Abstract
β-Arrestin-1 mediates agonist-dependent desensitization and internalization of G protein-coupled receptors (GPCRs) and is also essential for GPCR mitogenic signaling. In addition, insulin-like growth factor I receptor (IGF-IR) endocytosis is facilitated by β-arrestin-1, and internalization is necessary for IGF-I-stimulated mitogen-activated protein (MAP) kinase activation. Here, we report that treatment of cells for 12 h with insulin (100 ng/ml) induces an ∼50% decrease in cellular β-arrestin-1 content due to ubiquitination of β-arrestin-1 and proteosome-mediated degradation. This insulin-induced decrease in β-arrestin-1 content was blocked by inhibition of phosphatidylinositol-3 kinase (PI-3 kinase) and MEK with wortmannin and PD98059, respectively. We also found a marked decrease in the association of β-arrestin-1 with the IGF-IR and a 55% inhibition of IGF-I-stimulated MAP kinase phosphorylation. In insulin-treated, β-arrestin-1-downregulated cells, there was complete inhibition of lysophosphatidic acid (LPA) or isoproterenol (ISO)-stimulated MAP kinase phosphorylation. This was associated with a decrease in β-arrestin-1 association with the β2-AR as well as a decrease in β-arrestin-1-Src and Src-β2-AR association. Ectopic expression of wild-type β-arrestin-1 in insulin-treated cells in which endogenous β-arrestin-1 had been downregulated rescued IGF-I- and LPA-stimulated MAP kinase phosphorylation. In conclusion, we found the following. (i) Chronic insulin treatment leads to enhanced β-arrestin-1 degradation. (ii) This downregulation of endogenous β-arrestin-1 is associated with decreased IGF-I-, LPA-, and ISO-mediated MAP kinase signaling, which can be rescued by ectopic expression of wild-type β-arrestin-1. (iii) Finally, these results describe a novel mechanism for heterologous desensitization, whereby insulin treatment can impair GPCR signaling, and highlight the importance of β-arrestin-1 as a target molecule for this desensitization mechanism.
Heptahelical cell surface receptors are commonly referred to as G protein-coupled receptors (GPCRs), signifying their functional dependence on heterotrimeric G proteins to trigger biological effects (1, 26, 48). Receptor tyrosine kinases (RTKs) usually function as dimeric or tetrameric cell surface receptors and mediate signals through tyrosine phosphorylation events (10, 22, 52). However, recent evidence indicates that these two structural classes of receptors are not as functionally distinct as previously thought (5, 6, 13, 20, 27, 35). Thus, there is substantial evidence demonstrating that heptahelical receptors can mediate tyrosine kinase signaling events (6, 13, 26, 35). Lefkowitz and his colleagues have shown that after ligand binding and interaction with heterotrimeric G proteins, the β-adrenergic receptor (β2-AR) becomes phosphorylated on serine residues through the action of a specific G protein receptor kinase (GRK) (9, 26). At this point, β-arrestin-1, which serves as an adaptor or scaffold molecule and mediates receptor signaling and endocytosis, can bind to the phosphorylated β2-AR (4, 8, 11, 26, 28, 29, 37, 40). Since β-arrestin-1 is complexed with c-Src, this brings Src into physical association with β2-AR (31), resulting in activation of Src, initiating a tyrosine phosphorylation signaling cascade that leads to stimulation of the Ras/mitogen-activated protein (MAP) kinase pathway (31). Similar findings that other GPCRs initiate Src-mediated tyrosine phosphorylation signaling cascades have also been reported (30, 34). Therefore, heptahelical receptors do not simply function through heterotrimeric G proteins, but, in fact, mediate some of their signaling properties through tyrosine phosphorylation events involving the same downstream molecules engaged by RTKs (6, 13, 26, 35).
In a similar vein, there is now emerging evidence that RTKs can utilize heterotrimeric G proteins to subserve some of their biologic actions (5, 20, 27). For example, in many ways, the insulin-like growth factor I receptor (IGF-IR) fulfills the functional properties of a classical GPCR (5, 27). After ligand stimulation, it associates with a heterotrimeric G protein containing Gαi, as well as β-arrestin-1, and these molecules are essential for IGF-IR internalization, as well as IGF-I-stimulated mitogenic signaling (5, 27). Furthermore, recent evidence indicates that the insulin receptor can mediate metabolic events by signaling through a heterotrimeric G protein containing Gαq/11 (20), and earlier data suggested insulin receptor actions involving Gαi (33). These results indicate that the structurally distinct heptahelical and RTK receptors utilize many common downstream signaling molecules to fulfill their biologic repertoire.
Another common property of these two receptor classes is desensitization. There are many examples showing that ligand binding to its cognate receptor leads to homologous desensitization of that receptor's signaling pathway, and a great deal is known about some of the molecular mechanisms involved (1, 10, 22, 26, 39, 40). For example, it has been shown that the binding of β-arrestin to the cytoplasmic domain of the β2-AR mediates desensitization of that receptor to further ligand stimulation (26, 28, 40). This is because β-arrestin-1 prevents further binding of heterotrimeric G proteins to the GPCR and also mediates endocytosis of the receptor, and both of these events lead to desensitization (26, 28, 37, 40). Given the understanding that different classes of receptors can utilize common downstream signaling molecules, the possibility of heterologous desensitization emerges. Indeed, evidence exists that chronic stimulation with a particular ligand can lead to desensitization of a heterologous signaling pathway mediated by a different ligand (14, 21). In the present report, we have focused on heterologous desensitization mediated through β-arrestin-1, since this molecule can mediate both RTK and heptahelical receptor signals. Our studies show that insulin treatment leads to downregulation of β-arrestin-1, which can mediate desensitization of heptahelical as well as RTK receptor function.
MATERIALS AND METHODS
Materials.
Anti-β-arrestin-1, anti-ERK-1 antibody, anti-Shc protein antibodies, and IRS-1 antibodies were purchased from Transduction Laboratories (Lexington, Ky.). Horseradish peroxidase (HRP)-linked anti-rabbit anti- mouse antibodies, anti-insulin and anti-IGF-IR antibodies, antiubiquitin antibody, antiphosphotyrosine (PY-20) antibody, anti-Gαi and anti-Gβ antibodies, and protein A/G plus agarose were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Anti-Gβγ complex antibody was purchased from Calbiochem (San Diego, Calif.). Mouse monoclonal antiphosphotyrosine-p42/44 MAP (anti-phospho-p42/44) kinase antibody was purchased from New England Biolabs (Beverly, Mass.), and rabbit monoclonal anti-phospho-p42/44 MAP kinase antibody was obtained from Promega (Madison, Mass.). Sheep immunoglobulin G (IgG) and fluorescein isothiocyanate (FITC)- and tetramethyl rhodamine isothiocyanate (TRITC)-conjugated anti-rabbit, anti-mouse, and anti-sheep IgG antibodies were from Jackson Immunoresearch Laboratories, Inc. (West Grove, Pa.). Dulbecco's modified Eagle's medium (DMEM) and fetal calf serum (FCS) were purchased from Life Technologies (Grand Island, N.Y.). Polyvinylidene difluoride (PVDF) membranes (Immobilon-P) were purchased from Millipore (Bedford, Mass.). Radioisotopes were obtained from ICN (Costa Mesa, Calif.). For transfection experiments, SuperFECT was purchased from Qiagen (Valencia, Calif.). Cycloheximide and lactacystin were obtained from Calbiochem (San Diego, Calif.). Lysophosphatidic acid (LPA), isoproterenol (a β2-AR agonist), and all other reagents were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Cell culture.
Rat-1 fibroblasts overexpressing the human form of the insulin receptor (HIRcB cells) were maintained as previously described (32) in DMEM/F12 with 50 U of penicillin per ml, 50 μg of streptomycin per ml, 10% FCS, 0.5% Glutamax, and 0.5% methotrexate in a 5% CO2 environment at 37°C. Cultures were never allowed to become completely confluent. 3T3-L1 adipocytes were cultured and differentiated as previously described (16).
Western blotting.
Subconfluent HIRcB cells (70% confluent) were starved for 24 h in serum free DMEM/F12 with 0.1% bovine serum albumin (BSA) and incubated in the presence of insulin (100 ng/ml) for various time points (0 to 24 h) as indicated in the figure legends. At the end of the chronic incubation period, cells were extensively washed with phosphate-buffered saline (PBS) and then acutely stimulated with 100 ng of IGF-I per ml, 10 μM LPA, or 10 μM isoproterenol for 5 min at 37°C. After stimulation, cells were lysed for 30 min at 4°C in a solubilizing buffer containing 50 mM HEPES, 1 mM EDTA, 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride (PMSF), 30 mM PyroPO4, 10 mM NaF, and 1 mg of bacitracin per ml. Cell lysates were then centrifuged at 13,000 × g for 30 min to remove insoluble materials. For Western blot analysis, cell lysates (25 to 50 μg of protein per lane) were denatured by boiling in Laemmli sample buffer containing 100 mM dithiothreitol and 2-mercaptoethanol and resolved by SDS-polyacrylamide gel electrophoresis (PAGE). Gels were transferred to PVDF membrane with a Transblot apparatus (Bio-Rad, Hercules, Calif.). For immunoblotting, membranes were blocked and probed with specified antibodies. Blots were then incubated with HRP-linked second antibody followed by enhanced chemiluminescence (ECL) detection, according to the manufacturer's instructions (Pierce Chemical Co., Rockford, Ill.). For inhibitor treatments, serum-starved HIRcB cells were incubated with 100 ng of insulin per ml for 6 h or 100 ng of IGF-I per ml for 8 h in the presence of the phosphatidylinositol-3 kinase (PI-3 kinase) inhibitor wortmannin (100 nM), the MEK inhibitor PD98059 (20 μM), cycloheximide (10 μg/ml), or actinomycin D (2 μg/ml). The 26S proteasome inhibitor lactacystin (50 μM) was added for 1 h before the insulin treatment. All of the inhibitors used were tested alone and did not have an independent effect on β-arrestin-1 cell content. In all experiments, samples were normalized for protein content by Bradford protein assay.
Immunoprecipitation.
Serum-starved HIRcB cells were incubated for 12 h with 100 ng of insulin per ml and then acutely stimulated with 100 ng of IGF-I per ml at 37°C for 5 min. Cells were washed twice with ice-cold PBS and lysed for 30 min at 4°C in the solubilizing buffer, and lysates were subjected to immunoprecipitation. After centrifugation at 14,000 rpm for 30 min, supernatants (200 to 350 μg of total protein) were incubated with primary antibody for 4 h at 4°C. Immunocomplexes were then precipitated from the supernatant with protein A/G plus agarose, washed three times with ice-cold cell lysis buffer, boiled for 3 min in Laemmli sample buffer, and resolved by SDS-PAGE. Samples were normalized for protein content by Bradford protein assay in all experiments performed.
Immunostaining for phospho-p42/44 MAP kinase.
Cells were grown on a glass coverslip to 50% confluency and then starved in serum-free medium for 12 h and subjected to 12 h of insulin incubation (100 ng/ml). Cells were then stimulated with 100 ng of IGF-I per ml, 10 μM LPA, or 10 μM epidermal growth factor (EGF) for 5 min, fixed in 3.7% formaldehyde in PBS for 10 min, and washed and permeabilized with a mixture containing 0.1 M Tris-HCl (pH 7.4), 150 mM NaCl, and 0.1% Triton X-100 (TBST buffer). After incubation in blocking buffer (5% normal horse serum in TBST buffer) for 1 h at room temperature, cells were exposed to the anti-phospho-p44/42 MAP kinase primary antibody in 5% bovine serum albumin (BSA)-TBST buffer overnight at 4°C (1:400 dilution). After washing, anti-phospho-p44/42 MAP kinase antibody was incubated with HRP-conjugated donkey anti-mouse antibody in 1% BSA-TBST buffer (1:2,000 dilution) and visualized by diaminobenzidine (DAB)-based HRP reaction (DAB substrate kit; Pierce, Rockford, Ill.), followed by observation under a microscope. Mouse monoclonal and rabbit polyclonal phospho-p44/42 MAP kinase antibodies react specifically with phosphorylated MAP kinase and do not cross-react with the non-tyrosine-phosphorylated form of MAP kinase by Western blotting.
Transfection.
Transient transfection into HIRcB cells was performed with SuperFECT in accordance with the manufacturer's instructions. Cells were reseeded in complete culture medium. After 8 h, when the confluency of cells was nearly 30 to 40%, transfection was performed. Transfected vectors (control and wild-type) were removed 4 h later (time of transfection procedure, 4 h). Cells were then cultured for 12 h in complete culture medium to allow cell multiplication and wild-type β-arrestin-1 expression in this population of cells. Reaching the confluency of 60 to 70%, cells were starved, and insulin (100 ng/ml) was added for 12 h. Finally, cells were acutely stimulated with IGF-I (100 ng/ml) or LPA (10 μM) for 5 min.
Binding studies.
HIRcB cells (20% confluent), plated in 12-well plates, were incubated in a final volume of 500 μl with 0.03 nM 125I-IGF-I in binding buffer (500 mM HEPES, 0.13 M NaCl, 5 mM KCl, 1 mM MgSO4, 2.5 mM CaCl2 containing 1% BSA [pH 7.4]), with or without increased insulin concentration. After 3 h of incubation at 10oC, cells were washed three times with ice-cold PBS and solubilized with 1 N NaOH for determination of surface-bound radioactivity with a gamma counter. Nonspecific binding was determined in the presence of 0.1 μM IGF-I.
Microinjection experiments.
Serum-starved HIRcB cells were pretreated with insulin for 8 h and then microinjected with a reporter plasmid in which expression of β-galactosidase is driven by a multimerized AP-1 activation site (4× TRE) (23) at a concentration of 100 μg/ml mixed with TRITC-conjugated dextran for detection. The cells were subsequently stimulated with growth factors for 2 h to allow expression of the reporter gene. The cells were then fixed and stained as described by Rose et al. (44). Following staining, expression was quantitated with an epifluorescence microscope as described by Lavinsky et al. (24). Any degree of staining was counted as a positive result.
Statistical analyses.
Values are expressed as means ± standard errors. Results were analyzed by using the Student t test. A value of P < 0.05 was considered significant.
RESULTS
Insulin treatment decreases β-arrestin-1 expression.
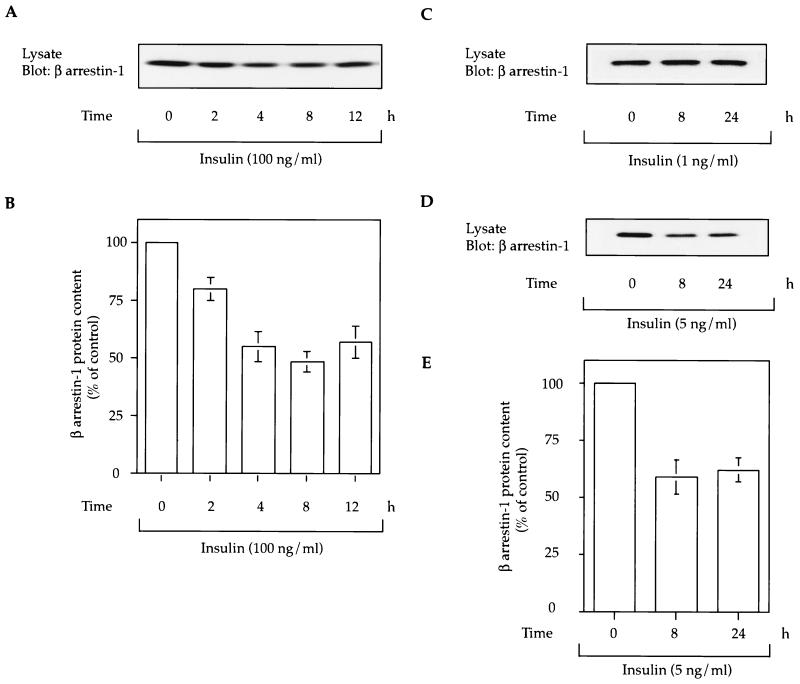
HIRcB cells were treated with insulin (1, 5, or 100 ng/ml) followed by Western blotting of cell lysates to measure β-arrestin-1 protein content. As seen in Fig. 1, insulin had a time- and dose-dependent effect in decreasing β-arrestin-1 levels. While a 1-ng/ml insulin pretreatment was without effect, 5 ng of insulin per ml led to an approximately 40% decrease in β-arrestin-1 cell content at 8 and 24 h, and 100 ng of insulin per ml led to an even greater effect, which was maximal at 8 to 12 h of treatment. To verify that insulin was acting through the insulin receptor rather than the IGF-IR, we determined whether insulin binds to and tyrosine phosphorylates the IGI-IR. We found that 100 ng of insulin per ml caused only a 10% displacement of 125I-IGF-I binding (not significant) and did not induce any measurable tyrosine phosphorylation of the IGF-IR (data not shown). These results suggest that the insulin-induced decrease in β-arrestin-1 is mediated by the insulin receptor and requires >4 h of cell exposure to insulin.
FIG. 1.
Insulin leads to a time-dependent decrease in cellular β-arrestin-1 content. (A, C, and D) HIRcB cells were starved for 24 h in serum-free culture medium with 0.1% BSA in the presence or absence of insulin (1, 5, or 100 ng/ml) for various times as indicated and lysed as described in Materials and Methods. Fifty micrograms of lysate was subjected to SDS-PAGE (10% polyacrylamide) and immunoblotted with anti-β-arrestin-1 monoclonal antibody. A typical exposure representative of five experiments is shown. (B and E) β-Arrestin-1 protein content at various times of insulin treatment, expressed as percentage of the basal β-arrestin-1 protein level (time 0 h). These values were obtained from quantitative results of three to five independent experiments. Gels were scanned and analyzed with the NIH Image program 6.0.
Insulin treatment decreases the association of β-arrestin-1 with the IGF-IR.
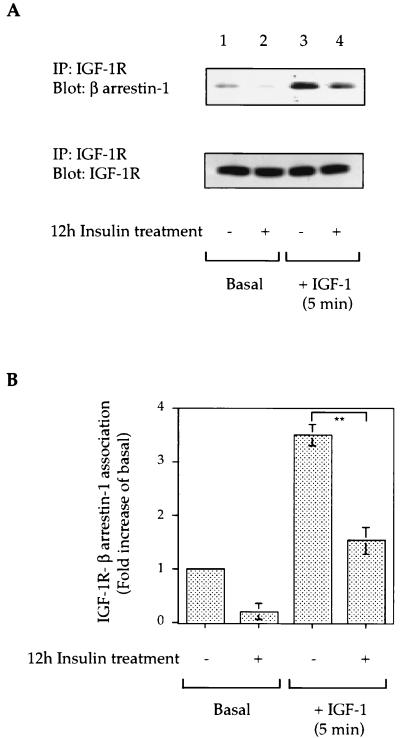
It is known that β-arrestin-1 associates with the IGF-IR in a ligand-dependent manner; this promotes receptor internalization and is essential for Shc phosphorylation and subsequent MAP kinase activation (2, 5, 27). Therefore, since insulin treatment for 12 h induced a 45 to 50% decrease in cellular β-arrestin-1 content (Fig. 1A and B), we assessed whether this inhibited the interaction between the IGF-IR and β-arrestin-1. As shown in Fig. 2, β-arrestin-1 is associated with the IGF-IR in the basal state (lane 1), and this is increased >10-fold at 5 min of IGF-I stimulation (lane 3). After 12 h of insulin treatment, the basal (lane 2) and agonist-stimulated IGF-IR-β-arrestin-1 interactions (lane 4) were decreased by 75 and 78%, respectively (Fig. 2). In control studies, we found that preincubation with insulin for 12 h had minimal effects on 125I-IGF-I binding to the cells and did not alter IGF-IR expression (Fig. 2A).
FIG. 2.
Effect of insulin treatment on IGF-IR-β-arrestin-1 interactions. (A) Serum-starved HIRcB cells were incubated with or without 100 ng of insulin per ml for 12 h as indicated, washed, and stimulated with 100 ng of IGF-I per ml at 37°C for 5 min. Cells were then lysed, and lysates (300 μg of protein) were subjected to immunoprecipitation (IP) under reducing conditions with an anti-IGF-IR β-subunit antibody. Immunoprecipitated proteins were resolved by SDS-PAGE (10% polyacrylamide) and blotted with anti-β-arrestin-1 antibody and anti-IGF-IR β-subunit antibody. Representative exposures are shown. (B) IGF-IR-β-arrestin-1 association deduced from quantitative results of three representative IGF-IR immunoprecipitation experiments. ∗∗, P < 0.01.
Effect of 12-h insulin treatment on MAP kinase phosphorylation.
Since β-arrestin-1 is important for IGF-I- and GPCR-induced mitogenic signaling (4, 5, 26, 27, 29), we measured IGF-I- and GPCR-stimulated MAP kinase activity in β-arrestin-1-downregulated cells. These experiments were performed in two ways. First, we assessed phospho-MAP kinase immunoblots of cell lysates. Second, we developed a visual cell assay by immunostaining of coverslips with the same antibody to determine the proportion of cells positive for phospho-MAP kinase. Studies were conducted before and after ligand stimulation in control and β-arrestin-1-downregulated (12-h insulin treatment) cells.
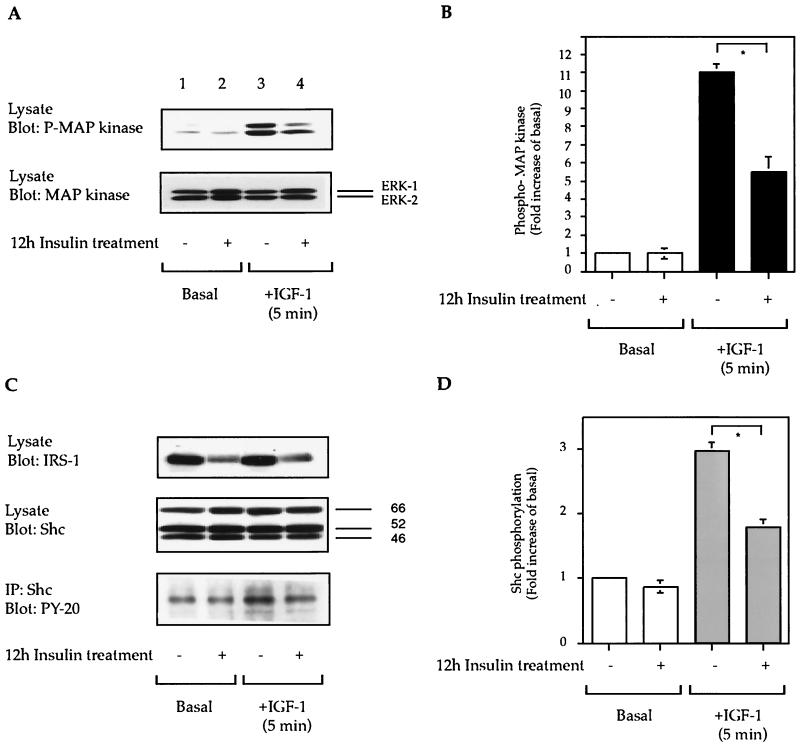
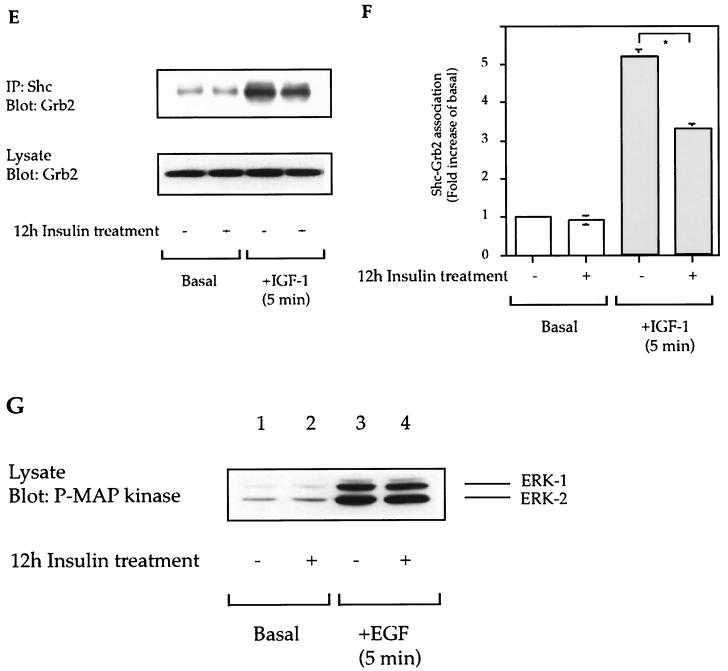
As shown in Fig. 3A and B, a 12-h preincubation period with insulin led to a 50% ± 5% inhibition of IGF-I-stimulated MAP kinase phosphorylation (lane 3 compared to lane 4). The total cellular levels of MAP kinase (Fig. 3A) and Shc (Fig. 3C) were unchanged by insulin treatment, whereas IRS-1 expression was decreased (Fig. 3C). Although the cellular levels of Shc were unchanged, IGF-I-stimulated tyrosine phosphorylation of Shc and Shc-Grb2 association were decreased in 12-h insulin-treated cells (Fig. 3C to F). Finally, we have recently shown that the mitogenic effects of EGF are independent of a β-arrestin-1 requirement (5). Thus, we measured EGF-stimulated MAP kinase phosphorylation and found it to be unaffected by insulin treatment and β-arrestin-1 downregulation (Fig. 3G), indicating that the other elements of the MAP kinase signaling pathway were intact.
FIG. 3.
Effect of insulin treatment on IGF-I- and EGF-stimulated MAP kinase phosphorylation. Starved HIRcB cells were treated or not for 12 h with 100 ng of insulin per ml as indicated, washed, and then stimulated with 100 ng of IGF-I per ml (A, C, and E) or 10 μM EGF (G) for 5 min at 37°C. Cell lysates were subjected to SDS-PAGE (7.5 to 10% polyacrylamide) and then immunoblotted with antiphosphotyrosine, anti-MAP kinase, anti-IRS-1, anti-Shc, and anti-Grb2 antibodies (A, C, E) or immunoprecipitated (IP) with anti-Shc antibody (C and E) as described in Materials and Methods. Typical autoradiographs representative of three to five experiments are shown. (B, D, and F) Level of MAP kinase phosphorylation (B), level of tyrosine phosphorylation of Shc (D), and Shc-Grb2 association (F), deduced from quantitative results of five representative experiments obtained with the NIH Image program. ∗, P < 0.05 versus IGF-I-stimulated MAP kinase phosphorylation (B), IGF-I-stimulated Shc tyrosine phosphorylation (D), or IGF-I-stimulated Shc-Grb2 association (F).
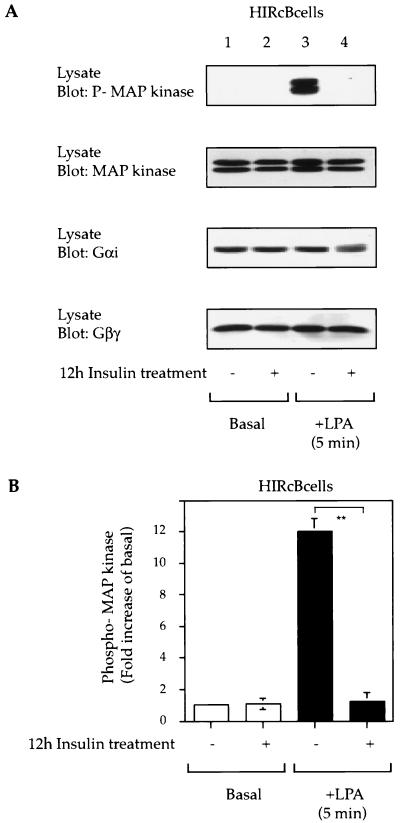
Insulin treatment inhibits GPCR-stimulated mitogenic signaling.
A wide variety of GPCRs can activate MAP kinase leading to mitogenic responses, and these signaling events are dependent on β-arrestin-1 function (4, 6, 13, 26, 29, 35, 53). Therefore, we measured the ability of the Gαi-coupled LPA receptor (30) to mediate MAP kinase phosphorylation in β-arrestin-1-downregulated cells. As shown in Fig. 4A, LPA stimulation led to MAP kinase phosphorylation in HIRcB cells, and chronic insulin treatment completely inhibited this response. Since Gαi and Gβγ subunits are essential for the activation of MAP kinase by LPA (30), we examined the expression of Gαi and Gβγ protein and found that the levels were the same with or without insulin treatment (Fig. 4A).
FIG. 4.
Chronic insulin treatment desensitizes LPA-mediated MAP kinase phosphorylation. (A) Serum-starved HIRcB cells were treated or not for 12 h with 100 ng of insulin per ml prior to stimulation with 10 μM LPA for 5 min at 37°C. Cell lysates were then subjected to SDS-PAGE (7.5 to 10% polyacrylamide) and immunoblotted with anti-phospho-MAP kinase, anti-MAP kinase protein (Erk 1/2), and anti-Gαi, and anti-Gβγ antibodies. Representative exposures are shown. (B) Graph showing MAP kinase phosphorylation from the results of five experiments obtained with the NIH Image program. ∗∗, P < 0.01.
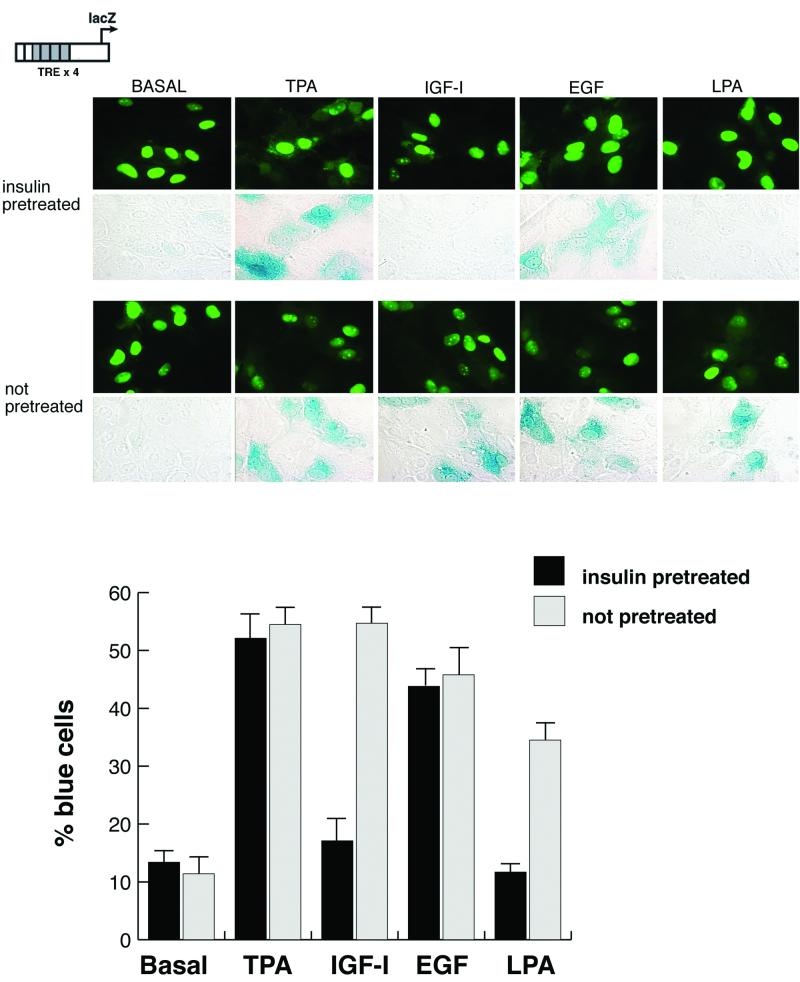
To extend these results by using a different approach, cells were microinjected with a promoter-reporter DNA construct that leads to the expression of β-galactosidase in cells in which AP-1, a direct nuclear target of MAP kinases, has been activated (23). The cells were starved overnight and then pretreated for 8 h with insulin. The reporter was then microinjected, and the cells were stimulated for 2 h with growth factors. Pretreatment of cells with insulin led to a dramatically reduced response of the reporter to both LPA and IGF-I, whereas the response to TPA and EGF was unaffected (Fig. 5). In quiescent cells not treated with insulin, there was a normal response to each of these agents, as has been reported previously (23). These results support the previous observations and represent an additional measure of MAP kinase activation at the level of gene expression.
FIG. 5.
AP-1 activity is downregulated in cells after chronic insulin treatment. Serum-starved HIRcB cells were microinjected with a reporter construct that indicates the activation of AP-1 (TRE ×4). Cells were pretreated with or without 100 ng of insulin per ml for 8 h. Fluorescent-labeled dextran was used as a carrier to indicate the injected cells. After a short recovery period, the cells were then stimulated with the indicated growth factors for 2 h. They were then fixed and stained for β-galactosidase expression. Quantitation is based upon the percentage of fluorescent cells in which there is detectable blue staining. Each parameter was performed at least twice, with approximately 300 cells injected in each experiment.
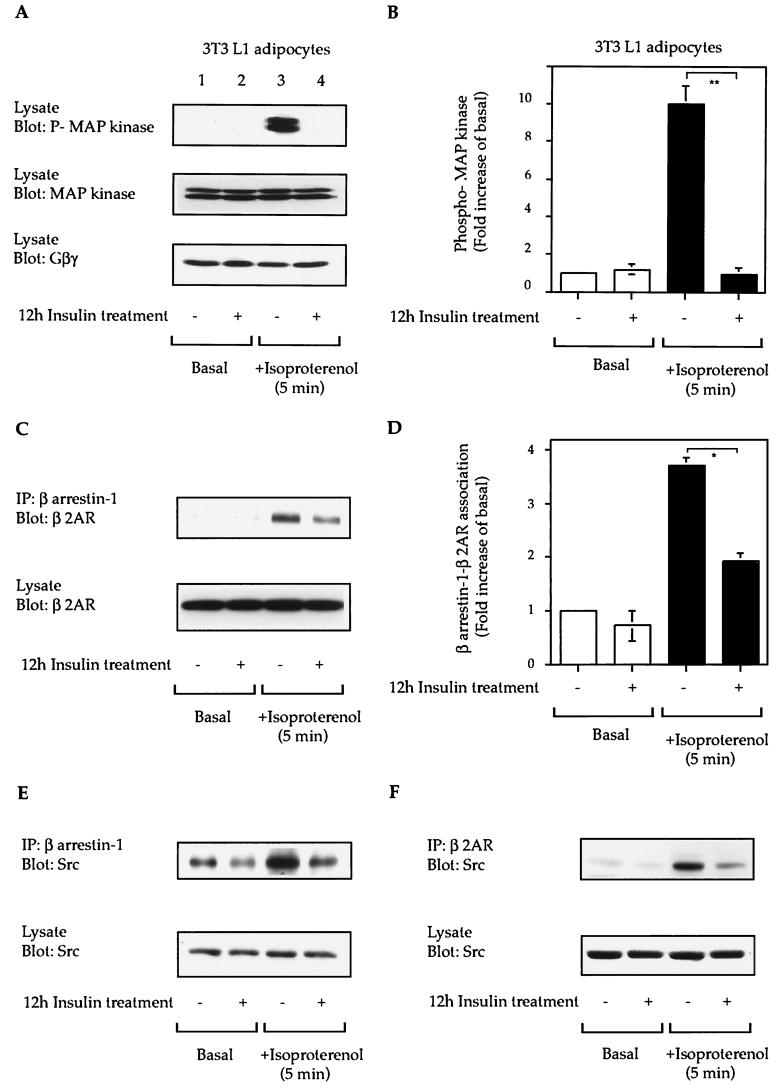
β-AR-mediated MAP kinase activation.
Using 3T3-L1 adipocytes, which express endogenous β2-AR and insulin receptors, we found that insulin treatment completely blocked isoproterenol-stimulated-MAP kinase phosphorylation (Fig. 6A and B). Agonist stimulation leads to the association of the β2-AR with β-arrestin-1 and then recruits Src into this complex, and these events are critical for β2-AR-mediated MAP kinase activation (29). We assessed this in insulin-treated cells and found a 60% decrease in β2-AR-β-arrestin-1 association following isoproterenol stimulation in the β-arrestin-downregulated cells (Fig. 6C and D). This decrease in β-arrestin-1-β2-AR association was accompanied by decreased β-arrestin-1-Src and Src-β2-AR associations (Fig. 6E, F, and G) in isoproterenol-stimulated β-arrestin-1-downregulated 3T3-L1 adipocytes.
FIG. 6.
Chronic insulin treatment desensitizes β2-AR-mediated MAP kinase phosphorylation. Starved 3T3-L1 adipocytes were treated or not for 12 h with 100 ng of insulin per ml prior to stimulation with 10 μM isoproterenol (C) for 5 min at 37°C. Cell lysates were then subjected to SDS-PAGE (7.5 to 10% polyacrylamide) and then immunoblotted with anti-phospho-MAP kinase, anti-MAP kinase protein (Erk 1/2), and anti-Gβγ antibodies (A) or immunoprecipitated with anti-β-arrestin-1 (C and D) or anti-β2-AR (F) antibodies as indicated. (B) MAP kinase phosphorylation from quantitative results of five experiments obtained with the NIH Image program. ∗∗, P < 0.01. (D) β-Arrestin-1-β2-AR association from quantitative results of three experiments. ∗, P < 0.05
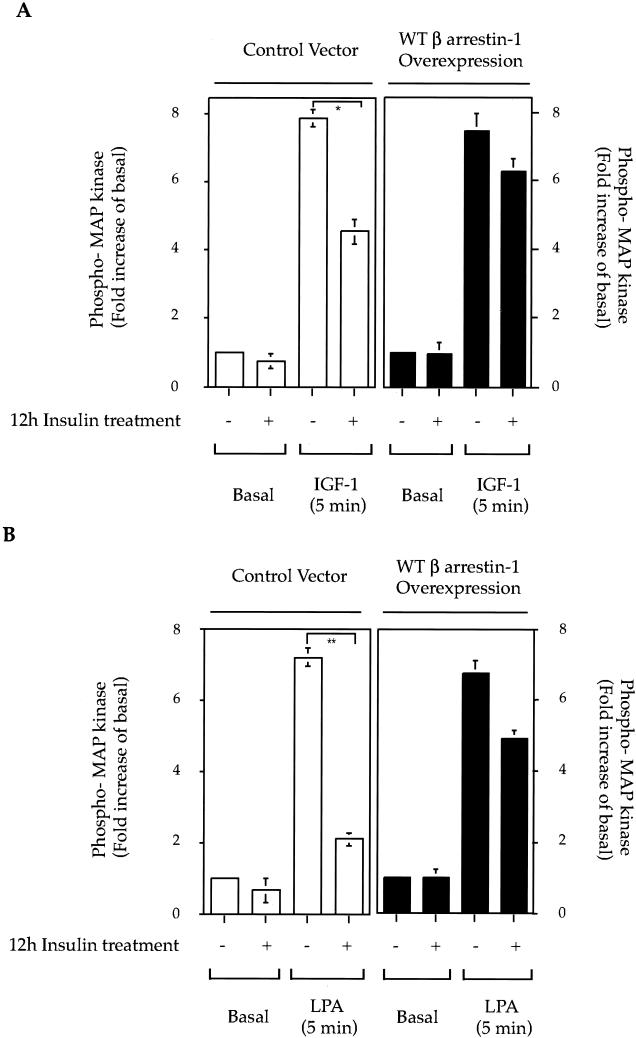
Overexpression of wild-type β-arrestin-1 rescues the signaling defect in β-arrestin-1-downregulated cells.
To test the hypothesis that the inhibition of IGF-I- and LPA-stimulated MAP kinase phosphorylation observed after insulin pretreatment is due to the lack of β-arrestin-1, we expressed wild-type β-arrestin-1 in insulin-treated HIRcB cells. As seen in Fig. 7A, IGF-I-stimulated MAP kinase phosphorylation was significantly decreased (45% ± 5%) in insulin-treated cells, while wild-type β-arrestin-1 overexpression largely restored this response. Comparable results were seen for LPA-stimulated MAP kinase phosphorylation. Thus, insulin treatment led to a 79% inhibition of LPA-induced MAP kinase phosphorylation, which was rescued by 75% with wild-type β-arrestin-1 overexpression. It should be noted that these were transient transfection experiments, and the transfection efficiency in the HIRcB cells is 60 to 80%, which can explain why the rescue of MAP kinase signaling was not complete.
FIG. 7.
Overexpression of wild-type β-arrestin-1 rescues IGF-I- and LPA-induced MAP kinase signaling in β-arrestin-1-downregulated cells. HIRcB cells (30 to 40% confluent) were transiently transfected with blank vector as control or wild type (WT) β-arrestin-1. Four hours later, cells were washed and cultured for 12 h in complete culture medium to allow cell multiplication and wild-type β-arrestin-1 expression. Cells were then starved, and insulin (100 ng/ml) was added for 12 h. After the insulin pretreatment, cells were then stimulated with IGF-I (100 ng/ml) (A) or LPA (10 μM) (B) for 5 min. Graphs depict MAP kinase phosphorylation from quantitative results obtained with the NIH Image program from three independent experiments. ∗, P < 0.05; ∗∗, P < 0.01.
Insulin-induced β-arrestin-1 degradation is mediated by the 26S proteasome pathway.
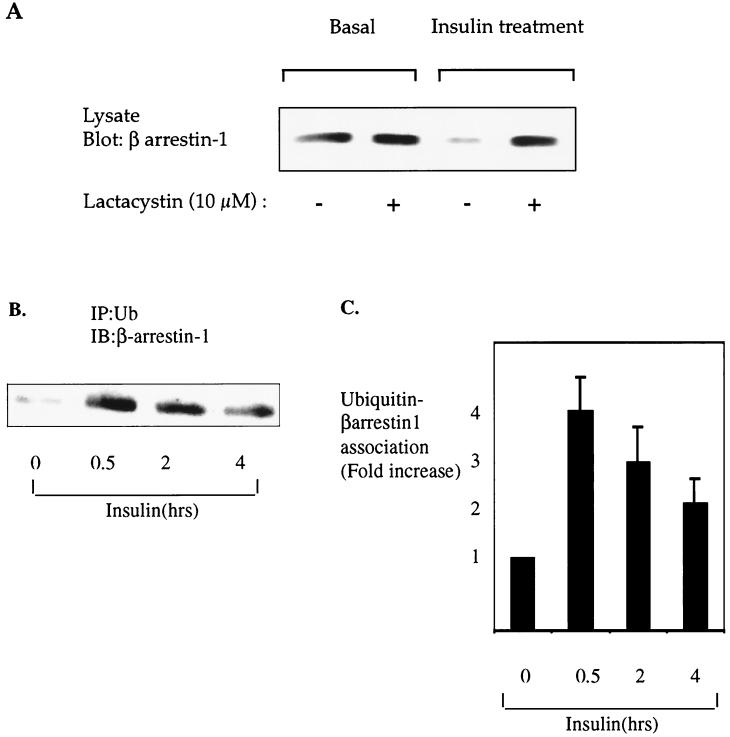
An important and highly regulated mechanism for degradation of signaling molecules involves the ubiquitin-mediated 26S proteasome (3, 7, 19, 36, 38). To investigate if insulin-induced β-arrestin-1 degradation is mediated by the 26S proteasome pathway, we preincubated HIRcB cells with or without the specific proteasome inhibitor lactacystin (7) before insulin treatment. As shown in Fig. 8A, lactacystin completely blocked β-arrestin-1 degradation induced by insulin treatment.
FIG. 8.
β-Arrestin-1 is degraded by the ubiquitin-mediated 26S proteasome pathway in response to insulin treatment. (A) Starved HIRcB cells were pretreated with dimethyl sulfoxide as a control or lactacystin (10 μM) for 1 h before a 12-h exposure to 100 ng of insulin per ml. Cells were then lysed as described in Materials and Methods, and 25 μg of protein was separated by SDS-PAGE (10% polyacrylamide) and immunoblotted with an anti-β-arrestin-1 monoclonal antibody. (B) Serum-starved HIRcB cells were incubated for the indicated times with 100 ng of insulin per ml and lysed, and 250 μg of total protein was subjected to immunoprecipitation (IP) in nonreduced conditions with an antiubiquitin (Ub) antibody. Immunoprecipitated proteins were resolved by SDS-PAGE (10% polyacrylamide) and immunoblotted with an anti-β-arrestin-1 antibody. A representative exposure is shown. (C) Ubiquitin and β-arrestin-1 association from quantitative results of three experiments obtained with the NIH Image program.
Since proteins are targeted for 26S proteasome-mediated degradation by covalent attachment of a 76-amino-acid polypeptide termed ubiquitin (38), we next sought to establish whether β-arrestin-1 becomes ubiquitinated upon insulin treatment. As seen in Fig. 8B and C, we found that insulin treatment of HIRcB cells increased the association of β-arrestin-1 and ubiquitin in a time-dependent manner.
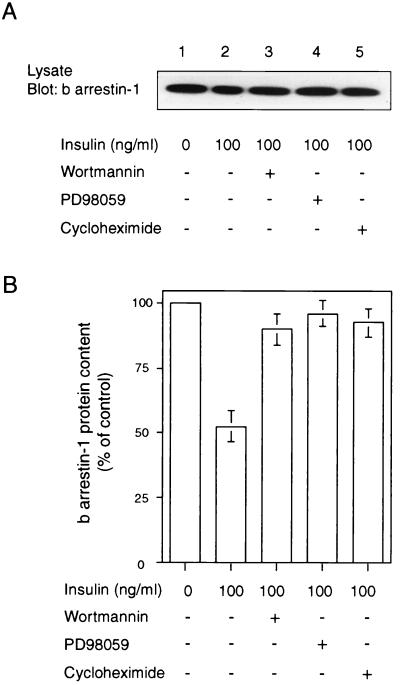
Mechanisms involved in insulin-induced β-arrestin-1 degradation.
To assess the signaling pathway or pathways that mediate insulin-induced β-arrestin-1 degradation, HIRcB cells were incubated with insulin in the presence of the PI-3 kinase inhibitor wortmannin (100 nM), the MEK inhibitor PD98059 (20 μM), and cycloheximide (10 μg/ml). The β-arrestin-1 protein level was then measured by immunoblot analysis. The PI-3 kinase inhibitor, the MEK inhibitor, and cycloheximide all prevented insulin-induced β-arrestin-1 degradation (Fig. 9A and B).
FIG. 9.
Insulin-induced β-arrestin-1 degradation signaling. (A) Serum-starved HIRcB cells were incubated for 6 h with 100 ng of insulin per ml without (control) or with wortmannin (100 nM), PD98059 (20 μM), or cycloheximide (10 μg/ml). Thirty micrograms of cell lysates was subjected to SDS-PAGE (10% polyacrylamide) and immunoblotted with an anti-β-arrestin-1, anti-MAP kinase, or anti-phospho-MAP kinase antibody. A typical autoradiograph representative of three to seven experiments is shown. (B) Quantitative results of three to five experiments obtained with the NIH Image program.
DISCUSSION
In this report, we show that insulin treatment leads to β-arrestin-1 downregulation through ubiquitination and proteasomal degradation in rat fibroblasts and 3T3-L1 adipocytes. Since β-arrestin-1 is a critical signaling molecule for the IGF-IR, as well as for a variety of heptahelical receptors, this insulin-induced decrease in β-arrestin-1 content has several important functional consequences. Thus, IGF-I-, LPA-, and isoproterenol-mediated MAP kinase stimulation are blunted in β-arrestin-1-downregulated cells, and this desensitization can be substantially restored by exogenous expression of wild-type β-arrestin-1. Taken together, these data indicate that insulin treatment leads to heterologous desensitization of IGF-I, LPA, and β2-AR signaling and that the insulin-induced decrease in β-arrestin-1 content is an important mechanism responsible for this effect.
Our studies demonstrate that insulin treatment of HIRcB cells or 3T3-L1 adipocytes leads to a time-dependent decrease in total cellular β-arrestin-1 content, which is maximal by 8 to 12 h. This effect of insulin is mediated through the classical insulin signaling molecules involved in the PI-3 kinase and MAP kinase pathways, since the effect is prevented by blocking PI-3 kinase and MEK with wortmannin and PD98059, respectively. The rapidity of the β-arrestin-1 downregulation suggests a process related to β-arrestin-1 protein turnover, and this is consistent with a mechanism involving enhanced β-arrestin-1 degradation. Indeed, our findings support this concept, since we observed that insulin treatment leads to an increase in ubiquitination of β-arrestin-1 and that inhibition of the proteasomal degradation pathway with lactacystin completely blocks the effect of insulin to cause β-arrestin-1 downregulation. Based on these results, it would seem that enhanced β-arrestin-1 degradation is at least one mechanism for the insulin-induced downregulation of this protein. It is interesting to note that isoproterenol stimulation can also lead to β-arrestin-1 ubiquitination, as recently reported by Shenoy et al. (47). Cycloheximide treatment also blocked the effect of insulin to downregulate β-arrestin-1, indicating the requirement for protein synthesis in this process. Since cycloheximide can also inhibit insulin receptor internalization and degradation (17), it is possible that the process of insulin receptor endocytosis might be involved in the effect of insulin to downregulate β-arrestin-1.
The major findings of this paper are that chronic insulin treatment leads to downregulation of β-arrestin-1, which is associated with a decrease in IGF-I, LPA, and β2-AR stimulation of MAP kinase activation, and this deficit can be substantially rescued by expression of exogenous wild-type β-arrestin-1 in insulin-treated cells. This indicates that the insulin-induced decrease in cellular β-arrestin-1 content plays a significant role in the impaired MAP kinase signaling, and there are several issues related to this possibility which should be noted. The effects of EGF to stimulate MAP kinase were not impaired in the insulin-treated β-arrestin-downregulated cells. Since we have previously shown that microinjection of anti-β-arrestin-1 antibodies blocks LPA, β2-AR, and IGF-I-stimulated DNA synthesis, but has no effect on EGF-induced mitogenesis (5), this would indicate that EGF does not utilize β-arrestin-1 in this signaling pathway. Therefore, the lack of an effect of insulin treatment on EGF-stimulated MAP kinase phosphorylation is fully consistent with the microinjection data (5), and demonstrates that the signaling molecules downstream of the EGF receptor involved in stimulation of MAP kinase are functionally unimpaired by insulin treatment, consistent with the role of decreased β-arrestin-1 content in this heterologous desensitization process. Expression of wild-type β-arrestin-1 in the β-arrestin-1-downregulated cells largely, but not fully, restored the effects of IGF-I and LPA to activate MAP kinase. The observation that the rescue is incomplete could be explained by the fact that β-arrestin-1 transfection efficiency is only 60 to 80%. Therefore, a certain proportion of the cells do not express the exogenous wild-type β-arrestin-1 and would still show impaired MAP kinase signaling. The β-arrestin-1 transfection studies were performed with HIRc cells, in which IGF-I and LPA, but not β2-AR, signaling can be readily measured. β2-AR action is assessed in 3T3-L1 adipocytes, but these cells cannot be efficiently transiently transfected, precluding β-arrestin-1 “rescue” experiments.
β-Arrestin-1 subserves a number of functions in cell surface signaling. For example, β-arrestin-1 is the key initiator of homologous agonist-mediated GPCR desensitization (8, 11, 26, 28, 37, 40). This occurs through a two-step process in which agonist-occupied GPCRs are phosphorylated by GRKs, which then allows association of β-arrestin-1 with the GPCR (8, 9, 11, 26, 28, 37, 40). The binding of β-arrestin-1 sterically hinders further association of heterotrimeric G proteins, inhibiting further GPCR signaling (8, 11, 26, 28, 37, 40). β-Arrestin-1 can also serve as an adaptor protein to clathrin-coated pits, mediating internalization and sequestration of the GPCR, leading to further desensitization (8, 11, 26, 28, 37, 40). In addition to association with the phosphorylated GPCR, β-arrestin-1 also physically binds to c-Src, resulting in the recruitment of Src kinase to the GPCR (29). In some way, this event activates c-Src, leading to phosphotyrosine signaling and activation of the Ras/MAP kinase pathway (4, 26, 29). Finally, while GPCR internalization desensitizes the classical G protein signals mediated through heterotrimeric G proteins, internalization is also a necessary step for complete activation of the MAP kinase pathway, as has been shown for β2-AR and IGF-IR (5, 8, 11, 26-29, 37, 40). β-Arrestin-1 couples these receptors to the clathrin-mediated internalization process and may also mediate endocytosis of additional upstream compartments of the MAP kinase signaling system, such as Shc, Ras, Raf, and MEK (27), which are essential for full MAP kinase phosphorylation.
It is interesting to note that the insulin-induced downregulation of β-arrestin-1 was approximately 50%, whereas the inhibition of β2-AR- and LPA-stimulated MAP kinase signaling was 90 to 100%. This greater inhibition of GPCR-mediated MAP kinase signaling compared to IGF-I signaling may relate to the different mechanisms of MAP kinase activation induced by GPCRs versus RTKs. For IGF-I, the receptor functions as a tyrosine kinase initiating MAP kinase signaling, and the importance of β-arrestin-1 in this process most likely relates to IGF-IR internalization, as well as internalization of other components of the Ras/MAP kinase pathway. For the action of GPCR, the concept that insulin-induced β-arrestin-1 downregulation is responsible for heterologous desensitization of LPA and β2-AR signaling seems straightforward. Thus, downregulation of β-arrestin-1 could inhibit internalization of the GPCR, as well as other MAP kinase signaling components, impairing the downstream signaling events that propagate MAP kinase activation. In addition, β-arrestin-1 downregulation leads to a decrease in isoproterenol-stimulated association of β-arrestin-1 with the β2-AR, which in turn impairs recruitment of Src to the GPCR, and this would inhibit tyrosine phosphorylation-mediated activation of the Ras/MAP kinase pathway. Since both of these actions (impaired internalization and inhibition of Src signaling) would apply to the LPA and β2-AR receptors, this could explain why the effects of insulin in decreasing MAP kinase signaling are greater for LPA and isoproterenol stimulation than for IGF-I. It is also possible that the residual β-arrestin-1 in the insulin-treated cells is not fully functional with respect to recruitment and activation of Src kinase. Indeed, this is suggested in Fig. 6, which shows a greater than 50% reduction in β-arrestin-1 association with Src and in Src recruitment to the β2-AR. The importance of decreased β-arrestin-1 in this heterologous desensitization mechanism is further supported by our findings that ectopic expression of wild-type β-arrestin-1 largely rescues the MAP kinase signaling defect for LPA stimulation in HIRc cells and that EGF stimulation of MAP kinase, which does not employ β-arrestin-1 (5), is unimpaired in β-arrestin-1-downregulated cells.
With respect to the inhibition of IGF-I-induced MAP kinase activation in insulin-treated cells, our data argue strongly that β-arrestin-1 downregulation also plays a role in this phenomenon. However, it is likely that other factors may be involved. For example, as shown in several previous reports (15, 25, 43, 50) and in Fig. 3, insulin treatment leads to a decrease in IRS-1 levels, and since IGF-I can signal through IRS-1, this may also contribute to impaired IGF-I-stimulated MAP kinase phosphorylation. However, previous reports have suggested that the connection of the IGF-IR to the Ras MAP kinase pathway is largely mediated through Shc (46). In this regard, our results show that 12 h of insulin treatment results in no change in cellular Shc content, but does lead to a striking decrease in IGF-I-mediated Shc phosphorylation, as well as a decrease in ligand-induced association of Shc with Grb2. Thus, it is possible that impaired signaling through Shc and IRS-1 contributes to the decreased IGF-I-stimulated MAP kinase activity in the β-arrestin-1-downregulated cells. Although decreased function of Shc and IRS-1 may participate in this desensitization process, we believe that the evidence presented in these studies indicates that β-arrestin-1 downregulation also plays an important role. Thus, it has been demonstrated that internalization of the IGF-IR is necessary for MAP kinase stimulation (2, 27) and that β-arrestin-1 is necessary for IGF-IR endocytosis (27). In addition, inhibition of cellular β-arrestin-1 function by microinjection of anti-β-arrestin-1 antibodies blocks IGF-I-stimulated MAP kinase phosphorylation and mitogenesis (5). Furthermore, ectopic expression of wild-type β-arrestin-1 in the insulin-treated β-arrestin-1-downregulated HIRc cells significantly improves the defect in MAP kinase signaling.
Taken together, these results indicate that insulin treatment leads to heterologous desensitization of the MAP kinase signaling pathway for IGF-I, LPA, and β2-AR, but not for EGF. It is likely that the effect of insulin to enhance β-arrestin-1 degradation is the cause of the defect in LPA and β2-AR signaling and is also an important contributory mechanism for the decrease in IGF-I-stimulated MAP kinase phosphorylation.
The IGF-I axis is clearly important for growth control and the process of carcinogenesis, and elevated serum IGF-I levels have been associated with prostate cancer, colorectal cancer, and lung cancer (12, 41, 42, 51). Much evidence indicates that IGF-I serves as an endocrine, paracrine, and autocrine regulator of mitogenesis, cell transformation, and survival (12, 41, 42, 49, 51), and the MAP kinase pathway is a point of integration for multiple mitogenic signals (41). Thus, insulin treatment, by inducing heterologous desensitization of IGF-I action, might affect the role of IGF-I in growth control and carcinogenesis.
The ability of insulin to mediate β-arrestin-1 ubiquitination is of interest, although insulin-stimulated ubiquitination of other proteins has already been reported (45). Ubiquitin-mediated degradation involves a two-step process in which multiple moieties of ubiquitin are covalently conjugated to proteins at specific sites, followed by degradation of the ubiquitin-conjugated protein by the 26S proteasome complex (18). Our data show that inhibition of the proteasome blocks insulin-mediated downregulation of β-arrestin-1, consistent with this process. A large family of ubiquitin protein ligases recognize specific motifs in different protein substrates, allowing ubiquitination to proceed. Modifications to these motifs, such as serine phosphorylation induced by protein kinases, may render them susceptible to recognition by the ubiquitin ligases. Thus, it is possible that an insulin-stimulated serine kinase or some other insulin-induced covalent modification of β-arrestin-1 allows ubiquitin ligase to act on β-arrestin-1 with subsequent proteasomal degradation.
In summary, these studies demonstrate new mechanisms of heterologous desensitization. We find that insulin treatment increases β-arrestin-1 degradation, leading to downregulation of cellular β-arrestin-1 levels. Since β-arrestin-1 is a common signaling molecule for a large number of cell surface receptors, this decrease in β-arrestin-1 has important functional consequences. Thus, LPA-, isoproterenol-, and IGF-I-stimulated MAP kinase activation is decreased in β-arrestin-1-downregulated cells, and this defect can be substantially rescued by ectopic expression of wild-type β-arrestin-1, consistent with the view that the decrease in cellular β-arrestin-1 content is a major mechanism for the desensitization effects of insulin. These studies also demonstrate the principle that heterologous desensitization can extend across receptor classes, since treatment with an RTK ligand, insulin, leads to desensitization not only of another RTK receptor, the IGF-IR, but also the heptahelical LPA and β2-AR.
Acknowledgments
We thank Elizabeth Hansen for editorial assistance. We also thank the Philippe Foundation.
This work was supported by a research grant from the National Institutes of Health (DK 33651), the Veterans Administration San Diego Health Care System, Research Service, and the Whittier Institute for Diabetes.
REFERENCES
- 1.Bourne, H. R. 1997. How receptors talk to trimeric G proteins. Curr. Opin. Cell Biol. 9:134-142. [DOI] [PubMed] [Google Scholar]
- 2.Chow, J. C., G. Condorelli, and R. J. Smith. 1998. Insulin-like growth factor-I receptor internalization regulates signaling via the Shc/mitogen-activated protein kinase pathway, but not the insulin receptor substrate-1 pathway. J. Biol. Chem. 273:4672-4680. [DOI] [PubMed] [Google Scholar]
- 3.Ciechanover, A., A. Orian, and A. L. Schwartz. 2000. The ubiquitin-mediated proteolytic pathway: mode of action and clinical implications. J. Cell. Biochem. 34:40-51. [DOI] [PubMed] [Google Scholar]
- 4.Daaka, Y., L. M. Luttrell, S. Ahn, G. J. Della Rocca, S. S. G. Ferguson, M. G. Caron, and R. J. Lefkowitz. 1998. Essential role of G protein-coupled receptor endocytosis in the activation of the mitogen-activated protein kinase. J. Biol. Chem. 273:685-688. [DOI] [PubMed] [Google Scholar]
- 5.Dalle, S., W. Ricketts, T. Imamura, P. Vollenweider, and J. M. Olefsky. 2001. Insulin and insulin-like growth factor I receptors utilize different G proteins signaling components. J. Biol. Chem. 276:15668-15695. [DOI] [PubMed] [Google Scholar]
- 6.Daub, H., C. Wallach, A. Lankenau, A. Herrlich, and A. Ullrich. 1997. Signal characteristics of G protein-transactivated EGF receptor. EMBO J. 16:7032-7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenteany, G., R. F. Standaert, W. S. Lane, S. Choi, E. J. Corey, and S. L. Schreiber. 1995. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science 268:726-731. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson, S. S. G., W. E. Downey III, A. M. Colapietro, L. S. Barak, L. Menard, and M. G. Caron. 1996. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 271:363-366. [DOI] [PubMed] [Google Scholar]
- 9.Fredericks, Z. L., J. Pitcher, and R. J. Lefkowitz. 1996. Identification of the G protein-coupled receptor kinase phosphorylation sites in human beta2-adrenergic receptor. J. Biol. Chem. 271:13796-13803. [DOI] [PubMed] [Google Scholar]
- 10.Froesch, E. R., M. Hussain, C. Schmid, and J. Zapf. 1997. Insulin-like growth factors, p. 95-114. In D Porte (ed.), Ellenberg and Rifkin's diabetes mellitus, 5th ed. McGraw-Hill Publishing Co., New York, N.Y.
- 11.Goodman, O. B., J. G. Krupnick, F. Santini, V. V. Gurevich, R. B. Penn, A. W. Gagnon, J. H. Keen, and J. L. Benovic. 1996. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature 383:447-450. [DOI] [PubMed] [Google Scholar]
- 12.Grimberg, A., and P. Cohen. 2000. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J. Cell Physiol. 183:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutkind, J. S. 1998. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J. Biol. Chem. 273:1839-1842. [DOI] [PubMed] [Google Scholar]
- 14.Haddad, T. C., and C. A. Conover. 1997. Insulin and interleukin-4 induce desensitization to the mitogenic effects of insulin-like growth factor-I. Pivotal role for insulin receptor substrate-2. J. Biol. Chem. 272:19525-19531. [DOI] [PubMed] [Google Scholar]
- 15.Haruta, T., T. Uno, J. Kawahara, A. Takano, K. Egawa, P. Sharma, J. M. Olefsky, and M. Kobayashi. 2000. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteosomal degradation of insulin receptor substrate-1. Mol. Endocrinol. 14:783-794. [DOI] [PubMed] [Google Scholar]
- 16.Haruta, T. A., A. J. Morris, D. W. Rose, J. G. Nelson, M. Mueckler, and J. M. Olefsky. 1995. Insulin-stimulated GLUT4 translocation is mediated by a divergent intracellular signaling pathway. J. Biol. Chem. 270:27991-27994. [DOI] [PubMed] [Google Scholar]
- 17.Heidenreich, K. A., D. Branderburg, P. Berhanu, and J. M. Olefsky. 1984. Metabolism of photoaffinity-labeled insulin receptors by adipocytes. Role of internalization, degradation, and recycling. J. Biol. Chem. 259:6511-6515. [PubMed] [Google Scholar]
- 18.Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67:425-479. [DOI] [PubMed] [Google Scholar]
- 19.Hochstrasser, M. 2000. Biochemistry. All in the ubiquitin family. Science 289:563-564. [DOI] [PubMed] [Google Scholar]
- 20.Imamura, T., P. Vollenweider, K. Egawa, M. Clodi, K. Ishibashi, N. Nakashima, S. Ugi, J. W. Adams, J. H. B. Brown, and J. M. Olefsky. 1999. G alpha-q/11 protein plays a key role in insulin-induced glucose transport in 3T3-L1 adipocytes. Mol. Cell. Biol. 19:6765-6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishibashi, K. J., T. Imamura, P. M. Sharma, J. Huang, S. Ugi, and J. M. Olefsky. 2001. Chronic endothelin-1 treatment leads to heterologous desensitization of insulin signaling in 3T3-L1 adipocytes. J. Clin. Investig. 107:1193-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klarlund, J., A. Cherniack, B. R. Conway, B. VanRenterghem, and M. P. Czech. 1997. Mechanisms of insulin action, p. 75-94. In D. Porte (ed.), Ellenberg and Rifkin's diabetes mellitus, 5th ed. McGraw-Hill Publishing Co., New York, N.Y.
- 23.Kolch, W., A. Philipp, H. Mischak, E. M. Dutil, T.-M. Mullen, J. M. Meinkoth, J. R. Feramisco, and D. W. Rose. 1996. Inhibition of Raf-1 signaling by a monoclonal antibody which interferes with Raf-1 activation and with Mek substrate binding. Oncogene 13:1305-1314. [PubMed] [Google Scholar]
- 24.Lavinsky, R. M., K. Jepsen, T. Heinzel, J. Torchia, T.-M. Mullen, R. Schiff, A. L. Del-Rio, M. Ricote, S. Ngo, J. Gemsch, S. G. Hilsenbeck, C. K. Osborne, C. K. Glass, M. G. Rosenfeld, and D. W. Rose. 1998. Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc. Natl. Acad. Sci. USA 95:2920-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, A. V., J. L. Gooch, S. Oesterreich, R. L. Guler, and D. Yee. 2000. Insulin-like growth factor I-induced degradation of insulin receptor substrate 1 is mediated by the 26S proteasome and blocked by phosphatidylinositol 3′-kinase inhibition. Mol. Cell. Biol. 20:1489-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lefkowitz, R. J. 1998. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J. Biol. Chem. 273:18677-18680. [DOI] [PubMed] [Google Scholar]
- 27.Lin, F. T., Y. Daaka, and R. J. Lefkowitz. 1998. Beta-arrestins regulate mitogenic signaling and clathrin-mediated endocytosis of the insulin-like growth factor I receptor. J. Biol. Chem. 273:31640-31643. [DOI] [PubMed] [Google Scholar]
- 28.Lohse, M. J., J. L. Benovic, J. Codina, M. G. Caron, and R. J. Lefkowitz. 1990. Beta-arrestin: a protein that regulates beta-adrenergic receptor function. Science 248:1547-1550. [DOI] [PubMed] [Google Scholar]
- 29.Luttrell, L. M., S. S. G. Ferguson, Y. Daaka, W. E. Miller, S. Maudsley, G. J. Della Rocca, F. T. Lin, H. Kawakatsu, K. Owada, D. K. Luttrell, M. G. Caron, and R. J. Lefkowitz. 1999. Beta-arrestin-dependent formation of the beta2 adrenergic receptor-Src protein kinase complexes. Science 283:655-660. [DOI] [PubMed] [Google Scholar]
- 30.Luttrell, L. M., B. E. Hawes, T. van Biesen, D. K. Luttrell, T. J. Lansing, and R. J. Lefkowitz. 1996. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gbetagamma subunit-mediated activation of the mitogen-activated protein kinases. J. Biol. Chem. 271:19443-19450. [DOI] [PubMed] [Google Scholar]
- 31.Maudsley, S., K. L. Pierce, A. M. Zamah, W. E. Miller, S. Ahn, Y. Daaka, R. J. Lefkowitz, and L. M. Luttrell. 2000. The beta(2)-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor. J. Biol. Chem. 275:9572-9580. [DOI] [PubMed] [Google Scholar]
- 32.McClain, D. A., H. Maegawa, J. Lee, T. J. Dull, A. Ullrich, and J. M. Olefsky. 1987. A mutant insulin receptor with defective tyrosine kinase displays no biologic activity and does not undergo endocytosis. J. Biol. Chem. 262:14663-14671. [PubMed] [Google Scholar]
- 33.Moxham, C. M., and C. C. Malbon. 1996. Insulin action impaired by deficiency of the G-protein subunit G ialpha2. Nature 379:840-844. [DOI] [PubMed] [Google Scholar]
- 34.Naor, Z., O. Benard, and R. Seger. 2000. Activation of MAPK cascades by G-protein-coupled receptors: the case of gonadotropin-releasing hormone receptor. Trends Endocrinol. Metab. 11:91-99. [DOI] [PubMed] [Google Scholar]
- 35.Pace, A. M., M. Faure, and H. R. Bourne. 1995. Gi2-mediated activation of the MAP kinase cascade. Mol. Biol. Cell 6:1685-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pagano, M. 1997. Cell cycle regulation by the ubiquitin pathway. FASEB J. 11:1067-1075. [DOI] [PubMed] [Google Scholar]
- 37.Parruti, G., F. Peracchia, M. Sallese, G. Ambrosini, M. Masini, D. Rotilio, and A. De Blasi. 1993. Molecular analysis of human beta-arrestin-1: cloning, tissue distribution, and regulation of expression. Identification of two isoforms generated by alternative splicing. J. Biol. Chem. 268:9753-9761. [PubMed] [Google Scholar]
- 38.Pickart, C. M. 1997. Targeting of substrates to the 26S proteasome. FASEB J. 11:1055-1066. [DOI] [PubMed] [Google Scholar]
- 39.Pierce, K. L., S. Maudsley, Y. Daaka, L. M. Luttrell, and R. J. Lefkowitz. 2000. Role of endocytosis in the activation of the extracellular signal-regulated kinase cascade by sequestering and nonsequestering G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 97:1489-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pippig, S., S. Andexinger, K. Daniel, M. Puzicha, M. G. Caron, R. J. Lefkowitz, and M. J. Lohse. 1993. Overexpression of beta-arrestin and beta-adrenergic receptor kinase augment desensitization of beta 2-adrenergic receptors. J. Biol. Chem. 268:3201-3208. [PubMed] [Google Scholar]
- 41.Prisco, M., G. Romano, F. Peruzzi, B. Valentinis, and R. Baserga. 1999. Insulin and IGF-I receptors signaling in protection from apoptosis. Horm. Metab. Res. 31:80-89. [DOI] [PubMed] [Google Scholar]
- 42.Remacle-Bonnet, M., F. Garrouste, S. Heller, F. Andre, J. L. Marvaldi, and G. J. Pommier. 2000. Insulin-like growth factor-I protects colon cancer cells from death factor-induced apoptosis by potentiating tumor necrosis factor alpha-induced mitogen-activated protein kinase and nuclear factor kappaB signaling pathways. Cancer Res. 60:2007-2017. [PubMed] [Google Scholar]
- 43.Rice, K. M., M. A. Turnbow, and C. W. Garner. 1993. Insulin stimulates the degradation of IRS-1 in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 190:961-967. [DOI] [PubMed] [Google Scholar]
- 44.Rose, D. W., T.-M. Mullen, M. G. Rosenfeld, and C. K. Glass. 1999. Functional characterization of coactivators using mammalian cell microinjection, p. 119-135. In D. Picard (ed.), Nuclear receptors: a practical approach. IRL Press, Oxford, United Kingdom.
- 45.Rui, L., Fisher, T. L., J. Thomas, and M. F. White. 2001. Regulation of insulin/insulin-like growth factor-1 signaling by proteasome-mediated degradation of insulin receptor substrate-2. J. Biol. Chem. 276:40362-40367. [DOI] [PubMed] [Google Scholar]
- 46.Sasaoka, T., M. Ishiki, T. Sawa, H. Ishihara, Y. Takata, T. Imamura, I. Usui, J. M. Olefsky, and M. Kobayashi. 1996. Comparison of the insulin and insulin-like growth factor 1 mitogenic intracellular signaling pathways. Endocrinology 137:4427-4434. [DOI] [PubMed] [Google Scholar]
- 47.Shenoy, S. K., P. H. McDonald, T. A. Kohout, and R. J. Lefkowitz. 2001. Regulation of receptor fat by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science 294:1307-1313. [DOI] [PubMed] [Google Scholar]
- 48.Sibley, D. R., and R. J. Lefkowitz. 1985. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor coupled-adenylate cyclase system as a model. Nature 31:124-129. [DOI] [PubMed] [Google Scholar]
- 49.Singleton, J., V. Dixit, and E. Feldman. 1996. Type I insulin-like growth factor receptor activation regulates apoptotic proteins. J. Biol. Chem. 271:31791-31794. [DOI] [PubMed] [Google Scholar]
- 50.Sun, X. J., J. L. Goldberg, L. Y. Qiao, and J. J. Mitchell. 1999. Insulin-induced insulin receptor substrate-1 degradation is mediated by the proteasome degradation pathway. Diabetes 48:1359-1364. [DOI] [PubMed] [Google Scholar]
- 51.Surmacz, E. 2000. Function of the IGF-I receptor in breast cancer. J. Mammary Gland Biol. Neoplasia 5:95-105. [DOI] [PubMed] [Google Scholar]
- 52.Ullrich, A., J. R. Bell, E. Y. Chen, R. Herrera, L. M. Petruzzeli, T. J. Dull, A. Gray, L. Coussens, Y. C. Liao, M. Tsubokawa, A. Mason, P. H. Seeburg, C. Grunfeld, O. M. Rosen, and J. Ramachandran. 1985. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature 313:756-761. [DOI] [PubMed] [Google Scholar]
- 53.van Biesen, T., L. M. Luttrell, B. E. Hawes, and R. J. Lefkowitz. 1996. Mitogenic signaling via G protein-coupled receptors. Endocr. Rev. 17:698-714. [DOI] [PubMed] [Google Scholar]