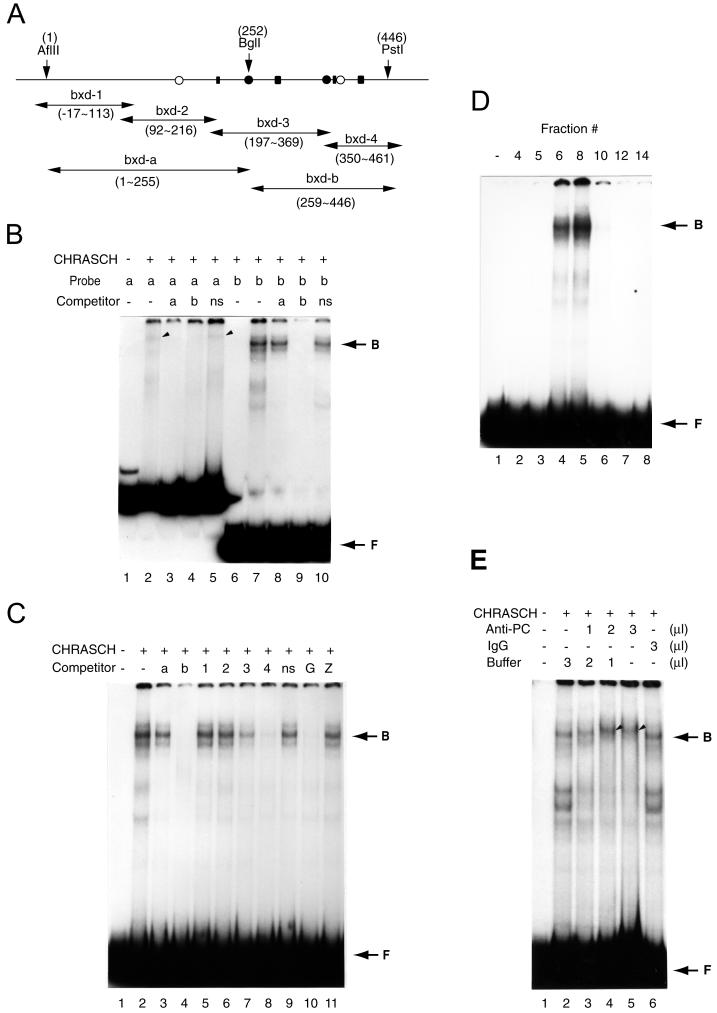
Since a functional Pc-G complex must act specifically on its response element (i.e., PRE), we examined whether CHRASCH can bind such sequences in vitro. PRE from several homeotic genes have been mapped, including the one from the upstream bxd region of Ubx (12, 13, 15). It was previously shown that an ∼440-bp fragment (B-151) from this region recapitulates transcriptional regulation by either Pc or trx in cultured cells (13). DNA fragments encompassing this region can also confer pairing-sensitive repression and are enriched for Pc proteins in chromatin immunoprecipitation experiments (12, 57). B-151 therefore contains physiologically relevant binding sites for the Pc-G complex. Using two subfragments from B-151 as probes for EMSAs, we found that CHRASCH binds strongly to the bxd-b fragment (Fig. 2B ), resulting in several slow-migrating bands. As will be shown later, these bands presumably reflect the binding to a reiterated motif in the bxd-b fragment. The binding of CHRASCH to the bxd-b fragment appeared to be specific, since it could be completely competed out by the addition of bxd-b but not by bxd-a or nonspecific vector sequences (Fig. 2B and C). A much weaker but specific binding of CHRASCH to bxd-a was also detected. The observation that bxd-b could compete for binding to bxd-a (Fig. 2B, lane 4) but that bxd-a could not compete effectively for binding to bxd-b (Fig. 2B, lane 8) suggested that these fragments might contain similar binding sequences, albeit with a lower affinity in the bxd-a fragment. Due to the difficulty in studying a weak binding activity with certainty, our subsequent studies focused on bxd-b.
FIG. 2.
CHRASCH binds the (GA)n motif. (A) Map of DNA fragments in B-151 used for binding and competition assays. The locations of the (GA)n motif (solid rectangular box), YY1 (solid circles), and ZESTE (open circles) binding sites are shown. The coordinates of restriction sites and various fragments are indicated. (B) Preferential binding of CHRASCH to the bxd-b fragment. Binding of labeled bxd-a (a) or bxd-b (b) probe was carried out with or without (−) an excess amount of unlabeled bxd-a or bxd-b or a DNA fragment from the polylinker of a pBluescribe vector(ns). Specific binding to bxd-a and bxd-b probes is indicated by the arrowhead and arrow, respectively. (C) Binding of CHRASCH to the (GA)n motif. Binding assays were carried out with the bxd-b probe. The competitors were bxd-a (a), bxd-b (b), bxd-1 to -4 (1 to 4), linker DNA (ns), or fragments containing multiple (GA)n (G) or ZESTE (Z) binding sites. (D) Elution profile of the DNA binding activity. Aliquots (2 μl) of eluted fractions (same fractions as shown in Fig. 1A) were assayed with the bxd-b probe. (E) Antibody supershift assay. Affinity-purified PC antibody (2.5 mg/ml), IgG (2.5 mg/ml), or buffer were preincubated with CHRASCH before the addition of a reaction mixture containing the bxd-b probe. The volume of antibody solution is indicated. The supershifted complex is indicated by the arrowhead. Note that fast-migrating bands appeared after 1 h of preincubation (lanes 2 and 6), suggesting instability of the complex. The EMSA shown here was done with 3.5% polyacrylamide gels. B, bound DNA-protein complex; F, free probe.
We used four partially overlapping fragments from B-151 in competition assays to further map the binding sites of CHRASCH. As shown in Fig. 2C, only the bxd-3 and bxd-4 fragments compete effectively for the binding to CHRASCH. In addition, bxd-4 appeared to compete better than bxd-3. Therefore, we deduced that the right half of B-151 must contain the primary binding sites for CHRASCH. Interestingly, transgenes containing small deletions in this region, but not in the left half of B-151, failed to silence the reporter gene effectively and could no longer respond to mutations in several Pc-G (60), indicating that this region is indeed relevant for Pc-G-mediated silencing. Three major sequence motifs can be identified in B-151. The first motif (C/T)GAG(C/T)G is the consensus binding site of the Zeste protein (47). Both the left and right halves of B-151 contain one Zeste binding site. The second motif ATGGC represents the binding site of a newly characterized member of Pc-G, pho, which encodes the Drosophila homologue of YY1 (8, 43). bxd-3 and bxd-4 each contain one copy of an almost identical YY1 site. The third motif is a (GA)n repeat, which represents the consensus binding site of GAF encoded by Trithorax-like (Trl) (21). Trl was originally identified as a member of trx-G (18); however, some recent studies suggest that it may also share some characteristics with Pc-G (22, 24). While both bxd-3 and bxd-4 contain 2 separate clusters of this motif, one cluster in bxd-4 is much further extended (GAGAGAGGGAGAG versus GAGAG). Since bxd-4 has been shown to be more effective in competition assays, it is likely that the (GA)n motif is most critical for CHRASCH binding. This possibility is further supported by the observation that CHRASCH binding to bxd-b was completely competed out by a fragment containing multiple (GA)n repeats (Fig. 2C, lane 10) but not by the one containing multiple Zeste repeats (Fig. 2C, lane 11) or an oligonucleotide containing a YY1 binding site (data not shown). Therefore, we conclude that the binding sequences of CHRASCH consist primarily of the (GA)n motif.
The association of the (GA)n binding activity with CHRASCH was further confirmed by the following observations. When the DNA binding activity was examined in peptide-eluted fractions, we found that fractions 6 and 8 had the strongest binding activities (Fig. 2D). These fractions also contained the highest amounts of PC and other associated proteins (compare Fig. 1B and 2D). Thus, the binding protein coeluted with CHRASCH in the immunoaffinity chromatography. In addition, we found that the DNA-protein complexes formed on bxd-b could be slightly supershifted by a preincubation with an affinity-purified PC antibody but not with a nonspecific IgG (Fig. 2E, lanes 4 and 5). The small supershift might be expected for a large complex in a gel composed of 3.5% polyacrylamide. Taken together, these results indicate that the (GA)n binding protein is physically associated with CHRASCH.
Psq proteins are responsible for (GA)n binding.
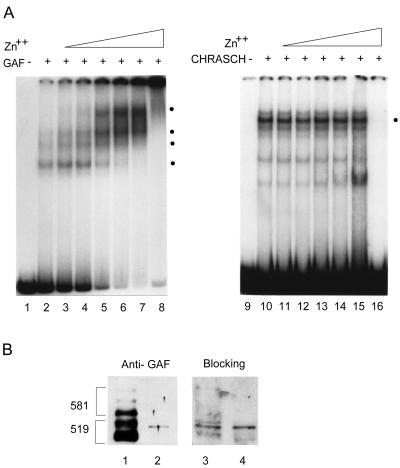
Since chromatin immunoprecipitation experiments have shown that GAF is enriched in the region encompassing B-151 and its vicinity (57), we examined whether the binding protein of CHRASCH is related to GAF. To our surprise, our results were not consistent with this notion. As expected, a purified recombinant GAF bound specifically to the bxd-b fragment. In addition, the binding activity of GAF was drastically stimulated by zinc ions over a wide range, resulting in a further retardation of the DNA-protein complexes in the EMSA (Fig. 3A). By contrast, the binding activity of CHRASCH was not significantly affected at intermediate concentrations of zinc ions and became completely inactivated at a high concentration (i.e., 0.5 mM). The differential effects of zinc ions on DNA binding properties argue that different DNA binding proteins are involved in the binding. Furthermore, we used a GAF-specific antiserum to examine our protein preparations. GAF antiserum detected multiple bands corresponding to two major classes of isoforms, GAF-519 and -581 (4), from the column input. A weak band migrating slightly slower than one GAF-519 isoform, however, was detected in the eluted fraction (Fig. 3B, compare lanes 1 and 2). To ascertain its identity, we asked whether the reactivity of GAF antiserum could be blocked by GAF. Preincubation of GAF antiserum with purified recombinant GAF could cause almost complete loss of its reactivity to GAF in the input fraction but had no effect on the reactivity to the band detected in the eluted fraction (Fig. 3B, lane 4). Therefore, this band most likely arises from a nonspecific cross-reactivity.
FIG. 3.
Lack of GAF in CHRASCH. (A) Effects of zinc ion on binding activities of GAF and CHRASCH. Different concentrations of ZnSO4 were included in binding reaction mixtures, followed by electrophoresis on 3.5% polyacrylamide gels. The concentrations used are as follows: none (lanes 2 and 10), 10 μM (lanes 3 and 11), 20 μM (lanes 4 and 12), 50 μM (lanes 5 and 13), 100 μM (lanes 6 and 14), 200 μM (lanes 7 and 15), and 500 μM (lanes 8 and 16). Specific bands are indicated by dots. In contrast to stronger binding for GAF at higher ZnSO4 concentrations, CHRASCH is refractory to intermediate concentrations and then becomes completely inactive. (B) Proteins from both column input (1 μl) (lanes 1 and 3) and fraction 8 (5 μl) (lanes 2 and 4) were resolved on an SDS-10% polyacrylamide gel, transferred, and assayed by GAF antiserum before (lanes 1 and 2) or after (lanes 3 and 4) a 1-h preincubation with purified recombinant GAF (∼16 μg/ml). Two major clusters of GAF isoforms, GAGA-519 and GAGA-581, are indicated. Preincubation of recombinant GAF strongly reduces the reactivity of the antiserum to GAF in the input but not to the unknown protein in the purified fraction, indicating that this protein is not related to GAF.
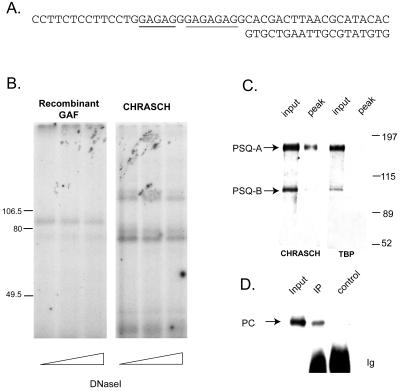
To further characterize the DNA binding protein of CHRASCH, we used a cross-linking method to specifically label this protein (2). An oligonucleotide probe was designed that allowed specific incorporation of both radioactive dCTP and a photoactivated cross-linking dTTP analogue (i.e., AB-dUTP) into the nucleotide sequences that correspond to the extended (GA)n motif in bxd-4 (Fig. 4A). Following DNA binding and UV irradiation, the cross-linked nucleoprotein complex was digested extensively with both DNase I and micrococcal nuclease to remove excessive DNA sequences. The indirectly labeled proteins were then resolved on an SDS-9% polyacrylamide gel. As shown in Fig. 4B, a major band of ∼85 kDa was identified for the purified recombinant GAF, whereas a different pattern was observed for CHRASCH, which consists of a doublet of ∼130 kDa and a doublet of ∼70 kDa. Taken together, our results provide strong evidence that the DNA binding activity of CHRASCH is not contributed by GAF but by a novel factor(s).
FIG. 4.
Psq proteins are directly involved in (GA)n binding. (A) Probe used for UV cross-linking of (GA)n binding protein. The DNA sequences of the upper and the lower strands are shown. Two stretches of the (GA)n motif are underlined, which are almost identical to the extended (GA)n motif in the bxd-4 fragment, except that their exact order is reversed. The sequences flanking both sides of the (GA)n motif in the probe bear no similarity to those in the bxd-4 fragment. Note that the incorporation of AB-dUTP and radioactive dCTP are restricted to the (GA)n motif only. (B) UV cross-linking of the binding protein in CHRASCH. Aliquots of recombinant GAF and CHRASCH that gave equivalent DNA binding activities, as judged by EMSA (data not shown), were used for UV cross-linking. After extensive digestion with various amounts of DNase I (10, 20, and 30 U) and 0.5 U of micrococcal nuclease, indirectly labeled proteins were resolved on an SDS-9% polyacrylamide gel. One major protein species of ∼85 kDa was found for purified GAF. Two sets of bands were found for CHRASCH. One set of bands was substantially larger than the 106.5-kDa mass marker, while the other one was ∼70 kDa. (C) Copurification of Psq proteins. The input and peak fractions from extracts containing tagged PC or TBP were resolved on a 7% polyacrylamide gel. After transfer to a nitrocellulose filter, the proteins were probed with an affinity-purified antibody specific for the C-terminal common half of PSQ. The left panel represents the immunoblot for CHRASCH, while the right panel represents that for TBP as a control. Note that PSQ-A and PSQ-B migrate as ∼150- and ∼95-kDa proteins, respectively. Note that the size of PSQ-B is larger than the one (∼80 kDa) reported by Horowitz and Berg (27). (D) Association of PSQ and PC in vivo. Embryonic nuclear extracts were immunoprecipitated by an affinity-purified PSQ antibody (IP) or nonimmune serum (control). The extracts (input) and immunoprecipitates were probed with an affinity-purified PC antibody. The positions for PC and Ig are indicated.
A new (GA)n-binding protein has been identified incidentally in Apis mellifera by screening an expression library with (GA)n repeats (40). The Drosophila homologue of this protein was found to be encoded by pipsqueak (psq), identified originally by its grandchildless phenotype and subsequently by its effect on eye development (27, 52, 61). By differential transcriptional and translational initiation, psq produces two major mRNAs containing open reading frames for 1,065 (PSQ-A) and 630 to 646 amino acids (PSQ-B). These two isoforms share a common C-terminal PSQ domain capable of binding to the (GA)n motif (40). The size similarity between one of the cross-linked proteins and PSQ-A prompted us to test whether Psq proteins are copurified with CHRASCH. Using an antibody that reacts with both PSQ-A and B (27), we found that PSQ-A and much less PSQ-B are clearly detectable in our sample (Fig. 4C). For a preparation of FLAG-tagged TATA-box binding protein (TBP), however, we found a large amount of PSQ-A in the input but not in the eluted fraction (Fig. 4C). These results demonstrate that PSQ-A and CHRASCH have indeed been copurified. To further examine the association between PC and PSQ in vivo, we performed coimmunoprecipitation with embryonic nuclear extracts. As shown in Fig. 4D, PC was precipitated by the PSQ antibody but not by the control serum. Although it is not clear whether the smaller ∼70-kDa proteins detected in the cross-linking experiments represent degradation products of PSQ or other unrelated proteins, our results strongly suggest that PSQ-A may play a major role in DNA binding.
Selective role of psq in vivo.
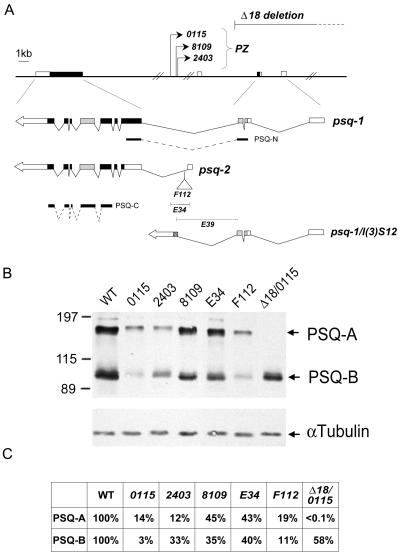
To determine the role of psq in vivo, we used a dosage-sensitive assay to test the genetic interactions between Pc and psq mutations (35). Two classes of psq mutations have been previously identified, each has been implicated in a distinct developmental function. For example, the 0115, 2403, and 8109 alleles (referred to as class I mutations) that result from P element insertion mutations in the ∼40-kb intron (see Fig. 7A) appear to primarily affect oogenesis, giving rise to the grandchildless phenotype (27, 52), whereas the F112, E34, and E39 alleles (referred to as class II mutations) that are clustered around the first exon of psq-B (or psq-2 by Horowitz and Berg [27]) appear to affect eye development (61). Although none of these psq mutations alone have been known to cause homeotic phenotypes in adults, it does not necessarily preclude their role in homeotic gene silencing, since an increasing number of Pc-G interacting genes have been found to cause little, if any, homeotic phenotype by themselves (14, 33, 56). Thus, we crossed females heterozygous for various psq alleles with male Pc4 heterozygotes and examined their effects on homeotic leg transformation, i.e., production of ectopic sex comb teeth on the second and third legs of F1 males. As shown in Table 1, the average number of ectopic sex comb teeth is strikingly enhanced for all alleles of class I mutations when doubly heterozygous with the Pc4 mutation (ranging from 9 to 10 teeth per leg), and the second and third legs were almost completely transformed into the first leg. In contrast, class II alleles showed relatively weak but significant effects on leg transformation, giving rise to 3 to 5 ectopic teeth per leg.
FIG. 7.
Relative abundance of Psq proteins in ovaries of psq mutants. (A) A map of the psq locus is redrawn on the basis of the information from Horowitz and Berg (27) and Weber et al. (61). The genomic structure of psq and the positions of class I mutations including three PZ insertion mutants (0115, 2403, and 8109) and a deletion mutant (Δ18) are shown above the map. The structures of two major cDNAs, psq-1 and psq-2, are shown below, with their splicing junctions indicated. The noncoding sequences (blank boxes) and the coding regions (solid boxes), including the N-terminal BTB (stippled boxes) and C-terminal PSQ domains (grey boxes), are also shown. Three class II mutations, F112, E34, and E39, are located around the first exon of psq-2. The approximate sites of insertion (F112) or deletion (E34 and E39) are indicated. psq-1/l(3)S12 represents a fusion product resulting from an aberrant splicing between psq-1 and l(3)S12 adventitiously present in the PZ transposons. The positions of two recombinant proteins, PSQ-N and PSQ-C, used for antibody purification are also indicated. The DNA fragments are drawn to scale except for the ∼40-kb intron. (B) Protein analysis of psq mutants. Ovaries were dissected from homozygous psq or transheterozygous mutant adults, and proteins were analyzed by SDS-polyacrylamide gel electrophoresis. psq proteins were detected with affinity-purified antibody against PSQ-C. Extracts derived from wild-type (WT) or mutants are indicated above the respective lanes. Homozygous psqE39 animals died during pupation. The positions for PSQ-A, PSQ-B, and an internal control (α-tubulin) are marked by arrows. The positions of mass markers (in kilodaltons) are marked. Note that trace amounts of PSQ-A could be detected in psqΔ18/psq0115 mutants when the film was exposed for a longer amount of time. (C) Relative abundance of psq proteins. The relative amounts of PSQ-A and PSQ-B in these mutants are derived by first calibrating against the internal control (i.e., α-tubulin) in each sample. Each value was then calculated by using wild-type proteins as references.
TABLE 1.
Genetic interaction between psq and Pc
| Expta | psq allele | Avg no. of ectopic sex comb teeth/leg for genotypeb:
|
|
|---|---|---|---|
| Bal Pc | psq Pc | ||
| A | 0115 | 4.08 (118) | 10.13 (167) |
| 2403 | 3.44 (148) | 8.58 (184) | |
| 8109 | 3.31 (140) | 10.02 (131) | |
| F112 | 3.67 (168) | 5.17 (216) | |
| E34 | 3.86 (175) | 4.41 (216) | |
| E39 | 3.44 (126) | 3.81 (108) | |
| B | 0115 | 3.77 (102) | 7.51 (135) |
| Δ18 | 2.52 (84) | 8.90 (132) | |
| 8109 | 3.00 (66) | 8.78 (136) | |
Virgin females of various psq mutant alleles were crossed with Pc4 mutant males. Two separate experiments were done to determine the allelic strength in interactions with the Pc4 mutation.
The average number of ectopic sex comb teeth per leg was calculated by dividing the total number of ectopic sex comb teeth by the number of second and third legs (values in parentheses) for each genotype. The number of sex comb teeth on the first leg of wild-type males usually varies between 10 and 12. A reciprocal cross between Pc4 females and psq2403 males gave a lower number (1.31) of ectopic sex comb teeth in Pc4 progeny but a similar number (8.30 versus 8.58) in double-mutant progeny, indicating a strong maternal effect of psq.
Since three major protein complexes containing different combinations of Pc-G proteins have been described previously (14, 24, 44, 51, 59), it is interesting to determine whether the function of psq is generally or specifically required for these complexes. Pc-G mutations that are representative of these protein complexes were tested. Our preliminary results showed that there was a remarkable increase in the number of ectopic sex comb teeth in the progeny carrying both Psc1 and psq2403 mutations compared to those carrying the Psc1 mutation alone. The ScmD1 mutation displays an intermediate level of interaction, giving rise to approximately two- to threefold increases, whereas no significant enhancement was found for the esc10 or E(z)63 mutations. These results suggest that psq is crucial for proper function of a subset of Pc-G proteins that are constituents of CHRASCH.
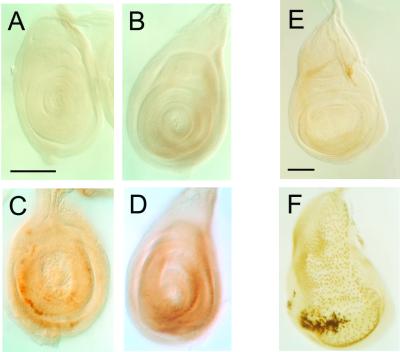
We further examined the effect of psq mutations on Ubx expression in imaginal discs. UBX proteins are normally expressed at high levels in the haltere- and third-leg discs but at low levels in the peripodial membranes of the wing discs. No significant change was observed in larvae heterozygous for either the Pc4 or psq mutation (data not shown). However, high levels of UBX proteins were observed in the medial sections of the wing discs from larvae doubly heterozygous for Pc4 and psq (Fig. 5F) In addition, substantial amounts of UBX proteins could be detected in the first- and second-leg discs (Fig. 5C and D).
FIG. 5.
Synergistic effect of psq and Pc mutations on ectopic UBX expression in imaginal discs. First (A and C)- and second (B and D)-leg discs and wing discs (E and F) from wild type (A, B, and E) or double heterozygotes (C, D, and F) of psq2403 and Pc4 were stained with a monoclonal UBX antibody. UBX is normally undetectable in the first-leg disk and detectable at a low level in second-leg and wing discs of wild-type larvae. A slight increase of UBX is observed in the wing discs of larvae heterozygous for Pc4. Strong UBX is observed in the discs of double mutants. The psq mutation alone has no detectable effect on UBX expression. Essentially the same results were observed for psq0115.
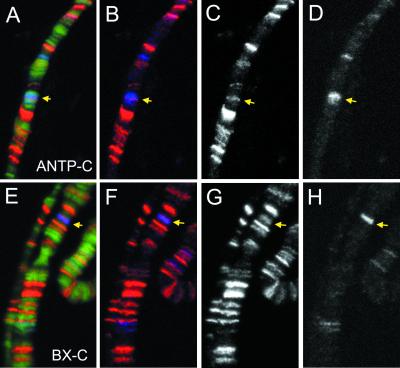
Given the copurification and genetic interactions between psq and Pc-G, one might expect PSQ to be detected on polytene chromosomes at sites corresponding to ANTP-C (at 84AB) and BX-C (at 89E), where strong Pc-G signals are normally observed. Using antibodies against PSC and PSQ for double immunofluorescent staining of polytene chromosomes under standard fixation conditions (i.e., 3.7% formaldehyde), we could not find consistent PSQ staining at ANTP-C and BX-C sites. It was possible, however, that the PSQ staining at these sites might have been masked by other proteins of the complex that are apparently layered upon it. Thus, we tested conditions to alleviate any potential masking effect. Using 2.5% formaldehyde for fixation, we found that PSQ signals can be detected at ANTP-C and BX-C sites (Fig. 6), with the PSC staining being consistently weaker than what was observed under standard conditions. The staining of PSC and PSQ at these sites was not always coextensive. For example, the PSQ signal represents only a part of the broader region of PSC staining at the ANTP-C site (Fig. 6A to D). The significance of this observation remains unclear.
FIG. 6.
Colocalization of PSC and PSQ on ANTP-C and BX-C. Polytene chromosomes from third-instar larvae were simultaneously stained with an affinity-purified rabbit PSQ antibody and a mouse monoclonal PSC antibody. The merged images are shown with DNA in green (A and E), PSQ staining in red (A, B, E, and F), and PSC staining in blue (A, B, E, and F). The images for single staining of PSQ (C and G) or PSC (D and H) are also shown. The sites for ANTP-C (A to D) and BX-C (E to H) are indicated by arrows.
We also examined the staining patterns on polytene chromosomes from transgenic flies containing insertions of small PRE fragments (e.g., PRE-D) (20). While these fragments showed ectopic PSC staining as expected, most of these fragments appeared to insert at sites that correspond to endogenous PSQ sites on wild-type chromosomes (data not shown). Thus, we could not use this approach to map the exact locations of PSQ binding sites within the PRE.
PSQ-A is essential for silencing.
Class I psq mutations have previously been shown to cause a more-severe reduction in the level of PSQ-A than in that of PSQ-B (27). It raised an interesting possibility that different classes of psq alleles might have differential effects on these two major protein species and that such effects might be correlated with their contribution to the silencing function. The relative abundance of these proteins from adult ovaries of several homozygous mutants was determined. As shown in Fig. 7B, there are indeed some fluctuations in the relative abundance of these two proteins, however, the fluctuations between different alleles within one class or between different classes do not seem to be consistent with the possibility that a specific Psq protein may be more critical for gene silencing. For example, while both psq0115 and psq2403 mutants contain much less PSQ than psq8109, psq2403 shows consistently weaker interaction with Pc than psq0115 and psq8109. In addition, although psq8109 and psqE34 contain similar amounts of PSQ, psq8109 shows substantially stronger interaction with Pc than psqE34. Thus, it seems unlikely that the homeotic phenotype can be attributed to the mere presence of different PSQ in a simple manner.
In addition to their effects at the level of gene expression, however, class I mutations can also result in the synthesis of a novel BTB-domain-containing fusion product [i.e., psq1-l(3)S12] due to aberrant splicing (26). It has further been shown that overexpression of this fusion protein in wild-type flies is sufficient to solicit phenotypes similar to those of class I mutations (27). It is believed that this fusion protein may interfere with the function of PSQ-A through the shared BTB protein interaction domain (1, 27) since this domain is present in PSQ-A protein but not in PSQ-B protein. Taking this possibility into consideration, the stronger interaction of class I mutations with the Pc mutation described above might result from a combination of reduced expression and an interference with PSQ-A protein, leading to a specific loss of PSQ-A function. To substantiate this possibility, we tested whether the loss of PSQ-A alone is sufficient to cause a strong interaction with the Pc mutation. psqΔ18 is a homozygous lethal mutation that deletes the 5′ exons of psq-1 and the intergenic region between psq and a divergently transcribed gene, lola. As expected, while the level of PSQ-B remains normal, very little PSQ-A is detected in ovaries from psq0115/psqΔ18 transheterozygotes (Fig. 7B), indicating that PSQ-A is specifically affected by the psqΔ18 mutation. When heterozygous psqΔ18 females were crossed with heterozygous Pc4 males, a very strong leg transformation was observed in their male progeny (Table 1, experiment B). Indeed, both the penetrance and expressivity caused by the psqΔ18 mutation are comparable to, if not stronger than, the two strongest psq alleles. Therefore, our results strongly support an essential role for PSQ-A in homeotic gene silencing.
DISCUSSION
The molecular mechanism responsible for sequence-specific targeting of Pc-G proteins has been elusive to date, partly because of the lack of strict consensus sequences of PRE and partly because of the plethora of proteins involved. In addition, given the identification of at least three physically separable Pc-G protein complexes (14, 24, 44, 51, 59) and the finding that different combinations of Pc-G proteins are detected on different target genes (58), it is likely that several strategies are employed by these complexes at different developmental stages, in different cell types, or at different target genes. Nevertheless, in this report, we provide several lines of evidence to show that a novel DNA binding factor encoded by psq is a constituent of CHRASCH, a previously characterized major Pc-G protein complex (14). Since CHRASCH also contains a histone modification factor, HDAC1, we suggest that this complex may represent a fully functional entity that can nucleate certain chromatin structures at and around specific sequences (i.e., PRE) of homeotic genes.
Biochemical purification of Pc-G protein complexes has been limited by their apparent instability (50; data not shown). Thus, a balance between biochemical purity and functional integrity might be considered. Different approaches are required subsequently to substantiate the physiological relevance of copurified proteins. To meet these criteria, we adopted the strategy of purifying Pc-G protein complexes to sufficient homogeneity mainly by immunoaffinity chromatography under moderate conditions, then examining the biochemical functions potentially relevant to these complexes, followed by identifying the functional constituents of the complex and corresponding genes, and finally validating their roles with genetic studies.
The bxd region has been extensively examined for PRE. Although different fragments ranging from ∼400 bp to ∼1 kb have been studied (12, 13, 24, 25, 60), they share a common region represented almost entirely by the B-151 fragment analyzed in this study. Among the three binding motifs of this fragment, we found that the (GA)n motif represents the most prominent binding site for CHRASCH. In recent studies, the role of this motif in silencing has been demonstrated in transgenic flies (24). Thus, we believe that this motif plays a critical role in anchoring one of the major Pc-G complexes (i.e., CHRASCH). Our results, however, are not mutually exclusive to the possibility that other motifs may be required for different functional aspects of PRE.
One of the most critical issues concerning the specific targeting of the Pc-G complex appears to reside in the identity of the DNA binding factor. Our results support the conclusion that PSQ-A plays a primary role in such a function for the following reasons. First, PSQ-A, but not GAF, is copurified with CHRASCH. Second, UV cross-linking studies strongly indicate that PSQ-A binds directly to the (GA)n motif. Additional proteins, however, were also evident in these studies. At present, we cannot distinguish between the possibilities that these proteins represent degradation products of PSQ-A, other novel binding proteins, or spurious cross-linking to sterically adjacent proteins in the complex. Nevertheless, it is clear that PSQ-A is involved in the binding of the (GA)n motif in vitro. Third, PSQ is colocalized with Pc-G protein at both ANTP-C and BX-C sites on polytene chromosomes. Fourth, there is a remarkably strong genetic interaction between Pc-G and psq that gives rise to leg transformation and ectopic Ubx expression. Finally, we show that the lack of PSQ-A in one mutant (i.e., psqΔ18) is sufficient to account for genetic interaction with Pc.
Recent studies have indicated that GAF (25) or a combination of novel forms of GAF and PSQ (24) is responsible for the binding of the Pc-G complex to the (GA)n motif. In one study, embryonic nuclear extracts were used to form the DNA-protein complex, followed by immunodetection with GAF antibody. Since multiple (GA)n motifs are present in the probes, it is difficult to exclude the possibility that GAF and Pc-G complexes might bind these motifs independently. Similar problems also arise from subsequent studies in which fusion proteins of LexA and Pc-G have been used to bind probes containing LexA binding sites (49), since the minimal GAF binding site, the GAG trinucleotide (63), is also present in the LexA probe. Although more purified fractions were used for DNA binding analysis in the other study (24), a combination of Bio-Rex 70 and Q-Sepharose may not provide sufficient resolving power to exclude the possibility that a large number of unrelated proteins are copurified. In addition, the final fractions appear to be enriched for a GAF of ∼54 kDa and to exclusively contain a PSQ of ∼70 kDa. Both proteins appear substantially smaller than the smallest forms detected in the original extracts (∼67 kDa for GAF and ∼95 kDa for PSQ). Since both GAF and PSQ antisera have nonspecific cross-reactivities (Fig. 3B for GAF) (see reference 27 for PSQ) (data not shown), the identities of these proteins remain obscure. Nonetheless, despite these uncertainties, it is possible that GAF may play a role in certain aspects of the silencing mechanism as suggested by genetic studies (22, 24).
Other sequence-specific DNA binding factors have also been implicated for Pc-G targeting by genetic and/or biochemical studies (8, 50). PHO is the only one that has been formally categorized as a Pc-G. Its binding sites are present in many PRE (43). In addition, mutations of the PHO binding site compromise the ability of PRE to silence reporter gene in larval tissues (20). However, PHO does not appear to be directly associated with many Pc-G proteins (49). Thus, despite its important role in homeotic gene silencing, it is not clear whether PHO is directly involved in the targeting of Pc-G complexes.
Another potential candidate involved in the binding of the Pc-G complex is the Zeste protein for its copurification with Pc repression complex 1 (50). It has been speculated that Zeste proteins act as the scaffold via self-multimerization to bring together regulatory sequences situated on the same chromosome or different chromosomes (3). Its binding site has also been found in several PRE. In contrast to the proposed role for silencing, however, previous molecular and genetic studies have shown that the Zeste protein is most likely an activator. For example, it stimulates transcription of the Ubx promoter in vitro (5). Expression of a Ubx-LacZ transgene is completely abolished by a zeste mutation (39). For its transactivating effect, zeste has been considered a trx-G (34). Consistent with this notion, direct physical interaction has recently been demonstrated between the Zeste protein and two trx-G proteins, MOIRE and OSA, of the BRAHMA nucleosome remodeling complex (30). Genetically, zeste has also been defined as a transactivator involved in transvection of several genes, including Ubx (47). In addition, several Pc-G have been identified as suppressors of zeste (7, 64). These observations cast some doubts on the physiological relevance of the Zeste protein in homeotic gene silencing. It is important to note that the two best characterized PRE (i.e., bxd and Fab7) also respond to trx-G (11, 13, 60). Thus, the mere existence of binding sites in PRE may not necessarily provide an unambiguous indication of their functions. While the manuscript was under review, however, a recent study has shown that zeste mutations result in an extended expression of a Ubx transgene containing a replacement of the proximal promoter with a combination of multiple Zeste and NTF-1 binding sites (28), suggesting a role for zeste in Ubx silencing. However, since extended expression was also observed for a Ubx transgene containing multiple NTF-1 binding sites at the proximal promoter region (28), the exact role of zeste may need to be more thoroughly examined.
In conclusion, we believe that our results provide direct evidence that a specific PSQ isoform is critically involved in the targeting of a major Pc-G protein complex CHRASCH to the (GA)n motifs that are commonly found in PRE. Earlier studies have demonstrated that a functional HDAC1 is associated with CHRASCH and is required for the silencing in vivo (14). We suggest a simple model for homeotic gene silencing that involves the assembly of multimeric complexes by known Pc-G proteins and other novel proteins yet to be identified, direct binding to specific sequences of PRE, and subsequent modification of N-terminal tails of core histones to establish a silencing code for stable maintenance of an inactive state.
It is also relevant to note that the functions of Pc-G silencing complexes may not be fully revealed by previous genetic or biochemical approaches because of the lack of suitable mutations, easily tractable phenotypes, or sufficient stability of the protein complexes. In the case of psq, a grandchildless class of mutations, sufficient amounts of PSQ remain detectable in most homozygous mutant adults (Fig. 7), yet embryos produced by these adults become severely defective before the manifestation of homeotic genes (27). In addition, the presence of more PSQ sites than Pc-G sites on polytene chromosomes suggests a much wider spectrum of target genes for PSQ. These effects altogether could conceivably obscure the homeotic effect caused by psq mutations, unless a more-sensitized genetic background (e.g., Pc mutations) is provided. The roles of MI-2 and HDAC1 in homeotic gene silencing also become apparent with similar approaches (14, 33). We speculate that some novel functions of the silencing complex may be defined by more-systematic studies.
Acknowledgments
We thank the Bloomington Stock Center for fly stocks and the Developmental Studies Hybridoma Bank at the University of Iowa (under the auspices of the National Institute of Child Health and Human Development) for the PSC antibody developed by P. Adler. We are grateful to C. Berg for class I psq alleles and PSQ antisera, to P. Geiduschek for AB-dUTP, to M. Koelle and D. Hogness for pMK33, to T. Tsukiyama and C. Wu for GAF cDNA and antisera, and to U. Weber and M. Mlodzik for class II psq alleles. We also thank the following people for help at different stages of the experiments: S.-C. Lin, I.-L. Chen, Y.-H. Peng, and P.-Y. Ho. We appreciate critical comments from Ken Deen on the manuscript.
This work was supported by grants from the Academia Sinica and the National Science Council (NSC 86-2316-B-001-009, 87-2311-B-001-118, 88-2311-B-001-126, and 89-2311-B-001-081).
REFERENCES
- 1.Bardwell, V. J., and R. Treisman. 1994. The POZ domain: a conserved protein-protein interaction motif. Genes Dev. 8:1664-1677. [DOI] [PubMed] [Google Scholar]
- 2.Bartholomew, B., R. L. Tinker, G. Kassavetis, and E. P. Geiduschek. 1995. Photochemical cross-linking assay for DNA tracking by replication proteins. Methods Enzymol. 262:476-494. [DOI] [PubMed] [Google Scholar]
- 3.Benson, M., and V. Pirrotta. 1988. The Drosophila zeste protein binds cooperatively to sites in many gene regulatory regions: implications for transvection and gene regulation. EMBO J. 7:3907-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benyajati, C., L. Mueller, N. Xu, M. Pappano, J. Gao, M. Mosammaparast, D. Conklin, D. Granok, C. Craig, and S. Elgin. 1997. Multiple isoforms of GAGA factor, a critical component of chromatin structure. Nucleic Acids Res. 25:3345-3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biggin, M. D., S. Bickel, M. Benson, V. Pirrotta, and R. Tjian. 1988. Zeste encodes a sequence-specific transcription factor that activates the Ultrabithorax promoter in vitro. Cell 53:713-722. [DOI] [PubMed] [Google Scholar]
- 6.Biggin, M. D., and R. Tjian. 1988. Transcription factors that activate the Ultrabithorax promoter in developmentally staged extracts. Cell 53:699-711. [DOI] [PubMed] [Google Scholar]
- 7.Bornemann, D., E. Miller, and J. Simon. 1998. Expression and properties of wild-type and mutant forms of the Drosophila sex comb on midleg (SCM) repressor protein. Genetics 150:675-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, J. L., D. Mucci, M. Whiteley, M.-L. Dirksen, and J. A. Kassis. 1998. The Drosophila Polycomb group gene pleiohomeotic encodes a DNA binding protein with homology to the transcription factor YY1. Mol. Cell 1:1057-1064. [DOI] [PubMed] [Google Scholar]
- 9.Bunch, T. A., Y. Grinblat, and L. S. B. Goldstein. 1988. Characterization and use of the Drosophila metallothionein promoter in cultured Drosophila melanogaster cells. Nucleic Acids Res. 16:1043-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavalli, G., and R. Paro. 1998. The Drosophila Fab-7 chromosomal element conveys epigenetic inheritance during mitosis and meiosis. Cell 93:505-518. [DOI] [PubMed] [Google Scholar]
- 11.Cavalli, G., and R. Paro. 1999. Epigenetic inheritance of active chromatin after removal of the main transactivator. Science 286:955-958. [DOI] [PubMed] [Google Scholar]
- 12.Chan, C.-S., L. Rastelli, and V. Pirrotta. 1994. A Polycomb response element in the Ubx gene that determines an epigenetically inherited state of repression. EMBO J. 13:2553-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang, Y.-L., B. O. King, M. O'Connor, A. Mazo, and D.-H. Huang. 1995. Functional reconstruction of trans regulation of the Ultrabithorax promoter by the products of two antagonistic genes, trithorax and Polycomb. Mol. Cell. Biol. 15:6601-6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang, Y.-L., Y.-H. Peng, I.-C. Pan, D.-S. Sun, B. King, and D.-H. Huang. 2001. Essential role of Drosophila Hdac1 in homeotic gene silencing. Proc. Natl. Acad. Sci. USA 98:9730-9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiang, A., M. O'Connor, R. Paro, J. Simon, and W. Bender. 1995. Discrete Polycomb-binding sites in each parasegmental domain of the bithorax complex. Development 121:1681-1689. [DOI] [PubMed] [Google Scholar]
- 16.Dawson, R. M. C., D. C. Elliott, W. H. Elliott, and K. M. Jones. 1986. Data for biochemical research, 3rd ed. Clarendon Press, Oxford, United Kingdom.
- 17.DeCamillis, M., N. Cheng, D. Pierre, and H. W. Brock. 1992. The polyhomeotic gene of Drosophila encodes a chromatin protein that shares polytene chromosome-binding sites with Polycomb. Genes Dev. 6:223-232. [DOI] [PubMed] [Google Scholar]
- 18.Farkas, G., J. Gausz, M. Galloni, G. Reuter, H. Gyurkovics, and F. Karch. 1994. The Trithorax-like gene encodes the Drosophila GAGA factor. Nature 371:806-808. [DOI] [PubMed] [Google Scholar]
- 19.Fauvarque, M.-O., and J.-M. Dura. 1993. Polyhomeotic regulatory sequences induce developmental regulator-dependent variegation and targeted P-element insertion in Drosophila. Genes Dev. 7:1508-1520. [DOI] [PubMed] [Google Scholar]
- 20.Fritsch, C., J. L. Brown, J. K. Kassis, and J. Mueller. 1999. The DNA-binding Polycomb group protein Pleiohomeotic mediates silencing of a Drosophila homeotic gene. Development 126:3905-3913. [DOI] [PubMed] [Google Scholar]
- 21.Granok, H., B. A. Leibovitch, C. D. Shaaffer, and S. C. R. Elgin. 1995. Ga-ga over GAGA factor. Curr. Biol. 5:238-241. [DOI] [PubMed] [Google Scholar]
- 22.Hagstrome, K., M. Muller, and P. Schedl. 1997. A Polycomb and GAGA dependent silencer adjoin the Fab-7 boundary in the Drosophila bithorax complex. Genetics 146:1365-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 24.Hodgson, J. W., B. Argiropoulos, and H. W. Brock. 2001. Site-specific recognition of a 70-base-pair element containing d(GA)n repeats mediates bithoraxoid Polycomb group response element-dependent silencing. Mol. Cell. Biol. 21:4528-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horard, B., C. Tatout, S. Poux, and V. Pirrotta. 2000. Structure of a Polycomb response element and in vitro binding of Polycomb group complexes containing GAGA factor. Mol. Cell. Biol. 20:3187-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horowitz, H., and C. A. Berg. 1995. Aberrant splicing and transcription termination caused by P element insertion into the intron of a Drosophila gene. Genetics 139:327-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horowitz, H., and C. A. Berg. 1996. The Drosophila pipsqueak gene encodes a nuclear BTB-domain-containing protein required early in oogenesis. Development 122:1859-1987. [DOI] [PubMed] [Google Scholar]
- 28.Hur, M.-W., J. D. Laney, S.-H. Jeon, J. Ali, and M. D. Biggin. 2002. Zeste maintains repression of Ubx transgenes: support for a new model of Polycomb repression. Development 129:1339-1343. [DOI] [PubMed] [Google Scholar]
- 29.Jurgens, G. 1985. A group of genes controlling the spatial expression of the bithorax complex in Drosophila. Nature 316:153-155. [Google Scholar]
- 30.Kal, A. J., T. Mahmoudi, N. B. Zak, and C. P. Verrijzer. 2000. The Drosophila Brahma complex is an essential coactivator for the trithorax group protein Zeste. Genes Dev. 14:1058-1071. [PMC free article] [PubMed] [Google Scholar]
- 31.Kassis, J. A. 1994. Unusual properties of regulatory DNA from the Drosophila engrailed gene: three “pairing-sensitive” sites within a 1.6 kb region. Genetics 136:1025-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufman, T. C., R. A. Lewis, and B. T. Wakimoto. 1980. Cytogenetic analysis of chromosome 3 in Drosophila melanogaster: the homeotic gene complex in polytene chromosome interval 84A-B. Genetics 94:115-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kehle, J., D. Beuchle, S. Treuheit, B. Christen, J. A. Kennison, M. Bienz, and J. Mueller. 1998. dMi-2, a hunchback-interacting protein that functions in Polycomb repression. Science 282:1897-1900. [DOI] [PubMed] [Google Scholar]
- 34.Kennison, J. A. 1995. The Polycomb and trithorax group proteins of Drosophila: trans-regulators of homeotic gene function. Annu. Rev. Genet. 29:289-303. [DOI] [PubMed] [Google Scholar]
- 35.Kennison, J. A., and J. W. Tamkun. 1988. Dosage-dependent modifiers of Polycomb and Antennapeidia mutations in Drosophila. Proc. Natl. Acad. Sci. USA 85:8136-8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerrigan, L. A., G. E. Croston, L. M. Lira, and J. T. Kadonaga. 1991. Sequence-specific transcriptional anti-repression of the Drosophila Kruppel gene by the GAGA factor. J. Biol. Chem. 266:574-582. [PubMed] [Google Scholar]
- 37.Koelle, M. R., W. S. Talbot, W. A. Segraves, M. T. Bender, P. Cherbas, and D. S. Hogness. 1991. The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell 67:59-77. [DOI] [PubMed] [Google Scholar]
- 38.Kyba, M., and H. W. Brock. 1998. The Drosophila Polycomb group protein Psc contacts ph and Pc through specific conserved domains. Mol. Cell. Biol. 18:2712-2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laney, J. D., and M. D. Biggin. 1997. Zeste-mediated activation by an enhancer is independent of cooperative DNA binding in vivo. Proc. Natl. Acad. Sci. USA 15:3602-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehmann, M., T. Siegmund, K. Lintermann, and G. Korge. 1998. The Pipsqueak protein of Drosophila melanogaster binds to GAGA sequences through a novel DNA-binding domain. J. Biol. Chem. 273:28504-28509. [DOI] [PubMed] [Google Scholar]
- 41.Lewis, E. B. 1978. A gene complex controlling segmentation in Drosophila. Nature 276:565-570. [DOI] [PubMed] [Google Scholar]
- 42.McGinnis, W., and R. Krumlauf. 1992. Homeobox genes and axial patterning. Cell 68:283-302. [DOI] [PubMed] [Google Scholar]
- 43.Mihaly, J., R. K. Mishra, and F. Karch. 1998. A conserved sequence motif in Polycomb-response elements. Mol. Cell 1:1065-1066. [DOI] [PubMed] [Google Scholar]
- 44.Ng, J., C. M. Hart, K. Morgan, and J. A. Simon. 2000. A Drosophila ESC-E(Z) protein complex is distinct from other Polycomb group complexes and contains covalently modified ESC. Mol. Cell. Biol. 20:3069-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peterson, A., M. Kyba, D. Borneman, K. Morgan, H. Brock, and J. A. Simon. 1997. A domain shared by the Polycomb group proteins Scm and ph mediates heterotypic and homotypic interactions. Mol. Cell. Biol. 17:6683-6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pirrotta, V. 1997. Chromatin silencing mechanisms in Drosophila maintain patterns of gene expression. Trends Genet. 13:314-318. [DOI] [PubMed] [Google Scholar]
- 47.Pirrotta, V. 1991. The genetics and molecular biology of zeste in Drosophila melanogaster. Adv. Genet. 29:301-348. [DOI] [PubMed] [Google Scholar]
- 48.Pirrotta, V. 1998. Polycombing the genome: Pc-G, trx-G and chromatin silencing. Cell 93:333-336. [DOI] [PubMed] [Google Scholar]
- 49.Poux, S., D. McCabe, and V. Pirrotta. 2001. Recruitment of components of Polycomb group chromatin complexes in Drosophila. Development 128:75-85. [DOI] [PubMed] [Google Scholar]
- 50.Saurin, A. J., Z. Shao, H. Erdjument-Bromage, P. Tempst, and R. E. Kingston. 2001. A Drosophila Polycomb group complex includes zeste and dTFII proteins. Nature 412:655-660. [DOI] [PubMed] [Google Scholar]
- 51.Shao, Z., F. Raible, R. Mollaaghababa, J. R. Guyon, C.-T. Wu, W. Bender, and R. E. Kingston. 1999. Stability of chromatin structure by PRC1, a Polycomb complex. Cell 98:37-46. [DOI] [PubMed] [Google Scholar]
- 52.Siegel, V., T. A. Jongens, L. Y. Jan, and Y. N. Jan. 1993. Pipsqueak, an early acting member of the posterior group of genes, affects vasa level and germ cell-somatic cell interaction in the developing egg chamber. Development 119:1187-1202. [DOI] [PubMed] [Google Scholar]
- 53.Simon, J., A. Chiang, W. Bender, M. J. Shimell, and M. O'Connor. 1993. Elements of the Drosophila Bithorax complex that mediate repression by Polycomb group products. Dev. Biol. 158:131-144. [DOI] [PubMed] [Google Scholar]
- 54.Simon, J., M. Peifer, W. Bender, and M. O'Connor. 1990. Regulatory elements of the bithorax complex that control expression along the anterior-posterior axis. EMBO J. 9:3945-3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soeller, W. C., W. E. Oh, and T. B. Kornberg. 1993. Isolation of cDNAs encoding the Drosophila GAGA transcription factor. Mol. Cell. Biol. 13:7961-7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soto, M. C., T. B. Chou, and W. Bender. 1995. Comparison of germ-line mosaics of genes in the Polycomb group of Drosophila melanogaster. Genetics 140:231-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strutt, H., G. Cavalli, and R. Paro. 1997. Co-localization of Polycomb protein and GAGA factor on regulatory elements responsible for the maintenance of homeotic gene expression. EMBO J. 16:3621-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strutt, H., and R. Paro. 1997. The Polycomb group protein complex of Drosophila melanogaster has different compositions at different target genes. Mol. Cell. Biol. 17:6773-6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tie, F., T. Furuyama, J. Prasad-Sinha, E. P. Jane, and P. J. Harte. 2001. The Drosophila Polycomb group proteins ESC and E(Z) are present in a complex containing the histone-binding protein p55 and the histone deacetylase RPD3. Development 128:275-286. [DOI] [PubMed] [Google Scholar]
- 60.Tillib, S., S. Petruk, Y. Sedkov, A. Kuzin, M. Fujioka, T. Goto, and A. Mazo. 1999. Trithorax- and Polycomb-group response elements within an Ultrabithorax transcription maintenance unit consist of closely situated but separable sequences. Mol. Cell. Biol. 19:5189-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weber, U., V. Siegel, and M. Mlodzik. 1995. Pipsqueak encodes a novel nuclear protein required downstream of seven-up for the development of photoreceptors R3 and R4. EMBO J. 14:6247-6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White, R. A. and M. Wilcox. 1984. Protein products of the Bithorax complex in Drosophila. Cell 39:163-171. [DOI] [PubMed] [Google Scholar]
- 63.Wilkins, R. C., and J. T. Lis. 1998. GAGA factor binding to DNA via a single trinucleotide sequence element. Nucleic Acids Res. 1:2672-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu, C., R. S. Jones, P. F. Lasko, and W. M. Gelbart. 1989. Homeosis and the interaction of zeste and white in Drosophila. Mol. Gen. Genet. 218:559-564. [DOI] [PubMed] [Google Scholar]
- 65.Zink, B., and R. Paro. 1989. In vivo binding of a trans-regulator of homeotic genes in Drosophila melanogaster. Nature 337:468-471. [DOI] [PubMed] [Google Scholar]