Abstract
Ca2+/calmodulin-dependent protein kinase IV-deficient (CaMKIV−/−) mice have been used to investigate the role of this enzyme in CD4+ T cells. We identify a functional defect in a subpopulation of CD4+ T cells, characterized by a cell surface marker profile usually found on memory phenotype CD4+ T cells. Upon T-cell receptor engagement, the mutant cells produce diminished levels of interleukin-2 (IL-2), IL-4, and gamma interferon protein and mRNA. The defect is secondary to an inability to phosphorylate CREB and to induce CREB-dependent immediate-early genes, including c-jun, fosB, fra2, and junB, which are required for cytokine gene induction. In contrast, stimulated naive CD4+ T cells from CaMKIV−/− mice show normal CREB phosphorylation, induction of immediate-early genes, and cytokine production. Thus, in addition to defining an important signaling role for CaMKIV in a subpopulation of T cells, we identify differential signaling requirements for cytokine production between naive T cells and T cells that express cell surface markers characteristic of the memory phenotype.
Ca2+ is a critical regulator of T-cell activation, the process initiated when the quiescent T cell undergoes T-cell receptor (TCR) engagement and which is characterized by the highly regulated expression of specific genes and concomitant cell cycle progression and proliferation (13). One of the critical Ca2+-dependent pathways activated is required to induce expression of the gene encoding interleukin-2 (IL-2), the major T-cell growth factor. Maximal induction of the IL-2 gene requires the inducible transcription factor AP-1, comprised of jun and fos immediate-early gene (IEG) family members, in combination with the constitutively expressed NFAT (22, 31). One well-characterized Ca2+-dependent event in this process is the dephosphorylation of NFAT by the Ca2+/calmodulin-dependent protein phosphatase calcineurin. This modification allows NFAT to translocate from cytoplasm to nucleus, where it forms a complex with the inducible factors to stimulate transcription (12, 36). It is this pathway which is blocked by the immunosuppressive drugs FK506 and cyclosporine (37). However, additional Ca2+-dependent pathways must exist since, even in the presence of FK506, IEG transcription is Ca2+ dependent (43). At least one critical Ca2+-dependent step is CREB phosphorylation. Barton et al. have demonstrated that, in resting T cells, CREB is in an unphosphorylated, transcriptionally inactive state but becomes rapidly phosphorylated after stimulation of the TCR and that this step is required for IEG and IL-2 induction during T-cell activation (6).
The identity of the signal transduction pathway responsible for CREB phosphorylation in T cells remains unclear. Of the many kinases able to phosphorylate CREB, Ca2+/calmodulin-dependent protein kinase IV (CaMKIV), a member of the Ca2+/calmodulin-dependent protein kinase family, is an especially good candidate (for reviews, see references 4 and 29). CaMKIV is highly expressed in only a few cell types, including brain, testes, and T cells, where it has been localized to the nucleus in a tight 1:1 stoichiometric association with protein phosphatase 2A (18, 44). Initially shown to be a CREB kinase, CaMKIV has been implicated in mediating the Ca2+-dependent transcriptional activation of IEGs of the fos and jun families and neurotrophin genes such as brain-derived neurotrophic factor (7, 15, 21, 27, 34, 38, 39). The kinase also stimulates activity of the transcription factors RORα and MEF2 (8, 23, 28).
In T cells, CaMKIV operates as a component of a CaM kinase cascade, undergoing rapid, transient activation as a consequence of signals that result in a rise in intracellular Ca2+ (5, 11, 20, 32, 42). Transgenic mice overexpressing a catalytically inactive form of CaMKIV in the T-cell lineage have been described. Thymocytes from these mice respond to TCR stimulation with dramatically reduced levels of CREB phosphorylation and IL-2 production, implicating the kinase as an important CREB kinase in T cells (3). TCR stimulation leading to CREB phosphorylation in T-cell-derived cell lines was also shown to require CaMK in combination with extracellular signal-regulated kinase and p38-dependent pathways (46). However, it has also been reported that TCR engagement leads to CREB phosphorylation through a single Ca2+-dependent pathway involving protein kinase C, Ras, and RSK2 (30).
In the present study, the role of CaMKIV in mediating CREB-dependent cytokine production during T-cell activation has been investigated by using a CaMKIV-deficient mouse previously generated by targeted gene disruption in murine embryonic stem cells (45). Focus has been limited in this study to the CD4+ helper T cell, since it is with this population that cytokine gene induction has been most thoroughly studied. We report that although stimulated naive CD4+ T cells from CaMKIV−/− mice are able to induce IL-2 and undergo normal cell proliferation, a CD4+ T-cell subset that expresses cell surface markers frequently associated with the memory phenotype is defective at IL-2 production. Closer examination shows that these memory phenotype cells from CaMKIV−/− mice also produce diminished levels of IL-4 and gamma interferon (IFN-γ) when stimulated and that these defects appear to be secondary to an inability to phosphorylate CREB and to induce the IEG required for cytokine gene induction in response to TCR stimulation.
MATERIALS AND METHODS
Mice.
The CaMKIV−/− mice used in this study were maintained on a C57BL/cj129 mixed genetic background. All animals were 5 to 10 weeks old.
T-cell isolation and stimulation.
Enrichment of CD4+ single positive T cells from the spleen was achieved by negative selection with mouse CD8 and B220 magnetic beads (Dynal, Lake Success, N.Y.). The purity of the resulting populations, as assessed by fluorescence-activated cell-sorting (FACS) analysis, was >90%. Initially, separation of CD4+ T cells into naive and memory T-cell populations was achieved by triple staining with antibodies against the NK1.1, l-selectin, and CD44 surface markers, followed by sorting with a Becton-Dickinson FACStar into NK1.1negative l-selectinhigh CD44low (naive T cells) and NK1.1negative l-selectinlow CD44high (defined here as memory phenotype T cells) populations (9, 10). It was subsequently discovered that the sorted cells behaved similarly whether NK1.1 was selected against or not. For this reason some separations were made based only on l-selectin and CD44 surface marker expression. Similar experimental results were obtained when naive and memory phenotype T-cell separation was achieved by staining with antibodies against the CD45RB and CD44 surface markers, followed by sorting into CD45RBhigh CD44low (naive) and CD45RBlow CD44high (memory) groups (24). The sorted cells were cultured in RPMI 1640 growth medium supplemented with 10% heat-inactivated fetal bovine serum (Gibco-BRL lot 1019504), 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 50 μM 2-mercaptoethanol. The cells were stimulated at a density of 0.25 × 106/ml for the indicated times in 96-well plates that had been coated with 100 μl of CD3 (clone 145-2C11; Pharmingen) and CD28 (clone 37.51; Pharmingen) antibody, each at 5 μg/ml. To promote Th1 or Th2 differentiation of naive CD4+ T cells, cells were primed for 4 days in standard growth medium supplemented with recombinant IL-12 (2 ng/ml) plus IL-4 neutralizing antibody (10 μg/ml) or with recombinant IL-4 (26, 41) (50 ng/ml), respectively. Restimulation consisted of pelleting, washing, counting by trypan blue exclusion, and reculturing the cells with plate-bound CD3 and CD28 antibodies for one additional day.
T-cell proliferation and survival assays.
T-cell proliferation was indirectly determined by measuring [3H]thymidine incorporation. CD4+ T cells, cultured in 96-well plates (0.25 × 106 cells/ml), received 1 μCi of [3H]thymidine/well during the last 6 h of either a 45- or a 72-h incubation. Incorporated radioactivity was determined by liquid scintillation counting. Cell survival was determined by incubating an aliquot of cells in 10% trypan blue dye for 5 min at room temperature. Living cells, which exclude dye, were counted on a hemocytometer.
Secreted cytokine determination.
Growth medium was assayed for secreted cytokines according to the PharMingen cytokine sandwich enzyme-linked immunosorbent assay protocol by using anti-mouse monoclonal capture and biotin-conjugated detection antibody pairs from PharMingen. Recombinant IL-2, IL-4, and IFN-γ proteins from PharMingen were used to generate standard curves.
Competitive RT-PCR.
Total RNA was isolated from 1 × 105 to 2 × 105 naive or memory CD4+ T cells with Ultraspec RNA reagent (Biotecx). First-strand cDNA was then generated by using the ProSTAR first-strand reverse transcription-PCR (RT-PCR) kit (Stratagene) with Moloney murine leukemia virus reverse transcriptase and random primers. Competitive RT-PCR has been described in detail elsewhere (33). Briefly, RT reaction mixtures were standardized for total cDNA levels by assaying aliquots for hypoxanthine phosphoribosyltransferase (HPRT) cDNA by PCR in the presence of a series of HPRT competitor construct dilutions. After the cDNA quantities were adjusted to equivalent levels, cytokine transcript levels were semiquantitated by PCR with cytokine-specific primer pairs and competitor cDNA. The IL-2 primers, 5′-CTGAAACTCCCCAGGATGCTC-3′ and 3′-CTGTCAAAGTATCATCTCAACAAGCC-5, amplified a 300-bp product; the IL-4 primers, 5′-ATCGGCATTTTGAACGAGGTC-3′ and 3′-GAATGAGTCCAAGTCCACATCACTG-5′, amplified a 272-bp product; the IFN-γ primers, 5′-AAAGGAGTCGCTGCTGATTCGG-3′ and 3′-GAAAACTGTGACTACACCCGATGAC-3′, amplified a 571-bp product; and the HPRT primers, 5′-GTTGGATACAGGCCAGACTTTGTTG-3′ and 3′-CTCCCATCCGACCGGATATCCGA-5′, amplified a 350-bp product. To create competitor constructs for HPRT, IL-2, IL-4, and IFN-γ, the wild-type PCR products were subcloned into pCR2 (Invitrogen) and digested with a unique-site restriction endonuclease within the murine cDNA. Spacer DNA was generated by parallel digestion of mouse genomic DNA, which was then size fractionated by agarose gel electrophoresis. DNA pieces of convenient size were extracted from the gel and ligated into the digested recombinant plasmids to increase the size of the wild-type genes. The HPRT PCR product was increased in size to ∼1,000 bp by an ∼650-bp HindIII fragment, the IL-2 PCR product was increased to ∼650 bp by an ∼350-bp BglII fragment, the IL-4 PCR product was increased to ∼750 bp by an ∼750-bp EcoRI fragment, and the IFN-γ PCR product was increased to ∼900 bp by an ∼351-bp fragment. The c-fos, Fra2, FosB, and junB transcript levels were determined by RT-PCR by using specific primer pairs but without competitor constructs. The c-fos primers, 5′-TTTCCTACTACCATTCCCCAGCCG-3′ and 3′-CTTGAAGATGAGAAGTCTGCGTTGC-5, amplified a 457-bp PCR product; the FosB primers, 5′-TGAGAGATTTGCCAGGGTCAAC-3′ and 3′-GTGCTCCGTCTCTGGTTTTCTG-5′, amplified a 496-bp product; the c-jun primers, ATGGGCACATCACCACTACACC-3′ and 3′-CCAACCTCAGCAACTTCAACCC-5′, amplified a 279-bp product; and the Fra2 primers, 5′-CAGAGAGAGAGAAAGAGAGCGAGC-3′ and 3′-CATTTTTCTCCAGCCCGTGG-5′, amplified a 452-bp product.
Western blotting.
Protein was electrophoresed through 10% polyacrylamide gels and transferred to Immobilon-P membranes. Anti-CaMKIV (Transduction Labs; monoclonal clone 26) was used at 1/1,000, anti-CREB NT (Upstate Biotechnology; polyclonal) was used at 1/2,000, anti-phospho-CREB (Upstate Biotechnology; polyclonal) was used at 1/1,000, and anti-RSK2 (Santa Cruz; monclonal clone E-1) was used at 1/250. Signal was formed with the use of horseradish peroxidase-labeled secondary antibodies and the ECL Detection System (Amersham).
NFAT translocation.
Naive and memory CD4+ T cells (106 cells/ml) were stimulated in growth medium with concanavalin A (ConA) at 10 μg/ml. Aliquots of 50 μl were spread onto prewarmed poly-l-lysine-coated slides and incubated at 37°C in 5% CO2 for 15 min. Cells were then fixed in formalin for 10 min at room temperature, washed in phosphate-buffered saline, and permeabilized at −20°C in acetone for 2 min. From this point, immunocytochemistry was carried out by using an immunoperoxidase detection system according to the manufacturer’s instructions (Vectastain elite ABC Kit; Vector Laboratories). The anti-NFATc (Santa Cruz; polyclonal sc-1149) was used at a 1/100 dilution.
RESULTS
CaMKIV is highly expressed in thymocytes, where it might be predicted to influence T-cell development (20). Therefore, before examination of CREB-dependent cytokine gene induction in activated T cells, it was first necessary to determine whether the formation of mature T cells occurs in CaMKIV−/− mice. The total number of T cells obtained from mutant thymus, spleen, and inguinal lymph node was normal, as was the CD4/CD8 surface marker distribution in each compartment, indicating that mature T cells are present in the CaMKIV−/− mice and are able to reach and survive in the periphery (data not shown).
An ∼90% pure CD4+ T-cell-enriched population was obtained from spleen by removing B cells and CD8+ T cells by negative selection. The CD4+-enriched T-cell population is comprised of both naive and memory T cells, each capable of producing cytokines in response to stimulation of the TCR. In order to assay the specific subsets, CD4+ cells were sorted into naive and memory phenotype populations based on l-selectin and CD44 surface marker expression (9, 10). It was first necessary, however, to consider the non-CD4+ T-cell contaminates present in the memory phenotype population, which include macrophages, dendritic cells, natural killer (NK) cells, and polymorphonuclear leukocytes. Of these, a subset of NK cells, NK1.1 cells, raises the most serious concern since it expresses the TCR and can respond to TCR stimulation by producing cytokines. Since NK1.1 cells could potentially interfere with interpretation of CD4+ T-cell function, cell sorting was set up to select only those cells negative for the NK1.1 surface marker. When this was carried out, the total number and l-selectin/CD44 staining profiles of wild-type and mutant CD4+ T cells appeared similar to previously reported patterns, with ca. 85 to 90% staining as l-selectinhigh CD44low (naive T cells) and 10 to 15% staining as l-selectinlow CD44high (memory T cells) (25; data not shown). It should be noted that surface marker expression cannot definitively differentiate between activated effector and long-lived memory T cells (25). Therefore, the l-selectinlow CD44high population will be referred to as memory phenotype CD4+ T cells.
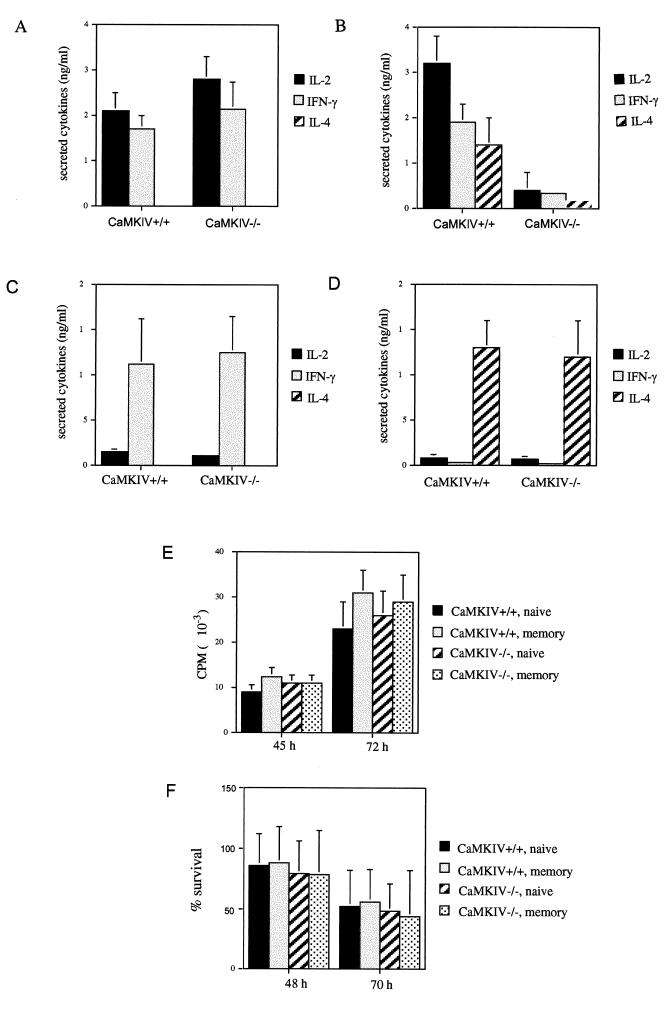
The naive T cells were assayed for IL-2 production after TCR stimulation with CD3 and CD28 antibodies under neutral conditions. The wild-type and mutant cells were found to secrete similar levels of IL-2, indicating that CaMKIV is not required for production of this cytokine (Fig. 1A). However, when memory phenotype T cells were stimulated with CD3 and CD28 antibodies, the CaMKIV−/− cells were found to secrete eightfold less IL-2 than did the wild type (Fig. 1B), indicating a possible functional defect in this T-cell subset.
FIG. 1.
CD4+ memory phenotype T cells from CaMKIV−/− mice exhibit defective cytokine secretion. After 3 days of stimulation with plate-bound CD3 and CD28 antibodies under neutral conditions, levels of IL-2, IFN-γ, and IL-4 from naive (A) and memory phenotype (B) CD4+ T cells from wild-type or CaMKIV−/− mice were determined. Bars represent the mean ± the standard error (SE) (n = 5). To assess helper cell differentiation, naive cells from wild-type and CaMKIV−/− mice were primed for 4 days in the presence of recombinant IL-12 and neutralizing IL-4 antibody, which promote Th1 differentiation (C), or with recombinant IL-4 to promote Th2 differentiation (D). The cells were then pelleted, washed, and restimulated for 1 day, and then the levels of secreted cytokines were determined. Bars represent the mean ± the SE (n = 3). Over the time course in which cytokine secretion was measured, cell proliferation, as assessed by measuring [3H]thymidine incorporation (E), and cell survival, based on trypan blue exclusion (F), were determined. Bars represent the mean ± the SE (n = 3 [E] or n = 7 [F]).
One of the distinguishing characteristics of memory phenotype T cells is their ability, collectively, to produce and secrete a broad range of cytokines. To determine whether the impaired cytokine production of the mutant was specific to IL-2, two other cytokines were examined. IFN-γ and IL-4 secretion from stimulated memory phenotype cells were reduced five- and eightfold, respectively, in cells from CaMKIV−/− mice (Fig. 1B). Next, to determine whether the impaired IFN-γ and IL-4 production was specific to the memory phenotype T-cell subset, naive cells were assayed for these cytokines. Stimulation by CD3 and CD28 antibodies, again under neutral conditions, led to a moderate level of IFN-γ secretion from both mutant and wild-type naive cells, while IL-4 secretion was not detectable from either (Fig. 1A). One distinguishing property of naive T cells is their ability to differentiate into Th1 or Th2 effector cells. Th1 effectors are defined by their tendency to produce large quantities of Th1 cytokines, including IFN-γ, while Th2 effectors selectively produce Th2 cytokines, such as IL-4. When cytokine production was determined after a 4-day priming period under conditions that favor Th1 differentiation, naive cells from wild-type and mutant mice produced similarly high levels of IFN-γ and no detectable IL-4 (Fig. 1C). Priming of naive cells under conditions that promote Th2 differentiation led to the secretion of high levels of IL-4 and low IFN-γ (Fig. 1D). Again, there was no significant difference in cytokine production between wild-type and mutant naive cells. In summary, stimulated naive T cells from CaMKIV−/− mice secrete normal levels of IL-2, IL-4, and IFN-γ, while the memory phenotype cells show impaired production of all three cytokines. Figure 1 also demonstrates that stimulated naive and memory phenotype cells from CaMKIV−/− mice display normal proliferation (Fig. 1E) and cell survival (Fig. 1F) over the time course in which cytokine production was measured. Thus, the diminished cytokine secretion by mutant memory phenotype cells is not due to increased apoptosis or to the failure of the cells to proliferate.
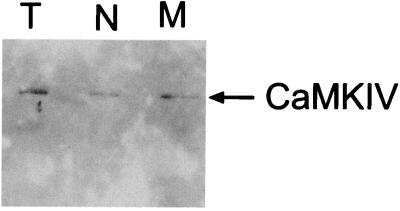
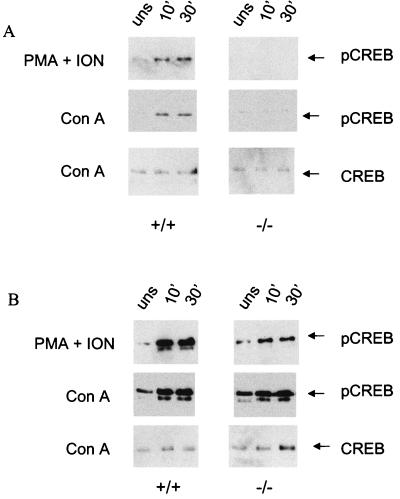
We next sought to determine whether the functional defect exhibited by the memory phenotype T cells was due to an acute signaling problem downstream of the TCR. Although CaMKIV expression in thymocytes and unfractionated CD4+ T cells has been previously reported, the presence of the kinase in memory cells was not examined (18, 20). Figure 2 shows that CaMKIV is expressed in both naive and memory phenotype CD4+ T cells. Based on the properties of CaMKIV, the most likely direct target of the kinase capable of influencing cytokine production is CREB Ser133. CREB phosphorylation in CaMKIV−/− memory phenotype T cells after acute stimulation either with phorbol myristate acetate (PMA) and ionomycin or with ConA was found to be dramatically reduced compared to cells from wild-type animals (Fig. 3). In contrast, the inducible CREB phosphorylation in naive T cells from CaMKIV−/− mice was similar to that observed in cells from wild-type animals (Fig. 3).
FIG. 2.
CaMKIV is expressed in CD4+ naive and memory phenotype T cells. CaMKIV protein expression was detected in thymocytes and in CD4+ naive and memory phenotype T cells by Western blotting of whole-cell lysates made from 0.3 × 106 cells. For each cell type the entire lysate was used in the assay, so that signals from equivalent numbers of cells are being compared. The results of one representative experiment (n = 2) are shown.
FIG. 3.
Stimulated CD4+ memory phenotype T cells from CaMKIV−/− mice display impaired CREB phosphorylation. The inducible CREB phosphorylation in CD4+ memory phenotype (A) and naive (B) T cells from wild-type and CaMKIV−/− mice was determined by Western blotting with antibody against CREB phospho-serine-133 (upper two panels of A and B). Cells were either unstimulated or stimulated with 10 ng of PMA/ml plus 1 μM ionomycin or with 10 μg ConA/ml for 10 and 30 min. Blotting with antibody against total CREB is shown in the lower panels of A and B. The results of one representative experiment (n = 6) are shown.
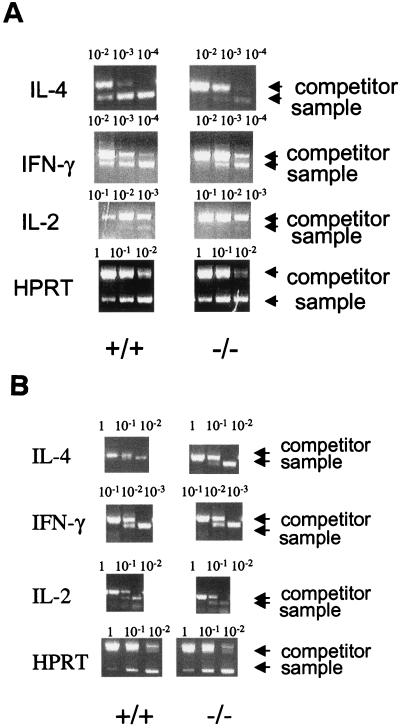
The decreased CREB phosphorylation and cytokine secretion observed in mutant memory phenotype cells suggested that IEG and cytokine gene induction would also be impaired. To determine whether cytokine gene induction was impaired, mRNA levels were indirectly measured by semiquantitative RT-PCR. The IL-4 cDNA from the mutant memory phenotype cells could be competed by 10- to 100-fold less competitor DNA compared to the wild type, indicating 10- to 100-fold less IL-4 mRNA induction in the mutant (Fig. 4A). The IL-2 and IFN-γ mRNA levels in the mutant memory phenotype cells were also reduced, each by ca. 10-fold compared to the wild type. Not shown are signals from unstimulated cells, which were always undetectable. Consistent with their ability to produce normal amounts of cytokines, the mutant naive T-cell mRNA levels were similar to those for the wild type (Fig. 4B).
FIG. 4.
CD4+ memory phenotype T cells from CaMKIV−/− mice display impaired cytokine gene induction. CD4+ memory phenotype (A) and naive (B) T cells from wild-type and CaMKIV−/− mice were stimulated with plate-bound CD3 and CD28 antibodies for 2 days. Total RNA was then isolated, and IL-2, IFN-γ, and IL-4 gene induction was measured by competitive RT-PCR as described in Materials and Methods. The numbers above the lanes indicate the picomoles of competitor construct included in the PCR. For each of the four genes examined, the PCR product arising from the competitor construct (upper arrow) runs higher in the gel compared to that from the endogenous gene (lower arrow). The results of one representative experiment (n = 4) are shown.
A number of IEGs implicated in cytokine gene induction, including c-jun, junB, fosB, and fra2, require CREB phosphorylation for their induction (6, 19, 35). The induction of these genes in mutant memory phenotype cells was examined by RT-PCR. c-jun mRNA was undetectable and junB, FosB, and Fra2 were markedly reduced (Fig. 5).
FIG. 5.
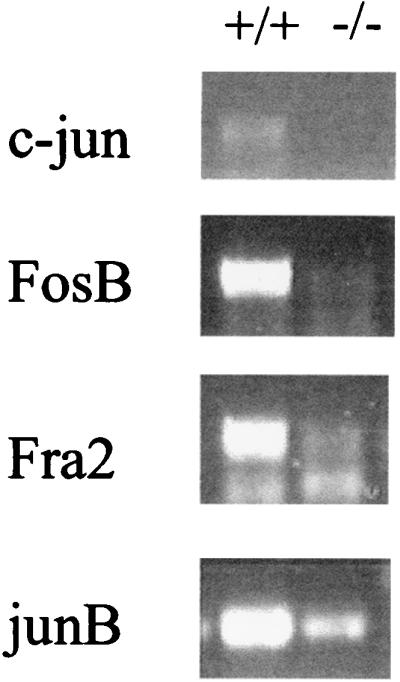
CD4+ memory phenotype T cells from CaMKIV−/− mice display impaired immediate early gene induction. CD4+ memory phenotype T cells from wild-type and CaMKIV−/− mice were stimulated with plate-bound CD3 and CD28 antibodies for 2 h. Total RNA was then isolated, and c-jun, fosB, fra2, and junB gene induction was determined by RT-PCR. The results of one representative experiment (n = 4) are shown.
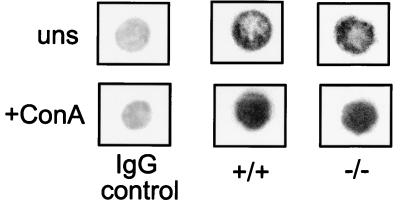
The results described thus far indicate that CaMKIV is required for the Ca2+-dependent CREB phosphorylation that leads to IEG and cytokine gene induction in stimulated memory phenotype cells. To verify that the Ca2+ signal upstream of CaMKIV is functional in the mutant cells, NFAT translocation was examined. NFAT translocation from cytoplasm to nucleus is a major Ca2+-dependent pathway critical in stimulated T cells. Immunostaining demonstrates that the movement of NFAT from the cytoplasm to the nucleus occurs in stimulated memory phenotype cells from mutant mice (Fig. 6).
FIG. 6.
NFAT translocation in CD4+ memory phenotype T cells from CaMKIV−/− mice is normal. CD4+ memory phenotype T cells from wild-type and CaMKIV−/− mice were either unstimulated or stimulated for 15 min with ConA. NFAT subcellular localization was then determined by immunocytochemistry. In the control, anti-mouse immunoglobulin G was substituted for anti-NFAT. Shown are representative cells from a single experiment that was repeated two times. In each experiment, 30 to 40 cells per treatment were scored. Magnification, ×500.
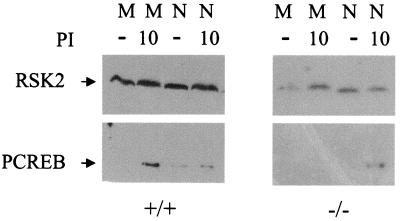
It is curious that memory phenotype, but not naive, T cells from CaMKIV−/− mice show a CREB phosphorylation signaling defect. RSK2 has been reported to phosphorylate CREB in unfractionated T cells and in lymphocyte-derived cell lines but has not been examined in purified naive and memory cells. Perhaps the RSK2 pathway is restricted to the naive T cell, where it regulates CREB phosphorylation, but is absent in the memory cell, leaving CaMKIV as the sole Ca2+-dependent CREB kinase in this T-cell subtype. To test this possibility, the presence and activation of RSK2 were assayed by Western blot in naive and memory phenotype T cells from wild-type and CaMKIV−/− mice. Figure 7 demonstrates that RSK2 is present in both wild-type naive and memory phenotype T cells and that it undergoes the expected shift to a slower-migrating, activated form in response to cell stimulation with PMA and ionomycin (similar results were obtained after stimulation with CD3 and CD28 antibodies [data not shown]). Shown also in Fig. 7 is the expected phosphorylation of CREB in response to cell stimulation. RSK2 is present and activatable in naive and memory phenotype T cells from CaMKIV−/− mice as well, although CREB phosphorylation is not detectable in the stimulated memory phenotype cells (Fig. 7). This later result indicates that, although present, RSK2 activation is not coupled to CREB phosphorylation in the memory phenotype T cell.
FIG. 7.
Expression and activation of RSK2 in CD4+ T cells. Naive (N) and memory phenotype (M) CD4+ T cells from wild-type and CaMKIV−/− mice were probed for RSK2 and for phospho-CREB by Western blot after stimulation with PMA (10 ng/ml) and ionomycin (1 μM) (PI) for 10 min. Activated RSK2 appears as a slower-migrating band.
DISCUSSION
In the absence of CaMKIV, stimulated CD4+ memory phenotype T cells are unable to produce cytokines. The number of cells recovered from the CaMKIV−/− mice and the distribution of surface markers indicative of the memory cell phenotype are normal, indicating that the development and survival of this population have not been compromised. However, when stimulated, the mutant cells fail to phosphorylate CREB and fail to induce the CREB-dependent IEGs required for cytokine gene induction. It thus appears that CaMKIV is required for CREB phosphorylation during memory phenotype CD4+ T-cell activation and, when absent, creates an acute signaling defect responsible for diminished cytokine production.
Stimulated naive T cells from CaMKIV−/− mice, in contrast, do not have an apparent problem producing cytokines. This is curious considering the similarities between the two cell types. Both are antigen-specific cells that express CaMKIV, and both respond to TCR stimulation by phosphorylating CREB, inducing IEG expression, and producing cytokines. Our results raise the question as to why CaMKIV is needed for CREB phosphorylation in one but not the other cell type. One reason may have to do with features that are distinct between naive and memory cells (1, 14). Naive T cells are mature cells that have never been activated. Once activated by the appropriate antigen, in the presence of additional costimulatory and cytokine signals, the cells are driven to differentiate into cytokine-producing effector cells. As antigen is eliminated, the majority of effectors die, but some further differentiate into long-lived memory cells (2). Memory cells differ from naive cells most clearly with respect to function. When activated, memory T cells immediately secrete large quantities of cytokines, without having to go through the process of differentiation. In addition, their requirements for activation, such as antigen dose and costimulation, that leads to proliferation and cytokine production are not as strict. In this respect, it seems logical that signals proximal to the TCR are differentially coupled to downstream events in naive and memory cells in order to bring about the proper response from the two cell types. There is precedence for differential TCR-mediated signaling in naive and memory CD4+ T cells. Farber et al. reported thatmemory T cells, after TCR stimulation, produce different patterns of tyrosine-phosphorylated substrates (17). In addition, several investigators have described significantly less Ca2+ signal generation in memory cells compared to naive cells (40). Our results indicate that the regulation of CREB phosphorylation may be a specific point of divergence between naive and memory cell signaling and raise the question as to how this may occur mechanistically.
In addition to CaMK, mitogen-activated protein kinase (MAPK)-dependent CREB phosphorylation has been shown to occur in activated T cells (30, 46). In one study carried out with unfractionated T cells, the MAPK likely to phosphorylate CREB directly was identified as RSK2 (30). Thus, one way that CREB phosphorylation may be differentially regulated in naive and memory cells is through differential dependence on RSK2 and CaMKIV pathways in the two cell types. We have demonstrated that RSK2 is expressed at the same level in naive and memory phenotype cells and that it can be upshifted to the activated form to similar extents when the cells are stimulated. Therefore, dominance of a CaMKIV pathway in memory phenotype cells cannot be attributed simply to the absence of an RSK2 pathway. There are reports that CaMKIV in some instances can stimulate MAPK pathways through an undefined mechanism (16). However, RSK2 expression and activation are unperturbed in memory phenotype cells from CaMKIV−/− mice, ruling out the possibility that the cells lacking CaMKIV fail to phoshorylate CREB because of a failure to activate RSK2. It is also possible that CaMIV-dependent CREB phosphorylation is important in both naive and memory cells but that naive cells are specifically able to compensate in the absence of the kinase. In this regard, it is relevant to note that thymocytes, which express high levels of CaMKIV, exhibit no obvious developmental or functional defect. They, too, may be able to compensate for the absence of the kinase. The precise mechanism responsible for the differences between naive and memory phenotype cells from CaMKIV−/− mice remains to be determined.
Acknowledgments
We are grateful to Bob Abraham for critically reading the manuscript. We also thank Mike Cook of the Duke Cancer Center Core Flow Cytometry Facility for performing the FACS analysis.
This work was supported by NIH grant HD-07503.
REFERENCES
- 1.Abbas, A. K., K. M. Kenneth, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787–793. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding their relation. Science 272:54–60. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, K. A., T. J. Ribar, M. Illario, and A. R. Means. 1997. Defective survival and activation of thymocytes in transgenic mice expressing a catalytically inactive form of Ca2+/calmodulin-dependent protein kinase IV. Mol. Endocrinol. 11:725–737. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, K. A., and C. D. Kane. 1998. Ca2+/calmodulin-dependent protein kinase IV and calcium signaling. BioMetals 11:331–343. [DOI] [PubMed] [Google Scholar]
- 5.Anderson, K. A., R. L. Means, Q. H. Huang, B. E. Kemp, E. G. Goldstein, M. A. Selbert, A. M. Edelman, R. T. Fremeau, and A. R. Means. 1998. Components of a calmodulin-dependent protein kinase cascade; molecular cloning, functional characterization, and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase B. J. Biol. Chem. 273:31880–31889. [DOI] [PubMed] [Google Scholar]
- 6.Barton, K., M. C. Muthusamy, C. Fischer, C. Clendenin, and J. M. Leiden. 1996. Defective thymocyte proliferation and IL-2 production in transgenic mice expressing a dominant-negative form of CREB. Nature 379:81–85. [DOI] [PubMed] [Google Scholar]
- 7.Bito, H., K. Deisseroth, and R. W. Tsien. 1996. CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell 87:1203–1214. [DOI] [PubMed] [Google Scholar]
- 8.Blaeser, F., N. Ho, R. Prywer, and T. A. Chatila. 2000. Ca2+-dependent gene expression mediated by MEF2 transcription factors. J. Biol. Chem. 275:197–209. [DOI] [PubMed] [Google Scholar]
- 9.Bradley, L. M., G. G. Atkins, and S. L. Swain. 1992. Long-term CD4+ memory T cells from the spleen lack mel-14, the lymph node homing receptor. J. Immunol. 148:324–331. [PubMed] [Google Scholar]
- 10.Budd, R. C., J. C. Cerottini, C. Horvath, C. Bron, T. Pedrazzini, R. C. Howe, and H. R. MacDonald. 1987. Distinction of virgin and memory T lymphocytes: stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J. Immunol. 138:3120–3129. [PubMed] [Google Scholar]
- 11.Chatila, T., K. A. Anderson, N. Ho, and A. R. Means. 1996. A unique phosphorylation-dependent mechanism for the activation of Ca2+/calmodulin-dependent protein kinase type IV/Gr. J. Biol. Chem. 271:21542–21548. [DOI] [PubMed] [Google Scholar]
- 12.Clipstone, N. A., and G. R. Crabtree. 1992. Identification of calcineurin as a key signaling enzyme in T lymphocyte activation. Nature 357:695–697. [DOI] [PubMed] [Google Scholar]
- 13.Crabtree, G. R., and N. Clipstone. 1994. Signal transmission between the plasma membrane and the nucleus of T lymphocytes. Annu. Rev. Biochem. 63:1045–1076. [DOI] [PubMed] [Google Scholar]
- 14.Dutton, R. W., L. M. Bradley, and S. L. Swain. 1998. T cell memory. Annu. Rev. Immunol. 16:201–223. [DOI] [PubMed] [Google Scholar]
- 15.Enslen, H., P. Sun, D. Brickey, S. H. Soderlin, E. Klamo, and T. R. Soderlin. 1994. Characterization of Ca2+/calmodulin-dependent protein kinase IV: role in transcription regulation. J. Biol. Chem. 269:15520–15527. [PubMed] [Google Scholar]
- 16.Enslen, H., H. Tokumitsu, P. J. S. Stork, R. J. Davis, and T. R. Soderling. 1996. Regulation of mitogen-activated protein kinases by a calcium/calmodulin-dependent protein kinase cascade. Proc. Natl. Acad. Sci. USA 93:10803–10808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farber, D. L., O. Acuto, and K. Bottomly. 1997. Differential T cell receptor-mediated signaling in naive and memory CD4 T cells. Eur. J. Immunol. 27:2094–2101. [DOI] [PubMed] [Google Scholar]
- 18.Frangakis, M. V., T. Chatila, E. R. Wood, and N. Sahyoun. 1991. Expression of a neuronal Ca2+/calmodulin-dependent protein kinase, CaM kinase-Gr, in rat thymus. J. Biol. Chem. 266:17592–17596. [PubMed] [Google Scholar]
- 19.Glimcher, L. H., and H. Singh. 1999. Transcription factors in lymphocyte development—T and B cells get together. Cell 96:13–33. [DOI] [PubMed] [Google Scholar]
- 20.Hanissian, S. H., M. Franakis, M. M. Bland, S. Jawahar, and T. A. Chatila. 1993. Expression of a Ca2+/calmodulin dependent protein kinase, CaM kinase-Gr, in human T lymphocytes. Regulation of kinase activity by T cell receptor signaling. J. Biol. Chem. 268:20055–20063. [PubMed] [Google Scholar]
- 21.Ho, N., J. A. Liauw, F. Blaeser, F. Wei, S. Hanissian, L. M. Muglia, D. F. Wozniak, A. Nardi, K. L. Arvin, D. M. Holtzman, D. J. Linden, M. Zhuo, L. J. Muglia, and T. A. Chatila. 2000. Impaired synaptic plasticity and cAMP response element-binding protein activation in Ca2+/calmodulin-dependent protein kinase type IV/Gr-deficient mice. J. Neurosci. 20:6459–6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain, J., P. G. McCaffrey, V. E. Valge-Archer, and A. Rao. 1992. Nuclear factor of activated T cells contains Fos and Jun. Nature 356:801–804. [DOI] [PubMed] [Google Scholar]
- 23.Kane, C. D., and A. R. Means. 2000. Activation of orphan receptor-mediated transcription by Ca2+/calmodulin-dependent protein kinase IV. EMBO J. 19:691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, W. T., X. M. Yin, and E. S. Vitetta. 1990. Functional and ontogenetic analysis of murine CD45Rhi and CD45Rlo CD4+ T cells. J. Immunol. 144:3288–3295. [PubMed] [Google Scholar]
- 25.Lee, W. T., and W. J. Pelletier. 1998. Visualizing memory phenotype development after in vitro stimulation of CD4+ T cells. Cell. Immunol. 188:1–11. [DOI] [PubMed] [Google Scholar]
- 26.Le Gros, G., S. Z. Ben-Sasson, R. Seder, F. D. Finkelman, and W. E. Paul. 1990. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J. Exp. Med. 172:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews, R. P., C. R. Guthrie, L. M. Wailes, X. Zhao, A. R. Means, and G. S. McKnight. 1994. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Mol. Cell. Biol. 14:6107–6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKinsey, T. A., C. L. Zhang, J. Lu, and E. N. Olson. 2000. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Means, A. R., T. J. Ribar, C. D. Kane, S. S. Hook, and K. A. Anderson. 1997. Regulation and properties of the rat Ca2+/calmodulin-dependent protein kinase IV gene and its protein products. Rec. Prog. Horm. Res. 52:389–407. [PubMed] [Google Scholar]
- 30.Muthusamy, N., and J. M. Leiden. 1998. A protein kinase C-, Ras-, and RSK2-dependent signal transduction pathway activate the cAMP-responsive element-binding protein transcription factor following T cell receptor engagement. J. Biol. Chem. 273:22841–22847. [DOI] [PubMed] [Google Scholar]
- 31.Northrop, J. P., S. N. Ho, L. Chen, K. F. Thomas, G. P. Nolan, A. Admon, and G. R. Crabtree. 1994. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature 369:497–502. [DOI] [PubMed] [Google Scholar]
- 32.Park, I. K., and T. J. Soderling. 1996. Activation of Ca2+/calmodulin-dependent protein kinase (CaM-kinase) IV by CaM-kinase kinase in Jurkat T lymphocytes. J. Biol. Chem. 270:30464–30469. [DOI] [PubMed] [Google Scholar]
- 33.Reiner, S. L., S. Zheng, D. B. Corry, and R. M. Locksley. 1993. Constructing polycompetitor cDNAs for quantitative PCR. J. Immunol. Methods 165:37–46. [DOI] [PubMed] [Google Scholar]
- 34.Ribar, T. J., R. M. Rodriguiz, L. Khiroug, W. C. Wetsel, G. J. Augustine, and A. R. Means. 2000. Cerebellar defects in Ca2+/calmodulin kinase IV-deficient mice. J. Neurosci. 20:RC107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rooney, J. W., T. Hoey, and L. H. Glimcher. 1995. Coordinate roles for NF-AT and AP-1 in the regulation of the murine IL-4 gene. Immunity 2:473–483. [DOI] [PubMed] [Google Scholar]
- 36.Ruff, V. A., and K. L. Leach. 1995. Direct demonstration of NFATp dephosphorylation and nuclear localization in activated HT-2 cells using a specific NFATp polyclonal antibody. J. Biol. Chem. 270:22602–22607. [DOI] [PubMed] [Google Scholar]
- 37.Shaw, K. T., A. M. Ho, A. Raghavan, J. Kim, J. Jain, J. Park, S. Sharma, A. Rao, and P. G. Hogan. 1995. Immunosuppressive drugs prevent a rapid dephosphorylation of transcription factor NFAT1 in stimulated immune cells. Proc. Natl. Acad. Sci. USA 92:11205–11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheng, M., M. A. Thompson, and M. E. Greenberg. 1991. CREB: a Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinase. Science 272:1427–1430. [DOI] [PubMed] [Google Scholar]
- 39.Shieh, P. B., S. C. Hu, K. Bobb, T. Timmusk, and A. Ghosh. 1998. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron 20:727–740. [DOI] [PubMed] [Google Scholar]
- 40.Sigova, A., E. Dedkova, V. Zinchenko, and I. Litvinov. 1999. A comparative study of the calcium system in memory T cells and naive T cells. FEBS Lett. 447:34–38. [DOI] [PubMed] [Google Scholar]
- 41.Swain, S. L., A. D. Weinberg, M. English, and G. Huston. 1990. IL-4 directs the development of Th2-like helper effectors. J. Immunol. 145:3796–3806. [PubMed] [Google Scholar]
- 42.Tokumitsu, H., H. Enslen, and T. J. Soderling. 1995. Characterization of a Ca2+/calmodulin-dependent protein kinase cascade; molecular cloning and expression of calcium/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 270:19320–19324. [DOI] [PubMed] [Google Scholar]
- 43.Ullman, K. S., J. P. Northrop, A. Admon, and G. R. Crabtree. 1993. Jun family members are controlled by a calcium-regulated, cyclosporin A-sensitive signaling pathway in activated T lymphocytes. Genes Dev. 7:188–196. [DOI] [PubMed] [Google Scholar]
- 44.Westphal, R. S., K. A. Anderson, A. R. Means, and B. E. Wadzinski. 1998. A signaling complex of protein kinase IV and protein phosphatase 2A. Science 280:1259–1261. [DOI] [PubMed] [Google Scholar]
- 45.Wu, J. Y., T. J. Ribar, D. E. Cummings, K. A. Burton, G. S. McKnight, and A. R. Means. 2000. Spermiogenesis and exchange of basic nuclear proteins are impaired in male germ cells lacking Camk4. Nature Gen. 25:448–452. [DOI] [PubMed] [Google Scholar]
- 46.Yu, C. T., H. M. Shih, and M. Z. Lai. 2001. Multiple signals required for cyclic AMP-responsive element binding protein (CREB) binding protein interaction induced by CD3/CD28 costimulation. J. Immunol. 166:284–292. [DOI] [PubMed] [Google Scholar]