Abstract
DNA methylation is commonly associated with gene silencing, and a link between histone deacetylation and DNA methylation has been established. However, the transcriptional impact of the position and length of methylated zones relative to the promoter and the coding region of a gene remains quite unclear. This study investigates the impact of regional methylation on transcription and the relationship between DNA methylation and histone acetylation. Using patch-methylated stable episomes in human cells, we establish the pivotal importance of the location of DNA methylation in the regulation of transcription. We further demonstrate that the size of the methylated patch is not a key determinant for transcriptional suppression. The impact of DNA methylation on transcription is greater when it is in the transcription unit, and it is primarily a local effect. However, methylation outside of the transcription unit may potentiate the effect of methylation within the transcription unit. Acetylated histones are associated with unmethylated DNA and are nearly absent from methylated DNA regions. This association appears to be local and does not propagate along the DNA.
It has been well established that DNA methylation can influence gene expression. In general, DNA methylation represses transcription, and loss of methylation is associated with gene activation (4). DNA methylation can directly interfere with transcription factor binding in some cases (6). In other cases, DNA methylation does not inhibit transcription factor binding directly, but transcriptional repression occurs nevertheless. In these cases, methyl-CpG binding proteins can mediate histone modification by recruiting histone deacetylases to methylated DNA (18, 19, 22). Although it is clear that this is an important mechanism in the silencing of genes, many aspects of this remain undefined. Specifically, the impact of the position and size of the methylated DNA segments on regional transcription has not been sufficiently characterized to permit inferences about the impact on transcription.
With fungi, it has been shown that methylation impacts transcriptional elongation more than initiation (2, 20). Consistent with this finding, we previously found that methylation of the coding region of a luciferase gene represses transcription more than does methylation of the Rous sarcoma virus long terminal repeat (RSV LTR) promoter (11). Several studies using nonreplicating plasmids have suggested that the size of the methylated region plays a major role in transcriptional suppression (15) and that methylation adjacent to the promoter may inhibit transcription (12, 14, 18). Interpreting the results of these studies is difficult because the experimental designs included the coding region in the methylated patch. Therefore, the impact of the size and location of the methylated patch on transcription may be more complicated than what was concluded in these studies.
This study was designed to examine the impact of the size and location of the methylated patch on transcription and to investigate the chromatin structure associated with methylated DNA. Episomes with methylated patches of various sizes (patch-methylated) were tested for transcriptional suppression. We also examined the histones associated with different regions of the patch-methylated episomes to determine whether DNA methylation affects the state of histone acetylation in the adjacent unmethylated DNA. It appears that DNA methylation has primarily a local effect on transcription and that acetylated histones are absent from the methylated region.
MATERIALS AND METHODS
Plasmids.
Unique restriction sites were engineered into plasmid pCLH22 (10) in order to clone methylated patches of various sizes at different locations in this plasmid. DNA fragments were purified with the GeneClean Kit (ISC BioExpress) after restriction digestion and fractionation by electrophoresis. Unmethylated and in vitro methylated fragments of the plasmid were ligated to generate patch-methylated constructs.
Plasmids with four nonoverlapping methylated patches 2 to 3 kb long at different locations outside of the transcription unit were generated by using the unique restriction sites available. Plasmids with methylation in more than one of these four patches were also constructed. These plasmids were designated OT (for methylation in the region outside of the luciferase transcription unit) and assigned numbers 1 through 4 to represent the order of the methylated patches downstream from the transcription unit. For example, plasmid pOT1 has a 2-kb methylated patch immediately downstream from the luciferase-coding region between the KpnI and RsrII restriction sites, whereas plasmid pOT2 has a 2.5-kb methylated patch between restriction sites RsrII and XmnI downstream from the OT1 patch (Fig. 1). The methylated patch on plasmid OT4 is farthest downstream from the luciferase-coding region, but it is immediately upstream from the RSV LTR promoter (Fig. 1). Plasmid pOT2+3 has methylation in both patches OT2 and OT3, and plasmid pOT1+2+3+4 has methylation in all four patches (Fig. 1).
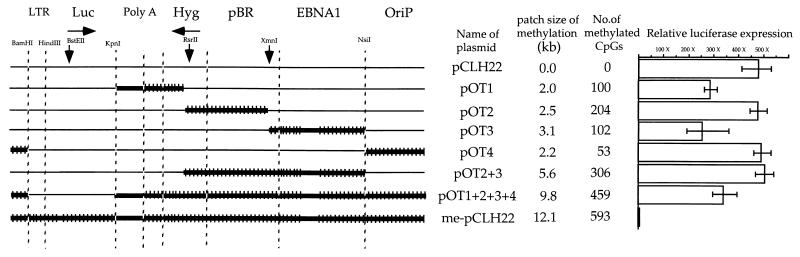
FIG. 1.
DNA methylation outside of the transcription unit has limited impact on transcription. The left side of the figure illustrates the regions of methylation on the plasmids. The names of the plasmids, the sizes of the methylated patches, and the numbers of methylation sites are listed to the right of each line. The components of the plasmids are indicated across the top, with vertical dashed lines dividing them. The unique restriction sites used to construct various patches are indicated by the arrows. The regions of methylation are marked by thick horizontal lines hatched with short vertical lines. Long stretches of sequences lacking CpG dinucleotides within the methylated regions are represented by the absence of vertical lines within the thick horizontal lines. The luciferase activities of the plasmids relative to that of pCLH22-me are presented on the right. The luciferase activity of pCLH22 is the highest among all plasmids, and the absolute luciferase readings range from 2.7 ×106 to 1.6 ×107 relative light units (RLU) in different experiments. The luciferase gene expression was normalized by the amount of DNA harvested from transfected cells as determined by Southern blot analysis. The luciferase activity of pCLH22-me was designated as 1 because it was the lowest. The bars represent the range of relative luciferase activity for the same construct from different transfections. Luc, luciferase gene; Hyg, hygromycin gene; OriP, EBV latent replication origin.
Plasmids with methylation only in the transcription unit are denoted with “me” hyphenated to the name of the patch that is methylated. These include pLTR-me, pLuc-me, and pLTR/Luc-me. Plasmids with a small methylation-free region are denoted with a minus sign before the name of that region; i.e., there is methylation in both the transcription unit and the rest of the plasmid but not in the region described in the name of the plasmid. These plasmids include pMe−LTR, pMe−Luc, pMe−OT1, and pMe−OT3. The fully unmethylated plasmid is designated pCLH22, and the fully methylated plasmid is designated pCLH22-me.
In vitro DNA methylation.
Plasmids or DNA fragments were methylated in vitro at all CpG sites by using the SssI methylase (New England Biolabs) under the conditions recommended by the manufacturer. Following methylation, DNA was dialyzed with nitrocellulose filters (Millipore) after phenol-chloroform extraction. The methylation status of each DNA fragment was confirmed by HhaI restriction enzyme digestion.
In vitro ligation.
The vector preparations were test ligated without the insert to identify any uncut or vector religation products. Only preparations with backgrounds less than 0.01% were used in the construction of the patch-methylated plasmids. Methylated and unmethylated portions of the plasmid were ligated in vitro to generate patch-methylated constructs. After ligation, the DNA was phenol-chloroform extracted and dialyzed with nitrocellulose filters. If the RSV LTR promoter and luciferase gene remain intact on the unligated vector, then these unligated molecules could possibly recircularize in mammalian cells after transfection, thereby generating undesired luciferase expression. Therefore, exonuclease V (US Biochemical) treatment was done after ligation of those constructions to eliminate any unligated DNA. Ligated DNA was quantitated by transformation of Escherichia coli with a known quantity of pCLH22 as a standard.
Cell line and transfection.
The calcium phosphate transfection method (10, 23) was used to transfect 293/EBNA1 cells (10) in this study. All transfections were done at least in duplicate in each experiment. All experiments were performed at least twice using products from independent ligations for confirmation. Further, each transfection was harvested at least three consecutive times over a 3-week interval after transfection to assess any possible changes occurring over time.
Episome recovery and analysis.
When transfected cells reached confluence, 1.25% of the cells were harvested for the luciferase assay, 2.5% of the cells were replated on a 100-mm-diameter tissue culture plate, and the remaining cells were harvested by the Hirt method for recovery of episomal DNA (8). To avoid possible selection bias, all experiments were done without hygromycin selection even though the selection marker is on the episome. The same fraction (10%) of the DNA harvested from each transfection was digested with XbaI to linearize the plasmid for DNA quantitation and was double-digested with XbaI and HhaI to determine the methylation status. Digested DNA was fractionated on a 0.8 or 1% agarose gel, Southern transferred, and probed with the entire plasmid. Southern blots were exposed to a phosphorimaging screen, and the radioactivity was quantitated with a phosphorimager (Bio-Rad GS525 or Bio-Rad FX).
Luciferase assay.
An aliquot (1.25%) of transfected cells was harvested and lysed for the luciferase assay. Luciferase activities were analyzed by using a Monolight 2020 luminometer (Analytical Luminescence) as described previously (10). The luciferase activities were normalized by the amount of plasmid recovered from the same harvest as described previously (10, 11). After subtraction of the background reading, the luciferase reading was divided by the relative amount of DNA from the same harvest as determined by Southern blot. This normalized luciferase activity from each transfection could then be compared with those from other transfections. This ensured that levels of luciferase expression from the same quantity of plasmids with different methylation states were being compared.
Chromatin immunoprecipitation.
Chromatin immunoprecipitation (ChIP) assays were performed by a modification of the protocol described by Braunstein et al. (3). Exponentially growing cultures of 293/EBNA1 cells transfected with patch-methylated stable episomes were fixed with 1% formaldehyde for 10 min at 25°C. Approximately 3 × 106 fixed cells were resuspended in 1 ml of radioimmunoprecipitation buffer (10 mM sodium phosphate [pH 7.2], 2 mM EDTA, 150 mM NaCl, 50 mM NaF, 0.2 mM Na3VO4, 1% sodium deoxycholate, 1% Nonidet P40, 0.1% sodium dodecyl sulfate) that contained mammalian protease inhibitors (Roche). The cell suspension was sonicated 20 times for 10 s each by using a Branson Sonifier 450 (output = 5, constant duty cycle). After sonication, soluble chromatin was obtained by centrifuging the cell lysate for 15 min at 14,000 rpm (16,000 × g) in a microcentrifuge to pellet the insoluble fraction. Aliquots of soluble chromatin, the total chromatin fraction (TCF), were reserved for quantitative PCR (Q-PCR) analysis and for monitoring of the DNA fragment size. For immunoprecipitation, 5 μg each of antiacetylated histone H3 immunoglobulin G (IgG) and antiacetylated histone H4 IgG (Upstate Biotechnology) were mixed with 0.5 ml of soluble chromatin and incubated at 4°C for 5 h. Then, 40 μg of sheared salmon sperm DNA and 100 μl of protein G-Sepharose slurry (50% in Tris-EDTA [TE], pH 8.0) were added, and the incubation was continued overnight at 4°C with rotary mixing. The Sepharose beads were collected by centrifugation and washed sequentially for 10 min each at 4°C, once with 0.5 ml of radioimmunoprecipitation buffer and twice with 0.5 ml of phosphate-buffered saline. Immunocomplexes were eluted by incubation with 1% sodium dodecyl sulfate in 0.1 M NaHCO3 for 30 min at 25°C with rotary mixing. Eluates were incubated at 65°C for 5 h to reverse the formaldehyde cross-links, and the released DNA was ethanol precipitated. Precipitated DNA was dissolved in 100 μl of TE (pH 8.0), digested with 50 μg of proteinase K at 55°C for 2 h, phenol-chloroform extracted, and ethanol precipitated. The final DNA pellet was dissolved in 50 μl of TE (pH 8.0) for Q-PCR analysis. Parallel experiments with equal amounts of soluble chromatin but without antibodies were done as negative controls for the immunoprecipitation experiments. Two independent immunoprecipitations were carried out for each patch-methylated episome.
Q-PCR.
Q-PCR was performed with a Bio-Rad iCycler using AmpliTaq Gold DNA polymerase (Applied Biosystems) and 1 μl of each DNA sample. Fluorescently labeled TaqMan probes for five regions of the plasmid pCLH22 were synthesized by Biosearch Technologies, and the primers were synthesized by Operon Technologies. The primer and probe sequences are listed in Table 1. All Q-PCRs were carried out with the same two-step program: 95°C for 15 s and 60°C for 1 min for 40 cycles. Within each set of Q-PCRs, titrations of known amounts of pCLH22 DNA were included as positive controls and for quantitation. DNA from the TCF, the ChIP sample immunoprecipitated with antiacetylated histone H3 and H4 antibodies (antiacetylated histone H3/H4), and the ChIP sample immunoprecipitated without antibodies (no Ab) were included in each set of Q-PCRs. All Q-PCRs were done in duplicate. The fraction of immunoprecipitated DNA was calculated by dividing the amount of DNA from the antiacetylated histone H3/H4 sample (after the background amplification from the no Ab control sample was subtracted) by the amount of DNA in the corresponding TCF sample. A genomic locus, XRCC1, was also quantitated to ensure that the immunoprecipitation was equally efficient in all tubes.
TABLE 1.
TaqMan probes and primers used in this study
| TaqMan probe sequence (probe no.) | Primers | PCR targetsa |
|---|---|---|
| 5′-AGCAGCGCAAAACGCCTAACCCTAAG-3′ (1) | 5′-GACCGACAATTGCATGAAGAATC-3′ | RSV LTR 1 |
| 5′-GCGTATATCTGGCCCGTACATC-3′ | ||
| 5′-ACTCCTAACCGCGTACAACCGAAGCC-3′ (2) | 5′-GGGTGTGTTTAGGCGAAAAGC-3′ | RSV LTR 2 |
| 5′-CCTATGCAAAAGCGAAACTACTATATCC-3′ | ||
| 5′-AGGCCCGGCGCCATTCTATCCT-3′ (3) | 5′-GGAAGACGCCAAAAACATAAAGA-3′ | Luc 1 |
| 5′-TCCAGCGGTTCCATCCTCTA-3′ | ||
| 5′-CGGGCGTGGCAGGTCTTCCC-3′ (4) | 5′-TACAACACCCCAACATCTTCGA-3′ | Luc 2 |
| 5′-AGTTCACCGGCGTCATCG-3′ | ||
| 5′-CAAGCCAACCACGGCCTCCAGAA-3′ (5) | 5′-CGCGTCTGCTGCTCCAT-3′ | Hyg |
| 5′-AATACGAGGTCGCCAACATCTT-3′ | ||
| 5′-CCTTACCTCCGGGAGGGCAGC-3′ (6) | 5′-TGGACTGTCACCGCATGC-3′ | XRCC1 |
| 5′-GCAGGGTTGGCGTGTGAG-3′ |
Luc 1, luciferase region 1; Luc 2, luciferase region 2; Hyg, hygromycin gene.
RESULTS
Methylation outside of the promoter and the coding region has little impact on transcription.
The impact of DNA methylation outside of the transcription unit on gene expression has not been directly evaluated previously. An earlier study reported that luciferase transcription was reduced 50-fold (relative to unmethylated DNA) when the RSV LTR promoter was the only methylation-free region on the plasmid, and it was reduced 100-fold when the luciferase-coding region was the only methylation-free region on the plasmid (11). While the fully methylated pCLH22 showed a 200- to 500-fold reduction of transcriptional activity, methylation in the RSV LTR and in the luciferase-coding region caused two- to fivefold and tenfold reductions in transcriptional activity, respectively (11). From these results, one would predict that when both the RSV LTR and the luciferase-coding region are free of methylation, the methylated vector would cause a five- to tenfold decrease in the luciferase transcription. We wanted to test whether this prediction is true and to evaluate the impact of DNA methylation outside of the transcription unit on gene expression. Six constructs with various sizes of methylated patches, pOT1, pOT2, pOT3, pOT4, pOT2+3, and pOT1+2+3+4, were generated (Fig. 1).
Each of these six plasmids was transfected individually into 293/EBNA1 cells. Fully unmethylated pCLH22 and fully methylated pCLH22-me were also individually transfected into 293/EBNA1 cells as controls. Transfected cells were harvested for the luciferase assay and for DNA analysis three to four times during a 3-week interval. As illustrated in Fig. 1, these plasmids have methylated patches of 2 to 9.8 kb positioned from 0 to 7.6 kb away from the 3′ edge of the luciferase-coding region (7.8 to 0 kb away from the beginning of the RSV LTR promoter). These six plasmids have 53 (pOT4) to 459 (pOT1+2+3+4) CpG sites in the methylated patches, with various CpG densities ranging from 1 of every 39 bases to 1 of every 12 bases (Fig. 1). After normalization, the luciferase gene expression from each of these six patch-methylated plasmids was no more than twofold lower than that from the fully unmethylated pCLH22, and it was more than 200-fold higher than that from the fully methylated plasmid, pCLH22-me (Fig. 1). The XbaI/HhaI double-digested DNA showed the expected pattern for each patch-methylated plasmid, and these patterns were maintained throughout the experimental interval (data not shown) as observed previously (10, 11). Furthermore, the subsequent harvests from the same transfections showed similar results (data not shown).
These findings suggest that transcription is not affected by DNA methylation outside of the promoter and outside of the coding region, regardless of the size, position, or density of the methylation patch. This lack of inhibition does not change over the length of time that the episomes are inside the mammalian cells.
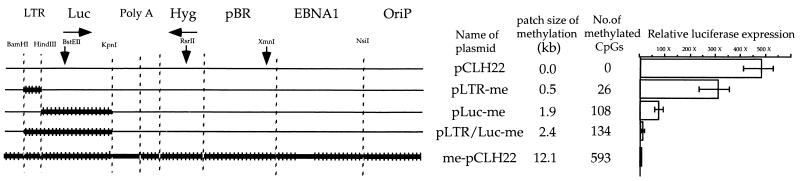
Methylation in the promoter and methylation in the coding region have a combined effect on transcriptional suppression.
Methylation in the RSV LTR inhibited luciferase gene expression by approximately two- to fivefold, and methylation in the luciferase-coding region was found in a previous study to reduce its expression by tenfold (11). To confirm these findings and to test whether methylation in the promoter and methylation in the coding region have a combined effect, plasmids pLTR-me, pLuc-me, and pLTR/Luc-me were tested. The methylation densities in the RSV LTR promoter and the luciferase-coding region were similar (Fig. 2). Each of these three plasmids, pCLH22, and pCLH22-me were transfected individually into the 293/EBNA1 cells. The normalized luciferase gene expression from pLTR-me was two- to threefold lower than that from pCLH22, and it was approximately 200- to 300-fold higher than that from pCLH22-me (Fig. 2). The luciferase gene expression from pLuc-me was approximately 10-fold lower than that from pCLH22, and it was about 70-fold higher than that from pCLH22-me (Fig. 2). These results confirm the findings of the previous report (11). The luciferase gene expression from pLTR/Luc-me was 100-fold lower than the expression from pCLH22 and fivefold higher than that from pCLH22-me (Fig. 2). The methylation patterns of pLTR-me and pLuc-me harvested from transfected cells (data not shown) were the same as those reported in a previous study (8). The methylation pattern of pLTR/Luc-me on the Southern blot of XbaI/HhaI double-digested DNA harvested from the transfected cells and probed with the entire plasmid was as expected (data not shown). The level of luciferase expression and the methylation pattern of the episome from each transfection were similar in cells harvested at different times throughout the experimental interval (data not shown).
FIG. 2.
DNA methylation in the transcription unit has a large impact on transcription. For details, see the legend to Fig. 1. The luciferase activity of pCLH22 is the highest among all plasmids, and the absolute luciferase readings range from 2.7 ×106 to 1.1 ×107 RLU in different experiments. Luc, luciferase gene; Hyg, hygromycin gene; OriP, EBV latent replication origin.
These findings indicate that DNA methylation in the transcription unit plays the major role in transcriptional inhibition. Furthermore, methylation of the promoter and that of the coding region have a combined effect on transcription. It appears that methylation of the coding region has a larger effect on transcription than does methylation of the promoter region (10-fold versus two- to threefold). However, the effect of the size of methylation cannot be ruled out because the luciferase-coding region is three times as large as the RSV LTR promoter. Again, the transcriptional inhibition was maintained throughout the 3-week experimental interval (data not shown).
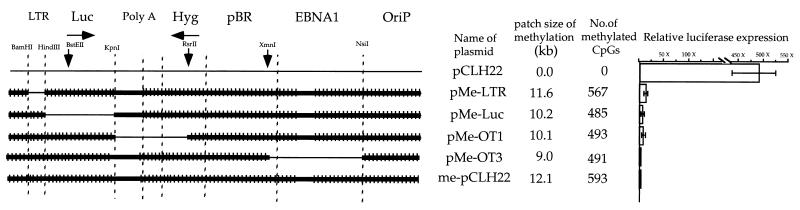
Methylation of regions outside of the transcription unit potentiates transcriptional repression when combined with methylation in the transcription unit.
No significant transcriptional inhibition was observed when both the RSV LTR promoter and the luciferase-coding region were free of methylation as described above. However, when either the promoter or the luciferase-coding region was methylated along with the rest of the plasmid, a much more dramatic effect on transcription (relative to that of methylation of either the promoter or the coding-region alone) was observed. Plasmids pMe−OT1 and pMe−OT3 have large patches of DNA methylation that include part or all of the transcription unit (Fig. 3). After these plasmids were transfected individually into 293/EBNA1 cells, the level of luciferase gene expression was 200- to 500-fold lower than the expression from pCLH22 (Fig. 3). The levels of luciferase expression from these two plasmids were similar to that from pCLH22-me (Fig. 3). The methylation patterns and the levels of luciferase gene transcription were well maintained throughout the course of the experiment (data not shown).
FIG. 3.
DNA methylation outside of the transcription unit can potentiate transcriptional suppression by methylation in the transcription unit. For details, see the legend to Fig. 1. The luciferase activity of pCLH22 is the highest among all plasmids, and the absolute luciferase readings range from 2.7 ×106 to 1.6 ×107 RLU in different experiments. The range of relative luciferase activities from different transfections was too small to illustrate with error bars. Luc, luciferase gene; Hyg, hygromycin gene; OriP, EBV latent replication origin.
If DNA methylation outside of the transcription unit plays no role in transcriptional repression, then the same level of inhibition should be observed when a given region of the transcription unit is methylated, regardless of whether the DNA outside of the transcription unit is methylated. This was clearly not the case for pMe−Luc and pMe−LTR in a previous study (11), and it was not the case for pMe−OT1 and pMe−OT3 in this study. This illustrates that methylation outside of the transcription unit contributes to the transcriptional inhibition much more when part of the transcription unit is also methylated. This finding suggests that the direct interference of transcription by DNA methylation in the transcription unit can be dramatically enhanced by the global chromatin structure. The global chromatin structure alone does not have a great impact on transcription as long as the transcription unit is entirely free of methylation.
Methylation does not change the association of acetylated histones with adjacent unmethylated DNA.
It has been proposed that methylated DNA can recruit histone deacetylase through methylation binding proteins and that the resulting repressed chromatin state may propagate into adjacent unmethylated DNA (12, 14, 18). However, the results of the experiments described above show that transcription is not greatly suppressed by methylated patches adjacent to the transcription unit. This finding suggests that the chromatin structure in the unmethylated DNA adjacent to methylated DNA may still be associated with acetylated histones and does not adopt a repressed state. To examine whether this is the case, ChIP assays using antiacetylated histone H3 and H4 antibodies were carried out. This assay, followed by Q-PCR, can determine the association of acetylated histones with various regions of the episome. Human cells harboring stable episomes with various patches of DNA methylation, pLTR-me, pLuc-me, pLTR/Luc-me, and pOT4 were studied. These episomes contain a fully unmethylated hygromycin selection gene. From the experiments described above, it is clear that cells can be selected with hygromycin to achieve homogeneous cell populations maintaining each of these episomes. Also, the hygromycin gene region can be used as an internal control for the assay as its chromatin structure may not be altered in this region of the episome by DNA methylation elsewhere on the same episome. Cells containing the fully unmethylated stable episome, pCLH22, were used as a control.
After hygromycin selection, plasmid DNA was harvested from 293/EBNA1 cells by the Hirt method (8) and the DNA methylation patterns were confirmed by restriction digestion and Southern blot analysis (data not shown). After the methylation patterns of the episomes were confirmed, cells were harvested for the ChIP assay. After immunoprecipitation, the endogenous XRCC1 locus in TCF, no Ab, and antiacetylated histone H3/H4 samples was studied by using Q-PCR to determine whether the immunoprecipitations were similarly efficient for each cell harvest. We found that the absolute amounts of DNA in all cell harvests were comparable and that approximately 3% of the DNA at the XRCC1 locus was immunoprecipitated by the antiacetylated histone H3 and H4 antibodies in all immunoprecipitation experiments (data not shown). This result indicates that the immunoprecipitation procedures were similarly efficient in all of the experiments and rules out potential differences in individual immunoprecipitations as artifacts.
Five regions of the episome were assessed for association with acetylated histones H3 and H4 by Q-PCR with the TaqMan assay. TaqMan probes 1, 2, 3, 4, and 5 were used to assay RSV LTR region 1, RSV LTR region 2, luciferase region 1, luciferase region 2, and the hygromycin gene, respectively (Table 1). The no Ab control served as a negative control for nonspecific pull-down of DNA with the protein G-Sepharose slurry. A typical real-time graph is shown in Fig. 4. The amount of DNA in each PCR was quantitated based on titrations of a known amount of plasmid pCLH22 DNA in the same set of PCRs. The percentage of DNA immunoprecipitated by the antibodies was calculated by dividing the amount of DNA in the precipitate by the amount of DNA in the TCF after the background (no Ab control) was subtracted from each. When the percentages of DNA immunoprecipitated in different regions of the plasmid are compared, the association of acetylated histones with various regions can be assessed.
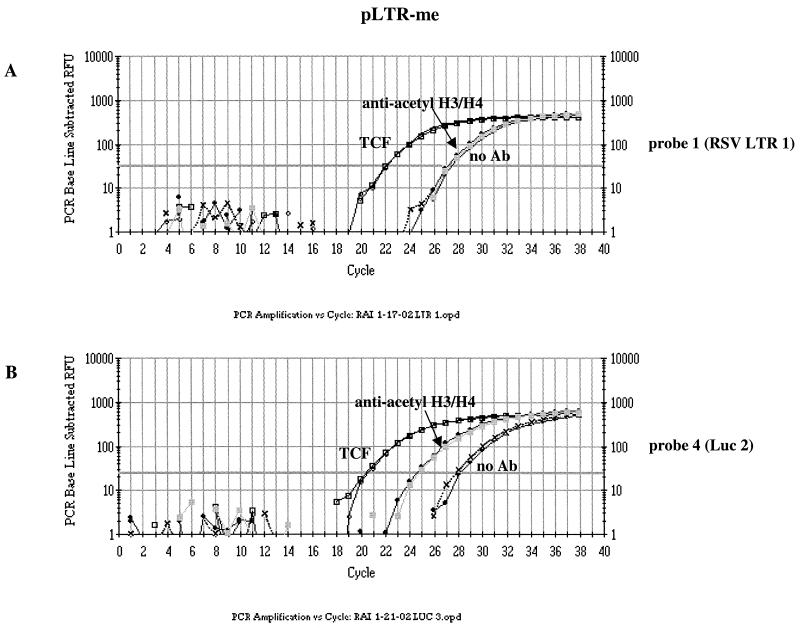
FIG. 4.
Representative Q-PCR graphs showing the relative amounts of regions RSV LTR 1 and Luc 2 immunoprecipitated from episome pLTR-me. (A) TaqMan traces showing that the antiacetylated H3/H4 sample and the no Ab sample contain similar amounts of DNA from RSV LTR region 1. Virtually none of the RSV LTR region 1 DNA on pLTR-me is associated with acetylated H3 and H4 histones in 293/EBNA1 cells. (B) TaqMan traces showing that a considerable amount of DNA from the Luc 2 region was immunoprecipitated by antiacetylated histone H3 and H4 IgG, demonstrating that this region of pLTR-me is associated with acetylated H3 and H4 histones in 293/EBNA1 cells. The arbitrary amplification threshold is depicted as a thick horizontal line in each graph. RFU, relative fluorescence units.
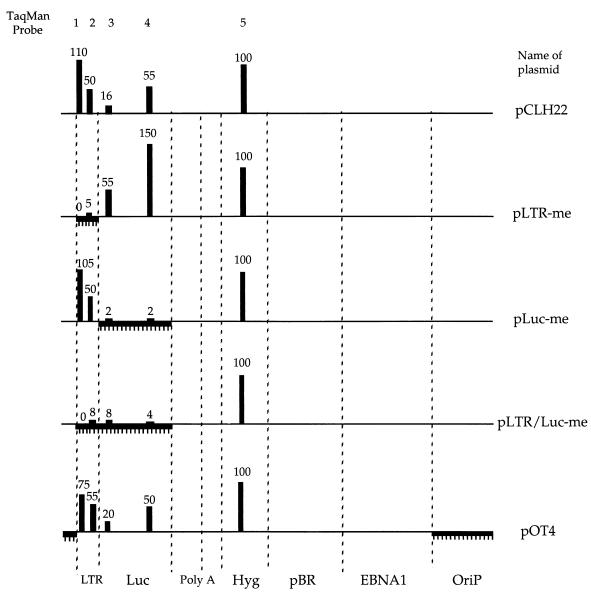
Fully unmethylated pCLH22 comprises three highly transcribed genes and should be associated mostly with acetylated histones; therefore, it was used as a positive control. The percentage of DNA immunoprecipitated in the hygromycin gene was used to normalize the percentages in other regions studied. For pCLH22, the hygromycin gene and region 1 of the RSV LTR showed similar results and yielded the highest association with acetylated histones (Fig. 5). On the same plasmid, region 1 of the luciferase gene showed the lowest association with acetylated histones and region 2 of the RSV LTR and region 2 of the luciferase gene showed intermediate levels of association (Fig. 5). This finding clearly reveals the association of acetylated histones with various regions of unmethylated pCLH22 and indicates that methylation status is not the sole determinant of chromatin structure. Interestingly, pOT4 showed nearly identical association with acetylated histones in these five regions (Fig. 5). The distance from Luc region 1 to the nearest methylated upstream CpG is 115 bases on pLTR-me, the distance from RSV LTR region 2 to the first methylated downstream CpG is 313 bases on pLuc-me, and the distance from RSV LTR region 1 to the nearest methylated upstream CpG is 73 bases on pOT4. Despite the close proximity of the zone of CpG methylation, the adjacent unmethylated DNA remains associated with acetylated histones. How the nucleosomes are positioned on these plasmids is unknown, but this observation suggests that methylation is not likely to impact chromatin structure in regions far away from the zone of methylation. Episomes pLTR-me, pLuc-me, and pLTR/Luc-me showed near-background levels of association with acetylated histones in the methylated regions and high levels of association with acetylated histones in the adjacent unmethylated regions (Fig. 5). These findings indicate that the relationship between DNA methylation and histone modification is most likely a local effect and that repressed chromatin structure due to DNA methylation does not spread into adjacent unmethylated regions. In addition, these findings are consistent with the results for luciferase gene expression from these patch-methylated episomes. The impact of DNA methylation on both transcription and the state of histone acetylation appears to be a local effect.
FIG. 5.
Methylated DNA patches are not associated with acetylated histones. Linear maps of pCLH22 and of each patch-methylated episome are shown. The vertical dotted lines divide the different regions of the plasmids indicated at the bottom of the graph. Methylated DNA patches are depicted by horizontal lines hatched with short vertical lines. Q-PCR was performed on TCF, no Ab, and antiacetylated H3/H4 ChIP samples with five different TaqMan probe and primer sets (Table 1). Approximate positions of the different TaqMan probes are indicated with numbers that correspond to the probe numbers listed in Table 1. Histograms with accompanying values represent averages of the percentages of DNA immunoprecipitated in two independent experiments normalized to that for the hygromycin gene (Hyg) region. Background amplification, determined with the corresponding no Ab control, was subtracted from each antiacetylated histone H3/H4 sample before the percentage of immunoprecipitated DNA was calculated. Luc, luciferase gene; OriP, EBV latent replication origin.
DISCUSSION
This is the first detailed study to dissect the impact of regional DNA methylation on transcription and on histone acetylation within cells. In this study, we established that the location of a methylated patch relative to the promoter and the coding region is extremely important in transcriptional regulation. We also clearly demonstrated that the size of the methylated patch is not essential for and is not proportional to transcriptional suppression. The impact of DNA methylation is primarily a local effect, and its impact on transcription is greater when it is in the transcription unit. Although methylation outside of the transcription unit has negligible impact on transcription by itself, it may enhance the effect of methylation within the transcription unit. These effects did not change over the 3-week interval during which these patch-methylated episomes were stably maintained in the human cells. In addition, the methylation pattern of these episomes was stable throughout the entire interval. We further showed that methylated DNA regions are devoid of acetylated histones and that this repressed chromatin may not spread far into adjacent methylation-free regions.
Our findings clearly rule out the hypothesis inferred by others using a nonreplicating plasmid system (15) that the size of the methylation patch is the key determinant for transcriptional repression. In addition, our data do not support the proposal that the repressed chromatin structure induced by DNA methylation propagates into adjacent DNA (5, 15, 16). It was also reported in a recent study using the Xenopus oocyte system that methylation of a CpG island can inhibit transcription from the herpes simplex virus tk promoter downstream (5). Our study indicates that the effect of the chromatin structure induced by DNA methylation outside of the transcription unit may be relevant only when a portion of the transcription unit is itself methylated. This discrepancy may be explained by the lack of replication of exogenous double-stranded DNA in the Xenopus oocyte system and in the mouse cells utilized in the earlier studies (5, 15, 16). In addition, some of the methylated DNA patches include a portion of or the entire transcription unit described in the studies by Kass et al. (15, 16). Although methylated plasmids acquire some form of chromatin structure after transfection into mammalian cells (9, 15), the chromatin structure changes after the first round of DNA replication in the mammalian cells, based on the results of a nuclease-sensitivity analysis (9). With the Xenopus oocyte system, it has been shown that the pathways for nucleosome assembly on nonreplicating and replicated templates may be different and that replication-coupled chromatin assembly is important for generating a repressed state of chromatin (1). Replicated and unreplicated plasmids may also be sequestered to different compartments of the nucleus. The episomes used in this study replicate once per cell cycle; therefore, they probably mimic the chromosomal events much more closely than do nonreplicating plasmids.
Our study shows that methylation of multiple CpG sites immediately upstream of the RSV LTR promoter does not significantly affect transcription. It also shows that methylation plays a minimal role in transcriptional suppression when it is outside of the transcription unit. When a 900-bp methylated patch with a CpG density of one CpG for every 9 bp was inserted at various sites outside of the transcription unit on the plasmid, transcription was not affected regardless of where the methylated patch was inserted (data not shown). These findings are inconsistent with the proposal that DNA methylation may affect transcription of genes downstream via histone deacetylase recruitment by methyl-CpG binding proteins (12, 16, 18). It has been shown that with five GAL4 binding sites immediately upstream of the promoter, a fusion protein with the GAL4 binding domain and the MeCP2 transcriptional-repression domain (TRD) can repress transcription in Xenopus oocytes and in mouse cells (12, 16). It is possible that GAL4 binds much tighter to DNA than does MeCP2, thereby making it more effective in anchoring the TRD of MeCP2 that was fused to the GAL4 binding domain in those previous experiments. Also, five GAL4 binding sites in tandem may be much more potent than a 2-kb methylated DNA fragment containing one CpG site for every 40 bases and positioned upstream of the promoter. An alternative possibility is that histone deacetylase interacts differently with the TRD of MeCP2 fused to the GAL4 binding domain than with the TRD in the native MeCP2 and that this may affect DNA at a greater distance from the GAL4 binding sites. Also, the lack of plasmid DNA replication in the Xenopus oocyte system and in the mouse cells used in the other studies may account for the contrasts between the previous findings and ours. Although methylated DNA may recruit factors, such as methyl-DNA binding proteins, regardless of DNA replication, the difference in the chromatin assemblies on nonreplicating versus replicating templates may alter the interaction of these factors with nucleosomes and the transcription machinery. In contrast to the findings with the Xenopus oocyte system, it has been demonstrated with Saccharomyces cerevisiae that Sin3-Rpd3-mediated histone deacetylation occurs over a range of one to two nucleosomes (13). It is likely that the deacetylation of histones mediated by methyl-CpG binding proteins also does not extend beyond one or two nucleosomes. Our findings with stable episomes in human cells support the view that DNA methylation affects transcription locally and that the impact on transcription is negligible when methylation is not in the promoter or the coding region.
It is evident that methylation of DNA outside of the RSV LTR promoter and the luciferase-coding region (pOT1+2+3+4) has little impact on transcription. In contrast, methylation of the same DNA plus methylation of a portion of the transcription unit (pMe−Luc and pMe−LTR) has a much larger impact than methylation in the promoter (pLTR-me) or the luciferase-coding region (pLuc-me) alone. Thus, methylation outside of the promoter and the coding region is important when a portion of or the entire transcription unit is also methylated. This finding suggests that global chromatin structure may facilitate the silencing of a gene when some methylation in the transcription unit exists but plays a minimal role in the absence of any methylation in the transcription unit. The inactivated X chromosome is heavily methylated (for a review, see reference 7); however, a small number of genes that escape X-chromosome inactivation remain unmethylated and expressed (17). If the DNA methylation-induced chromatin structure can affect nearby unmethylated genes, then one would expect these unmethylated genes to be transcriptionally silent. The fact that these genes are being transcribed while most of the X chromosome is methylated and silent may be more easily understood in light of our findings here.
In our experimental system, methylation of the luciferase-coding region has a major impact on its expression. In the genome, a majority of the genes have introns that may harbor methylated sequences while the associated exons may remain unmethylated. Do the methylated sequences in the intron suppress transcription? It is possible that different portions of the coding regions have different impacts on transcription. We are currently studying this question.
In the present study, decreased acetylation of histones H3 and H4 was observed within the methylated patches on episomes even though it was not observed in the adjacent unmethylated DNA. These findings do not support the proposal that DNA methylation can repress transcription from a distance, possibly through the mechanism of histone deacetylation (18, 22). However, this result is consistent with the finding in yeast that DNA binding repressors can cause localized decreases in histone acetylation (13). It is also consistent with the finding that histone acetylation is correlated with the methylation density of transgenes (21). Although histone modification has been reported to cover large regions, histone acetylation and deacetylation at the promoters may be more locally confined (for a review, see reference 24). The chromatin analyses reported here are within the zone of transcription. Our findings strongly indicate that the impact of DNA methylation on chromatin structure is highly localized at least when DNA methylation is within the transcription unit.
Acknowledgments
We thank David VanDenBerg for providing technical assistance for the TaqMan assays and for providing the TaqMan probes and primers for the XRCC1 analysis. We also thank M. R. Lieber, Y. Ma, and K. Yu for critical reading of the manuscript.
This work was supported by NIH grants RO1 GM54781 and RO1 GM60237. R.A.I. was supported by the ARCS Foundation.
REFERENCES
- 1.Almouzni, G., and A. P. Wolffe. 1993. Replication-coupled chromatin assembly is required for the repression of basal transcription in vivo. Genes Dev. 7:2033-2047. [DOI] [PubMed] [Google Scholar]
- 2.Barry, C., G. Faugeron, and J.-L. Rossignol. 1993. Methylation induced premeiotically in Ascobolus: coextension with DNA repeat lengths and effect on transcript elongation. Proc. Natl. Acad. Sci. USA 90:4557-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braunstein, M., A. B. Rose, S. G. Holmes, C. D. Allis, and J. R. Broach. 1993. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev. 7:592-604. [DOI] [PubMed] [Google Scholar]
- 4.Cedar, H. 1988. DNA methylation and gene activity. Cell 53:3-4. [DOI] [PubMed] [Google Scholar]
- 5.Curradi, M., A. Izzo, G. Badaracco, and N. Landsberger. 2002. Molecular mechanisms of gene silencing mediated by DNA methylation. Mol. Cell. Biol. 22:3157-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrlich, M., and K. C. Ehrlich. 1993. Effect of DNA methylation on the binding of vertebrate and plant proteins to DNA, p. 145-168. In J. P. Jost and H. P. Saluz (ed.), DNA methylation: molecular biology and biological significance. Birkhauser Verlag, Basel, Switzerland. [DOI] [PubMed]
- 7.Goto, T., and M. Monk. 1998. Regulation of X-chromosome inactivation in development in mice and humans. Microbiol. Mol. Biol. Rev. 62:362-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirt, B. 1967. Selective extraction of polyoma DNA from infected mouse cultures. J. Mol. Biol. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh, C.-L., and M. R. Lieber. 1992. CpG methylated minichromosomes become inaccessible for V(D)J recombination after undergoing replication. EMBO J. 11:315-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh, C.-L. 1994. Dependence of transcriptional repression on CpG methylation density. Mol. Cell. Biol. 14:5487-5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh, C.-L. 1997. Stability of patch methylation and its impact in regions of transcriptional initiation and elongation. Mol. Cell. Biol. 17:5897-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones, P. L., G. J. Veenstra, P. A. Wade, D. Vermaak, S. U. Kass, N. Landsberger, J. Strouboulis, and A. P. Wolffe. 1998. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 19:187-191. [DOI] [PubMed] [Google Scholar]
- 13.Kadosh, D., and K. Struhl. 1998. Targeted recruitment of the Sin3-Rpd3 histone deacetylase complex generates a highly localized domain of repressed chromatin in vivo. Mol. Cell. Biol. 18:5121-5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaludov, N. K., and A. P. Wolffe. 2000. MeCP2 driven transcriptional repression in vitro: selectivity for methylated DNA, action at a distance and contacts with the basal transcription machinery. Nucleic Acids Res. 28:1921-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kass, S. U., J. P. Goddard, and R. L. P. Adams. 1993. Inactive chromatin spreads from a focus of methylation. Mol. Cell. Biol. 13:7372-7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kass, S. U., N. Landsberger, and A. P. Wolffe. 1997. DNA methylation directs a time dependent repression of transcription initiation. Curr. Biol. 7:157-165. [DOI] [PubMed] [Google Scholar]
- 17.Miller, A. P., and H. F. Willard. 1998. Chromosomal basis of X chromosome inactivation: identification of a multigene domain in Xp11.21-p11.22 that escapes X inactivation. Proc. Natl. Acad. Sci. USA 95:8709-8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nan, X., H. H. Ng, C. A. Johnson, C. D. Laherty, B. M. Turner, R. N. Eisenman, and A. Bird. 1998. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393:386-389. [DOI] [PubMed] [Google Scholar]
- 19.Ng, H.-H., Y. Zhang, B. Hendrich, C. A. Johnson, B. M. Turner, H. Erdjument-Bromage, P. Tempst., D. Reinberg, and A. Bird. 1999. MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex. Nat. Genet. 23:58-61. [DOI] [PubMed] [Google Scholar]
- 20.Rountree, M. R., and E. U. Selker. 1997. DNA methylation inhibits elongation but not initiation of transcription in Neurospora crassa. Genes Dev. 11:2383-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schubeler, D., M. C. Lorincz, D. M. Cimbora, A. Telling, Y. Q. Feng, E. E. Bouhassira, and M. Groudine. 2000. Genomic targeting of methylated DNA: influence of methylation on transcription, replication, chromatin structure, and histone acetylation. Mol. Cell. Biol. 20:9103-9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wade, P. A., A. Gegonne, P. L. Jones, E. Ballestar, F. Aubry, and A. P. Wolffe. 1999. Mi-2 complex couples DNA methylation to chromatin remodeling and histone deacetylation. Nat. Genet. 23:62-66. [DOI] [PubMed] [Google Scholar]
- 23.Wigler, M., R. Sweet, G. K. Sim, B. Wold, A. Pellicer, E. Lacy, T. Maniatis, S. Silverstein, and R. Axel. 1979. Transformation of mammalian cells with genes from prokaryotes and eukaryotes. Cell 16:777-785.222468 [Google Scholar]
- 24.Wu, J., and M. Grunstein. 2000. 25 years after the nucleosome model: chromatin modifications. Trends Biochem. Sci. 25:619-623. [DOI] [PubMed] [Google Scholar]