Abstract
Gene targeting was used to create mice lacking sperm-associated antigen 6 (Spag6), the murine orthologue of Chlamydomonas PF16, an axonemal protein containing eight armadillo repeats predicted to be important for flagellar motility and stability of the axoneme central apparatus. Within 8 weeks of birth, approximately 50% of Spag6-deficient animals died with hydrocephalus. Spag6-deficient males surviving to maturity were infertile. Their sperm had marked motility defects and was morphologically abnormal with frequent loss of the sperm head and disorganization of flagellar structures, including loss of the central pair of microtubules and disorganization of the outer dense fibers and fibrous sheath. We conclude that Spag6 is essential for sperm flagellar motility and that it is important for the maintenance of the structural integrity of mature sperm. The occurrence of hydrocephalus in the mutant mice also implicates Spag6 in the motility of ependymal cilia.
Fertilization is the process whereby sperm and eggs interact reciprocally to begin development. To initiate fertilization, mammalian sperm cells rely on the propulsive forces generated by their flagella to reach the site of fertilization in the oviduct and to penetrate the investments of the egg (8). All flagella contain an axoneme composed of structural elements and motor proteins that work in a coordinated and regulated fashion to produce wave forms that produce progressive movement (3, 4, 6, 8, 15, 21). The axoneme consists of a central pair of microtubules (central apparatus) surrounded by nine doublets of microtubules with the associated force-generating dynein arms. The basic axonemal structure among cilia and flagella is conserved across species, and much of our understanding of the structure and function of the axoneme has been derived from the study of model organisms. Genetic studies on the green alga, Chlamydomonas, have revealed the importance of several genes for flagellar assembly, stability of specific axonemal structures, and motility (2-6, 15, 21). Inactivation of PF16, one of these Chlamydomonas genes, results in flagellar paralysis (2, 20, 21). Moreover, when the flagella from the pf16 mutant are demembranated to produce axonemes, the C1 microtubule is destabilized and C1-associated polypeptides are lost. We cloned the human and murine orthologues of PF16, named sperm-associated antigen 6 (Spag6), and found that the amino acid sequences of the mammalian and algal proteins were highly conserved, including the eight armadillo repeats required for the assembly of PF16 onto the C1 microtubule and for flagellar function (11, 16, 20, 21). To determine if Spag6 plays a critical role in the function of the mammalian axoneme, we inactivated mouse Spag6. Males lacking Spag6 were infertile because their sperm had striking motility defects and were frequently decapitated and had disorganized flagellar structures. Approximately 50% of nullizygous males and females have enlarged heads and smaller bodies and die prematurely with hydrocephalus, presumably reflecting abnormalities in the function of cilia of ependymal cells that facilitate circulation of cerebral spinal fluid. Our findings indicate that Spag6 is essential for sperm flagellar motility and that it may serve as a scaffold protein that maintains the structural integrity of the sperm flagella. The occurrence of hydrocephalus strongly suggests a role for Spag6 in ependymal ciliary motility.
MATERIALS AND METHODS
Targeted mutation of Spag6.
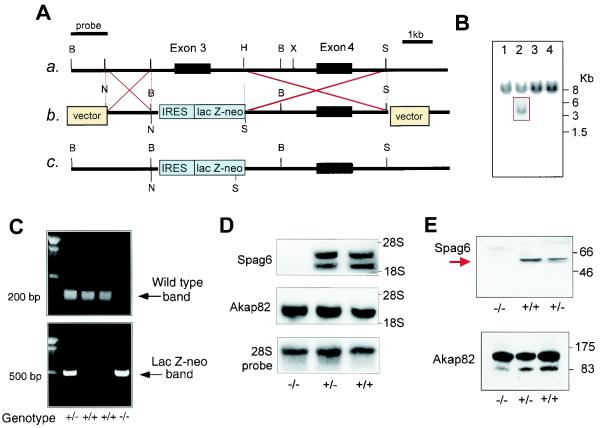
We screened a 129/Sv mouse genomic mouse library and obtained clones covering approximately 10 kb of the ∼80-kb Spag6 gene containing putative exons 3 and 4. We constructed a targeting vector by substitution of the exon encoding amino acid residues 40 to 96 (GenBank accession number AF486266) with an internal ribosome entry site (IRES)-lacZ-Neor fusion gene (9). If the preceding coding sequences were to be expressed, a 39-amino-acid peptide would be produced that lacks the eight contiguous armadillo repeats believed to be essential for Spag6 function (16, 22). For the purpose of screening, we inserted a BamHI site at the end of the short arm. Embryonic stem cells derived from 129/Sv mice were transfected with the linearized βgeo targeting vector, selected in medium supplemented with G-418, and analyzed by Southern blotting to identify correctly targeted clones. For Southern blotting, genomic DNA was digested with BamHI and the blots were probed with a 1.5-kb cDNA containing genomic sequence upstream from the targeted genomic sequence. A correctly targeted embryonic stem cell clone was used to generate chimeric mice, which were crossed with C57BL/6J females to obtain heterozygous mutants. Mice used in these studies were the offspring of crosses between the F1 and/or F2 generations (129/SvJ/C57BL/6J genetic background). Mice were genotyped by PCR. Two sets of primers were used in the PCRs. One set of primers corresponded to the Neo gene: 5′-CGTGTTCCGGCTGTCAGCGCA-3′ and 5′-CAACGCTATGTCCTGATAGCGGTC-3′. The other set of primers corresponded to the deleted region of the Spag6 gene: 5′-GACTTAGCAGAAGCAGTCGTG-3′ and 5′-CGGAGA GAAGCTGCTACCAAG-3′.
Assessment of fertility and fecundity.
To assess fertility and fecundity, littermate males (>6 weeks old) were placed in cages with two mature wild-type females for 2 months or more. Littermate females were caged with a wild-type fertile male for a similar period. The number of mice achieving a pregnancy and the number of offspring from each mating set or pregnancy were recorded.
Northern blot analysis.
Northern blots containing total testicular RNA (30 μg/lane) were probed with a full-length Spag6 cDNA and a cDNA comprising 700 bp of sequence downstream of the targeted exon (16). Similar results were obtained with both probes. Blots were stripped and reprobed for mouse Akap82 (1) and 28S rRNA.
Western blot analysis.
Equal amounts of testicular protein (40 μg/lane) were subjected to Western analysis using antibodies against Spag6 (11, 16) and Akap82 (1).
Motility assays.
Sperm isolation and motility analyses were carried out as previously described (18). For each observation, four fields from each of two dilutions of the original sperm suspension were pooled. The IVOS Sperm Analyzer (Hamilton-Thorne Research, Beverly, Mass.) was used for all motility analyses. Only cells with ≥16 points in their track and a mean curvilinear velocity (VCL) of ≥50 μm/s were analyzed. Sperm populations were analyzed as soon as possible after release from the epididymis.
Histology and immunoelectron microscopy and transmission electron microscopy.
Cauda epididymal sperm, testes, reproductive tracts, tracheal tissue, and ependymal tissue were prepared for light and electron microscopy using standard methods. For immunoelectron microscopy, anti-Spag6 antibody was labeled with 10-nm gold particles as previously described (19).
RESULTS
Targeted disruption of Spag6.
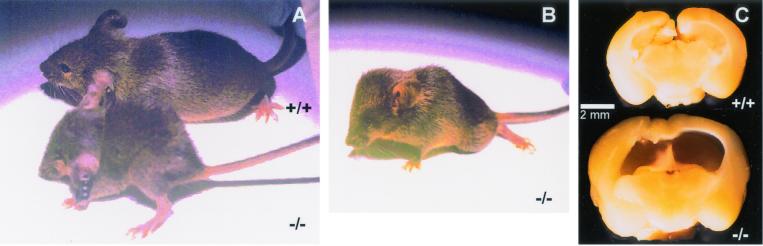
We disrupted the Spag6 gene in murine embryonic stem cells by replacing the third exon with the fusion gene βgeo (Fig. 1A). This manipulation prevents expression of protein containing the eight contiguous armadillo repeats that, by analogy to PF16, are predicted to be essential for Spag6 function (22). To generate chimeras, embryonic stem cells carrying a mutant copy of the Spag6 gene (Fig. 1B) were injected into blastocysts and implanted into pseudopregnant mice. Mutant mice were produced from the chimeric offspring. Disruption of the Spag6 gene was confirmed by PCR analysis (Fig. 1C) and Southern blotting (data not shown). The proportion of wild-type (55 of 189, 29%), heterozygous (89 of 189, 51%), and nullizygous (36 of 189, 19%) offspring from mating of heterozygous males and females was not significantly different from the expected Mendelian pattern of inheritance (chi-square test result = 4.08; P = 0.131). Approximately 50% of the Spag6−/− mice were smaller than their heterozygous and wild-type littermates, and these animals died before 2 months of age with enlarged heads and hydrocephalus, reflected in dilated lateral and third ventricles observed in sagittal sections of the brains (Fig. 2). Hydrocephalus resulting from impaired circulation of cerebral spinal fluid is associated with immotile cilia of ependymal cells (7, 10). The mutant mice displayed no gross abnormalities in organ structure (e.g., polycystic kidneys) or laterality (situs inversus), an abnormality associated with defects in ciliogenesis and immotile cilia syndrome (14, 17).
FIG. 1.
Targeted disruption of the mouse Spag6 gene. (A) Schematic representation of the strategy used to disrupt Spag6. (a) Partial genomic structure of the Spag6 gene. (b) Structure of the targeting vector. (c) Structure of the mutated allele. Restriction sites: H = HpaI, X = XhoI, B = BamHI, N = NotI, and S = SalI. IRES, internal ribosome entry site. (B) Southern blot analysis of transfected embryonic stem cell clones. An external probe gave rise to a single 8-kb band in wild-type genomic DNA digested with BamHI and a 4-kb band in the mutant allele (box). (C) Genotyping by PCR. The wild-type allele yielded a 200-bp amplicon that is absent in the homozygous mutant. A 500-bp amplicon representing the lacZ-Neo cassette was detectable only when a targeted allele was present. (D) Spag6 mRNA is absent in nullizygous mice. Northern blot shows absence of the two Spag6 transcripts in testicular RNA from Spag6−/− mice (upper panel) but also the presence of Akap82 message (middle panel). The lower panel shows the probing for 28S rRNA to assess RNA loading. (E) Spag6 protein was not detectable in the testes of Spag6−/− mice. Western blot demonstrating that the 55,000-Mr Spag6 protein is absent from the testis of nullizygous mice and present at approximately half the level in heterozygous mice (upper panel). Akap82 protein was detected in testes extracts from all genotypes (lower panel). Numbers to the right of panel E indicate the molecular weights (103) of protein standards.
FIG. 2.
Photographs of Spag6−/− mice (A and B) and a wild-type littermate (A) showing small body with a disproportionately large head in the Spag6-deficient animal. (C) Hydrocephalus in Spag6−/− mice. Sagittal sections of brains from a Spag6-deficient mouse and wild-type mouse revealing dilatation of the lateral and third ventricles in the mutant brain.
Northern (Fig. 1D) and Western blot analysis (Fig. 1E) confirmed the absence of Spag6 mRNA and 55,000-Mr protein in testes of Spag6−/− mice that survived to sexual maturity. The polyclonal antibody used to perform the Western blot shown in Fig. 1E, generated against full-length recombinant protein, did not detect lower-molecular-weight immunoreactive bands that could have represented truncated Spag6 resulting from translation of mRNA containing coding sequence upstream from the targeted exon. The testes of Spag6−/− mice did contain pro- and mature protein for Akap82, also known as Akap4, the major component of the sperm flagellum fibrous sheath, and Akap82 mRNA (Fig. 1D and E).
Spag6 is required for male but not female fertility.
Spag6−/− males mated with wild-type females produced no pregnancies after more than 2 months of continuous cohabitation even though vaginal plugs were observed in the females (Table 1). The Spag6+/− littermates were all fertile, producing as many offspring per pregnancy as wild-type littermates. Eight of the 10 Spag6−/− females achieved a pregnancy during the observation period, but the time to establishing a pregnancy was several weeks longer than for wild-type and heterozygous littermates.
TABLE 1.
Fertility and fecundity of Spag6+/+, Spag6+/−, and Spag6−/− micea
| Spag genotype | Male fertilityb | Litter size | Female fertilityb | Litter size |
|---|---|---|---|---|
| +/+ | 8/8 | 6.8 ± 2.4* | 6/6 | 5.2 ± 1 |
| +/− | 10/10 | 7 ± 1.3 | 7/7 | 7 ± 2 |
| −/− | 0/12 | 0 | 8/10 | 5.8 ± 1.2 |
Mice of the indicated genotypes were caged with wild-type C57BL/6J mice of the opposite sex for 2 months or more. The number of fertile animals and litter sizes were recorded. *, Means ± standard errors.
Shown are the number of fertile mice/total number of mice.
Spag6-deficient sperm have motility and structural defects.
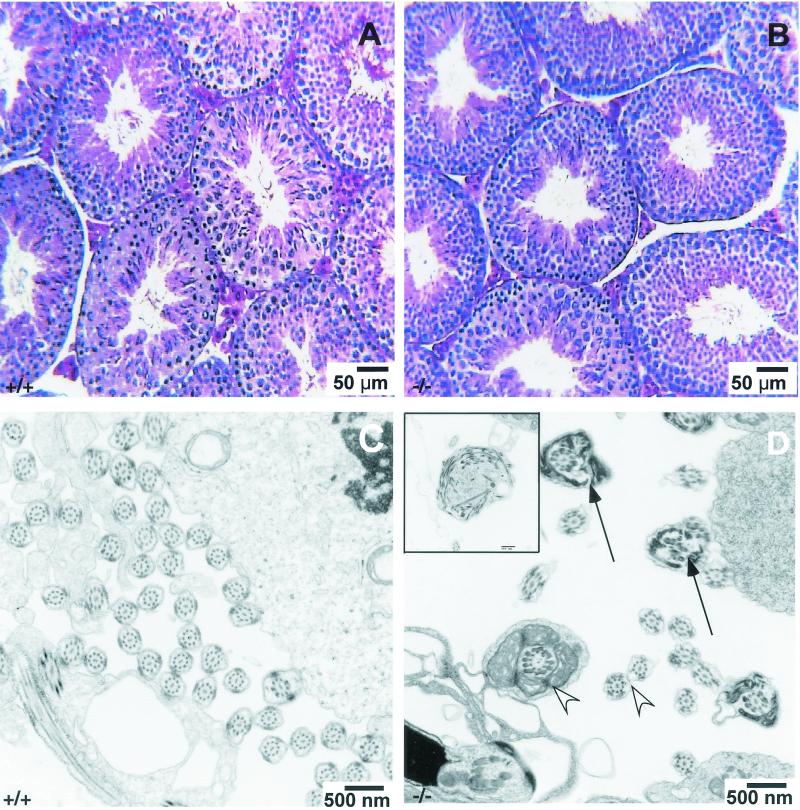
The testes of Spag6−/− males surviving to sexual maturity were of a weight similar to that of wild-type littermates (Spag6−/−: 0.37 ± 0.05 g/100 g of body weight, n = 5; Spag6+/+: 0.38 ± 0.04, n = 5, mean ± standard deviation). Likewise, the seminal vesicles were similar in weight (Spag6−/−: 0.82 ± 0.01 g/100 g, n = 5; Spag6+/+: 0.77 ± 0.10, n = 5, mean ± standard deviation). The reproductive organs were grossly normal, and light microscopy analysis of histological sections of the testes and reproductive tract revealed normal architecture of the seminiferous tubules and interstitium (Fig. 3A and B). Sperm were present in the testes and efferent ducts. The three genotypes were not statistically different in terms of the concentration of sperm that could be recovered from the caudae epididymides (Table 2), although the concentration recovered tended to be lower from Spag6−/− mice, because the recovery process is aided by sperm motility.
FIG. 3.
Histology and ultrastructure of the testis of Spag6−/− and wild-type mice. (A) Histology of wild-type testis. (B) Histology of Spag6-deficient mouse testis revealing normal architecture of the seminiferous tubules and interstitial tissue. (C) Ultrastructure of the wild-type testis seminiferous tubule showing normal sperm flagellar structure. (D) Ultrastructure of a seminiferous tubule of a Spag6-deficient mouse showing sperm debris (inset) some abnormal sperm tails (arrows) and some normal-appearing sperm tails (arrowheads).
TABLE 2.
Motility characteristics of sperm from Spag+/+, Spag+/−, and Spag6−/− micea
| Spag genotype | n | Sperm concn (106/ml) (mean ± SD) | % Motile sperm (mean ± SD) (range of mean) | VCL (mean ± SD) (range of mean) | LIN (mean ± SD) (range of mean) |
|---|---|---|---|---|---|
| +/+ | 3 | 82 ± 31b | 52 ± 6b (46-58) | 341 ± 33b (313-378) | 35 ± 5b, c (30-40) |
| +/− | 3 | 65 ± 35b | 56 ± 5b (52-62) | 252 ± 37c (218-292) | 38 ± 4c (34-42) |
| −/− | 3 | 26 ± 9b | 8 ± 9c (0.3-18) | 136 ± 8d (127-142) | 27 ± 3b (24-30) |
Computer-assisted sperm analysis was performed as described in the text. The number of motile sperm samples analyzed for each genotype ranged from 289 to 1,107 for wild-type mice, 425 to 635 for heterozygous mice, and 11 to 366 for nullizygous mice. n = number of males tested; VCL, mean curvilinear velocity; LIN = linearity, the best estimate of the straightness of a sperm cell's track (100 = a straight line). Means with different superscripts (b, c, and d) in the same column are statistically significantly different: P < 0.05 by analysis of variance and Newman-Keuls multiple-comparison test.
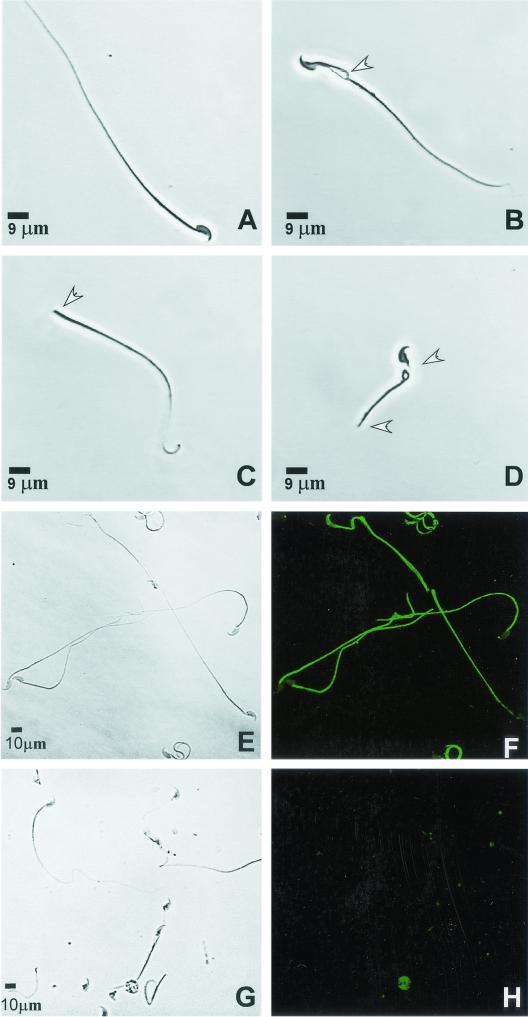
There were striking differences between the mutant and wild-type sperm morphology and motility. Light microscopy examination revealed that 42% of the epididymal sperm from Spag6−/− was abnormal, as reflected in fragmentation of the midpiece, truncated flagella, or decapitation, whereas 7% of heterozygous and wild-type sperm had an abnormal morphology (Fig. 4). As expected, Spag6 protein was not detectable in the tails of permeabilized sperm from Spag6−/− mice (Fig. 4).
FIG. 4.
Morphology of epididymal sperm from Spag6−/− mice. (A) Wild-type sperm. (B) Sperm cell from a Spag6−/− mouse showing a disruption of the midpiece. (C) Headless sperm from a Spag6−/− mouse. (D) Sperm cell with a truncated flagellum and abnormal midpiece from a Spag6−/− mouse. (E) Phase-contrast figure of wild-type sperm. (F) Immunostaining of Spag6 in Triton X-100-permeabilized wild-type sperm showing staining along the tail. (G) Phase-contrast image of Spag6-deficient sperm. (H) Absence of Spag6 staining in Spag6-deficient sperm. Arrowheads indicate abnormalities.
Sperm from wild-type littermates displayed vigorous flagellar activity and progressive forward movement (Table 2 and movie that can be viewed at http://www.med.upenn.edu/crrwh/movies/sapiro.mov). In contrast, only a small percentage of the mutant sperm showed progressive forward motion; flagellar activity was generally limited to a quaking or twitching motion (Table 2 and movie on website). Computer-assisted sperm analysis confirmed that motility parameters were severely impaired in the Spag6−/− mutant (Table 2). The percentage of motile sperm recovered from the epididymis and the VCL of sperm that were motile, an estimate of instantaneous sperm swimming speed, were significantly less in Spag6−/− mice. A mean of 8% of recovered Spag6−/− sperm was motile compared to >50% motile in heterozygous and wild-type mice. Interestingly, sperm from Spag6+/− mice had an intermediate value for VCL, suggesting that, although these animals were fertile, the inactivation of one Spag6 allele impairs flagellar activity due to a reduction in Spag6 protein. There were no differences in linearity of motile sperm, an estimate of the straightness of the sperm track, between wild-type sperm and mutant sperm.
The structural integrity of sperm of Spag6-deficient mice.
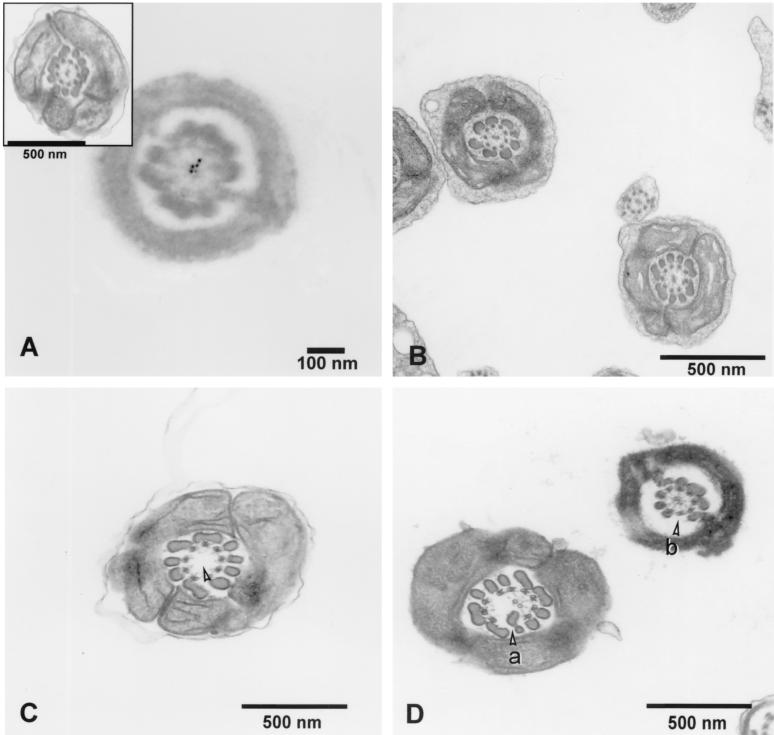
The frequent loss of the sperm head in Spag6−/− mice indicated that the absence of Spag6 affected sperm structural integrity. Immunoelectron microscopy localized Spag6 to the central apparatus of wild-type sperm (Fig. 5). However, we could not determine whether Spag6 was confined to one of the central apparatus microtubules, as is the case in Chlamydomonas. Transmission electron microscopy analysis of epididymal sperm revealed that numerous Spag6−/− sperm samples lacked the central pair of microtubules of the axoneme and that the external microtubule doublets and outer dense fibers were disorganized (Fig. 5). Analysis of 179 transverse sections of Spag6−/− epididymal sperm revealed an abnormal morphology in approximately 60% of the flagella (Table 3). The midpiece was most prominently affected with the central pair of microtubules missing and/or alterations in the fibrous sheaths or outer dense fibers. Only ∼2% of 264 transverse sections of sperm flagella from wild-type animals had an abnormal ultrastructure. The flagella of testicular Spag6−/− sperm had fewer morphological abnormalities and did not display loss of the central pair of microtubules (Table 3 and Fig. 5), but sacs of sperm debris were observed in the seminiferous tubules, apparently the result of phagocytosis by Sertoli cells (Fig. 3). This was not seen in wild-type testes.
FIG. 5.
Ultrastructure of epididymal and testicular sperm from Spag6−/− mice. (A) Immunoelectron microscopy localization of Spag6 in wild-type epididymal sperm using colloidal gold-labeled antibody. Spag6 is localized in the central apparatus. Control sections processed in the absence of primary antibody showed no specific localization (not shown). Inset, transverse section through a wild-type epididymal sperm viewed by transmission electron microscopy. (B) Ultrastructure of Spag6−/− testicular sperm revealing normal flagellar architecture (C) Transverse section from a Spag6−/− epididymal sperm lacking a central apparatus (arrowhead). (D) Transverse sections through Spag6−/− epididymal sperm showing intact central apparatus microtubules but supernumerary (a) and disorganized outer dense fibers (b).
TABLE 3.
Structural abnormalities in the flagella of wild-type and Spag6−/− sperma
| Sperm type | Genotype | % Normal flagella (mean ± SE) for:
|
% Flagella lacking central pair (mean ± SE) for:
|
% Flagella with FS and/or ODF alterations (mean ± SE) for:
|
|||
|---|---|---|---|---|---|---|---|
| Spag6+/+ | Spag6−/− | Spag6+/+ | Spag6−/− | Spag6+/+ | Spag6−/− | ||
| Epididymal sperm | MP | 98 ± 2 | 40 ± 15 | 2.3 ± 2 | 32 ± 11* | 0 | 28 ± 4 |
| PP | 99 ± 1 | 68 ± 12 | 0 | 8 ± 6* | 1 ± 2 | 24.3 ± 3 | |
| Testicular sperm | MP | 100 | 77 ± 11 | 0 | 0 | 0 | 23 ± 11 |
| PP | 100 | 86 ± 3 | 0 | 0 | 0 | 14 ± 3 | |
Transverse sections of flagella from wild-type and Spag6−/− sperm were examined by transmission electron microscopy. Each cross-section was scored for flagellar abnormalities (absence of central pair of microtubules or alterations in the fibrous sheath and/or outer dense fibers). Sperm from three wild-type and three Spag6−/− mice was examined. A total of 264 Spag6+/+ and 179 Spag6−/− epididymal sperm and 247 Spag6+/+ and 166 Spag6−/− testicular sperm were examined. *, P < 0.001, chi-square test. MP, midpiece; PP, principal piece; FS, fibrous sheath; and ODF, outer dense fibers.
DISCUSSION
Spag6, the murine orthologue of Chlamydomonas PF16, is detectable in the flagella of permeabilized sperm (11, 16). Immunoelectron microscopy localized the protein to the central apparatus, a finding consistent with the known residence of PF16 (2, 11). A key feature of the domain structure of Spag6 and PF16 is eight contiguous armadillo repeats, motifs that are involved in protein-protein interaction (15, 20, 21). Smith and Lefebvre suggested that the unstable C1 microtubule in the pf16 Chlamydomonas mutant resulted from impairment of critical protein interactions in the central apparatus (20, 21). Our observations on sperm of Spag6-deficient mice substantiate a role for this protein in the maintenance of structural integrity of the flagella. However, in mice lacking Spag6, the disorganization of sperm structure extends beyond instability of the central apparatus microtubules and includes the fragile attachment of the sperm head as well as disarray of the outer microtubule doublets and outer dense fibers. This may imply that Spag6 has a broader role in maintaining the architecture of the sperm flagellum or that stability of the mouse sperm central apparatus is essential for the integrity of other flagellar structures. However, we cannot formally exclude the possibility that a putative truncated amino-terminal fragment of Spag6 was generated in our mutant mice and that this truncated protein may have led to structural abnormalities in the sperm.
The motility defects in sperm lacking Spag6 probably result from dysfunction of the central apparatus rather than from the structural abnormalities in the flagella. The fact that only 8% of recovered epididymal sperm was motile while ∼60% of transverse sections showed ultrastructural abnormalities in the flagella suggests that the absence of Spag6 does impair motility even when the flagella have a normal architecture. Moreover, the intermediate VCL value for sperm from Spag6+/− mice, which did not have ultrastructural flagellar abnormalities, also indicates an important role for Spag6 in sperm motility. However, because our ultrastructural analyses did not encompass serial sectioning through the full length of the flagella, we cannot exclude the possibility that a higher percentage of mutant sperm had regional structural defects that were not detected.
The fact that the ultrastructure of >75% of testicular sperm of Spag6−/− mice appeared to be generally normal, whereas 60% of the epididymal sperm was abnormal, suggests that Spag6 is not absolutely essential for flagellar assembly or intraflagellar transport. However, the presence of abnormalities in the organization of the outer dense fibers and fibrous sheath and debris, reflecting phagocytosis of presumably abnormal sperm in the testes of Spag6−/− mice, indicates that there is impairment in spermatogenesis or the maintenance of structural integrity of testicular sperm flagella. Thus, Spag6 appears to be important for maintaining the architecture of the central apparatus of sperm after their release from the testis. This observation is consonant with the fact that the central apparatus is structurally normal in the Chlamydomonas pf16 mutant and that instability of the C1 microtubule is found when the axonemes of the mutant flagella are isolated (2, 20, 21).
A role for Spag6 as a scaffold protein mediating protein-protein interactions and structural stability is consistent with our observations that Spag6 and fusion Spag6-green fluorescent protein associate with high affinity to microtubules in transfected COS-1 and Chinese hamster ovary cells and that microtubules decorated with Spag6-green fluorescent protein are bundled and stabilized (i.e., resistant to nocodazole or cooling to 4°C) [16; R. Sapiro, J. M. Murray, M. Zhang, E. J. Blanchette-Mackie, and J. F. Strauss III, 34th Annu. Meet. Soc. Study Reprod., 28 July to 1 August 2001, Ottawa, Canada; Biol. Reprod. 64(Suppl. 1):106, 2001]. The identities of the proteins other than polymerized tubulin that could be directly or indirectly associated with Spag6, remain to be determined. Interestingly, three polypeptides are missing from pf16 flagella (2), and these molecules, if orthologues exist in mice, are candidates for interacting proteins.
We deduce that Spag6 is important for the motility of cilia, as reflected in the occurrence of hydrocephalus in Spag6-deficient mice, an indication of a motility defect in ependymal cell cilia (7, 10). Low levels of Spag6 mRNA are present in the lung (11), and the mRNA is expressed in the medulla as documented in expressed-sequence-tag data for UniGene Cluster Hs.158213 (Spag6). Hydrocephalus, retarded postnatal growth, and early death have been previously described in mice with mutations affecting axonemal structure and function (7). While the ultrastructural appearance of tracheal and ependymal cell cilia of Spag6−/− mice was normal, we did not directly examine the motility of ependymal cell cilia and can only infer from the occurrence of hydrocephalus that ciliary motility is affected. Mice with defects in Tg737, the homologue of the Chlamydomonas IFT88 intraflagellar transport protein, die shortly after birth from polycystic kidney disease, resulting from shorter than normal primary cilia in the kidneys of these mice (14). This renal lesion was not observed in Spag6-deficient mice.
Some epithelial cells of the female reproductive tract are ciliated, and normal beating of these organelles could be important for gamete and embryo transport. The fact that females lacking Spag6 were fertile suggests that either ciliary function is not impaired in Spag6-deficient females or that normal ciliary function is not essential for female reproduction. However, the finding that only 80% of the nullizygous females conceived in our study and that the time to pregnancy was delayed suggest that there may be subtle deficits in reproductive function in Spag6−/− females. Moreover, the nullizygous mice that died early with severe hydrocephalus may have had more profound defects in ciliary function and could have demonstrated impaired fertility had they survived to maturity.
Human infertility associated with absence of the central pair of microtubules, “9 + 0” immotile sperm, has been reported by several authors (12, 13, 23). Other ultrastructural defects have been described in the flagella of these cases. The underlying causes of these abnormalities remain largely unknown. Our findings suggest that mutations in the SPAG6 gene could be one cause.
In summary, we have shown that a conserved orthologue of a Chlamydomonas central apparatus protein plays a key role in regulating the function of ependymal cilia and in maintaining the motility and organization of the mouse sperm flagellum and, consequently, male fertility.
Acknowledgments
This research was supported by NIH grants HD37416 (J.F.S.), HD06274 (J.F.S. and G.L.G.), and HD15045 (P.O.-C.). R.S. was a visiting scholar from the Department of Histology and Embryology, Faculty of Medicine, University of the Republic, Montevideo, Uruguay, and supported by the Fogarty International Center (D43-TW/HD00671).
We thank Stuart Moss and Vargheese Chennathukuzhi and Melanie Lieberman for their technical advice and comments on this work. The Biomedical Imaging Core Laboratory of the University of Pennsylvania Diabetes Center supported by DK19525 is also recognized for assistance with the ultrastructural analysis. We acknowledge the advice and helpful comments of Pete Lefebvre (University of Minnesota) during the course of this work.
REFERENCES
- 1.Carrera, A., G. L. Gerton, and S. B. Moss. 1994. The major fibrous sheath polypeptide of mouse sperm: structural and functional similarities to A-kinase anchoring proteins. Dev. Biol. 165:272-284. [DOI] [PubMed] [Google Scholar]
- 2.Dutcher, S. K., B. Huang, and D. J. L. Luck. 1984. Genetic dissection of the central pair microtubules of the flagella of Chlamydomonas reinhardtii. J. Cell Biol. 98:229-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodenough, U. W., and J. E. Heuser. 1985. Substructure of the inner dynein arms, radial spokes, and the central pair projection complex of cilia and flagella. J. Cell Biol. 100:2008-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haimo, L. T., and J. L. Rosenbaum. 1981. Cilia, flagella, and microtubules. J. Cell Biol. 91:125s-130s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang, B., G. Piperno, and D. J. L. Luck. 1979. Paralyzed flagella mutants of Chlamydomonas reinhardtii. J. Biol. Chem. 254:3091-3099. [PubMed] [Google Scholar]
- 6.King, S. M. 2000. The dynein microtubule motor. Biochim. Biophys. Acta 1496:60-75. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi, Y., M. Watanabe, Y. Okada, H. Sawa, H. Takai, M. Nakanishi, Y. Kawase, H. Suzuki, K. Nagashima, K. Ikeda, and N. Motoyama. 2002. Hydrocephalus, situs inversus, chronic sinusitis, and male infertility in DNA polymerase λ-deficient mice: possible implication for the pathogenesis of immotile cilia syndrome. Mol. Cell. Biol. 22:2769-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mortimer, S. T. 1997. A critical review of the physiological importance and analysis of sperm movement in mammals. Hum. Reprod. Update 3:403-439. [DOI] [PubMed] [Google Scholar]
- 9.Mountford, P., B. Zevnik, A. Duwel, J. Nichols, M. Li, C. Dani, M. Robertson, I. Chambers, and A. Smith. 1994. Dicistronic targeting construct: reporters and modifiers of mammalian gene expression. Proc. Natl. Acad. Sci. USA 91:4303-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura, Y., and K. Sato. 1993. Role of disturbance of ependymal ciliary movement in development of hydrocephalus in rats. Child's Nerv. Syst. 9:65-71. [DOI] [PubMed] [Google Scholar]
- 11.Neilson, L. I., P. L. Schneider, P. G. Van Deerlin, M. Kiriakidou, D. A. Driscoll, M. C. Pellegrini, S. Millinder, K. K. Yamamoto, C. K. French, and J. F. Strauss III. 1999. cDNA cloning and characterization of a human sperm antigen (SPAG6) with homology to the product of the Chlamydomonas PF16 locus. Genomics 60:272-280. [DOI] [PubMed] [Google Scholar]
- 12.Neugebauer, D. C., J. Neuwinger, F. Jockenhovel, and E. Nieschlag. 1990. ′9 + 0′ axoneme in spermatozoa and some nasal cilia of a patient with totally immotile spermatozoa associated with thickened sheath and short midpiece. Hum. Reprod. 5:981-986. [DOI] [PubMed] [Google Scholar]
- 13.Okada, H., A. Hayashi, H. Tanaka, et al. 1993. Ultrastructure of immotile spermatozoa obtained from infertile male patients. Jpn. J. Urol. 84:1879-1882. [DOI] [PubMed] [Google Scholar]
- 14.Pazour, G. J., B. L. Dickert, Y. Vucica, E. S. Seeley, J. L. Rosenbaum, G. B. Witman, and D. G. Cole. 2000. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 151:709-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter, M. E., and W. S. Sale. 2000. The 9+2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J. Cell Biol. 151:F37-F42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sapiro, R., L. M. Tarantino, F. Velazquez, M. Kiriakidou, N. B. Hecht, M. Bucan, and J. F. Strauss III. 2000. Sperm antigen 6 is the murine homologue of the Chlamydomonas reinhardtii central apparatus protein encoded by the PF16 locus. Biol. Reprod. 62:511-518. [DOI] [PubMed] [Google Scholar]
- 17.Schneider, H., and M. Brueckner. 2000. Of mice and men: dissecting the genetic pathway that controls left-right asymmetry in mice and humans Am. J. Med. Genet. 97:258-270. [PubMed] [Google Scholar]
- 18.Si, Y., and P. Olds-Clarke. 2000. Evidence for the involvement of calmodulin in mouse sperm capacitation. Biol. Reprod. 62:1081-1087. [DOI] [PubMed] [Google Scholar]
- 19.Smith, R. M., and L. Jarett. 1993. Electron microscopic immunocytochemical approaches to the localization of ligands, receptors, transducers, and transporters, p. 227-264. In F. de Pablo, C. G. Scanes, and B. D. Weintraub (ed.), Handbook of endocrine research techniques. Academic Press, New York, N.Y.
- 20.Smith, E. F., and P. A. Lefebvre. 1996. PF16 encodes a protein with armadillo repeats and localizes to a single microtubule of the central apparatus in Chlamydomonas flagella. J. Cell Biol. 132:359-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith, E. F., and P. A. Lefebvre. 1997. The role of central apparatus components in flagellar motility and microtubule assembly. Cell Motil. Cytoskelet. 38:1-8. [DOI] [PubMed] [Google Scholar]
- 22.Smith, E. F., and P. A. Lefebvre. 2000. Defining functional domains within PF16: a central apparatus component required for flagellar motility. Cell Motil. Cytoskelet. 46:157-165. [DOI] [PubMed] [Google Scholar]
- 23.Torikata, C., T. Kawai, S. Nogawa, K. Ikeda, K. Shimizu, and C. Kijimoto. 1991. Nine Japanese patients with immotile-dyskinetic cilia syndrome: ultrastructural study using tannic acid-containing fixation. Hum. Pathol. 22:830-836. [DOI] [PubMed] [Google Scholar]