Abstract
Cdc7, a conserved serine/threonine protein kinase, controls initiation of DNA replication. A regulatory subunit, Dbf4, stimulates the kinase activity of Cdc7 and recruits it to the replication origins. Schizosaccharomyces pombe has a homologous kinase complex, composed of Hsk1 and Dfp1/Him1. Here, we report a novel protein kinase of S. pombe, Spo4, which shares common structural features with the Cdc7 kinases. In spite of the structural similarities, Spo4 is dispensable for mitotic growth and premeiotic DNA replication. Intriguingly, spo4 null mutants are defective in initiation and progression of the second meiotic division. Spindles for meiosis II are often fragmented. Spo4 kinase activity is markedly enhanced when the enzyme is associated with its regulatory subunit, Spo6, a Dbf4-like protein. Expression of Spo4 is specifically induced during meiosis. Spo4 is preferentially present in nuclei, but this nuclear localization does not require Spo6. These results suggest that Spo4 is a Cdc7 kinase whose primary role is in meiosis, not in DNA replication. This is the first report of an organism which has two Cdc7-related kinase complexes with different biological functions.
The fission yeast Schizosaccharomyces pombe initiates a sporulation program when starved of nutrients, particularly nitrogen sources (14, 16, 71). Ascospore formation is a process of gametogenesis, because it is linked to meiotic nuclear divisions. Yeast meiosis shares a fundamental mechanism with the meiosis in higher eukaryotes. Two meiotic divisions are preceded by a round of premeiotic DNA replication. The first division is characterized by reductional chromosome segregation. Between the first and second meiotic divisions, no DNA replication occurs. The mechanism for skipping the S phase relates to the premature reactivation of maturation-promoting factors after meiosis I in Xenopus oocytes (20, 53), but it has not been established whether the same mechanism also exists in fission yeast. The second meiotic division is similar to mitotic division because the sister chromatids are separated, and thus it is called equational chromosome segregation (9, 15, 16, 71).
In both budding and fission yeasts, meiosis has been analyzed genetically. Many meiosis- and sporulation-deficient S. pombe mutants have been isolated and analyzed (5, 41). The study of genomic or cDNA clones which complement the meiosis-defective mutation has identified several meiotic genes, such as the mei2+ gene encoding a crucial inducer of meiosis and mei4+ encoding a meiosis-specific transcription factor (29, 66, 67). S. pombe mutants deficient in meiotic recombination have also been extensively explored. Some of the rec+ genes are essential for normal progression of meiosis. For example, rec8+ codes for the meiosis-specific cohesin and plays an essential role in sister chromatid cohesion during meiosis I (56, 68). The spo+ genes were originally defined as those that are not required for mitotic proliferation and meiotic division (5, 41). The traits of many spo genes have recently been reexamined by cloning and disruption. We have isolated spo2, spo3, spo4, spo5, spo6, spo13, spo14, spo15, spo18, spo19, and spo20 (31, 51; M. Nakamura-Kubo, T. Nakamura, and C. Shimoda, unpublished data). At least two spo genes (spo14+ and spo20+) were shown to be necessary not only for sporulation but also for vegetative growth (52; Nakamura-Kubo et al., unpublished). spo20+ encodes a structural and functional homologue of budding yeast Sec14 (52). The Saccharomyces cerevisiae Sec14 is a phosphatidylinositol transfer protein and is crucial to post-Golgi vesicle traffic (39). Furthermore, some spo mutants fail to undergo sporulation as a consequence of a defect in meiosis. The spo6+ gene regulates the progression of meiosis II, because most spo6 mutant cells fail to complete meiosis II (50). Interestingly, the predicted Spo6 protein has high similarity to budding yeast Dbf4, which is a regulatory subunit of Cdc7 serine/threonine kinase (32, 36, 42). Thus, it is likely that an S. pombe Cdc7 homologue is involved in meiosis and sporulation.
The S. cerevisiae Cdc7 kinase is composed of Cdc7 (a catalytic subunit) and Dbf4 (a regulatory subunit). A Cdc7-Dbf4 kinase complex controls initiation of DNA replication (4, 12, 13, 27, 28, 35, 43, 57, 62, 63). Like Cdks, Cdc7 kinase activity is regulated by association with Dbf4 (32, 42). The abundance of Dbf4 periodically fluctuates, peaking at S phase (10, 11, 17, 55), while Cdc7 levels remain constant during the cell cycle (61). The Cdc7-Dbf4 kinase complex is evolutionarily conserved among eukaryotic organisms (34, 35, 37, 40, 43, 46, 59). The fission yeast S. pombe has a homologous kinase complex, composed of Hsk1 and Dfp1 (also known as Him1), which is also essential for the onset of DNA replication (6, 7, 45, 63).
Spo6 and Dfp1 are encoded by different genes, indicating that S. pombe has two Dbf4-like proteins. spo6+ is dispensable for both mitotic and premeiotic DNA synthesis. Spo6 and Hsk1 show no physical interaction. These findings suggest that the partner of Spo6 is not Hsk1 but a novel Cdc7-like kinase (50). In this article, we report a gene termed spo4+, which encodes a second Cdc7-like protein. Spo4 forms an active kinase complex with Spo6. Unlike known Cdc7 kinases of budding yeast and other eukaryotes, spo4+ is essential for neither mitotic nor premeiotic DNA replication. However, spo4+ is required for the initiation and progression of a second meiotic division. In addition, spo4+ activity is regulated at the transcriptional level. We discuss a model in which Spo4 regulates the initiation and progression of meiosis II.
MATERIALS AND METHODS
Yeast strains, media, and culture conditions.
The S. pombe strains used in this study are listed in Table 1. A sporulation-deficient spo4-B4 mutant of S. pombe was isolated by Bresch et al. (5) and genetically analyzed by Kishida and Shimoda (41). The complete medium YEA supplemented with 75 μg of adenine sulfate/ml and 50 μg of uracil/ml was used for growth. The synthetic medium MM was used. The malt extract medium MEA and the synthetic sporulation media SSA, SSL−N, and MM−N were used for mating and sporulation. These media were described by Egel and Egel-Mitani (15), Gutz et al. (22), and Moreno et al. (49). S. pombe cells were grown and sporulated at 28°C.
TABLE 1.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| S. pombe | ||
| L968 | h90 | U. Leupold |
| B4 | h90spo4-B4 ade6-M210 | 5 |
| TN29 | h90leu1-32 ura4-D18 | 31 |
| TN47 | h+sade6-M216 | 51 |
| TN53 | h−ade6-M210 | 51 |
| MK4L | h90spo4-B4 leu1-32 | This study |
| TN33 | h90spo4Δ::ura4+leu1-32 ura4-D18 | This study |
| HA46-11B | h90spo6Δ::ura4+leu1-32 ura4-D18 | H. Asakawa |
| TN68 | h+sspo4Δ::ura4+ade6-M216 ura4-D18 | This study |
| TN69 | h−spo4Δ::ura4+ade6-M210 ura4-D18 | This study |
| TN72 | h+sspo6Δ::ura4+ade6-M210 ura4-D18 | This study |
| TN73 | h−spo6Δ::ura4+ade6-M216 ura4-D18 | This study |
| TN92 | h+sspo4Δ::ura4+spo6Δ::ura4+ade6-M210 ura4-D18 | This study |
| TN93 | h−spo4Δ::ura4+spo6Δ::ura4+ade6-M216 ura4-D18+ | This study |
| JZ670 | h−/h−pat1-114/pat1-114 ade6-M210/ade6-M216 leu1-32/leu1-32 | M. Yamamoto |
| AB4 | h−/h−mei4Δ::ura4+/mei4OΔ::ura4+pat1-114/pat1-114ade6-M210/ade6-M216 leu1-32/leu1-32 ura4-D18/ura4-D18 | 1 |
| TN74 | h+s/h−spo4Δ::ura4+/spo4Δ::ura4+ade6-M210/ade6-M216 ura4-D18/ura4-D18 | This study |
| TN75 | h+s/h−ade6-M210/ade6-M216 | This study |
| TN76 | h+s/h−spo6Δ::ura4+/spo6Δ::ura4+ade6-M210/ade6-M216 ura4-D18/ura4-D18 | This study |
| TN77 | h+s/h−spo4Δ::ura4+/spo4Δ::ura4+spo6Δ::ura4+/spo6Δ::ura4+ade6-M210/ade6-M216 ura4-D18/ura4-D18 | This study |
| TN151 | h−/h−spo4Δ::ura4+/spo4Δ::ura4+pat1-114/pat1-114 ade6-M210/ade6-M216 leu1-32/leu1-32 ura4-D18/ura4-D18 | |
| TN188 | h−/h−pat1-114/pat1-114 ade6-M210/ade6-M216 leu1-32/leu1-32 spo4-HA≪LEU2/spo4+ | This study |
| TN194 | h90spo4-HA≪LEU2 leu1-32 | This study |
| TN195 | h90spo4-HA≪LEU2 spo6Δ::ura4+leu1-32 ura4-D18 | This study |
| TN229 | h90spo4-B4 leu1-32 ura4-D18 | This study |
| S. cerevisiae | ||
| Y187 | matα ura3-52 his3-200 ade2-101 trp1-901 leu2-3, 112 gal4Δ mef gal80Δ URA3::GAL1UAS-GAL1TATA-lacZ | 26 |
Cloning of spo4+.
A homothallic spo4-B4 mutant (MK4L) was transformed with an S. pombe genomic library containing partial Sau3AI fragments (a gift from Y. Watanabe) constructed in a multicopy plasmid, pDB248′ (3), and with a cDNA library, pTN-RC5 (this study), containing meiotic cDNA fragments constructed in the expression vector pREP42 (47). One of 15,000 transformants from the genomic library and four of 100,000 transformants from the cDNA library were able to sporulate. Plasmid DNA molecules were recovered from these transformants, and the plasmids obtained from the genomic library were further analyzed.
Plasmid construction.
Plasmid pIL2 was constructed as follows. A 2.2-kb fragment containing LEU2 was ligated into the SspI site of pBluescript KS(−) to create pIL2. A 2-kb NotI-SacI fragment, which contains three tandem repeats of the hemagglutinin (HA) epitope and the nmt1 terminator of pSLF272 (19), was ligated into the same sites of pIL2 to create pTN218. pREP41(NotI) was constructed by inserting a NotI linker into the SmaI site of pREP41. Two oligonucleotides, spo4N and pREP-AS (see below), were used to amplify spo4+ cDNA. As a template, pREP42(spo4), containing spo4+ cDNA, was used. The 1.4-kb fragment was digested with SalI and NotI and inserted into the corresponding site of pREP41(NotI) and pTN218, yielding pREP41(spo4) and pIL2(spo4-HA), respectively. pREP41(GST) was constructed by inserting the PstI-SacI fragment of glutathione S-transferase (GST) from pDS473 (19) into the same site of pREP41. For expression of GST-Spo4, pREP41(GST-Spo4) was used. This plasmid was constructed by inserting the full length of spo4+ cDNA into plasmid pREP41(GST). pTN63 was constructed using two oligonucleotides, 5′-GGCCGCGACTATAAGGACGACGATGACAAGTGACCGCGG-3′ and 5′-TCGACCGCGGTCACTTGTCATCGTCGTCCTTATAGTCGC-3′. pREP42(spo4) was used as a template. These DNAs were annealed and inserted into the NotI-SalI site of pSLF272 (19), yielding pTN63. For expression of Spo6-FLAG, pTN63(spo6) was used. This plasmid was constructed as follows. Two oligonucleotides, 5′-CCCCTCGAGTATGGACTTCTATTCAGTGAAG-3′ (the XhoI site is underlined) and 5′-GATGCGGCCGCCATTTGTCCGAATTGGGCG-3′ (the NotI site is underlined), were used to amplify the spo6+ cDNA by PCR. pREP42(spo6), containing spo6+ cDNA, was used as a template. The PCR product was digested with XhoI and NotI and then inserted into the same site of pTN63, yielding pTN63(spo6). For two-hybrid analysis, a DNA fragment encoding full-length spo4+ cDNA was cloned into pGAD424 to express Spo4 fused with the Gal4 transcriptional activator domain pGAD(spo4). A DNA fragment encoding full-length spo6+ cDNA was cloned into pGBT9 to express Spo6 fused with the Gal4 DNA binding domain (50).
Disruption of spo4.
spo4+ was disrupted by inserting ura4+ into its coding region. A 4.9-kb SphI-SalI fragment was subcloned into pHSG396 (TaKaRa). A 0.9-kb HindIII-StuI fragment was replaced with the 1.8-kb ura4+ cassette (21) (Fig. 1B). The 5.8-kb HindIII-SalI fragment containing the interrupted spo4 allele (spo4::ura4+) was used to transform the strain, TN29. Disruption was confirmed by Southern hybridization of genomic DNA (data not shown).
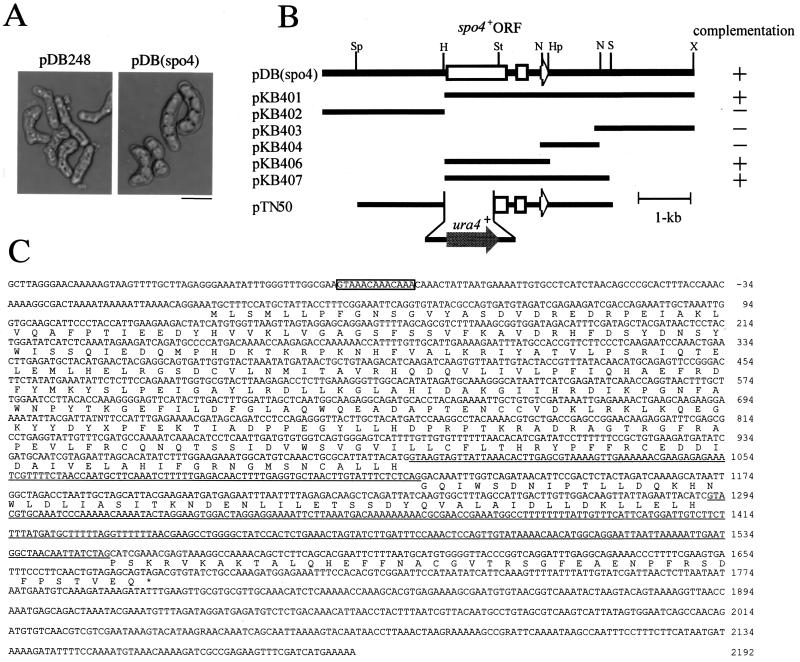
FIG. 1.
Isolation, subcloning, and sequencing of the spo4+ gene. (A) Isolation of the spo4+ gene. Strain MK4L (spo4-B4) was transformed with either pDB248′ or pDB(spo4). The Leu+ transformants were incubated on synthetic sporulation medium (SSA) at 28°C for 2 days. (B) Restriction map and construction of null mutants. The open arrows indicate the region and direction of the spo4+ open reading frame, which encodes a protein composed of 429 amino acid residues. All subclones were derived from pDB(spo4). Complementation by each subclone is indicated as follows: +, complements; −, does not complement. Restriction enzyme sites: H, HindIII; Hp, HpaI; N, NsiI; S, SalI; Sp, SphI; St, StuI; X, XhoI. (C) Nucleotide and predicted amino acid sequences of spo4+. Two introns are marked by underlining. A putative FLEX sequence in the promoter region is boxed.
Site-directed mutagenesis.
The four kinds of mutation (K95A, D182N, T264A, and T264E) in the spo4 gene were created by PCR with the following primers: for the N-terminal fragment, 5′-CCCGTCGACAATGCTTTCCATGCTATTACC-3′ (spo4N) (the SalI site is underlined), 5′-CGGTGGCATAAATTCTGGCCAATGCAACAAAATGGTTTTTTG-3′ (K95A-as), 5′-GTTACCTGGTTTGATATTTCGATGAATTATGCCCTTTGCATC-3′ (D182N-as), 5′-CGAAATCCTCTTGCTCCGGCTCGGTCAGCA-3′ (T264A-as), and 5′-CGAAATCCTCTTTCTCCGGCTCGGTCAGCA-3′ (T264E-as); for the C-terminal fragment, 5′-GTTGCATTGGCCAGAATTTATGCCACCGTTCTTCCC-3′ (K95A-s), 5′-GGGCATAATTCATCGAAATATCAAACCAGGTAACTTTGC-3′ (D182N-s), 5′-GACCGAGCCGGAGCAAGAGGATTTCGAGCG-3′ (T264A-s), 5′-GACCGAGCCGGAGAAAGAGGATTTCGAGCG-3′ (T264E-s), and 5′-CAAAATCGTAATATGCAGCTTGAATGGGC-3′ (pREP-AS). The mutated alleles were obtained by PCR with spo4N-K95A-as and K95A-s-pREP-AS for K95A, spo4N-D182N-as and D182N-s-pREP-AS for D182N, spo4N-T264A-as and T264A-s-pREP-as for T264A, and spo4N-T264E-as and T264E-s-pREP-as for T264E. In the PCRs, pREP42(spo4) was used as a template. In every case, the fragments produced were subjected to a second PCR with a pair of primers, spo4N and pREP-AS. The products were restricted by SalI and NotI, and the resulting 1.4-kb fragment was inserted into the same site of pREP41(NotI) and pREP41(N-HA), yielding pREP41(spo4K95A) and pREP41(HA-spo4K95A), pREP41(spo4D182N) and pREP41(HA-spo4D182N), pREP41(spo4T264A) and pREP41(HA-spo4T264A), or pREP41(spo4T264E) and pREP41(HA-spo4T264E), respectively. Plasmid pREP41(GST-spo4K95A) was constructed by inserting the SalI-NotI fragment of pREP41(spo4K95A) into the corresponding site of pREP41(GST).
Southern and Northern analyses.
Genomic DNA was restricted, fractionated in a 1.0% agarose gel, and then transferred to nylon membranes (Biodyne A; Nihon Pall Co). Total RNA was prepared from S. pombe cultures (33) and fractionated on a 1.0% gel containing 3.7% formaldehyde as previously reported (65).
Western blotting.
The pIL2(spo4)-HA plasmid was linearized by restricting it with StuI near the center of the spo4 sequences and was introduced into TN8. Since Leu+ transformants which were competent for sporulation were obtained, we concluded that Spo4-HA is a functional protein. Likewise, this plasmid was integrated into HA46-11B and JZ670. A wild-type strain (TN194) and a spo6 mutant strain (TN195) were cultured in liquid sporulation medium (SSL−N). At intervals, culture aliquots were collected and crude cell extracts were prepared as described by Masai et al. (45). Polypeptides were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% gels and then transferred to polyvinylidene difluoride membranes (Millipore). The filters were probed with either mouse anti-HA antibody 12CA5 (Boehringer Mannheim), mouse anti-FLAG M2 antibody (Sigma), rat anti-HA antibody 3F10 (Boehringer Mannheim), or goat anti-GST antibody (Pharmacia) at a 1:1,000, 1:1,000, 1:1,000, or 1:200 dilution, respectively. Blots were also probed with anti-α-tubulin antibody, TAT-1 (70), to normalize the protein load. Immunoreactive bands were visualized by staining them with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG; Promega), goat anti-rat IgG (BioSource), or rabbit anti-goat antibody (ICN/CAPPEL) and by chemiluminescence (NEN Life Sciences).
Immunofluorescence microscopy.
For cell fixation, we followed the procedure of Hagan and Hyams (24), using glutaraldehyde and paraformaldehyde. The spindle pole body (SPB) was visualized by indirect immunofluorescence microscopy using rabbit anti-Sad1 antibody (a gift from O. Niwa) and Alexa 546-conjugated goat anti-rabbit IgG (Molecular Probes) (23). For microtubule staining, TAT-1 anti-α-tubulin antibody (70) and Alexa 488-conjugated goat anti-mouse IgG (Molecular Probes) were used. Spo4-HA was visualized using rat anti-HA antibody 3F10 and Alexa 488-conjugated goat anti-rat IgG (Molecular Probes). The nuclear chromatin region was stained with 4′,6-diamidino-2-phenylindole (DAPI) at 1 μg/ml. Stained cells were observed under a fluorescence microscope (model BX50; Olympus) and a Cool SNAP charge-coupled device camera (Roper Scientific).
Interaction of GST-Spo4 with Spo6-FLAG in S. pombe cells.
The strain TN29 was cotransformed with either pREP41(GST), pREP41(GST-spo4), or pREP(GST-spo4K95A) and pREP42(spo6-FLAG). Transformants were grown in liquid MM to mid-log phase. Isolations of GST-Spo4 and Spo6-FLAG were performed according to the method described by Brown and Kelly (6).
Kinase assays using purified GST-Spo4.
Either GST-Spo4 or GST-Spo4K95A was expressed in wild-type cells with or without Spo6-FLAG and purified using glutathione beads from wild-type cells carrying the appropriate plasmid according to the method described by Brown and Kelly (6). Kinase reactions were performed as described previously (6) using a recombinant S. pombe Mcm2 protein (18, 48). To express His6-Mcm2 fusion protein in Escherichia coli, a DNA fragment encoding amino acids 1 to 220 of Mcm2 (63) was amplified by PCR and cloned into pQE30 (Qiagen). The fusion protein was purified using Ni-nitrilotriacetic acid resin as directed by the manufacturer (Qiagen).
Nucleotide sequence accession number.
The sequence data for spo4+ are available from EMBL-GenBank-DDBJ under accession no. AB036342 (for the genome) and AB036343 (for the cDNA).
RESULTS
spo4+ encodes a novel Cdc7-like protein.
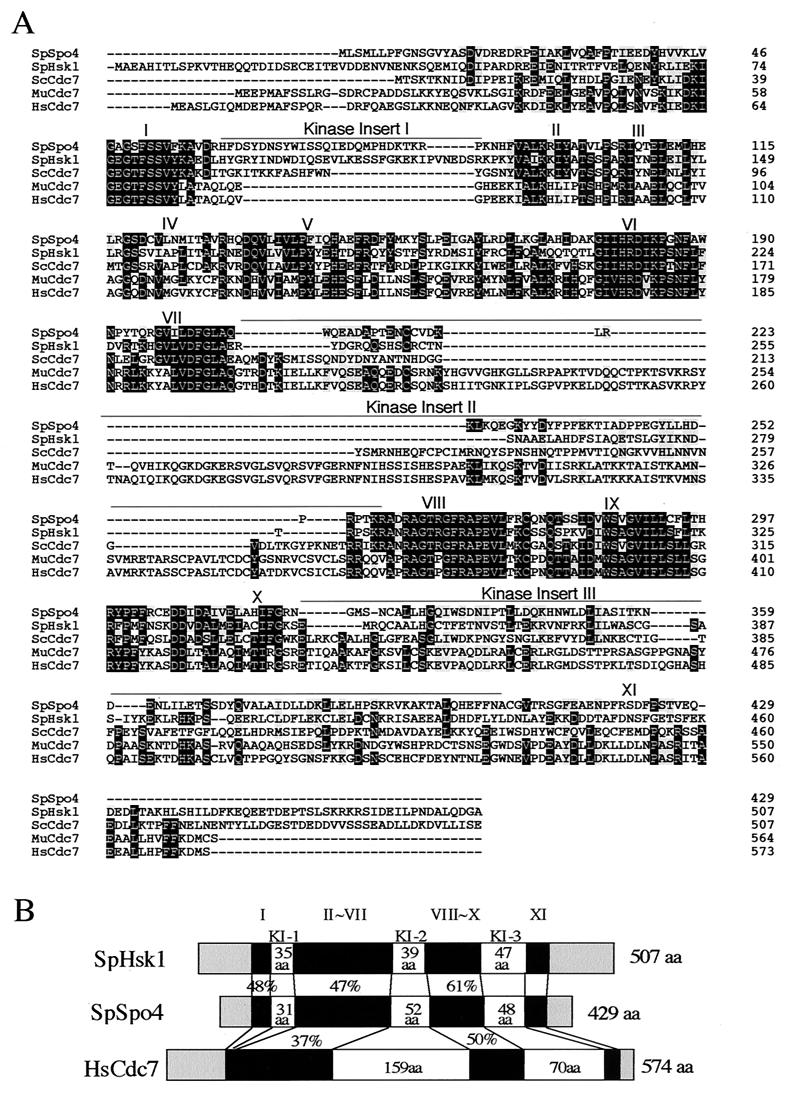
To identify the spo4+ gene product and its biological function, we isolated spo4+ by functional complementation. From a screen of about 15,000 colonies transformed with an S. pombe genomic library constructed in pDB248′ (3), we recovered one plasmid which rescued the sporulation deficiency of the spo4-B4 mutant (Fig. 1A). Subcloning indicated that the complementing activity of the plasmid was associated with the 2.0-kb HindIII-HpaI fragment (Fig. 1B). Sequencing of this fragment of pKB406 revealed one open reading frame of 1,287 nucleotides split by two potential introns (Fig. 1C). The same gene was isolated in independent complementation screens using a cDNA library (see Materials and Methods). A comparison of both genomic and cDNA sequences confirmed two introns of 123 and 261 bp (Fig. 1C). The cloned DNA fragment complementing spo4 mutation carries the genetically defined spo4+ itself as described below. BLAST searches showed that the predicted Spo4 protein had significant sequence similarity to budding yeast Cdc7 kinase, fission yeast Hsk1 kinase, and mammalian Cdc7-related kinases (34, 40, 45, 58, 59) (Fig. 2A). The sequence similarity is striking in most of the kinase subdomains. Moreover, Spo4 contains three “kinase insert” stretches, which is characteristic of Cdc7-related kinases (Fig. 2B). The positions of insertion are identical to those of other Cdc7-related kinases, although mammalian Cdc7 does not have kinase insert 1 (34, 40, 59) (Fig. 2C). Budding yeast Cdc7 and fission yeast Hsk1 contain a C-terminal region rich in acidic amino acid residues, which seems essential for interaction with their regulatory subunit, Dbf4 (45, 54, 58). We note that Spo4 lacks this “acidic tail,” as do the human and Xenopus Cdc7 homologues (34, 40, 59) (Fig. 2B). These structural features imply that Spo4 is a novel Cdc7 family kinase.
FIG. 2.
spo4+ encodes a novel protein which has a high degree of similarity to Cdc7-related kinase. (A) Comparison of the amino acid sequences of Spo4 and fission yeast Hsk1 (SpHsk1; GenBank accession number D50493), budding yeast Cdc7 (ScCdc7; GenBank accession number M12624), mouse Cdc7 (MuCdc7; GenBank accession number AB018574), and human Cdc7(HsCdc7; GenBank accession number NM003503). Dark and light shaded boxes indicate identical and similar residues in three of five proteins, respectively. Dashes represent gaps in the sequences. (B) Structural similarity of Spo4 with two other Cdc7-related kinases. I to XI represent kinase subdomains. KI-1 to KI-3 represent kinase inserts. The percentages of identical amino acids (aa) in the respective regions are also shown. (C) Four spo4 mutations and their function effects on sporulation. (Top) Positions of point mutations. All the point mutants (K95A, D182N, T264A, and T264E) contain amino acid substitutions in the kinase domains. (Bottom) Strain TN33 bearing either pREP41(spo4), pREP41(spo4K95A), pREP41(spo4D182N), pREP41(spo4T264A), or pREP41(spo4T264E) was sporulated on SSA medium for 2 days at 28°C. Bar, 10 μm. (D) Expression of spo4 mutant proteins. Strain TN33 bearing either pREP41(HA-spo4+), pREP41(HA-spo4K95A), pREP41(HA-spo4D182N), pREP41(HA-spo4T264A), or pREP41(HA-spo4T264E) was sporulated in SSL-N medium for 8 h at 28°C. Protein extracts were subjected to Western blot analysis with the rat anti-HA (α-HA), as well as with anti-α-tubulin (tubulin) antibody, as the loading control.
Spo4 is essential for initiation and progression of meiosis II.
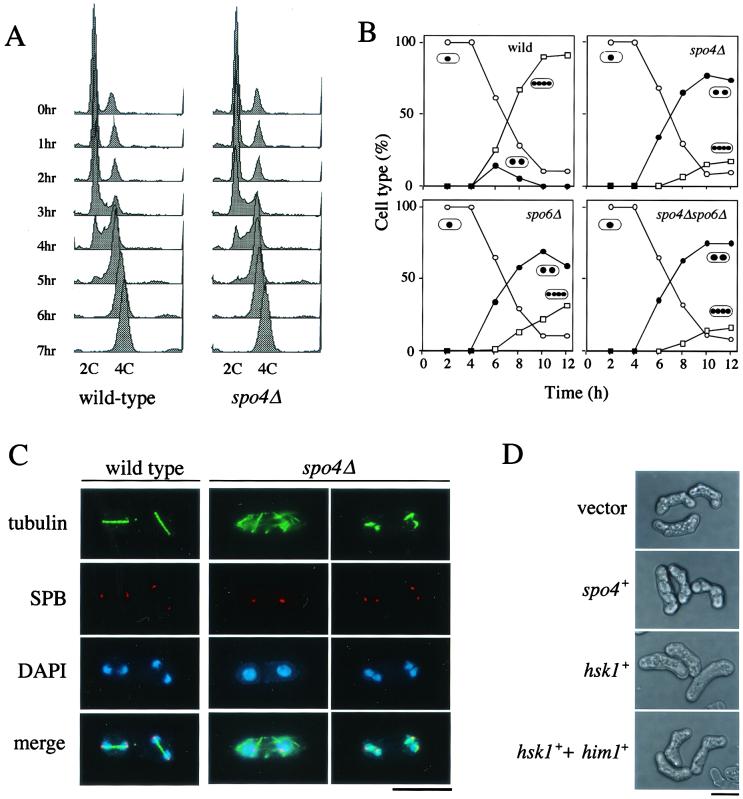
To investigate the biological role of spo4+, a null mutant was created by conventional gene disruption using ura4+ as a marker (Fig. 1B). The spo4 deletion mutant (spo4Δ) was viable but displayed sporulation defects like the original spo4-B4 mutant (data not shown). In various organisms, the Cdc7-related kinases are required for the initiation of DNA replication, suggesting that Spo4 is necessary for the initiation of premeiotic S phase. To test this possibility, we conducted flow cytometric analyses of propidium iodide-stained pat1-114 cells that synchronously underwent meiosis at a restrictive temperature (30). The DNA content of spo4Δ cells roughly doubled prior to meiotic nuclear division (Fig. 3A). We observed a similar result with spo4Δ homozygous diploid cells cultured in nitrogen-free medium for the induction of meiosis (data not shown). We thus conclude that spo4Δ cells complete premeiotic DNA replication normally.
FIG. 3.
spo4+ is required for progression of meiosis II but not for the onset of premeiotic DNA synthesis. (A) Fluorescence-activated cell sorter analysis of premeiotic DNA replication in spo4Δ cells. Synchronous meiosis is induced in the diploid strains JZ670 (pat1-114/pat1-114) (wild type) and TN151 (spo4Δ pat1-114/spo4Δ pat1-114) (spo4Δ). Samples were taken every hour, and the DNA content was measured by FACScan. (B) Kinetics of meiosis in various strains. Mid-log-phase cells of wild-type (TN75), spo4Δ (TN74), spo6Δ (TN76), and spo4Δ spo6Δ (TN77) homozygous diploid strains were cultured in MM-N medium at 28°C. The progression of meiosis was monitored by DAPI staining. One, two, and four dots in ovals represent mononucleate, binucleate, and tetranucleate cells, respectively. (C) Microtubules during meiosis visualized by immunofluorescence microscopy. Diploid cells of the wild-type (TN75) and spo4Δ (TN74) strains were incubated in SSL-N sporulation medium at 28°C, sampled, and fixed. Indirect immunofluorescence microscopy was conducted using an anti-α-tubulin and an anti-Sad1 antibody to visualize microtubules and SPB (23), respectively. Bar, 10 μm. (D) Inability of Hsk1 to complement sporulation defect of the spo4 mutant. Strain TN229 (spo4-B4) was transformed with plasmids in the following combinations: pREP41 plus pREP2, pREP41(spo4) plus pREP2, pREP41(hsk1) plus pREP2, or pREP41(hsk1) plus pREP2(HA-Him1). The transformants were sporulated on SSA medium for 2 days at 28°C. HA-tagged Him1 is functional, as described by Takeda et al. (63). Bar, 10 μm.
We next examined the precise arrest phenotype of a homozygous spo4Δ diploid strain during meiosis. Meiosis was induced by transferring a log-phase culture to nitrogen-free medium. spo4Δ cells proceeded through meiosis I with kinetics similar to those of the wild type. However, approximately 80% of spo4Δ cells arrested at the binucleate stage (Fig. 3B). Most of the spo4Δ cells (approximately 90%) arrested at the binucleate stage exhibited cytoplasmic microtubules (Fig. 3C), suggesting that these cells were in the interkinesis between meiosis I and meiosis II. A small number of spo4Δ cells had pairs of chromatin regions insufficiently separated (Fig. 3C). At anaphase II, wild-type cells assembled spindles of interdigitated microtubules between SPBs (Fig. 3C). However, few intact spindles were seen in spo4Δ cells. Instead, these cells contained short fragments of microtubules associated with the SPB, indicating that the integrity of spindle microtubules would be impaired (Fig. 3C). We conclude that Spo4 is required for both entry into meiosis II and the progression through anaphase II.
S. pombe has another Cdc7-related kinase, Hsk1 (45). Hsk1 forms a complex with the Dbf4-related protein Dfp1/Him1 (6, 63). This complex is required for the initiation of DNA replication. To assess the level of functional relatedness between Spo4 and Hsk1, hsk1+ was ectopically expressed under the control of the nmt1 promoter in the spo4 mutant. Such overexpression of hsk1+ could not rescue the sporulation defect of the mutant (Fig. 3D). The same result was obtained when Hsk1 and Dfp1/Him1 were coexpressed in the spo4 mutant (Fig. 3D). These observations suggest that Hsk1 cannot be substituted for Spo4.
Expression of Spo4.
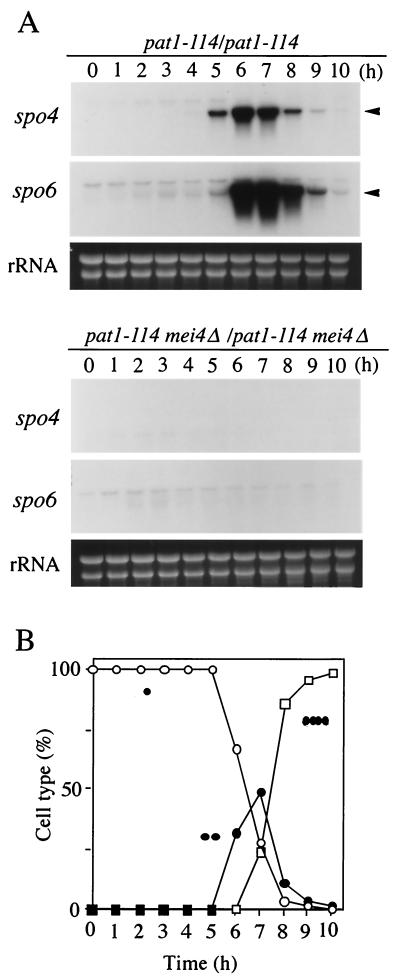
In budding yeast and other organisms, the abundance of either the transcript or protein of the Cdc7 kinase is constant throughout the cell cycle (61). We next explored whether the expression of spo4+ was controlled in a similar manner. Synchronous meiosis was induced using JZ670 strains (pat1-114) (30), and the spo4 mRNA levels were monitored by Northern blot analysis. spo4 mRNA was barely detectable in vegetative cells, though it abruptly increased during meiosis. The timing of a burst of spo4 transcription during meiosis was then more precisely determined with cells undergoing meiosis synchronously. Figure 4 shows that the induction of spo4+ occurs with or just before the appearance of binucleate cells, that is, at the end of meiosis I. spo4+ has a consensus sequence (GTAAACAAACA) named FLEX (1, 29) in the 5′ upstream region by which a meiosis-specific transcription factor, Mei4, recognizes its targets (Fig. 1C). In fact, transcription of spo4+ was completely abolished in mei4Δ (Fig. 4A). In conclusion, the transcription of spo4+ is induced during meiosis under the regulation of Mei4.
FIG. 4.
(A) Transcription of the spo4+ gene in pat1-driven synchronous meiosis (30). Meiosis of the diploid strains harboring homozygous pat1-114, JZ670 (mei4+), and AB4 (mei4Δ) was synchronous, and at intervals total RNA was prepared and analyzed by Northern blot hybridization as described in Materials and Methods. Arrowheads, spo4 and spo6 mRNAs. The approximate quantity of RNA was checked by staining the gels with ethidium bromide, which reveals rRNA. (B) Meiotic nuclear division of JZ670 (mei4+) was monitored by DAPI staining. Frequencies of mononucleate cells (single dot; open circles), binucleate cells (two dots; solid circles), and tetranucleate cells (four dots; open squares) were determined.
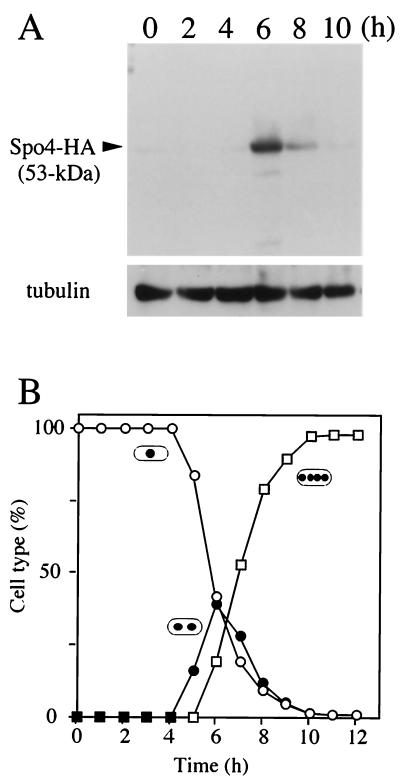
To identify the Spo4 protein, Spo4 was tagged with an HA epitope at the carboxyl terminus. This construct is fully functional, because it rescues meiotic defects of a spo4Δ mutant (data not shown). SDS-PAGE analysis showed that the Spo4-HA protein was not detectable in cell extract from vegetative cells, while it appeared as a 53-kDa polypeptide in meiotic-cell extract (Fig. 5A). Spo4-HA protein was most abundant in binucleate cells which completed meiosis I (Fig. 5B).
FIG. 5.
Time course of expression of Spo4 during meiosis. (A) Cells homozygous for a gene replacement of Spo4-HA (TN188) were induced to undergo synchronous meiosis (30). Aliquots were removed every 2h, and protein extracts were subjected to immunoblot analysis with the mouse anti-HA antibody 12CA5, as well as with anti-α-tubulin antibody as the loading control. (B) Meiotic nuclear division was monitored by counting the number of nuclei. Frequencies of mononucleate cells (single dot; open circles), binucleate cells (two dots; closed circles), and tetranucleate cells (four dots; open squares) were determined.
To address the question of whether protein kinase activity is a prerequisite for the meiotic function of Spo4, we introduced a series of amino acid substitutions, such as K95A, D182N, T264A, and T264E, according to the method of Ohtoshi et al. (54) (Fig. 2C). These are mutations at an ATP-binding site (K95A) and a conserved kinase domain (D182) (25). Threonine 264 (T264) might correspond to T167 of S. pombe Cdc2, which is phosphorylated by Cdk activation kinase (8). We expressed these mutant genes under the control of the nmt1 promoter in a spo4Δ mutant. As Fig. 2C shows, none of these mutant spo4 genes complement the sporulation defect of the spo4Δ mutant. Similarly, overexpression of the HA-tagged mutant proteins could not rescue the defect, although the HA-tagged wild-type Spo4 suppressed the spo4 mutation (data not shown). As the abundance of the mutant proteins was roughly the same as that of the wild-type HA-Spo4, the inability to complement spo4Δ is not due to the reduction of their expression levels (Fig. 2D). These results suggest that the protein kinase activity is probably essential for the Spo4 function and that posttranslational regulation might be implicated in the activity.
Spo4 forms an active kinase complex with a Dbf4-like protein, Spo6.
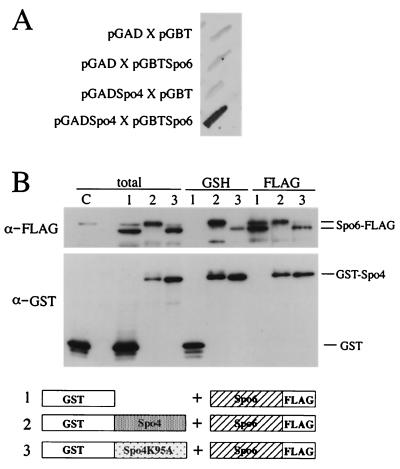
The activity of Cdc7 kinase is controlled by its regulatory subunit, Dbf4. The abundance of Dbf4 fluctuates during the cell cycle, peaking at S phase. Recently, we identified a sporulation-specific gene, spo6+, which encodes a Dbf4-like protein in fission yeast (50). Therefore, it is possible that Spo4 is complexed with Spo6. spo6 mutants exhibit defective phenotypes in the second meiotic division and in sporulation similar to spo4 mutants, though the phenotype of spo4Δ was slightly more severe than that of spo6Δ (50) (Fig. 3B). Furthermore, the spo4 defect in the initiation of the second meiotic division was not exaggerated by the null mutation of spo6. Additionally, the transcriptional induction of spo6+ coincided with that of spo4+ and was regulated in a Mei4-dependent manner (29) (Fig. 4A). These results are consistent with the idea that Spo4 and Spo6 form an active complex to execute the function essential for meiosis. This possibility was further confirmed by a yeast two-hybrid assay (2). The entire lengths of Spo4 and Spo6 were fused to a Gal4 activation domain and a Gal4 DNA binding domain, respectively. Both chimeric proteins were expressed in S. cerevisiae strain Y187. Apparently, the assay indicated a positive interaction between the two proteins (Fig. 6A).
FIG. 6.
Spo4 interacts with Spo6. (A) Yeast two-hybrid analysis. The interaction between Spo4 and Spo6 is positive, as judged by the blue coloring of the host cells. The entire lengths of Spo4 and Spo6 were fused to a Gal4 activation domain and a Gal4 DNA binding domain, respectively. Both chimeric proteins were expressed in S. cerevisiae strain Y187. The intensity of the blue is reproduced in black and white. (B) Spo4 interacts with Spo6 in vivo. (Top) GST (lanes 1), GST-Spo4 (lanes 2), and GST-Spo4K95A (lanes 3) were coexpressed with FLAG-tagged Spo6. As a control, only GST was expressed (lane C). Total cell lysates (total) and proteins bound to glutathione beads (GSH) or anti-FLAG M2 affinity gel (FLAG) were analyzed by immunoblotting using anti-GST and anti-FLAG antibodies (α-GST and α-FLAG, respectively). Note that nonspecific cross-reaction was observed near the band of Spo6-FLAG. (Bottom) A diagrammatic representation of the fusion proteins expressed in cells. The numbers correspond to lanes.
To assess the association of Spo4 and Spo6 in vivo, a chimeric protein of Spo4 fused to GST was coexpressed with FLAG-tagged Spo6 in S. pombe. GST-Spo4 was pulled down with glutathione beads, and copurification of Spo6 was verified by immunoblotting using anti-FLAG antibody. Spo6-FLAG was copurified with GST-Spo4 but not with unfused GST (Fig. 6B). Conversely, the immunoprecipitate obtained with anti-FLAG antibody contained GST-Spo4 (Fig. 6B). A kinase-negative Spo4 (GST-Spo4K95A) was also able to associate with Spo6-FLAG. These results indicate that Spo4-FLAG and GST-Spo6 are physically associated and do not require the Spo4 kinase activity. It is noteworthy that the mobility of both GST-Spo4 and Spo6-FLAG on SDS-PAGE was retarded when wild-type Spo4 was expressed. Such mobility shifting was eliminated by treatment with phosphatase (data not shown), indicating that the shifted bands were hyperphosphorylated forms of Spo4 and Spo6. Such phosphorylated forms were not observed in cells expressing GST-Spo4K95A (Fig. 6B).
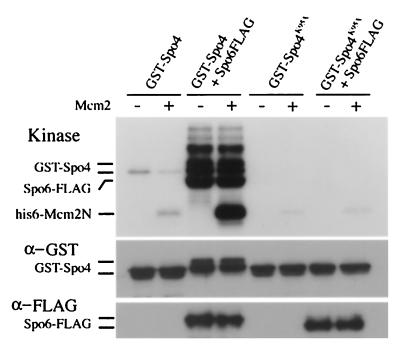
Next we addressed the question of whether Spo4 has kinase activity in vitro. GST-Spo4 and GST-Spo4K95A were expressed in either spo6+ or spo6Δ strains and partially purified using glutathione beads from cell extracts. The N-terminal region of S. pombe Mcm2 (Nda1/Cdc19) protein was used as a potential substrate (18, 48), because known Cdc7-related kinases phosphorylate Mcm2 in vitro (6, 44, 63). Autokinase and Mcm2 kinase activities were detected in the isolated GST-Spo4, whereas GST-Spo4K95A abolished both activities (Fig. 7). The control extract from the transformant bearing pREP41(GST) gave no activity (data not shown). In the presence of Spo6, both autokinase and Mcm2 kinase activities of Spo4 were markedly enhanced (Fig. 7). We conclude that Spo4 and Spo6 form an active kinase complex like Cdc7-Dbf4-related kinase complexes of other eukaryotes.
FIG. 7.
Protein kinase assays of Spo4 protein. A homothallic strain, HA46-11B (spo6Δ), harboring either pREP41(GST-spo4) or pREP41(GST-spo4 K95A), and TN29 (wild type) harboring pREP42(spo6-FLAG) and either pREP41(GST-spo4) or pREP41(GST-spo4 K95A) were cultured in MM for 12 h at 28°C. Extracts were prepared from each culture, proteins bound to glutathione beads were incubated with [γ-32P]ATP in kinase assay buffer, and the products were examined by SDS-PAGE followed by autoradiography. The positions of Spo4, Spo6, and Mcm2 (added as a substrate) are shown. The Spo4 and Spo6 proteins were detected by immunoblotting using anti-GST and anti-FLAG antibodies, respectively. +, present; −, absent.
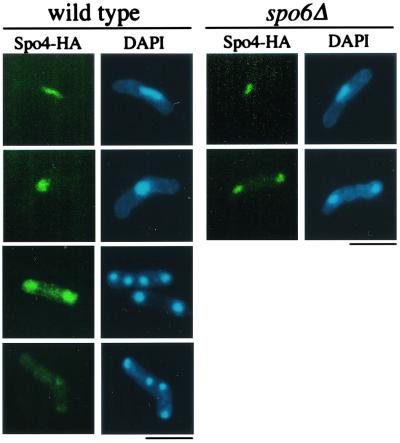
Spo4 localizes to the nucleus during early meiosis.
Finally, we investigated the subcellular localization of Spo4-HA protein by indirect immunofluorescence microscopy. As expected, the Spo4-HA signal was not detectable in vegetative cells (data not shown). During meiosis, Spo4-HA preferentially localized in the nucleus (Fig. 8). The Spo4 signal in the nucleus, however, became less intense as cells proceeded through meiosis II. Noticeably, Spo6, a partner of Spo4, localized in the nucleus during meiosis (50). In S. cerevisiae, Dbf4 recruits Cdc7 to replication initiation complexes (13, 57, 64, 69). To clarify whether Spo6 determines the nuclear localization of Spo4, we observed the localization of Spo4 in the spo6Δ mutant. However, the nuclear localization of Spo4-HA was not affected by the spo6 mutation (Fig. 8), suggesting that localization of Spo4-HA is independent of Spo6 function.
FIG. 8.
Subcellular localization of Spo4-HA during meiosis. The homothallic haploid strains TN194 (wild type) and TN195 (spo6Δ) harboring chromosomally integrated Spo4-HA were cultured in SSL-N to induce meiosis. Spo4-HA was observed at different stages of meiosis after fixation using Rat anti-HA antibody 3F10. Bars, 10 μm.
DISCUSSION
A serine/threonine protein kinase complex, Cdc7-Dbf4, plays a critical role in the initiation of S phase (4, 12, 13, 27, 28, 35, 43, 45, 57, 62, 63). The regulatory subunit, Dbf4, is a cyclin-like protein, in that its abundance periodically fluctuates, peaking at S phase, while Cdc7 levels remain constant throughout the cell cycle (10, 11, 17, 55). Dbf4 stimulates the kinase activity of the catalytic subunit, Cdc7 (32, 42). As Dbf4 interacts with the origin recognition complex, it may be possible that Dbf4 recruits Cdc7 to the chromosomal replication origins (13, 49, 57, 64). The Cdc7 kinase is characterized by a well-conserved primary structure and the presence of kinase insert regions, as well as by complex formation with the Dbf4-like protein. The Cdc7-Dbf4 kinase complex is evolutionarily conserved among eukaryotic organisms (34, 35, 37, 40, 43, 46, 59). The fission yeast S. pombe has a homologous kinase complex, composed of Hsk1 and Dfp1/Him1, which is also essential for the onset of DNA replication (6, 7, 45, 63). The present and previous studies revealed that Spo4-Spo6 is a novel Cdc7-related kinase complex. First, Spo4 protein has significant sequence similarity to known Cdc7 kinases. In addition, it contains three kinase insert sequences at positions identical to those of S. cerevisiae and other Cdc7-related kinases. Furthermore, Spo4 forms a complex with the Dbf4-like protein, Spo6, which markedly enhances the Spo4 kinase activity. This interaction is essential for its function. We conclude from these features that the fission yeast Spo4-Spo6 complex is a novel Cdc7-Dbf4 kinase.
In spite of these common features, Spo4-Spo6 kinase has some unique characteristics. Spo4 did not have the C-terminal tail rich in acidic amino acids found in budding yeast Cdc7 and fission yeast Hsk1. This C-terminal tail is essential for the functions of the Cdc7 kinase (54). Two-hybrid assays indicated that Dbf4 protein interacted with the C-terminal acidic tail of Cdc7 protein in budding yeast (54). However, human and Xenopus Cdc7 proteins did not contain such C-terminal tails. Spo4 might interact with Spo6 in a manner analogous to human and Xenopus Cdc7 proteins.
The kinase activity of some conventional Cdc7 proteins is regulated by association with Dbf4, whose level is cell cycle regulated. Spo4 kinase activity was markedly enhanced by Spo6. Therefore, we conclude that the catalytic subunit, Spo4, is controlled by its associated subunit, Spo6. Cdc7 kinase has been suggested to be regulated by phosphorylation, though the kinase responsible is still unknown. The threonine residue at 264 in Spo4 is equivalent to the threonine residue at 167 of S. pombe Cdc2. Phosphorylation of the corresponding residue of budding yeast Cdc7 is required to activate the kinase (8). Both Spo4T264A and Spo4T264E mutant proteins turned out to be nonfunctional, suggesting that phosphorylation of this threonine residue is necessary for the function. The expression of known Cdc7-related kinases is not regulated throughout the cell cycle. In contrast, spo4+ was transcriptionally regulated; it is barely transcribed in vegetative cells but is induced under the control of a meiosis-specific transcription factor, Mei4, during meiosis. Interestingly, the expression of spo6+ is also regulated by Mei4. Thus, both spo4+ and spo6+ are coordinately expressed by the same transcription mechanism. This is in contrast to other eukaryotic members of the Cdc7-Dbf4 kinase family.
Cdc7-related proteins are known to localize to the nucleus (34, 59, 72). Likewise, Spo4 was detected in nuclei of meiotic cells, relatively abundantly in mono- and binucleate cells and less so in tetranucleate cells. This result was consistent with the fact that Spo4 has an essential function in the initiation and progression of meiosis II. In S. cerevisiae, as well as activating Cdc7, Dbf4 recruits Cdc7 to the replication initiation complex. Spo6 has a putative nuclear localization signal (50), in contrast to Spo4, which has no such signal. However, localization of Spo4 to the nucleus does not depend on Spo6. Of course, we cannot exclude the possibility that Spo6 recruits Spo4 to a specific site within the nucleus.
All known Cdc7-related kinases are involved in the initiation of DNA replication. However, we found no obvious defects in DNA replication in the spo4 mutant. What is the cellular function of the Spo4-Spo6 kinase complex? Most of the spo4 mutant cells arrest at the interkinetic stage between meiosis I and II. A small fraction of the spo4 cells that entered into meiosis II, however, exhibited poorly separated sister chromatids with fragmented spindles. These results indicate that spo4+ is indispensable for initiation of the second meiotic division and probably for the maintenance of spindle integrity during meiosis II. Schild and Byers (60) reported that the budding yeast CDC7 gene is also required for meiosis. cdc7 mutants arrest at a pachytene stage of meiosis I, and the genetic recombination is severely impaired. Although both the S. cerevisiae cdc7 mutant and S. pombe spo4 mutant show meiotic defects, their phenotypes are totally different. Physiological substrates of Cdc7 kinase for meiotic function have not been identified. Like other Cdc7 kinases, Spo4 efficiently phosphorylates Mcm2 in vitro. Phosphorylation of Mcm2 by the Cdc7 kinases, including Hsk1, may regulate the essential function of the MCM complex in initiation of DNA replication. It is unlikely that in vivo phosphorylation of Mcm2, if it occurs, plays a physiological role in meiosis. Identification of the in vivo substrate of Spo4 is necessary for understanding the function of Spo4-Spo6 kinase in S. pombe.
In conclusion, S. pombe has at least two Cdc7-Dbf4 kinase complexes. One is Hsk1-Dfp1, and the other is Spo4-Spo6. Despite the sequence similarity, swapping of the partner results in loss of function. The Hsk1-Dfp1 complex has a cellular function very similar to those of other known Cdc7-related kinases, and its involvement in mitotic nuclear division has not been reported. In contrast, Spo4-Spo6 has a function different from those of any reported Cdc7-related kinases. This nonconventional type of Cdc7 homologue may expand the concept of the biological role of this family of kinases. It reminds us of the case of the cyclin-dependent kinases (Cdks). Cdk was first isolated as a cell cycle regulator. Recently a novel type of Cdk-related protein kinase, Pho85, was reported not to be involved in cell cycle regulation (38). To date, the fission yeast S. pombe is the only organism known to have two sets of Cdc7 kinase complexes, each with a different biological role. We could not find a second gene encoding Cdc7 and Dbf4 proteins in genome sequence databases of model organisms such as S. cerevisiae and Caenorhabditis elegans. Further progress in genome sequencing projects for other eukaryotes may elucidate organisms which contain multiple Cdc7 kinase complexes, like S. pombe.
Acknowledgments
We thank K. Gull of the University of Manchester for anti-α-tubulin antibody TAT-1, O. Niwa of Kazusa DNA Research Institute for affinity-purified antibodies against Sad1, and S. Forsburg of the Salk Institute for plasmids. We also thank M. Yamamoto and Y. Watanabe of the University of Tokyo for the S. pombe genomic library and H. Masai of the University of Tokyo for plasmids and useful discussion.
This work was supported by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan to C.S. and T.N. and from the Saneyoshi Scholarship Foundation to T.N.
REFERENCES
- 1.Abe, H., and C. Shimoda. 2000. Autoregulated expression of Schizosaccharomyces pombe meiosis-specific transcription factor Mei4 and a genome-wide search for its target genes. Genetics 154:1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai, C., and S. J. Elledge. 1996. Gene identification using the yeast two-hybrid system. Methods Enzymol. 273:331–347. [DOI] [PubMed] [Google Scholar]
- 3.Beach, D., and P. Nurse. 1981. High-transformation of the fission yeast Schizosaccharomyces pombe. Nature 290:140–142. [DOI] [PubMed] [Google Scholar]
- 4.Bousset, K., and J. F. Diffley. 1998. The Cdc7 protein kinase is required for origin firing during S phase. Genes Dev. 12:480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bresch, C., G. Muller, and R. Egel. 1968. Genes involved in meiosis and sporulation of a yeast. Mol. Gen. Genet. 102:301–306. [DOI] [PubMed] [Google Scholar]
- 6.Brown, G. W., and T. J. Kelly. 1998. Purification of Hsk1, a minichromosome maintenance protein kinase from fission yeast. J. Biol. Chem. 273:22083–22090. [DOI] [PubMed] [Google Scholar]
- 7.Brown, G. W., and T. J. Kelly. 1999. Cell cycle regulation of Dfp1, an activator of the Hsk1 protein kinase. Proc. Natl. Acad. Sci. USA 96:8443–8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buck, V., A. White, and J. Rosamond. 1991. CDC7 protein kinase activity is required for mitosis and meiosis in Saccharomyces cerevisiae. Mol. Gen. Genet. 227:452–457. [DOI] [PubMed] [Google Scholar]
- 9.Byers, B. 1981. Cytology of the yeast life cycle, p. 59–96. In J. N. Strathern, E. W. Jones, and J. R. Broach (ed.), The molecular biology of the yeast Saccharomyces cerevisiae: life cycle and inheritance. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 10.Chapman, J. W., and L. H. Johnston. 1989. The yeast gene, DBF4, essential for entry into S phase, is cell cycle regulated. Exp. Cell Res. 180:419–428. [DOI] [PubMed] [Google Scholar]
- 11.Cheng, L., T. Collyer, and C. F. Hardy. 1999. Cell cycle regulation of DNA replication initiator factor Dbf4p. Mol. Cell. Biol 19:4270–4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donaldson, A. D., W. L. Fangman, and B. J. Brewer. 1998. Cdc7 is required throughout the yeast S phase to activate replication origins. Genes Dev. 12:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowell, S. J., P. Romanowski, and J. F. Diffley. 1994. Interaction of Dbf4, the Cdc7 protein kinase regulatory subunit, with yeast replication origins in vivo. Science 265:1243–1246. [DOI] [PubMed] [Google Scholar]
- 14.Egel, R. 1971. Physiological aspects of conjugation in fission yeast. Planta 98:89–96. [DOI] [PubMed] [Google Scholar]
- 15.Egel, R., and M. Egel-Mitani. 1974. Premeiotic DNA synthesis in fission yeast. Exp. Cell Res. 88:127–134. [DOI] [PubMed] [Google Scholar]
- 16.Egel, R. 1989. Mating-type genes, meiosis and sporulation, p. 31–73. In A. Nasim, P. Young, and B. F. Johnson (ed.), Molecular biology of the fission yeast. Academic Press, San Diego, Calif.
- 17.Ferreira, M. F., C. Santocanale, L. S. Drury, and J. F. Diffley. 2000. Dbf4p, an essential S phase-promoting factor, is targeted for degradation by the anaphase-promoting complex. Mol. Cell. Biol. 20:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forsburg, S. L., and P. Nurse. 1994. The fission yeast cdc19+ gene encodes a member of the MCM family of replication proteins. J. Cell Sci. 107:2779–2788. [DOI] [PubMed] [Google Scholar]
- 19.Forsburg, S. L., and D. A. Sherman. 1997. General purpose tagging vectors for fission yeast. Gene 191:191–195. [DOI] [PubMed] [Google Scholar]
- 20.Furuno, N., M. Nishizawa, K. Okazaki, H. Tanaka, J. Iwashita, N. Nakajo, Y. Ogawa, and N. Sagata. 1994. Suppression of DNA replication via Mos function during meiotic divisions in Xenopus oocytes. EMBO J. 13:2399–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimm, C., J. Kohli, J. Murray, and K. Maundrell. 1988. Genetic engineering of Schizosaccharomyces pombe: a system for gene disruption and replacement using the ura4 gene as a selectable marker. Mol. Gen. Genet. 215:81–86. [DOI] [PubMed] [Google Scholar]
- 22.Gutz, H., H. Heslot, U. Leupold, and N. Loprieno. 1974. Schizosaccharomyces pombe, p. 395–446. In R. C. King (ed.), Handbook of genetics. Plenum Press, New York, N.Y.
- 23.Hagan, I., and M. Yanagida. 1995. The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J. Cell Biol. 129:1033–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagan, I. M., and J. S. Hyams. 1988. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 89:343–357. [DOI] [PubMed] [Google Scholar]
- 25.Hanks, S. K., A. M. Quinn, and T. Hunter. 1988. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241:42–52. [DOI] [PubMed] [Google Scholar]
- 26.Harper, J. W., G. R. Adami, N. Wei, K. Keyomarsi, and S. J. Elledge. 1993. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75:805–816. [DOI] [PubMed] [Google Scholar]
- 27.Hartwell, L. 1973. Genetic control of the cell division cycle in yeast: V. Genetic analysis of cdc mutants. Genetics 74:267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartwell, L. 1973. Three additional genes required for deoxynucleic acid synthesis in Saccharomyces cerevisiae. J. Bacteriol. 115:966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horie, S., Y. Watanabe, K. Tanaka, S. Nishiwaki, H. Fujioka, H. Abe, M. Yamamoto, and C. Shimoda. 1998. The Schizosaccharomyces pombe mei4+ gene encodes a meiosis-specific transcription factor containing a forkhead DNA-binding domain. Mol. Cell. Biol. 18:2118–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iino, Y., Y. Hiramine, and M. Yamamoto. 1995. The role of cdc2 and other genes in meiosis in Schizosaccharomyces pombe. Genetics 140:1235–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikemoto, S., T. Nakamura, M. Kubo, and C. Shimoda. 2000. S. pombe sporulation-specific coiled-coil protein Spo15p is localized to the spindle pole body and essential for its modification. J. Cell Sci. 113:545–554. [DOI] [PubMed] [Google Scholar]
- 32.Jackson, A. L., P. M. Pahl, K. Harrison, J. Rosamond, and R. A. Sclafani. 1993. Cell cycle regulation of the yeast Cdc7 protein kinase by association with the Dbf4 protein. Mol. Cell. Biol. 13:2899–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen, R., G. F. Sprague, Jr., and I. Herskowitz. 1983. Regulation of yeast mating-type interconversion: feedback control of HO gene expression by the mating-type locus. Proc. Natl. Acad. Sci. USA 80:3035–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang, W., and T. Hunter. 1997. Identification and characterization of a human protein kinase related to budding yeast Cdc7p. Proc. Natl. Acad. Sci. USA 94:14320–14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang, W., D. McDonald, T. J. Hope, and T. Hunter. 1999. Mammalian Cdc7-Dbf4 protein kinase complex is essential for initiation of DNA replication. EMBO J. 18:5703–5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnston, L. H., and A. P. Thomas. 1982. A further two mutants defective in initiation of the S phase in the yeast Saccharomyces cerevisiae. Mol. Gen. Genet. 186:445–448. [DOI] [PubMed] [Google Scholar]
- 37.Johnston, L. H., H. Masai, and A. Sugino. 1999. First the CDKs, now the DDKs. Trends Cell Biol. 9:249–252. [DOI] [PubMed] [Google Scholar]
- 38.Kaffman, A., I. Herskowitz, R. Tjian, and E. K. O’Shea. 1994. Phosphorylation of the transcription factor PHO4 by a cyclin-CDK complex, PHO80-PHO85. Science 263:1153–1156. [DOI] [PubMed] [Google Scholar]
- 39.Kearns, B. G., J. G. Alb, Jr., and V. Bankaitis. 1998. Phosphatidylinositol transfer proteins: the long and winding road to physiological function. Trends Cell Biol. 8:276–282. [DOI] [PubMed] [Google Scholar]
- 40.Kim, J. M., N. Sato, M. Yamada, K. Arai, and H. Masai. 1998. Growth regulation of the expression of mouse cDNA and gene encoding a serine/threonine kinase related to Saccharomyces cerevisiae CDC7 essential for G1/S transition. Structure, chromosomal localization, and expression of mouse gene for S. cerevisiae Cdc7-related kinase. J. Biol. Chem. 273:23248–23257. [DOI] [PubMed] [Google Scholar]
- 41.Kishida, M., and C. Shimoda. 1986. Genetic mapping of eleven spo genes essential for ascospore formation in the fission yeast Schizosaccharomyces pombe. Curr. Genet. 10:443–447. [DOI] [PubMed] [Google Scholar]
- 42.Kitada, K., L. H. Johnston, T. Sugino, and A. Sugino. 1992. Temperature-sensitive cdc7 mutations of Saccharomyces cerevisiae are suppressed by the DBF4 gene, which is required for the G1/S cell cycle transition. Genetics 131:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumagai, H., N. Sato, M. Yamada, D. Mahony, W. Seghezzi, E. Lees, K. Arai, and H. Masai. 1999. A novel growth- and cell cycle-regulated protein, ASK, activates human Cdc7-related kinase and is essential for G1/S transition in mammalian cells. Mol. Cell. Biol. 19:5083–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lei, M., Y. Kawasaki, M. R. Young, M. Kihara, A. Sugino, and B. K. Tye. 1997. Mcm2 is a target of regulation by Cdc7-Dbf4 during the initiation of DNA synthesis. Genes Dev. 11:3365–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masai, H., T. Miyake, and K. Arai. 1995. hsk1+, a Schizosaccharomyces pombe gene related to Saccharomyces cerevisiae CDC7, is required for chromosomal replication. EMBO J. 14:3094–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masai, H., E. Matsui, Z. You, Y. Ishimi, K. Tamai, and K. Arai. 2000. Human Cdc7-related kinase complex. In vitro phosphorylation of MCM by concerted actions of Cdks and Cdc7 and that of a criticial threonine residue of Cdc7 by Cdks. J. Biol. Chem. 275:29042–29052. [DOI] [PubMed] [Google Scholar]
- 47.Maundrell, K. 1993. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123:127–130. [DOI] [PubMed] [Google Scholar]
- 48.Miyake, S., N. Okishio, I. Samejima, Y. Hiraoka, T. Toda, I. Saitoh, and M. Yanagida. 1993. Fission yeast genes nda1+ and nda4+, mutations of which lead to S-phase block, chromatin alteration and Ca2+ suppression, are members of the CDC46/MCM2 family. Mol. Biol. Cell 4:1003–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moreno, S., A. Klar, and P. Nurse. 1990. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe Methods Enzymol. 194:793–823. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura, T., M. Kishida, and C. Shimoda. 2000. The Schizosaccharomyces pombe spo6+ gene encoding a nuclear protein with sequence similarity to budding yeast Dbf4 is required for meiotic second division and sporulation. Genes Cells 5:463–479. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura, T., M. Nakamura-Kubo, A. Hirata, and C. Shimoda. 2001. The Schizosaccharomyces pombe spo3+ gene is required for assembly of the forespore membrane and genetically interacts with psy1+ encoding syntaxin-like protein. Mol. Biol. Cell, in press. [DOI] [PMC free article] [PubMed]
- 52.Nakase, Y., T. Nakamura, A. Hirata, S. M. Routt, H. B. Skinner, V. A. Bankaitis, and C. Shimoda. 2001. The Schizosaccharomyces pombe spo20+ gene encoding a homologue of Saccharomyces cerevisiae Sec14 plays an important role in forespore membrane formation. Mol. Biol. Cell 12:901–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohsumi, K., W. Sawada, and T. Kishimoto. 1994. Meiosis-specific cell cycle regulation in maturing Xenopus oocytes. J. Cell Sci. 107:3005–3013. [DOI] [PubMed] [Google Scholar]
- 54.Ohtoshi, A., T. Miyake, K. Arai, and H. Masai. 1997. Analyses of Saccharomyces cerevisiae Cdc7 kinase point mutants: dominant-negative inhibition of DNA replication on overexpression of kinase-negative Cdc7 proteins. Mol. Gen. Genet. 254:562–570. [DOI] [PubMed] [Google Scholar]
- 55.Oshiro, G., J. C. Owens, Y. Shellman, R. A. Sclafani, and J. J. Li. 1999. Cell cycle control of Cdc7p kinase activity through regulation of Dbf4p stability. Mol. Cell. Biol. 19:4888–4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parisi, S., M. J. McKay, M. Molnar, M. A. Thompson, P. J. van der Spek, E. van Drunen-Schoenmaker, R. Kanaar, E. Lehmann, J. H. Hoeijmakers, and J. Kohli. 1999. Rec8p, a meiotic recombination and sister chromatid cohesion phosphoprotein of the Rad21p family conserved from fission yeast to humans. Mol. Cell. Biol. 19:3515–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pasero, P., B. P. Duncker, E. Schwob, and S. M. Gasser. 1999. A role for the Cdc7 kinase regulatory subunit Dbf4p in the formation of initiation-competent origins of replication. Genes Dev. 13:2159–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patterson, M., R. A. Sclafani, W. L. Fangman, and J. Rosamond. 1986. Molecular characterization of cell cycle gene CDC7 from Saccharomyces cerevisiae. Mol. Cell. Biol. 6:1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sato, N., K. Arai, and H. Masai. 1997. Human and Xenopus cDNAs encoding budding yeast Cdc7-related kinases: in vitro phosphorylation of MCM subunits by a putative human homologue of Cdc7. EMBO J. 16:4340–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schild, D., and B. Byers. 1978. Meiotic effects of DNA-defective cell division cycle mutations of Saccharomyces cerevisiae. Chromosoma 70:109–130. [DOI] [PubMed] [Google Scholar]
- 61.Sclafani, R. A., M. Patterson, J. Rosamond, and W. L. Fangman. 1988. Differential regulation of the yeast CDC7 gene during mitosis and meiosis. Mol. Cell. Biol. 8:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sclafani, R. A. 2000. Cdc7p-Dbf4p becomes famous in the cell cycle. J. Cell Sci. 113:2111–2117. [DOI] [PubMed] [Google Scholar]
- 63.Takeda, T., K. Ogino, E. Matsui, M. K. Cho, H. Kumagai, T. Miyake, K. Arai, and H. Masai. 1999. A fission yeast gene, him1+/dfp1+, encoding a regulatory subunit for Hsk1 kinase, plays essential roles in S-phase initiation as well as in S-phase checkpoint control and recovery from DNA damage. Mol. Cell. Biol. 19:5535–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanaka, T., and K. Nasmyth. 1998. Association of RPA with chromosomal replication origins requires an Mcm protein, and is regulated by Rad53, and cyclin- and Dbf4-dependent kinases. EMBO J. 17:5182–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomas, P. S. 1980. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc. Natl. Acad. Sci. USA 77:5201–5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watanabe, Y., Y. Lino, K. Furuhata, C. Shimoda, and M. Yamamoto. 1988. The S. pombe mei2 gene encoding a crucial molecule for commitment to meiosis is under the regulation of cAMP. EMBO J. 7:761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watanabe, Y., S. Shinozaki-Yabana, Y. Chikashige, Y. Hiraoka, and M. Yamamoto. 1997. Phosphorylation of RNA-binding protein controls cell cycle switch from mitotic to meiotic in fission yeast. Nature 386:187–190. [DOI] [PubMed] [Google Scholar]
- 68.Watanabe, Y., and P. Nurse. 1999. Cohesin Rec8 is required for reductional chromosome segregation at meiosis. Nature 400:461–464. [DOI] [PubMed] [Google Scholar]
- 69.Weinreich, M., and B. Stillman. 1999. Cdc7p-Dbf4p kinase binds to chromatin during S phase and is regulated by both the APC and the RAD53 checkpoint pathway. EMBO J. 18:5334–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woods, A., T. Sherwin, R. Sasse, T. H. MacRae, A. J. Baines, and K. Gull. 1989. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J. Cell Sci. 93:491–500. [DOI] [PubMed] [Google Scholar]
- 71.Yamamoto, M., Y. Imai, and Y. Watanabe. 1997. Mating and sporulation in Schizosaccharomyces pombe, p. 1037–1106. In J. R. Pringle, J. B. Broach, and E. W. Jones (ed.), Molecular and cellular biology of the yeast Saccharomyces. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 72.Yoon, H. J., and J. L. Campbell. 1991. The CDC7 protein of Saccharomyces cerevisiae is a phosphoprotein that contains protein kinase activity. Proc. Natl. Acad. Sci. USA 88:3574–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]