Abstract
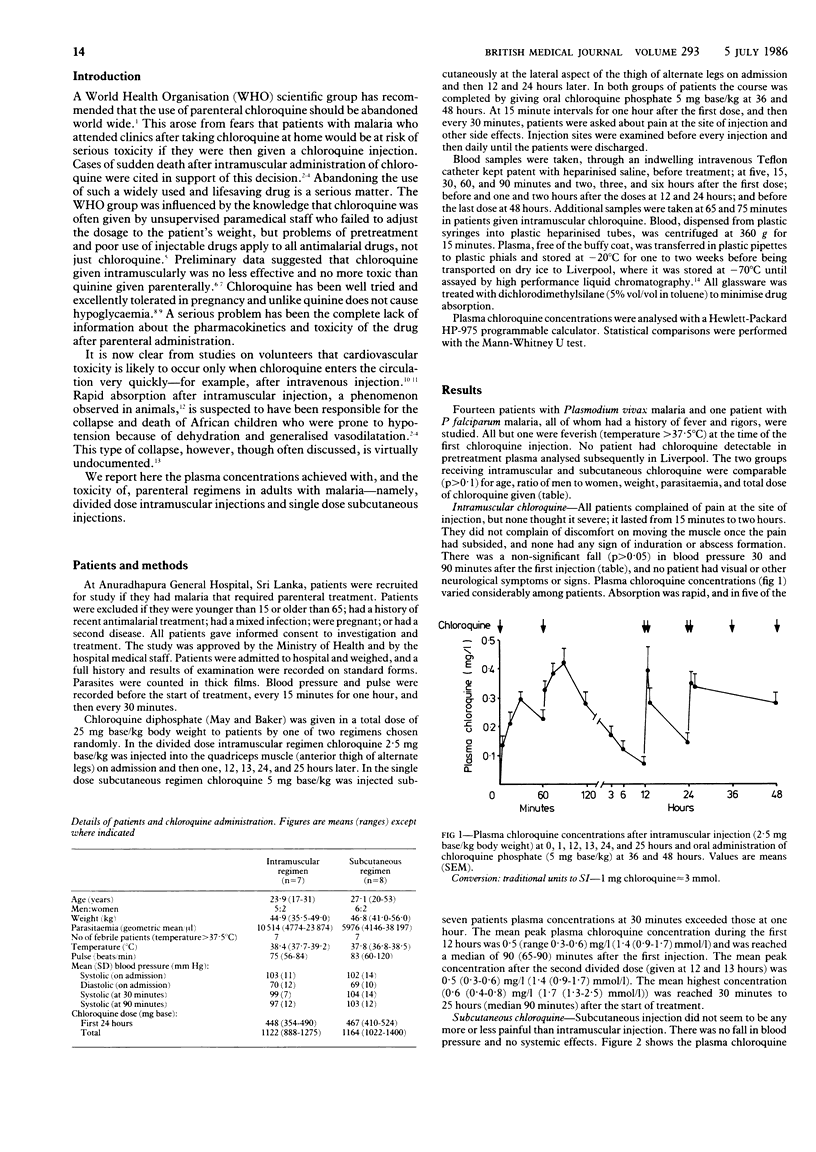
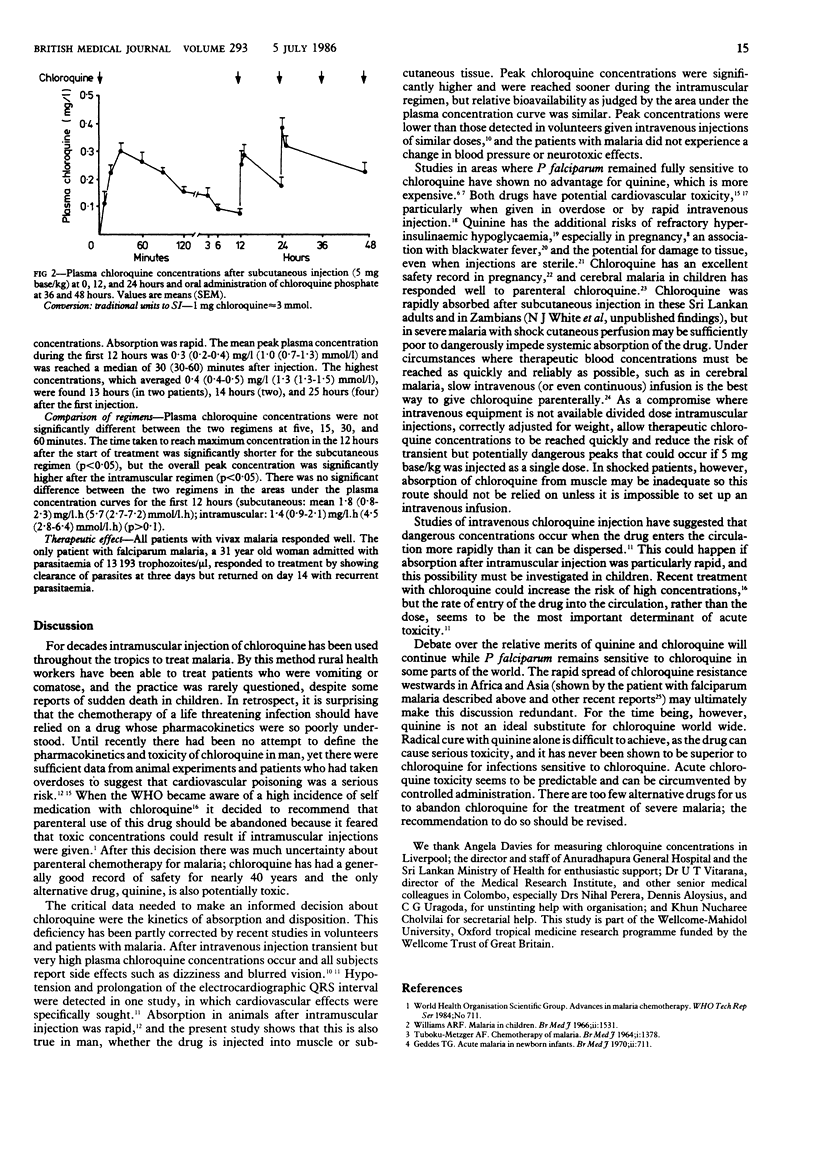
Adults with malaria in Sri Lanka were treated with parenteral chloroquine diphosphate, either 2.5 mg base/kg intramuscularly at 0, 1, 12, 13, 24, and 25 hours or 5 mg base/kg subcutaneously at 0, 12, and 24 hours. Both regimens were completed with oral chloroquine phosphate, 5 mg base/kg, at 36 and 48 hours. Mean peak chloroquine concentrations in the first 12 hours, which were 0.5 (range 0.3-0.6) mg/l (1.4 (0.9-1.7) mu mol/l) [corrected] with the intramuscular regimen and 0.3 (0.2-0.4) mg/l (1.0 (0.7-1.3) mu mol/l) [corrected] with the subcutaneous regimen (p less than 0.05), were reached in median times of 90 (65-90) minutes and 30 (30-60) minutes respectively (p less than 0.05) after the start of treatment. The mean area under the plasma concentration curve for the first 12 hours was 1.4 (0.9-2.1) mg/l.h (4.5 (2.8-6.4) mu mol/l.h) [corrected] after intramuscular administration and 1.8 (0.8-2.3) mg/l.h (5.7 (2.7-7.2) mu mol/l.h) [corrected] after subcutaneous administration (p greater than 0.1). Mean maximum plasma concentrations were higher after intramuscular administration (0.6 (0.4-0.8) mg/l (1.7 (1.3-2.5) mu mol/l)) [corrected] than after subcutaneous administration (0.4 (0.4-0.5) mg/l (1.3 (1.3-1.5) mu mol/l)) [corrected] (p less than 0.05), but both regimens produced satisfactory plasma profiles. Chloroquine resistance was found in the only case of Plasmodium falciparum malaria. Chloroquine is absorbed rapidly after divided dose intramuscular injection and single dose subcutaneous injection and does not cause hypotension or neurotoxicity in adults. Similar regimens should be evaluated in children before the parenteral use of this drug is abandoned.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alván G., Ekman L., Lindström B. Determination of chloroquine and its desethyl metabolite in plasma, red blood cells and urine by liquid chromatography. J Chromatogr. 1982 Apr 16;229(1):241–247. doi: 10.1016/s0378-4347(00)86059-4. [DOI] [PubMed] [Google Scholar]
- Gustafsson L. L., Walker O., Alván G., Beermann B., Estevez F., Gleisner L., Lindström B., Sjöqvist F. Disposition of chloroquine in man after single intravenous and oral doses. Br J Clin Pharmacol. 1983 Apr;15(4):471–479. doi: 10.1111/j.1365-2125.1983.tb01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looareesuwan S., Phillips R. E., White N. J., Kietinun S., Karbwang J., Rackow C., Turner R. C., Warrell D. A. Quinine and severe falciparum malaria in late pregnancy. Lancet. 1985 Jul 6;2(8445):4–8. doi: 10.1016/s0140-6736(85)90056-x. [DOI] [PubMed] [Google Scholar]
- Maguire M. J. The management of cerebral malaria in African children. East Afr Med J. 1983 Apr;60(4):260–265. [PubMed] [Google Scholar]
- Phillips R. E., Looareesuwan S., White N. J., Chanthavanich P., Karbwang J., Supanaranond W., Turner R. C., Warrell D. A. Hypoglycaemia and antimalarial drugs: quinidine and release of insulin. Br Med J (Clin Res Ed) 1986 May 17;292(6531):1319–1321. doi: 10.1136/bmj.292.6531.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R. E., Warrell D. A., Looareesuwan S., Turner R. C., Bloom S. R., Quantrill D., Moore A. R. Effectiveness of SMS 201-995, a synthetic, long-acting somatostatin analogue, in treatment of quinine-induced hyperinsulinaemia. Lancet. 1986 Mar 29;1(8483):713–716. doi: 10.1016/s0140-6736(86)91103-7. [DOI] [PubMed] [Google Scholar]
- ROTHE H. 100 cases of cerebral malaria. East Afr Med J. 1956 Oct;33(10):405–407. [PubMed] [Google Scholar]
- Salako L. A., Adelusi S. A. Plasma, whole blood and red blood cell kinetics of chloroquine after oral and parenteral administration in rabbits. Arch Int Pharmacodyn Ther. 1983 Jan;261(1):4–15. [PubMed] [Google Scholar]
- Trigg P. I., Wernsdorfer W. H., Sheth U. K., Onori E. Intramuscular chloroquine in children. Lancet. 1984 Aug 4;2(8397):288–288. doi: 10.1016/s0140-6736(84)90328-3. [DOI] [PubMed] [Google Scholar]
- Van Poucke G. A comparative study on the use of intramuscular chloroquine and quinine (as Quinimax) in the treatment of uncomplicated falciparum malaria in adults. East Afr Med J. 1979 Apr;56(4):158–162. [PubMed] [Google Scholar]
- White N. J., Chanthavanich P., Krishna S., Bunch C., Silamut K. Quinine disposition kinetics. Br J Clin Pharmacol. 1983 Oct;16(4):399–403. doi: 10.1111/j.1365-2125.1983.tb02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N. J., Looareesuwan S., Warrell D. A. Quinine and quinidine: a comparison of EKG effects during the treatment of malaria. J Cardiovasc Pharmacol. 1983 Mar-Apr;5(2):173–175. doi: 10.1097/00005344-198303000-00001. [DOI] [PubMed] [Google Scholar]
- Wolfe M. S., Cordero J. F. Safety of chloroquine in chemosuppression of malaria during pregnancy. Br Med J (Clin Res Ed) 1985 May 18;290(6480):1466–1467. doi: 10.1136/bmj.290.6480.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]


