Abstract
The linker region of Syk and ZAP70 tyrosine kinases plays an important role in regulating their function. There are three conserved tyrosines in this linker region; Tyr317 of Syk and its equivalent residue in ZAP70 were previously shown to negatively regulate the function of Syk and ZAP70. Here we studied the roles of the other two tyrosines, Tyr342 and Tyr346 of Syk, in FcɛRI-mediated signaling. Antigen stimulation resulted in Tyr342 phosphorylation in mast cells. Syk with Y342F mutation failed to reconstitute FcɛRI-initiated histamine release. In the Syk Y342F-expressing cells there was dramatically impaired receptor-induced phosphorylation of multiple signaling molecules, including LAT, SLP-76, phospholipase C-γ2, but not Vav. Compared to wild-type Syk, Y342F Syk had decreased binding to phosphorylated immunoreceptor tyrosine-based activation motifs and reduced kinase activity. Surprisingly, mutation of Tyr346 had much less effect on FcɛRI-dependent mast cell degranulation. An anti-Syk-phospho-346 tyrosine antibody indicated that antigen stimulation induced only a very minor increase in the phosphorylation of this tyrosine. Therefore, Tyr342, but not Tyr346, is critical for regulating Syk in mast cells and the function of these tyrosines in immune receptor signaling appears to be different from what has been previously reported for the equivalent residues of ZAP70.
Aggregation of the high-affinity immunoglobulin E (IgE) receptor (FcɛRI) on mast cells initiates a biochemical cascade that ultimately results in degranulation and release of inflammatory mediators (1, 19, 49, 50). Among these biochemical changes, protein tyrosine phosphorylation is one of the earliest detectable events. Since FcɛRI itself has no intrinsic tyrosine kinase activity, the sequential activation of the nonreceptor protein tyrosine kinases (PTKs) such as Syk is essential for this signal transduction pathway (2, 3, 12, 15, 22, 24, 41, 47, 58). Because of the importance of Syk in signaling, there is much interest in understanding its regulation.
Syk is a member of the Syk and ZAP70 PTK family and is expressed in most hematopoietic cells (30). The tandem Src homology 2 region (SH2) domains in the N-terminal half of Syk are involved in its association with subunits of FcɛRI after receptor aggregation (3, 5, 26, 47). This interaction is mediated by the SH2 domains of Syk binding to the tyrosine phosphorylated immunoreceptor tyrosine-based activation motif (ITAM) especially of the γ subunit of FcɛRI. Binding of Syk to a diphosphorylated ITAM results in a conformational change and an increase of its kinase activity (27). The linker region of Syk and ZAP70, located between the second SH2 and the kinase domain, has been reported to play an important role in regulating the enzymatic function of the molecule (64). SykB, which lacks a 23-amino-acid (aa) sequence in this linker region of Syk, is inefficient at coupling stimulation of FcɛRI or T-cell antigen receptor to the early and late events of cellular activation (32). There are three conserved tyrosines in the linker region of both Syk and ZAP70 (aa 317, 342, and 346 in rat Syk and the orthologous aa 292, 315, and 319 in human ZAP70). The putative Cbl interaction site, Tyr317 of Syk (Tyr292 in human ZAP70), negatively regulates Syk and ZAP70 signaling (9, 25, 34, 45). The other two tyrosines in the linker region have been reported to be involved in the interaction and tyrosine phosphorylation of phospholipase C-γ1 and Vav. Thus, in COS cells the expression of Syk with both Tyr342 and Tyr346 mutated to Phe results in the loss of the association of Syk with phospholipase C-γ1 and decrease in the tyrosine phosphorylation of phospholipase C-γ1 (PLC-γ1) (33). Other experiments using the two-hybrid system suggest that Tyr342 of Syk is important for the interaction of Syk with Vav (10).
The roles of the two orthologous tyrosines in the linker region of ZAP70 (Tyr315 or Tyr319) have been extensively investigated (11, 18, 35, 42, 54, 56). Both tyrosines are phosphorylated after T-cell receptor (TCR) cross-linking. Tyr319 of ZAP70 is essential for downstream propagation of signals such as tyrosine phosphorylation of PLC-γ1 and calcium influx (11, 54). However, there have been more-variable results in studies with the Y315F mutant of ZAP70. Experiments with Syk−/− chicken B cells suggest that Tyr315 is essential for the interaction of Vav with ZAP70 and critical for antigen receptor-mediated signal transduction (56). In contrast, Y315F ZAP70 has only minimal inhibitory effects on TCR signaling when expressed in Jurkat T cells (11). Studies of transgenic or knockin mice demonstrate that Tyr315 of ZAP70 plays an important role in the positive and negative selection of T cells (18, 35). Although Syk and ZAP70 have similar functions in antigen receptor signaling; there are still differences in their regulation and their capacity to mediate receptor-mediated signaling (6, 14, 17, 20, 31, 55, 65). Therefore, these Tyr residues in the linker region of Syk may have a function different from that of their orthologues in ZAP70.
The purpose of the present study was to characterize the roles of Tyr342 and Tyr346 of Syk in mast cell signaling. Therefore, Y342F and Y346F mutant Syk were stably expressed in a Syk-deficient variant of the RBL-2H3 mast cells. Compared with wild-type Syk, mutation of Tyr342 resulted in a dramatic reduction of IgE-stimulated mast cell degranulation. However, Y346F mutant Syk still reconstituted FcɛRI-initiated histamine release. Detailed analysis suggested that IgE-receptor aggregation induced a clear increase in the phosphorylation of Tyr342 but not Tyr346 of Syk. Furthermore, the Y342F mutant Syk had decreased binding to a phosphorylated ITAM peptide based on FcɛRIγ, which also resulted in decreased Syk activation.
MATERIALS AND METHODS
Materials and antibodies.
The horseradish peroxidase-conjugated antiphosphotyrosine antibody PY-20 was from ICN Immunobiologics (Lisle, Ill). The horseradish peroxidase-conjugated antiphosphotyrosine antibody 4G-10, anti-PLC-γ1, anti-SLP-76, Rac1 activation assay kit, and anti-LAT antibodies were from Upstate Biotechnology (Lake Placid, N.Y.). The anti-PLC-γ2, anti-Syk (N-19), anti-Vav, and anti-PKD/PKCμ antibodies were from Santa Cruz Biotechnology (Santa Cruz, Calif.); the anti-phospho-PKD/PKCμ (Ser916), anti-phospho-p44/42 MAP kinase, and anti-p44/42 MAP kinase antibodies were from Cell signaling (Beverly, Mass.).
The anti-Syk antibodies have been described previously; the anti-SykI was raised to a sequence in the linker region between the second SH2 domain and the kinase region, and the anti-SykC is an antibody to a peptide corresponding to the carboxyl-terminal amino acids (3). The anti-phospho-AL-Syk antibody was to a phosphopeptide corresponding to the activation loop of rat Syk (62). The diphosphorylated synthetic peptide based on FcɛRIγ (γPP) has been described previously. The sources of other materials not indicated were as described previously (3).
Anti-Syk phospho-342 (anti-pTyr342) and anti-Syk phospho-346 (anti-pTyr346) specific antibodies.
Peptides were synthesized with a Milligen model 9500 peptide synthesizer. The parent peptide NH2-ALPMDTEVYESPYADPE-C-COOH corresponding to the rat Syk aa 334 to 350 was made by using 9-fluorenylmethoxycarbony chemistry, with a cysteine C-terminal resin. For monophosphorylated peptides, a phosphorylated derivative of tyrosine was used instead of the Tyr residues. Rabbits were immunized with the conjugate of the monophosphorylated peptides coupled to keyhole limpet hemocyanin (Sigma) via the COOH-terminal cysteine residue. The phosphopeptide-specific sera were purified by negative absorption with affinity beads containing the native, nonphosphorylated peptides.
Construction of cDNA and cell transfections.
The rat wild-type Syk expression vector pSVL-Syk has been described previously (61). Tyr342 and Tyr346 of rat Syk were each mutated to Phe individually by using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.) according to the manufacturer's directions. Mutations were verified by nucleotide sequencing. For stable transfection, 20 μg of linearized mutant Syk expression plasmids and 2 μg of pSV2-neo vector were cotransfected into 5 × 106 Syk-negative TB1A2 cells by electroporation (310 V, 960 μF) as described previously (61). The stable transfected clones were selected with 400 μg of active G418 (Life Technologies, Gaithersburg, Md.)/ml. By fluorescence-activated cell sorting analysis, FcɛRI expression levels were similar for all the clones used in these experiments. The expression of mutated Syk was confirmed by immunoblotting using anti-Syk antibody.
Cell culture and activation.
The Syk-negative variant of RBL-2H3 and its wild-type Syk transfectants have been described previously (61). For cell activation, Syk-negative TB1A2 cells and their wild-type or mutant Syk transfectants were cultured overnight as monolayers either with or without antigen-specific IgE. The cells cultured with IgE were stimulated with antigen at concentrations ranging from 0.01 to 1.0 μg/ml. The cells cultured without IgE were incubated with calcium ionophore A23187 at 0.25 to 2 μM or with 0.4 mM pervanadate for 30 min at 37°C. After stimulation for the indicated times, the supernatants were removed for histamine analysis.
Immunoprecipitation and immunoblotting.
After stimulation, cell monolayers were rinsed with ice-cold phosphate-buffered saline containing 2 mM Na3VO4 and protease inhibitors (2 mM phenylmethylsulfonyl fluoride, 90 mU of aprotinin/ml, 50 μg of leupeptin/ml, 50 μg of pepstatin/ml) and solubilized in Triton lysis buffer (1% Triton X-100, 20 mM Tris [pH 7.4], 100 mM NaCl, 50 mM NaF, plus protease inhibitors and Na3VO4). The postnuclear supernatants were immunoprecipitated with antibodies bound to protein A-agarose beads. After rotation at 4°C for 1 h, the beads were washed four times with ice-cold lysis buffer and the proteins were eluted by boiling for 5 min with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer as described previously (26). Whole-cell lysates or immunoprecipitated proteins were separated by SDS-PAGE and electrotransferred to polyvinylidene difluoride membranes (Millipore, Bedford, Mass.). The blots were probed with antiphosphotyrosine or other specific antibodies. All the blots probed by anti-phospho-antibodies were stripped and reblotted with the corresponding specific antibody to verify equal loading. In all blots, proteins were visualized by enhanced chemiluminescence (Renaissance). Immunoprecipitation with the phosphorylated γ−ITAM peptide was performed as described previously (63).
In vitro kinase assay.
Syk immunoprecipitated as described above was further washed with kinase buffer (30 mM HEPES [pH 7.5], 10 mM MgCl2, and 2 mM MnCl2) and resuspended in 40 μl of the same buffer. The kinase reactions were for 18 min at room temperature with 3 μCi of [γ-32P]ATP and 4 μM ATP. The reactions were stopped by the addition of 40 μl of 2× Laemmli sample buffer and boiling for 10 min. The eluted proteins were separated under reducing conditions by SDS-PAGE, electrotransferred to membranes, and visualized by autoradiography. The membranes were immunoblotted with anti-Syk antibody as described above.
Rac1 activation assay.
Active Rac1 was detected with the Rac1 activation assay kit following the instructions of the manufacturer. Briefly, cells cultured overnight with IgE were transferred for 2 h to a culture medium in which the serum was replaced by 1% bovine serum albumin (BSA) and IgE. The monolayers were then washed and activated for 12 min with antigen (0.1 μg/ml). The activated Rac1 was precipitated with the p21-binding domain of PAK-1 (PAK-1 PBD) agarose and analyzed by immunoblotting with anti-Rac1 antibody.
Measurement of calcium influx.
Cells (2 × 105/well) were cultured overnight with or without IgE in 24-well culture plates. The monolayers were then washed twice with loading medium (medium 199 [Biofluid, Inc, Rockville, Md.], supplemented with 2 mM CaCl2 and 0.1% BSA) and loaded with 2 μM Fura-2 AM for 1 h at 37°C. After loading, cells were transferred to room temperature for 1 h and then washed four times with working medium (medium 199 containing 2 mM CaCl2, 10 mM Tris [pH 7.4], and 0.01% BSA). Fura-2 fluorescence in single cells was measured with a Tillvision System (Till Photononic GmbH, Grafelfing, Germany) attached to an inverted Olympus microscope with a Zeiss Fluar 10× objective. A pair of fluorescence images was acquired every 2 s at excitation wavelengths of 340 and 380 nm. For data analysis, 60 cells were chosen at random and the ratio of the fluorescence intensities (with background subtracted) excited at the two wavelengths was calculated and plotted for individual cells.
RESULTS
Tyr342 of Syk is phosphorylated after FcɛRI aggregation.
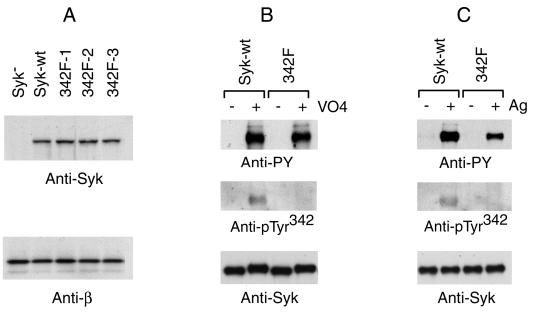
To investigate the function of Tyr342 of Syk in mast cell signal transduction, a variant of RBL-2H3 cells deficient in Syk was transfected with Y342F mutant Syk. Three cloned lines were selected based on their expression of Syk at levels approximating that in the cells transfected with wild-type Syk (Fig. 1A). The in vivo phosphorylation of Tyr342 was tested with a specific antibody generated by using a phosphopeptide based on the sequence surrounding Tyr342 of Syk. The antiserum was adsorbed by the corresponding nonphosphorylated peptide to deplete antibodies reactive with the native Syk molecule. To test the specificity of this antibody, the cells expressing wild-type or Y342F mutant Syk were treated with pervanadate to induce maximum tyrosine phosphorylation of cellular proteins. Syk was then immunoprecipitated from the cell lysates and analyzed by immunoblotting with either an antibody that binds general phosphotyrosine residues (monoclonal antibody 4G10) or with the anti-Syk 342-phosphotyrosine antibody (anti-pTyr342). As shown in Fig. 1B, a strong signal was observed with the antiphosphotyrosine 4G10 antibody for both the wild-type and the Tyr342 mutant Syk after incubation with pervanadate. However, when using the anti-pTyr342 antibody a signal was observed only with wild-type but not with the Y342F mutant Syk. Also, this antibody did not react with the nonphosphorylated molecule. Therefore, this anti-pTyr342 antibody is specific for the phosphorylated Tyr342 of Syk.
FIG. 1.
Tyr342 of Syk was phosphorylated in vivo. (A) Generation of stable mast cell lines expressing Y342F mutant Syk. The Syk-negative TB1A2 cells were transfected with cDNA encoding Y342F mutant Syk and selected with G418. Cell lysates of cloned lines were screened by immunoblotting with anti-Syk antibody, and three positive clones were selected for further study. Lysates from these cells were immunoblotted with anti-Syk antibody and with anti-FcɛRIβ antibodies. (B) Specificity of anti-pTyr342. Syk-negative TB1A2 cells transfected with wild-type or Y342F mutant Syk were incubated with 0.4 mM pervanadate for 30 min at 37°C. Syk was immunoprecipitated with rabbit anti-Syk and analyzed by immunoblotting with antiphosphotyrosines 4G10, anti-pTyr342, or anti-Syk antibodies. (C) FcɛRI aggregation induced increased Tyr342 phosphorylation. Cells were cultured overnight with antigen-specific IgE and stimulated with antigen at a concentration of 0.1 μg/ml for 20 min. Syk was immunoprecipitated with rabbit anti-Syk and analyzed by immunoblotting with antiphosphotyrosines, anti-pTyr342, or anti-Syk antibodies.
Whether Tyr342 is phosphorylated after IgE antigen stimulation is a critical question for interpreting its function in FcɛRI-induced signal transduction. To clarify this, the cells expressing wild-type Syk or Y342F mutant Syk were stimulated with IgE plus specific antigen. Immunoprecipitated Syk was tested with anti-pTyr342 antibody. As shown in Fig. 1C, receptor aggregation did induce increased phosphorylation of Tyr342 of Syk.
Syk Y342F mutation markedly impairs FcɛRI signal transduction in mast cells.
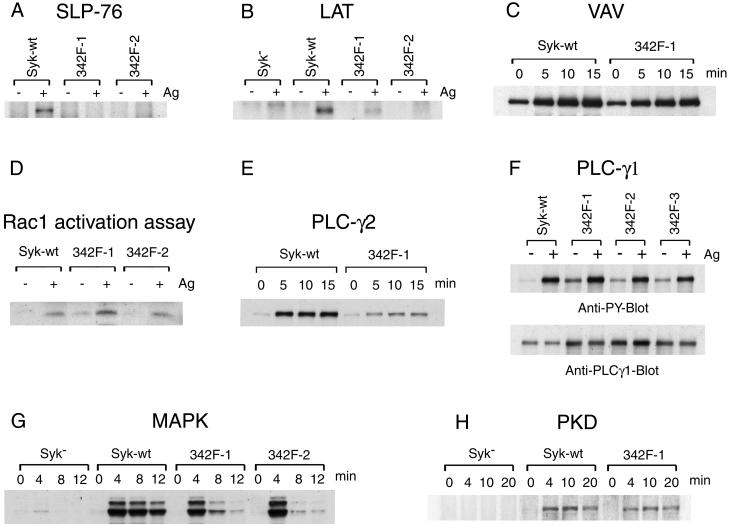
Cellular protein tyrosine phosphorylation is one of the earliest events following FcɛRI stimulation. Most of the protein tyrosine phosphorylations observed in total cell lysates require the expression of Syk. Surprisingly, in the cells expressing Y342F mutant Syk, FcɛRI aggregation still induced an obvious increase in cellular protein tyrosine phosphorylation, although not to the same extent as wild-type Syk (data not shown). To study the effect of the Y342F mutation on the FcɛRI pathway, we examined the antigen-induced tyrosine phosphorylation of several important signaling molecules. SLP-76 is a cytosolic adapter protein that is tyrosine phosphorylated downstream of Syk and regulates the antigen-induced tyrosine phosphorylation of PLC-γ1, calcium mobilization, and degranulation (21, 43). As shown in Fig. 2A, Syk Y342F mutation dramatically impaired the antigen-induced tyrosine phosphorylation of SLP-76. LAT, another adapter protein, becomes tyrosine phosphorylated after antigen stimulation and plays an important role in IgE-mediated calcium influx and mast cell degranulation (46). The lack of the FcɛRI-induced LAT tyrosine phosphorylation in the Syk-negative mast cells was reconstituted by wild-type Syk transfection (Fig. 2B). This antigen-induced LAT tyrosine phosphorylation was also dramatically reduced by the Y342F mutation of Syk (Fig. 2B).
FIG. 2.
Syk Y342F mutation impairs multiple steps in the FcɛRI signaling pathway. The indicated cell lines were cultured overnight with antigen-specific IgE and stimulated by antigen at 0.1 μg/ml. Lysates were immunoprecipitated with anti-SLP-76 (A), anti-LAT (B), anti-Vav (C), anti-PLC-γ2 (E), and anti-PLC-γ1 (F) antibodies and analyzed by immunoblotting with antiphosphotyrosine antibody. (D) Active Rac1 was precipitated with PAK-1 PBD-agarose and blotted with anti-Rac1 antibody. Lysates were also analyzed by immunoblotting with anti-phospho-p44/42 MAP kinase (G) and anti-phospho-PKD/PKCμ (Ser916) (H). Stimulation was for the indicated times except for 30 min for panels A and B and 12 min for panels D and F. All the blots probed by anti-phospho-antibodies were stripped and reblotted with the corresponding specific antibody and had equal loading (data not shown) except for PLC-γ1 (F). The results shown are representative of at least three independent experiments.
Tyr315 in ZAP70 (equivalent to Tyr342 of rat Syk) plays a critical role for B-cell receptor-induced Vav tyrosine phosphorylation (56). Therefore, we examined the phosphorylation of Vav in cells expressing wild-type or Y342F mutant Syk. Unexpectedly, antigen stimulation still induced an increase in Vav tyrosine phosphorylation in Y342F mutant transfectants (Fig. 2C). The time courses of Vav tyrosine phosphorylation in the cells expressing wild-type Syk and those expressing Y342F Syk were similar. Immunoprecipitation experiments with anti-Syk or anti-Vav antibodies compared the association of these two proteins in the cells expressing wild-type and Y342F mutant Syk. Both antibodies demonstrate that the association of Syk and Vav in the cells expressing wild-type Syk was similar to that in the cells expressing Y342F Syk (data not shown). Tyrosine phosphorylation of Vav results in the activation of Rac1. As expected, there were similar levels of activation of Rac1 in the cells expressing wild-type Syk and in those expressing Y342F mutant Syk (Fig. 2D).
FcɛRI-stimulation induces the activation of phospholipase C, which results in the formation of inositol 1,4,5-trisphosphate and 1,2-diacylglycerol. These secondary messengers are responsible for releasing Ca2+ and activating protein kinase C. As has been observed previously, the receptor-initiated tyrosine phosphorylation of PLC-γ2 required the expression of Syk and therefore is downstream of Syk (data not shown). However, the expression of Y342F mutant Syk in the Syk-deficient cells reconstituted only minimal tyrosine phosphorylation of PLC-γ2; by densitometry the 10-fold increase in the FcɛRI-induced tyrosine phosphorylation of PLC-γ2 in wild-type Syk-expressing cells was decreased by ∼70% in the cells transfected with the Y342F mutant (Fig. 2E). The effect of Y342F mutation on PLC-γ1 was also tested. Surprisingly, in cells expressing Y342F Syk there was ∼2.3-fold higher expression of PLC-γ1 than in the wild-type Syk transfectants (Fig. 2F and data not shown). This increased expression was associated with higher PLC-γ1 tyrosine phosphorylation in the nonstimulated Y342-expressing cells, although the fraction of the total PLC-γ1 that was phosphorylated was similar to that in the cells expressing wild-type Syk. Antigen stimulation induced increased PLC-γ1 tyrosine phosphorylation in both wild-type and mutant Syk transfectants; by densitometry this receptor-induced increase in PLC-γ1 phosphorylation was ∼7-fold in the cells expressing wild-type Syk and ∼2.7-fold in the Y342F cell lines, a ∼60% decrease. Therefore the Y342F mutation of Syk results in similar decreases in receptor-induced tyrosine phosphorylation of PLC-γ1 and PLC-γ2 with similar fractions of both that are phosphorylated and presumably activated.
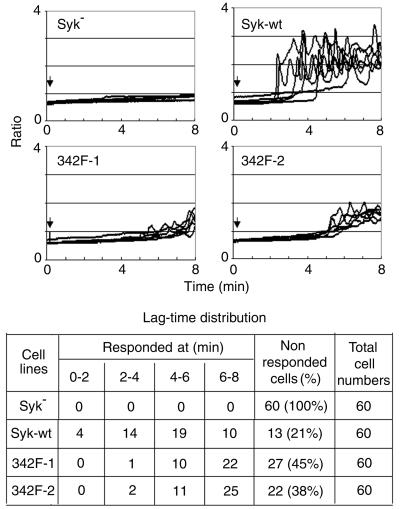
Syk is critical for antigen-induced calcium mobilization in mast cells, which is regulated by several molecules including LAT, SLP-76, Vav, and PLC-γ, whose tyrosine phosphorylations were changed by the Y342F mutation of Syk. Therefore the single-cell responses of the different cell lines were examined to test the effect of the Y342F mutation of Syk on Ca2+ responses (Fig. 3). Surprisingly, even though the Y342F mutation impaired multiple steps of the FcɛRI-signal pathway, receptor stimulation still initiated some calcium response in these cells. However, compared to cells expressing wild-type Syk, the antigen-induced calcium response in Y342F Syk-expressing cells was of smaller amplitude and delayed in onset. Furthermore, the fraction of cells that responded to antigen stimulation was much lower in Y342F Syk transfectants than in the cells reconstituted with wild-type Syk. In contrast, thrombin stimulation induced a fast response with similar magnitudes in all the different cell lines (data not shown).
FIG. 3.
Comparison of antigen-induced calcium response between wild-type and Y342F mutant Syk transfectants. Cells were cultured overnight with IgE, washed, and loaded with Fura-2 and then stimulated with antigen (0.1 μg/ml) at the time indicated by the arrow. Calcium responses of 6 individual cells from each cell line, representative of the 60 cells within each experiment, are shown. The distributions of lag-time (time between antigen addition and initiation of the calcium response) of all cells within each experiment are in the table. The result shown is representative of two independent experiments.
Several other molecules are activated after FcɛRI aggregation. The activation of Erk1/2 of the MAPK after antigen stimulation is also under the control of Syk. As shown in Fig. 2G, the transfection of Y342F mutant Syk restored only transient phosphorylation of Erk 1/2 in Syk-deficient cells. PKD is a serine/threonine protein kinase that is activated by antigen receptors in T cells, B cells, and mast cells downstream of protein kinase C (37, 39). Anti-PKD-phospho-Ser916 specifically recognizes the phosphorylated activation loop residues and therefore can be used to monitor the catalytic activity of this kinase (38). By using this antibody, we studied whether Syk plays a role on FcɛRI-stimulated PKD activation and the effect of Syk Y342F mutation on this response (Fig. 2H). Receptor-induced PKD activation was clearly downstream of Syk. Surprisingly, Y342F mutation of Syk only slightly reduced the FcɛRI-induced phosphorylation of PKD. Therefore, FcɛRI-induced PKD and thus PKC activation are downstream of Syk but are not dramatically impaired in the Y342F Syk-expressing cells.
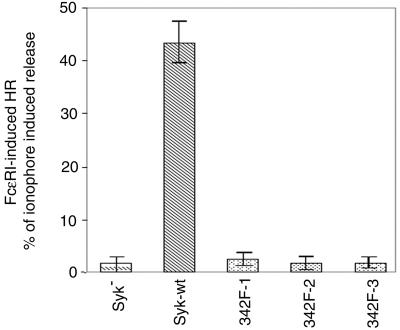
Degranulation is one of the major functional responses of mast cells to FcɛRI stimulation, a reaction in which Syk is essential (7, 61). We therefore tested the effect of Y342F mutation on FcɛRI-induced histamine release. Syk-negative cells and cells transfected with wild-type or mutated Syk were activated by either IgE plus antigen or by calcium ionophore A23187 (Fig. 4). As observed previously, wild-type Syk reconstituted antigen-induced histamine release in Syk-deficient cells. However, in cells expressing Y342F mutant Syk, receptor aggregation induced essentially no degranulation. The dose responses obtained by using antigen from 0.01 to 1 μg/ml also showed similar results (data not shown). Therefore, Tyr342 of Syk is critical for propagating the intracellular signals that lead to mast cell degranulation.
FIG. 4.
FcɛRI-induced degranulation. Cells were stimulated with antigen or with calcium ionophore A23187 for 45 min at 37°C. The antigen (0.1 μg/ml)-induced release is normalized by expression as a percentage of that induced with 1 μM A23187. The result shown is the average (± standard deviation) of at least four different experiments. The A23187-induced average release as a percentage of the total cellular histamine content in the different cell lines was 54 to 68%.
The Y342F mutation impairs multiple functions of Syk.
The observation that Syk Y342F had minimal effects on FcɛRI-induced Vav tyrosine phosphorylation suggests that the mechanism for the effects of Y342F mutation in mast cells may be different from that of Y315F mutant ZAP70 in B cells. In the Y342F Syk-expressing cells, antigen-induced tyrosine phosphorylation of LAT, SLP-76, and PLC-γ2 were all decreased, suggesting that the defect was probably at or upstream of Syk. Therefore, the next series of experiments investigated the changes in Syk.
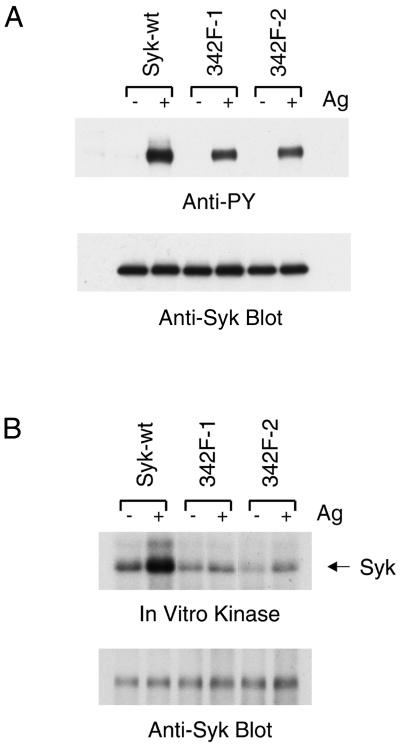
We first examined the antigen-stimulated tyrosine phosphorylation and kinase activities of the different forms of Syk expressed in these cells. As shown in Fig. 5A, although FcɛRI aggregation did induce tyrosine phosphorylation of Y342F mutant Syk, the phosphorylation signal of the mutant Syk was clearly weaker than that of wild-type Syk. The in vitro kinase assay showed interesting results (Fig. 5B). Compare to wild-type Syk, Y342F mutation reduced both the basal and the antigen-stimulated kinase activities of Syk. The loss of one phosphorylation site due to the Y342F mutation may have contributed to this decrease, but this result also suggested that Y342F mutation might reduce the structural flexibility of the Syk molecule.
FIG. 5.
FcɛRI-induced tyrosine phosphorylation and enzymatic activity of Y342F mutant Syk. (A) FcɛRI-induced tyrosine phosphorylation of Syk. Cells were cultured overnight with antigen specific IgE and either nonstimulated or stimulated with antigen (0.1 μg/ml) (− or + Ag) for 20 min. Lysates were immunoprecipitated with rabbit anti-Syk antibody. The immunoprecipitates were analyzed by immunoblotting with antiphosphotyrosine (4G10) and mouse anti-Syk antibody. (B) In vitro kinase assay of Syk. Syk was immunoprecipitated from lysates of either nonstimulated or stimulated cells as in panel A and then incubated with [γ-32P]ATP in a protein kinase assay. The proteins were separated by SDS-PAGE, electrotransferred, and visualized by autoradiography. The membranes were then blotted with anti-Syk antibody. The results shown are representative of at least three independent experiments.
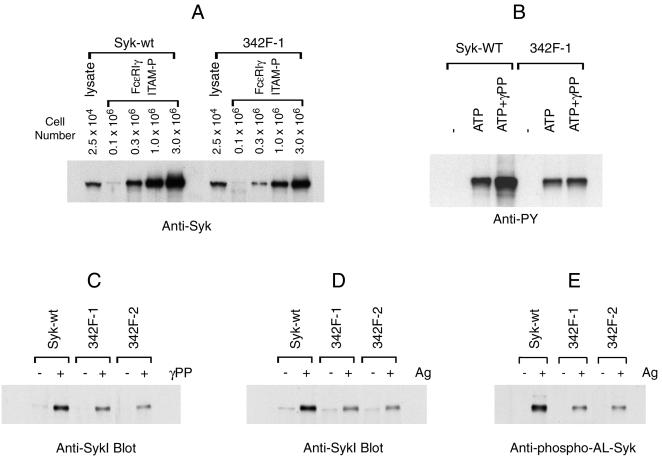
SykB, which lacks a 23-aa sequence in the linker region, has reduced capacity to bind phosphorylated ITAMs (32). To test if Tyr342 was also involved in the association of Syk with FcɛRI, we compared the abilities of wild-type and Y342F mutant Syk to bind with the ITAM of FcɛRIγ. A biotinylated peptide corresponding to diphosphorylated γ-ITAM of FcɛRI was prebound to streptavidin beads and incubated with various amounts of nonstimulated cell lysates from cells expressing wild-type or Y342F mutant Syk. As shown in Fig. 6A, both wild-type Syk and Y342F mutant Syk bound to the diphosphorylated γ-ITAM in a concentration-dependent manner. However, with the same concentration of Syk and peptide there was twice as much binding of wild-type as of Y342F mutant Syk.
FIG. 6.
Y342F mutation impairs multiple functions of Syk. (A) The Y342F mutation reduces the binding of Syk with phosphorylated γ-ITAM. Lysates from the indicated number of nonstimulated, wild-type, or Y342F mutant Syk transfectants were precipitated with the diphosphorylated ITAM peptide based on FcɛRIγ that had been prebound to streptavidin beads. For comparison, one lane contains lysates from 2.5 × 104 cell equivalents prepared from the two cell types. The precipitated proteins were analyzed by immunoblotting with anti-Syk antibody. (B) The Y342F mutation impairs phospho-γ-ITAM-induced Syk kinase activity. Syk was immunoprecipitated from nonstimulated cells and used for immune complex kinase assay without (−) or with 4 μM of ATP in the presence or absence 1 μM diphosphorylated FcɛRI γ-ITAM peptide (γPP) at 4°C for 80 min. The precipitates were analyzed by immunoblotting with antiphosphotyrosine antibody or anti-Syk antibody and have equal loading (data not shown). (C) Diphosphorylated γITAM-initiated Syk conformational change. Lysates from nonstimulated cells expressing the indicated forms of Syk were incubated with or without tyrosine-phosphorylated peptide based on the ITAM of FcɛRIγ (at 1 μM) and then immunoprecipitated with rabbit anti-SykC antibody. The immunoprecipitates were analyzed by immunoblotting with anti-SykI antibody. (D) Antigen-induced conformational changes of Syk. Cells were either nonstimulated or stimulated with antigen for 18 min (0.1 μg/ml). Lysates were immunoprecipitated with rabbit anti-SykC antibody and analyzed by immunoblotting with anti-SykI antibody. (E) The Y342F mutation impairs FcɛRI-induced phosphorylation of Syk activation loop tyrosines. Cells were cultured overnight with antigen-specific IgE and then stimulated with antigen (0.1 μg/ml) for 20 min. Syk was immunoprecipitated and analyzed by immunoblotting with anti-phospho-AL-Syk or anti-Syk antibody and have equal loading (data not shown). The results shown are representative of at least two independent experiments.
The binding of Syk to tyrosine-phosphorylated ITAM peptides enhances the kinase activity of Syk (27). Since Y342F mutant Syk had a lower binding affinity to diphosphorylated γ-ITAM, this mutation should also reduce this enhanced kinase activity. As shown in Fig. 6B, there was much greater enhancement of the kinase activity of wild-type than of the Y342F Syk by the addition of diphosphorylated γ-ITAM peptide.
The interaction of Syk with tyrosine-phosphorylated ITAM peptides also results in a conformational change that allows precipitation of this protein by anti-SykC antibody (27). Therefore, we tested whether Y342F mutant Syk still retained this capacity for conformational change on ITAM binding. As reported previously, incubating the lysates from cells expressing wild-type Syk with diphosphorylated synthetic γITAM peptide strongly enhanced precipitation of Syk by anti-SykC antibody (Fig. 6C). However, this precipitation was considerably decreased with the Y342F mutant Syk. Next we examined whether Y342F mutation had a similar effect on the in vivo antigen-induced conformational change of Syk. As shown in Fig. 6D, FcɛRI-aggregation induced a dramatic increase in the amount of wild-type Syk that was precipitated by the anti-SykC antibody, while there was much less precipitation of the Y342F mutant Syk. Altogether, these results suggested that the Y342F mutation decreased both the in vitro and in vivo phosphor-ITAM-induced conformational changes of Syk.
The phosphorylation of Syk activation loop tyrosines, predominantly due to auto- or transphosphorylation, plays a critical role in propagating FcɛRI signal transduction (60, 62). Since Y342F mutant Syk has decreased binding capacity to γ-ITAM peptide (Fig. 6A) and reduced structural flexibility (Fig. 6C and D), we tested whether this mutation had any effect on receptor-induced phosphorylation of Syk activation loop tyrosines. As shown in Fig. 6E, FcɛRI aggregation resulted in strong phosphorylation of activation loop tyrosines in wild-type Syk. However, this phosphorylation was dramatically decreased in Y342F mutant Syk, even though the mutant protein still retains the intact tyrosines at this site. There was similar decreased phosphorylation of the activation loop tyrosines in the in vitro kinase reaction (data not shown). Therefore, Y342F mutant Syk has reduced capacity for activation.
Tyr346 is not essential for antigen-induced mast cell degranulation.
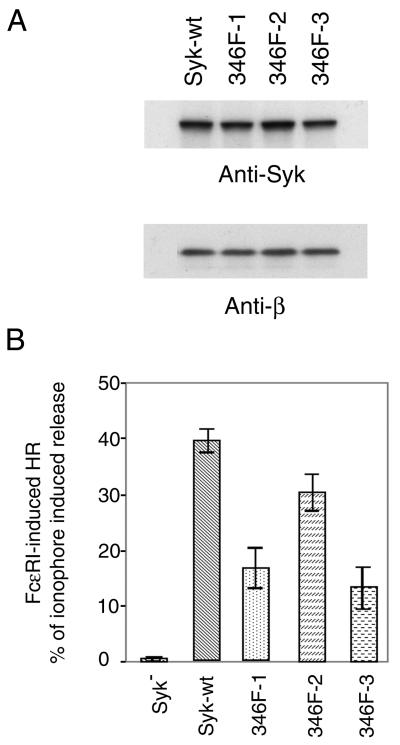
The other tyrosine in the linker region, Tyr319 of ZAP70, plays an important role in T-cell antigen receptor signaling (11, 54). To test whether the equivalent tyrosine in Syk played a similar function, Tyr346 of rat Syk was mutated to Phe. This Y346F mutant Syk was stably transfected into Syk-deficient mast cells, and three clones that expressed mutated Syk at a level similar to that in cells transfected with wild-type Syk were amplified (Fig. 7A). Antigen- and ionophore-induced histamine releases were compared among Syk-deficient cells and the cells transfected with wild-type Syk or Y346F mutant Syk (Fig. 7B). Surprisingly, Y346F mutant Syk did reconstitute antigen-induced mast cell degranulation in Syk deficient cells, although the extent of the release by transfectants with this Syk mutant was lower than that in cells expressing wild-type Syk.
FIG. 7.
The Y346F mutant Syk reconstituted FcɛRI-induced histamine release. (A) Generation of stable mast cell lines expressing Y346F mutant Syk. The Syk-negative TB1A2 cells were transfected with cDNA encoding Y346F mutant Syk and were screened as in Fig. 1. Three positive clones were selected for further study. Lysates from these cells were immunoblotted with anti-Syk antibody and with anti-FcɛRIβ antibodies. (B) Histamine release results. Cells were cultured overnight with antigen-specific IgE, washed, and then stimulated with antigen (0.1 μg/ml) or with calcium ionophore A23187 for 45 min at 37°C. The antigen-induced release is normalized by expression as a percentage of that induced with 1 μM of A23187. The result shown is the average (± standard deviation) of at least three different experiments. The A23187-induced average releases as percentages of the total cellular histamine content in the different cell lines were 50% for Syk negative or wild type and 69 to 73% for the Y346F lines.
Phosphorylation of Tyr346 of Syk was not dramatically increased after FcɛRI aggregation.
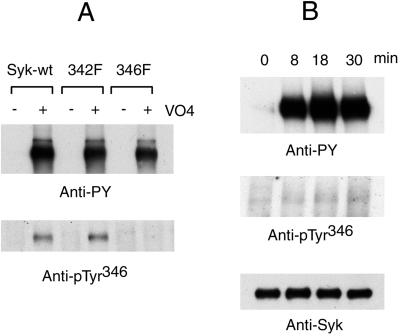
To understand why Y346F mutant Syk still reconstituted antigen-induced mast cell degranulation, an anti-Syk phospho-346 tyrosine antibody (anti-pTyr346) was generated to evaluate if this tyrosine was phosphorylated in vivo. The specificity of anti-pTyr346 was tested with cells stably transfected by wild-type Syk or Y342F or Y346F mutant Syk. These cells were incubated with pervanadate to induce maximum cellular protein tyrosine phosphorylation. Syk was immunoprecipitated from the cell lysates and blotted by anti-pTyr346. As shown in Fig. 8A, positive signals were observed in stimulated cells expressing wild-type Syk or Y342F mutant Syk but not in the cells transfected with Y346F mutant Syk. Therefore, the anti-pTyr346 is specific for phosphorylated Tyr346 of Syk.
FIG. 8.
Phosphorylation of Tyr346 was not dramatically increased after antigen stimulation. (A) Specificity of anti-pTyr346 antibodies. Syk-negative TB1A2 cells transfected with wild-type Syk, Y342F mutant Syk, or Y346F mutant Syk were incubated with 0.4 mM pervanadate for 30 min at 37°C. Syk was immunoprecipitated with rabbit anti-Syk and analyzed by immunoblotting with antiphosphotyrosines and anti-pTyr346 antibodies. (B) Phosphorylation of Tyr346 before and after FcɛRI aggregation. Wild type RBL-2H3 cells were cultured overnight with IgE and then stimulated with antigen (0.1 μg/ml) for the indicated times. Syk was immunoprecipitated and analyzed by immunoblotting with antiphosphotyrosines, anti-pTyr346, or mouse anti-Syk antibodies. The results shown are representative of three independent experiments.
To find out if FcɛRI-aggregation could induce phosphorylation of Tyr346 of Syk, RBL-2H3 wild-type cells were stimulated with IgE plus specific antigen, and immunoprecipitated Syk was blotted with anti-general phosphotyrosine antibody or anti-pTyr346. Although there was a strong signal by immunoblotting with an anti-general phosphotyrosine antibody, the anti-pTyr346 detected only very weak signals with Syk from activated cells (Fig. 8B). There was still the same minor signal with this antibody with different antigen concentrations (data not shown). Since FcɛRI aggregation induced only a very minor increase in the phosphorylation of Tyr346 of Syk, it is reasonable to expect that Y346F mutant Syk would still retain the capacity to reconstitute antigen-initiated mast cell degranulation.
DISCUSSION
The linker region of Syk and ZAP70 plays an important role in regulating their kinase function. There are three conserved tyrosines present in this region. In this study, we demonstrate that in mast cells, FcɛRI stimulation induced a clear increase in the phosphorylation of Tyr342, but not Tyr346, of Syk. Correlated with this in vivo phosphorylation, mutation of Tyr342, but not Tyr346, of Syk had dramatic effects on FcɛRI signal transduction. The expression of Y342F mutant Syk failed to reconstitute FcɛRI-stimulated histamine release, with impaired antigen-induced tyrosine phosphorylation of SLP-76, LAT, PLC-γ2, but not Vav. These defects in signaling are due to reduced binding capacity of the mutated Syk with phospho-γITAM, decreased Syk kinase activity, and conformational change, which finally results in decreased phosphorylation of Syk activation loop tyrosines.
Phosphopeptide mapping has identified 6 tyrosine phosphorylation sites in ZAP70 and 10 in Syk (16, 53). Mutagenesis of different tyrosines has demonstrated that these residues are important in up- or down-regulating Syk and ZAP70 function. For example, mutation of the three adjacent tyrosines at the COOH-terminal region of Syk or ZAP70 results in a gain of function in T-cell lines (59). In contrast, the two adjacent activation loop tyrosines in the catalytic domain of Syk (Tyr519 and Tyr520 in rat Syk) are essential for propagating receptor-induced downstream signaling (13, 29, 60). These results suggest that tyrosine phosphorylation is a critical feature of the regulation of Syk and ZAP70 enzymatic activity and function.
Experiments have investigated the functional role of the three conserved tyrosines in the linker region of Syk and ZAP70. The putative Cbl binding site, Tyr292 in ZAP70 or Tyr317 in Syk, is a negative regulator of signal transduction in cells (9, 25, 34, 45). The functions of the other two-linker region tyrosines in Syk, however, have not been clearly defined.
The role of Tyr342 and Tyr346 of Syk was first investigated by expressing a chimeric molecule with the extracellular and transmembrane domain of mCD8 fused with Syk (33). Substitution of these two tyrosines with Phe in mCD8-Syk reduced in vitro interaction of the fusion protein with the SH2 domain of PLC-γ1 and eliminated its capacity to induce tyrosine phosphorylation of PLC-γ1 in vivo. In the yeast two-hybrid system, the Tyr342 of Syk was the binding site for the SH2-domain of Vav (10). In vitro, mutation of this site reduced the interaction of Syk with a fusion protein containing the SH2 domain of Vav and in transiently transfected COS cells, Syk with this mutation did not tyrosine phosphorylate Vav. Furthermore, by overexpression in Jurkat cells, Syk with this tyrosine mutated lost its capacity to enhance NFAT activation after TCR stimulation. However, the present in vivo data from mast cells suggest that Tyr342 but not Tyr 346 is functionally critical for downstream signal transduction.
There have been a number of studies of the two orthologous tyrosines in the linker region of ZAP70 (Tyr315 or Tyr319). Both of these tyrosines are phosphorylated after immune receptor activation (11, 54). In mice, Tyr315 of ZAP70 (equivalent to Tyr342 of Syk) is involved in regulating the positive and negative selections of T cells (18, 35). In Syk−/− chicken B cells, the expression of Y315F mutant ZAP70 eliminates Vav-ZAP70 interaction and profoundly alters the capacity of ZAP70 to reconstitute the antigen receptor-signaling pathway (56). However, overexpression of this mutated ZAP70 in Jurkat T cells is not very effective as a negative dominant inhibitor of TCR-induced NFAT activation (11). Tyr319 of ZAP70 (equivalent to Tyr346 of rat Syk) is critical for antigen-receptor signaling in T cells and is important for the interaction of ZAP70 with the SH2 domain of PLC-γ1 and Lck (11, 42, 54). In contrast, in the present study, we observed that Tyr342 but not Tyr346 of rat Syk was essential for FcɛRI signaling in mast cells. The functional importance of Tyr342 of Syk in mast cell signaling is similar to the role of its equivalent tyrosine in ZAP70, Tyr315, in B-cell signaling. However, our finding that Tyr346 of Syk plays a minor role in mast cells is in contrast with the results obtained with the orthologous site, Tyr319 of ZAP70.
Although both Syk and ZAP70 belong to the same PTK family, there are differences in their signaling capacity and the mechanism of their regulation (8, 14, 55). Binding to phosphorylated ITAM can activate Syk, while ZAP70 requires additional stimulatory input from Lck (23, 28, 44, 65). In T cells, Syk, but not ZAP70, can transduce TCR signals independent of CD45 and Lck (6). In Syk and CD45 double-deficient mast cells, the expression of Syk but not ZAP70 reconstitutes FcɛRI signaling (63). Site-directed mutagenesis of the activation loop tyrosines of ZAP70 and Syk suggests that the orthologous sites on the two kinases might play different roles. For ZAP70, mutation of the first activation loop tyrosine increases in vitro kinase activity and enhances in vivo signal transduction in a Syk-negative avian B-cell line (4, 28, 52). In contrast, mutation of either one of these two tyrosines in Syk has no effect on in vitro kinase activity but results in a loss of the capacity of Syk to function in the IgE receptor signaling pathway (60). Therefore, it is possible that the equivalent tyrosines in Syk or ZAP70 may have different functions. Nevertheless, our result further highlights the observation that the linker region of Syk is critical for regulating the function of the enzyme in mast cell signaling.
The linker region Tyr342 of Syk has been considered a putative binding site for Vav. This site is required for the interaction of Syk and Vav in the two-hybrid system. Mutation of this tyrosine also reduced the binding of Syk with a Vav SH2 fusion protein in vitro and eliminated the capacity of Syk to phosphorylate Vav in vitro and in vivo (10). Similarly, the equivalent tyrosine in ZAP70, Tyr315, was reported to play an important role in Vav interaction with ZAP70 and the mutation to Phe resulted in a marked reduction in the tyrosine phosphorylation of Vav in B cells (56). However, in other systems this Tyr may not regulate Vav phosphorylation. For example, in a transformed T-cell line or in T cells obtained from Y315F knockin mice, the Y315F mutation does not dramatically decrease the receptor-induced Vav tyrosine phosphorylation (35, 40). In the present experiments we found that Y342F mutant Syk reconstitutes FcɛRI-induced Vav tyrosine phosphorylation in mast cells. The discrepancy among the different results may be due to the different cell types used. Nevertheless, our results suggest that the impaired signal transduction in cells expressing Y342F mutant Syk is not due to decreased phosphorylation of Vav.
The Y342F mutation of Syk reduced its capacity to bind diphosphorylated γ-ITAM and the capacity of Syk to undergo a conformational change, which results in a reduction in autophosphorylation and phosphorylation of its own activation loop tyrosines. Binding of diphosphorylated γ-ITAM stimulates Syk kinase activity, thus increasing autophosphorylation and the phosphorylation of the activation loop tyrosines (27, 44, 48, 62). In the present study, Y342F mutant Syk had decreased binding to diphosphorylated ITAM and decreased capacity for autophosphorylation and phosphorylation of the activation loop tyrosines both in vitro and in vivo. This defect in binding to phospho-ITAM is reminiscent of the observations that the alternatively spliced variant of Syk, termed SykB, which lacks a 23-aa sequence in the linker domain, has reduced capacity to bind phosphorylated ITAMs and is inefficient at coupling immune receptors such as FcɛRI to the early and late events of cellular activation (32). Phosphorylation of Syk activation loop tyrosines is essential for Syk to mediate antigen-induced mast cell degranulation (62). The decreased tyrosine phosphorylation of the activation loop in the Y342F mutant Syk, therefore, may be the major reason for the loss of downstream signal transduction caused by this mutation.
The FcɛRI-induced increase in [Ca2+]i is downstream of Syk and regulated by several enzymes and adapters including PLC-γ, LAT, SLP-76, and Vav (36, 43, 46, 51). As there were changes in the tyrosine phosphorylation of several of these proteins, it is not surprising that there were also profound changes in the receptor-induced increase in [Ca2+]i. The bone marrow-derived mast cells from PLC-γ2−/− mice are essentially negative for FcɛRI-induced degranulation, and receptor activation of the B cells from these mice does not induce an increase in [Ca2+]i. Therefore, PLC-γ2 is essential for signaling from the FcɛRI. The defects in signaling downstream of Y342F Syk that we observed therefore could be due to the decreased activation of the phospholipases, especially PLC-γ2. The partial functioning of downstream signals in Y342F mutant Syk-expressing cells was supported by the results of antigen-initiated transient phosphorylation of MAPK and a slightly reduced PKD phosphorylation.
The higher expression level of PLC-γ1 in the cells with the Y342F mutant Syk was unexpected. However, we have previously observed a similar phenomenon. The overexpression of Gab2 in RBL-2H3 cells impairs FcɛRI-signal transduction and results in reduced Syk activation loop tyrosine phosphorylation (57). These Gab2-overexpressing cells also have a higher expression of PLC-γ1, and FcɛRI aggregation results in an increased tyrosine phosphorylation of PLC-γ1 but not PLC-γ2. Therefore, the overexpression of PLC-γ1 under both of these conditions may be a self-adjusting response when normal signaling pathways are interrupted. Clearly, further experiments will be necessary to understand the mechanism of the higher expression of PLC-γ1.
In summary, we found that in mast cells aggregation of FcɛRI initiates a clear increase in the phosphorylation of Tyr342 but only a very minor, if any, increase in the phosphorylation of Tyr346. Furthermore, Tyr342 but not Tyr346 is essential for Syk to mediate FcɛRI signal transduction in mast cells. Tyr342, by regulating the binding capacity of Syk with phosphorylated ITAMs and Syk conformational change, controls the extent of Syk activation, loop tyrosine phosphorylation, and signal transduction from immunoreceptors. Therefore, this tyrosine in the linker region is critical in regulating the biological function of Syk.
Acknowledgments
We thank M. Haddad and K. Suzuki for reviewing the manuscript. We also thank Greta Bader and Lynda Weedon for excellent technical help and Suzanne Houser for secretarial assistance.
We thank Melvin Billingsley and Randall Kincaid for the preparation of the anti-Syk phospho-specific antibodies under National Institutes of Health Contract NO1-DE-62614.
REFERENCES
- 1.Beaven, M. A., and H. Metzger. 1993. Signal transduction by Fc receptors: the FcɛRI case. Immunol. Today 14:222-226. [DOI] [PubMed] [Google Scholar]
- 2.Benhamou, M., J. S. Gutkind, K. C. Robbins, and R. P. Siraganian. 1990. Tyrosine phosphorylation coupled to IgE receptor-mediated signal transduction and histamine release. Proc. Natl. Acad. Sci. USA 87:5327-5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benhamou, M., N. J. P. Ryba, H. Kihara, H. Nishikata, and R. P. Siraganian. 1993. Protein-tyrosine kinase p72syk in high affinity IgE receptor signaling. Identification as a component of pp72 and association with the receptor γ chain. J. Biol. Chem. 268:23318-23324. [PubMed] [Google Scholar]
- 4.Chan, A. C., M. Dalton, R. Johnson, G. H. Kong, T. Wang, R. Thoma, and T. Kurosaki. 1995. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 14:2499-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, T., B. Repetto, R. Chizzonite, C. Pullar, C. Burghardt, E. Dharm, A. C. Zhao, R. Carroll, P. Nunes, M. Basu, W. Danho, M. Visnick, J. Kochan, D. Waugh, and A. M. Gilfillan. 1996. Interaction of phosphorylated FcɛRIγ immunoglobulin receptor tyrosine activation motif-based peptides with dual and single SH2 domains of p72syk. Assessment of binding parameters and real time binding kinetics. J. Biol. Chem. 271:25308-25315. [DOI] [PubMed] [Google Scholar]
- 6.Chu, D. H., H. Spits, J. F. Peyron, R. B. Rowley, J. B. Bolen, and A. Weiss. 1996. The Syk protein tyrosine kinase can function independently of CD45 or Lck in T cell antigen receptor signaling. EMBO J. 15:6251-6261. [PMC free article] [PubMed] [Google Scholar]
- 7.Costello, P. S., M. Turner, A. E. Walters, C. N. Cunningham, P. H. Bauer, J. Downward, and V. L. J. Tybulewicz. 1996. Critical role for the tyrosine kinase Syk in signalling through the high affinity IgE receptor of mast cells. Oncogene 13:2595-2605. [PubMed] [Google Scholar]
- 8.Couture, C., S. Williams, N. Gauthier, P. Tailor, and T. Mustelin. 1997. Role of Tyr518 and Tyr519 in the regulation of catalytic activity and substrate phosphorylation by Syk protein-tyrosine kinase. Eur. J. Biochem. 246:447-451. [DOI] [PubMed] [Google Scholar]
- 9.Deckert, M., C. Elly, A. Altman, and Y. C. Liu. 1998. Coordinated regulation of the tyrosine phosphorylation of Cbl by Fyn and Syk tyrosine kinases. J. Biol. Chem. 273:8867-8874. [DOI] [PubMed] [Google Scholar]
- 10.Deckert, M., S. Tartare-Deckert, C. Couture, T. Mustelin, and A. Altman. 1996. Functional and physical interactions of Syk family kinases with the Vav proto-oncogene product. Immunity 5:591-604. [DOI] [PubMed] [Google Scholar]
- 11.Di Bartolo, V., D. Mege, V. Germain, M. Pelosi, E. Dufour, F. Michel, G. Magistrelli, A. Isacchi, and O. Acuto. 1999. Tyrosine 319, a newly identified phosphorylation site of ZAP-70, plays a critical role in T cell antigen receptor signaling. J. Biol. Chem. 274:6285-6294. [DOI] [PubMed] [Google Scholar]
- 12.Eiseman, E., and J. B. Bolen. 1992. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature 355:78-80. [DOI] [PubMed] [Google Scholar]
- 13.El-Hillal, O., T. Kurosaki, H. Yamamura, J. P. Kinet, and A. M. Scharenberg. 1997. Syk kinase activation by a src kinase-initiated activation loop phosphorylation chain reaction. Proc. Natl. Acad. Sci. USA 94:1919-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzer-Attas, C. J., D. G. Schindler, T. Waks, and Z. Eshhar. 1998. Harnessing Syk family tyrosine kinases as signaling domains for chimeric single chain of the variable domain receptors: optimal design for T cell activation. J. Immunol. 160:145-154. [PubMed] [Google Scholar]
- 15.Fukamachi, H., Y. Kawakami, M. Takei, T. Ishizaka, K. Ishizaka, and T. Kawakami. 1992. Association of protein-tyrosine kinase with phospholipase C-γ1 in bone marrow-derived mouse mast cells. Proc. Natl. Acad. Sci. USA 89:9524-9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furlong, M. T., A. M. Mahrenholz, K. H. Kim, C. L. Ashendel, M. L. Harrison, and R. L. Geahlen. 1997. Identification of the major sites of autophosphorylation of the murine protein-tyrosine kinase Syk. Biochim. Biophys. Acta 1355:177-190. [DOI] [PubMed] [Google Scholar]
- 17.Futterer, K., J. Wong, R. A. Grucza, A. C. Chan, and G. Waksman. 1998. Structural basis for Syk tyrosine kinase ubiquity in signal transduction pathways revealed by the crystal structure of its regulatory SH2 domains bound to a dually phosphorylated ITAM peptide. J. Mol. Biol. 281:523-537. [DOI] [PubMed] [Google Scholar]
- 18.Gong, Q., X. H. Jin, A. M. Akk, N. Foger, M. White, Q. Q. Gong, J. B. Wardenburg, and A. C. Chan. 2001. Requirement for tyrosine residues 315 and 319 within ζ chain-associated protein 70 for T cell development. J. Exp. Med. 194:507-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon, J. R., P. R. Burd, and S. J. Galli. 1990. Mast cells as a source of multifunctional cytokines. Immunol. Today 11:458-464. [DOI] [PubMed] [Google Scholar]
- 20.Hatada, M. H., X. Lu, E. R. Laird, J. Green, J. P. Morgenstern, M. Lou, C. S. Marr, T. B. Phillips, M. K. Ram, and K. Theriault. 1995. Molecular basis for interaction of the protein tyrosine kinase ZAP-70 with the T-cell receptor. Nature 377:32-38. [DOI] [PubMed] [Google Scholar]
- 21.Hendricks-Taylor, L. R., D. G. Motto, J. Zhang, R. P. Siraganian, and G. A. Koretzky. 1997. SLP-76 is a substrate of the high affinity IgE receptor stimulated protein tyrosine kinases in RBL-2H3 cells. J. Biol. Chem. 272:1363-1367. [DOI] [PubMed] [Google Scholar]
- 22.Hutchcroft, J. E., R. L. Geahlen, G. G. Deanin, and J. M. Oliver. 1992. FcɛRI-mediated tyrosine phosphorylation and activation of the 72-kDa protein-tyrosine kinase, PTK72, in RBL-2H3 rat tumor mast cells. Proc. Natl. Acad. Sci. USA 89:9107-9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isakov, N., R. L. Wange, J. D. Watts, R. Aebersold, and L. E. Samelson. 1996. Purification and characterization of human ZAP-70 protein-tyrosine kinase from a baculovirus expression system. J. Biol. Chem. 271:15753-15761. [DOI] [PubMed] [Google Scholar]
- 24.Jouvin, M. H., R. P. Numerof, and J. P. Kinet. 1995. Signal transduction through the conserved motifs of the high affinity IgE receptor FcɛRI. Semin. Immunol. 7:29-35. [DOI] [PubMed] [Google Scholar]
- 25.Keshvara, L. M., C. C. Isaacson, T. M. Yankee, R. Sarac, M. L. Harrison, and R. L. Geahlen. 1998. Syk- and Lyn-dependent phosphorylation of Syk on multiple tyrosines following B cell activation includes a site that negatively regulates signaling. J. Immunol. 161:5276-5283. [PubMed] [Google Scholar]
- 26.Kihara, H., and R. P. Siraganian. 1994. Src homology 2 domains of Syk and Lyn bind to tyrosine-phosphorylated subunits of the high affinity IgE receptor. J. Biol. Chem. 269:22427-22432. [PubMed] [Google Scholar]
- 27.Kimura, T., H. Sakamoto, E. Appella, and R. P. Siraganian. 1996. Conformational changes induced in the protein tyrosine kinase p72syk by tyrosine phosphorylation or by binding of phosphorylated immunoreceptor tyrosine-based activation motif peptides. Mol. Cell. Biol. 16:1471-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong, G. H., M. Dalton, J. B. Wardenburg, D. Straus, T. Kurosaki, and A. C. Chan. 1996. Distinct tyrosine phosphorylation sites in ZAP-70 mediate activation and negative regulation of antigen receptor function. Mol. Cell. Biol. 16:5026-5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurosaki, T., S. A. Johnson, L. Pao, K. Sada, H. Yamamura, and J. C. Cambier. 1995. Role of the Syk autophosphorylation site and SH2 domains in B cell antigen receptor signaling. J. Exp. Med. 182:1815-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurosaki, T., M. Takata, Y. Yamanashi, T. Inazu, T. Taniguchi, T. Yamamoto, and H. Yamamura. 1994. Syk activation by the Src-family tyrosine kinase in the B cell receptor signaling. J. Exp. Med. 179:1725-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Latour, S., L. M. L. Chow, and A. Veillette. 1996. Differential intrinsic enzymatic activity of Syk and ZAP-70 protein-tyrosine kinases. J. Biol. Chem. 271:22782-22790. [DOI] [PubMed] [Google Scholar]
- 32.Latour, S., J. Zhang, R. P. Siraganian, and A. Veillette. 1998. A unique insert in the linker domain of Syk is necessary for its function in immunoreceptor signalling. EMBO J. 17:2584-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Law, C. L., K. A. Chandran, S. P. Sidorenko, and E. A. Clark. 1996. Phospholipase C-γ1 interacts with conserved phosphotyrosyl residues in the linker region of Syk and is a substrate for Syk. Mol. Cell. Biol. 16:1305-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lupher, M. L., N. Rao, N. L. Lill, C. E. Andoniou, S. Miyake, E. A. Clark, B. Druker, and H. Band. 1998. Cbl-mediated negative regulation of the Syk tyrosine kinase. A critical role for Cbl phosphotyrosine-binding domain binding to Syk phosphotyrosine 323. J. Biol. Chem. 273:35273-35281. [DOI] [PubMed] [Google Scholar]
- 35.Magnan, A., V. Di Bartolo, A. M. Mura, C. Boyer, M. Richelme, Y. L. Lin, A. Roure, A. Gillet, C. Arrieumerlou, O. Acuto, B. Malissen, and M. Malissen. 2001. T cell development and T cell responses in mice with mutations affecting tyrosines 292 or 315 of the ZAP-70 protein tyrosine kinase. J. Exp. Med. 194:491-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manetz, T. S., C. Gonzalez-Espinosa, R. Arudchandran, S. Xirasagar, V. Tybulewicz, and J. Rivera. 2001. Vav1 regulates phospholipase Cγ activation and calcium responses in mast cells. Mol. Cell. Biol. 21:3763-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthews, S. A., T. Iglesias, E. Rozengurt, and D. Cantrell. 2000. Spatial and temporal regulation of protein kinase D (PKD). EMBO J. 19:2935-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matthews, S. A., E. Rozengurt, and D. Cantrell. 1999. Characterization of serine 916 as an in vivo autophosphorylation site for protein kinase D/protein kinase Cμ. J. Biol. Chem. 274:26543-26549. [DOI] [PubMed] [Google Scholar]
- 39.Matthews, S. A., E. Rozengurt, and D. Cantrell. 2000. Protein kinase D: a selective target for antigen receptors and a downstream target for protein kinase C in lymphocytes. J. Exp. Med. 191:2075-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michel, F., L. Grimaud, L. Tuosto, and O. Acuto. 1998. Fyn and ZAP-70 are required for Vav phosphorylation in T cells stimulated by antigen-presenting cells. J. Biol. Chem. 273:31932-31938. [DOI] [PubMed] [Google Scholar]
- 41.Minoguchi, K., M. Benhamou, W. D. Swaim, Y. Kawakami, T. Kawakami, and R. P. Siraganian. 1994. Activation of protein tyrosine kinase p72syk by FcɛRI aggregation in rat basophilic leukemia cells: p72syk is a minor component but the major protein tyrosine kinase of pp72. J. Biol. Chem. 269:16902-16908. [PubMed] [Google Scholar]
- 42.Pelosi, M., V. Di Bartolo, V. Mounier, D. Mege, J. M. Pascussi, E. Dufour, A. Blondel, and O. Acuto. 1999. Tyrosine 319 in the interdomain B of ZAP-70 is a binding site for the Src homology 2 domain of Lck. J. Biol. Chem. 274:14229-14237. [DOI] [PubMed] [Google Scholar]
- 43.Pivniouk, V. I., T. R. Martin, J. M. Lu-Kuo, H. R. Katz, H. C. Oettgen, and R. S. Geha. 1999. SLP-76 deficiency impairs signaling via the high-affinity IgE receptor in mast cells. J. Clin. Investig. 103:1737-1743. [PMC free article] [PubMed] [Google Scholar]
- 44.Rowley, R. B., A. L. Burkhardt, H. G. Chao, G. R. Matsueda, and J. B. Bolen. 1995. Syk protein-tyrosine kinase is regulated by tyrosine-phosphorylated Igα/Igβ immunoreceptor tyrosine activation motif binding and autophosphorylation. J. Biol. Chem. 270:11590-11594. [DOI] [PubMed] [Google Scholar]
- 45.Sada, K., J. Zhang, and R. P. Siraganian. 2000. Point mutation of a tyrosine in the linker region of Syk results in a gain of function. J. Immunol. 164:338-344. [DOI] [PubMed] [Google Scholar]
- 46.Saitoh, S., R. Arudchandran, T. S. Manetz, W. G. Zhang, C. L. Sommers, P. E. Love, J. Rivera, and L. E. Samelson. 2000. LAT is essential for FcɛRI-mediated mast cell activation. Immunity 12:525-535. [DOI] [PubMed] [Google Scholar]
- 47.Shiue, L., J. Green, O. M. Green, J. L. Karas, J. P. Morgenstern, M. K. Ram, M. K. Taylor, M. J. Zoller, L. D. Zydowsky, J. B. Bolen, and J. S. Brugge. 1995. Interaction of p72syk with the γ and β subunits of the high-affinity receptor for immunoglobulin E, FcɛRI. Mol. Cell. Biol. 15:272-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shiue, L., M. J. Zoller, and J. S. Brugge. 1995. Syk is activated by phosphotyrosine-containing peptides representing the tyrosine-based activation motifs of the high affinity receptor for IgE. J. Biol. Chem. 270:10498-10502. [DOI] [PubMed] [Google Scholar]
- 49.Siraganian, R. P. 1993. Mechanism of IgE-mediated hypersensitivity, p. 105-134. In E. J. Middleton, C. E. Reed, E. F. Ellis, N. F. J. Adkinson, J. W. Yunginger, and W. W. Busse (ed.), Allergy. Principles and practice. Mosby-Year Book, Inc., St. Louis, Mo.
- 50.Stevens, R. L., and K. F. Austen. 1989. Recent advances in the cellular and molecular biology of mast cells. Immunol. Today 10:381-386. [DOI] [PubMed] [Google Scholar]
- 51.Wang, D., J. Feng, R. R. Wen, J. C. Marine, M. Y. Sangster, E. Parganas, A. Hoffmeyer, C. W. Jackson, J. L. Cleveland, P. J. Murray, and J. N. Ihle. 2000. Phospholipase Cγ2 is essential in the functions of B cell and several Fc receptors. Immunity 13:25-35. [DOI] [PubMed] [Google Scholar]
- 52.Wange, R. L., R. Guitian, N. Isakov, J. D. Watts, R. Aebersold, and L. E. Samelson. 1995. Activating and inhibitory mutations in adjacent tyrosines in the kinase domain of ZAP-70. J. Biol. Chem. 270:18730-18733. [DOI] [PubMed] [Google Scholar]
- 53.Watts, J. D., M. Affolter, D. L. Krebs, R. L. Wange, L. E. Samelson, and R. Aebersold. 1994. Identification by electrospray ionization mass spectrometry of the sites of tyrosine phosphorylation induced in activated Jurkat T cells on the protein tyrosine kinase ZAP-70. J. Biol. Chem. 269:29520-29529. [PubMed] [Google Scholar]
- 54.Williams, B. L., B. J. Irvin, S. L. Sutor, C. C. S. Chini, E. Yacyshyn, J. B. Wardenburg, M. Dalton, A. C. Chan, and R. T. Abraham. 1999. Phosphorylation of Tyr319 in ZAP-70 is required for T-cell antigen receptor-dependent phospholipase C-γ1 and Ras activation. EMBO J. 18:1832-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams, B. L., K. L. Schreiber, W. G. Zhang, R. L. Wange, L. E. Samelson, P. J. Leibson, and R. T. Abraham. 1998. Genetic evidence for differential coupling of Syk family kinases to the T-cell receptor: reconstitution studies in a ZAP-70-deficient Jurkat T-cell line. Mol. Cell. Biol. 18:1388-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu, J., Q. H. Zhao, T. Kurosaki, and A. Weiss. 1997. The Vav binding site (Y315) in ZAP-70 is critical for antigen receptor-mediated signal transduction. J. Exp. Med. 185:1877-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie, Z. H., I. Ambudkar, and R. P. Siraganian. 2002. The adapter molecule Gab2 regulates FcɛRI-mediated signal transduction in mast cells. J. Immunol. 168:4682-4691. [DOI] [PubMed] [Google Scholar]
- 58.Yamashita, T., S.-Y. Mao, and H. Metzger. 1994. Aggregation of the high-affinity IgE receptor and enhanced activity of p53/56lyn protein-tyrosine kinase. Proc. Natl. Acad. Sci. USA 91:11251-11255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeitlmann, L., T. Knorr, M. Knoll, C. Romeo, P. Sirim, and W. Kolanus. 1998. T cell activation induced by novel gain-of-function mutants of Syk and ZAP-70. J. Biol. Chem. 273:15445-15452. [DOI] [PubMed] [Google Scholar]
- 60.Zhang, J., T. Kimura, and R. P. Siraganian. 1998. Mutations in the activation loop tyrosines of protein tyrosine kinase Syk abrogate intracellular signaling but not kinase activity. J. Immunol. 161:4366-4374. [PubMed] [Google Scholar]
- 61.Zhang, J., E. H. Berenstein, R. L. Evans, and R. P. Siraganian. 1996. Transfection of Syk protein tyrosine kinase reconstitues high affinity IgE receptor mediated degranulation in a Syk negative variant of rat basophilic leukemia RBL-2H3 cells. J. Exp. Med. 184:71-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, J., M. L. Billingsley, R. L. Kincaid, and R. P. Siraganian. 2000. Phosphorylation of Syk activation loop tyrosines is essential for Syk function. An in vivo study using a specific anti-Syk activation loop phosphotyrosine antibody. J. Biol. Chem. 275:35442-35447. [DOI] [PubMed] [Google Scholar]
- 63.Zhang, J., and R. P. Siraganian. 1999. CD45 is essential for FcɛRI signaling by ZAP70, but not Syk, in Syk-negative mast cells. J. Immunol. 163:2508-2516. [PubMed] [Google Scholar]
- 64.Zhao, Q. H., B. L. Williams, R. T. Abraham, and A. Weiss. 1999. Interdomain B in ZAP-70 regulates but is not required for ZAP-70 signaling function in lymphocytes. Mol. Cell. Biol. 19:948-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zoller, K. E., I. A. MacNeil, and J. S. Brugge. 1997. protein tyrosine kinases Syk and ZAP-70 display distinct requirements for Src family kinases in immune response receptor signal transduction. J. Immunol. 158:1650-1659. [PubMed] [Google Scholar]