Abstract
The mitotic polo-like kinases have been implicated in the formation and function of bipolar spindles on the basis of their respective localizations and mutant phenotypes. To date, this putative regulation has been limited to a kinesin-like motor protein, a centrosomal structural protein, and two microtubule-associated proteins (MAPs). In this study, another spindle-regulating protein, the mammalian non-MAP microtubule-binding and -stabilizing protein, the translationally controlled tumor protein (TCTP), was identified as a putative Plk-interacting clone by a two-hybrid screen. Plk phosphorylates TCTP on two serine residues in vitro and cofractionates with the majority of kinase activity toward TCTP in mitotic cell lysates. In addition, these sites were demonstrated to be phosphorylated in vivo. Overexpression of a Plk phosphorylation site-deficient mutant of TCTP induced a dramatic increase in the number of multinucleate cells, rounded cells with condensed ball-like nuclei, and cells undergoing cell death, similar to both the reported anti-Plk antibody microinjection and the low-concentration taxol treatment phenotypes. These results suggest that phosphorylation decreases the microtubule-stabilizing activity of TCTP and promotes the increase in microtubule dynamics that occurs after metaphase.
Viability of daughter cells requires the proper segregation of chromosomes during mitosis. The segregation of sister chromatids during anaphase is dependent on the assembly of a functional bipolar spindle and the regulation of spindle dynamics. For proper spindle function to occur, the proper balance of microtubule-stabilizing and -destabilizing activities for each stage of mitosis is critical. Reversible protein phosphorylation plays an important role in the control of the assembly and function of the mitotic spindle, generally through the regulation of microtubule binding proteins and microtubule motors (3, 18, 54, 72). Phosphorylation directly affects microtubule binding proteins by increasing or decreasing their binding affinities for microtubules (15, 60), changing their stabilization activities without affecting their binding affinities (56), altering their non-tubulin-binding affinities such as for cross-linking actin microfilaments (61), and affecting the degree of steric hindrance to the binding sites of motor proteins (52). In addition to this indirect regulation of microtubule motility, kinase activities can directly affect motor proteins by regulating their binding to microtubules (38), their ATPase activity (63), their localization (6), and their binding to other proteins and cargoes (7, 63).
Among the protein kinases that regulate spindle function, the mitotic polo-like kinases have been implicated by genetic, biochemical, and cytological evidence to play a significant role. The polo-like kinases are a family of serine/threonine kinases that have a high degree of homology in their amino-terminal catalytic domains. In addition, regions of homology are present in the carboxy-terminal noncatalytic domains, which include the highly conserved stretch of ∼30 amino acids called the polo box (21) and two other regions of homology dubbed polo boxes 2 and 3 (31). Members of this family include the mammalian Plk (31, 32, 36, 37, 45, 47), Snk (65), and Prk/Fnk (24, 49), Xenopus laevis Plx1, Plx2, and Plx3 (25, 44), Drosophila melanogaster polo (51), Schizosaccharomyces pombe plo1 (55), Saccharomyces cerevisiae Cdc5p (42), and Caenorhabditis elegans plc1 and plc2 (57). Closely related to this family are mammalian Sak-a and Sak-b, which possess homology to the catalytic domain of the polo family members but lack the conserved carboxy terminus and characteristic polo boxes (28). All of these members, except for the G1-specific Snk and perhaps the putative Xenopus Snk homolog Plx2 proteins, regulate a variety of M-phase-specific events. These include centrosome maturation (34, 46), bipolar spindle formation (46, 51, 55), microtubule motor regulation (1, 17, 47), activation of Cdc2 via the Cdc25C phosphatase (44, 58), DNA damage checkpoint adaptation (69), regulation of anaphase-promoting complex and 26S proteasome activity (22, 26, 39, 43, 64), and regulation of cytokinesis (4, 17, 66).
The Drosophila polo1 mutant phenotype was the first to be characterized and displays extensive spindle abnormalities. polo mutants display a high mitotic index and spindle defects that include mono- and multipolar spindles and disorganized spindle poles. These defects are thought to contribute to the high degree of abnormal chromosome segregation which leads to the observed aneuploid and polyploid states (67). Lane and Nigg established the mammalian loss-of-function phenotype by microinjecting anti-Plk antibodies into HeLa cells. These injected cells displayed mitotic arrest, monopolar spindles with duplicated but not separated centrosomes, and abnormal nuclear states such as micro- and multinucleation and ball-like condensed chromatin (46). Monopolar spindles were also observed in the plo1 loss-of-function phenotype in S. pombe (55) as well as in Xenopus embryos microinjected with anti-Plx1 antibodies (62). Work with Drosophila shows that exogenous polo added to heat-inactivated lysates preferentially phosphorylated microtubule-associated substrates, which were identified as β-tubulin, an 85-kDa microtubule-associated protein (MAP), and a 220-kDa protein later identified as the centrosomal abnormal spindle protein (asp). Genetic evidence of the synergy of the polo1 aspE3 double-mutant phenotype, as well as recent biochemical evidence, supports a role of polo in regulating centrosomal function through asp (23, 34, 68). Phosphorylation of DMAP-85 by polo was later shown to regulate its in vitro microtubule-binding activity (16). In addition, work with Xenopus egg extracts has suggested that Plx1 can phosphorylate the MAP Op18 and regulate its destabilizing activity (14). These substrates suggest possible pathways through which polo family members may function in regulating microtubule dynamics and controlling the formation of a bipolar spindle.
In this study, the yeast two-hybrid system identified a Plk substrate, the translationally controlled tumor protein (TCTP), which was recently shown to be a tubulin-binding protein that dynamically interacts with microtubules during the cell cycle (30). TCTP was originally identified as a serum-inducible 23-kDa protein band that undergoes an early and prominent increase upon serum stimulation in tissue culture cells (5). The TCTP mRNA is expressed at constant levels in both growing and nongrowing cells, and the translation is regulated by its polypyrimidine-rich 5′ untranslated region (8). TCTP localizes to microtubules from G1 until metaphase and then detaches from the spindle at the metaphase-to-anaphase transition. Both in vitro tubulin binding by TCTP and sequence homology to the tubulin-binding domain of MAP-1B support these localization data. In addition, TCTP levels in overexpressing cells were correlated with microtubule stabilization and reduced growth rate in vivo (30). Here, TCTP was shown to be directly phosphorylated in mitosis in vivo and by Plk in vitro. The Plk phosphorylation sites were mapped to two serine residues, and overexpression of a double serine-to-alanine mutant led to an increase of phenotypes associated with mitotic catastrophe such as multinucleation and rounded cells with ball-like condensed chromatin very similar to a subset of those phenotypes reported by Lane and Nigg in the Plk loss-of-function study and by Mundt et al. in experiments studying the overexpression of wild-type and kinase-dead Plk (46, 53). The kinase-substrate relationship between Plk and TCTP and the correlation between the effects of neutralizing Plk activity and of expressing a Plk phosphorylation site-deficient mutant protein are consistent with the idea that TCTP is a key mitotic target of Plk for regulating anaphase progression.
MATERIALS AND METHODS
Two-hybrid screen and cloning of TCTP cDNA
To search for proteins that interact with the conserved carboxy terminus and polo box of Plk, the XmaI-XmaI fragment of murine Plk (encoding amino acids 356 to 499) was cloned into the pGBT9 vector and fused to the DNA-binding domain of GAL4. This bait was transformed into yeast strain Y153, which carries two reporter genes, HIS3 and lacZ. The Y153 strain carrying the bait was transformed with an oligo(dT)-primed HeLa cDNA library, and the transformants were plated on Trp− Leu− His− plus 3-amino-1,2,4-triazole (a histidine biosynthesis inhibitor) selection plates and incubated at 30°C. After 7 to 10 days, the yeast colonies were subjected to β-galactosidase filter assays as described elsewhere (11). Plasmids from positive clones were sequenced on both strands by using the T7 DNA polymerase sequencing kit (Life Sciences). The sequences indicated that five of the nine positives in this screen were partial clones of human TCTP. To clone the full-length murine gene, one of the longer partial clones was used to probe a Lambda-Zap mouse neonatal brain library (Stratagene). Six of the eight positive plaques were full-length TCTP cDNA. Primers were designed to add a 5′ EcoRI site and a 3′ XhoI site for subcloning purposes and to remove the UTRs which contain translational inhibitory signals. The oligonucleotides 5′-GGAATTCATGATCATCTACCGGGACCTC-3′ and 5′-CCGCTCGAGTTAACATTTCTCCATCTCTAAGC-3′ and Taq polymerase (Promega) were used to amplify the TCTP cDNA by PCR.
Western blot analyses
Proteins resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were transferred to polyvinylidene difluoride (PVDF) blotting membranes (Immobilon-P; Millipore) at 80 V for 2 h using either a standard methanol or ethanol plus SDS Tris-glycine transfer buffer. Western blot analyses were performed with a PBST (phosphate-buffered saline plus 0.1% Tween 20) plus 3% bovine serum albumin blocking buffer, incubated with primary antibody in blocking buffer, washed three times with PBST, incubated with horseradish peroxidase-conjugated secondary antibodies (Amersham) in PBST plus 5% nonfat dry milk (Nestlé-Carnation), and finally washed four times with PBST. Bands were visualized by chemiluminescence using ECL detection reagents (Amersham-Pharmacia).
In vitro binding and phosphorylation assays.
To make glutathione S-transferase (GST)-TCTP fusion protein, the TCTP cDNA was cloned into the pGEX-KG vector. JM109 bacteria containing the resultant plasmid were diluted 1:10 from an overnight culture, grown for 1 h at 37°C, induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 4 h, and harvested. The bacteria were lysed by freezing at −70°C followed by lysozyme treatment, and GST-TCTP was purified and eluted by standard methods. HA-Plk and HA-Snk proteins were made by infecting Sf9 cells with the appropriate baculovirus (47; M. Liu and R. Erikson, unpublished data) and used either as a crude lysate or as an immunoprecipitate with 12CA5 anti-HA monoclonal antibody. For the in vitro binding assay, Sf9 cells infected with HA-Plk baculovirus for 2 days were lysed in a buffer containing 100 mM NaCl, 20 mM Tris-Cl (pH 7.5), 1 mM EDTA, 1% Triton X-100, 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF), 10 μg of pepstatin per ml, 10 μg of leupeptin per ml, and 5 μg of aprotinin per ml. Supernatants from a 10-min microcentrifuge step were incubated for 1 h with GST or GST-TCTP prebound to glutathione-agarose, and the beads were washed four times in the lysis buffer. For the in vitro kinase assays, Plk immunoprecipitated with a Plk C-13 polyclonal antibody (47) from nocodazole-treated MEL cells, HA-Plk and HA-Snk immunoprecipitated with 12CA5 monoclonal antibody from Sf9 cells infected with the appropriate baculovirus, GST-Plk purified with glutathione-agarose from Sf9 cells infected with the pAcGHLT-Plk baculovirus, or 50-μg crude lysates of random and nocodazole-treated HeLa cells were used as kinase sources to phosphorylate TCTP and casein. Plk and crude lysate kinase reactions were carried out in TBMD cocktail as described previously (47). Snk kinase reactions were carried out in a kinase cocktail containing 25 mM Tris-Cl (pH 7.5), 10 mM MgCl2, 0.1 μg of bovine serum albumin per ml, 1 mM dithiothreitol, 50 μM ATP, and 5 μCi of [γ-32P]ATP and terminated with Laemmli SDS sample buffer.
Phosphoamino acid, deletion, and point mutant analyses.
GST-TCTP phosphorylated by immunoprecipitated Plk, purified GST-Plk, and crude lysates were subjected to phosphoamino acid analysis as described previously (10). Since all of the phosphorylation localized to serine residues, deletion mutants were created to determine which of the eight serines in TCTP were phosphorylated by Plk. pGEX KG-TCTP was digested with either ClaI-XhoI or KpnI-XhoI, filled in with Klenow and T4 polymerase, respectively, and ligated to form carboxy-terminal deletions. pGEX KG-TCTP was also digested with either EcoRI-ClaI or EcoRI-KpnI, filled in with Klenow and T4 polymerase, respectively, and ligated to form amino-terminal deletions. Potential serine phosphorylation sites in TCTP were mutated to alanine by the Dut− Ung− method of mutagenesis (71).
Cell culture.
HeLa, HEK293, and MEL cells were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (Intergen), penicillin G (100 U/ml), and streptomycin (100 μg/ml) (Gibco-BRL Life Technologies). Sf9 cells were cultured in suspension at 27°C in IPL-41 medium supplemented with 10% fetal bovine serum, yeastolate, lactalbumin hydrolysate (VWR Scientific), and 0.1% pluronic acid F-68 (Gibco-BRL Life Technologies). Baculovirus infections were performed either on plates or in suspension.
Column chromatography and immunodepletion.
Shake-off cells from plates of nocodazole-treated (400 ng/ml) (Sigma) HeLa cells were harvested and lysed by Dounce homogenization in TEV (20 mM Tris [pH 7.4], 1 mM EGTA, 100 μM sodium orthovanadate) plus 10 mM β-glycerol phosphate, 20 mM p-nitrophenyl phosphate, 1 mM AEBSF, 10 μg of pepstatin per ml, 10 μg of leupeptin per ml, and 5 μg of aprotinin per ml. After centrifugation at 100,000 × g at 4°C, the supernatant was assayed for protein content by the Bradford method. A 10-mg portion of protein was then loaded onto a 1-ml MonoQ column (Amersham-Pharmacia) preequilibrated with TEV. A gradient of 0.0 to 1.0 M NaCl over 40 ml (with 5 ml of TEV supplemented with 0 and 1 M NaCl before and after the gradient, respectively) was used to elute protein from the column, and 1-ml fractions were collected. Aliquots of every other fraction as well as crude lysate were subjected to SDS-PAGE, transferred to PVDF, and Western blotted as described above using anti-Plk antibodies to determine where the Plk protein eluted. Kinase activity toward TCTP was measured for every other fraction and crude lysate by using either GST-wild-type (wt)-TCTP or GST-S46A S64A (AA)-TCTP as a substrate and utilizing kinase assay conditions described previously. The fractions containing kinase activity toward GST-TCTP were combined, dialyzed overnight in buffer A (50 mM sodium phosphate [pH 7.0], 1 mM EGTA), and loaded onto a 1-ml MonoS column (Amersham-Pharmacia) preequilibrated with buffer A. A gradient of 0.0 to 0.5 M NaCl over 35 ml followed by a gradient of 0.5 to 1.0 M NaCl over 5 ml (with 5 ml of buffer A supplemented with 0 M and 1 M NaCl before and after the gradient, respectively) was used to elute the protein from the column. All further analysis was the same as in the MonoQ run. Immunodepletion experiments were preformed by lysing nocodozole-arrested mitotic shake-off cells by Dounce homogenization in Plk kinase buffer with 150 mM NaCl. An equal amount of lysate was incubated with protein G beads, which were prebound with either anti-Plk antibodies (Zymed) or control antibodies (anti-Mek1 or anti-Myc). The lysate was then used in kinase assays with GST-wt-TCTP or GST-AA-TCTP bound to beads as substrates. The NaCl was diluted fourfold with kinase buffer to a final NaCl concentration of 37.5 mM. The GST-TCTP beads were washed and processed for SDS-PAGE.
In vivo labeling.
HEK293 cells were transfected with pCDNA-AmpI HA-TCTP (wt and AA mutant) by a standard calcium phosphate precipitation method. At ∼70% confluence, the cells were treated with nocodazole (400 ng/ml) for 16 h in standard medium, washed with PBS, and incubated in 4 ml of phosphate-free medium without serum or antibiotics and supplemented with nocodazole and 3 mCi of 32Pi per 10-cm plate. The cells were labeled for 3 h and harvested. The cell pellets were lysed in a modified RIPA buffer containing 20 mM Tris (pH 8.0), 50 mM NaCl, 1 mM MgCl2, 10% glycerol, 1% NP-40, 20 mM p-nitrophenyl phosphate, 100 μM sodium orthovanadate, 1 mM AEBSF, 10 μg of pepstatin per ml, 10 μg of leupeptin per ml, and 5 μg of aprotinin per ml. The lysates were microcentrifuged for 10 min at 4°C, and the supernatants were incubated with 4 μg of anti-HA monoclonal antibody followed by protein A-Sepharose beads. The beads were washed and prepared with Laemmli SDS sample buffer. Purified GST-TCTP was phosphorylated by purified GST-Plk using 100 μCi of [γ-32P]ATP, and the reaction was stopped with sample buffer. The samples were subjected to SDS-PAGE and transferred to PVDF. Part of each sample was loaded separately for Western analysis to confirm protein expression. The membrane was then subjected to autoradiography, and the appropriate bands were excised with a scalpel. The standard tryptic mapping protocol (10) was followed. The electrophoresis was performed in pH 1.9 buffer, and the chromatography was performed in phosphochromatography buffer. The thin-layer chromatography plates were then subjected to autoradiography.
Microscopy.
HEK293 cells were grown on collagen (Sigma)-coated coverslips and transfected with green fluorescent protein (GFP), GFP-wt-TCTP, GFP-AA-TCTP, HA-wt-TCTP, and HA-AA-TCTP expression vectors by the standard calcium phosphate protocol. The cells were fixed with 4% paraformaldehyde for 10 min and permeabilized with methanol for 2 min. They were stained with anti-β-tubulin and anti-γ-tubulin primary antibodies (Sigma) and donkey anti-mouse fluorescein isothiocyanate-conjugated secondary antibodies (Jackson ImmunoResearch) by a standard method. DNA was stained with propidium iodide. Stained cells were viewed either under a Zeiss LSM-410 confocal microscope equipped with a krypton-argon laser or under a Nikon Optiphat 2 microscope and Diagnostic Instruments Spot camera.
Polyclonal antibody generation.
Polyclonal antibodies were generated in rabbits by Cocalico Biologicals, Inc. (Reamstown, Pa.), using bacterially produced and purified GST-TCTP as the antigen. Antibodies were immunopurified by first preclearing with GST covalently linked to CNBr-activated Sepharose 4B (Amersham-Pharmacia), binding to GST-TCTP Sepharose 4B beads, washing with 100 mM Tris (pH 8.0)-500 mM NaCl, and eluting with 100 mM glycine (pH 2.5).
RESULTS
TCTP clones interacted with a carboxy-terminal polo box-containing Plk bait in the two-hybrid screen.
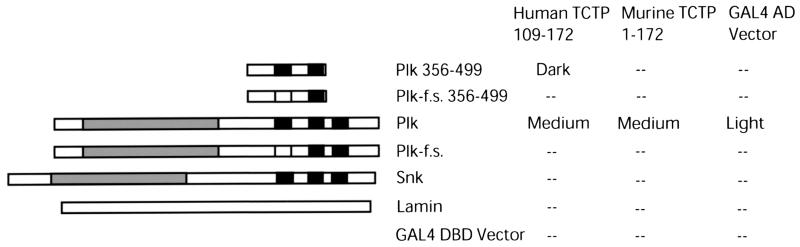
The polo box is a hallmark of this family of kinases; however, very little is known about the function of this conserved noncatalytic domain. It is essential in Plk and Cdc5p for localization to substructures such as the spindle pole body and cytokinetic neck filaments and for mitotic progression in S. cerevisiae, while having no detectable influence on the protein's in vitro casein kinase activity (48, 66). It had also been hypothesized that the polo box may influence substrate specificity, as was later shown with GRASP65 (50). To further elaborate on the role of the conserved carboxy terminus and polo box of Plk and to illuminate possible Plk signaling pathways, a 144-amino-acid region of Plk containing the polo box was used as a bait for a two-hybrid screen. This bait was used to screen an oligo(dT)-primed HeLa library. Five of the nine positives from the screen were partial clones of human TCTP. The clones ranged from the carboxy-terminal 34 to 143 of the full-length 172 amino acids, with the shorter clones binding more strongly to the Plk polo box bait. Clone 53.1 (amino acids 107 to 172) interacted with full-length Plk but not with full-length Snk, which also contains a polo box domain, and did not interact with the lamin and vector controls. The interactions with both full-length Plk and the original 144-amino-acid noncatalytic baits were demonstrated to be polo box dependent via a polo box frameshift mutation (50) (Fig. 1).
FIG. 1.
Two-hybrid analysis of the TCTP-Plk interaction. A Plk carboxy-terminal bait containing the polo box interacts with HeLa partial clones of TCTP in the two-hybrid screen. Five of the nine positives from the screen were partial clones of human TCTP. One partial human clone and full-length murine TCTP were shown to interact with full-length Plk but not full-length Snk, and this interaction was shown to be dependent on the polo box of Plk (f.s. is the frameshift mutation). Lamin and GBT9 (GAL4 DNA-binding domain vector) were used as negative controls. Interactions were determined by assaying for β-galactosidase activity, with interaction or activity strength denoted by dark, medium, light, and (--), which roughly correlated to turning blue in under 1 h, between 1 and 4 h, longer than 4 h, and not detectable after 24 h, respectively. The Plk bait showed a slight degree of autoactivation; however, the Plk-TCTP (both partial and full-length) interactions were consistently much stronger (detectably blue in 1.5 h versus 6 h for the control) as determined by β-galactosidase activity.
The full-length murine gene was cloned from a mouse neonatal brain lambda phage library using a [α-32P]dCTP-labeled probe of clone 56.1 (coding for amino acids 97 to 172). Eight positives in duplicate from 106 plaques were picked, and six phages were determined to contain full-length coding sequences. PCR was used to subclone the TCTP open reading frame and remove the translational inhibitory signals (9, 20). The full-length murine TCTP gene was subcloned in frame with the GAL4 DNA-binding domain to determine if full-length Plk, Snk, and TCTP interact in the two-hybrid system in the same fashion as the Plk polo box bait and partial TCTP clones. Full-length TCTP interacted with Plk in a polo box-dependent manner, but not with Snk, as determined by the β-galactosidase assay (Fig. 1). The interaction is specific for Plk even though both Plk and Snk contain almost identical 30-amino-acid polo boxes. Perhaps the slight variability in the polo boxes themselves or the divergence in the polo box-flanking regions could explain the specificity of binding to TCTP.
Plk interacts with and phosphorylates TCTP in vitro.
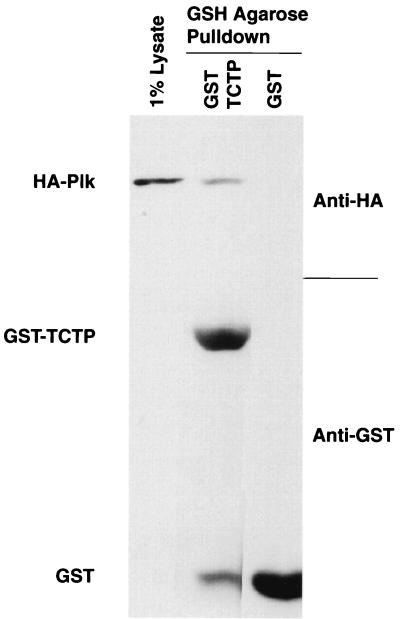
To confirm that Plk and TCTP interact in vitro as they do in the two-hybrid system, TCTP was expressed as a GST fusion protein. GST-TCTP and GST immobilized on glutathione-agarose beads were incubated with lysates from baculovirus-infected Sf9 cells expressing HA-Plk. After extensive washing, GST-TCTP, but not the GST control, was found to retain HA-Plk from these lysates (Fig. 2).
FIG. 2.
In vitro binding of HA-Plk to GST-TCTP. Lysates from baculovirus-infected Sf9 cells expressing HA-Plk were used in a pull-down assay with either GST or GST-TCTP prebound to glutathione (GSH)-agarose beads. HA-Plk and GST/GST-TCTP were detected by Western immunoblotting with anti-HA monoclonal and anti-GST polyclonal antibodies, respectively.
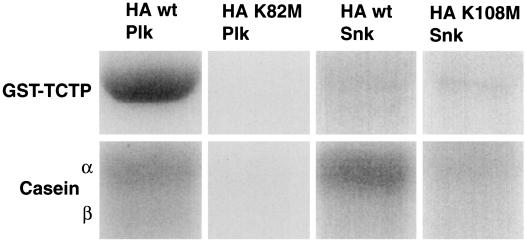
Since Plk was shown to bind TCTP in the two-hybrid system and in vitro, kinase reactions were carried out to determine if TCTP is also a Plk substrate. HA-wt-Plk and HA-wt-Snk, as well as their respective kinase-defective mutants HA-K82M-Plk and HA-K108M-Snk, were immunoprecipitated from baculovirus-infected Sf9 cell lysates with anti-HA antibody. Immunocomplex kinase reactions were performed using [γ-32P]ATP and either GST-TCTP or casein as substrates. As shown in Fig. 3, HA-wt-Plk could efficiently phosphorylate both GST-TCTP and the exogenous substrate casein. Thrombin cleavage of GST-TCTP, as well as kinase reactions using GST alone as a substrate, indicated that Plk phosphorylates residues in TCTP and not in the GST moiety (data not shown). HA-wt-Snk displayed kinase activity toward casein but could not phosphorylate TCTP to any appreciable degree. In addition, kinase-defective mutants of both HA-Plk and HA-Snk could not phosphorylate GST-TCTP, indicating that the phosphorylation of GST-TCTP by HA-wt-Plk was due to the catalytic activity of Plk and not to a Plk-associated kinase.
FIG. 3.
Plk but not Snk phosphorylates TCTP. HA-wt-Plk, HA-wt-Snk, and their respective kinase-defective mutants from baculovirus-infected Sf9 cells were used in anti-HA immunocomplex kinase reactions with either GST-TCTP or casein as substrates. The exogenous Plk and Snk substrate casein was used as a control for kinase activities.
Plk phosphorylates TCTP on two serine residues.
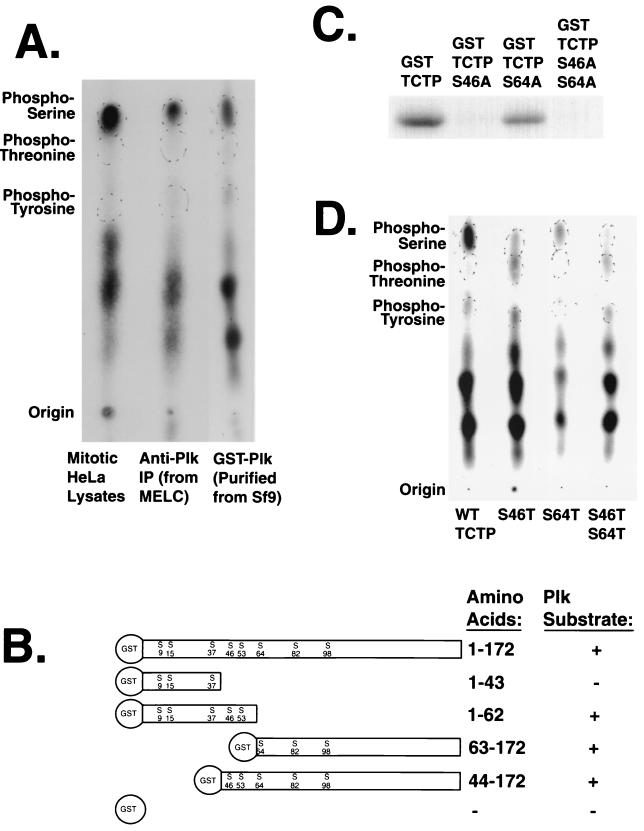
To determine the TCTP sites phosphorylated by Plk, phosphoamino acid analysis was performed to first identify which types of residues were phosphorylated. In vitro kinase reactions were performed with endogenous Plk immunoprecipitated from nocodazole-treated MEL cells or GST-Plk purified from baculovirus-infected Sf9 cells using GST-TCTP as a substrate. The reactions were processed for partial amino acid hydrolysis followed by electrophoresis on thin-layer chromatography plates. GST-TCTP was phosphorylated exclusively on serine residues by purified and immunoprecipitated Plk. In addition, this phosphoamino acid composition matched that of GST-TCTP phosphorylated by crude nocodazole-arrested HeLa cell lysates in vitro (Fig. 4A).
FIG. 4.
Plk phosphorylates TCTP on two serine residues. (A) Both Plk and mitotic lysates phosphorylate TCTP on serine residues. Partial acid hydrolysis followed by phosphoamino acid analysis was performed on GST-TCTP phosphorylated by GST-Plk purified from baculovirus-infected Sf9 cells, immunoprecipitated Plk from nocodazole-treated MEL cells, and crude lysate from nocodazole-arrested HeLa cells. (B) Deletion mapping of in vitro Plk phosphorylation sites in TCTP indicates more than one phosphorylation site. (C) Serine-to-alanine point mutant analysis indicates that Plk phosphorylates TCTP on serines 46 and 64 in a hierarchical fashion. GST-TCTP and mutant fusion proteins were phosphorylated by Sf9-produced GST-Plk in separate in vitro kinase reactions. (D) Serine-to-threonine mutations in TCTP support the two-Plk-phosphorylation-site hypothesis. TCTP mutants with combinations of serines and threonines at resides 46 and 64 were phosphorylated by GST-Plk and subjected to phosphoamino acid analysis. The lack of apparent Plk phosphorylation on the Ser64Thr residue indicates a specificity for serine at this site.
A combination of deletion mutant and point mutant analyses was performed to determine which of the eight serines in TCTP were phosphorylated by Plk. Two amino- and two carboxy-terminal deletion mutants were generated using internal and external restriction sites. Deletion mutant analysis indicated that there were at least two phosphorylation sites, with at least one serine phosphorylated in a peptide containing Ser46 and Ser53 and at least one serine phosphorylated in a peptide containing Ser64, Ser82, and Ser98 (Fig. 4B). Site-directed mutagenesis to convert serines to alanines was performed, and two alanine substitutions, Ser46 and Ser64, affected the degree to which Plk could incorporate radioactive phosphate on GST-TCTP. The Ser64Ala mutation reduced the phosphorylation by Plk to about half that of GST-wt-TCTP. Unexpectedly, the Ser46Ala mutation abrogated phosphorylation by Plk, as did the Ser46Ala Ser64Ala double mutant (AA-TCTP). This suggests a hierarchical mechanism in which the phosphorylation of Ser64 requires the prior phosphorylation of Ser46 in the full-length TCTP protein (Fig. 4C). Phosphoamino acid analysis of serine-to-threonine mutants phosphorylated by Plk confirmed that both of these sites and only these two sites in TCTP are phosphorylated by Plk in vitro (Fig. 4D).
Kinase activity toward TCTP is upregulated in mitosis, and the majority of activity cofractionates with Plk.
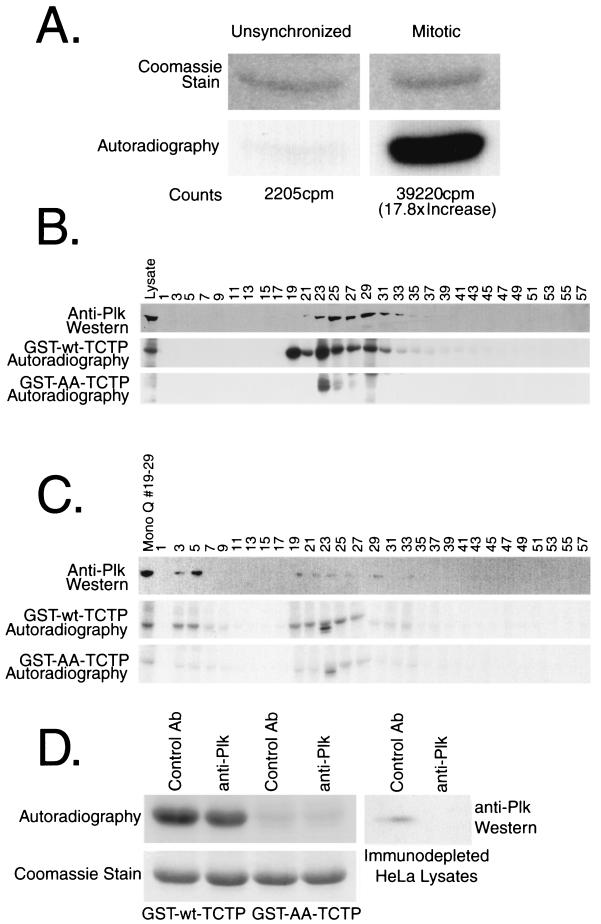
Since the kinase activity of Plk peaks in mitosis, crude lysates were assayed for kinase activity toward TCTP to determine if both kinase activities coincided in a cell cycle-dependent manner. Cell lysates from growing and nocodazole-arrested HeLa cells were lysed, and 50 μg of each lysate was incubated with [γ-32P]ATP and GST-TCTP immobilized on glutathione-agarose. The GST-TCTP beads were washed, the TCTP was cleaved from the GST moiety with thrombin, the supernatants were resolved by SDS-PAGE, and TCTP phosphorylation was visualized by autoradiography. In vitro kinase activity toward TCTP was elevated approximately 18-fold in nocodazole-arrested cell lysates compared to unsynchronized cell lysates (Fig. 5A).
FIG. 5.
Kinase activity toward TCTP is high in mitotic lysates, and the majority cofractionates with Plk on successive MonoQ and MonoS columns. (A) Unsynchronized and nocodazole-arrested HeLa lysates were incubated with GST-TCTP on beads in an in vitro kinase reaction. The washed GST-TCTP was thrombin cleaved, and subjected to SDS-PAGE and then to autoradiography. (B) Nocodazole-arrested HeLa lysates were fractionated over a MonoQ column. The top panel is an anti-Plk blot showing the Plk elution pattern over the salt gradient. The middle and bottom panels are kinase reactions using GST-wt-TCTP and GST-AA-TCTP, respectively, to monitor the elution pattern of kinase activities toward TCTP. (C) MonoQ fractions 19 to 29 were loaded onto a MonoS column and subjected to a similar analysis as performed on the MonoQ fractions. The middle and bottom panels depict kinase reactions with each column fraction and either GST-wt-TCTP or GST-AA-TCTP, respectively, as a substrate. The top panel shows Plk fractionation as determined by Western blotting. The slightly faster-migrating band phosphorylated in fraction 23 from the MonoQ and fraction 23 from the MonoS column correspond to a contaminating protein from the lysate and not phosphorylation on GST-TCTP. (D) Immunodepletion experiments show that at least one-third of the kinase activity toward TCTP in HeLa cells is due to Plk or a Plk complex in vitro. The top panel shows kinase reactions with an equal amount of crude lysate that were immunodepleted with a control antibody (anti-Myc or anti-Mek1) or with anti-Plk antibodies. The bottom panel shows the Coomassie-stained bands of GST-TCTP with the wild-type fusion protein in the left two lanes and the double-alanine mutant in the right two lanes. The right-hand panel shows the effectiveness of the Plk immunodepletions.
Since the elevation of kinase activity toward TCTP corresponds to the point in the cell cycle when Plk is active, nocodazole-arrested HeLa lysates were fractionated by column chromatography to determine if the kinase activity toward TCTP cofractionates with the Plk protein. Nocodazole-arrested HeLa cells were lysed, and a soluble supernatant was prepared. A 10-mg portion of protein was loaded on a MonoQ column, and odd-numbered column fractions were assayed for kinase activity toward TCTP. Kinase reactions using GST-AA-TCTP as a substrate were also run to show the specificity of the phosphorylation to serine residues 46 and 64. To determine the Plk elution profile, odd-numbered fractions were probed with anti-Plk monoclonal antibodies. Kinase activity toward TCTP eluted in fractions 19 through 37. GST-AA-TCTP was phosphorylated to a slight degree in fractions 23 though 29, which indicates that there may be a kinase that can phosphorylate alternative sites in TCTP or perhaps the GST moiety, in vitro. The radiolabeled band below both GST-wt-TCTP and GST-AA-TCTP in fraction 23 appears to correspond to a Coomassie-stainable band that remained bound to the bead complex after the washes. Plk protein coeluted with the kinase activity toward TCTP in fractions 21 through 37; the kinase activity that elutes in fraction 19 did not coelute with Plk and appears to be another kinase which can phosphorylate GST-TCTP on the same two serine residues as Plk can (Fig. 5B).
Fractions 19 through 29 were loaded on a MonoS column, and analyses similar to those done in the MonoQ run were performed on the MonoS fractions. The kinase activity toward TCTP eluted in the flowthrough in fractions 3 through 5 and in the salt gradient in fractions 19 through 29. A non-serine 46- and serine 64-directed kinase activity appears relatively faintly in fractions 23 through 27. Plk protein coeluted with the kinase activity toward TCTP in both the flowthrough and salt gradient fractions. The second kinase activity as observed in the MonoQ run was not distinct from the Plk elution pattern and may be coeluting with Plk in some of the fractions (Fig. 5C).
Thus, Plk appeared to cofractionate with the majority of the kinase activity toward TCTP over two successive column runs. The majority of all kinase activity toward TCTP was specific for the two serine residues phosphorylated by Plk in vitro. There appears to be at least one other in vitro kinase activity toward Ser46 and Ser64 in TCTP that does not coelute with Plk in some of the fractions. The presence of this secondary kinase activity is supported by in vitro kinase assay data obtained using anti-Plk immunodepleted cell lysates (Fig. 5D). Purified cdc2-cyclin B1 and anti-cyclin B1 immunocomplexes, both with high histone H1 kinase activities, were not able to phosphorylate TCTP to any detectable level in vitro, which indicates that cdc2-cyclin B1 is unlikely to be the other kinase activity observed in vitro (data not shown).
In vivo phosphorylation sites on TCTP include those phosphorylated by Plk in vitro.
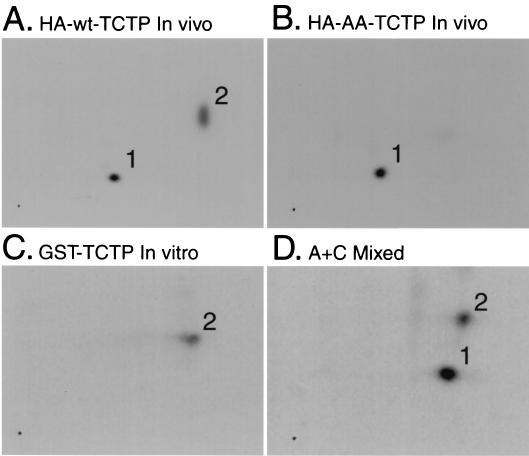
To show that the sites on TCTP phosphorylated by Plk in vitro are relevant sites in vivo, HEK293 cells were transfected with cytomegalovirus (CMV) promoter-driven HA-TCTP constructs (both wt and AA), treated with nocodazole for 16 h, and labeled with 3 mCi of 32Pi for 3 h in the presence of nocodazole. The products of an in vitro kinase reaction with GST-Plk and GST-TCTP as well as the anti-HA immunoprecipitates from the in vivo-labeled cell lysates were processed for two-dimensional tryptic analysis.
HA-wt-TCTP was phosphorylated on two different tryptic peptides (Fig. 6A), while the double-alanine mutant was phosphorylated on only one tryptic peptide (Fig. 6B). GST-TCTP was phosphorylated by Plk on one tryptic peptide in vitro (Fig. 6C), which matches the phosphorylated tryptic peptide present in HA-wt-TCTP but absent in HA-AA-TCTP in vivo, as confirmed by a mixing experiment (Fig. 6D).
FIG. 6.
The Plk in vitro phosphorylation sites on TCTP are phosphorylated in vivo in mitosis. Tryptic mapping of in vivo-labeled overexpressed HA-wt-TCTP (A) and HA-AA-TCTP (B) of GST-TCTP phosphorylated by GST-Plk in vitro (C) and of in vivo HA-wt-TCTP and in vitro GST-TCTP mixed (D). The horizontal axis represents electrophoresis in pH 1.9 buffer, and the vertical axis represents chromatography in phosphochromatography buffer.
The Plk phosphorylation site-deficient TCTP mutant interferes with karyokinesis.
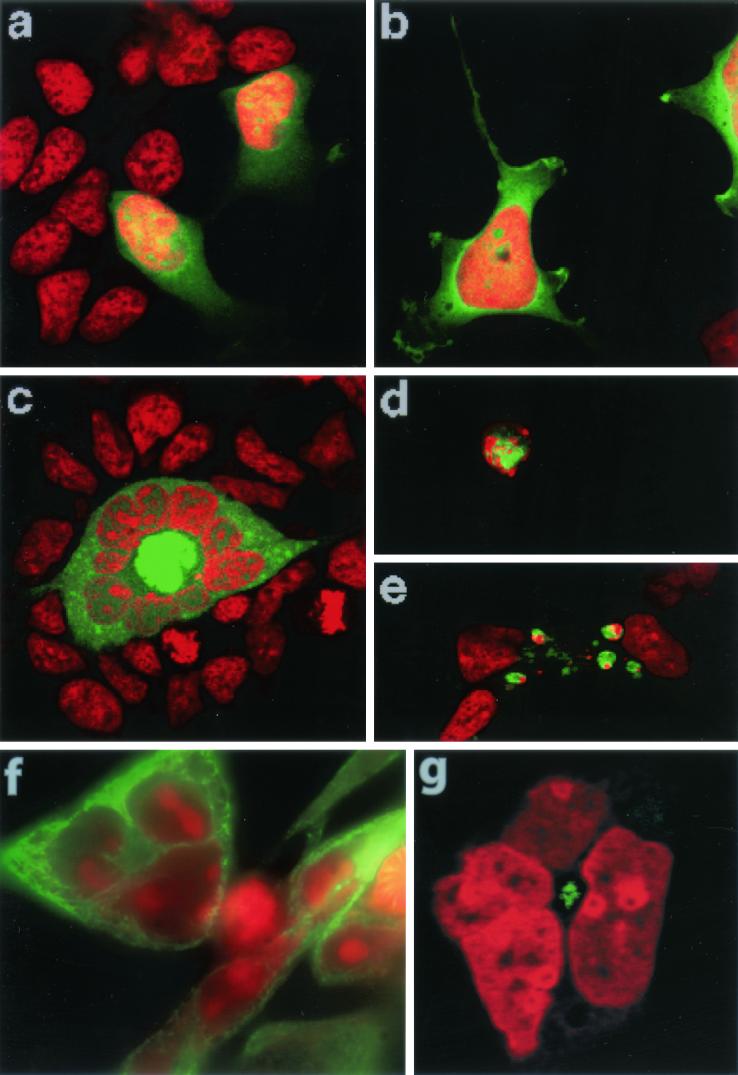
Since serine 46 and serine 64 appear to be phosphorylated in vivo during mitosis, the overexpression of a double-alanine mutant of TCTP was tested for its effect on mitosis and the cell cycle. HEK293 cells grown on collagen-treated coverslips were transfected with a CMV promoter-driven GFP (Fig. 7a), GFP-wt-TCTP (Fig. 7b), and GFP-AA-TCTP constructs (Fig. 7c). At 40 h posttransfection, the cells were fixed and the DNA was stained with propidium iodide. Three independent observers, testing blindly with respect to the construct transfected, each scored approximately 280 green fluorescent cells per construct. The cells were scored for those that contained one nucleus (Fig. 7a and b) or several nuclei (Fig. 7c), mitotic cells, rounded cells with ball-like condensed chromosomes (Fig. 7d), and cells undergoing cell death (Fig. 7e). The cells undergoing cell death showed nuclear and cytoplasmic shrinkage, membrane blebbing, and cell fragmentation.
FIG. 7.
Overexpression of AA-TCTP leads to an increase in the number of multinucleated cells, and overexpression of wt-TCTP and AA-TCTP leads to an increase in the number of cell deaths and cells with ball-like condensed DNA. (a to c) GFP (a), GFP-wt-TCTP (b), and GFP-AA-TCTP (c) were overexpressed in HEK293 cells. Panel c shows an example of the multinucleate phenotype. (d and e) Overexpression of either form of TCTP leads to an increase in the number of rounded cells with ball-like condensed DNA (d) and the number of cells undergoing cell death (e). DNA is stained with propidium iodide (red) and transfected cells fluoresce green in these images. (f) The microtubule network in these multinucleate HA-tagged AA-TCTP-expressing cells appears interphase-like and is concentrated in the center of the ring of nuclei (upper left). Interphase nontransfected cells possess a microtubule network throughout the cytoplasm (right). The green signal is β-tubulin staining, and the red signal is DNA (propidium iodide staining). (g) A pair of centrosomes also localizes to the center of the nuclear annulus in AA-TCTP-transfected cells, as determined by γ-tubulin staining (green) and DNA staining (red).
Overexpression of GFP-AA-TCTP led to a 2.8-fold increase in the number of multinucleate cells compared to overexpression of both GFP and GFP-wt-TCTP. The multinucleation observed in both cells expressing the GFP and the GFP-wt-TCTP proteins was at the basal level of binucleated cells characteristic of many tissue culture cell lines, and only the GFP-AA-TCTP-expressing cells routinely contained three or more nuclei. In fact, the GFP-AA-TCTP-expressing cells had as many as 13 nuclei, some of which appeared to be as small as a single chromosome, often referred to as satellite DNA (Table 1). Fluorescence-activated cell sorter analysis indicates that these mutant TCTP-expressing cells had a DNA content between 2N and 4N DNA, with no detectable peaks at higher ploidy levels (data not shown). In addition, the nuclei in these multinucleate cells generally appeared less intensely stained by propidium iodide and more hollow in appearance than the nuclei of the neighboring cells. The multinucleate cells were often arranged in a partial or complete annulus around an intensely fluorescent GFP-AA-TCTP core (Fig. 7c). Staining with anti-α- or anti-β-tubulin antibodies indicates that this core is enriched with interphase-like microtubules and that these microtubules are radiating out from the center of the annulus. On the other hand, interphase nontransfected cells have microtubules throughout the cytoplasm, with a slight enrichment at the cell periphery (Fig. 7f). The observed intensely fluorescent GFP-AA-TCTP cores may be due to the enrichment of microtubules in this region and the ability of TCTP to bind tubulin (Fig. 7c and f). Staining with anti-γ-tubulin antibodies suggests that a pair of centrosomes is in the center of the nuclear ring (Fig. 7g). In addition, over a 9-day observation period, these transfected multinucleate cells continued to grow to well over 10-fold larger than the untransfected neighboring cells, and these multinucleate cells failed to enter mitosis or undergo cell death (Fig. 7c and data not shown).
TABLE 1.
Phenotypes of 40-h GFP, GFP-wt-TCTP, and GFP-AA-TCTP overexpression in HEK293 cellsa
| Cell type | % of cells expressing:
|
||
|---|---|---|---|
| GFP vector | GFP-wt-TCTP | GFP-AA-TCTP | |
| Mononucleate | 85.6 ± 7.2 | 81.6 ± 7.4 | 69.6 ± 7.6 |
| Multinucleate | 5.28 ± 2.79 | 5.31 ± 0.37 | 14.6 ± 5.8 |
| Mitotic | 1.99 ± 0.72 | 1.03 ± 0.29 | 1.76 ± 1.23 |
| Condensed+cell death | 7.11 ± 5.40 | 11.8 ± 7.4 | 14.1 ± 7.8 |
| Mean no. of cells counted | 274 ± 30 | 289 ± 27 | 277 ± 33 |
Asynchronously growing HEK293 cells on coverslips were transfected with CMV-driven GFP, GFP-wt-TCTP, and GFP-AA-TCTP plasmids. After a 40-h incubation, the cells were fixed, permeabilized, stained with propidium iodide, and analyzed by confocal and immunofluorescence microscopy. Three independent observers testing blindly scored the phenotypes of the green fluorescent cells. Values are means ± standard deviations.
Besides the increase in the multinucleate phenotype, there was a 1.7- and 2.0-fold increase in the number rounded cells with ball-like condensed chromosomes and cells undergoing cell death for cells expressing GFP-wt-TCTP and GFP-AA-TCTP, respectively. The total degree of cell death and ball-like condensed chromosomes per transfectant is most probably much greater due to the transient nature of these phenotypes before detachment from the coverslip. In addition, there was no discernible effect on the mitotic index observed for expression of any of the constructs (Table 1). The phenotypes observed by expressing the double-alanine mutant TCTP in cells are indicative of a mitotic catastrophe, with the phenotypes of cell cycle arrest and abnormal nuclear and microtubule structures being most striking.
Low-level taxol treatment and expression of double-alanine TCTP have an additive effect on karyokinesis.
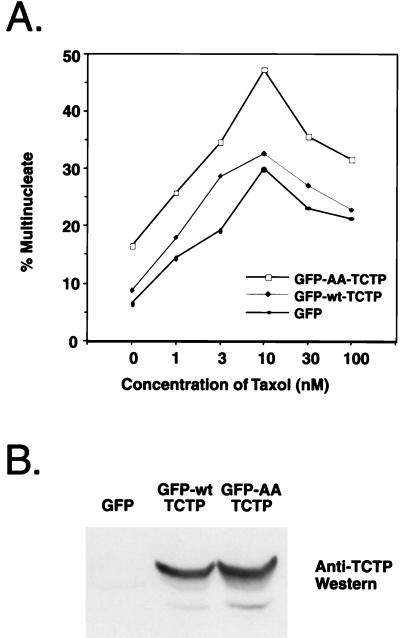
The phenotype of cells expressing TCTP with alanine substitutions in the Plk phosphorylation sites in HEK293 cells was quite similar to the observations made by Jordan et al. for HeLa cells treated with low (∼10 nM) concentrations of the microtubule-stabilizing drug taxol. These phenotypes include spindle abnormalities as well as a similar multinucleate terminal state (40). To determine if the karyokinesis defects induced by taxol treatment and the AA-TCTP mutant expression could be additive, HEK293 cells were transfected with either GFP, GFP-wt-TCTP, or GFP-AA-TCTP and treated with 0, 1, 3, 10, 30, or 100 nM taxol. The cells were fixed and stained for fluorescence microscopy 24 h after transfection and drug treatment. Green fluorescent cells were scored for being either mono- or multinucleate. The experiment was performed in triplicate, with approximately 100 cells scored for each condition per trial. The effect of the taxol treatment on cells expressing the double-alanine TCTP mutant protein in terms of inducing multinucleation was additive and at some concentrations was synergistic relative to both the cells expressing wild-type TCTP and vector the alone control (Fig. 8A). An anti-TCTP Western blot shows comparable levels of exogenous wt- and AA-TCTP expression (Fig. 8B). Therefore, it appears that the double-alanine TCTP mutant and taxol may be working in a similar pathway to disrupt spindle function and mitotic progression.
FIG. 8.
Taxol and expression of AA-TCTP have additive effects to the multinucleate-cell index. (A) Cells grown on coverslips were transfected and taxol treated for 24 h, fixed, and scored for a multinucleate phenotype (average of three separate transfections). (B) An anti-TCTP Western blot showing that the phenotype is not due to differential stability or expression levels of GFP-wt-TCTP and GFP-AA-TCTP.
DISCUSSION
Plk but not Snk interacts with and phosphorylates the microtubule-stabilizing protein TCTP in vitro.
Examination of the mutant phenotypes of polo1, loss of function of plo1, and the effect of anti-Plk antibody microinjection shows that the most apparent cytological effect is that of aberrations in the mitotic spindle. In this study, the Plk substrate TCTP was identified by a two-hybrid screen using a bait containing the highly conserved polo box domain found in the carboxy terminus of Plk. While Plk does bind to TCTP in vitro, the most notable biochemical aspect of this interaction is that Plk can robustly phosphorylate TCTP in vitro. The mammalian polo family member Snk did not interact with TCTP in the two-hybrid system and did not phosphorylate TCTP in vitro. The binding and two-hybrid interaction is specific for Plk even though Plk and Snk have 52% overall identity and 73% identity in the polo box domain (21). The strongest TCTP clone, which is also the shortest, is a very hydrophobic region in the carboxy terminus, with more than half of the 34 residues being hydrophobic. In addition, these hydrophobic residues are concentrated in two discrete and evolutionarily conserved patches of five or six residues each. Both the nuances in the polo box sequences and, more likely, the divergent flanking regions around the polo boxes might dictate the binding specificity of these kinases; interestingly, the seven residues preceding the Plk polo box are all hydrophobic whereas the same region in Snk is rather hydrophilic. The specificity of the results was surprising due to the expression patterns of Plk, Snk, and TCTP. Both Snk and TCTP are immediate-early genes, and their respective proteins appear within an hour after serum stimulation, while the M-phase-specific Plk protein does not begin to appear until 14 h after serum stimulation or during the late S phase of NIH 3T3 cells (8, 47, 65). The disparity between the timing of TCTP translation at the beginning of interphase and the timing of Plk kinase activity toward the end of the cell cycle seemed to suggest that nonphosphorylated TCTP could be functioning as an inhibitor to mitotic progression that is either resynthesized or dephosphorylated at the initiation of the next cell cycle.
To gain some insight into the Plk-TCTP interaction and TCTP function in general, a mutant that cannot be phosphorylated by Plk was generated. The Plk phosphorylation sites on TCTP were mapped by deletion and point mutant analysis to two serines located at residues 46 and 64. These two sites are similar in that the serine residues are preceded by one or two aspartate or glutamate residues and followed by several residues with uncharged side chains [E-G-(A/E)-(I/G)-(D/T)-(D/E)-S-(L/T)-(I/V)]. This consensus site is similar to that predicted by Golsteyn et al. By comparing specific residues in the conserved kinase domain of Plk to those in protein kinase A which have been analyzed by X-ray crystallography, the authors predicted that the second and third residues amino-proximal to the phosphorylated serine or threonine in the Plk substrate would not be positively charged and that the residue following the serine or threonine would be hydrophobic (32). This prediction matches the evidence from serine-to-alanine and serine-to-threonine mutations than Ser46 (IDDSL) is a stronger Plk phosphorylation site than Ser64 (GTEST). In addition, Plk appears to prefer negatively charged regions and substrates such as casein, α-, β-, and γ-tubulin, and TCTP (pI = 4.5) over positively charged ones such as histone H1 (27, 30, 33, 47). Along with the sites mapped in S. cerevisiae Cdc5p phosphorylation of Scc1p and in Xenopus Plx1 and human Plk phosphorylation of human cyclin B1, a polo kinase phosphorylation site consensus sequence can be derived as a stretch of six uncharged or negatively charged residues preceding a serine (or threonine). All but Ser147 of cyclin B1 are directly preceded by one or two aspartate or glutamate residues in the two positions amino-proximal to the phosphorylated residue, and the phosphorylated residue is followed by an uncharged residue (2, 70).
The vast majority of phosphorylation by crude lysates on TCTP in vitro matches these two Plk phosphorylation sites. In addition, column chromatography demonstrated that a large proportion of the kinase activity toward TCTP cofractionates with Plk over two columns. The contribution by Plk in crude lysates toward phosphorylating TCTP in vitro was further demonstrated by anti-Plk immunodepletion experiments. The other mitotically active polo family members and related members, Fnk/Prk (19, 59) and Sak-a (29), might be responsible for this mitotic non-Plk kinase activity toward TCTP. Support for the possibility of overlapping regulation of mitotic substrates by polo family members is drawn from the fact that both Plx1 (44) and Prk (58) have been reported to be able to phosphorylate Cdc25C and upregulate its phosphatase activity in vitro.
TCTP is phosphorylated in vivo on the in vitro Plk phosphorylation sites and may transiently colocalize with Plk during the metaphase-to-anaphase transition.
Two-dimensional mapping shows that two different tryptic peptides are phosphorylated in vivo, and one of the peptides appears to contain the two sites phosphorylated by Plk in vitro. The identity of the second peptide, as well as the kinase responsible for the phosphorylation and the functional significance of this phosphorylation, is not known. The in vivo phosphorylation on this second peptide could be due to the same activity which is responsible for the in vitro phosphorylation detected in both the MonoQ and MonoS columns runs while using the double-alanine TCTP mutant protein as a substrate.
These two in vitro Plk phosphorylation sites are just amino-terminal to the basic domain in TCTP, which has similarity to the tubulin-binding domain of MAP-1B. TCTP is a tubulin-binding protein in vitro, and it interacts with microtubules in interphase through metaphase and detaches during the metaphase-to-anaphase transition (30). It is possible that when Plk translocates at the metaphase-to-anaphase transition from the centrosomes to the midzone by way of the spindle microtubules (33) in a hypothesized MKLP-1/CHO1-dependent fashion (1, 17, 47), it can phosphorylate any TCTP bound to these microtubules. The binding affinity of TCTP for tubulin may be phosphorylation dependent, similar to that of several MAPs including MAP2 (13), E-MAP-77 (12), and tau (35).
The Plk phosphorylation site mutant of TCTP appears to induce an abortive mitosis by overstabilizing microtubules during the metaphase-to-anaphase transition.
The multinucleate phenotype observed in cells overexpressing the double-alanine mutant of TCTP is quite similar to that seen in other p53+ cells treated with low concentrations of the microtubule-stabilizing drug taxol. These taxol-treated cells arrest at the metaphase-to-anaphase transition after sister chromatid separation and proceed to undergo an aberrant mitotic exit without the occurrence of anaphase chromosome-to-pole movement or cytokinesis. The cells re-form nuclear membranes around subgenomic chromosome aggregations, giving rise to a multinucleate state, and enter a G1-like arrested state with interphase-like microtubule arrays and decondensed chromatin. More than 55% of these taxol-treated cells become multinucleate and later undergo apoptosis after 3 to 5 days (40, 41, 73). Similarly, overexpression of wt-TCTP during interphase has been reported to show an increase of microtubule bundling and stabilization against depolymerization by nocodazole treatment (30). Perhaps this microtubular stabilization activity of TCTP during interphase is carried over into mitosis in cells expressing the double-alanine mutant and thereby interferes with the delicate balance of microtubule-stabilizing and -destabilizing proteins needed to ensure a proper karyokinesis. Low-level taxol treatment also yields monopolar spindles as well as abnormal bipolar metaphase-like spindles with lagging chromosomes near one or both poles (40). Preliminary data from double-alanine TCTP mutant-expressing tetracycline-repressible HeLa cells showed similar phenotypes of monopolar spindles as well as bipolar spindles with chromosomes dispersed from the midzone to each pole only in the absence of tetracycline (data not shown). In addition, taxol treatment and the expression of the double-alanine TCTP mutant in HEK293 cells, which are generally assumed to be p53+, induced a greater number of multinucleate cells after 24 h than did either treatment alone or with the combined expression of wild-type TCTP and treatment with taxol. The effect of multinucleation in taxol treatment is thought to be a result of an abortive mitosis due to a block specifically at the metaphase-to-anaphase transition. This block has been proposed to be caused by taxol increasing the stability of microtubules and decreasing their dynamic nature, which is required to apply tension between sister chromatids (40). Similarly, TCTP has been reported to increase the stability of microtubules and has been shown to dissociate from the spindle at the metaphase-to-anaphase transition (30).
TCTP may be a key target substrate of Plk, since this multinucleate phenotype as well as the increase in cells undergoing cell death and rounded cells with ball-like condensed chromatin show a remarkable similarity to a subset of the effects of microinjecting anti-Plk antibodies into tissue culture cells. Injection of anti-Plk antibodies into HeLa cells and overexpression of AA-TCTP in HEK293 cells caused a fivefold and a threefold increase, respectively, in the number of multinucleate cells compared to the control conditions (46). The similarities in the stabilization of microtubules by taxol and by interphase TCTP, the effect of taxol and expression of the double-alanine mutant on anaphase progression, and the temporal correlation of Plk traversing the microtubules from the centrosome to the midzone and of TCTP detaching from the microtubules seem to suggest that Plk plays a regulatory role through TCTP in controlling spindle dynamics at the metaphase-to-anaphase transition. Furthermore, the similarities between removing Plk kinase activity from the cell and expressing a nonphosphorylatable in vitro Plk substrate indicate that TCTP could be an important downstream signaling component of Plk, regulating the cell cycle at both the metaphase-to-anaphase and the mitosis-to-interphase transitions.
Acknowledgments
I am grateful to Raymond Erikson, Timothy Mitchison, David Pellman, Brian Dynlacht, John Chant, Kyung Lee, Alessandro Alessandrini, Barbara Brott, Yasuhiko Terada, Lori Taylor, Chin-Yo Lin, and Benjamin Pinsky for critical discussions and reading of the manuscript.
This research was supported by NIH grants CA62580 and GM59172.
REFERENCES
- 1.Adams, R. R., A. A. Tavares, A. Salzberg, H. J. Bellen, and D. M. Glover. 1998. pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev. 12:1483-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexandru, G., F. Uhlmann, K. Mechtler, M. A. Poupart, and K. Nasmyth. 2001. Phosphorylation of the cohesin subunit Scc1 by Polo/Cdc5 kinase regulates sister chromatid separation in yeast. Cell 105:459-472. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, S. S. 1999. Balanced regulation of microtubule dynamics during the cell cycle: a contemporary view. Bioessays 21:53-60. [DOI] [PubMed] [Google Scholar]
- 4.Bahler, J., A. B. Steever, S. Wheatley, Y. I. Wang, J. R. Pringle, K. L. Gould, and D. McCollum. 1998. Role of polo kinase and Mid1p in determining the site of cell division in fission yeast. J. Cell Biol. 143:1603-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benndorf, R., P. Nurnberg, and H. Bielka. 1988. Growth phase-dependent proteins of the Ehrlich ascites tumor analyzed by one- and two-dimensional electrophoresis. Exp. Cell Res. 174:130-138. [DOI] [PubMed] [Google Scholar]
- 6.Blangy, A., H. A. Lane, P. d'Herin, M. Harper, M. Kress, and E. A. Nigg. 1995. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 83:1159-1169. [DOI] [PubMed] [Google Scholar]
- 7.Blangy, A., L. Arnaud, and E. A. Nigg. 1997. Phosphorylation by p34cdc2 protein kinase regulates binding of the kinesin-related motor HsEg5 to the dynactin subunit p150. J. Biol. Chem. 272:19418-19424. [DOI] [PubMed] [Google Scholar]
- 8.Bohm, H., B. Gross, M. Gaestel, U. A. Bommer, G. Ryffel, and H. Bielka. 1991. The 5′-untranslated region of p23 mRNA from the Ehrlich ascites tumor is involved in translation control of the growth related protein p23. Biomed. Biochim. Acta 50:1193-1203. [PubMed] [Google Scholar]
- 9.Bohm, H., R. Benndorf, M. Gaestel, B. Gross, P. Nurnberg, R. Kraft, A. Otto, and H. Bielka. 1989. The growth-related protein P23 of the Ehrlich ascites tumor: translational control, cloning and primary structure. Biochem. Int. 19:277-286. [PubMed] [Google Scholar]
- 10.Boyle, W. J., P. van der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201:110-149. [DOI] [PubMed] [Google Scholar]
- 11.Breeden, L., and K. Nasmyth. 1985. Regulation of the yeast HO gene. Cold Spring Harbor Symp. Quant. Biol. 50:643-650. [DOI] [PubMed] [Google Scholar]
- 12.Brisch, E., M. A. Daggett, and K. A. Suprenant. 1996. Cell cycle-dependent phosphorylation of the 77 kDa echinoderm microtubule-associated protein (EMAP) in vivo and association with the p34cdc2 kinase. J. Cell Sci. 109:2885-2893. [DOI] [PubMed] [Google Scholar]
- 13.Brugg, B., and A. Matus. 1991. Phosphorylation determines the binding of microtubule-associated protein 2 (MAP2) to microtubules in living cells. J. Cell Biol. 114:735-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Budde, P. P., A. Kumagai, W. G. Dunphy, and R. Heald. 2001. Regulation of Op18 during spindle assembly in Xenopus egg extracts. J. Cell Biol. 153:149-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns, R. G., K. Islam, and R. Chapman. 1984. The multiple phosphorylation of the microtubule-associated protein MAP2 controls the MAP2:tubulin interaction. Eur. J. Biochem. 141:609-615. [DOI] [PubMed] [Google Scholar]
- 16.Cambiazo, V., E. Logarinho, H. Pottstock, and C. E. Sunkel. 2000. Microtubule binding of the Drosophila DMAP-85 protein is regulated by phosphorylation in vitro. FEBS Lett. 483:37-42. [DOI] [PubMed] [Google Scholar]
- 17.Carmena, M., M. G. Riparbelli, G. Minestrini, A. M. Tavares, R. Adams, G. Callaini, and D. M. Glover. 1998. Drosophila polo kinase is required for cytokinesis. J. Cell Biol. 143:659-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassimeris, L. 1999. Accessory protein regulation of microtubule dynamics throughout the cell cycle. Curr. Opin. Cell Biol. 11:134-141. [DOI] [PubMed] [Google Scholar]
- 19.Chase, D., Y. Feng, B. Hanshew, J. A. Winkles, D. L. Longo, and D. K. Ferris. 1998. Expression and phosphorylation of fibroblast-growth-factor-inducible kinase (Fnk) during cell-cycle progression. Biochem. J. 333:655-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chitpatima, S. T., S. Makrides, R. Bandyopadhyay, and G. Brawerman. 1988. Nucleotide sequence of a major messenger RNA for a 21 kilodalton polypeptide that is under translational control in mouse tumor cells. Nucleic Acids Res. 16:2350.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clay, F. J., S. J. McEwen, I. Bertoncello, A. F. Wilks, and A. R. Dunn. 1993. Identification and cloning of a protein kinase-encoding mouse gene, Plk, related to the polo gene of Drosophila. Proc. Natl. Acad. Sci. USA 90:4882-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Descombes, P., and E. A. Nigg. 1998. The polo-like kinase Plx1 is required for M phase exit and destruction of mitotic regulators in Xenopus egg extracts. EMBO J. 17:1328-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.do Carmo Avides, M., A. Tavares, and D. M. Glover. 2001. Polo kinase and Asp are needed to promote the mitotic organizing activity of centrosomes. Nat. Cell Biol. 3:421-424. [DOI] [PubMed] [Google Scholar]
- 24.Donohue, P. J., G. F. Alberts, Y. Guo, and J. A. Winkles. 1995. Identification by targeted differential display of an immediate early gene encoding a putative serine/threonine kinase. J. Biol. Chem. 270:10351-10357. [DOI] [PubMed] [Google Scholar]
- 25.Duncan, P. L., N. Pollet, C. Niehrs, and E. A. Nigg. 2001. Cloning and characterization of Plx2 and Plx3, two additional Polo-like kinases from Xenopus laevis. Exp. Cell Res. 270:78-87. [DOI] [PubMed] [Google Scholar]
- 26.Feng, Y., D. L. Longo, and D. K. Ferris. 2001. Polo-like kinase interacts with proteasomes and regulates their activity. Cell Growth Differ. 12:29-37. [PubMed] [Google Scholar]
- 27.Feng, Y., D. R. Hodge, G. Palmieri, D. L. Chase, D. L. Longo, and D. K. Ferris. 1999. Association of polo-like kinase with alpha-, beta- and gamma-tubulins in a stable complex. Biochem. J. 339:435-442. [PMC free article] [PubMed] [Google Scholar]
- 28.Fode, C., B. Motro, S. Yousefi, M. Heffernan, and J. W. Dennis. 1994. Sak, a murine protein-serine/threonine kinase that is related to the Drosophila polo kinase and involved in cell proliferation. Proc. Natl. Acad. Sci. USA 91:6388-6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fode, C., C. Binkert, and J. W. Dennis. 1996. Constitutive expression of murine Sak-a suppresses cell growth and induces multinucleation. Mol. Cell. Biol. 16:4665-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gachet, Y., S. Tournier, M. Lee, A. Lazaris-Karatzas, T. Poulton, and U. A. Bommer. 1999. The growth-related, translationally controlled protein P23 has properties of a tubulin binding protein and associates transiently with microtubules during the cell cycle. J. Cell Sci. 112:1257-1271. [DOI] [PubMed] [Google Scholar]
- 31.Glover, D. M., H. Ohkura, and A. Tavares. 1996. Polo kinase: the choreographer of the mitotic stage? J. Cell Biol. 135:1681-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golsteyn, R. M., S. J. Schultz, J. Bartek, A. Ziemiecki, T. Ried, and E. A. Nigg. 1994. Cell cycle analysis and chromosomal localization of human Plk1, a putative homologue of the mitotic kinases Drosophila polo and Saccharomyces cerevisiae Cdc5. J. Cell Sci. 107:1509-1517. [DOI] [PubMed] [Google Scholar]
- 33.Golsteyn, R. M., K. E. Mundt, A. M. Fry, and E. A. Nigg. 1995. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J. Cell Biol. 129:1617-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez, C., C. E. Sunkel, and D. M. Glover. 1998. Interactions between mgr, asp, and polo: asp function modulated by polo and needed to maintain the poles of monopolar and bipolar spindles. Chromosoma 107:452-460. [DOI] [PubMed] [Google Scholar]
- 35.Gustke, N., B. Steiner, E. M. Mandelkow, J. Biernat, H. E. Meyer, M. Goedert, and E. Mandelkow. 1992. The Alzheimer-like phosphorylation of tau protein reduces microtubule binding and involves Ser-Pro and Thr-Pro motifs. FEBS Lett. 307:199-205. [DOI] [PubMed] [Google Scholar]
- 36.Hamanaka, R., S. Maloid, M. R. Smith, C. D. O'Connell, D. L. Longo, and D. K. Ferris. 1994. Cloning and characterization of human and murine homologues of the Drosophila polo serine-threonine kinase. Cell. Growth Differ. 5:249-257. [PubMed] [Google Scholar]
- 37.Holtrich, U., G. Wolf, A. Brauninger, T. Karn, B. Bohme, H. Rubsamen-Waigmann, and K. Strebhardt. 1994. Induction and down-regulation of PLK, a human serine/threonine kinase expressed in proliferating cells and tumors. Proc. Natl. Acad. Sci. USA 91:1736-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hosoya, H., S. Komatsu, T. Shimizu, M. Inagaki, M. Ikegami, and K. Yazaki. 1994. Phosphorylation of dynamin by cdc2 kinase. Biochem. Biophys. Res. Commun. 202:1127-1133. [DOI] [PubMed] [Google Scholar]
- 39.Jaspersen, S. L., J. F. Charles, R. L. Tinker-Kulberg, and D. O. Morgan. 1998. A late mitotic regulatory network controlling cyclin destruction in Saccharomyces cerevisiae. Mol. Biol. Cell 9:2803-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jordan, M. A., K. Wendell, S. Gardiner, W. B. Derry, H. Copp, and L. Wilson. 1996. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 56:816-825. [PubMed] [Google Scholar]
- 41.Jordan, M. A., R. J. Toso, D. Thrower, and L. Wilson. 1993. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc. Natl. Acad. Sci. USA 90:9552-9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitada, K., A. L. Johnson, L. H. Johnston, and A. Sugino. 1993. A multicopy suppressor gene of the Saccharomyces cerevisiae G1 cell cycle mutant gene dbf4 encodes a protein kinase and is identified as CDC5. Mol. Cell. Biol. 13:4445-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotani, S., S. Tugendreich, M. Fujii, P. M. Jorgensen, N. Watanabe, C. Hoog, P. Hieter, and K. Todokoro. 1998. PKA and MPF-activated polo-like kinase regulate anaphase-promoting complex activity and mitosis progression. Mol. Cell 1:371-380. [DOI] [PubMed] [Google Scholar]
- 44.Kumagai, A., and W. G. Dunphy. 1996. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science 273:1377-1380. [DOI] [PubMed] [Google Scholar]
- 45.Lake, R. J., and W. R. Jelinek. 1993. Cell cycle- and terminal differentiation-associated regulation of the mouse mRNA encoding a conserved mitotic protein kinase. Mol. Cell. Biol. 13:7793-7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lane, H. A., and E. A. Nigg. 1996. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J. Cell Biol. 135:1701-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee, K. S., Y. L. Yuan, R. Kuriyama, and R. L. Erikson. 1995. Plk is an M-phase-specific protein kinase and interacts with a kinesin-like protein, CHO1/MKLP-1. Mol. Cell. Biol. 15:7143-7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee, K. S., T. Z. Grenfell, F. R. Yarm, and R. L. Erikson. 1998. Mutation of the polo-box disrupts localization and mitotic functions of the mammalian polo kinase Plk. Proc. Natl. Acad. Sci. USA 95:9301-9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li, B., B. Ouyang, H. Pan, P. T. Reissmann, D. J. Slamon, R. Arceci, L. Lu, and W. Dai. 1996. Prk, a cytokine-inducible human protein serine/threonine kinase whose expression appears to be down-regulated in lung carcinomas. J. Biol. Chem. 271:19402-19408. [DOI] [PubMed] [Google Scholar]
- 50.Lin, C. Y., M. L. Madsen, F. R. Yarm, Y. J. Jang, X. Liu, and R. L. Erikson. 2000. Peripheral Golgi protein GRASP65 is a target of mitotic polo-like kinase (Plk) and Cdc2. Proc. Natl. Acad. Sci. USA 97:12589-12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Llamazares, S., A. Moreira, A. Tavares, C. Girdham, B. A. Spruce, C. Gonzalez, R. E. Karess, D. M. Glover, and C. E. Sunkel. 1991. polo encodes a protein kinase homolog required for mitosis in Drosophila. Genes Dev. 5:2153-2165. [DOI] [PubMed] [Google Scholar]
- 52.Lopez, L. A., and M. P. Sheetz. 1995. A microtubule-associated protein (MAP2) kinase restores microtubule motility in embryonic brain. Biol. Chem. 270:12511-12517. [DOI] [PubMed] [Google Scholar]
- 53.Mundt, K. E., R. M. Golsteyn, H. A. Lane, and E. A. Nigg. 1997. On the regulation and function of human polo-like kinase 1 (PLK1): effects of overexpression on cell cycle progression. Biochem. Biophys. Res. Commun. 239:377-385. [DOI] [PubMed] [Google Scholar]
- 54.Nigg, E. A., A. Blangy, and H. A. Lane. 1996. Dynamic changes in nuclear architecture during mitosis: on the role of protein phosphorylation in spindle assembly and chromosome segregation. Exp. Cell Res. 229:174-180. [DOI] [PubMed] [Google Scholar]
- 55.Ohkura, H., I. M. Hagan, and D. M. Glover. 1995. The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 9:1059-1073. [DOI] [PubMed] [Google Scholar]
- 56.Ookata, K., S. Hisanaga, J. C. Bulinski, H. Murofushi, H. Aizawa, T. J. Itoh, H. Hotani, E. Okumura, K. Tachibana, and T. Kishimoto. 1995. Cyclin B interaction with microtubule-associated protein 4 (MAP4) targets p34cdc2 kinase to microtubules and is a potential regulator of M-phase microtubule dynamics. J. Cell Biol. 128:849-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ouyang, B., Y. Wang, and D. Wei. 1999. Caenorhabditis elegans contains structural homologs of human prk and plk. DNA Seq. 10:109-113. [DOI] [PubMed] [Google Scholar]
- 58.Ouyang, B., W. Li, H. Pan, J. Meadows, I. Hoffmann, and W. Dai. 1999. The physical association and phosphorylation of Cdc25C protein phosphatase by Prk. Oncogene 18:6029-6036. [DOI] [PubMed] [Google Scholar]
- 59.Ouyang, B., H. Pan, L. Lu, J. Li, P. Stambrook, B. Li, and W. Dai. 1997. Human Prk is a conserved protein serine/threonine kinase involved in regulating M phase functions. J. Biol. Chem. 272:28646-28651. [DOI] [PubMed] [Google Scholar]
- 60.Pedrotti, B., L. Ulloa, J. Avila, and K. Islam. 1996. Characterization of microtubule-associated protein MAP1B: phosphorylation state, light chains, and binding to microtubules. Biochemistry 35:3016-3023. [DOI] [PubMed] [Google Scholar]
- 61.Pedrotti, B., and K. Islam. 1996. Dephosphorylated but not phosphorylated microtubule associated protein MAP1B binds to microfilaments. FEBS Lett. 388:131-133. [DOI] [PubMed] [Google Scholar]
- 62.Qian, Y. W., E. Erikson, C. Li, and J. L. Maller. 1998. Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol. Cell. Biol. 18:4262-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Runnegar, M. T., X. Wei, and S. F. Hamm-Alvarez. 1999. Increased protein phosphorylation of cytoplasmic dynein results in impaired motor function. Biochem. J. 342:1-6. [PMC free article] [PubMed] [Google Scholar]
- 64.Shirayama, M., W. Zachariae, R. Ciosk, and K. Nasmyth. 1998. The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J. 17:1336-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simmons, D. L., B. G. Neel, R. Stevens, G. Evett, and R. L. Erikson. 1992. Identification of an early-growth-response gene encoding a novel putative protein kinase. Mol. Cell. Biol. 12:4164-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song, S., T. Z. Grenfell, S. Garfield, R. L. Erikson, and K. S. Lee. 2000. Essential function of the polo box of Cdc5 in subcellular localization and induction of cytokinetic structures. Mol. Cell. Biol. 20:286-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sunkel, C. E., and D. M. Glover. 1988. polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J. Cell Sci. 89:25-38. [DOI] [PubMed] [Google Scholar]
- 68.Tavares, A. A., D. M. Glover, and C. E. Sunkel. 1996. The conserved mitotic kinase polo is regulated by phosphorylation and has preferred microtubule-associated substrates in Drosophila embryo extracts. EMBO J. 15:4873-4883. [PMC free article] [PubMed] [Google Scholar]
- 69.Toczyski, D. P., D. J. Galgoczy, and L. H. Hartwell. 1997. CDC5 and CKII control adaptation to the yeast DNA damage checkpoint. Cell 90:1097-1106. [DOI] [PubMed] [Google Scholar]
- 70.Toyoshima-Morimoto, F., E. Taniguchi, N. Shinya, A. Iwamatsu, and E. Nishida. 2001. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature 410:215-220. [DOI] [PubMed] [Google Scholar]
- 71.Wang, L. M., D. K. Geihl, G. G. Choudhury, A. Minter, L. Martinez, D. K. Weber, and A. Y. Sakaguchi. 1989. Site-directed deletion mutagenesis using phagemid vectors and genetic selection. BioTechniques 7:1000-1006, 1008-1010. [PubMed] [Google Scholar]
- 72.Wittmann, T., A. Hyman, and A. Desai. 2001. The spindle: a dynamic assembly of microtubules and motors. Nat. Cell Biol. 3:E28-E34. [DOI] [PubMed] [Google Scholar]
- 73.Woods, C. M., J. Zhu, P. A. McQueney, D. Bollag, and E. Lazarides. 1995. Taxol-induced mitotic block triggers rapid onset of a p53-independent apoptotic pathway. Mol. Med. 1:506-526. [PMC free article] [PubMed] [Google Scholar]