Abstract
Background
Exercise programs improve balance, strength and agility in elderly people and thus may prevent falls. However, specific exercise programs that might be widely used in the community and that might be “prescribed” by physicians, especially for patients with osteoporosis, have not been evaluated. We conducted a randomized controlled trial of such a program designed specifically for women with osteoporosis.
Methods
We identified women 65 to 75 years of age in whom osteoporosis had been diagnosed by dual-energy X-ray absorptiometry in our hospital between 1996 and 2000 and who were not engaged in regular weekly programs of moderate or hard exercise. Women who agreed to participate were randomly assigned to participate in a twice-weekly exercise class or to not participate in the class. We measured baseline data and, 20 weeks later, changes in static balance (by dynamic posturography), dynamic balance (by a timed figure-eight run) and knee extension strength (by dynamometry).
Results
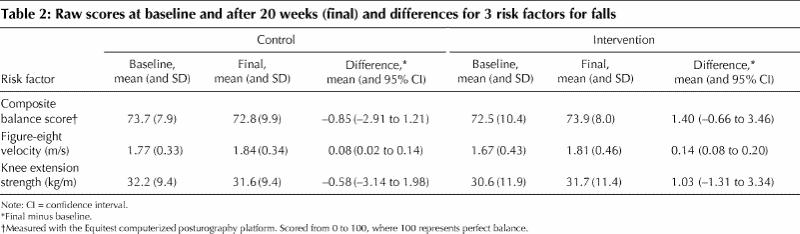
Of 93 women who began the trial, 80 completed it. Before adjustment for covariates, the intervention group tended to have greater, although nonsignificant, improvements in static balance (mean difference 4.8%, 95% confidence interval [CI] –1.3% to 11.0%), dynamic balance (mean difference 3.3%, 95% CI –1.7% to 8.4%) and knee extension strength (mean difference 7.8%, 95% CI –5.4% to 21.0%). Mean crude changes in the static balance score were –0.85 (95% CI –2.91 to 1.21) for the control group and 1.40 (95% CI –0.66 to 3.46) for the intervention group. Mean crude changes in figure-eight velocity (dynamic balance) were 0.08 (95% CI 0.02 to 0.14) m/s for the control group and 0.14 (95% CI 0.08 to 0.20) m/s for the intervention group. For knee extension strength, mean changes were –0.58 (95% CI –3.02 to 1.81) kg/m for the control group and 1.03 (95% CI –1.31 to 3.34) kg/m for the intervention group. After adjustment for age, physical activity and years of estrogen use, the improvement in dynamic balance was 4.9% greater for the intervention group than for the control group (p = 0.044). After adjustment for physical activity, cognitive status and number of fractures ever, the improvement in knee extension strength was 12.8% greater for the intervention group than for the control group (p = 0.047). The intervention group also had a 6.3% greater improvement in static balance after adjustment for rheumatoid arthritis and osteoarthritis, but this difference was not significant (p = 0.06).
Interpretation
Relative to controls, participants in the exercise program experienced improvements in dynamic balance and strength, both important determinants of risk for falls, particularly in older women with osteoporosis.
In people with osteoporosis, exercise may reduce the risk of fracture by its effect on maintenance of bone mass and, probably more important, by improving postural stability and thus decreasing rates of falling.1 Numerous studies have examined the effect of exercise on bone mineral density in women with normal bone mass. Meta-analyses have revealed that either aerobic or resistance training can confer a 1% to 2% advantage relative to control participants, largely by slowing the loss of bone mineral.2,3,4,5,6 Few exercise interventions have been undertaken in women with osteoporosis,7 but even the limited data available make it clear that antiresorptive therapy augments bone mineral more effectively than does exercise alone.8,9
There is, however, increasing evidence that specific exercise interventions can reduce risk factors for falls and actual falls in older people.10,11,12 Further investigation in women with osteoporosis is therefore warranted, as these subjects are at particular risk of fracture if they fall. The response to exercise programs could very well be similar for women with osteoporosis and those with normal bone health, but this assumption needs to be tested. There may be disease-related, physiological, or biomechanical and posture-related differences between women with osteoporosis and the women in whom exercise and risk factors for falls have been studied previously.
In a randomized controlled trial of 10 weeks of physiotherapy in 53 women with vertebral osteoporosis and back pain, Malmros and colleagues13 showed that static balance (measured by computerized posturography) improved significantly in the treatment group. In another randomized clinical trial, physiotherapy-directed exercise in 30 patients with osteoporosis (not defined) significantly improved static balance measured by functional reach and quadriceps strength determined with an isokinetic dynamometer.14 Although both studies showed that exercise programs could improve known risk factor profiles for falls, they were limited by the small number of subjects and their short duration (maximum 12 weeks). Neither study measured both static and dynamic balance, both of which are predictors of falls.10,11,12,13,15 Lastly, both studies employed hospital-based physiotherapists as instructors and thus could not be widely used for patients living in the community.
A large number of tools are available to measure risk factors for falls, such as static and dynamic balance and strength.10 A sophisticated tool for measuring static balance, the Equitest computerized posturography platform (Neurocom International, Clackamas, Ore.), is considered by many the gold standard for measuring sway.16 It is reliable and is designed to distinguish the contributions of the visual, proprioceptive and vestibular systems in maintaining balance,17 but the device measures sway only in the anteroposterior plane, even though most falls occur to the side. In contrast, a measure of dynamic balance, the figure-eight run,18 which has previously been used in older people19,20 is simple to perform and does not require special equipment or training. Quadriceps strength is another independent predictor of both falls21 and fracture risk,10,22 and it can be measured reliably, simply and cheaply with a strain gauge dynamometer.21
The Osteoporosis Program at the BC Women's Hospital and Health Centre developed Osteofit, a community-centre-based exercise program suitable for people with osteoporosis.23 The program aims to improve participants' static and dynamic balance, strengthen key muscle groups and ameliorate quality of life. Since its inception in 1998, over 500 women have participated in the program in over 50 community centres. Similar programs exist in the United States, Australia and Europe, but to our knowledge there have been no reports of the efficacy of any readily accessible community-based exercise programs on risk factors for falls in women with osteoporosis.
We tested the primary hypothesis that a 20-week Osteofit exercise program, provided in a community centre setting with classes of 12 participants per certified instructor, would improve measures of balance and knee extension strength in community-dwelling women aged 65 to 75 years in whom osteoporosis had been diagnosed by dual-energy X-ray absorptiometry. Our secondary hypothesis was that the intervention would also improve quality of life24,25 as measured by an osteoporosis-specific quality-of-life index.26 A planned interim report of the trends observed after 10 weeks of intervention has been published elsewhere.27
Methods
We identified all women aged 65 to 75 who were residents of greater Vancouver and in whom osteoporosis had been diagnosed at the BC Women's Hospital and Health Centre (as bone mineral density T-score at the total hip or spine at least 2.5 standard deviations below the young normal sex-matched bone mineral density of the Lunar reference database28) as potential participants. We excluded women who were less than 5 years after their menopause, weighed more than 130% of ideal body weight, had other contraindications to participating in an exercise program, were already doing moderate or hard exercise for more than 8 hours per week or were planning to be absent from the city for more than 4 weeks during the 20-week trial. The women who met the inclusion criteria were invited by letter to participate in an exercise intervention study.
The participants were randomly assigned (by a computer- generated program) to either the exercise intervention or the control group.
The protocol was approved by the University of British Columbia Clinical Research Ethics Board and the Research Committee of the Children's and Women's Hospital of British Columbia. All subjects gave written informed consent.
Osteofit, a twice-weekly exercise program offered in British Columbia community centres to people with osteoporosis, targets posture, balance, gait, coordination, and hip and trunk stabilization. The emphasis on improving posture and balance may differentiate this program from other exercise programs for seniors, some of which include an aerobic exercise component. The BC Women's Hospital and Health Centre Osteoporosis Program certifies all instructors. The classes for participants in this research study were typical of those in the regular Osteofit program. Participants in our intervention group constituted 4 Osteofit classes in 2 community centres.
The Osteofit exercise program is described in detail elsewhere,23,27 and the program manual is available on request. In brief, the 40-minute main workout consists of 8 to 16 strengthening and stretching exercises to combat medially rotated shoulders, the “chin poke” posture (protrusion of the mandible with extension of the cervical spine), thoracic kyphosis and loss of lumbar lordosis. Thera-band elastic bands (KAS Enterprises, Covington, La.) and small free weights (2 to 5 lbs [1 to 2 kg]) are used for resistance in strength training.
For the purposes of our study, instructors encouraged participants to report any injury or illness (including injuries occurring during the class or arising after a previous class). Any such injuries were to be noted in the instructor's journal and passed on weekly to the Osteofit coordinator (C.W.).
Subjects assigned to the control group were asked to continue their routine daily activities. Both the experimental and control subjects were invited to bimonthly social seminars, primarily intended to encourage the control group to stay involved in the study between measurement sessions (described below). These seminars were given separately to the 2 groups to avoid contamination.
At baseline and at 20 weeks, all participants attended the hospital-based laboratory to complete questionnaires and undergo physical testing. All data were collected by trained researchers blinded to group assignment.
We assessed general health with a subset of questions from the Canadian Multicentre Osteoporosis Study questionnaire29 relating to current medication use (number of medications), associated medical conditions that are risk factors for falls (arthritis, stroke, depression, low blood pressure, Parkinson's disease and epilepsy), lifetime tobacco use (pack years), years of menopausal estrogen therapy, and history of falls and fractures. Cognitive status was documented with the mini-mental state test.30
We evaluated total physical activity (mild, moderate and hard) with the 7-day physical activity recall questionnaire of Blair and colleagues.31 Quality of life was measured with the osteoporosis-specific health-related quality-of-life questionnaire of the European Foundation for Osteoporosis.26,32,33
An experienced neuroscientist-clinician (A.M.) measured static balance using the Equitest computerized posturography platform, a specialized force platform that measures sway34,35 and provides a composite equilibrium score (0 to 100, where 100 represents perfect static balance, i.e., no sway). For each subject, balance was measured twice to overcome any learning effects.36 The test–retest reliability of the composite score for computerized posturography (Pearson correlation coefficient, r) was 0.98.
Dynamic balance was tested by timing participants as they traversed, as quickly as possible, 2 laps of a standard 10-m figure-eight course from a standing start.18,20,37,38 The fastest time of 2 trials was recorded, and the result was converted to velocity (metres per second).
Knee extension strength of the dominant leg was tested with a strap assembly incorporating a strain gauge according to the method of Lord and associates.39 Test–retest reliability in our laboratory for an age-matched population of 8 subjects was 0.92 (Pearson r). For the analysis reported here, adjusted knee extension strength is expressed per unit of leg length to compensate for the length of the lever arm.
After the baseline measurements, all participants were given a fall calendar to be completed and returned to the investigators monthly. A fall was defined in the standard way — inadvertently coming to rest on the ground or another lower level with or without loss of consciousness and other than as the consequence of sudden onset of paralysis, epileptic seizure, excess alcohol intake or overwhelming external force.40
The characteristics of the participants are reported as means with standard deviation or 95% confidence interval (CI). Unpaired t-tests were used to compare baseline characteristics between groups and to assist in the choice of covariates. Analysis of covariance (ANCOVA) was used to examine differences between groups in percent change in various measures after adjustment for confounding factors.
Potential confounding factors were those that are established biological determinants of balance or that were statistically associated with dependent variables in univariate correlations (p < 0.05) and were different between control and intervention groups by paired t-test (p < 0.15). On the basis of these criteria, the following variables were considered potential confounding factors: baseline value (for static balance score, figure-eight velocity or knee extension strength), height, weight, weight change during the study, years of estrogen use, number of current medications, tobacco use (pack years), baseline physical activity, age, mini-mental state score, total quality-of-life score, lifetime number of fractures, falls in the past year, and presence of physician-diagnosed osteoarthritis or rheumatoid arthritis. In a multivariate analysis, the independent variables were added to the model containing the group variable (intervention or control). Variables that produced a significant change in the adjusted R2 for the group variable were used as covariates in the final ANCOVA models. Those variables were as follows: for change in static balance score, rheumatoid arthritis and osteoarthritis; for change in figure-eight velocity (dynamic balance), age, physical activity and years of estrogen use; and for change in knee extension strength, physical activity, cognitive status and lifetime number of fractures. Effect sizes of 0.4 to 0.6 have been reported for exercise interventions measuring similar outcomes in healthy older women.41 Our sample size of 40 per group had 80% power or greater to detect a difference of 20% from baseline with type I (α) error set at p = 0.05.
Results
Of the 456 women identified as potential participants, 36 (8%) could not be contacted. A further 79 (17%) ruled themselves out because of the entry criteria relating to weight (5 [1%]), absence from the city (37 [8%]), medical contraindications to exercise (23 [5%]) and exercising more than 8 hours per week (14 [3%]). Fifty women (11%) were too busy to attend classes, and for 108 (24%) it was not feasible to travel to the classes. Twenty-nine women (6%) said they felt too unwell to exercise, and another 29 (6%) were not interested in an exercise intervention. Seventeen women (4%) declined to participate without giving a reason. The remaining 108 women (24%) volunteered to participate and attended a group information session (Fig. 1). After this session, 8 women declined to participate in the exercise intervention because of time constraints, and 3 declined because they wished to take an exercise class rather than risk being assigned to the control group.
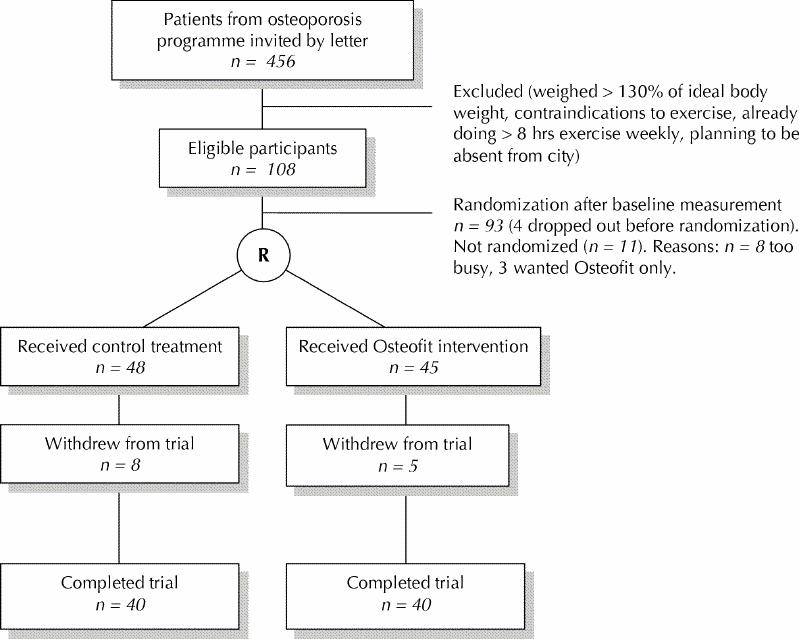
Fig. 1: Flow of subjects through the study. R = randomization.
Of the 97 women who underwent baseline measurements, 2 chose not to participate after completing this step and 2 were excluded because their responses on the baseline questionnaire showed that they exceeded the entry criterion for baseline physical activity. Therefore, 93 women were randomly assigned to either the exercise intervention group (n = 45) or the control group (n = 48). Five women in the intervention group and 8 women in the control group lost interest in the study and declined follow-up measurements. Of the intervention participants, 2 dropped out because they moved away from the area, 2 had language barriers and hence had difficulty participating in the class, and 1 developed shingles. One participant in the control group dropped out because of a fall and fracture, 4 because of loss of interest, 2 because their spouses died and 1 because of a need to care for grandchildren.
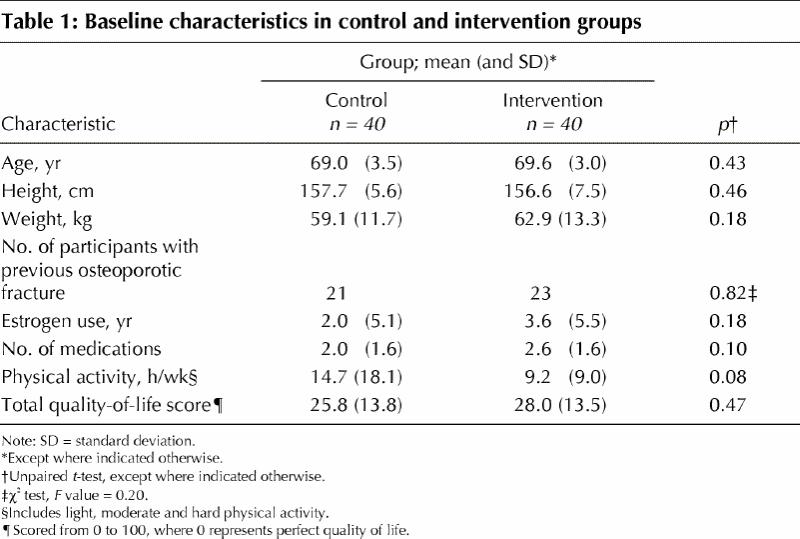
The intervention and control groups did not differ at baseline in terms of age, height or weight (Table 1). The control group reported fewer years of estrogen therapy, fewer current medications and higher physical activity at baseline, although the differences were not significant. Nonetheless, these variables were considered potential covariates in the analyses. Participants who completed the study attended 89% of all possible classes.
Table 1
Before adjustment for covariates, the intervention group had greater (but nonsignificant) improvements in static balance score (mean difference 4.8%, 95% CI –1.3% to 11.0%), dynamic balance (mean difference 3.3%, 95% CI –1.7% to 8.4%) and knee extension strength (mean difference 7.8%, 95% CI –5.4% to 21.0%). Mean unadjusted scores at baseline and 20 weeks, as well as the differences, are presented in Table 2.
Table 2
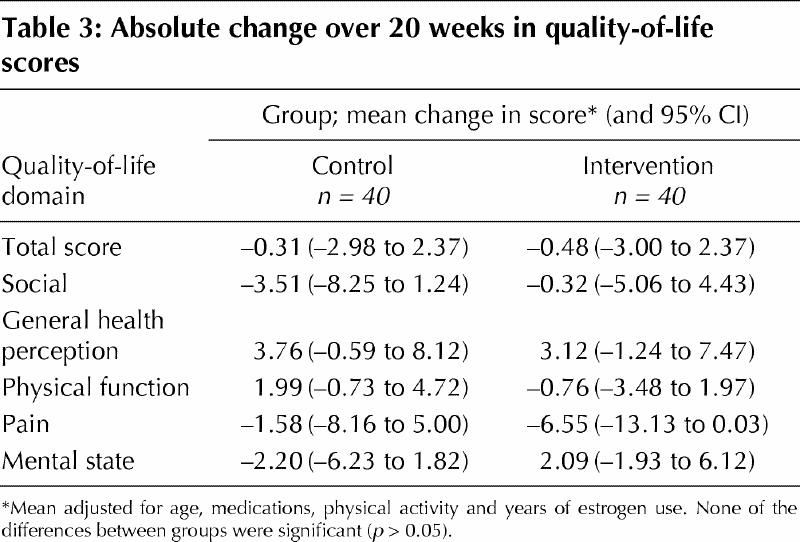
Percent changes in static balance score, dynamic balance and knee extension strength for each group after adjustment for confounding factors are shown in Fig. 2. The intervention group improved significantly more than the control group in terms of dynamic balance (4.9% greater improvement, 95% CI 0.3% to 9.7%; p = 0.044) and knee extension strength (12.8% greater improvement, 95% CI 0.2% to 25.8%; p = 0.047). The intervention group also tended to have greater (although not statistically significant) improvement for static balance (6.3% greater improvement, 95% CI –0.9% to 11.6%; p = 0.06). There were no differences between the groups in total quality-of-life score or subscale scores at baseline or after the 20-week intervention (Table 3).
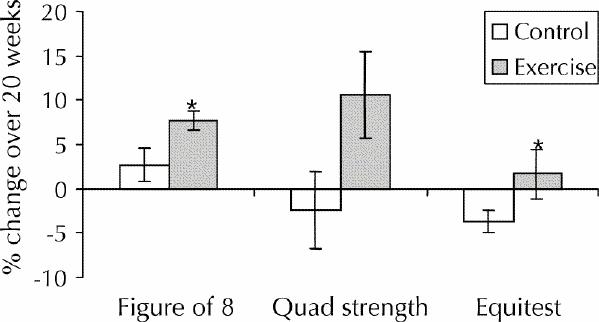
Fig. 2: Percent change over 20 weeks for static balance score, dynamic balance (as figure-eight velocity) and knee extension strength for control and intervention groups. Bars represent standard errors. Asterisks indicate significant differences from the control group (p < 0.05). The mean values have been adjusted for the following covariates: for static balance score, rheumatoid arthritis and osteoarthritis; for figure-eight velocity, age, physical activity and years of estrogen use; and for knee extension strength, activity, cognitive status (as mini-mental state score) and lifetime number of fractures.
Table 3
One participant in the control group sustained a grade I quadriceps muscle strain during baseline measurement, which resolved in 3 days. She was able to complete the follow-up assessment and had no persisting symptoms. No injuries resulting from the Osteofit clases were recorded in the logbooks of instructors or the participants' fall calendars. Participants in the intervention group experienced a total of 7 falls, and those in the control group a total of 8 falls. No participants dropped out because of injury associated with the intervention.
Interpretation
This randomized controlled trial, involving women with proven osteoporosis, demonstrated that a 20-week community-based exercise program provided by certified fitness instructors improved participants' dynamic balance as measured by speed traversing a figure-eight circuit. It also improved knee extension strength, an independent risk factor for falls21 and osteoporotic fracture.22 There was also a clear though nonsignificant trend toward a reduction in sway (a measure of static balance) for women in the intervention group. Thus, the intervention appeared to have no adverse effects and ameliorated at least 2 major risk factors for falls, dynamic balance and knee extension strength. Specific exercises in the Osteofit program that may improve balance include exercises such as walking in circles, single-leg standing with arms held out, tandem walking and standing, and balancing with eyes closed.
We reported preliminary results for these variables after 10 weeks;27 at that stage the trends were evident but not statistically significant. The control group's performance in terms of knee extension strength continued to deteriorate between 10 and 20 weeks, whereas that of the intervention group continued to improve. In a previous study, 10 weeks proved long enough for improvements in neuromuscular facilitation in healthy women of this age,42 but the women in that study gained between 28% and 115% of baseline values. These improvements represent 2 to 9 times the strength gain in our group, which suggests that these healthy women undertook more intense strength training than did the women in the Osteofit classes. The more gradual intervention of the Osteofit program appears to provide benefit beyond 10 weeks, whereas physiological losses were seen in the control group between 10 and 20 weeks. However, further studies with measurements for more subjects at all 3 time points (baseline, 10 weeks and 20 weeks) are needed to address this question directly.
Compared with the control group, the intervention group experienced no improvement in quality of life, perhaps because women who volunteered for the research study had rather high quality of life at baseline. Although more than half of the subjects had established osteoporosis with significant past fractures, the mean quality-of-life score was similar to that of similarly aged osteoporotic women without any prevalent vertebral fractures.33 This suggests that our study population had better function than is typical for women with this degree of osteoporosis. Although this type of ascertainment bias (recruitment of healthier patients into a study) is a well-recognized problem,43 it has the corollary that the effect size for change in balance and strength was probably lower in this population than it would have been in a frailer group. This speculation is supported by data from other studies showing greater changes in strength44 and suggests that a home program targeting frail subjects may be warranted. Home programs of exercise intervention have prevented falls in those over 80 years of age.11,12,45,46,47
Our study extends previous findings of exercise-related improvement in static balance in women with vertebral osteoporosis13 by demonstrating, in a larger cohort of women, that an exercise intervention can improve dynamic balance and knee extension strength. More important, the intervention was administered by certified instructors in the community setting, which suggests that it is feasible to offer the program widely.
Half of the participants had a previous low-trauma fracture, in addition to the diagnosis of osteoporosis by dual-energy X-ray absorptiometry. Such women might usually eschew exercise programs for fear of injury, but this program proved effective in a population particularly at risk of fracture. We also emphasize that the exercise protocol for Osteofit is clearly detailed in the course manual, that classes are delivered by certified instructors and that instructors are periodically evaluated during actual classes as part of the provincial-level quality control component of the program. Similar programs exist in some other Canadian centres, and we believe our findings are generalizable to comparable exercise programs for women of comparable demographic background.
The study had several limitations. As discussed above, exercise intervention studies appeal to more healthy and motivated individuals,43 and our clinical impression was that some of the participants were particularly fit for their age. Especially frail women (in addition to having osteoporosis) may not be able to attend community-based classes, even though they might benefit even more than healthy women from an exercise intervention. Because motivation can contribute to each of our main outcome measures, the Hawthorne effect48,49 should be considered. This effect would reduce the apparent effectiveness of the intervention. Also, we limited the weight range of the population studied to reduce the variance of our key outcome measures, which limits the generalizability of our findings. It would be valuable to undertake an exercise intervention to reduce risk factors for falls in older women with osteoporosis (e.g., 75 years and older), as that population would be at greater risk of falls (and fracture) than the women we studied.
We acknowledge that reducing the risk of falling does not guarantee fewer falls. There is a need for a larger study evaluating the effect of this type of intervention in a high-risk group with sufficient power to detect a treatment effect on falls and, ideally, injurious falls and fractures.
From a public health perspective, Osteofit is an inexpensive program to operate, since participants take the classes in groups of up to 12 people per certified instructor in community centres, not health care facilities. Participants in the Osteofit community program each pay $60 to $100 for a 10-week program of 20 classes, a cost of about $3 to $5 per class. Classes were provided free to participants in our research study, and the total cost of the 20-week intervention for 48 subjects was $4800. Our data add weight to the argument that there may be a benefit for public health authorities to pay for, or at least subsidize, programs such as Osteofit. Participant enjoyment was evidenced by the 89% program adherence.
Exercise should generally be part of the treatment plan in patients with osteoporosis.50,51 We conclude that an exercise program offered in community centres is a simple, inexpensive strategy that physicians can suggest to at least some patients with osteoporosis to ameliorate at least 2 important risk factors for falls. Clearly such programs represent an adjunct to, not a replacement for, pharmacotherapy for bone health, appropriate nutrition and fall prevention strategies in this population.
β See related article page 1005
Acknowledgments
This study was supported by the BC Medical Services Foundation of the Vancouver Foundation (grant 990074) and by the British Columbia Sports Medicine Foundation. In addition, the RBC Foundation supports the Osteofit program. None of these supporting organizations had any input into the conduct or reporting of this research study.
Footnotes
This article has been peer reviewed.
Contributors: Nick Carter and Constance Waterman were responsible for study coordination and data management and contributed to interpreting the data and writing the manuscript. Karim Khan was the principal investigator on the grant that funded the project and, together with Heather McKay, Moira Petit Ari Heinonen, conceived and designed the study. These 6 authors were involved in all aspects of data analysis and manuscript writing. Patti Janssen was responsible for research methodology and statistical analysis and, with Moira Petit, undertook statistical management. Meghan Donaldson, Arthur Mallinson and Lenore Riddell contributed to the study design, undertook data collection and contributed to editing the paper. Karen Kruse, Jerilynn Prior and Leon Flicker contributed to clinical matters related to patient safety in the study design, contributed to the analysis, edited the manuscript and revised it critically for important intellectual content.
Dr. Khan is a Canadian Institutes of Health Research New Investigator (Institute of Musculoskeletal Health and Arthritis). Dr. McKay is a British Columbia Health Research Foundation Scholar. Dr. Heinonen is an International Visiting Research Fellow of the Finnish Ministry of Education.
The data from this study were presented in abstract form at the 23rd annual meeting of the American Bone and Mineral Society, Oct. 12-16, 2001, Phoenix, Ariz.
Competing interests: None declared.
Correspondence to: Dr. Karim Khan, UBC Bone Health Research Group, University of British Columbia, Room 175, James Mather Building, 5804 Fairview Ave., Vancouver BC V6T 1Z3; fax 604 822-6950; kkhan@interchange.ubc.ca
References
- 1.Close JCT, Glucksman E. Falls in the elderly: What can be done? Med J Aust 2000; 173:176-7. [DOI] [PubMed]
- 2.Wolff I, van Croonenborg JJ, Kemper HCG, Kostense PJ, Twisk JWR. The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int 1999; 9:1-12. [DOI] [PubMed]
- 3.Kelley G. Aerobic exercise and lumbar spine bone mineral density in postmenopausal women: a meta-analysis. J Am Geriatr Soc 1998;46(2):143-52. [DOI] [PubMed]
- 4.Kelley GA, Kelley KS, Tran ZV. Resistance training and bone mineral density in women: a meta-analysis of controlled trials. Am J Phys Med Rehabil 2001; 80(1):65-77. [DOI] [PubMed]
- 5.Kelley GA. Aerobic exercise and bone density at the hip in postmenopausal women: a meta-analysis. Prev Med 1998;27(6):798-807. [DOI] [PubMed]
- 6.Kelley GA. Exercise and regional bone mineral density in postmenopausal women: a meta-analytic review of randomized trials. Am J Phys Med Rehabil 1998; 77(1):76-87. [PubMed]
- 7.Prince RL, Smith M, Dick IM, Price RI, Webb PG, Henderson NK, et al. Prevention of postmenopausal osteoporosis: a comparative study of exercise, calcium supplementation and hormone-replacement therapy. N Engl J Med 1991; 325:1189-95. [DOI] [PubMed]
- 8.Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab 2000;85(11):4118-24. [DOI] [PubMed]
- 9.Wallace BA, Cumming RG. Systematic review of randomized trials of the effect of exercise on bone mass in pre- and postmenopausal women. Calcif Tissue Int 2000;67(1):10-8. [DOI] [PubMed]
- 10.Lord SR, Sherrington C, Menz HB. Falls in older people: risk factors and strategies for prevention. New York: Cambridge University Press; 2001.
- 11.Robertson MC, Devlin N, Gardner MM, Campbell AJ. Effectiveness and economic evaluation of a nurse delivered home exercise programme to prevent falls. 1: Randomised controlled trial. BMJ 2001;322(7288):697-701. [DOI] [PMC free article] [PubMed]
- 12.Robertson MC, Gardner MM, Devlin N, McGee R, Campbell AJ. Effectiveness and economic evaluation of a nurse delivered home exercise programme to prevent falls. 2: Controlled trial in multiple centres. BMJ 2001; 322 (7288): 701-4. [DOI] [PMC free article] [PubMed]
- 13.Malmros B, Mortenson L, Jensen MB, Charles P. Positive effects of physiotherapy on chronic pain and performance in osteoporosis. Osteoporos Int 1998; 8: 215-21. [DOI] [PubMed]
- 14.Mitchell SL, Grant S, Atchison T. Physiological effects of exercise on post-menopausal osteoporotic women. Physiotherapy 1988;84:157-63.
- 15.Lord SR, Ward JA, Williams P, Anstey KJ. Physiological factors associated with falls in older community-dwelling women. JAm Geriatr Soc 1994; 42: 1110-7. [DOI] [PubMed]
- 16.Nashner LM. Computerized dynamic posturography. In: Jacobson GP, Newman CW, Kartush JM, editors. Handbook of balance function testing. St Louis: Mosby-Year Book; 1993. p. 280-307.
- 17.Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol A Biol Sci Med Sci 1995; 50 (Spec No.): 64-7. [DOI] [PubMed]
- 18.Tegner Y, Lysholm J, Gillquist J. A performance test to monitor rehabilitation and evaluate anterior cruciate ligament injuries. Am J Sports Med 1986; 14: 156-9. [DOI] [PubMed]
- 19.Heinonen A, Oja P, Sievänen H, Pasanen M, Vuori I. Effect of two training regimens on bone mineral density in healthy perimenopausal women: a randomized controlled trial. J Bone Miner Res 1998;13:483-90. [DOI] [PubMed]
- 20.Uusi-Rasi K, Sievanen H, Vuori I, Heinonen A, Kannus P, Pasanen M, et al. Long-term recreational gymnastics, estrogen use, and selected risk factors for osteoporotic fractures. J Bone MinerRes 1999;14(7):1231-8. [DOI] [PubMed]
- 21.Lord SR, Clark RD. Simple physiological and clinical tests for accurate prediction of falling in older people. Gerontology 1996;42:199-203. [DOI] [PubMed]
- 22.Nguyen TV, Sambrook PN, Kelly PJ, Jones G, Lord S, Freund J, et al. Prediction of osteoporotic fractures by postural instability and bone density. BMJ 1993; 307:1111-5. [DOI] [PMC free article] [PubMed]
- 23.Osteofit [Web site]. Vancouver: Osteoporosis Program of BC Women's Hospital and Health Centre and OSTOP, Osteoporosis Society of Canada (BC Division); 2000. Available: www.osteofit.org (accessed 2002 Sep 16).
- 24.Gold DT, Shipp KM, Lyles KW. Managing patients with complications of osteoporosis. Endocrinol Metab Clin North Am 1998;27(2):485-96. [DOI] [PubMed]
- 25.Gold DT. The nonskeletal consequences of osteoporotic fractures. Psychologic and social outcomes. Rheum Dis Clin North Am 2001;27(1):255-62. [DOI] [PubMed]
- 26.Lips P, Cooper C, Agnusdei D, Caulin F, Egger P, Johnell O, et al. Quality of life as outcome in the treatment of osteoporosis: the development of a questionnaire for quality of life by the European Foundation for Osteoporosis. Osteoporos Int 1997;7(1):36-8. [DOI] [PubMed]
- 27.Carter ND, Khan KM, Petit MA, Heinonen A, Waterman C, Donaldson MG, et al. Results of a 10 week community based strength and balance training program to reduce fall risk factors: a randomised controlled trial in 65–75 year old women with osteoporosis. Br J Sports Med 2001;35:348-51. [DOI] [PMC free article] [PubMed]
- 28.World Health Organization Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Geneva: The Organization; 1994. [PubMed]
- 29.Krieger N, Tenenhouse A, Joseph L, Mackenzie M, Poliquin S, Brown J. The Canadian Multicentre Osteoporosis Study (CaMOS): background, rationale, methods. Can J Aging 1999;18:376-87.
- 30.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. [DOI] [PubMed]
- 31.Blair SN, Jacobs DR, Powell KE. Relationships between exercise or physical activity and other health behaviours. Public Health Rep 1985;100:173-80. [PMC free article] [PubMed]
- 32.Lips P, Cooper C, Agnusdei D, Caulin F, Egger P, Johnell O, et al. Quality of life in patients with vertebral fractures: validation of the Quality of Life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO). Working Party for Quality of Life of the European Foundation for Osteoporosis. OsteoporosInt 1999;10(2):150-60. [DOI] [PubMed]
- 33.Oleksik A, Lips P, Dawson A, Minshall ME, Shen W, Cooper C, et al. Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J BoneMiner Res 2000;15(7):1384-92. [DOI] [PubMed]
- 34.Whipple R, Wolfson L, Derby C, Singh D, Tobin J. Altered sensory function and balance in older persons. J Gerontol 1993;48(Spec No.):71-6. [DOI] [PubMed]
- 35.Carter N, Khan KM, Waterman C, Mallinson A, Janssen P, Petit M, et al. Quadriceps strength is a significant determinant of static and dynamic balance as well as quality of life in older women with osteoporosis. Gerontology 2002; 48: 360-8. [DOI] [PubMed]
- 36.Camicioli R, Panzer VP, Kaye J. Balance in the healthy elderly. Posturography and clinical assessment. Arch Neurol 1997;54:976-81. [DOI] [PubMed]
- 37.Heinonen A, Kannus P, Sievanen H, Oja P, Pasanen M, Rinne M, et al. Randomised controlled trial of effect of high-impact exercise on selected risk factors for osteoporotic fractures. Lancet 1996;348:1343-7. [DOI] [PubMed]
- 38.Uusi-Rasi K, Sievanen H, Vuori I, Pasanen M, Heinonen A, Oja P. Associations of physical activity and calcium intake with bone mass and size in healthy women at different ages. J Bone Miner Res 1999;13:133-42. [DOI] [PubMed]
- 39.Lord SR, Ward JA, Williams P, Strudwick M. The effect of a 12-month exercise trial on balance, strength, and falls in older women: a randomized controlled study. J Am Geriatr Soc 1995;43:1198-206. [DOI] [PubMed]
- 40.Close J, Ellis M, Hooper R, Glucksman E, Jackson S, Swift C. Prevention of falls in the elderly trial (PROFET): a randomized controlled trial. Lancet 1999; 353:93-7. [DOI] [PubMed]
- 41.Nelson ME, Fiatarone MA, Morganti CM, Trice I, Greenberg RA, Evans WJ. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures: a randomized controlled trial. JAMA 1994;272:1909-14. [DOI] [PubMed]
- 42.Charette SL, McEvoy L, Pyka G, Snow-Harter C, Guido D, Wiswell RA, et al. Muscle hypertrophy response to resistance training in older women. J Appl Physiol 1991;70(5):1912-6. [DOI] [PubMed]
- 43.Pacala JT, Judge JO, Boult C. Factors affecting sample selection in a randomized trial of balance enhancement: the FICSIT Study. J Am Geriatr Soc 1996;44(4):377-82. [DOI] [PubMed]
- 44.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 1990;263:3029-34. [PubMed]
- 45.Campbell AJ, Robertson MC, Gardner MM, Norton RN, Tilyard MW, Buchner DM. Randomised controlled trial of a general practice programme of home based exercise to prevent falls in elderly women. BMJ 1997; 315: 1065-9. [DOI] [PMC free article] [PubMed]
- 46.Campbell AJ, Roberton MC, Gardner MM, Norton RN, Buchner DM. Psychotropic medicine withdrawal and a home-based exercise program to prevent falls: a randomized controlled trial. J Am Geriatr Soc 1999;47:850-3. [DOI] [PubMed]
- 47.Gardner MM, Buchner DM, Robertson MC, Campbell AJ. Practical implementation of an exercise-based falls prevention programme. Age Ageing 2001; 30 (1):77-83. [DOI] [PubMed]
- 48.De Amici D, Klersy C, Ramajoli F, Brustia L, Politi P. Impact of the Hawthorne effect in a longitudinal clinical study: the case of anesthesia. Control Clin Trials 2000;21(2):103-14. [DOI] [PubMed]
- 49.Hart CWM. The Hawthorne experiments. In: White KL, editor. Health services research: an anthology. Washington: Pan American Health Organization; 1992. p. 29-37.
- 50.Kannus P. Preventing osteoporosis, falls, and fractures among elderly people. Promotion of lifelong activity is essential. BMJ 1999;318:205-6. [DOI] [PMC free article] [PubMed]
- 51.Khan K, McKay H, Kannus P, Wark J, Bailey D. Exercise prescription for people with osteoporosis. In: Physical activity and bone health. Champaign (IL): Human Kinetics; 2001. p. 181-98.


