Abstract
Tumor necrosis factor (TNF) signaling through the TNF receptors involves the recruitment of key signaling factors, leading to the activation of both the transcription factor NF-κB and the stress-activated Jun kinase (JNK). In most cells, TNF signaling leads to a rapid and transient increase in JNK activity. However, we show that TNF treatment leads to the sustained activation of JNK in cells that are null for the p65/RelA subunit of NF-κB as well as in cells expressing the super-repressor form of IκB. In addition, the data indicate that the ability of p65/RelA to regulate gene expression is required to suppress the persistent activation of JNK. Interestingly, this suppression occurs upstream of JNK, within the signal transduction cascade leading to JNK activation, without affecting the stress-activated kinase p38. Since NF-κB has previously been shown to be involved in the suppression of TNF-induced apoptosis, we were interested in determining the role of deregulated JNK activity, induced by the loss of NF-κB, in controlling the cell death response. Through the use of different approaches for inhibition of JNK, we show that the suppression of JNK activity in cells that lack active NF-κB enhances the apoptotic response to TNF. These data suggest that the activity of JNK in cells blocked for NF-κB function provides an antiapoptotic signal and explains, at least partly, why a significant number of NF-κB null cells remain viable following TNF treatment.
The transcription factor NF-κB is a dimeric complex composed of members of the Rel family of proteins (12, 29). The classic form of NF-κB is a dimer consisting of the transcriptionally inactive p50 subunit and the p65/RelA (p65) subunit, which contains a potent, C-terminal transactivation domain (12, 28). NF-κB activity is suppressed primarily through interactions with various IκB proteins that promote the cytoplasmic localization of NF-κB (12). The activation of NF-κB is achieved when signals, such as those elicited by tumor necrosis factor (TNF), activate a kinase known as IKK that causes the phosphorylation of IκB proteins (16, 43). This phosphorylation triggers the ubiquitination and subsequent degradation of the IκB proteins, allowing for the nuclear accumulation of NF-κB (12, 16). Gene-specific transcription by NF-κB is achieved through the recognition of distinct DNA binding sites in genes typically associated with inflammatory and immune responses as well as cell cycle regulation and apoptosis (2, 12, 29).
Recently it was demonstrated that NF-κB is a potent regulator of apoptosis. Evidence from a number of groups has indicated that NF-κB suppresses apoptosis induced by TNF and other apoptotic stimuli by inhibiting the activation of the cell death caspase cascade as well as by inhibiting the release of cytochrome c from the mitochondria (3, 23, 35, 38, 40). The inhibition of apoptosis by NF-κB appears to be largely transcriptional, since several antiapoptotic genes, such as those encoding Bcl-xL, c-IAP1, c-IAP2, A1/Bfl-2, and the death domain proteins TRAF1 and TRAF2, have been shown to be transcriptionally regulated by NF-κB (7, 36, 37, 44). Therefore, when cells that lack functional NF-κB are treated with TNF, a significant proportion of cells undergo apoptosis (3, 35, 38). Interestingly, cell death under these circumstances never reaches 100%, so it has been speculated that an additional pathway activated by TNF may play an antiapoptotic role when NF-κB is suppressed.
TNF is a key inflammatory cytokine that regulates signal transduction cascades, gene expression, and apoptosis. The biological effects of TNF are mediated through two distinct cell surface receptors, TNF-RI and TNF-RII (1). Ligand-induced trimerization of the TNF receptors leads to the recruitment of the TNF-receptor-associated death domain protein, TRADD, which serves to recruit additional proteins involved in the activation of specific signal transduction pathways (1). For example, when FADD is recruited to TRADD, a caspase cascade is initiated through the recruitment of caspase-8. In addition, binding of TRAF2 to TRADD leads to the recruitment of additional proteins that can activate both the stress-activated protein kinase cascade, which includes Jun kinase (JNK) (9, 18), and as the NF-κB signaling pathway (1, 25). Therefore, the stimulation of cells with TNF can have both apoptotic and antiapoptotic consequences and can lead to the simultaneous activation of different pathways. How these pathways interact and potentially regulate each other may have a profound effect on the activity of a particular signaling pathway as well as on the outcome of cell death.
Like NF-κB, JNK and its downstream target, c-Jun, have each been implicated in the control of cell death, although their roles have proven to be complex. In particular, JNK has been shown to either positively or negatively regulate cell death depending on the biological context. For instance, JNK-deficient (JNK1 and JNK2 null) embryonic fibroblasts are blocked in UV-induced apoptosis, while animals that are null for JNK exhibit enhanced forebrain cell apoptosis (17, 33). Also, JNK has been shown to be involved in the protection of cells against taxol-induced cell death by epidermal growth factor (22). Importantly, c-Jun, the downstream effector of JNK, has also proven to play both pro- and antiapoptotic roles. c-Jun can prevent apoptosis during hepatogenesis, but it is necessary for excitotoxin-induced cell death in neurons (13, 14). Furthermore, depending on the differentiation state of PC12 cells, c-Jun can function in a positive or negative manner towards cell death (20). It is these disparities that led us to ask whether the activation status of NF-κB might determine the pro- or antiapoptotic status of JNK or other members of the stress kinase signaling pathway.
We show, as other groups have, that TNF leads to the rapid and transient activation of JNK in wild-type cells. However, the transient nature of this activation depends on the p65 subunit of NF-κB, since JNK activity in response to TNF is sustained in cells that lack functional NF-κB. In addition, this regulation requires the transcription function of the p65 subunit and appears to involve the inhibition of upstream signals that control JNK activity but not of those that regulate another stress-activated kinase, p38. Therefore, based on the involvement of JNK in cell death, we speculated that the sustained activation of JNK in NF-κB null cells could play an antiapoptotic role, possibly explaining the lack of complete TNF-induced cell death in p65−/− fibroblasts. In accordance, the use of inhibitors to suppress sustained JNK activity in cells that lack functional NF-κB results in enhanced cell death in response to TNF. Therefore, the JNK pathway appears to inhibit cell death when the antiapoptotic role for NF-κB is suppressed. Recently it has been shown that NF-κB negatively modulates JNK activity (15, 30, 32). However, in contrast to our studies, those studies indicate that deregulated JNK activity, resulting from inhibition of NF-κB, provides a proapoptotic signal in response to TNF. Two of these papers (30, 32) indicate that the ability of NF-κB to regulate either XIAP or GADD45β suppresses JNK activity. However, a recent paper indicates that XIAP antiapoptotic function requires JNK1 activation (27). Our data provide new evidence of functional cross-regulation between NF-κB and the JNK pathway at a level upstream of JNK-specific activation and provide a rationale to explain why loss of NF-κB does not fully sensitize cells to the apoptotic potential of TNF.
MATERIALS AND METHODS
Cell culture.
32D myeloid cells were maintained in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS) (Life Technologies Inc., Gaithersburg, Md.), 10% WEHI-conditioned medium as a source of interleukin 3 (IL-3), and 100 μg (each) of penicillin and streptomycin (P/S) (Sigma-Aldrich, St. Louis, Mo.)/ml. 32D cells stably expressing IκBα super-receptor (IκBα-SR) have been previously described (26). 32D-IκBα-SR cells were additionally supplemented with 0.5 mg of Geneticin (Life Technologies, Inc.)/ml. p65+/+ and p65−/− mouse embryo fibroblast cells were maintained in Dulbecco's modified Eagle medium H supplemented with 10% FCS and P/S. 293T cells were maintained in RPMI 1640 supplemented with 10% FCS and P/S. p65−/−-TAM67 cells were additionally supplemented with 1 μg of puromycin/ml.
Cell extract preparation and reagents.
For Western analysis, p65−/− and p65+/+ cells were plated at 1 × 105 to 3 × 105 cells in a six-well dish and incubated at 37°C. The following day, cells were treated with 1 to 25 ng of mouse TNF-α (Roche Molecular Biochemicals, Indianapolis, Ind.)/ml or with 5 ng of IL-1β (Life Technologies, Inc.)/ml for the indicated times. 32D and 32D-IκBα-SR cells were plated at 2 × 105 to 3 × 105 cell/ml in a 10-ml volume and incubated at 37°C for 2 h prior to treatment with 1 to 25 ng of mouse TNF-α/ml for the indicated times. For experiments involving actinomycin D, cells were pretreated with 5 μg of actinomycin D (Sigma-Aldrich)/ml for 5 min prior to the addition of TNF. For experiments involving SP600125, cells were pretreated with 10 to 20 μm SP600125 (4) (Celgene, San Diego, Calif.) 1 h prior to treatment with TNF. All cell protein extracts were obtained by collecting total cells, washing them with 1× phosphate-buffered saline (PBS), resuspending them in four volumes of sample buffer (125 mM Tris [pH 6.8], 20% glycerol, 4% sodium dodecyl sulfate, 1.44 mM β-mercaptoethanol, bromphenol blue), and boiling them for 5 min.
Western analysis.
Cell protein extracts, approximately equivalent to 2 × 105 p65 fibroblast cells or 1 × 106 32D myeloid cells, were separated on a sodium dodecyl sulfate-10% polyacrylamide gel and transferred to nitrocellulose. For all Western experiments, equal protein loading was confirmed by staining the nitrocellulose membrane with Ponceau S. Western analysis with the anti-phospho-JNK, anti-phospho-SEK-1, anti-phospho-ATF-2, and anti-phospho-p38 polyclonal antibodies (Cell Signaling Technologies, Beverly, Mass.) was performed as per the manufacturer's instructions. Western analysis with the anti-phospho-c-Jun polyclonal antibody (Upstate Biotechnology, Lake Placid, N.Y.) was performed as per the manufacturer's instructions. Western analyses with the anti-carboxy-terminal IκBα polyclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) and the anti-carboxy-terminal p65 polyclonal antibody (Rockland, Boyertown, Pa.) were performed identically and have been previously described (26).
Transfections. (i) Luciferase reporter assays.
293T cells (3 × 105) were plated in six-well dishes and incubated at 37°C. The following day, transfections were performed by the calcium phosphate method by diluting 15 ng of Gal4-c-Jun fusion protein, 300 ng of Gal5-luciferase reporter construct, and 150 ng of either pCMV4, pCMV-p65, pCMV-IκBα, pCMV-p52, or pCMV-c-Rel to a total of 2 μg of DNA into 250 μl of HEPES-buffered saline, pH 7.05 (137 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4·7H20, 6 mM dextrose, 20 mM HEPES). To this mix, 25 μl of 1.25 M CaCl2 was quickly added, vortexed for 10 s, and incubated for 5 min at room temperature. Transfection mixes were subsequently added drop-wise to cells and incubated at 37°C for 3 h. Cells were then washed once with 1× PBS and replenished with complete media. Cells were collected 48 h posttransfection for luciferase activity analysis.
(ii) Generation of p65−/−-TAM67 cells.
p65−/− cells were transfected with the TAM67 expression vector or the control vector and subsequently selected with 1 μg of puromycin/ml for 12 days. The presence of TAM67 in these p65−/− resistant clones was verified by Western blot analysis with an anti-c-Jun/AP-1 (Ab-1) antibody (Calbiochem-Novabiochem Corp., San Diego, Calif.). Positive clones expressing similar levels of TAM67 protein were pooled and utilized for subsequent experiments.
(iii) Luciferase reporter assays.
Cell extracts were prepared by washing cells with 1× PBS and resuspending in 100 μl of M-PER Mammalian Protein Extraction Reagent (Pierce, Rockford, Ill.) for 10 min. The cell lysate was collected by centrifugation, and 50 μl was used in a Luciferase reporter assay that has been previously described (26).
Cell death assays. (i) Immunohistochemistry.
The day before TNF treatment, 0.5 × 105 p65−/− cells were plated on coverslips in a six-well dish. Cells were treated with 20 ng of TNF/ml for the indicated times, rinsed with PBS, and placed in fixative (3% paraformaldehyde, 2% sucrose in PBS) for 1 h at room temperature. Fixed cells were washed with PBS and stained for 1 h at 37°C using the cell death detection kit-TMR Red as per the manufacturer's instructions (Roche Molecular Biochemicals). Subsequently, cells were washed twice with PBS, incubated with Hoechst 33342 (Molecular Probes, Eugene, Oreg.) for 2 min, washed with PBS, and mounted for visualization by microscopy.
(ii) Cell death enzyme-linked immunosorbent assay (ELISA).
32D-IκBα-SR cells were plated at 3 × 105 cells/ml and incubated at 37°C for 2 h. Cells were subsequently treated with TNF for the indicated times and analyzed utilizing the cell Death detection ELISAPLUS kit as per the manufacturer's directions (Roche Molecular Biochemicals).
(iii) Annexin V staining.
p65−/− and p65−/−-vector cells were plated at 2 × 105 to 3 × 105 cells in a six-well dish and incubated at 37°C. The following day, cells were treated with 1 or 5 ng of TNF/ml for the indicated times with or without SP600125 and stained utilizing the ApoAlert Annexin V-FITC apoptosis kit as per the manufacturer's instructions (Clontech, Palo Alto, Calif.). Stained cells were analyzed on a FACScalibur (Becton Dickinson, Franklin Lakes, N.J.), and the data were compiled using Summit version 3.0 (Cytomation, Fort Collins, Colo.).
(iv) TAM-67 cell death assay.
p65−/−-TAM67 cells were plated at 2 × 105 to 3 × 105 cells in a six-well dish and incubated at 37°C. The following day, cells were treated with TNF for the indicated times and cell viability was determined utilizing the trypan blue exclusion assay.
RESULTS
Inhibition of NF-κB in a variety of cell lines leads to sustained JNK activation in response to TNF or IL-1β treatment.
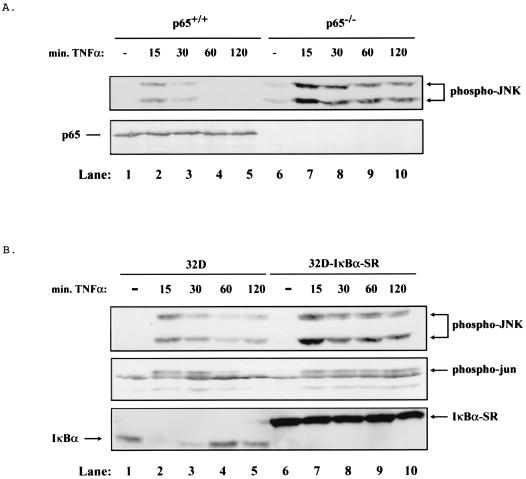
In order to address the potential for cross-regulation between NF-κB and JNK activity, we analyzed JNK activation in response to TNF treatment with p65 wild-type (p65+/+) cells and with p65−/− null fibroblast cells. In wild-type fibroblasts, TNF treatment caused a transient induction of JNK activity as measured by the enhanced phosphorylation of JNK. The activation of JNK peaked 15 min post-TNF treatment, with complete loss of activity within 1 h of cell stimulation (Fig. 1A, lanes 1 to 5, upper panel). In contrast, TNF treatment of p65−/− fibroblasts led to a persistent activation of JNK, with sustained activity extending past the 2-h time point (Fig. 1A, lanes 6 to 10, upper panel). The absence of p65 in p65−/− cells is verified by Western analysis (Fig. 1A, lower panel). Since we have observed that c-Rel is strongly activated in response to TNF treatment in p65−/− cells (data not shown), these data indicate that p65 is the primary mediator of the suppression of JNK activity in response to TNF.
FIG. 1.
In the absence of NF-κB, TNF leads to sustained activation of JNK. (A) TNF leads to sustained activation of JNK in p65−/− cells. Cell extracts were prepared from p65+/+ and p65−/− cells treated with 10 ng of TNF/ml for 0, 15, 30, 60, or 120 min by lysing an equal number of cells in sample buffer. Extracts were analyzed by Western blotting with an anti-phospho-JNK or an anti-carboxy-terminal p65 antibody. Mobilities of phosphorylated JNK and p65 protein are indicated with arrows. (B) TNF leads to sustained activation of JNK in 32D-IκBα-SR cells. Cell extracts from 32D and 32D-IκBα-SR cells treated with 10 ng of TNF/ml for 0, 15, 30, 60, or 120 min were prepared as for panel A. Extracts were analyzed by Western blotting with an anti-phospho-JNK, anti-phospho-Jun, or an anti-carboxy-terminal IκBα antibody. Mobilities of phosphorylated JNK, phosphorylated Jun, IκBα, and IκBα-SR are indicated with arrows.
In order to extend these findings, we analyzed additional cell types that were inhibited for NF-κB activation to determine whether TNF induced persistent JNK activity in these cells as well. In particular, cells expressing a dominant inhibitory form of IκB (IκB super-repressor, IκB-SR), a potent inhibitor of NF-κB complexes, were analyzed. Using 32D and 32D-IκBα-SR cells (26), we measured the phosphorylation status of JNK as well as the phosphorylation status of the JNK substrate, c-Jun. As with wild-type fibroblasts, control 32D myeloid cells showed a transient stimulation of JNK activity peaking within 15 min of TNF treatment (Fig. 1B, lanes 1 to 5, upper and middle panels). However, 32D-IκBα-SR cells exhibited sustained activation of JNK as well as phosphorylated c-Jun up to 2 h following TNF treatment (Fig. 1B, lanes 6 to 10, upper and middle panels). The presence of IκBα-SR in 32D-IκBα-SR cells was verified by Western analysis (Fig. 1B, lower panel). Virtually identical results were obtained with HT1080 fibrosarcoma cells expressing the IκBα-SR (data not shown). In addition, we were interested in determining whether IL-1β, which can activate both the JNK and NF-κB pathways, would also lead to the sustained activation of JNK in the absence of NF-κB. As with TNF, IL-1β leads to a transient phosphorylation of c-Jun in p65+/+ cells and in 32D cells, while this phosphorylation is sustained in both p65−/− cells and 32D-IκBα-SR cells (data not shown). These results demonstrate that inhibition of NF-κB in several cell types leads to the sustained activation of JNK and the inability to down regulate this activity following TNF or IL-1β treatment.
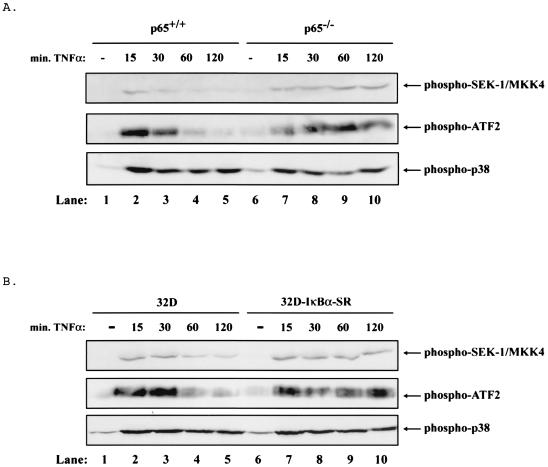
Induction of sustained JNK activity is associated with enhanced SEK-1/MKK4 activation.
The inhibition of JNK activity by NF-κB could be controlled at the level of JNK, possibly through the control of a phosphatase (see Discussion) or through the inhibition of upstream proteins within the JNK signaling pathway. Two different mitogen-activated protein kinases (SEK-1/MKK4 and MKK7) have been implicated as direct, upstream activators of JNK (8, 34). Therefore, we tested whether SEK-1/MKK4 was persistently activated in cells lacking NF-κB. In p65−/− cells and 32D-IκBα-SR cells, TNF treatment led to the sustained phosphorylation of SEK-1/MKK4 (Fig. 2A and B, lanes 6 to 10, top panel) but only transient activation in control cells (Fig. 2A and B, lanes 1 to 5, top panels). Interestingly, another stress-activated kinase, p38, exhibited sustained activity in the presence or absence of NF-κB in response to TNF treatment (Fig. 2A and B, bottom panels). In addition, the transcription factor ATF-2, another substrate for JNK, was persistently phosphorylated in the p65−/− cells and 32D-IκBα-SR cells but only transiently phosphorylated in control cells (Fig. 2A and B, middle panels). These results indicate that one mechanism for sustained JNK activity is through the loss of control of the down regulation of SEK-1/MKK4 activity or of a regulatory factor upstream of SEK-1/MKK4 when NF-κB is inhibited.
FIG. 2.
In the absence of NF-κB, TNF leads to sustained activation of the JNK pathway. (A) In p65−/− cells, TNF leads to sustained activation of several members of the JNK pathway. p38 is not differentially regulated in the p65+/+ and p65−/− cells. Cell extracts were prepared from p65+/+ and p65−/− cells treated with 10 ng of TNF/ml for 0, 15, 30, 60, or 120 min by lysing an equal number of cells in sample buffer. Extracts were analyzed by Western blotting with an anti-phospho-SEK-1/MKK4, anti-phospho-ATF2, or anti-phospho-p38 antibody. Mobilities of phosphorylated SEK-1/MKK4, ATF2, and p38 are indicated with arrows. (B) In 32D-IκBα-SR cells, TNF leads to sustained activation of several members of the JNK pathway but p38 is not differentially affected. Cell extracts were prepared from 32D and 32D-IκBα-SR cells and analyzed as in panel A. Mobilities are indicated with arrows as in panel A.
Transcriptional activity of NF-κB/p65 is required for the inhibition of JNK activity.
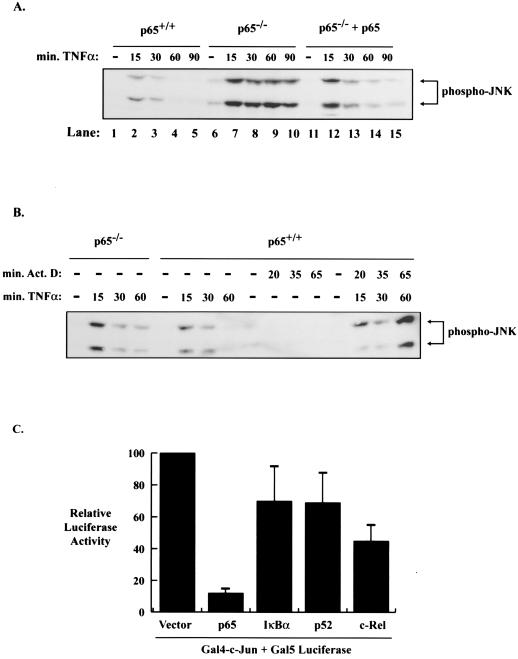
It can be envisioned that NF-κB would regulate JNK activity through the ability of p65 to regulate the transcription of a gene or a set of genes (30, 32). First, to demonstrate that JNK activity is regulated specifically by p65, we reconstituted p65−/− cells with p65 and examined whether transient stimulation of JNK is restored in these cells. Again, p65−/− cells show persistent JNK phosphorylation (Fig. 3A, lanes 6 to 10), whereas p65+/+ cells and p65−/− cells reconstituted with p65 showed only transient activation of JNK (Fig. 3A, lanes 11 to 15). It was then important to determine whether NF-κB transcriptional activity is required for the suppression of JNK activity. The first approach was to examine whether actinomycin D, a general inhibitor of transcription, would modulate the activation of JNK. As seen in Fig. 3B, the transient phosphorylation of JNK that occurs in p65+/+ cells in response to TNF is increased in p65+/+ cells pretreated with the transcription inhibitor actinomycin D. The concentration of actinomycin D used did not, on its own, induce JNK activation. These data suggest that transcriptional activity is required for the down regulation of JNK activity following TNF stimulation. Second, we examined whether expression of p65 was sufficient to block the activation of a Gal4-c-Jun fusion protein known to be regulated by JNK (9, 18). As seen in Fig. 3C, luciferase reporter assays reveal that transient expression of p65 strongly suppressed Gal4-c-Jun activity whereas the p52 and c-Rel subunits of NF-κB and IκBα are significantly less effective at blocking this activity (although c-Rel exhibits more activity in blocking Gal-4-c-Jun than p52). Combined, these results are consistent with the model that p65 is the primary NF-κB regulator that serves to suppress sustained JNK activity following TNF stimulation and that this suppression is controlled through the ability of NF-κB to transcriptionally regulate an unknown target gene.
FIG. 3.
The transcriptional activity of the p65 subunit of NF-κB is responsible for the regulation of the JNK pathway. (A) Cell extracts were prepared from p65+/+, p65−/−, and p65−/− cells reconstituted with p65 treated with 10 ng of TNF/ml for 0, 15, 30, 60, or 90 min by lysing equal numbers of cells in sample buffer. Extracts were analyzed by Western blotting with an anti-phospho-JNK antibody. The mobility of phosphorylated JNK is indicated with an arrow. (B) The down regulation of JNK activity by NF-κB requires transcription. Cell extracts were prepared from p65+/+ cells treated with 10 ng of TNF/ml for 0, 15, 30, or 60 min with or without a 5-min pretreatment with 5 μg of actinomycin D/ml and prepared and analyzed as for panel A. For control purposes, p65+/+ cells were also treated with 5 μg of actinomycin D/ml for 0, 20, 35, or 65 min, and p65−/− cells were treated with 10 ng of TNF/ml for 0, 15, 30, or 60 min. The mobility of phosphorylated JNK is indicated with an arrow. (C) The p65 subunit of NF-κB is sufficient to suppress Jun-dependent Gal4 transcriptional activity. Luciferase reporter assays were performed with cell extracts from 293T cells cotransfected with a luciferase reporter containing five Gal4-DNA binding sites (Gal5 luciferase) and a c-Jun/Gal4 fusion protein (Fal4-c-Jun). In addition, all cotransfections contained a pCMV empty vector, pCMV-p65, pCMV-IκBα, pCMV-p52, or a pCMV-c-Rel expression vector. Transfections were incubated for 48 h. The data shown are representative of three experiments, each performed in triplicate. Relative luciferase activity is indicated ± the standard deviation.
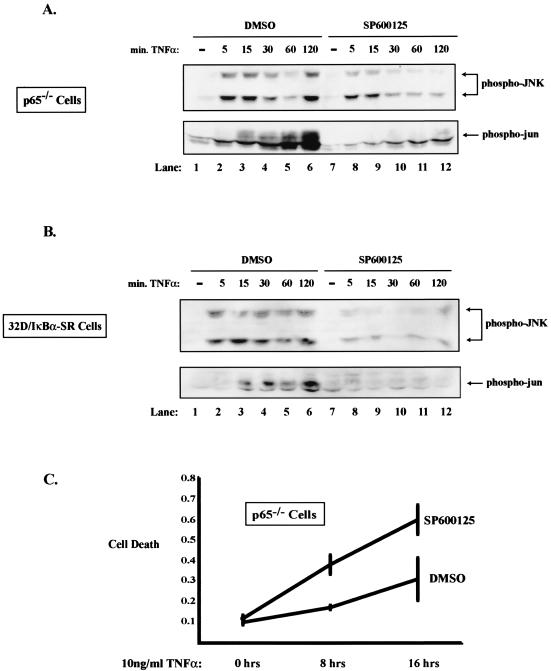
SP600125 inhibits sustained JNK activity and promotes TNF-induced apoptosis.
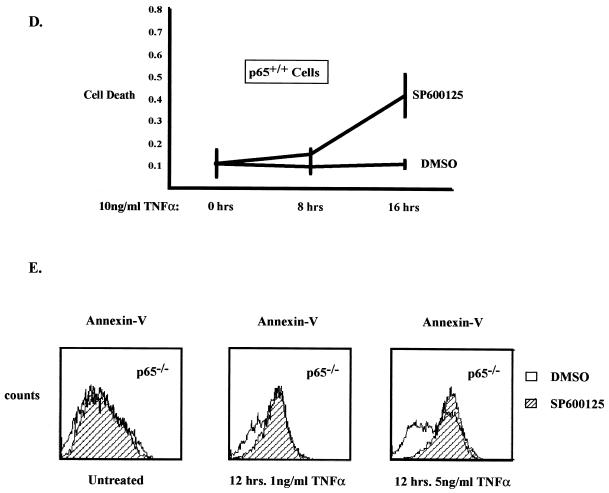
Since we have established that NF-κB plays a role in regulating JNK activity, we were interested in determining what potential biological consequences this regulation may have with respect to cell death in response to TNF stimulation. In order to answer this question, it was necessary to ask what happens when persistent JNK activity is blocked in cells that lack NF-κB. One approach involves a recently described compound (SP600125) that has been shown to block JNK activity (4). We demonstrate, as expected, that SP600125 inhibits sustained JNK activity and sustained phospho-Jun in p65−/− cells (Fig. 4A, lanes 7 to 12) and in 32D-IκBα-SR cells (Fig. 4B, lanes 7 to 12). We then tested what effect the pretreatment of cells with SP600125 had on TNF-induced cell death using a cell death ELISA assay. As shown in Fig. 4C, treatment of p65 null cells with SP600125 caused an approximately twofold increase in cell death at 8 and 16 h post-TNF treatment over that for cells given dimethyl sulfoxide (DMSO) alone. Similar results were obtained using 32D/IκBα-SR cells (data not shown). In order to address whether JNK plays an antiapoptotic role in p65+/+ cells, we performed an experiment similar to that performed for Fig. 4C. Treatment of these wild-type mouse embryo fibroblasts with TNF alone did not induce cell death, as expected (Fig. 4D). However, treatment with TNF and SP600125 led to a significant increase in death at the 16-h time point, suggesting that JNK plays an antiapoptotic role in response to TNF. In addition, we measured cell death with Annexin V staining and flow cytometry analysis. p65−/− cells were pretreated with SP600125 or DMSO and subsequently treated with 1 or 5 ng of TNF/ml. After 12 h, cells were collected and stained with antibodies to Annexin V. Once apoptosis is initiated in a cell, phosphatidylserine is often translocated from the inner to the outer membrane (11, 24). Annexin V specifically stains phosphatidylserine on the outer membrane, and an increase in this staining along the x axis indicates an increase in cell death. As seen in Fig. 4E, Annexin V staining increases (as indicated with a shift to the right), and therefore cell death increases, in cells pretreated with SP600125. It is important to note that 1 ng of TNF/ml is sufficient to cause the sustained phosphorylation of c-Jun in p65−/− cells (data not shown). These data suggest that in the absence of NF-κB, sustained activation of JNK can serve to suppress apoptosis induced by TNF.
FIG. 4.
SP600125 effectively blocks JNK activation and promotes apoptosis in cells that lack functional NF-κB. (A) Sp600125 effectively blocks the sustained activation of JNK in p65−/− cells treated with TNF. Cell extracts were prepared from p65−/− cells pretreated with 20 μM SP600125 or DMSO for 1 h and subsequently treated with 10 ng of TNF/ml for 0, 5, 15, 30, 60, or 120 min by lysing equal numbers of cells in sample buffer. Extracts were analyzed by Western blotting with an anti-phospho-JNK antibody or an anti-phospho-Jun antibody. Mobilities of phosphorylated JNK and phosphorylated Jun are indicated with arrows. (B) Sp600125 effectively blocks the sustained activation of JNK in 32D-IκBα-SR cells treated with TNF. Cell extracts were prepared from 32D-IκBα-SR cells treated and analyzed as in panel A. Mobilities are indicated with an arrow. (C) Inhibition of JNK activity leads to an increase in TNF-induced apoptosis in p65−/− cells. p65 null cells were pretreated with 20 μM SP600125 or with DMSO for 2 h and subsequently treated with 25 ng of TNF for 8 and 16 h. Cellular death was determined by analyzing the cell lysate with a cell death ELISA kit. Relative cell death is indicated on the y axis. (D) Inhibition of JNK in p65+/+ cells augmented cell death. The experiment was identical to that performed in Fig. 4C. (E) Inhibition of JNK activity leads to an increase in TNF-induced apoptosis in p65−/− cells. p65−/− cells were pretreated with 20 μM SP600125 or with DMSO and subsequently treated with 1 or 5 ng of TNF/ml for 12 h. Total cells were collected and incubated with Annexin V-FITC for 15 to 30 min and analyzed by flow cytometry.
Expression of an inhibitor of JNK enhances TNF-induced cell death in NF-κB null cells.
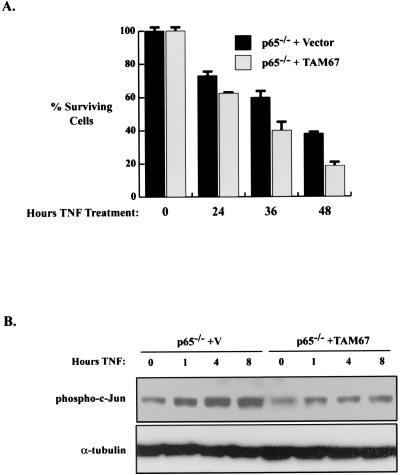
To further address the potential antiapoptotic role for JNK in TNF-stimulated NF-κB null cells, we tested the effects of a second inhibitor of JNK activity. TAM67 is a mutant form of c-Jun that binds JNK but is not able to be phosphorylated or released from the kinase, thus rendering JNK inactive (5, 6). Therefore, p65 null cells were generated that stably express TAM67. Treatment of the p65−/−-TAM67 cells with TNF for 24, 36, or 48 h led to an approximately twofold enhancement in cell death compared to results for p65−/− control cells (Fig. 5A). In order to show that TAM67 suppressed the sustained activation of c-Jun induced by TNF in p65 −/− cells, we assayed for phospho-c-Jun in the vector control p65 null cells and in the p65 null TAM67 cells. The data indicate that TAM67 blocks the sustained activation of c-Jun (Fig. 5B), consistent with its ability to augment apoptosis in p65 null cells in response to TNF treatment.
FIG. 5.
Inhibition of JNK activity leads to an increase in apoptosis cells that lack functional NF-κB. (A) TAM67 enhances TNF-induced apoptosis in p65−/− cells. p65−/− cells expressing vector or TAM67 were treated with 20 ng of TNF/ml for 0, 24, 36, or 48 h. Cell viability was analyzed by the trypan blue exclusion assay. Fold activation is indicated ± the standard deviation. (B) TAM67 blocks sustained c-jun phosphorylation. Whole-cell extracts from p65 null/vector control cells or from p65 null cells stably expressing TAM67 (either untreated or treated) with TNF for the indicated times were probed with the phospho-c-Jun antibody.
DISCUSSION
These data reveal a surprising negative cross talk mechanism between the NF-κB response pathway and the JNK pathway. Our experiments demonstrate that a feedback control mechanism exists downstream of NF-κB, involving a presumed transcriptional target of NF-κB, which functions to inhibit JNK activity. The block appears to be upstream of JNK, since inhibition of NF-κB leads to sustained SEK-1/MKK4 phosphorylation without affecting p38 regulation. Additionally, it appears as if the p65 subunit is the dominant regulator of the suppressive response, since c-Rel is strongly activated in p65−/− cells in response to TNF and because transfection of c-Rel only partially blocked the activity of Gal4-c-Jun. Consistent with our findings, other groups recently published experiments showing that inhibition of NF-κB led to a similar sustained JNK response (15, 30, 32). Furthermore, our data indicate that deregulated JNK activity functions in an antiapoptotic manner to block TNF-induced cell death.
The transcriptional target(s) of NF-κB to suppress JNK is presently unclear. One candidate is A20, which is NF-κB regulated and which inhibits TNF induction of JNK (19). de Smaele et al. (30) indicate that GADD45β induction is regulated by NF-κB and expression of this protein blocked JNK activity. Tang et al. (32) provided evidence that the antiapoptotic protein XIAP blocked the sustained activity of JNK. Others, however, have indicated that XIAP activity requires JNK activity (27), so it is unclear if XIAP is the relevant protein. Thus, it is possible that multiple activities regulated by NF-κB might block TNF signaling to the Jun kinase pathway. We found (data not shown), as did others, that NF-κB does not control the JNK phosphatase, MKP-1 (15), which has the ability to block JNK activity. This result is consistent with our observation that the control of JNK lies upstream of JNK in the signaling cascade.
As discussed previously, the role of JNK in apoptosis is complex. Our data indicate that JNK plays an antiapoptotic role when its activation is sustained in cells lacking NF-κB. We have shown that two inhibitors of JNK or the JNK pathway (namely, SP600125 and TAM67) lead to enhanced apoptosis in response to TNF signaling when NF-κB is inhibited (Fig. 4 and 5). Additionally, SP600125 enhanced apoptosis in wild-type cells treated with TNF, indicating that JNK activity is antiapoptotic downstream of TNF. Expression of JIP, a scaffold protein involved in stress-activated protein kinase signaling that blocks JNK activity when it is overexpressed (10, 39, 42), enhanced cell death in p65−/− cells (data not shown). However, we were unable to maintain these cells for further study. Our results are in contrast with those of other publications (15, 30, 32), which have used dominant-negative inhibitors of the JNK pathway to provide evidence for the pro-apoptotic role of JNK. It is unclear at this time whether the differences in our data are due to the use of different cell types or to the use of different approaches to block JNK. It is worth noting that JNK has been shown to function both pro- and antiapoptotically, depending on the cell type and the differentiation state of the cell (20). It is also intriguing to consider that differences in NF-κB activation levels between different cell types or in response to different inductive stimuli could potentially explain why JNK may function to promote either cell survival or cell death. Additionally, it has been shown that ERK stimulation can inhibit the pro-apoptotic function of JNK (41); however, we did not observe an increase in ERK activity when cells inhibited for NF-κB were treated with TNF (data not shown). These data, taken together with the results obtained utilizing SP600125 and TAM67, indicate that deregulated JNK plays an antiapoptotic role in certain cells that are treated with TNF and that lack NF-κB.
Another interesting point is that NF-κB and AP-1 have been shown to function together in regulating gene expression and promoting transformation. For example, AP-1 and NF-κB activities are required to induce transformation of cells in response to tumor promoters (21). Additionally, NF-κB and AP-1 subunits have been shown to interact, at least in vitro, and to coregulate gene expression (31). Whether the ability of NF-κB to suppress JNK activity and the phosphorylation of c-Jun has any influence on a functional or physical interaction between these transcription factors remains to be determined.
Our data obtained with respect to apoptosis have significant clinical applications. Since NF-κB activation typically serves to protect cells from cancer therapy-induced cell death, it will be interesting to determine if the parallel inhibition of NF-κB in response to chemotherapy treatment leads to JNK activation (2). If sustained JNK activation occurs under these conditions, it will be important to determine whether therapy-induced JNK activity modulates cell death in an antiapoptotic manner and whether inhibition of JNK in conjunction with NF-κB inhibitors and chemotherapy may augment cancer therapy-induced apoptosis.
Acknowledgments
This research was supported by NIH grants AI35098, CA73756, and CA75080 to A.S.B, DE13848 and DE13788 to C.-Y.W., and CA81503 to H.S.E. Support for R.J.D. was derived from the Howard Hughes Medical Institute.
We thank Amer Beg for the p65−/− fibroblasts.
REFERENCES
- 1.Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 281:1305-1308. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin, A. S. 2001. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J. Clin. Investig. 107:241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beg, A. A., and D. Baltimore. 1996. An essential role for NF-kappaB in preventing TNF alpha-induced cell death. Science 274:782-784. [DOI] [PubMed] [Google Scholar]
- 4.Bennett, B. L., D. Sasaki, B. Murray, E. O'Leary, Y. Satoh, W. Xu, A. Motiwala, S. Pierce, J. Leisten, S. Bhagwat, A. Manning, and D. Anderson. 2001. SP600125, an anthrapyrazolone inhibitor of Jun-N-terminal kinase. Proc. Natl. Acad. Sci. USA 98:13681-13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, P. H., R. Alani, L. H. Preis, and M. J. Birrer. 1993. Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene 8:877-886. [PubMed] [Google Scholar]
- 6.Brown, P. H., T. K. Chen, and M. J. Birrer. 1994. Mechanism of action of a dominant-negative mutant of c-Jun. Oncogene 9:791-799. [PubMed] [Google Scholar]
- 7.Chen, C., L. C. Edelstein, and C. Gelinas. 2000. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L). Mol. Cell. Biol. 20:2687-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derijard, B., J. Raingeaud, T. Barrett, I. H. Wu, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 267:682-685. [DOI] [PubMed] [Google Scholar]
- 9.Derijard, B., M. Hibi, I. H. Wu, T. Barrett, B. Su, T. Deng, M. Karin, and R. J. Davis. 1994. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76:1025-1037. [DOI] [PubMed] [Google Scholar]
- 10.Dickens, M., J. S. Rogers, J. Cavanagh, A. Raitano, Z. Xia, J. R. Halpern, M. E. Greenberg, C. L. Sawyers, and R. J. Davis. 1997. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science 277:693-696. [DOI] [PubMed] [Google Scholar]
- 11.Fadok, V. A., D. R. Voelker, P. A. Campbell, J. J. Cohen, G. L. Bratton, and P. M. Henson. 1992. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148:2207-2216. [PubMed] [Google Scholar]
- 12.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 13.Ham, J., A. Eilers, J. Whitfield, S. J. Neame, and B. Shah. 2000. c-Jun and the transcriptional control of neuronal apoptosis. Biochem. Pharmacol. 60:1015-1021. [DOI] [PubMed] [Google Scholar]
- 14.Hilberg, F., A. Aguzzi, N. Howells, and E. F. Wagner. 1993. c-jun is essential for normal mouse development and hepatogenesis. Nature 365:179-181. [DOI] [PubMed] [Google Scholar]
- 15.Javelaud, D., and F. Besançon. 2001. NF-κB activation results in rapid inactivation of JNK in TNF-treated Ewing sarcoma cells: a mechanism for the anti-apoptotic effect of NF-κB. Oncogene 20:4365-4372. [DOI] [PubMed] [Google Scholar]
- 16.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 17.Kuan, C. Y., D. D. Yang, D. R. Samanta Roy, R. J. Davis, P. Rakic, and R. A. Flavell. 1999. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron 22:667-676. [DOI] [PubMed] [Google Scholar]
- 18.Kyriakis, J. M., P. Banerjee, E. Nikolakaki, T. Dai, E. A. Rubie, M. F. Ahmad, J. Avruch, and J. R. Woodgett. 1994. The stress-activated protein kinase subfamily of c-Jun kinases. Nature 369:156-160. [DOI] [PubMed] [Google Scholar]
- 19.Lademann, U., T. Kallunki, and M. Jaattela. 2001. A20 zinc finger protein inhibits TNF-induced apoptosis and stress response early in the signaling cascades and independently of binding to TRAF2 or 14-3-3 proteins. Cell Death Differ. 8:265-272. [DOI] [PubMed] [Google Scholar]
- 20.Leppä, S., M. Eriksson, R. Saffrich, W. Ansorge, and D. Bohmann. 2001. Complex functions of AP-1 transcription factors in differentiation and survival of PC12 Cells. Mol. Cell. Biol. 21:4369-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, J. J., C. Westergaard, P. Ghosh, and N. H. Colburn. 1997. Inhibitors of both nuclear factor-kappaB and activator protein-1 activation block the neoplastic transformation response. Cancer Res. 57:3569-3576. [PubMed] [Google Scholar]
- 22.Liu. B., M. Fang, Y. Lu, Y. Lu, G. B. Mills, and Z. Fan. 2001. Involvement of JNK-mediated pathway in EGF-mediated protection against paclitaxel-induced apoptosis in SiHa human cervical cancer cells. Br. J. Cancer 85:303-311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, Z. G., H. Hsu, D. V. Goeddel, and M. Karin. 1996. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell 87:565-576. [DOI] [PubMed] [Google Scholar]
- 24.Martin, S. J., C. P. Reutelingsperger, A. J. McGahon, J. A. Rader, R. C. van Schie, D. M. LaFace, and D. R. Green. 1995. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 182:1545-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinhard, C., B. Shamoon, V. Shyamala, and L. T. Williams. 1997. Tumor necrosis factor-induced activation of c-jun N-terminal kinase is mediated by TRAF2. EMBO J. 16:1080-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reuther, J. Y., G. W. Reuther, D. Cortez, A. M. Pendergast, and A. S. Baldwin, Jr. 1998. A requirement for NF-κB activation in Bcr-Abl-mediated transformation. Genes Dev. 12:968-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanna, M., J. da Silva Correia, O. Ducrey, J. Lee, K. Nomoto, N. Schranz, Q. Deveraux, and R. Ulevitch. 2002. IAP suppression of apoptosis involves distinct mechanisms: the TAK1/JNK1 signaling cascade and caspase inhibition. Mol. Cell. Biol. 22:1754-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmitz, M. L., and P. A. Baeuerle. 1991. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J. 10:3805-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverman, N., and T. Maniatis. 2001. NF-kappaB signaling pathways in mammalian and insect innate immunity. Genes Dev. 15:2321-2342. [DOI] [PubMed] [Google Scholar]
- 30.de Smaele, E., F. Zazzeroni, S. Papa, D. Nguyen, R. Jin, J. Jones, R. Cong, and G. Franzoso. 2001. Induction of GADD45β by NF-κB downregulates pro-apoptotic JNK signaling. Nature 414:308-313. [DOI] [PubMed] [Google Scholar]
- 31.Stein, B., A. S. Baldwin, Jr., D. W. Ballard, W. C. Greene, P. Angel, and P. Herrlich. 1993. Cross-coupling of the NF-kappa B p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J. 12:3879-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang, G., Y. Minemoto, B. Dibling, N. Purcell, Z. Li, M. Karin, and A. Lin. 2001. Inhibition of JNK activation through NF-κB target genes. Nature 414:313-317. [DOI] [PubMed] [Google Scholar]
- 33.Tournier, C., P. Hess, D. D. Yang, J. Xu, T. K. Turner, A. Nimnual, D. Bar-Sagi, S. N. Jones, R. A. Flavell, and R. J. Davis. 2000. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 288:870-874. [DOI] [PubMed] [Google Scholar]
- 34.Tournier, C., A. J. Whitmarsh, J. Cavanagh, T. Barrett, and R. J. Davis. 1997. Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc. Natl. Acad. Sci. USA 94:7337-7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Antwerp, D. J., S. J. Martin, T. Kafri, D. R. Green, and I. M. Verma. 1996. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science 274:787-789. [DOI] [PubMed] [Google Scholar]
- 36.Wang, C. Y., D. C. Guttridge, M. W. Mayo, and A. S. Baldwin, Jr. 1999. NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol. Cell. Biol. 19:5923-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, C. Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin, Jr. 1998. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680-1683. [DOI] [PubMed] [Google Scholar]
- 38.Wang, C. Y., M. W. Mayo, and A. S. Baldwin, Jr. 1996. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science 274:784-787. [DOI] [PubMed] [Google Scholar]
- 39.Whitmarsh, A. J., C. Y. Kuan, N. J. Kennedy, N. Kelkar, T. F. Haydar, J. P. Mordes, M. Appel, A. A. Rossini, S. N. Jones, R. A. Flavell, P. Rakic, and R. J. Davis. 2001. Requirement of the JIP1 scaffold protein for stress-induced JNK activation. Genes Dev. 15:2421-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu, M., H. Lee, R. Bellas, S. Schauer, M. Arsura, D. Katz, M. Fitzgerald, T. Rothstein, and G. Sonenshein. 1996. Inhibition of NF-kappaB/Rel induces apoptosis of murine B cells. EMBO J. 15:4682-4690. [PMC free article] [PubMed] [Google Scholar]
- 41.Xia, Z., M. Dickens, J. Raingeaud, R. J. Davis, and M. E. Greenberg. 1995. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326-1331. [DOI] [PubMed] [Google Scholar]
- 42.Yasuda, J., A. J. Whitmarsh, J. Cavanagh, M. Sharma, and R. J. Davis. 1999. The JIP group of mitogen-activated protein kinase scaffold proteins. Mol. Cell. Biol. 19:7245-7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zandi, E., D. M. Rothwarf, M. Delhase, M. Hayakawa, and M. Karin. 1997. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell 91:243-252. [DOI] [PubMed] [Google Scholar]
- 44.Zong, W. X., L. C. Edelstein, C. Chen, J. Bash, and C. Gelinas. 1999. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 13:382-387. [DOI] [PMC free article] [PubMed] [Google Scholar]