Abstract
To examine the physiological functions of mannose-binding lectin A (MBL-A), we generated mice that were deficient in MBL-A and examined their susceptibilities to the microbial pathogens Candida albicans and Plasmodium yoelii, an accepted experimental malaria model in mouse. We found no differences in the survival rates and fungal burdens of wild-type and MBL-A−/− mice with disseminated C. albicans infection. The two mouse strains were also similar in their abilities to resist hepatic accumulation of P. yoelii parasites. We conclude that MBL-A deficiency does not alter resistance to disseminated candidiasis or initial hepatic invasion by P. yoelii.
Mannose-binding lectin (MBL), a serum protein belonging to the collectin family, is considered to be a pattern recognition molecule designed to detect pathogen-associated molecular patterns (5). MBL exists in serum as multimeric trimers of 32-kDa polypeptide chains; each chain has a carbohydrate recognition domain (CRD) and a collagen-like region for trimeric assembly. The CRD has been shown to bind mannose, fucose, and N-acetylglucosamine (4), and the cluster of CRDs rendered by oligomerization is thought to be suitable for binding polymeric sugars found on microbes (19, 42). Indeed, MBL has been shown to bind a host of pathogens, including subsets of gram-positive and gram-negative bacteria, Mycobacterium tuberculosis, and Candida albicans (5, 31). Once bound to microbes, MBL is believed to act as an opsonin for phagocytosis (17) and to activate the complement cascade via mannose-binding lectin-associated protein 2 (MASP-2) for microbial lysis (28, 34, 39).
MBL is encoded by a single gene in humans, but in rodents it is encoded by two genes, the MBL-A and the MBL-C genes. It was initially believed that MBL-A and MBL-C were found in serum and the liver, respectively, but recently it was shown that both proteins are found in serum in comparable amounts and that both have similar capacities to activate complement (12). Although it has been shown that the two proteins are distinct in binding-site architecture and multimeric complexity, with some fine differences in specificities for monosaccharides (3, 20, 21, 32), it is currently unknown whether they have nonoverlapping functions or recognize different sets of microbes.
MBL deficiency is a common genetic defect in humans. Three single nucleotide substitutions that impair oligomerization of MBL polypeptide chains have been described previously (40). The frequencies of these three point mutations differ among human populations (23), and there are also polymorphisms within the promoter region that affect the level of MBL in serum (25, 26).
MBL deficiency may be linked to susceptibility to recurrent infection (37). Children suffering from frequent infections were observed to have sera that poorly opsonized Saccharomyces cerevisiae. A significant correlation was found between low levels of MBL and this opsonization defect, which was corrected by the addition of purified MBL. Other studies have shown that a significantly higher proportion of MBL-deficient homozygosity was found in patients with increased susceptibilities to infections (7, 36).
C. albicans is a common cause of morbidity and mortality in immunocompromised individuals. C. albicans is part of the normal commensal flora in the human gastrointestinal and female lower genital tracts, but in patients with immunodeficiency it can establish invasive fungal infection. Th1 cells are believed to confer resistance against candidiasis, and the induction of Th1 CD4+ cells requires the activation of phagocytes (30). Phagocytosis of C. albicans cells is thought to be an important first-line mechanism for host defense and is enhanced by serum opsonization (22).
MBL has the capacity to function as an opsonin and has been shown to strongly bind C. albicans, which displays mannan, a candidate ligand for MBL, on its surface (14). Following intravenous injection of mice with C. albicans, levels of MBL-A in serum decreased and levels of MBL-A in mRNA increased, suggesting that MBL-A is involved in the initial phases of host defense (38).
Plasmodium falciparum is another pathogen against which MBL may have a protective contribution, but a controversy surrounds this proposal. A case-control study of Gabonese children has shown that the severity of malarial infection was associated with lower levels of MBL in serum and higher frequencies of MBL mutations than those of the group with mild disease (24). However, a retrospective study conducted in Gambia found that MBL deficiency was not a risk factor for severe malaria (1).
Infection of mice by Plasmodium yoelii sporozoites is a highly sensitive, widely accepted experimental malaria model. Although little is known about the specific interaction of the sporozoite with its target cells, namely, hepatocytes, two sporozoite proteins, circumsporozoite (CS) and thrombospondin-related adhesive protein, have been shown to play important roles in hepatic invasion (29, 35). Both proteins contain potential C mannosylation (13) and N and O glycosylation sites (11, 27). Furthermore, CS protein contains a putative C-terminal glycosylphosphatidylinositol (GPI) anchoring signal; GPI anchor moieties represent the major carbohydrate modification in Plasmodium species (10). Thus, the sporozoite proteins, thrombospondin-related adhesive protein and CS, may interact with MBL protein in the serum, modifying sporozoite infectivity.
To examine the role of MBL-A in host defense against the microbial pathogens C. albicans and P. yoelii, we generated mice that were deficient in the expression of MBL-A.
MATERIALS AND METHODS
Gene targeting of MBL-A.
A targeting vector for MBL-A was constructed by using a phage clone isolated from a 129SVJ-Lambda Fix II mouse genomic library (Stratagene, La Jolla, Calif.). The library was screened with a DNA probe containing MBL-A exons 1 through 4. The targeting vector contained a 1.5-kb fragment downstream of exon 1 as the short arm and an 8-kb fragment comprising part of exon 4 and exon 5 as the long arm.
For positive selection of G418, a neo gene flanked by loxP sites from the pGEM7-Neo5′3′loxP vector (a generous gift from P. Mombaerts, Rockefeller University) was inserted downstream of the short-arm fragment. A negative selection gene, the diphtheria toxin A gene from pKO SelectDT V840 (Lexicon Genetics, The Woodlands, Tex.), was subcloned upstream of the short-arm fragment.
For Southern analysis of the targeted disruption of MBL-A, a HindIII restriction site was introduced downstream of the neo gene to create a distinctive restriction map after homologous recombination.
The targeting vector was linearized and transfected into embryonic stem (ES) cells (9) by electroporation. Viable G418-resistant clones were subsequently screened by Southern blotting for homologous recombination. HindIII-digested genomic DNA was probed with a 420-bp fragment containing exon 1.
Positive clones were injected into C57BL/6 mouse blastocysts to generate chimeric mice. Heterozygous offspring of the chimera were bred to the C57BL/6 Cre transgenic mice (18) to flox the Neo gene. The mice carrying the floxed allele were then mated with C57BL/6 mice (Jackson Laboratory, Bar Harbor, Maine) to generate Cre-negative and floxed-allele-positive heterozygotes. These mice were subsequently interbred to generate homozygotes.
Northern analysis.
Mice were injected with 1 ml of 4% brewer's yeast-modified thioglycolate medium (Becton Dickinson). Twenty-four hours later, the total RNA was extracted from the livers with TRIzol LS reagent (Gibco, Grand Island, N.Y.) according to the manufacturer's instructions. Northern blot analysis of total RNA samples (30 μg/lane) was performed as previously described (33).
Mice.
Mannose-binding-lectin-A-knockout (MBL-A−/−) mice were generated on the 129SVJ × C59BL/6 background and backcrossed to the C57BL/6 strain for seven generations. The wild-type controls for all experiments were the offspring of backcrossed MBL-A−/− littermates.
C. albicans infection and survival curves.
Wild-type and MBL-A−/− mice were matched by gender and age, and more than 30 mice per genotype were used for each experiment. C. albicans (ATCC 18804) was cultured in 2% Sabouraud's dextrose broth (Difco Laboratories, Becton Dickinson, Sparks, Md.) in a shaker at 30°C for 24 h. The fungi were washed in phosphate-buffered saline (Gibco BRL) two times before injection. Six- to 9-week-old mice were challenged intraperitoneally with 8 × 107 or 1 × 108 C. albicans blastoconidia and observed for 28 days.
Determination of blood and tissue fungal burden.
The fungal burdens in the blood and organs of infected mice were determined by quantitating the CFU as described previously (15). On day 3 after infection, wild-type and MBL-A−/− mice were sacrificed by CO2 asphyxiation. Blood samples were aseptically obtained, and 100 μl of each sample was plated onto Sabouraud's dextrose agar (Difco Laboratories) in duplicate. Livers and kidneys were excised and weighed before homogenization in 0.1% Triton X-100 (LabChem, Pittsburgh, Pa.). Serial dilutions of organ homogenates were plated in duplicate. After 24 to 36 h of incubation at 37°C, yeast colonies were counted and numbers of CFU were determined for each sample. Numbers of CFU from seven to eight mice per experimental group were used to calculate the mean log10 CFU per ml of blood, and four mice per group were used to determine the mean log10 CFU per gram of organ.
Parasites.
P. yoelii (strain 17XNL) was maintained by repeated cyclic passage of the parasites in Anopheles stephensi mosquitoes and BALB/c mice. Sporozoites were collected by dissecting the mosquito salivary glands in medium 199 at 2 weeks after the third infective blood meal. Mice were injected intravenously with 3 × 104 sporozoites in a volume of 200 μl.
Quantification of P. yoelii rRNA in the livers of sporozoite-inoculated mice by real-time PCR.
The quantification of P. yoelii rRNA was performed as described previously (2). Briefly, mice were sacrificed 42 h after intravenous injection of 105 P. yoelii sporozoites, and total RNA was isolated from the livers of infected mice by the method of Chomczynski and Sacchi (3a). The amount of RNA in the samples was determined by spectrophotometric readings at 260 nm. After reverse transcription of the extracted RNA, the cDNA obtained was analyzed by real-time PCR with the ABI Prism 5700 sequence detection system (Perkin-Elmer). Primers and a fluorogenic probe with the following sequences were custom designed by using the ABI Prism Primer Express software (PE Biosystems, Foster City, Calif.) and the P. yoelii (17XNL) 18S rRNA sequence. The primers, a forward primer (5′-GGGGATTGGTTTTGACGTTTTTGCG-3′) and a reverse primer (5′-AAGCATTAAATAAAGCGAATACATCCTTAT-3′), were purchased from Operon Technologies Inc., Alameda, Calif. The specific fluorogenic probe, PyNYU (5′-FAM-CAATTGGTTTACCTTTTGCTCTTT-TAMRA-3′; PE Biosystems), was generated with 5-propyne-2′-deoxyuridine (a turbo TaqMan probe) to achieve a proper thermal denaturation. The reaction mixture contained 5 μl of 10× TaqMan buffer A (PE Biosystems), 3.5 mM MgCl2, 200 μM deoxynucleoside triphosphate, 0.3 μM forward primer, 0.3 μM reverse primer, 50 nM turbo TaqMan probe PyNYU, 1.25 U of AmpliTaq Gold DNA polymerase, and enough water to produce a final reaction volume of 50 μl. The temperature profile included one cycle at 95°C for 10 min, 35 cycles of denaturation at 95°C for 15 s, and annealing and extension at 60°C for 1 min. The amount of parasite-derived 18S cDNA molecules detected in this assay was determined by linear regression analysis with a standard curve generated with known amounts of plasmid 18S cDNA and threshold cycle (CT) values obtained from liver samples.
Statistics.
StataQuest 4.0 (Stata Corporation, College Station, Tex.) was used to determine the statistical significance (P < 0.05) of differences between the wild-type and knockout groups. A two-sample test of proportions for the survival rates and a two-sample t test for the quantification of organ CFU and P. yoelii rRNA were used.
RESULTS
Generation of MBL-A−/− mice.
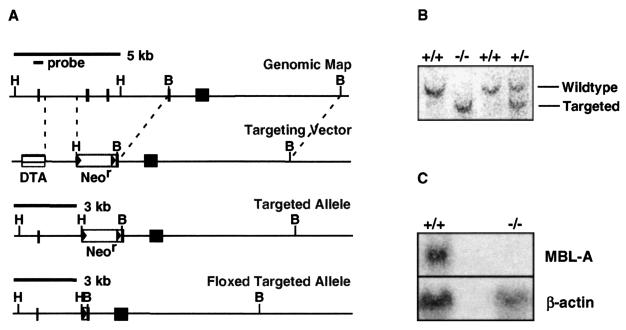
The gene targeting vector for the MBL-A locus (Fig. 1A) was designed to delete exons 2 and 3 and part of exon 4. Exon 2 encodes the start codon and the signal peptide, without which MBL-A cannot be expressed. The neomycin resistance (Neor) gene, a positive selection marker, was flanked by loxP sites to effect deletion of the Neor gene by Cre recombinase after gene targeting.
FIG. 1.
Generation of MBL-A−/− mice. (A) Maps of the genomic MBL-A locus and MBL-A targeting vector. DTA, diphtheria toxin A; H, HindIII; B, BamHI. (B) ES cell screening for homologous recombination. A 420-bp probe containing exon 1 was used for hybridization to HindIII-digested genomic DNA. The wild-type (5-kb) and targeted (3-kb) fragments are indicated. (C) Detection of MBL-A mRNA by Northern blotting. Total RNA (30 μg/lane) from the livers of thioglycolate-stimulated mice was probed with a DNA fragment containing exon 5. A β-actin probe was used as a control.
The targeting vector was introduced into ES cells (9), and homologous recombinants were used to produce chimeric mice. Chimeric mice were mated with C57BL/6 Cre transgenic mice (18), and heterozygotes with the Neor gene deletion were subsequently interbred to generate MBL-A−/− homozygotes. Genotyping was done by Southern blotting on HindIII-digested tail genomic DNA (Fig. 1B). To examine MBL-A expression, mice were injected with 4% thioglycolate broth to induce MBL-A and total RNA was obtained from the livers. A DNA fragment containing MBL-A exon 5 was used as a probe for Northern blots. A β-actin probe was used for loading control. Northern blots showed that MBL-A−/− mice did not produce MBL-A mRNA (Fig. 1C).
Heterozygous breeding showed a Mendelian transmission of the targeted allele. MBL-A−/− mice were viable and exhibited normal development and fertility. Histological examination of major organs, including brain, heart, lungs, liver, kidneys, and gut, showed no abnormalities (data not shown). In pathogen-free conditions, MBL-A−/− mice had a normal life span and did not show an increased propensity for infections.
Role of MBL-A in C. albicans infection.
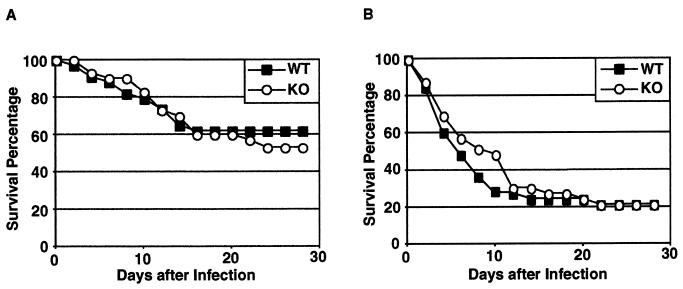
To assess the role of MBL-A in host defense against C. albicans, mice were challenged intraperitoneally with 8 × 107 C. albicans blastoconidia and were observed for 28 days (Fig. 2A). In the 28-day period after infection, 62% of wild-type and 53% of MBL-A−/− mice survived. The difference in survival rates was not statistically significant. Wild-type and MBL-A−/− mice were also equally sensitive to infection with 108 blastoconidia (Fig. 2B). We conclude that wild-type and MBL-A−/− mice have comparable levels of resistance to systemic candidiasis.
FIG. 2.
Survival of C. albicans-infected wild-type and MBL-A−/− mice. Mice were infected intraperitoneally with 8 × 107 (A) or 1 × 108 (B) blastoconidia and observed for 28 days. Thirty or more mice were used per genotype in each experiment. WT, wild-type mice; KO, MBL-A−/− mice.
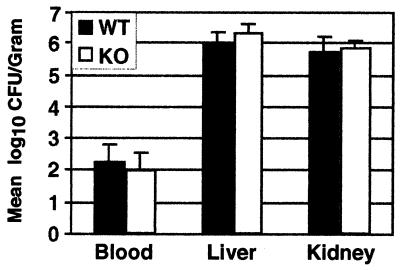
To determine whether the MBL-A deficiency was associated with higher fungal burdens during the early phase of infection, the blood, livers, and kidneys from C. albicans-infected mice were examined on day 3 after infection. The livers and kidneys were excised and homogenized, and serial dilutions of organ homogenates, along with blood samples, were plated to measure CFU. On day 3, no significant differences in the mean numbers of CFU in blood samples or organ homogenates were detected between the wild-type and MBL-A−/− groups (Fig. 3).
FIG. 3.
Blood and organ fungal burdens in C. albicans-infected mice. Fungal burdens in blood, liver, and kidneys were assessed at 3 days postinfection. Seven to eight mice and four mice per genotype were used for assessing blood fungal levels and organ fungal burdens, respectively. The results are expressed as the mean log10 CFU per milliliter of blood or per gram of organ. Error bars indicate standard deviation.WT, wild-type mice; KO, MBL-A−/− mice.
Role of MBL-A in hepatic entry and replication by P. yoelii sporozoites.
In order to test the susceptibility of MBL-deficient mice to infection by P. yoelii sporozoites, six mice per group were infected with 3 × 104 of these sporozoites. Forty-two hours later, by which time sporozoites fully develop in infected livers, the livers were excised and the levels of hepatic parasite RNA in wild-type and MBL-deficient mice were determined by quantitative real-time PCR.
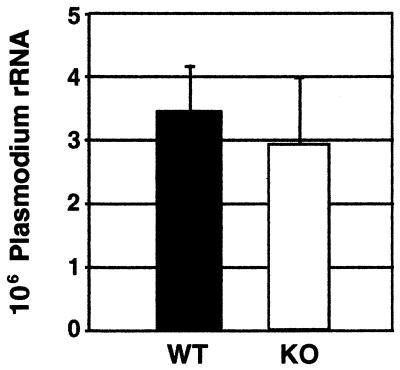
The mean level of parasite RNA in the livers of MBL-deficient mice was equivalent to that in the livers of control mice (Fig. 4), indicating that mice lacking MBL-A protein are comparable to wild-type mice in their susceptibility to P. yoelii sporozite liver infection and subsequent development.
FIG. 4.
Parasite RNA loads in the livers of wild-type and MBL-A−/− mice infected with P. yoelii sporozoites. Mice were inoculated with highly infective P. yoelii sporozoites, and the levels of parasite rRNA present in their livers were determined 42 h after infection, as described in Materials and Methods. The results are expressed as the mean values ± standard deviations for six mice.
DISCUSSION
Like antibodies, MBL opsonizes microbes and activates complement. MBL-mediated complement activation has been shown to be a component of innate mucosal defense against sporozoites of the Cryptosporidium parvum species (16). Plasmodium sporozoites are known to synthesize GPI anchors used for glycosylation, and the sporozoite proteins thought to be required for liver infection contain potential mannosylation sites in their sequences (13). However, very little or no information is available on the nature and extent of glycosylation in the sporozoite stages of Plasmodium species. Given the possibility that these potential glycoproteins may interact with MBL, we infected MBL-A-deficient mice with P. yoelii. Our results showed that P. yoelii sporozoite infection and subsequent parasite development in the liver are not affected by MBL-A deficiency. But, we cannot rule out the possibility of a role for this protein at the erythrocytic stages of Plasmodium infection, as has been proposed for humans.
Functional redundancy may compensate for MBL deficiency during an innate immune response. It has been proposed that MBL activity may be critical only in early life, when a limited repertoire of antibodies exists, or in early phases of infection before the onset of immunoglobulin M production (40). The remarkably high frequency of mutated MBL alleles (23, 40) in humans also suggests that indeed this protein may not be essential for host defense. The most common mutations occur in codons 52, 54, and 57 in exon 1 of the MBL gene. The codon 54 mutation is estimated to occur in approximately 26% of Caucasians; the codon 57 mutation was present in 58% of the Gambian adults tested; and the codon 52 mutation occurs in approximately 5% of both Caucasian and African populations (40). These mutations are thought to disrupt the structure of the MBL protein and, consequently, its interaction with MASPs (41). Interference with MASP binding would functionally impair MBL, as MBL induces a complement cascade via MASP activation (28, 39).
Aside from structural mutations, polymorphisms have also been found in the promoter region of the human MBL gene, producing various levels of MBL protein in serum. These polymorphisms exist in linkage disequilibrium with the structural mutations described and appear to further modulate the levels of MBL in serum. These genetic studies suggest that a delicate balance is required to regulate the function of MBL and that limiting its activity level may be important. In fact, hypotheses have been made that MBL deficiency may be advantageous for host survival. Lack of MBL function may reduce the severity of inflammatory responses and thus limit damage to the host. It may also increase resistance against intracellular parasites that use C3 opsonization and C3 receptors to gain entry into the host cell (8). Supporting evidence for the latter theory came from a study of Ethiopian patients infected with Mycobacterium leprae; infected patients had significantly higher levels of MBL in their sera than the control group did (6).
Our study demonstrates that MBL-A deficiency in mouse does not alter resistance to disseminated candidiasis or hepatic malarial infection. This does not exclude the possibility that MBL may modulate host defense, since the mouse genome encodes two forms of MBL: MBL-A and MBL-C. The mouse MBL-C gene is found within the collectin gene cluster on chromosome 14, a region that is syntenic for the human collectin gene cluster containing the gene for MBL. This gene clustering information has led some to believe that MBL-C may be functionally more similar to the human counterpart. Whether MBL-C deficiency or a double deficiency of MBL-A and MBL-C results in altered immune response to these organisms remains to be determined.
Acknowledgments
This work was supported by NIH Medical Scientist Training Program grant GM07739 (S.J.L.), by the NIH, and by the Howard Hughes Institute (M.C.N.).
We are indebted to C. Nathan for many helpful discussions and gifts of thioglycolate broth and to T. Eisenreich for technical assistance with infection.
REFERENCES
- 1.Bellamy, R., C. Ruwende, K. P. McAdam, M. Thursz, M. Sumiya, J. Summerfield, S. C. Gilbert, T. Corrah, D. Kwiatkowski, H. C. Whittle, and A. V. Hill. 1998. Mannose binding protein deficiency is not associated with malaria, hepatitis B carriage nor tuberculosis in Africans. QJM 91:13-18. [DOI] [PubMed] [Google Scholar]
- 2.Bruna-Romero, O., J. C. Hafalla, G. Gonzalez-Aseguinolaza, G. Sano, M. Tsuji, and F. Zavala. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 31:1499-1502. [DOI] [PubMed] [Google Scholar]
- 3.Childs, R. A., T. Feizi, C. T. Yuen, K. Drickamer, and M. S. Quesenberry. 1990. Differential recognition of core and terminal portions of oligosaccharide ligands by carbohydrate-recognition domains of two mannose-binding proteins. J. Biol. Chem. 265:20770-20777. [PubMed] [Google Scholar]
- 3a.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thoicyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 4.Drickamer, K. 1992. Engineering galactose-binding activity into a C-type mannose-binding protein. Nature 360:183-186. [DOI] [PubMed] [Google Scholar]
- 5.Epstein, J., Q. Eichbaum, S. Sheriff, and R. A. Ezekowitz. 1996. The collectins in innate immunity. Curr. Opin. Immunol. 8:29-35. [DOI] [PubMed] [Google Scholar]
- 6.Garred, P., M. Harboe, T. Oettinger, C. Koch, and A. Svejgaard. 1994. Dual role of mannan-binding protein in infections: another case of heterosis? Eur. J. Immunogenet. 21:125-131. [DOI] [PubMed] [Google Scholar]
- 7.Garred, P., H. O. Madsen, B. Hofmann, and A. Svejgaard. 1995. Increased frequency of homozygosity of abnormal mannan-binding-protein alleles in patients with suspected immunodeficiency. Lancet 346:941-943. [DOI] [PubMed] [Google Scholar]
- 8.Garred, P., H. O. Madsen, J. A. Kurtzhals, L. U. Lamm, S. Thiel, A. S. Hey, and A. Svejgaard. 1992. Diallelic polymorphism may explain variations of the blood concentration of mannan-binding protein in Eskimos, but not in black Africans. Eur. J. Immunogenet. 19:403-412. [DOI] [PubMed] [Google Scholar]
- 9.Gong, S., and M. C. Nussenzweig. 1996. Regulation of an early developmental checkpoint in the B cell pathway by Ig beta. Science 272:411-414. [DOI] [PubMed] [Google Scholar]
- 10.Gowda, D. C., and E. A. Davidson. 1999. Protein glycosylation in the malaria parasite. Parasitol. Today 15:147-152. [DOI] [PubMed] [Google Scholar]
- 11.Han, K. K., and A. Martinage. 1992. Possible relationship between coding recognition amino acid sequence motif or residue(s) and post-translational chemical modification of proteins. Int. J. Biochem. 24:1349-1363. [DOI] [PubMed] [Google Scholar]
- 12.Hansen, S., S. Thiel, A. Willis, U. Holmskov, and J. C. Jensenius. 2000. Purification and characterization of two mannan-binding lectins from mouse serum. J. Immunol. 164:2610-2618. [DOI] [PubMed] [Google Scholar]
- 13.Hofsteenge, J., K. G. Huwiler, B. Macek, D. Hess, J. Lawler, D. F. Mosher, and J. Peter-Katalinic. 2001. C-mannosylation and O-fucosylation of the thrombospondin type 1 module. J. Biol. Chem. 276:6485-6498. [DOI] [PubMed] [Google Scholar]
- 14.Jack, D. L., N. J. Klein, and M. W. Turner. 2001. Mannose-binding lectin: targeting the microbial world for complement attack and opsonophagocytosis. Immunol. Rev. 180:86-99. [DOI] [PubMed] [Google Scholar]
- 15.Káposzta, R., P. Tree, L. Maródi, and S. Gordon. 1998. Characteristics of invasive candidiasis in gamma interferon- and interleukin-4-deficient mice: role of macrophages in host defense against Candida albicans. Infect. Immun. 66:1708-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly, P., D. L. Jack, A. Naeem, B. Mandanda, R. C. Pollok, N. J. Klein, M. W. Turner, and M. J. Farthing. 2000. Mannose-binding lectin is a component of innate mucosal defense against Cryptosporidium parvum in AIDS. Gastroenterology 119:1236-1242. [DOI] [PubMed] [Google Scholar]
- 17.Kuhlman, M., K. Joiner, and R. A. Ezekowitz. 1989. The human mannose-binding protein functions as an opsonin. J. Exp. Med. 169:1733-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakso, M., J. G. Pichel, J. R. Gorman, B. Sauer, Y. Okamoto, E. Lee, F. W. Alt, and H. Westphal. 1996. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl. Acad. Sci. USA 93:5860-5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, R. T., Y. Ichikawa, T. Kawasaki, K. Drickamer, and Y. C. Lee. 1992. Multivalent ligand binding by serum mannose-binding protein. Arch. Biochem. Biophys. 299:129-136. [DOI] [PubMed] [Google Scholar]
- 20.Lee, R. T., and Y. C. Lee. 1997. Difference in binding-site architecture of the serum-type and liver-type mannose-binding proteins. Glycoconj. J. 14:357-363. [DOI] [PubMed] [Google Scholar]
- 21.Lee, R. T., Y. Shinohara, Y. Hasegawa, and Y. C. Lee. 1999. Lectin-carbohydrate interactions: fine specificity difference between two mannose-binding proteins. Biosci. Rep. 19:283-292. [DOI] [PubMed] [Google Scholar]
- 22.Lehrer, R. I., and M. J. Cline. 1969. Interaction of Candida albicans with human leukocytes and serum. J. Bacteriol. 98:996-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipscombe, R. J., M. Sumiya, A. V. Hill, Y. L. Lau, R. J. Levinsky, J. A. Summerfield, and M. W. Turner. 1992. High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene. Hum. Mol. Genet. 1:709-715. [DOI] [PubMed] [Google Scholar]
- 24.Luty, A. J., J. F. Kun, and P. G. Kremsner. 1998. Mannose-binding lectin plasma levels and gene polymorphisms in Plasmodium falciparum malaria. J. Infect. Dis. 178:1221-1224. [DOI] [PubMed] [Google Scholar]
- 25.Madsen, H. O., P. Garred, S. Thiel, J. A. Kurtzhals, L. U. Lamm, L. P. Ryder, and A. Svejgaard. 1995. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J. Immunol. 155:3013-3020. [PubMed] [Google Scholar]
- 26.Madsen, H. O., M. L. Satz, B. Hogh, A. Svejgaard, and P. Garred. 1998. Different molecular events result in low protein levels of mannan-binding lectin in populations from southeast Africa and South America. J. Immunol. 161:3169-3175. [PubMed] [Google Scholar]
- 27.Marshall, R. D. 1972. Glycoproteins. Annu. Rev. Biochem. 41:673-702. [DOI] [PubMed] [Google Scholar]
- 28.Matsushita, M., and T. Fujita. 1992. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J. Exp. Med. 176:1497-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menard, R., A. A. Sultan, C. Cortes, R. Altszuler, M. R. van Dijk, C. J. Janse, A. P. Waters, R. S. Nussenzweig, and V. Nussenzweig. 1997. Circumsporozoite protein is required for development of malaria sporozoites in mosquitoes. Nature 385:336-340. [DOI] [PubMed] [Google Scholar]
- 30.Mencacci, A., E. Cenci, F. Bistoni, A. Bacci, G. Del Sero, C. Montagnoli, C. Fe d'Ostiani, and L. Romani. 1998. Specific and non-specific immunity to Candida albicans: a lesson from genetically modified animals. Res. Immunol. 149:352-361. (Discussion, 149:517-519.) [DOI] [PubMed] [Google Scholar]
- 31.Neth, O., D. L. Jack, A. W. Dodds, H. Holzel, N. J. Klein, and M. W. Turner. 2000. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect. Immun. 68:688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng, K. K., K. Drickamer, and W. I. Weis. 1996. Structural analysis of monosaccharide recognition by rat liver mannose-binding protein. J. Biol. Chem. 271:663-674. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 34.Schweinle, J. E., R. A. Ezekowitz, A. J. Tenner, M. Kuhlman, and K. A. Joiner. 1989. Human mannose-binding protein activates the alternative complement pathway and enhances serum bactericidal activity on a mannose-rich isolate of Salmonella. J. Clin. Investig. 84:1821-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sultan, A. A., V. Thathy, U. Frevert, K. J. Robson, A. Crisanti, V. Nussenzweig, R. S. Nussenzweig, and R. Menard. 1997. TRAP is necessary for gliding motility and infectivity of plasmodium sporozoites. Cell 90:511-522. [DOI] [PubMed] [Google Scholar]
- 36.Summerfield, J. A., M. Sumiya, M. Levin, and M. W. Turner. 1997. Association of mutations in mannose binding protein gene with childhood infection in consecutive hospital series. BMJ 314:1229-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Super, M., S. Thiel, J. Lu, R. J. Levinsky, and M. W. Turner. 1989. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet ii:1236-1239. [DOI] [PubMed]
- 38.Tabona, P., A. Mellor, and J. A. Summerfield. 1995. Mannose binding protein is involved in first-line host defence: evidence from transgenic mice. Immunology 85:153-159. [PMC free article] [PubMed] [Google Scholar]
- 39.Thiel, S., T. Vorup-Jensen, C. M. Stover, W. Schwaeble, S. B. Laursen, K. Poulsen, A. C. Willis, P. Eggleton, S. Hansen, U. Holmskov, K. B. Reid, and J. C. Jensenius. 1997. A second serine protease associated with mannan-binding lectin that activates complement. Nature 386:506-510. [DOI] [PubMed] [Google Scholar]
- 40.Turner, M. W., and R. M. Hamvas. 2000. Mannose-binding lectin: structure, function, genetics and disease associations. Rev. Immunogenet. 2:305-322. [PubMed] [Google Scholar]
- 41.Wallis, R., and R. B. Dodd. 2000. Interaction of mannose-binding protein with associated serine proteases: effects of naturally occurring mutations. J. Biol. Chem. 275:30962-30969. [DOI] [PubMed] [Google Scholar]
- 42.Weis, W. I., and K. Drickamer. 1994. Trimeric structure of a C-type mannose-binding protein. Structure 2:1227-1240. [DOI] [PubMed] [Google Scholar]