Abstract
Transketolase (TKT) is a ubiquitous enzyme used in multiple metabolic pathways. We show here by gene targeting that TKT-null mouse embryos are not viable and that disruption of one TKT allele can cause growth retardation (≈35%) and preferential reduction of adipose tissue (≈77%). Other TKT+/− tissues had moderate (≈33%; liver, gonads) or relatively little (≈7 to 18%; eye, kidney, heart, brain) reductions in mass. These mice expressed a normal level of growth hormone and reduced leptin levels. No phenotype was observed in the TKT+/− cornea, where TKT is especially abundant in wild-type mice. The small female TKT+/− mice mated infrequently and had few progeny (with a male/female ratio of 1.4:1) when pregnant. Thus, TKT in normal mice appears to be carefully balanced at a threshold level for well-being. Our data suggest that TKT deficiency may have clinical significance in humans and raise the possibility that obesity may be treated by partial inhibition of TKT in adipose tissue.
Transketolase (EC 2.2.1.1) (TKT) is a thiamine diphosphate-dependent enzyme linking the nonoxidative branch of the pentose phosphate pathway (PPP) to the glycolytic pathway. The PPP generates sugar phosphates for intermediary biosynthesis and nucleic acid synthesis and NADPH for reductive biosynthesis (26, 31). Fatty acid biosynthesis and steroid biosynthesis utilize large amounts of NADPH. TKT is expressed in most tissues throughout the animal and plant kingdoms (27). It is prevalent in proliferating tumor cells, and its repression can inhibit tumor cell growth (2).
Our interest in TKT was stimulated by its abundance in the cornea of mice (25) and rabbits (9). In mice, TKT comprises approximately 10% (25) and aldehyde dehydrogenase class 3 (ALDH3) comprises approximately 40% (4, 16) of the water-soluble protein of the corneal epithelial cells. By analogy with the use of enzymes as lens crystallins, we have speculated that the abundant metabolic enzymes in the cornea may serve a structural as well as metabolic role (4, 21, 22).
In the present investigation we disrupted the TKT gene by homologous recombination to test whether corneal clarity is dependent on the concentration of this enzyme. We were only able to obtain viable TKT+/− mice. While their corneas appeared normal, the TKT haploinsufficient mice were often smaller and the small females were relatively infertile. The sizes of the organs were differentially affected, with adipose tissue being markedly reduced in the TKT+/− mice. It thus appears that in mice, as found earlier in plants (8), a reduction in TKT leads to multiple phenotypes and metabolic disturbances. Moreover, our results suggest that TKT haploinsufficiency or a reduction in TKT activity may have clinical implications in humans.
MATERIALS AND METHODS
Generation and mating of TKT+/− mice.
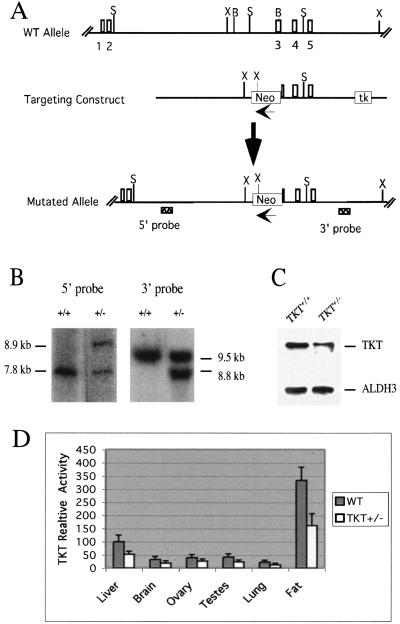
A 12.5-kb genomic clone containing the mouse TKT gene was isolated from a 129Sv library (Stratagene, La Jolla, Calif.). The targeting construct was created by replacing a 2.8-kb BamHI-BamHI fragment (24) with a 1.6-kb PGK/neo cassette for positive selection and inserting a PGK/HSVtk cassette at the 3′ end for negative selection. Homologous recombination of this targeting construct with the wild-type (WT) gene results in deletion of part of intron 2 and exon 3, as illustrated in Fig. 1A. The AscI-linearized targeting construct was electroporated into 129/SvJ embryonic stem (ES) cells (GenomeSystems, Inc.), and colonies were selected with G418 and ganciclovir. Surviving colonies were screened by Southern blotting for correct targeting of TKT. Three correctly targeted ES cell clones were microinjected into C57BL/6NCr blastocysts, and chimeric mice produced from two of these clones were mated to C57BL/6J females. Offspring were analyzed by Southern blot hybridization using genomic DNA to ensure that the appropriate targeted disruption was present. Genomic DNA was cut with SacII or XbaI, and the blots were probed with the 5′ probe and 3′ probe, respectively, identified in Fig. 1A. Deletion of a SacII site causes an increase from 7.8 to 8.9 kb of the probe-containing SacII fragment, and insertion of a new XbaI site within the PGK/neo cassette causes a decrease from 9.5 to 8.8 kb of the probe-containing XbaI fragment. TKT heterozygotes were maintained in a mixed genetic background (129Sv/J × C57BL/6J) by breeding TKT+/− mice to TKT+/− or C57BL/6J mice.
FIG. 1.
TKT+/− mice. (A) Knockout strategy. Numbered rectangles, exons; Neo, neomycin resistance gene cassette; tk, herpes simplex virus thymidine kinase gene cassette. Restriction sites: B, BamHI; S, SacII; X, XbaI. (B) Southern blots of SacII- and XbaI-digested tail DNAs. (C) Immunoblots of WT and TKT+/− cornea extracts probed with a TKT (6) or ALDH3 antibody. (D) TKT activity per microgram of protein from various tissues of WT and TKT+/− mice. Bars represent the amount of TKT activity in the specified tissue relative to that in WT liver.
Immunoblotting.
Corneas were dissected from TKT+/− and WT mice and homogenized in 50 mM triethanolamine-diethanolamine-HCl buffer (18) on ice with a microtissue grinder. Extracts were centrifuged at 10,000 × g for 15 min at 4°C and the protein concentration of each supernatant fraction was determined using the Bio-Rad protein assay kit (Bio-Rad Laboratories). Equal amounts of protein from WT and TKT+/− corneas were resolved on 10 to 20% sodium dodecyl sulfate (SDS)-polyacrylamide gels, electrotransferred to nitrocellulose membrane, and probed with anti-TKT antibody (25) by using an ECL Western blotting detection kit (Amersham) according to the manufacturer's instructions. The blot was stripped by incubating it with the stripping buffer (phosphate-buffered saline with 7 μl of β-mercaptoethanol/ml and 2% SDS) for 30 min at room temperature with agitation and then reprobed with anti-ALDH3 antibody (gift of Ronald Lindahl, University of South Dakota, Vermillion, S.Dak.).
Enzyme assays.
Cell extracts were prepared from tissues of WT and TKT+/− mice in 50 mM triethanolamine-diethanolamine-HCl buffer (18). The enzyme assay was performed by the method of Takeuchi et al. (28), in which the production of 7-sedoheptulose phosphates is measured by a colorimetric assay. The enzyme activities were normalized to protein concentrations.
In vitro embryo culture.
Six- to 8-week-old normal-sized female TKT+/− mice were superovulated by intraperitoneal injection of 5 IU of pregnant mare serum gonadotropin (Sigma) followed 48 h later by 5 IU of human chorionic gonadotropin (hCG; Sigma). The females were bred with TKT+/− or WT males immediately after hCG injection. One-cell embryos were isolated from the ampulla tubae of superovulated females 20 h after hCG injection. The embryos were cultured in M16 medium (Sigma) and incubated with 5% CO2 at 37°C, and the culture medium was changed every 24 h. Photographs were taken every day with a Zeiss Axiovert microscope equipped with a digital camera.
Growth hormone and leptin quantitation.
Approximately 1 ml of blood was collected from the heart of anesthetized WT and TKT+/− mice and placed into a clean tube. After standing at room temperature for 20 to 30 min, the coagulated blood was centrifuged at 900 × g for 10 min at 4°C The serum was immediately stored at −70°C in a freezer and shipped on dry ice to Ani Lytics Laboratory (Gaithersburg, Md.) for the growth hormone assay. Leptin was measured using a commercially available leptin enzyme-linked immunosorbent assay (ELISA) kit (Crystal Chem Inc.). Samples (5 μl) were measured in duplicate.
RESULTS
Creation and mating of TKT+/− mice.
We replaced part of intron 2 and exon 3 of the mouse TKT gene (24) with the neomycin resistance gene cassette (neo) in ES cells (Fig. 1A), made chimeric mice, and verified the recombination event in F1 progeny by Southern blot analysis of genomic DNA (Fig. 1B). Immunoblot analyses demonstrated that the cornea (Fig. 1C) and other tissues (fat, heart, kidney, liver, and testes [data not shown]) of the TKT+/− mice contained half the amount of TKT as in the WT mice. Finally, enzymatic assays confirmed the approximate 50% reductions of TKT activity in liver, brain, ovary, testes, lung, and adipose tissue of the TKT+/− mice (Fig. 1D). As expected, very high TKT levels were found in adipose tissue.
The initial intercross mating of the TKT+/− mice produced litters with a non-Mendelian distribution of the TKT knockout allele of WT (n = 56), heterozygous (n = 61), and homozygous (n = 0) mice. Moreover, PCR genotyping showed no TKT-null blastocysts (WT/heterozygotes/homozygotes ratio = 1:1:0; n = 10) or embryonic day 6.5 to embryonic day 12.5 embryos. In addition to the absence of TKT-null mice, the expected 2:1 ratio of heterozygous to WT progeny was not observed, although the expected 1:1 ratio was observed in the progeny of matings between TKT+/− and WT mice (Table 1).
TABLE 1.
Number of progeny produced from breeding of TKT+/− mice
| Mating type | TKT+/+ pups | TKT+/− pups | Total pups | Male/female ratio All pups | TKT+/− pups |
|---|---|---|---|---|---|
| TKT+/− × TKT+/+ | 60 | 63 | 123 | 60:63 (= 1.00:1.05) | 31:32 (= 1.00:1.03) |
| TKT+/− × TKT+/− | 56 | 61 | 117 | 68:49 (= 1.39:1.00) | 36:25 (= 1.44:1.00) |
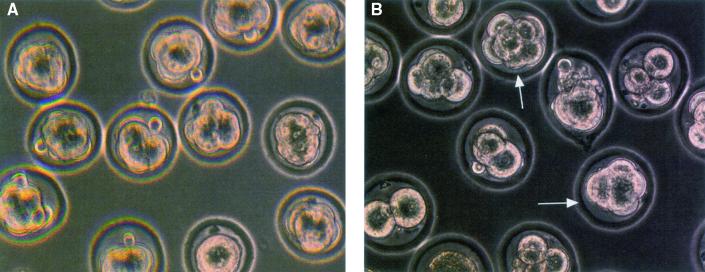
We next cultured fertilized eggs collected from TKT+/− and TKT+/+ females mated to TKT+/− males. After 3 days, 40 to 50% of the fertilized eggs from TKT+/− × TKT+/− crosses were morulas, while the remainder were at the 4- to 16-cell stage of development; by contrast, at least 90% of the fertilized eggs from the TKT+/− females mated with WT males were at the morula stage (Fig. 2). Taken together, these data indicate that TKT-null embryos die at or before the morula stage.
FIG. 2.
In vitro culture of fertilized eggs. Heterozygous TKT+/− female mice were mated with WT (A) or TKT+/− (B) males. Eggs collected from plugged females were cultured in M16 medium with 5% CO2 at 37°C. Pictures were taken 84 h after ovulation. All eggs (in the view) are at morula stage in panel A, and only two morulas were observed in panel B. Arrows indicate morula-stage embryos.
Growth and organ sizes of TKT+/− mice.
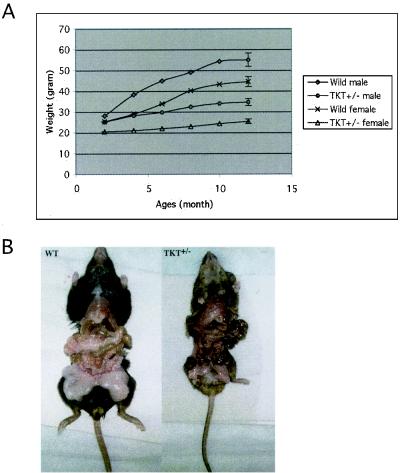
Approximately 40% of the TKT+/− mice exhibited postnatal growth retardation compared to the WT mice (Fig. 3A). The average weights of these smaller TKT+/− mice were 60% (for females) and 64% (for males) that of the WT mice after 1 year. Markedly reduced white adipose tissue was observed in the small TKT+/− mice (Fig. 3B).
FIG. 3.
Growth of WT and small TKT+/− mice. (A) Six WT mice (three males and three females) and six small heterozygous TKT+/− mice (three males and three females) were weighed every month for a year. Growth curves represent the average weight in each group. (B) Production of fat tissues in sibling, 8-month-old female WT (left) and TKT+/− (right) mice. The whole-body weight of the TKT+/− mouse (24.1g) was 62% of that of the WT mouse (38.6g), but the weight of fat tissues in the TKT+/− mouse was only 22% of that of the WT mouse.
To test if the reduced TKT enzyme activity was associated with organ-specific growth retardation, we weighed various tissues from 5 pairs of males (one WT mouse and one small TKT+/− mouse delivered from the same female) from four different litters at 6 months of age. The relative wet weights of adipose tissue, brain, heart, liver, testes, and kidney were compared within each pair. The small TKT+/− mice (35% body weight reduction) showed an approximately 77% weight reduction in adipose tissue, 33% reduction in liver and testes, and 7 to 18% reduction in eye, brain, heart, and kidney (Table 2). All the organs from the small TKT+/− mice appeared histologically normal (data not shown). Serum growth hormone levels were similar in the small TKT+/− and WT adult mice (Table 3), suggesting that the reduced growth phenotypes of the TKT+/− mice are due to metabolic rather than hormonal differences. Table 3 also shows that the leptin level was reduced 10-fold compared to the WT or normal-sized TKT+/− mice. The normally sized TKT+/− mice had a normal amount of body fat. The reduced leptin level in the small TKT+/− mice was probably due to the reduced adipose tissue (1, 7).
TABLE 2.
Organ-specific growth defects of the small TKT+/− micea
| Organ | Organ wt (% of WT) |
|---|---|
| Whole body | 64.1 ± 5.3 |
| Fat | 27.2 ± 10.2 |
| Brain | 92.0 ± 1.6 |
| Heart | 85.0 ± 6.3 |
| Liver | 67.2 ± 9.2 |
| Testes | 67.1 ± 11.5 |
| Kidney | 82.0 ± 10.8 |
| Eye | 92.7 ± 5.7 |
Fat, brain, heart, liver, testes, and kidney tissues from male TKT+/− and TKT+/+ mice were dissected and weighed. The organ weights and total body weights of TKT+/− mice are expressed as a percentage of the WT values (± standard error of the mean).
TABLE 3.
Growth hormone and leptin levels in WT and small TKT+/− mice
| Genotype | Growth hormone (ng/ml) | Leptin (ng/ml) |
|---|---|---|
| WT | 42.7 ± 3.2 | 13.7 ± 2.3 |
| TKT+/− | 48.3 ± 16.8 | 1.3 ± 1.8 |
Mating and fertility of TKT+/− females.
The small TKT+/− females mated infrequently, even when kept in the same cage with WT or TKT+/− males for 2 months. The remaining 60% of the TKT+/− females, which were of normal size, had variably reduced fertility as judged by the number of pups delivered. By contrast, all the TKT+/− males displayed normal mating behavior. Unexpectedly, the male/female ratio among the progeny of TKT+/− × TKT+/− matings was 1.4:1. However, the normal 1:1 gender ratio was observed in progeny of TKT+/− × WT matings. Histological analysis of the ovaries of the small TKT+/− females showed mature corpora lutea and all stages of primary and antral follicles, although the overall mass of the ovaries was reduced (data not shown). Superovulation of TKT+/− females by injection of pregnant mare serum gonadotropin and hCG failed to increase mating frequency and pregnancy.
Corneas of TKT+/− mice.
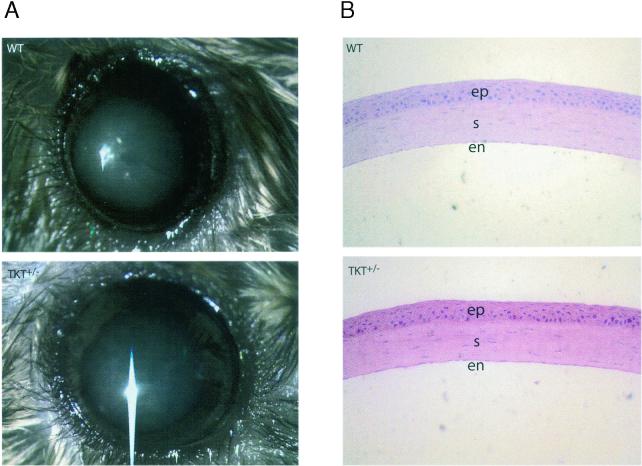
Corneal clarity of the TKT+/− mice was indistinguishable from that of the WT mice as judged by visual inspection (Fig. 4A) and slit-lamp biomicroscopy, despite a 50% reduction in the amount of TKT (Fig. 1C). Immunocytochemistry also showed an approximate 50% reduction in the amount of TKT in the corneal epithelial cells (data not shown). The TKT+/− corneas appeared histologically normal (Fig. 4B).
FIG. 4.
Visual inspection and cornea sections. (A) Dilated eyes from WT (upper) and TKT heterozygous (TKT+/−) (lower) mice are shown. The lens (blue area) and iris (surrounding the lens) are observable through the clear corneas. The slight opacity of the lens is a typical reaction to the anesthetic. Images were taken with a Zeiss Stemi SV11 stereomicroscope. (B) Histological analysis of corneas from WT and heterozygous TKT knockout mice (hematoxylin and eosin stain). Magnification, ×20. The corneal epithelium (ep), stroma (s), and endothelium (en) of the TKT+/− mice appear to be normal.
DISCUSSION
The present experiments indicate that TKT is required for early mouse development, consistent with its metabolic importance for energy production and nucleic acid synthesis (26, 31). The unexpected 1:1 ratio of TKT+/− to TKT+/+ progeny resulting from TKT+/− × TKT+/− crosses suggests that, in addition to the complete loss of TKT−/− embryos, about half of the TKT+/− embryos also die. This is probably due to maternal TKT haploinsufficiency, as the expected 1:1 ratio of TKT+/− to TKT+/+ progeny is observed in TKT+/− × TKT+/+ crosses. Of the viable TKT+/− mice, approximately 40% exhibited a severe growth retardation. The possibility that this was due to their mixed genetic background requires further study. The present experiments showing significant growth retardations in TKT haploinsufficient mice indicate that TKT may be a rate-limiting step in the PPP, consistent with results in glucose-6-phosphate dehydrogenase (G6PD)-deficient mice (17).
The reason for the 1.4:1 male to female ratio among the TKT+/− progeny is not known. One possibility is that the female TKT+/− embryos are vulnerable during early development. If so, this may be related to the precise time or level at which the endogenous gene begins to be expressed during embryogenesis, which may be influenced by the sex-related genes (11). G6PD, the first enzyme in the PPP, is encoded on the X chromosome. During embryogenesis, it is possible that female embryos express higher levels of G6PD prior to X chromosome inactivation than do the male embryos (11, 20). This, combined with decreased TKT activity, could result in accumulation of PPP intermediates that may be toxic or fail to produce sufficient end products.
Since TKT-null mice were not obtained, the present experiments did not provide insight into the reason for the abundance of TKT in the mouse cornea (21, 22). TKT is concentrated in the corneal epithelium of WT mice (25) as well as enriched in the corneal stroma and endothelium (6) of WT mice. The fact that a 50% reduction in corneal TKT had no detectable effect is analogous to our earlier finding that elimination of ALDH3, the most abundant corneal enzyme and which is also concentrated in the corneal epithelium, had no detectable effect on corneal clarity or structure (16). There was no compensatory protein that accumulated in the corneas of the ALDH3−/− (16) or TKT+/− (present report; data not shown) mice. Thus, it remains an enigma why these metabolic enzymes are so abundant in the cornea.
Fertility problems are a major medical issue affecting more than 10% of married couples in the population (29). The present finding that TKT haploinsufficiency preferentially affects female fertility in mice may provide a new insight into this important medical issue in humans.
We are unaware of TKT mutations associated with human pathologies. However, Alzheimer's disease has been associated with abnormally cleaved TKT (19), and Wernicke-Korsakoff syndrome (13), as well as other pathologies, has been associated with thiamine deficiencies (12, 26). Direct analysis of human TKT cDNAs has indicated that allelic variants of the TKT gene cannot account for Wernicke-Korsakoff syndrome (14). Since TKT appears to be near the threshold level for the well-being of the present TKT+/− mouse, it is possible that these haploinsufficient mice will be useful for generating an animal model for Wernicke-Korsakoff syndrome and other disorders associated with TKT activity.
We do not know whether the reduction in adipose tissue of the small TKT+/− mice is due to an NADPH deficiency that limits production of lipids (10), or to the reduced cell proliferation. The average serum leptin levels were decreased 10-fold in the small TKT+/− male and female mice (data not shown), consistent with the direct relationship between adiposity and leptin in humans (5). The preferential loss of adipose tissue in the haploinsufficient mice suggests that pharmacological lowering of TKT activity might have clinical relevance for treatment of obesity in humans.
Other fat-deficient mouse models exist (15, 23). The white fatless mouse was generated by adipose-specific expression of a dominant-negative b-ZIP transgene (15). In contrast to our TKT+/− mice, the white fatless mice had larger body size and organs than the WT mice.
Finally, our results are consistent with mouse TKT being encoded in a single-copy gene (24) expressed at a threshold level for normal homeostasis. Similarly, a small decrease in TKT activity in tobacco results in decreased ribulose-1,5-diphosphate, photosynthesis, sugars, aromatic amino acids, phenylpropenoid metabolism, chlorophyll, and carotene (8). Our experiments indicate that there is no compensation for loss of a TKT allele by the TKT-like gene (Tktl1) on the X chromosome in mice (30). Mouse Tktl1 is expressed specifically in the gonads, yet we found a 50% reduction in testes TKT activity in the TKT+/− mice. Humans too have one TKT gene (14) and an X-linked TKT-like gene (TKR) that apparently lacks enzyme activity (3). Thus, our findings raise the possibility that mutations of human TKT affecting enzyme activity may affect obesity and female fertility disorders.
Acknowledgments
We thank Christoph Westphal (Harvard Medical School) for providing us with the PGK-neo-polyA cassette for the TKT gene-targeting construct and Zbynek Kozmik, Shivalingpappa K. Swamynathan, Jyotshnbaba Kanungo, David Nees, and Janine Davis for constructive criticisms on the manuscript.
REFERENCES
- 1.Bunger, L., M. Nicolson, and W. G. Hill. 1999. Leptin levels in lines of mice developed by long-term divergent selection on fat content. Genet. Res. 73:37-44. [DOI] [PubMed] [Google Scholar]
- 2.Cascante, M., J. J. Centelles, R. L. Veech, W.-N. P. Lee, and L. G. Boros. 2000. Role of thiamin vitamin B-1 and transketolase in tumor cell proliferation. Nutr. Cancer 36:150-154. [DOI] [PubMed] [Google Scholar]
- 3.Coy, J. F., S. Dubel, P. Kioschis, K. Thomas, G. Micklem, H. Delius, and A. Poustka. 1996. Molecular cloning of tissue-specific transcripts of a transketolase-related gene: implications for the evolution of new vertebrate genes. Genomics 32:309-316. [DOI] [PubMed] [Google Scholar]
- 4.Cuthbertson, R. A., S. I. Tomarev, and J. Piatigorsky. 1992. Taxon-specific recruitment of enzymes as major soluble proteins in the corneal epithelium of three mammals, chicken, and squid. Proc. Natl. Acad. Sci. USA 89:4004-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farooqi, I. S., J. M. Keogh, S. Kamath, S. Jones, W. T. Gibson, R. Trussell, S. A. Jebb, G. Y. Lip, and S. O'Rahilly. 2001. Partial leptin deficiency and adiposity. Nature 414:34-35. [DOI] [PubMed] [Google Scholar]
- 6.Guo, J., C. M. Sax, J. Piatigorsky, and F. X. Yu. 1997. Heterogeneous expression of transketolase in ocular tissues. Curr. Eye Res. 16:467-474. [DOI] [PubMed] [Google Scholar]
- 7.Havel, P. J. 2000. Role of adipose tissue in body-weight regulation: mechanisms regulating leptin production and energy balance. Proc. Nutr. Soc. 59:359-371. [DOI] [PubMed] [Google Scholar]
- 8.Henkes, S., U. Sonnewald, R. Badur, R. Flachmann, and M. Stitt. 2001. A small decrease of plastid transketolase activity in antisense tobacco transformants has dramatic effects on photosynthesis and phenylpropanoid metabolism. Plant Cell 13:535-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jester, J. V., T. Moller-Pedersen, J. Huang, C. M. Sax, W. T. Kays, H. D. Cavangh, W. M. Petroll, and J. Piatigorsky. 1999. The cellular basis of corneal transparency: evidence for “corneal crystallins.” J. Cell Sci. 112:613-622. [DOI] [PubMed] [Google Scholar]
- 10.Kather, H., M. Rivera, and K. Brand. 1972. Interrelationship and control of glucose metabolism and lipogenesis in isolated fat-cells. Effect of the amount of glucose uptake on the rates of the pentose phosphate cycle and of fatty acid synthesis. Biochem. J. 128:1089-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kochhar, H. P. S., J. Peippo, and W. A. King. 2001. Sex related embryo development. Theriogenology 55:3-14. [DOI] [PubMed] [Google Scholar]
- 12.Kril, J. J. 1996. Neuropathology of thiamine deficiency disorders. Metab. Brain Dis. 11:9-17. [DOI] [PubMed] [Google Scholar]
- 13.Martin, P. R., B. A. McCool, and C. K. Singleton. 1995. Molecular genetics of transketolase in the pathogenesis of the Wernicke-Korsakoff syndrome. Metab. Brain Dis. 10:45-55. [DOI] [PubMed] [Google Scholar]
- 14.McCool, B. A., S. G. Plonk, P. R. Martin, and C. K. Singleton. 1993. Cloning of human transketolase cDNAs and comparison of the nucleotide sequence of the coding region in Wernicke-Korsakoff and non-Wernicke-Korsakoff individuals. J. Biol. Chem. 268:1397-1404. [PubMed] [Google Scholar]
- 15.Moitra, J., M. M. Mason, M. Olive, D. Krylov, O. Krylov, B. Marcus-Samuels, L. Feigenbaum, E. Lee, T. Aoyama, M. Eckhaus, M. L. Reitman, and C. Vinson. 1998. Life without white fat: a transgenic mouse. Genes Dev. 12:3168-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nees, D. W., E. F. Wawrousek, W. G. Robison, Jr., and J. Piatigorsky. 2002. Structurally normal corneas in aldehyde dehydrogenase 3a1-deficient mice. Mol. Cell. Biol. 22:849-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicol, C. J., J. Zielenski, L. C. Tsui, and P. G. Wells. 2000. An embryoprotective role for glucose-6-phosphate dehydrogenase in developmental oxidative stress and chemical teratogenesis. FASEB J. 14:111-127. [DOI] [PubMed] [Google Scholar]
- 18.Paoletti, F. 1983. Purification and properties of transketolase from fresh rat liver. Arch. Biochem. Biophys. 222:489-496. [DOI] [PubMed] [Google Scholar]
- 19.Paoletti, F., A. Mocali, and D. Tombaccini. 1997. Cysteine proteinases are responsible for characteristic transketolase alterations in Alzheimer fibroblasts. J. Cell Physiol. 172:63-68. [DOI] [PubMed] [Google Scholar]
- 20.Peters, J., S. T. Ball, D. J. Charles, W. Pretsch, G. Bulfield, D. Miller, and V. M. Chapman. 1988. The localization of G6pd, glucose-6-phosphate dehydrogenase, and mdx, muscular dystrophy in the mouse X chromosome. Genet. Res. 52:195-201. [DOI] [PubMed] [Google Scholar]
- 21.Piatigorsky, J. 1998. Gene sharing in lens and cornea: facts and implications. Prog. Retin. Eye Res. 17:145-174. [DOI] [PubMed] [Google Scholar]
- 22.Piatigorsky, J. 2001. Enigma of the abundant water-soluble cytoplasmic proteins of the cornea: the “refracton” hypothesis. Cornea 20:853-858. [DOI] [PubMed] [Google Scholar]
- 23.Reue, K., and M. Peterfy. 2000. Mouse models of lipodystrophy. Curr. Atheroscler. Rep. 2:390-396. [DOI] [PubMed] [Google Scholar]
- 24.Salamon, C., M. Chervenak, J. Piatigorsky, and C. M. Sax. 1998. The mouse transketolase (TKT) gene: cloning, characterization, and functional promoter analysis. Genomics 48:209-220. [DOI] [PubMed] [Google Scholar]
- 25.Sax, C. M., C. Salamon, W. T. Kays, J. Guo, F. X. Yu, R. A. Cuthbertson, and J. Piatigorsky. 1996. Transketolase is a major protein in the mouse cornea. J. Biol. Chem. 271:33568-33574. [DOI] [PubMed] [Google Scholar]
- 26.Schenk, G., R. G. Duggleby, and P. F. Nixon. 1998. Properties and functions of the thiamin diphosphate dependent enzyme transketolase. Int. J. Biochem. Cell Biol. 30:1297-1318. [DOI] [PubMed] [Google Scholar]
- 27.Schenk, G., R. Layfield, J. M. Candy, R. G. Duggleby, and P. F. Nixon. 1997. Molecular evolutionary analysis of the thiamine-diphosphate-dependent enzyme, transketolase. J. Mol. Evol. 44:552-572. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi, T., K. Nishino, and Y. Itokawa. 1984. Improved determination of transketolase activity in erythrocytes. Clin. Chem. 30:658-661. [PubMed] [Google Scholar]
- 29.Templeton, A. 2000. Infertility and the establishment of pregnancy—overview. Br. Med. Bull. 56:577-587. [DOI] [PubMed] [Google Scholar]
- 30.Wang, P. J., J. R. McCarrey, F. Yang, and D. C. Page. 2001. An abundance of X-linked genes expressed in spermatogonia. Nat. Genet. 27:422-426. [DOI] [PubMed] [Google Scholar]
- 31.Wood, T. 1985. The pentose phosphate pathway. Academic Press/Harcourt Brace Jovanovich, New York, N.Y.