Abstract
The GCN2 eIF2α kinase is essential for activation of the general amino acid control pathway in yeast when one or more amino acids become limiting for growth. GCN2's function in mammals is unknown, but must differ, since mammals, unlike yeast, can synthesize only half of the standard 20 amino acids. To investigate the function of mammalian GCN2, we have generated a Gcn2−/− knockout strain of mice. Gcn2−/− mice are viable, fertile, and exhibit no phenotypic abnormalities under standard growth conditions. However, prenatal and neonatal mortalities are significantly increased in Gcn2−/− mice whose mothers were reared on leucine-, tryptophan-, or glycine-deficient diets during gestation. Leucine deprivation produced the most pronounced effect, with a 63% reduction in the expected number of viable neonatal mice. Cultured embryonic stem cells derived from Gcn2−/− mice failed to show the normal induction of eIF2α phosphorylation in cells deprived of leucine. To assess the biochemical effects of the loss of GCN2 in the whole animal, liver perfusion experiments were conducted. Histidine limitation in the presence of histidinol induced a twofold increase in the phosphorylation of eIF2α and a concomitant reduction in eIF2B activity in perfused livers from wild-type mice, but no changes in livers from Gcn2−/− mice.
All organisms utilize externally available amino acids in lieu of synthesizing amino acids de novo. Consequently, amino acid biosynthetic pathways are generally repressed until external sources become limiting. The bacterium Escherichia coli is capable of assessing specific amino acid limitations and responding selectively to express enzymes involved in the synthesis of the limiting amino acid (36, 42). In contrast, the eukaryotic yeast Saccharomyces cerevisiae relies upon a general amino acid control system, whereby starvation for any single amino acid elicits derepression of all of the amino acid biosynthetic pathways (10, 41). Activation of the yeast general amino acid control system is mediated by GCN2 protein kinase induction of GCN4 translational expression. GCN4 is a transcriptional activator of genes involved in amino acid synthesis and related metabolic pathways (12, 24, 25, 39-41). Amino acid limitation results in an increase in uncharged tRNAs, which in turn activates the GCN2 eIF2α kinase. Phosphorylation of Ser51 of eIF2α by GCN2 inhibits the activity of the eIF2B guanylate exchange factor, resulting in reduced formation of the eIF2α-GTP-Met-tRNAi ternary complex necessary for translation initiation (22). Although decreased availability of the eIF2α-GTP-Met-tRNAi ternary complex can result in the repression of global protein synthesis, the yeast GCN4 mRNA contains a unique cluster of upstream open reading frames (uORFs) that mediate translational derepression of the GCN4 coding sequence only when the ternary complex is limiting. Thus, it is clear that phosphorylation of eIF2α by GCN2 under conditions of amino acid starvation allows yeast to adapt to this stress by down-regulating global protein synthesis while promoting the translation of a transcription factor, which in turn activates the transcription of genes encoding amino acid biosynthetic enzymes.
Clues to the mechanism by which amino acid starvation is coupled to GCN2 activation were found in the predicted domain structure of the GCN2 protein. GCN2 contains a domain homologous to histidyl-tRNA synthetases (HisRS), an enzyme normally responsible for charging histidyl-tRNA with histidine. The HisRS-related domain of GCN2 lacks this normal synthetase activity, and residues critical for histidine-specific binding are missing in the GCN2 HisRS domain (40). Wek and coworkers (40) proposed that uncharged tRNAs, which increase in concentration concomitant with amino acid deprivation, may activate the eIF2α kinase activity of GCN2 through binding the modified GCN2 HisRS-related domain. This hypothesis is supported by the demonstration that a variety of uncharged tRNAs can bind the modified HisRS domain of GCN2, resulting in activation of the catalytic domain (6, 28, 38, 41, 44).
Regulation of amino acid biosynthetic pathways in metazoans, including mammals, entails a further complication in that they do not have the biosynthetic capacity to synthesize 10 of the amino acids (i.e., the so-called “essential amino acids”). Regulation of the biosynthesis of the nonessential amino acids appears to be dependent upon a general amino acid control system similar to that of yeast (14), but also appears to be independent of eIF2B activity and eIF2α phosphorylation. In contrast, deprivation of essential amino acids induces phosphorylation of eIF2α, reduction in eIF2B activity, and repression of global protein synthesis (13, 21, 37). Recently, homologues of yeast GCN2 have been discovered and characterized in Neurospora (31), Drosophila (26, 30), and mice (2, 34). The major domains of GCN2, including catalytic and HisRS-related domains, are conserved among these species. However, analysis of the complete genomic sequence of Drosophila, Caenorhabditis elegans, mice, and humans has failed to detect an apparent homolog of GCN4, the regulatory target of yeast GCN2 (D.R.C., unpublished observations). Nonetheless, upon introduction in yeast cells, Drosophila and mouse GCN2 are capable of rescuing Gcn2 mutants by phosphorylating yeast eIF2α and thereby derepressing the translation initiation of GCN4 (26, 34).
Yeast GCN2 has been implicated as an important regulator of growth in response to the stress of limiting amino acids. GCN2 mutants not only are incapable of mounting the general control response when starved for amino acids, but also are unable to grow when amino acids are limiting or when inhibitors of amino acid biosynthesis are present in the growth medium (5, 12, 41). The role of GCN2 in growth and development in higher eukaryotes under conditions of nutritional stress is currently unknown.
To determine the potential role of GCN2 in the regulation of amino acid biosynthesis in higher eukaryotes, we have isolated the mouse Gcn2 gene and have generated a Gcn2−/− knockout (loss-of-function) mutant mouse line. Gcn2−/− mice are viable and fertile when reared under standard conditions, but have a decreased probability of completing development under conditions in which amino acids are deprived. Limiting specific amino acids is correlated with an increase in phosphorylation of eIF2α and a concomitant reduction of eIF2B activity in wild-type liver and cultured cells; however, these responses are ablated in the absence of GCN2.
MATERIALS AND METHODS
Cloning and targeted disruption of the Gcn2 gene.
Genomic clones of the Gcn2 gene were isolated from a BAC genomic library (Genome Systems, St. Louis, Mo.) and from a lambda genomic library constructed from genomic DNA isolated from the mouse TL1 129 SvEvTac strain (a gift from Christopher Wright, Vanderbilt University School of Medicine). Extensive DNA sequencing was performed throughout the 39 exons and flanking intronic regions of GCN2. Exon-intron boundaries were determined by comparison of a GCN2 cDNA clone (34) with the genomic DNA sequence determined herein. To generate a targeted substitution or deletion of essential domains of the Gcn2 gene, the neomycin resistance (Neor) gene was substituted for most of exon 12 (corresponding to residues 606 to 748 of the mGCN2β isoform [GenBank accession no. AF193343]). Specifically, the targeting vector was constructed in the pPNT-1 plasmid and contained a 3.0-kb HindIII genomic GCN2 fragment comprised of intron 11 and the first 15 nucleotides of exon 12, a 1.1-kb HindIII-XhoI fragment containing the Neor gene, and a 9-kb XhoI-HindIII genomic GCN2 fragment comprised of introns 12 to 15 and exons 13 to 15. The genomic DNA fragments of GCN2 were all derived from the lambda genomic library of the TL1 129 SvEvTac mouse strain. The GCN2-neo targeting vector was electroporated into TL1 129 SvEvTac mouse embryonic stem (ES) cells (Vanderbilt University Medical Center, Transgenic Mouse Core Facility). Homologous recombinants were identified by SacI digestion followed by Southern blot analysis and further verified by PCR. Primer 1 (5′-GGTCTGAAGTAGAAGGATGTCAAGTTC-3′) and primer 2 (5′-TCCATCAGTTTGCTTCTCTC-3′) will amplify an 890-bp fragment from the wild-type Gcn2 allele, while primer 1 and the neo-specific primer 3 (5′-TACTGTGGTTTCCAAATGTGTCAGTTT-3′) will amplify a 550-bp fragment when exon 12 has been replaced by the Neor marker. Homologous recombinant cells were transplanted into C57BL/6 blastocysts. Two chimeric founder mice were shown to have the Gcn2-neo gene in their germ lines (data not shown). The heterozygous F1 hybrid mice were inbred to produce F2 progeny.
Homozygous Gcn2−/− ES cells were generated by incubating Gcn2+/− heterozygous ES cells with a high (1 μg/ml) concentration of G418 (neomycin) and selecting for resistant colonies. One highly resistant colony was subjected to further molecular analysis and shown to be homozygous for the target Neor substitution of exon 12 of mGCN2.
RT-PCR analysis of Gcn2 mRNA.
Total RNA was isolated with TRI reagent (Sigma). The following primers were used for reverse transcription-PCR (RT-PCR) analysis of the mGCN2 isoforms: RCW 150 (3′ primer for all three isoforms), 5′-ATGGAGGATGTCACACGAGCCAGGAGAG; RCW 285, 5′-AAGTTGAGTCTGGTTGTTACACTGT; RCW 244, 5′-ATACCCAGATGTAGTTCCCGAAA; and RCW 201, 5′-GACCAGGTGGTACAGGGTT. The 5′ primer for mGCN2α was RCW 285, corresponding to sequences in the extended 5′-exon specific for the α isoform. The 5′ primer for mGCN2β was RCW 244, corresponding to sequences contiguous between exons 1 and 2 of the β isoform. In addition, RCW 201, the 5′ primer for mGCN2γ, was within a portion of the 314-bp exon unique of the γ isoform, located between exons 1 and 2 of mGCN2β (34).
For analysis of Gcn2 knockout mutants, 1 μg of total RNA was subjected to RT-PCR with the Access RT-PCR kit (Promega) with primers flanking the targeted exon. Forward primer from the upstream exon 11 (positions 1768 to 1787, 5′-ACCGTCATTCCCAGCAACCA-3′) and reverse primer from the downstream exon 15 (positions 2509 to 2488, 5′-GCAGCGTGCTCTTCTCGCAGTA-3′) will amplify a 742-bp product from the wild-type Gcn2 allele, whereas both Gcn2−/− and Gcn2+/− display an 812-bp fragment. RT-PCR products were cloned for sequencing. Sequence analysis revealed that the 812-bp mutant Gcn2 mRNA corresponded to the RNA generated from a cryptic splice located on the antisense strand of the Neor gene, whereas the other splicing events remain intact. RT-PCR products were further analyzed by Southern hybridization with part of exon 12 as the probe to demonstrate that the Gcn2−/− mice completely lacked the RT-PCR product (742 bp) containing exon 12 coding sequences.
Amino acid limitation diet experiments.
For 10 days prior to the beginning of dietary experiments, male and female mice were provided with a low-fat synthetic diet for 3 h. Food was weighed both prior to and after the 3-h feeding period. Water was provided ad libitum throughout the experiment. Mice were weighed daily, and their food intake and weight were plotted versus time. After the 10-day meal-training regimen, animals were switched to the synthetic control diet containing a complete complement of amino acids, and matings were established between several replicate cages each containing one Gcn2+/− and two Gcn2−/− females. After 4 days, the males were removed. On day 12 of gestation, each cage was randomly assigned one of the four diets: glycine-deficient, leucine-deficient, tryptophan-deficient, or complete-synthetic diet. Pregnant females remained on the diet until day 17 of gestation or until weight loss exceeded 1 g for multiple days and then switched back to the complete amino acid control diet. Preliminary experiments indicated that this was the longest period of time amino-acid-limited diets could be imposed during gestation without causing an unacceptable rate of spontaneous abortions. Cages were checked daily for new litters, which were removed immediately. The date of birth, genotype of parents, general health, and number of pups were recorded for each litter. Live pups were euthanized, and tail DNA was isolated from all pups for the purpose of genotyping. The genotype and numbers of alive and dead pups were recorded for each diet.
Liver perfusion.
Livers were perfused in situ as described previously (3, 16), with the following modifications. Livers were perfused for 15 min with a nonrecirculating medium delivered at a flow rate of 4 ml/min. The perfusate contained amino acids present at 10 times the concentrations in rat arterial plasma (control medium) (35) or 10 times the concentration found in rat arterial plasma except for histidine and additionally containing 4 mM histidinol (histidinol medium). Livers were then removed, rinsed in ice-cold saline, and homogenized as described below.
Determination of eIF2α phosphorylation state.
Livers were homogenized in 7 volumes of buffer consisting of 20 mM HEPES (pH 7.4), 100 mM KCl, 0.2 mM EDTA, 2 mM EGTA, 1 mM dithiothreitol, 50 mM NaF, 50 mM β-glycerolphosphate, 0.1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, and 0.5 mM sodium vanadate and then centrifuged at 10,000 × g for 10 min at 4°C. The relative amount of eIF2α in the phosphorylated form was quantitated by protein immunoblot analysis with an affinity-purified antibody that specifically recognizes eIF2α phosphorylated at Ser51 (eIF2α[P]; Biosource International). The total amount of eIF2α in the samples was determined by reprobing the blot with a monoclonal antibody that recognizes equally the phosphorylated and unphosphorylated forms of eIF2α followed by an antimouse secondary antibody. Values obtained with the anti-eIF2α[P] antibody were normalized for the total amount of eIF2α present in the sample as described previously (20).
Measurement of eIF2B activity.
Livers were homogenized in 4 volumes of buffer consisting of 45 mM HEPES (pH 7.4), 95 mM potassium acetate, 375 μM magnesium acetate, 75 μM EDTA, 10% glycerol, 2.5 mg of digitonin per ml, and 1 μM microcystin and then centrifuged at 10,000 × g for 10 min at 4°C. The guanine nucleotide exchange activity of eIF2B was measured in the 10,000 × g supernatant exactly as described previously (17). Briefly, eIF2 purified from rat liver (18) was incubated with [3H]GDP to form the substrate for the reaction. Liver supernatant was mixed with assay buffer containing nonradiolabeled GDP at a final concentration of 1 μM and incubated at 30°C for 1 min. The eIF2-[3H]GDP complex was added, and at various times, aliquots of the reaction mixture were removed and collected on nitrocellulose filter disks. Radioactivity bound to the disks was quantitated by liquid scintillation spectrometry. eIF2B activity was calculated as the rate of loss of [3H]GDP from the eIF2-[3H]GDP complex.
Measurements of rates of protein synthesis.
Rates of protein synthesis were determined by the incorporation of [3H]leucine into protein during the final 10 min of liver perfusion as described previously (7).
GCN2 immunoblot analysis.
Liver or kidney tissue derived from Gcn2+/+ or Gcn2−/− mice was frozen in liquid nitrogen, pulverized, and homogenized in 10 volumes (wt/vol) of 50 mM Tris-HCl (pH 7.5), 1% Nonidet P-40, 1 mM dithiothreitol, 10% glycerol, 1 mM EDTA, and protease inhibitors (100 μM phenylmethylsulfonyl fluoride [PMSF], 0.15 μM aprotinin, 1 μM pepstatin, 1 μM leupeptin). The homogenate was subjected to a 5-s burst with a Polytron homogenizer and clarified by centrifugation at 14,000 × g. Seventy micrograms of protein for each sample were fractionated on 7.5% SDS-polyacrylamide gels and transferred to a nitrocellulose filter, which was then incubated with an antibody prepared against the carboxy terminus of mouse GCN2 (1/1,000 dilution). After incubation with a horseradish peroxidase-coupled antirabbit secondary antibody, the antibody-protein complexes were visualized with a chemiluminescent substrate.
Stress analysis of ES cells.
ES cells were cultured in Dulbecco's modified Eagle's medium (BioWhittaker), supplemented with 2 mM glutamine, 0.1 mM each nonessential amino acid, 50 μg of gentamicin per ml, 5.5 μM β-mercaptoethanol, 10% fetal bovine serum (HyClone), and 10 ng of mouse leukemia inhibitory factor (LIF) per ml in humidified air with 5% CO2 at 37°C. ES cells were maintained on irradiated mouse embryonic fibroblast feeder cells and passed twice without feeders on gelatinized plates before each experiment. Cells were cultured to about 50% confluence and subjected to the presence or absence of stress for the indicated times. Stress conditions were brought about by growth in complete medium supplemented with 1 μM thapsigargin or in media depleted of leucine. In the leucine depletion study, the medium was supplemented with dialyzed fetal bovine serum. Lysates were prepared in a solution of 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% NP-400, and 0.5% sodium deoxycholate supplemented with protease inhibitors (100 μM PMSF, 0.15 μM aprotinin, 1 μM pepstatin, 1 μM leupeptin), 50 mM NaF, and 40 mM β-glycerolphosphate for Western blot analysis to detect the phosphorylated form of eIF2α at Ser51 (antisera from Research Genetics) or total eIF2α with a monoclonal antibody that recognizes either phosphorylated or nonphosphorylated forms.
RESULTS
Isolation and characterization of the mouse Gcn2 gene.
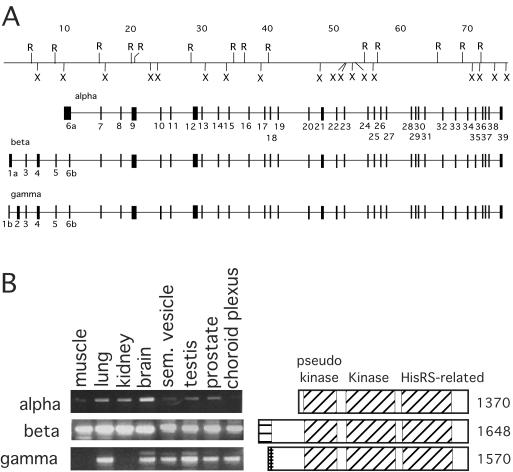
The Gcn2 gene was isolated from mouse genomic libraries, and all of the exons were identified by sequencing and comparison to cDNA clones of GCN2 (Fig. 1A). Three major mRNAs (α, β, and γ) that arise from three alternative promoters (34) were identified. Mouse Gcn2 is comprised of 39 exons that span 75 kb of the mouse genome. Drosophila Gcn2 is composed of 10 exons (26) and does not share any exon-intron boundaries with the mouse Gcn2 gene, suggesting that the extant introns were inserted into the Gcn2 genes of each of these species after they diverged from a common ancestor. The β GCN2 mRNA is the most abundant GCN2 isoform and is expressed in a variety of tissues (Fig. 1B). The α GCN2 mRNA isoform is most abundantly expressed in the brain, whereas the γ isoform is expressed in nearly equivalent levels in six of the eight tissues examined, but is completely absent in skeletal muscle and kidney. All three GCN2 mRNA isoforms encode the critical catalytic and HisRS regulatory domains, but are predicted to have distinct amino-terminal domains. The amino terminus of the GCN2 β isoform contains an evolutionarily conserved domain that interacts with GCN1 in yeast (8, 23). Recent studies show that GCN1 is required to mediate the activation of GCN2 by uncharged tRNAs (8). The α and γ isoforms of GCN2 are missing all or part of the GCN1 binding domain, suggesting that they may be activated by a different mechanism.
FIG. 1.
Structure and expression of the mouse Gcn2 gene. (A) Three isoforms of mouse GCN2 RNA transcripts are encoded within a 75-kb region of the mouse. The first exon of the α isoform (denoted 6a) is inclusive of exon 6b present in the β and γ isoforms and shares a common 3′ end with exon 6b. The first exon of the β isoform (denoted 1a) is inclusive of exon 1b present in the γ isoform and shares a common 3′ end with exon 1b. Each of the three isoforms contains a unique translation initiation site (34), but terminates translation at a common UGA stop codon in exon 39. All of the exon-intron junctions conform to the eukaryotic consensus splice sites. Restriction sites mapped in genomic DNA: X, XbaI; and R, EcoRI. (B) Expression of mGCN2 mRNA isoforms. Primers specific for each of the three GCN2 mRNA isoforms were used in RT-PCRs with RNA isolated from various mouse tissues to detect their relative tissue-specific expression. Further quantitative RT-PCR experiments indicated that the β isoform is considerably more abundant than the α and γ isoforms. Diagrams depicting the domains included in each isoform are shown to the right.
Generation of Gcn2 knockout mutant mice.
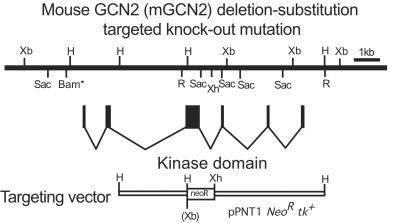
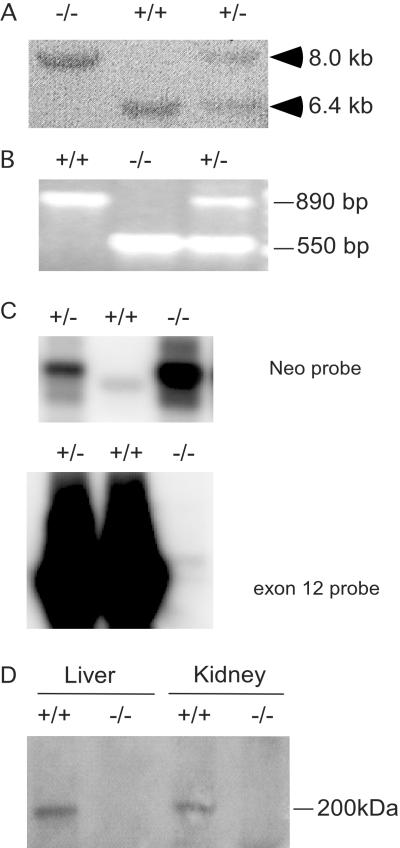
To generate a loss-of-function mutation of GCN2, we created a targeted disruption of exon 12, which encodes the critical kinase subdomains II to IV and part of the insert region between subdomains IV and V (Fig. 2). Targeted disruption of exon 12 was verified by Southern hybridization (Fig. 3A) and PCR (Fig. 3B). Several Gcn2−/− mutant strains were isolated and shown to produce a single-mutant GCN2 mRNA (Fig. 3C), and they do not express GCN2 protein (Fig. 3D). Sequence analysis of RT-PCR product from mutant GCN2 mRNA showed that cryptic splicing sites exist in the Neor gene, and the splicing product contains a frameshift mutation. Matings between heterozygous Gcn2+/− mice result in the expected frequency of viable Gcn2−/− mutants. Similar results were obtained from another Gcn2 mutant mouse strain, in which exon 12 was entirely deleted by using the Cre/loxP system (unpublished data).
FIG. 2.
Gcn2 targeting knockout vector. The approximate locations of the mouse Gcn2 exons 10 to 15 are shown below a restriction endonuclease map of genomic DNA. Xb, XbaI; Sac, SacI; Bam, BamHI; R, EcoRI; H, HindIII; and Xh, XhoI. The left arm of the target vector included a 3.0-kb HindIII fragment including a small part of exon 12, whereas the right arm included a 5.0-kb XhoI-HindIII fragment. The Neor gene was inserted between the right arm and left arm of the targeting vector, resulting in a deletion and substitution of most of exon 12.
FIG. 3.
Targeted disruption of the mouse Gcn2 gene. (A) Southern hybridization was performed on SacI-restricted genomic DNA to verify the targeted disruption. The Southern blot was probed with a combination of mGCN2 cDNA derived from exons 9 to 12 and a BamHI 1.3-kb genomic fragment corresponding to the 3′ end of the left arm of the targeting vector. The sizes of the fragments are indicated. +/+, wild type; +/−, heterozygote; −/−, homozygous mutant. (B) Transgenic animals were verified by PCR amplification of genomic DNA. The wild-type mGCN2 allele was detected as a 550-bp fragment from exon 12, whereas the mutant allele was detected as an 890-bp product from Neor gene substitution. (C) RT-PCR products (exons 11 to 15) derived from liver RNA were analyzed by Southern hybridization with Neor gene or a fragment of exon 12 as the probe. Gcn2−/− and Gcn2+/− mice display an 812-bp fragment detected by the Neor probe. Sequence analysis of this fragment revealed that it corresponded to an RNA generated from a cryptic splice located on the antisense strand of the Neor gene. As expected, the Gcn2−/− mice completely lacked the RT-PCR product (742 bp) containing exon 12 coding sequences. (D) Western blot analysis of mouse GCN2 with antisera prepared against the carboxyl terminus of mouse GCN2 indicated the absence of GCN2 in the homozygous mutant liver and kidney.
Mutation of Gcn2 has significant developmental affects when essential amino acids are absent in the maternal diet.
In yeast, GCN2 is required for growth in the absence of amino acids or in the presence of inhibitors of amino acid biosynthesis (5, 12, 41). Prenatal and postnatal growth in mammals is influenced by maternal health and diet, as well as the genetic composition of the progeny. To determine if GCN2 influences mouse growth and development under conditions of limiting amino acids, Gcn2−/− homozygous mutant females were mated with Gcn2+/− heterozygous males to produce litters with an expected Mendelian 1:1 ratio of Gcn2−/− and Gcn2+/− genotypes. During days 12 to 17 of gestation, pregnant females were fed one of three synthetic diets lacking a single amino acid. Two of the diets lacked the essential amino acids leucine or tryptophan, while the other lacked the nonessential amino acid glycine. Control groups were fed a synthetic diet replete with all amino acids.
Mice fed a complete synthetic diet during gestation showed the expected 1:1 ratio of Gcn2−/− to Gcn2+/− progeny. In contrast, mice fed a leucine-deficient diet showed a nearly threefold reduction in the number of Gcn2−/− progeny (Table 1). The number of Gcn2−/− progeny was also reduced in the glycine- and tryptophan-deficient diet experiments, although the reduction did not quite reach statistical significance in either case. Furthermore, the amino acid-deficient diets adversely affect prenatal viability, as shown by the relatively large number of stillborn pups. The negative impact of the amino acid-deficient diets was most pronounced in the leucine-deficient diet, with which 24% of the pups were stillborn.
TABLE 1.
Affects of amino acid deprivation on progeny number and genotype
| Diet | No. of progeny
|
Probability | No. of still born progeny
|
||
|---|---|---|---|---|---|
| Gcn2−/− | Gcn2+/− | Gcn2−/− | Gcn2+/− | ||
| Control | |||||
| Observed | 15 | 17 | 0 | 0 | |
| Expected | 16 | 16 | 0.723 | ||
| Minus glycine | |||||
| Observed | 12 | 22 | 3 | 0 | |
| Expected | 17 | 17 | 0.086 | ||
| Minus leucine | |||||
| Observed | 7 | 19 | 5 | 3 | |
| Expected | 17 | 17 | 0.013∗ | ||
| Minus tryptophan | |||||
| Observed | 21 | 30 | 2 | 3 | |
| Expected | 25.5 | 25.5 | 0.207 | ||
Probability by chi-square test. ∗, significantly deviates from expected number based upon chi-square goodness-of-fit test.
Gcn2−/− mice exhibit normal liver glycogen content.
Scheuner and coworkers (32) have mutated the regulatory phosphorylation site of eIF2α in mice by substituting the Ser51 residue with alanine. Ser51Ala knock-in mice die within the first day after birth due to hypoglycemia associated with the lack of glycogen storage in the liver, leading Scheuner et al. (32) to speculate that the Ser51Ala lethal phenotype was caused by blocking the regulatory activity of GCN2. However, as noted, our Gcn2−/− mice are normal at birth and have survived for more than 1 year under standard laboratory conditions. We have examined the liver glycogen content of adult mice (4 to 10 months old) and found that no difference occurs between Gcn2−/− knockout mice and wild-type mice (Table 2).
TABLE 2.
Liver glycogen levels in Gcn2+/+ versus Gcn2−/− mice
| Genotype | No. of mice | Mean glycogen content (μmol/mg [wet wt]) | SE |
|---|---|---|---|
| Gcn2+/+ | 9 | 145.6 | 26.1 |
| Gcn2−/− | 12 | 146.2 | 17.1 |
Gcn2−/− mice fail to show induction of eIF2α[P] and repression of eIF2B activity during histidine deprivation.
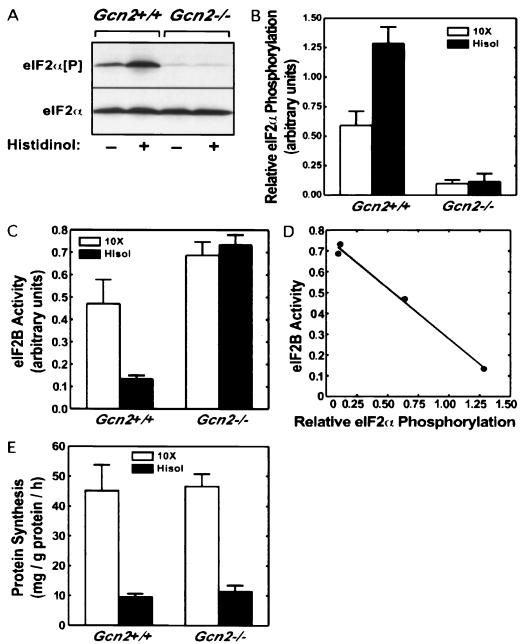
In a previous study, it was shown that perfusion of rat livers with medium lacking histidine and containing the amino acid analog histidinol resulted in an increase in the levels of phosphorylated eIF2α and a reduction in eIF2B activity in liver extracts (16). In the present study, the effect of histidinol on eIF2α phosphorylation in livers from wild-type and Gcn2−/− mice was examined. In the absence of stress, the relative phosphorylation of eIF2α in Gcn2+/+ mice was significantly higher than that in Gcn2−/− littermates. Upon perfusion with histidinol, eIF2α phosphorylation in the Gcn2+/+ livers was increased about twofold, with no detectable phosphorylation of this initiation factor in the Gcn2−/− tissue (Fig. 4A and B).
FIG. 4.
Effect of histidinol treatment on relative eIF2α phosphorylation, eIF2B activity, and protein synthesis. (A) Mouse livers were perfused with histidinol in situ as described in Materials and Methods. Total eIF2α and eIF2α[P] were then detected by Western blot analysis. (B) Quantitative analysis of eIF2α[P] relative to total eIF2α is shown as the mean of seven livers per condition. (C) The guanine nucleotide exchange activity of eIF2B in mouse liver homogenates was measured as described in Materials and Methods. The results represent the mean ± standard error of three to seven livers per condition. (D) The eIF2α phosphorylation and eIF2B activity data presented in panels B and C were subjected to linear regression analysis and are presented in graphic form. (E) Rates of protein synthesis were determined by the incorporation of [3H]leucine into protein during the final 10 min of the perfusion and are expressed as milligrams of protein synthesized per gram of tissue protein per hour. The values represent the mean ± standard error for three to six livers per condition: a complete mixture of amino acids (open bars) or medium containing all amino acids except histidine and additionally containing 4 mM histidinol (solid bars). *, P < 0.01 versus complete amino acid condition. The perfusate contained amino acids present at 10 times the concentrations typically found in arterial plasma (10X control medium) or 10 times the concentration found in arterial plasma except for histidine and additionally containing 4 mM histidinol (Hisol medium).
The guanine nucleotide exchange activity of eIF2B was measured in extracts of livers from wild-type and Gcn2−/− mice perfused in the presence or absence of histidinol. The eIF2B activity was reduced to approximately 30% of the control value in livers from wild-type mice perfused with histidinol medium (Fig. 4C). In contrast, histidinol had no affect on eIF2B activity in livers from Gcn2−/− mice. Interestingly, eIF2B activity was significantly greater in livers from Gcn2−/− mice perfused with control medium than in livers from wild-type animals (P = 0.05). The latter finding correlates with the reduction in eIF2α phosphorylation observed in livers from Gcn2−/− mice compared to wild-type mice. In fact, linear regression analysis revealed that eIF2α phosphorylation and eIF2B activity were inversely proportional (r2 = 0.99; Fig. 4D) for the four conditions examined here. Despite the fact that eIF2B activity remained high in Gcn2−/− livers perfused with histidinol, global protein synthesis in Gcn2−/− livers was repressed by histidinol treatment to the same extent as that seen in Gcn2+/+ mice (Fig. 4E).
GCN2 phosphorylates eIF2α in response to leucine depletion.
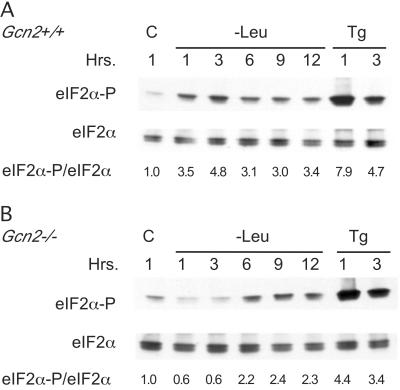
Leucine deprivation results in the repression of global protein synthesis via the reduction in eIF2B activity (19). The reduction in eIF2B activity is caused in part by the phosphorylation of Ser51 of eIF2α by one or more of the eIF2α kinases. To assess the importance of GCN2 in the induction of eIF2α phosphorylation during leucine deprivation, Gcn2−/− ES cells were incubated in leucine-deficient media for 1 to 12 h. A 3.5-fold enhancement of eIF2α phosphorylation in Gcn2+/+ ES cells was observed after the first hour of leucine deprivation and was sustained throughout the 12-h incubation period (Fig. 5). In contrast, Gcn2−/− ES cells failed to show any induction in eIF2α phosphorylation during the first 3 h of leucine deprivation, and only after a 6-h stress was there a two- to fourfold induction of eIF2α phosphorylation. In contrast, thapsigargin, an endoplasmic reticulum stress agent that inhibits Ca2+-ATPase and activates the PERK eIF2α kinase, showed a strong and rapid induction of eIF2α phosphorylation. This increase in eIF2α phosphorylation was observed independent of the Gcn2 genotype.
FIG. 5.
Phosphorylation of eIF2α in leucine-deprived ES cell is dependent upon GCN2. Gcn2+/+ (A) and Gcn2−/− (B) ES cells were grown in leucine-replete (lane C) or leucine-deficient (−Leu) media as described in Materials and Methods. Cells were harvested at 1, 3, 6, 9, and 12 h and analyzed by immunoblotting with antisera directed against either eIF2α or eIF2α[P]. The ratio of eIF2α[P] to eIF2α is indicated at the bottom of each panel. For comparison, Gcn2+/+ and Gcn2−/− ES cells were also treated for 1 or 3 h with 1 μM thapsigargin (Tg) to induce eIF2α phosphorylation via the PERK-dependent unfolded protein response.
DISCUSSION
The control of translation in response to external signaling is an important mechanism by which cells and organisms respond to changes in their environment. Yeast cells have evolved a mechanism exquisitely adapted to sense amino acid deprivation that results in the selective translational derepression of GCN4, a transcriptional activator of the amino acid biosynthetic genes (12). Yeast has a single eIF2α kinase, GCN2, which acts as the sensor in this regulatory pathway. Regulation of amino acid biosynthesis in multicellular higher eukaryotes is considerably more complex owing to the diverse needs of different tissues, the transport of amino acids through the circulatory system, and the inability of higher eukaryotes to synthesize 10 of the amino acids. Nonetheless, GCN2 is also present in higher eukaryotes and is functionally equivalent to yeast GCN2 (26, 34).
Similar to loss-of-function mutations in yeast Gcn2, mouse Gcn2 knockout mutants have no observable mutant phenotype in a nonstressed state. The loss of GCN2 function, however, negatively impacts fetal development when specific amino acids, particularly leucine, are missing in the maternal diet. This developmental defect is analogous to the slow-growth phenotype of yeast Gcn2 mutants grown on amino acid-deprived media or in the presence of inhibitors of amino acid biosynthesis. The developmental defects that we observe in the Gcn2−/− knockout mice reared on leucine-deficient diets are likely to be due to the loss of signaling via the phosphorylation of eIF2α. Gcn2−/− ES cells do not exhibit the normal rapid induction of phosphorylation of eIF2α when shifted to media lacking leucine. In contrast, eIF2α phosphorylation is induced in Gcn2−/− cells by thapsigargin, a known inducer of the unfolded protein response and an indirect activator of the PERK (PEK) eIF2α kinase (10, 33). Leucine deprivation does eventually result in the induction of eIF2α phosphorylation in Gcn2−/− ES cells, suggesting that one or more of the other eIF2α kinases are activated by a secondary effect of the leucine deprivation. We have observed a similar effect in Perk−/− cells treated with thapsigargin (D.R.C., unpublished data), which suggests that although PERK is the major eIF2α kinase responding to Ca2+ depletion in the endoplasmic reticulum, one or more of the other eIF2α kinases can also respond. We propose that although each of the eIF2α kinases may play a dominant role in responding to a specific physiological change or stress, each may also contribute to eIF2α phosphorylation signaling in response to a diverse array of perturbations.
Deprivation of essential amino acids in cultured mammalian cells and in liver perfusion experiments has shown that phosphorylation of eIF2α is induced concomitant with a decrease in eIF2B activity and global protein synthesis (15, 37). We show that phosphorylation of eIF2α and reduction of eIF2B activity is strikingly dependent upon GCN2 when the liver is deprived of histidine. Surprisingly, however, global protein synthesis is still repressed in Gcn2−/− mutant liver under histidine deprivation, suggesting that the repression of global protein synthesis is not mediated by the phosphorylation of eIF2α and the reduction in eIF2B activity. The repression of global protein synthesis under these conditions may instead be mediated by changes in eIF4EBP, as has been suggested previously (15). In yeast, deprivation of a single amino acid does not result in the repression of global protein synthesis, although GCN2 is activated, phosphorylates eIF2α, and leads to the reduction in eIF2B activity and the translational derepression of the amino acid biosynthetic transcriptional activator GCN4. Although mammalian cells apparently lack an orthologue of GCN4, we propose that mammalian GCN2 is likely to be important in translational control of specific mRNAs. Over half of the mRNAs encoding regulatory proteins of diverse nature in mammals contain multiple uORFs in their 5′ untranslated leader sequence (29), and these transcripts are candidates for similar translational control as described for yeast GCN4. One candidate for such regulation in mammals is the transcription factor ATF4 (also called CREB-2), which contains conserved uORFs. Harding and coworkers (10) have shown that derepression of ATF4 in mouse ES cells deprived of leucine is dependent upon GCN2 and the 5′ leader of ATF4 mRNA.
GCN2 exhibits a distinct tissue-specific pattern of expression, suggesting that it may have specific developmental or physiological functions (reference 34 and data shown herein). The highest level of GCN2 mRNA is seen in the brain of both mouse (reference 34 and data shown herein) and Drosophila (26, 30). ATF4, a known target of GCN2 regulation in cell culture, is intimately involved in regulating long-term memory in mammals and invertebrates, including Drosophila (1). In addition to their primary function in protein synthesis, specific amino acids, including glutamate and aspartate, function as excitatory signals in the central nervous system. In part because of these dual functions, amino acid pools in the brain are rigorously regulated (4, 9, 27).
In addition to amino acid deprivation, yeast GCN2 is known to be activated by purine and glucose deprivation. In mammalian cells, glucose-induced secretion of insulin is dependent upon amino acids, and individual amino acids such as arginine are potent stimulators of insulin secretion and thereby play important roles in glucose homeostasis. Regulation of translation initiation via phosphorylation of eIF2α appears to play multiple roles in glucose homeostasis, as revealed by mutating the regulatory phosphorylation site (Ser51) of eIF2α and by knockout mutations of the PERK eIF2α kinase. Ser51Ala knock-in mutant mice exhibit neonatal loss of insulin-secreting pancreatic β cells and deficiency in liver glycogen storage. These mice typically die within the first day after birth because of severe hypoglycemia associated with defects in gluconeogenesis. The defect in insulin-secreting pancreatic β cells is also seen in the Perk knockout mutant mice, which succumb to severe hyperglycemia after the third postnatal week (11, 43). Zhang and coworkers have also shown that the glucagon-secreting pancreatic α cells are lost in the Perk−/− mutants after the loss of the insulin-secreting β cells (43). Scheuner and coworkers (32) speculated that the severe hypoglycemia observed in their Ser51Ala knock-in mice was due to the lack of regulation of eIF2α phosphorylation in the liver, as mediated by GCN2. However, we show herein that Gcn2−/− mice exhibit normal glycogen levels in the liver. Inasmuch as none of the single-knockout mice for the four eIF2α kinases (GCN2, PERK, HRI, and PKR) display the severe neonatal hypoglycemia seen in the Ser51Ala knock-in mutant, we suggest that two or more of the eIF2α kinases participate jointly in liver glycogen metabolism. We are currently generating double- and triple-mutant combinations of Gcn2−/−, Perk−/−, and Pkr−/− mice to test this hypothesis.
Acknowledgments
We thank Mark Magnuson and Cathy Pettepher for help in establishing the mouse Gcn2 knockout strain and Christopher Wright for providing the TL1 129 SvEvTac lambda genomic library. We thank Scott Myers, Keri Merritt, Zhao Lin, Rui Peng, and Adam Harris for technical assistance in isolation and characterization of the Gcn2 gene and Gcn2 knockout mice.
This work was supported by the Culpeper Foundation; the Ingram Cancer Center and Clinical Nutrition Research Unit of the Vanderbilt University School of Medicine; the Pennsylvania State University; and National Institutes of Health grants GM56957 to D.R.C., DK13499 to L.S.J., and GM49164 and GM643540 to R.C.W.
REFERENCES
- 1.Abel, T., and E. Kandel. 1998. Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res. Rev. 26:360-378. [DOI] [PubMed] [Google Scholar]
- 2.Berlanga, J. J., J. Santoyo, and C. De Haro. 1999. Characterization of a mammalian homolog of the GCN2 eukaryotic initiation factor 2α kinase. Eur. J. Biochem. 265:754-762. [DOI] [PubMed] [Google Scholar]
- 3.Chan, T. M., K. M. Young, N. J. Hutson, F. T. Brumley, and J. H. Exton. 1975. Hepatic metabolism of genetically diabetic (db/db) mice. I. Carbohydrate metabolism. Am. J. Physiol. 229:1702-1712. [DOI] [PubMed] [Google Scholar]
- 4.Colombo, J. P., H. Cervantes, M. Kokorovic, U. Pfister, and R. Perritaz. 1992. Effect of different protein diets on the distribution of amino acids in plasma, liver and brain in the rat. Ann. Nutr. Metab. 36:23-33. [DOI] [PubMed] [Google Scholar]
- 5.Dever, T. E., L. Feng, R. C. Wek, A. M. Cigan, T. F. Donahue, and A. G. Hinnebusch. 1992. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell 68:585-596. [DOI] [PubMed] [Google Scholar]
- 6.Dong, J., H. Qiu, M. Garcia-Barrio, J. Anderson, and A. G. Hinnebusch. 2000. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol. Cell 6:269-279. [DOI] [PubMed] [Google Scholar]
- 7.Flaim, K. E., W. S. Liao, D. E. Peavy, J. M. Taylor, and L. S. Jefferson. 1982. The role of amino acids in the regulation of protein synthesis in perfused rat liver. II. Effects of amino acid deficiency on peptide chain initiation, polysomal aggregation, and distribution of albumin mRNA. J. Biol. Chem. 257:2939-2946. [PubMed] [Google Scholar]
- 8.Garcia-Barrio, M., J. Dong, S. Ufano, and A. G. Hinnebusch. 2000. Association of GCN1-GCN20 regulatory complex with the N-terminus of eIF2α kinase GCN2 is required for GCN2 activation. EMBO J. 19:1887-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gustafson, J. M., S. J. Dodds, R. C. Burgus, and L. P. Mercer. 1986. Prediction of brain and serum free amino acid profiles in rats fed graded levels of protein. J. Nutr. 116:1667-1681. [DOI] [PubMed] [Google Scholar]
- 10.Harding, H. P., I. Novoa, I., Y. Zhang, H. Zeng, R. Wek, M. Schapira, and D. Ron. 2000. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6:1099-1108. [DOI] [PubMed] [Google Scholar]
- 11.Harding, H. P., H. Zeng, Y. Zhang, R. Jungries, P. Chung, H. Plesken, D. D. Sabatini, and D. Ron. 2001. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell 7:1153-1163. [DOI] [PubMed] [Google Scholar]
- 12.Hinnebusch, A. G. 1990. Transcriptional and translational regulation of gene expression in the general control of amino-acid biosynthesis in Saccharomyces cerevisiae. Prog. Nucleic Acid Res. Mol. Biol. 38:195-240. [DOI] [PubMed] [Google Scholar]
- 13.Jefferson, L. S., and S. R. Kimball. 2001. Amino acid regulation of gene expression. J. Nutr. 131:2460S-2466S, 2486S-2487S. [DOI] [PubMed] [Google Scholar]
- 14.Kilberg, M. S., R. G. Hutson, and R. O. Laine. 1994. Amino acid-regulated gene expression in eukaryotic cells. FASEB J. 8:13-19. [DOI] [PubMed] [Google Scholar]
- 15.Kimball, S. R. 2001. Regulation of translation initiation by amino acids in eukaryotic cells. Prog. Mol. Subcell. Biol. 26:155-184. [DOI] [PubMed] [Google Scholar]
- 16.Kimball, S. R., D. A. Antonetti, R. M. Brawley, and L. S. Jefferson. 1991. Mechanism of inhibition of peptide chain initiation by amino acid deprivation in perfused rat liver. Regulation involving inhibition of eukaryotic initiation factor 2 alpha phosphatase activity. J. Biol. Chem. 266:1969-1976. [PubMed] [Google Scholar]
- 17.Kimball, S. R., W. V. Everson, K. E. Flaim, and L. S. Jefferson. 1989. Initiation of protein synthesis in a cell-free system prepared from rat hepatocytes. Am. J. Physiol. 256:C28-C34. [DOI] [PubMed] [Google Scholar]
- 18.Kimball, S. R., W. V. Everson, L. M. Myers, and L. S. Jefferson. 1987. Purification and characterization of eukaryotic initiation factor 2 and a guanine nucleotide exchange factor from rat liver. J. Biol. Chem. 262:2220-2227. [PubMed] [Google Scholar]
- 19.Kimball, S. R., R. L. Horetsky, and L. S. Jefferson. 1998. Implication of eIF2B rather than eIF4E in the regulation of global protein synthesis by amino acids in L6 myoblasts. J. Biol. Chem. 273:30945-30953. [DOI] [PubMed] [Google Scholar]
- 20.Kimball, S. R., A. M. Karinch, R. C. Feldhoff, H. Mellor, and L. S. Jefferson. 1994. Purification and characterization of eukaryotic translational initiation factor eIF-2B from liver. Biochim. Biophys. Acta 1201:473-481. [DOI] [PubMed] [Google Scholar]
- 21.Kimball, S. R., H. Mellor, K. M. Flowers, and L. S. Jefferson. 1996. Role of translation initiation factor eIF-2B in the regulation of protein synthesis in mammalian cells. Prog. Nucleic Acid Res. Mol. Biol. 54:165-196. [DOI] [PubMed] [Google Scholar]
- 22.Krishnamoorthy, T., G. D. Pavitt, F. Zhang, T. E. Dever, and A. G. Hinnebusch. 2001. Tight binding of the phosphorylated α subunit of initiation factor 2 (eIF2α) to the regulatory subunits of guanine nucleotide exchange factor eIF2B is required for inhibition of translation initiation. Mol. Cell. Biol. 21:5018-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubota, H., Y. Sakaki, and T. Ito. 2000. GI domain-mediated association of the eukaryotic initiation factor 2α kinase GCN2 with its activator GCN1 is required for general amino acid control in budding yeast. J. Biol. Chem. 275:20243-20246. [DOI] [PubMed] [Google Scholar]
- 24.Miller, P. F., and A. G. Hinnebusch. 1989. Sequences that surround the stop codons of upstream open reading frames in GCN4 mRNA determine their distinct functions in translational control. Genes Dev. 3:1217-1225. [DOI] [PubMed] [Google Scholar]
- 25.Mueller, P. P., and A. G. Hinnebusch. 1986. Multiple upstream AUG codons mediate translational control of GCN4. Cell 45:201-207. [DOI] [PubMed] [Google Scholar]
- 26.Olsen, D. S., B. Jordan, D. Chen, R. C. Wek, and D. R. Cavener. 1998. Isolation of the gene encoding the Drosophila melanogaster homolog of the Saccharomyces cerevisiae GCN2 eIF-2α kinase. Genetics 149:1495-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters, J. C., and A. E. Harper. 1985. Adaptation of rats to diets containing different levels of protein: effects on food intake, plasma and brain amino acid concentrations and brain neurotransmitter metabolism. J. Nutr. 115:382-398. [DOI] [PubMed] [Google Scholar]
- 28.Qiu, H., J. Dong, C. Hu, C. S. Francklyn, and A. G. Hinnebusch. 2001. The tRNA-binding moiety in GCN2 contains a dimerization domain that interacts with the kinase domain and is required for tRNA binding and kinase activation. EMBO J. 20:1425-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogozin, I. B., A. V. Kochetov, F. A. Kondrashov, E. V. Koonin, and L. Milanesi. 2001. Presence of ATG triplets in 5′ untranslated regions of eukaryotic cDNAs correlates with a "weak' context of the start codon. Bioinformatics 17:890-900. [DOI] [PubMed] [Google Scholar]
- 30.Santoyo, J., J. Alcalde, R. Mendez, D. Pulido, and C. de Haro. 1997. Cloning and characterization of a cDNA encoding a protein synthesis initiation factor-2alpha (eIF-2α) kinase from Drosophila melanogaster. Homology to yeast GCN2 protein kinase. J. Biol. Chem. 272:12544-12550. [DOI] [PubMed] [Google Scholar]
- 31.Sattlegger, E., A. G. Hinnebusch, and I. B. Barthelmess. 1998. cpc-3, the Neurospora crassa homologue of yeast GCN2, encodes a polypeptide with juxtaposed eIF2α kinase and histidyl-tRNA synthetase-related domains required for general amino acid control. J. Biol. Chem. 273:20404-20416. [DOI] [PubMed] [Google Scholar]
- 32.Scheuner, D., B. Song, E. McEwen, C. Liu, R. Laybutt, P. Gillespie, T. Saunders, S. Bonner-Weir, and R. J. Kaufman. 2001. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7:1165-1176. [DOI] [PubMed] [Google Scholar]
- 33.Sood, R., A. C. Porter, K. Ma, L. A. Quilliam, and R. C. Wek. 2000. Pancreatic eukaryotic initiation factor-2α kinase (PEK) homologues in humans, Drosophila melanogaster and Caenorhabditis elegans that mediate translational control in response to endoplasmic reticulum stress. Biochem. J. 346:281-293. [PMC free article] [PubMed] [Google Scholar]
- 34.Sood, R., A. C. Porter, D. A. Olsen, D. R. Cavener, and R. C. Wek. 2000. A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2α. Genetics 154:787-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tolman, E. L., C. M. Schworer, and L. S. Jefferson. 1973. Effects of hypophysectomy on amino acid metabolism and gluconeogenesis in the perfused rat liver. J. Biol. Chem. 248:4552-4560. [PubMed] [Google Scholar]
- 36.Umbarger, H. E. 1969. Regulation of amino acid metabolism. Annu. Rev. Biochem. 38:323-370. [DOI] [PubMed] [Google Scholar]
- 37.Vary, T. C., L. S. Jefferson, and S. R. Kimball. 1999. Amino acid-induced stimulation of translation initiation in rat skeletal muscle. Am. J. Physiol. 277:E1077-E1086. [DOI] [PubMed] [Google Scholar]
- 38.Vazquez de Aldana, C. R., R. C. Wek, P. S. Segundo, A. G. Truesdell, and A. G. Hinnebusch. 1994. Multicopy tRNA genes functionally suppress mutations in yeast eIF-2α kinase GCN2: evidence for separate pathways coupling GCN4 expression to unchanged tRNA. Mol. Cell. Biol. 14:7920-7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wek, R. C. 1994. eIF-2 kinases: regulators of general and gene-specific translation initiation. Trends Biochem. Sci. 19:491-496. [DOI] [PubMed] [Google Scholar]
- 40.Wek, R. C., B. M. Jackson, and A. G. Hinnebusch. 1989. Juxtaposition of domains homologous to protein kinases and histidyl-tRNA synthetases in GCN2 protein suggests a mechanism for coupling GCN4 expression to amino acid availability. Proc. Natl. Acad. Sci. USA 86:4579-4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wek, S. A., S. Zhu, and R. C. Wek. 1995. The histidyl-tRNA synthetase-related sequence in the eIF-2α protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol. Cell. Biol. 15:4497-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanofsky, C. 1981. Attenuation in the control of expression of bacterial operons. Nature 289:751-758. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, P., B. McGrath, S. Li, A. Frank, F. Zambito, J. Reinert, M. Gannon, K. Ma, K. McNaughton, and D. R. Cavener. 2002. The PERK eukaryotic initiation factor 2α kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol. Cell. Biol. 22:3864-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu, S., A. Y. Sobolev, and R. C. Wek. 1996. Histidyl-tRNA synthetase-related sequences in GCN2 protein kinase regulate in vitro phosphorylation of eIF-2. J. Biol. Chem. 271:24989-24994. [DOI] [PubMed] [Google Scholar]