Abstract
Under low-oxygen conditions, cells develop an adaptive program that leads to the induction of several genes, which are transcriptionally regulated by hypoxia-inducible factor 1 (HIF-1). On the other hand, there are other factors which modulate the HIF-1-mediated induction of some genes by binding to cis-acting motifs present in their promoters. Here, we show that c-Jun functionally cooperates with HIF-1 transcriptional activity in different cell types. Interestingly, a dominant-negative mutant of c-Jun which lacks its transactivation domain partially inhibits HIF-1-mediated transcription. This cooperative effect is not due to an increase in the nuclear amount of the HIF-1α subunit, nor does it require direct binding of c-Jun to DNA. c-Jun and HIF-1α are able to associate in vivo but not in vitro, suggesting that this interaction involves the participation of additional proteins and/or a posttranslational modification of these factors. In this context, hypoxia induces phosphorylation of c-Jun at Ser63 in endothelial cells. This process is involved in its cooperative effect, since specific blockade of the JNK pathway and mutation of c-Jun at Ser63 and Ser73 impair its functional cooperation with HIF-1. The functional interplay between c-Jun and HIF-1 provides a novel insight into the regulation of some genes, such as the one for VEGF, which is a key regulator of tumor angiogenesis.
Tissue oxygen tension is an essential regulatory factor in many physiological as well as pathological processes. Under low-oxygen conditions, cells develop a series of adaptive responses, including changes in their patterns of gene expression. Thus, hypoxia has been shown to induce the expression of erythropoietin (45), VEGF (15), tyrosine hydroxylase (37), transferrin receptor (50), glycolytic enzymes and glucose transporters (46), LDH A (14), and others.
The expression of the genes for most of these proteins is transcriptionally regulated by hypoxia-inducible factor 1 (HIF-1). HIF-1 is a heterodimer comprising α and β (also called ARNT) subunits, which belong to the bHLH PAS family of transcription factors (23). Both HIF-1α and HIF-1β mRNAs are constitutively transcribed; the HIF-1β protein level is not modified by oxygen tension, whereas HIF-1α protein is rapidly degraded by the proteasome under normoxic conditions (42) in a process in which the VHL tumor supressor protein is directly involved (32). The mechanism for HIF-1α stabilization by hypoxia is beginning to be elucidated. In this regard, it has been reported that prolyl hydroxilation in normoxia targets HIF-1α for VHL-mediated degradation (19, 21). Phosphatidic acid production is involved in HIF-1α stabilization (1), and hypoxia-induced generation of reactive species at mitochondria has also been shown to participate in this process, though this is currently a controversial issue (10, 48, 54). HIF-1α translocates into the nucleus, where it dimerizes with the β subunit and binds to cognate sequences in the regulatory regions of its target genes (the hypoxia response element [HRE]). HIF-1 binding to DNA and transcriptional activity have been proposed to be regulated by phosphorylation. In this regard, it has recently been shown that HIF-1 is a substrate for p42 mitogen-activated protein kinase (39). In addition, the p300 transcriptional coactivator has been shown to associate with HIF-1 and seems to be essential for its transcriptional activity (2, 24). HIF-1 has been demonstrated to be crucial in tumor angiogenesis and embryonic vascularization (8, 20, 31, 41), processes in which endothelial cells play a key role, since they are able to form new vascular structures upon stimulation by proangiogenic factors, such as VEGF (47).
Low oxygen tension has also been shown to induce the expression of several AP-1 proteins, including c-Jun, JunB, and c-Fos (3), and it modulates their DNA binding activity in some cases (4, 59). On the other hand, members of the AP-1 family of transcription factors bind to cis-acting elements present in the promoters of the VEGF, tyrosine hydroxylase, and endothelin-1 genes, where they potentiate their HIF-1-mediated hypoxic induction (12, 37, 58). This seems to be a recurrent feature in hypoxia-regulated genes, since ATF/CREB proteins bind constitutively to the HIF-1 consensus sequence in the EPO 3′ enhancer (27) and have been shown to cooperate with HIF-1 in the induction of the lactate dehydrogenase A gene (14). IRF-1 and HIF-1 can also cooperate in the induction of the NOS2 gene in macrophages (51).
c-Jun is a member of the basic region-leucine zipper (bLZ) family of transcription factors. This protein can form a variety of dimeric complexes with other bLZ factors, such as Jun-Jun and Jun-Fos dimers (which recognize AP-1 sites), as well as Jun-ATF dimers (which bind CRE-like sequences) (17). c-Jun has also been shown to associate with and enhance the transcriptional activity of other transcription factors, such as p65, Sp1, and PU.1 (6, 25, 49). In addition, several proteins, including Rb and members of the Smad family, are able to interact with c-Jun and potentiate AP-1-dependent transcription (36, 62).
c-Jun is involved in many cellular processes, including proliferation, transformation, differentiation, apoptosis, and stress-adaptive responses (53, 57). This transcription factor is regulated by different extracellular stimuli, such as growth factors, cytokines, heat shock, oxidative and osmotic stresses, and UV irradiation, which can act through transcriptional and posttranscriptional mechanisms. In this regard, the phosphorylation of c-Jun at Ser63 and Ser73 by kinases of the JNK/SAPK family increases its DNA binding and transcriptional activity and contributes to the stability of this factor (18, 35). Among the variety of stimuli which can regulate the JNK pathway, hypoxia and/or reoxygenation have been described as activating some of these kinases; however, this effect seems to depend largely on the cell type and the degree of oxygen deprivation (29, 34, 44).
In this report, we show that the c-Jun transcription factor functionally cooperates with HIF-1, potentiating its transcriptional activity. This effect is not mediated by an increase in HIF-1 nuclear protein content and does not depend on direct binding of c-Jun to DNA. On the other hand, c-Jun and HIF-1 associate with each other in vivo, and this interaction probably requires an additional protein or a posttranslational modification of these factors. In addition, we have found that phosphorylation of c-Jun at Ser63 by JNK in hypoxia seems to be involved in the transcriptional cooperation between these factors.
MATERIALS AND METHODS
Cell cultures and hypoxic conditions.
Human umbilical vein cells (HUVEC) were isolated from normal human umbilical cord veins. The umbilical vein was cannulated and incubated with 1% collagenase for 15 min at 37°C. Following removal of the collagenase, cells were pooled and established as primary cultures in medium 199 (Biowhittaker, Walterville, Md.) containing 20% fetal calf serum (FCS). HUVEC were serially passaged and maintained using medium 199 supplemented with 20% FCS, 50 μg of bovine brain extract/ml, and 100 μg of heparin (Sigma, St. Louis, Mo.)/ml in tissue culture dishes precoated with 0.5% gelatin. The cells were used within the first three passages. The human dermal microvascular endothelial cell line HMEC-1 was kindly provided by E. W. Ades and T. J. Lawly (Centers for Disease Control and Prevention and Emory University School of Medicine, Atlanta, Ga.). HMEC-1 cells were grown in MCDB 131 medium (Life Technologies, Paisley, Scotland) supplemented with 20% FCS, 10 ng of epidermal growth factor (Promega, Madison, Wis.)/ml, and 1 mg of hydrocortisone (Sigma)/ml. The F9 murine teratocarcinoma cell line was maintained in Dulbecco’s modified Eagle’s medium (Biochrom KG, Berlin, Germany) supplemented with 10% FCS, and COS-7 cells were cultured in RPMI 1640 (Life Technologies) with 10% FCS. The murine hepatoma Hepa-1c1c7 cell line and mutant Hepa-1c4 cells were a gift of O. Hankinson (Department of Pathology and Laboratory Medicine, UCLA Medical Center, Los Angeles, Calif.), and were grown in minimal essential medium alpha (Life Technologies) supplemented with 10% FCS.
To achieve a hypoxic atmosphere, cells were placed in an airtight chamber, infused with a mixture of 1% O2-5% CO2-94% N2 (S. E. Carburos Metalicos S.A., Madrid, Spain) for 30 min and then cultured under these conditions for different periods of time.
Plasmids.
To generate the p1HIF1Luc and p9HIF1Luc reporter plasmids, one or nine copies, respectively, of an oligonucleotide corresponding to the HIF-1 binding sequence located between the −984 and −950 positions of the 5′ human VEGF gene promoter (−984 ccacagtgcaTACGTGGGctccaacaggtcctctt −950; the core HIF-1 binding sequence is in capital letters) were cloned in front of the rat prolactin minimal promoter, followed by the luciferase cDNA. p1HIF1mLuc was generated as described above, but the oligonucleotide sequence was 5′ ccacagtgcaTAAAAGGGctccaacaggtcctctt 3′ (mutated bases at the HIF-1 binding site are underlined). pcDNA3α1 and pARNT were kindly provided by E. Huang (Brigham and Women’s Hospital, Harvard Medical School, Boston, Mass.). pRSV c-Jun was a gift of P. Angel (Deutsches Krebsforschungszentrum, Heidelberg, Germany). pKBF-Luc and 2XAP1 Luc were kindly provided by A. Israel (Institut Pasteur, Paris, France) and M. Rincón (Yale University School of Medicine, New Haven, Conn.), respectively. The human NFATc expression vector pSH107c was a gift of G. R. Crabtree (Howard Hughes Medical Institute, Stanford University School of Medicine, Palo Alto, Calif.). pCMV TAM67 and pcDNA3-Flag-JBD (JIP1) were kindly provided by R. Pope (Department of Medicine, Northwestern University Medical School, Chicago, Ill.) and Pura Muñoz (Institut de Reserca Oncologica, Barcelona, Spain), respectively. pRSV c-Jun S63A/S73A was a gift of M. Karin (Department of Pharmacology, University of California).
Transfections and analysis of luciferase activity.
In transient-transfection experiments, 1.75 × 105 F9 cells were plated on 35-mm-diameter culture dishes and grown overnight. Then, the cells were transfected in OPTI-MEM medium (Life Technologies) containing 7.8 μg of Lipofectin (Life Technologies), 0.5 μg of the different reporter plasmids, and 1 μg of each expression vector or empty control plasmid to a total DNA amount of 2.6 μg. Transfection efficiency was normalized by cotransfection of Renilla expression vector. After 5 h, the Lipofectin-containing medium was removed, and the cells were incubated in normoxia (21% O2) or hypoxia (1% O2) for 12 h. Then, the cells were lysed, and luciferase activity was measured and normalized using the Dual-Luciferase Reporter assay system (Promega) with a Lumat LB9501 luminometer (Berthold, Wildbad, Germany). HMEC-1, Hepa-1c1c7, and Hepa-1c4 cells were plated at near confluence on 24-well culture plates and transfected in Dulbecco’s modified Eagle’s medium-10% fetal bovine serum using a standard calcium phosphate method. The amounts of the different reporter plasmids were 0.2 μg for HMEC-1 and 0.25 μg for Hepa cells, and 0.2 (HMEC-1 cells) or 0.5 μg (Hepa cells) of each expression vector or empty control plasmid was used. After 8 h in the presence of DNA precipitates, the cells were washed with phosphate-buffered saline and cultured in fresh medium for 16 h. The transfected cells were then cultured in normoxia or hypoxia for 8 h and processed as for F9 cells. COS-7 cells were electroporated (960 μF; 200 V) with 8 μg of each expression vector or control empty vector and carrier DNA to a total amount of 25 μg per 107 cells. After 40 h, the cells were incubated in normoxia or hypoxia for 5 h, and nuclear extracts were obtained. Transfection efficiency was estimated by cotransfecting the pEGFP-C1 plasmid (Clontech, Palo Alto, Calif.) and analyzing the percentage of positive cells by flow cytometry.
Expression of proteins in vitro.
Expression of proteins in vitro was performed using the coupled in vitro transcription-translation system (TNT) (Promega) according to the manufacturer’s instructions. In some cases, in vitro-expressed proteins were labeled by including [35S]methionine in the TNT reaction mixture.
Nuclear extracts.
After different treatments, nuclear extracts were prepared according to a procedure described elsewhere (1) with some modifications. Briefly, cell plates were washed once with ice-cold phosphate-buffered saline and incubated with 1 ml of buffer A (10 mM HEPES [pH 7.3], 10 mM KCl, 0.1 mM EDTA, 0.75 mM spermidine, 0.15 mM spermine, 1 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride [PMSF], 10 mM Na2MoO4, 1 μg of pepstatin/ml, 6 μg of aprotinin/ml, and 3 μg of leupeptin/ml) on an orbital platform. Then 62.5 μl of Nonidet P-40 (10%) was added, and the cell nuclei were harvested with a cell scraper and collected by centrifugation for 30 s at 14,000 × g. The nuclear pellet was washed twice with buffer A, and then nuclear protein was extracted with 25 to 30 μl of buffer C (20 mM HEPES [pH 7.3], 400 mM NaCl, 1 mM EDTA, 1 mM DTT, 0.5 mM PMSF, 10 mM Na2MoO4, 1 μg of pepstatin/ml, 6 μg of aprotinin/ml, and 3 μg of leupeptin/ml). After 30 min on a rocking platform and further centrifugation at 14,000 × g for 10 min, the supernatants were collected and stored at −80°C. All steps were performed on ice or at 4°C. Nuclear extract protein concentrations were determined by Bradford assay.
Electrophoretic mobility shift assays.
Nuclear extracts (3 μg per lane) or in vitro-translated proteins were incubated with 0.5 μg of poly(dI-dC), 100 ng of calf thymus DNA, and 2.5 μl of 5× DNA binding buffer (50 mM Tris buffer [pH 7.5], 130 mM KCl, 5 mM MgCl2, 2.5 mM ZnCl2, 25 mM DTT, 5 mM EDTA, 25% [vol/vol] glycerol) for 10 min at room temperature. For supershift assays, we generated a polyclonal antibody raised against the first 13 amino acids of the human HIF-1α protein following standard methods; 1 μl of this antibody or polyclonal antibodies against c-Jun (D) and c-Fos (K-25) (Santa Cruz Biotechnology, Santa Cruz, Calif.) was added to the binding reaction and incubated for 10 min. Next, 0.5 to 1 ng of avian myeloblastosis virus reverse transcriptase (Promega) 32P-labeled double-stranded oligonucleotide were added. After 15 min of incubation at room temperature, DNA-protein complexes were resolved by electrophoresis on a 4% nondenaturing polyacrylamide gel in 0.5× Tris-borate-EDTA buffer. The sequences of the oligonucleotides used as probes in these assays were as follows (5′ to 3′; HIF-1 and AP-1 consensus binding sites are underlined): TCGACCACAGTGCATACGTGGGCTCCAACAGGTCCTCTTC and its complementary strand (the nucleotides spanning from −984 to −950 of the human VEGF 5′ gene promoter), and GCCCCCTCTGACTCATGCTGACA and its complementary strand (nucleotides −68 to −46 of the CD11c gene promoter).
Western blot and immunoprecipitation assays.
For Western blot experiments, 10 to 20 μg of protein per lane was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. The membranes were probed with anti-HIF-1α (Transduction Laboratories, Lexington, Ky.), anti-c-Jun (D or H-79; Santa Cruz Biotechnology), or anti-phospho c-Jun (KM-1; Santa Cruz Biotechnology) antibody. Immunoreactive bands were detected by an enhanced chemiluminiscent substrate (Supersignal; Pierce, Rockford, Ill.). For immunoprecipitation assays, COS-7 cells were transfected as described above, and nuclei were extracted and lysed at 4°C in lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 0.2% NP-40, 1 mM DTT, 10 mM β-glycerophosphate, 1 mM sodium vanadate, 1 mM NaF, 1 mM PMSF, and 1 mg each of aprotinin, leupeptin, and pepstatin/ml). The lysates were precleared with rabbit preimmune serum for 30 min at 4°C and then incubated with either anti-HIF-1α, anti c-Jun (D or H-79), or control polyclonal antibodies coupled to protein A-Sepharose (Amersham Pharmacia Biotech, Uppsala, Sweden) for 3 h at 4°C. After being washed, immunocomplexes were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and blotted with anti-HIF-1α or anti-c-Jun monoclonal antibody (Transduction Laboratories). As a control, aliquots of cell lysates were subjected to direct immunoblotting. In some cases, in vitro-translated, [35S]methionine-labeled proteins were immunoprecipitated as described above, and immunocomplexes were resolved by SDS-PAGE and detected by autoradiography.
Statistical analysis.
Experimental data were analyzed by the analysis of variance test followed by the Newman-Keuls test; the P values obtained are indicated in the text and/or in the figure legends when statistically significant.
RESULTS
c-Jun potentiates HIF-1-dependent transcription.
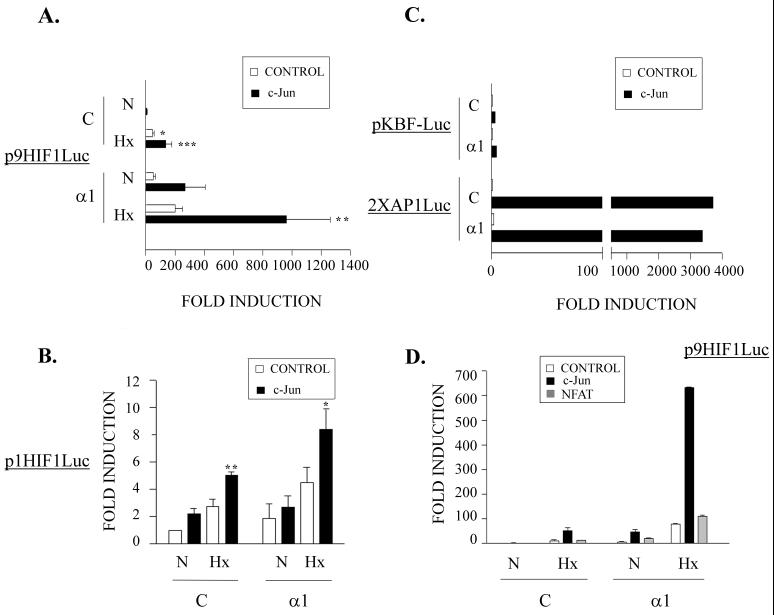
To analyze the effect of c-Jun on HIF-1 transcriptional activity, the c-Jun-defective F9 cell line was transiently transfected with the p9HIF1Luc reporter plasmid (which contains nine copies of the HRE from the VEGF 5′ untranslated region [UTR]) along with expression vectors for c-Jun and the α1 subunit of HIF-1 or their corresponding control vectors. After transfection, the cells were incubated in either a normoxic (21% O2) or a hypoxic (1% O2) atmosphere (Fig. 1A). The overexpression of c-Jun alone had little effect under normoxic conditions, but it significatively enhanced transcription from the HIF-1-dependent construct in hypoxia (P < 0.001). The transfection of the α1 subunit was sufficient to transactivate p9HIF1Luc in F9 cells, both in normoxia and in hypoxia (Fig. 1A), probably because they have enough endogenous β subunit to dimerize with overexpressed α1. Interestingly, c-Jun was able to increase HIF-1 transcriptional activity when cotransfected with the α1 subunit of this factor under hypoxic conditions (P < 0.01). Similar experiments were performed with p1HIF1Luc, which contains a single copy of the HIF-1 DNA binding sequence from the VEGF 5′ UTR (Fig. 1B). Despite the lower levels of induction, the results were similar to those obtained with p9HIF1Luc, ruling out a cis-acting cooperation between these two transcription factors due to the use of a multimerized sequence within the reporter plasmid.
FIG. 1.
Cooperative effect of c-Jun on HIF-1 transcriptional activity. (A) c-Jun-defective F9 teratocarcinoma cells were transiently transfected with 0.5 μg of the p9HIF1Luc luciferase reporter plasmid together with 1 μg of pcDNA3α1 (α1) or the empty vector pcDNA3 (C) and pRSV c-Jun (solid bars) or control pUCRSV empty vector (open bars). After transfection, the cells were incubated in 1% O2 (Hx) or 21% O2 (N) for 12 h and then analyzed for luciferase activity. (B) F9 cells were transfected and processed as for panel A, but the luciferase reporter plasmid was p1HIF1Luc. The data in panels A and B are the means + standard errors of the mean of five independent experiments performed in duplicate. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 compared to controls in normoxia. (C) F9 cells were transfected as described above, but the luciferase reporter plasmids were pKBF-luc and 2XAP1Luc. (D) F9 cells were transiently transfected with the p9HIF1Luc luciferase reporter plasmid (0.5 μg) together with 1 μg of pcDNA3α1 (α1) or control pcDNA3 (C) and expression vectors for c-Jun (solid bars), NFATc (shaded bars), or control empty vector (open bars) and processed as for panel A. The data are the means + standard deviations of a representative experiment out of three performed in duplicate.
The cooperative effect between c-Jun and α1 was not due to a nonspecific activation of transcription, since it could not be observed when these expression vectors were cotransfected with other reporter plasmids, such as pKBF-Luc (with a trimer of the NFKB motif of the H-2Kb gene) and 2xAP1 Luc (which contains two AP-1 sites from the human collagenase promoter) (Fig. 1C). In addition, cotransfection of an NFATc expression vector, alone or in combination with the HIF-1 α subunit, did not have any effect on HIF-1-dependent transcription (Fig. 1D). These results strongly suggest that c-Jun is able to functionally cooperate with HIF-1, markedly enhancing its transcriptional activity.
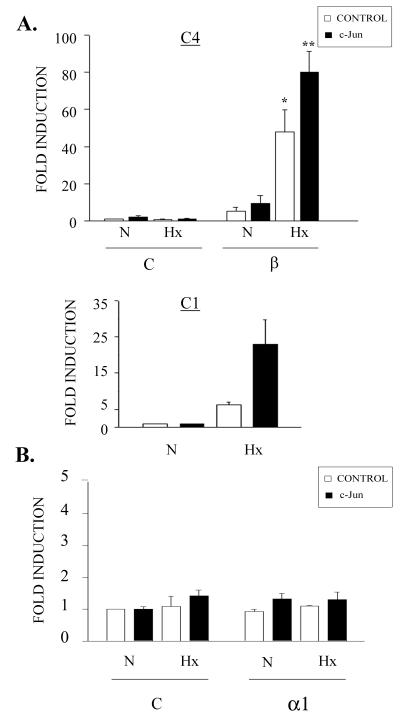
In order to assess whether the effect of c-Jun was dependent on the presence of HIF-1, transient-transfection experiments were carried out in mouse hepatoma Hepa-1c4 cells. This cell line is derived from Hepa-1c1c7 cells and contains a mutant HIF-1 β subunit which fails to form functional HIF-1 heterodimers (16). As expected, when the p9HIF1Luc reporter vector was transfected alone, there was no transcriptional activation of this construct in hypoxia (Fig. 2A, top). In contrast, cotransfection of wild-type HIF-1β, which allows the formation of functional HIF-1 heterodimers in hypoxia, restored hypoxic induction of p9HIF1Luc in these cells. Transient transfection of the c-Jun expression vector alone did not give rise to any transcriptional activation of the p9HIF1Luc reporter plasmid, under either normoxic or hypoxic conditions. Interestingly, cotransfection of both HIF-1β and c-Jun expression vectors significantly restored c-Jun cooperative effect (P < 0.01). To discard any nonspecific effect due to HIF-1β overexpression, similar experiments were performed with parental Hepa-1c1c7 cells, in which c-Jun was able to potentiate HIF-1 transcriptional activity in hypoxia (Fig. 2A, bottom). In order to investigate whether the cooperative effect of c-Jun requires the presence of a functional HIF-1 DNA binding site, we performed experiments with a construct containing a mutated version of the HRE (p1HIF1mLuc). As shown in Fig. 2B, the mutagenesis of the HIF-1 DNA binding site also abrogated the cooperative effect of c-Jun, suggesting that this factor acts in an HRE-dependent fashion. Altogether, these data indicate that c-Jun transcription factor enhances HIF-1-dependent transcription and that this effect requires the presence of a functional HIF-1 and the integrity of its DNA binding site to promote this cooperative effect.
FIG. 2.
Lack of c-Jun cooperative effect in the absence of a functional HIF-1 or a preserved HIF-1-response element. (A) (Top) HIF-1β-defective Hepa-1c4 mouse hepatoma cells (C4) were transfected with 0.25 μg of p9HIF1Luc reporter plasmid together with 0.5 μg of pARNT (β) or the empty vector pcDNA3 (C) and pRSV c-Jun (solid bars) or control pUCRSV empty vector (open bars). Sixteen hours after transfection, the cells were grown under normoxic (N) or hypoxic (Hx) conditions for 8 h, and luciferase activity was determined. The histogram shows the means + standard errors of the mean of three independent experiments performed in duplicate; ∗, P < 0.05, and ∗∗, P < 0.001 compared to control in normoxia. (Bottom) The Hepa-1c1c7 mouse hepatoma cell line (C1) was transfected with the p9HIF1Luc reporter plasmid together with pRSV c-Jun (solid bars) or control pUCRSV empty vector (open bars) and processed as C4 cells. The data are means + standard deviations of a representative experiment out of four performed in triplicate. (B) F9 cells were transfected with 0.5 μg of p1HIF1mLuc luciferase reporter plasmid (which contains a mutated HRE) together with 1 μg of pcDNA3 α1 (α1) or empty vector pcDNA3 (C) and pRSV c-Jun (solid bars) or control pUCRSV empty vector (open bars). After transfection, the cells were processed as for panel A. The data are means + standard errors of the mean of three independent experiments performed in duplicate.
A dominant-negative form of c-Jun partially inhibits HIF-1-dependent transcriptional activation.
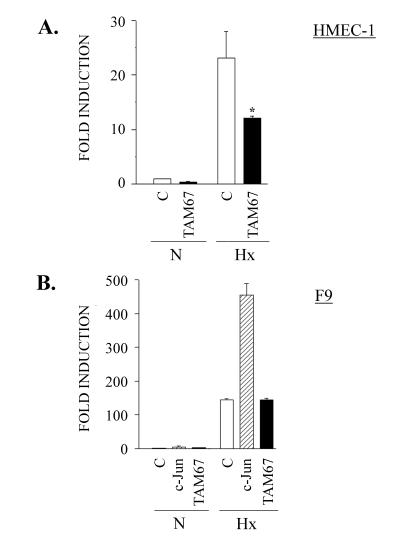
HIF-1 has been demonstrated to be crucial in tumor angiogenesis and embryonic vascularization, processes in which endothelial cells play an essential role (41). Once it was established that overexpressed c-Jun functionally cooperates with HIF-1, we wanted to investigate whether, in this cell type, endogenous c-Jun was also able to modulate HIF-1 transcriptional response in a similar fashion. For this purpose, the HMEC-1 endothelial cell line was transiently transfected with the p9HIF1Luc reporter plasmid, together with an expression vector for the dominant-negative form of c-Jun, TAM67, which lacks its transactivation domain (60). As shown in Fig. 3A, this c-Jun mutant was capable of partially inhibiting hypoxic induction of the p9HIF1Luc reporter plasmid (P < 0.05). In order to confirm the specificity of this effect, we transiently transfected c-Jun-defective F9 cells with p9HIF1Luc, along with expression vectors for wild-type c-Jun or its dominant-negative version, TAM67 (Fig. 3B). In the absence of c-Jun, the dominant-negative construct did not significantly affect the endogenous HIF-1 transcriptional activity in hypoxia, whereas wild-type c-Jun again had a cooperative effect. These findings are in concordance with those shown in Fig. 1A and indicate that endogenous c-Jun transcription factor is involved in the induction of HIF-1-dependent genes in hypoxia. In addition, these results suggest that the transactivation domain of c-Jun is necessary for enhancing HIF-1 transcriptional response.
FIG. 3.
Effect of c-Jun dominant-negative mutant on HIF-1-dependent transcription. (A) The HMEC-1 endothelial cell line was transiently transfected with 0.2 μg of p9HIF1Luc reporter plasmid together with 0.2 μg of pCMV TAM67 (TAM 67), or control vector pCMV (C). Sixteen hours after transfection, the cells were either left untreated (N) or grown in 1% O2 for 8 h (Hx) and analyzed for luciferase activity. The data are means + standard errors of the mean of four independent experiments performed in duplicate. ∗, P < 0.05 compared to control in hypoxia. (B) F9 cells were transiently transfected with 0.5 μg of p9HIF1Luc together with 1 μg of pRSVc-Jun (c-Jun), pCMV TAM67, or control empty vector. After transfection, the cells were incubated under normoxic (N) or hypoxic (Hx) conditions for 12 h, and then luciferase activity was measured. The data are means plus standard errors of the mean of three independent experiments performed in duplicate.
c-Jun overexpression does not modify nuclear HIF-1 protein level.
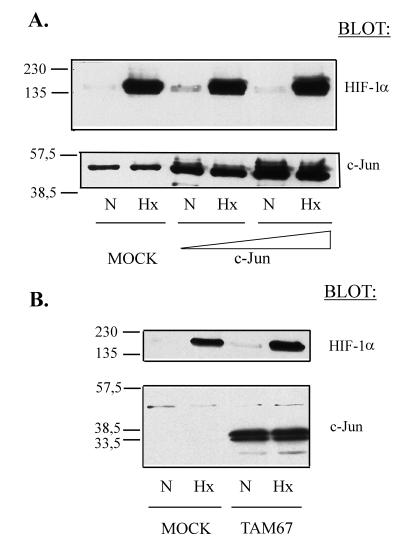
We next decided to analyze whether the overexpression of wild-type c-Jun or its dominant-negative version, TAM67, could modulate the endogenous HIF-1α protein level as a mechanism for their functional cooperation. COS-7 cells were used as a model, because transient-transfection assays yielded a high efficiency (70 to 80%) and functional cooperation between c-Jun and HIF-1 was also observed in this cell line (data not shown). COS-7 cells were transiently transfected with control empty vector or increasing amounts of wild-type c-Jun expression vector. After transfection, the cells were grown under normoxic or hypoxic conditions, and nuclear extracts were obtained. Western blot analysis of these extracts showed that the overexpression of c-Jun does not significantly modify the amount of endogenous HIF-1α (Fig. 4A). Parallel experiments showed that overexpression of the c-Jun dominant-negative form, TAM67, did not alter the endogenous HIF-1α protein level either, indicating that the inhibitory effect of this mutant on HIF-1-dependent transcription is not due to a decrease in the nuclear amount of the transcription factor (Fig. 4B).
FIG. 4.
Effect of c-Jun or its dominant-negative mutant on HIF-1α expression. (A) COS-7 cells were transfected with control empty vector (MOCK) or increasing amounts of c-Jun expression vector (c-Jun). After transfection, the cells were grown under normoxic (N) or hypoxic (Hx) conditions for 5 h. Nuclear extracts were obtained and subjected to immunoblotting (20 μg per lane) with an anti-HIF-1α antibody (top) or an anti-c-Jun antibody (bottom). (B) COS-7 cells were transfected with 8 μg of control empty vector (MOCK) or pCMV TAM67 (TAM67) and processed as for panel A. Molecular weight markers are shown on the left.
c-Jun is present in the DNA-protein complexes obtained with the HIF-1 consensus sequence of the VEGF 5′ UTR.
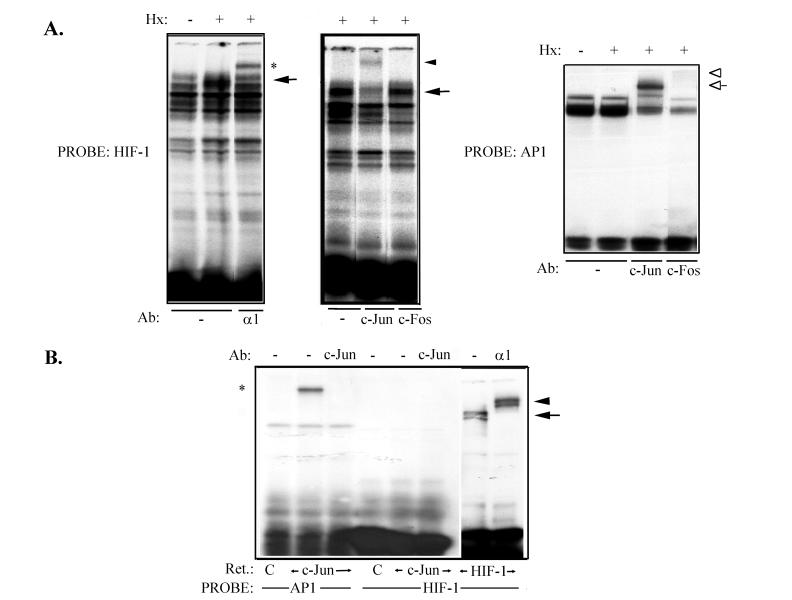
We next decided to investigate whether the functional cooperation between c-Jun and HIF-1 required c-Jun binding to the HIF-1 recognition site present in the VEGF 5′ UTR, used as a target sequence in our functional assays. To address this issue, electrophoretic mobility shift assays were performed with nuclear extracts from HUVEC grown under normoxic or hypoxic conditions. These extracts were incubated with a 32P-labeled probe containing the above-mentioned sequence (Fig. 5A). A hypoxia-inducible complex was observed, which could be identified as HIF-1 in supershift experiments with a specific polyclonal antibody against the α1 subunit of this factor. Interestingly, a specific antibody against c-Jun also supershifted a DNA-protein complex, whereas a control antibody against c-Fos did not have any effect (Fig. 5A). Parallel assays were carried out with a 32P-labeled probe containing a consensus AP-1 site from the CD11c promoter as a control (Fig. 5A). A constitutive DNA-protein complex was formed, in which both c-Jun and c-Fos could be detected with specific antibodies. Despite these results, no apparent AP-1 consensus site (5′-TGAC/GTCA-3′) is present in the sequence used for functional assays and binding experiments (see Materials and Methods). Electrophoretic mobility shift assays carried out with in vitro-translated c-Jun (Fig. 5B) showed that c-Jun alone is not able to bind directly to the HIF-1 recognition site of the VEGF 5′ UTR or its flanking sequences, though it does form a specific complex with an AP-1 site-containing probe, suggesting that it requires interaction with another protein(s) to bind this sequence in vivo.
FIG. 5.
c-Jun binding to VEGF 5′ UTR HIF-1 consensus sequence in endothelial cells. (A) Electrophoretic mobility shift assay of nuclear extracts obtained from primary endothelial cells either untreated or subjected to hypoxia (Hx) for 4 h; these extracts were incubated (3 μg per lane) with a 32P-labeled probe which contains the HIF-1 DNA binding consensus sequence of the VEGF 5′ UTR (HIF-1; 0.5 ng per lane), showing a hypoxia-inducible DNA-protein complex (arrows). In some cases, nuclear extracts were incubated with specific antibodies (Ab) against the HIF-1α subunit (α1) (left) or c-Jun or c-Fos (middle) before the probe was added. The autoradiographs show supershifted complexes with anti-HIF-1α (asterisk) and c-Jun (arrowhead) antibodies, whereas anti-c-Fos had no effect; (right) electrophoretic mobility shift assay performed as described above but with a 32P-labeled probe containing an AP-1 consensus sequence from the CD11c promoter (AP-1) used as a positive control. Supershifted complexes can be observed with both anti-c-Jun (open arrow) and anti c-Fos (open arrowhead) antibodies. (B) In vitro-translated (Ret.) pcDNA3 (C), c-Jun, or HIF-1 (α1 plus β subunits) was incubated with the same labeled probes as for panel A (1 ng per lane). Specific complexes with AP-1 (asterisk) and HIF-1 (arrow) probes could be observed, which could be identified, respectively, as c-Jun and the HIF-1 α subunit (arrowhead) with specific antibodies (Ab) against these factors. +, present; −, absent.
Association between c-Jun and HIF-1α.
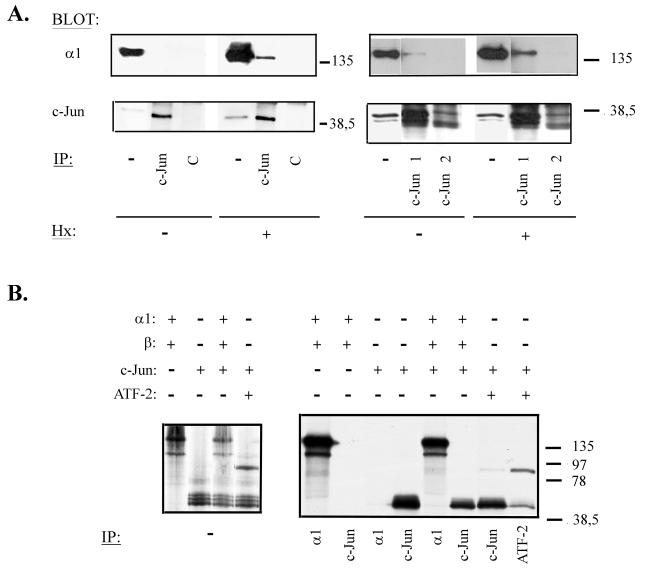
In order to investigate whether functional cooperation between c-Jun and HIF-1α requires a physical interaction of these proteins in vivo, coimmunoprecipitation experiments were conducted with COS-7 cells transiently transfected with expression vectors for both transcription factors. After transfection, the cells were cultured in normoxia or hypoxia. Then, nuclear extracts were obtained and immunoprecipitated with a polyclonal antibody against c-Jun or a control antibody and subjected to Western blot analysis. Under hypoxic conditions, HIF-1α could be detected in c-Jun immunocomplexes (Fig. 6A, left). These findings indicate that c-Jun and HIF-1 can associate in vivo in hypoxic cells and suggest that this association can be involved in the functional cooperation between the factors. On the other hand, coimmunoprecipitation assays performed with COS-7 cells transiently transfected with TAM67 and HIF-1α expression vectors (Fig. 6A, right) showed that TAM67 also associates with this transcription factor, suggesting that the c-Jun domain(s) involved in its interaction with HIF-1 is preserved in this truncated form.
FIG. 6.
Association between c-Jun and HIF-1α. (A) COS-7 cells were cotransfected with 8 μg of pcDNA3α1 and pRSV c-Jun (left) or pCMV TAM67 (right) expression vector. After 40 h, the cells were incubated in normoxia or hypoxia (Hx) for 4 h, and nuclear extracts were obtained. These extracts were immunoprecipitated (IP) with polyclonal antibodies against the DNA binding domain (c-Jun and c-Jun 1) or the transactivation domain (c-Jun 2) of c-Jun or a control antibody (C) and immunoblotted with anti-HIF-1α (top) and anti-c-Jun (bottom) antibodies; as a control for protein expression, aliquots of the lysates (representing 1/10 of each immunoprecipitation reaction) (−) were also subjected to Western blotting with antibodies against HIF-1α and c-Jun. (B) HIF-1α (α1), c-Jun, HIF-1β (β), and ATF-2 were in vitro translated or cotranslated in the presence of [35S]methionine and immunoprecipitated with antibodies against HIF-1α (α1), c-Jun, or ATF-2. The immunocomplexes were analyzed by SDS-PAGE (right); the autoradiograph on the left shows 1/10 of each translation reaction as a control. +, present; −, absent. Molecular weight markers are shown on the right.
These experiments indicate that HIF-1α and c-Jun associate with each other, but they do not demonstrate a direct interaction between the factors. To elucidate this issue, coimmunoprecipitation experiments were carried out with in vitro-translated proteins and polyclonal antibodies specific to c-Jun and HIF-1α (Fig. 6B). Neither c-Jun antibody was able to immunoprecipitate cotranslated HIF-1α, nor was associated c-Jun detected in HIF-1α immunocomplexes. As controls for the experimental conditions, the HIF-1 β subunit and ATF-2 transcription factor were cotranslated in some of the reactions, and they could be coimmunoprecipitated with their dimerization partners, HIF-1α and c-Jun, respectively. The presence of HIF-1β did not interfere with the interaction between HIF-1α and c-Jun, since similar results were obtained when HIF-1β was not included in the reaction (data not shown). These results indicate that the association of c-Jun and HIF-1α found in in vivo experiments is probably mediated through another protein(s) or that it requires a posttranslational modification of one or both transcription factors, which takes place only in the cell.
Hypoxia-induced phosphorylation of c-Jun at Ser63 is involved in the functional cooperation between HIF-1 and c-Jun.
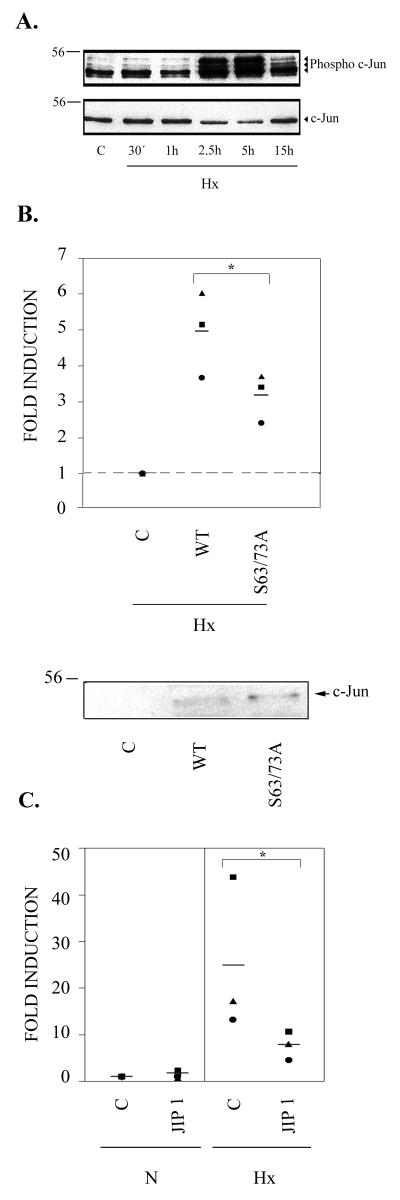
There are previous reports that JNK can be activated by hypoxia and/or reoxygenation in several cell types (29, 34, 44). In order to analyze whether hypoxia induced the phosphorylation of endogenous c-Jun in endothelial cells, Western blot assays were carried out with nuclear extracts of HUVEC, blotted with an antibody which specifically recognizes c-Jun phosphorylated at Ser63. Hypoxia induced a transient c-Jun phosphorylation, with a peak between 2.5 and 5 h (Fig. 7A). To investigate whether this phosphorylation of c-Jun in hypoxia was involved in the enhancement of HIF-1-dependent transcription, F9 cells were transiently transfected with the p9HIF1Luc reporter vector, together with expression vectors for wild-type c-Jun or a construct in which Ser63 and Ser73 were mutated to Ala residues. As shown in Fig. 7B, a significant (P < 0.05) reduction in the cooperative effect of c-Jun on HIF-1 transcriptional activity in hypoxia was found in c-Jun S63A-S73A compared with the wild type. Western blot assays of nuclear extracts from F9 cells transfected as described above showed no differences in the levels of expression of both constructs which could be responsible for this transcriptional effect. To confirm the involvement of the JNK pathway in the c-Jun cooperative effect, we carried out transient-transfection experiments with HMEC-1 cells with an expression vector for the JNK-binding domain (JBD) of the JIP1 scaffold protein. Overexpression of this protein has been shown to specifically block JNK-regulated signal transduction (13). As shown in Fig. 7C, JIP1 partially inhibited hypoxic induction of the p9HIF1Luc reporter plasmid (P < 0.05). These data indicate that JNK-mediated phosphorylation of c-Jun can contribute to the cooperative effect of c-Jun and HIF-1 under hypoxic conditions.
FIG. 7.
Involvement of JNK pathway in functional cooperation between c-Jun and HIF-1. (A) Western blot analysis of phosphorylated c-Jun in endothelial cells under hypoxic conditions. (Top) Nuclear extracts were obtained from HUVEC grown in 1% O2 (Hx) for 30 min (30′) and 1, 2.5, 5, and 15 h or under normoxic conditions (C) and subjected to immunoblotting with an antibody which specifically recognizes c-Jun phosphorylated at Ser63. (Bottom) The same membrane was stripped and reblotted with an anti-c-Jun antibody to show equivalent amounts of protein in each lane. (B) (Top) F9 cells were transfected with 0.5 μg of p9HIF1Luc luciferase reporter plasmid, together with 1 μg of pRSVc-Jun (WT), pRSVc-Jun S63A-S73A (S63/73A), or pUCRSV control vector (C); after transfection, the cells were grown under hypoxic conditions (Hx) for 16 h, and luciferase activity was determined. Data from three separate experiments are shown, normalized to control in hypoxia (set as 1); the bars represent the means of the different values for each experimental condition. ∗, P < 0.05. (Bottom) F9 cells were transfected as described above, and nuclear extracts were obtained and subjected to immunoblotting (20 μg per lane) with a specific antibody against c-Jun. Molecular weight markers are shown on the left. (C) The HMEC-1 endothelial cell line was transiently transfected with 0.2 μg of p9HIF1Luc reporter plasmid together with 0.2 μg of JIP1 or control vector (C). Sixteen hours after transfection, the cells were either left untreated (N) or grown in 1% O2 for 8 h (Hx) and analyzed for luciferase activity. Data from three separate experiments are shown, normalized to control in normoxia; the bars represent the means of the different values for each experimental condition. ∗, P < 0.05.
DISCUSSION
Here, we show that c-Jun is able to functionally cooperate with HIF-1, enhancing transcription from a HIF-1-dependent construct in hypoxia. This construct contains the HIF-1 binding sequence of the VEGF 5′ UTR. Three AP-1 binding elements which have been involved in the regulation of the VEGF gene in hypoxia have been described throughout this promoter sequence (12). For this reason, we decided to restrict the target sequence for functional and binding experiments (see Materials and Methods) so that we could analyze the modulatory effect of c-Jun only on HIF-1-dependent transcription.
Though both factors functionally cooperate in hypoxia, in some cases c-Jun seems to transactivate the HIF-1-dependent constructs in normoxia also (Fig. 1A). The statistical analysis indicates that the data on c-Jun cooperation in normoxia are not significant. Nevertheless, this effect could be due to the presence of residual amounts of HIF-1 under normoxic conditions; in this regard, targeted disruption of HIF-1α or -β results in a reduced basal transcription level of some hypoxia-inducible genes; it has been proposed that α1β dimers present in normoxia would maintain a basal expression of a series of genes which are necessary for cellular metabolism (20, 31). In addition, it has been reported that some culture conditions, such as confluence status and cytokine stimulation, can modulate HIF-1α expression in different cell types (40, 56, 61). In agreement with this possibility, experiments with Hepa-1c4 cells (Fig. 2A) indicate that c-Jun effect can only be observed in the presence of a functional HIF-1. In fact, these assays show that HIF-1 β-subunit expression is able to transactivate p9HIF1Luc in normoxia, suggesting that there might be some basal HIF-1α under these conditions, since HIF-1β dimers are unable to bind to the HIF-1 consensus sequence (55). Alternatively, c-Jun could act in an HRE-independent manner, but experiments with p1HIF1mLuc indicate that c-Jun needs the integrity of the HIF-1 DNA binding site to display its cooperative effect (Fig. 2B).
A key step in the regulation of HIF-1 transcriptional activity is the stabilization of its α subunit in hypoxia. The tumor suppressor proteins VHL and p53 are involved in the HIF-1 degradation process (32, 38). It has been reported that c-Jun acts as a negative regulator of p53 expression (43). In addition, the HIF-1α promoter region contains several AP-1 binding elements (33). Thus, it was possible that c-Jun could modulate the HIF-1α protein level by transcriptional or posttranscriptional events as a mechanism for its cooperative effect on HIF-1 transcriptional activity. Transfection experiments with COS-7 cells (Fig. 4) show that c-Jun does not affect the endogenous HIF-1α protein level. An additional regulatory point is the active nuclear translocation of the HIF-1 α subunit to the nucleus induced by hypoxia (24). The fact that the nuclear HIF-1α amount is not modified in c-Jun-transfected COS-7 cells suggests that it does not interfere with this process either, though further experiments will be necessary to demonstrate this point.
Several reports reveal the importance of some transcription factors that functionally cooperate with HIF-1 by binding to cis regulatory regions of some genes, such as the tyrosine hydroxylase, lactate dehydrogenase A, endothelin-1, and NOS2 genes (14, 37, 51, 58). In contrast, our experiments (Fig. 5) show that direct binding of c-Jun to DNA is not necessary to achieve a functional cooperation with HIF-1. A similar mechanism has been proposed for macrophage colony-stimulating factor receptor regulation and p21 promoter transactivation, where c-Jun acts as a coactivator of PU.1 and Sp1, respectively, without binding to DNA (6, 25). Another member of the bLZ family of transcription factors, CHOP, has also been demonstrated to potentiate AP-1-mediated transcription by “tethering” CHOP to preexisting AP-1 DNA-protein complexes (52). On the other hand, c-Jun has been shown to enhance the DNA binding activity of NF-κB p65 (49); however, we have been unable to demonstrate a similar mechanism for HIF-1 (data not shown). Some articles report an increase in AP-1 binding to DNA under hypoxic conditions in certain cell types (59) that could be mediated by the nuclear redox factor Ref-1, which is induced in hypoxia and has also been shown to participate in HIF-1 transcriptional activity (9). We have not found any significant variation in AP-1 binding in hypoxia (Fig. 5A), which is in agreement with a number of other reports (3, 4).
In this study, we show that c-Jun and HIF-1α are able to associate in vivo, but we have not detected any interaction between them when carrying out coimmunoprecipitation experiments with in vitro-translated proteins (Fig. 6B). This suggests either that the association between the factors may be dependent on posttranslational events which only occur in a cellular context or that it is mediated by an additional protein(s). In this regard, it has been recently reported that HIF-1α is targeted for degradation in normoxia by the hydroxylation of a conserved proline residue. This proline hydroxylase activity requires molecular oxygen and Fe2+ and has been shown to be present in the rabbit reticulocyte lysate used to generate the in vitro-translated proteins (19, 21). Given the fact that c-Jun associates with HIF-1α under hypoxic conditions, it is possible that this interaction could be negatively regulated by proline hydroxilation of HIF-1α. It will be of great interest to perform additional experiments to confirm this possibility. On the other hand, the dominant-negative mutant of c-Jun, TAM67, also interacts with this factor in transfected COS-7 cells (Fig. 6A). This construct lacks the c-Jun transactivation domain and has been shown to inhibit the function of endogenous AP-1 proteins through a “quenching” mechanism (7). In the case of HIF-1, it could act in a similar fashion and sequester this factor in low-activity complexes, thus impairing HIF-1-dependent transcription.
JNK has been demonstrated to phosphorylate c-Jun at residues Ser63 and Ser73 and to enhance its transcriptional activity (18). Activation of the stress kinases JNK and p38 by hypoxia has been widely described (11, 29, 34, 44), though there are important discrepancies depending on the cell type and the experimental conditions utilized. The induction of MKP-1 phosphatase by low oxygen tension has also been reported as a regulatory step for JNK activity under these conditions (28). In our hands, hypoxia induces an increase in endogenous c-Jun phosphorylation at Ser63 (Fig. 7A) which could contribute to the cooperative effect of c-Jun and HIF-1 in hypoxia. In fact, experiments with c-Jun S63A-S73A (Fig. 7B) show an important reduction in c-Jun potentiation of HIF-1 transcriptional activity versus the wild type. The JIP1 scaffold protein aggregates components of the mitogen-activated protein kinase cascade to form a functional JNK signaling module. Its overexpression causes cytoplasmic retention of JNK and inhibits JNK-regulated gene expression (13). As shown in Fig. 7C, JIP1 partially inhibited hypoxic induction of the p9HIF1Luc reporter plasmid, suggesting a modulatory role of JNK-dependent phosphorylation of c-Jun on the expression of hypoxia-regulated genes.
Both c-Jun and HIF-1α bind to the transcriptional coactivators CBP/p300 and SRC-1, which are essential for their transcriptional activities (2, 9, 30). In the case of HIF-1α, the recruitment of CBP/p300 and SRC-1 has been shown to be redox regulated. These transcription factors bind to different regions of the p300 molecule (the C/H1 domain for HIF-1α and the CREB binding region for c-Jun), so it is feasible that both proteins could bind simultaneously to this coactivator. It is also possible that c-Jun could positively modulate HIF-1α binding to CBP/p300 or SRC-1, thus enhancing its transcriptional activity. On the other hand, c-Jun association with CBP/p300 has been shown to depend on the integrity of residues Ser63 and Ser73 in a phosphorylation-independent manner (5), so that the impairment of the cooperative effect in the experiments with c-Jun S63A-S73A (Fig. 7B) could also be due to an inefficient interaction with such transcriptional coactivators. Preliminary experiments show that p300 enhances the functional cooperation between c-Jun and HIF-1 (data not shown), but further research will be necessary to address this issue.
HIF-1 plays a pivotal role in the development of many cancers, mainly by regulating the expression of VEGF and promoting the neovascularization of solid tumors (8, 41). HIF-1 is upregulated in the hypoxic areas of these tumors, but it can also be induced by the inactivation of some tumor suppressor genes, such as VHL, p53, and PTEN genes, or by the action of some oncogenes, such as the v-SRC gene (22, 32, 38, 63). c-Jun participates in cellular growth control and is also able to transform cells by itself. It has been shown that some tumors derived from c-Jun-transformed cells exhibit a higher level of neovascularization than those derived from other oncogenes (26). The findings reported in this study provide an additional mechanism for solid-tumor development in which c-Jun not only would facilitate cell proliferation but also would potentiate VEGF induction in tumor hypoxic areas by functional cooperation with HIF-1.
Acknowledgments
We thank E. Huang and R. Davis for their generous gifts of HIF-1 expression vectors and GST constructs; we also thank P. Angel, R. Pope, and M. Karin for kindly providing us their c-Jun plasmids and A. Israel, M. Rincón, P. Muñoz, and G. R. Crabtree for their p 75 BFLuc, 2XAP1Luc, pcDNA3-Flag-JBD (JIP1), and pSH107c plasmids, respectively. We are very grateful to L. del Peso, R. Ramos, J. M. Redondo, M. López-Cabrera, F. Sánchez-Madrid, and M. C. Castellanos for their critical reading of the manuscript.
This work was supported by grants from the Ministerio de Educación y Cultura (PM 98/0024), from Fondo de Investigaciones Sanitarias (Fis 98/1382), and from Comunidad Autónoma de Madrid (CAM 08.3/0022/00). A.A. and F.V. were supported by fellowships from the Ministerio de Educación y Cultura; M.D.G. and J.A. were supported by fellowships from Comunidad Autónoma de Madrid.
REFERENCES
- 1.Aragones, J., D. R. Jones, S. Martin, M. A. San Juan, A. Alfranca, F. Vidal, A. Vara, I. Merida, and M. O. Landazuri. 2001. Evidence for the involvement of diacylglycerol kinase in the activation of hypoxia-inducible transcription factor-1 by low oxygen tension. J. Biol. Chem. 276:10548–10555. [DOI] [PubMed] [Google Scholar]
- 2.Arany, Z., L. E. Huang, R. Eckner, S. Bhattacharya, C. Jiang, M. A. Goldberg, H. F. Bunn, and D. M. Livingston. 1996. An essential role for p300/CBP in the cellular response to hypoxia. Proc. Natl. Acad. Sci. USA 93:12969–12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausserer, W. A., B. Bourrat-Floeck, C. J. Green, K. R. Laderoute, and R. M. Sutherland. 1994. Regulation of c-jun expression during hypoxic and low-glucose stress. Mol. Cell. Biol 14:5032–5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandyopadhyay, R. S., M. Phelan, and D. V. Faller. 1995. Hypoxia induces AP-1-regulated genes and AP-1 transcription factor binding in human endothelial and other cell types. Biochim. Biophys. Acta 1264:72–78. [DOI] [PubMed] [Google Scholar]
- 5.Bannister, A. J., T. Oehler, D. Wilhelm, P. Angel, and T. Kouzarides. 1995. Stimulation of c-Jun activity by CBP: c-Jun residues Ser63/73 are required for CBP induced stimulation in vivo and CBP binding in vitro. Oncogene 11:2509–2514. [PubMed] [Google Scholar]
- 6.Behre, G., A. J. Whitmarsh, M. P. Coghlan, T. Hoang, C. L. Carpenter, D. E. Zhang, R. J. Davis, and D. G. Tenen. 1999. c-Jun is a JNK-independent coactivator of the PU.1 transcription factor. J. Biol. Chem. 274:4939–4946. [DOI] [PubMed] [Google Scholar]
- 7.Brown, P. H., T. K. Chen, and M. J. Birrer. 1994. Mechanism of action of a dominant-negative mutant of c-Jun. Oncogene 9:791–799. [PubMed] [Google Scholar]
- 8.Carmeliet, P., Y. Dor, J. M. Herbert, D. Fukumura, K. Brusselmans, M. Dewerchin, M. Neeman, F. Bono, R. Abramovitch, P. Maxwell, C. J. Koch, P. Ratcliffe, L. Moons, R. K. Jain, D. Collen, E. Keshert, and E. Keshet. 1998. Role of HIF-1 alpha in hypoxia-mediated apoptosis, cell proliferation and tumor angiogenesis. Nature 394:485–490. [DOI] [PubMed] [Google Scholar]
- 9.Carrero, P., K. Okamoto, P. Coumailleau, S. O’Brien, H. Tanaka, and L. Poellinger. 2000. Redox-regulated recruitment of the transcriptional coactivators CREB-binding protein and SRC-1 to hypoxia-inducible factor 1alpha. Mol. Cell. Biol. 20:402–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandel, N. S., D. S. McClintock, C. E. Feliciano, T. M. Wood, J. A. Melendez, A. M. Rodriguez, and P. T. Schumacker. 2000. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 275:25130–25138. [DOI] [PubMed] [Google Scholar]
- 11.Conrad, P. W., R. T. Rust, J. Han, D. E. Millhorn, and D. Beitner-Johnson. 1999. Selective activation of p38alpha and p38gamma by hypoxia. Role in regulation of cyclin D1 by hypoxia in PC12 cells. J. Biol. Chem. 274:23570–23576. [DOI] [PubMed] [Google Scholar]
- 12.Damert, A., E. Ikeda, and W. Risau. 1997. Activator-protein-1 binding potentiates the hypoxia-inducible factor-1-mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells. Biochem. J. 327:419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickens, M., J. S. Rogers, J. Cavanagh, A. Raitano, Z. Xia, J. R. Halpern, M. E. Greenberg, C. L. Sawyers, and R. Davis. 1997. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science 277:693–696. [DOI] [PubMed] [Google Scholar]
- 14.Firth, J. D., B. L. Ebert, and P. J. Ratcliffe. 1995. Hypoxic regulation of lactate dehydrogenase A. Interaction between hypoxia-inducible factor 1 and cAMP response elements. J. Biol. Chem. 270:21021–21027. [DOI] [PubMed] [Google Scholar]
- 15.Forsythe, J. A., B. H. Jiang, N. V. Iyer, F. Agani, S. W. Leung, R. D. Koos, and G. L. Semenza. 1996. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16:4604–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gassmann, M., I. Kvietikova, A. Rolfs, and R. H. Wenger. 1997. Oxygen- and dioxin-regulated gene expression in mouse hepatoma cells. Kidney Int. 51:567–574. [DOI] [PubMed] [Google Scholar]
- 17.Hai, T., and T. Curran. 1991. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc. Natl. Acad. Sci. USA 88:3720–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hibi, M., A. Lin, T. Smeal, A. Minden, and M. Karin. 1993. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 7:2135–2148. [DOI] [PubMed] [Google Scholar]
- 19.Ivan, M., K. Kondo, H. Yang, W. Kim, J. Valiando,, M. Ohh, A. Salic, J. M. Asara, W. S. Lane, and W. G. Kaelin, Jr. 2001. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292:464–468. (First published 5 April 2001; 10.1126/science.1059817.) [DOI] [PubMed] [Google Scholar]
- 20.Iyer, N. V., L. E. Kotch,, F. Agani, S. W. Leung, E. Laughner,, R. H. Wenger, M. Gassmann, J. D. Gearhart, A. M. Lawler, A. Y. Yu, and G. L. Semenza. 1998. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 12:149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakkola, P., D. R. Mole, Y. Tian, M. I. Wilson, J. Gielbert, S. J. Gaskell, A. Kriegsheim, H. F. Hebestreit, M. Mukherji, C. J. Schofield, P. H. Maxwell, C. W. Pugh, and P. J. Ratcliffe. 2001. Targeting of HIF-1α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468–472. [DOI] [PubMed] [Google Scholar]
- 22.Jiang, B. H., F. Agani, A. Passaniti, and G. L. Semenza. 1997. V-SRC induces expression of hypoxia-inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF-1 in tumor progression. Cancer Res. 57:5328–5335. [PubMed] [Google Scholar]
- 23.Jiang, B. H., E. Rue, G. L. Wang, R. Roe, and G. L. Semenza. 1996. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J. Biol. Chem. 271:17771–17778. [DOI] [PubMed] [Google Scholar]
- 24.Kallio, P. J., K. Okamoto, S. O’Brien, P. Carrero, Y. Makino, H. Tanaka, and L. Poellinger. 1998. Signal transduction in hypoxic cells: inducible nuclear translocation and recruitment of the CBP/p300 coactivator by the hypoxia-inducible factor-1alpha. EMBO J. 17:6573–6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kardassis, D., P. Papakosta, K. Pardali, and A. Moustakas. 1999. c-Jun transactivates the promoter of the human p21(WAF1/Cip1) gene by acting as a superactivator of the ubiquitous transcription factor Sp1. J. Biol. Chem. 274:29572–29581. [DOI] [PubMed] [Google Scholar]
- 26.Kraemer, M., R. Tournaire, V. Dejong, N. Montreau, D. Briane, C. Derbin, and B. Binetruy. 1999. Rat embryo fibroblasts transformed by c-Jun display highly metastatic and angiogenic activities in vivo and deregulate gene expression of both angiogenic and antiangiogenic factors. Cell Growth Differ. 10:193–200. [PubMed] [Google Scholar]
- 27.Kvietikova, I., R. H. Wenger, H. H. Marti, and M. Gassmann. 1995. The transcription factors ATF-1 and CREB-1 bind constitutively to the hypoxia-inducible factor-1 (HIF-1) DNA recognition site. Nucleic Acids Res. 23:4542–4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laderoute, K. R., H. L. Mendonca, J. M. Calaoagan, A. M. Knapp, A. J. Giaccia, and P. J. Stork. 1999. Mitogen-activated protein kinase phosphatase-1 (MKP-1) expression is induced by low oxygen conditions found in solid tumor microenvironments. A candidate MKP for the inactivation of hypoxia-inducible stress-activated protein kinase/c-Jun N-terminal protein kinase activity. J. Biol. Chem. 274:12890–12897. [DOI] [PubMed] [Google Scholar]
- 29.Laderoute, K. R., and K. A. Webster. 1997. Hypoxia/reoxygenation stimulates Jun kinase activity through redox signaling in cardiac myocytes. Circ. Res. 80:336–344. [DOI] [PubMed] [Google Scholar]
- 30.Lee, S., H. Kim, S. Na, T. Kim, H. Choi, S. Im, and L. Lee. 1998. Steroid receptor coactivator-1 coactivates activating protein-1-mediated transactivations through interaction with the c-Jun and c-Fos subunits. J. Biol. Chem. 273:16651–16654. [DOI] [PubMed] [Google Scholar]
- 31.Maltepe, E., J. V. Schmidt, D. Baunock, C. A. Bradfield, and M. C. Simon. 1997. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 386:403–407. [DOI] [PubMed] [Google Scholar]
- 32.Maxwell, P. H., M. S. Wiesener, G. W. Chang, S. C. Clifford, E. C. Vaux, M. E. Cockman, C. C. Wykoff, C. W. Pugh, E. R. Maher, and P. J. Ratcliffe. 1999. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271–275. [DOI] [PubMed] [Google Scholar]
- 33.Minet, E., I. Ernest, G. Michel, I. Roland, J. Remacle, M. Raes, and C. Michiels. 1999. HIF1A gene transcription is dependent on a core promoter sequence encompassing activating and inhibiting sequences located upstream from the transcription initiation site and cis elements located within the 5′UTR. Biochem. Biophys. Res. Commun. 261:534–540. [DOI] [PubMed] [Google Scholar]
- 34.Mizukami, Y., K. Yoshioka, S. Morimoto, and K. Yoshida. 1997. A novel mechanism of JNK1 activation. J. Biol. Chem. 272:16657–16662. [DOI] [PubMed] [Google Scholar]
- 35.Musti, A., M. Treier, and D. Bohmann. 1997. Reduced ubiquitin-dependent degradation of c-Jun after phosphorylation by MAP kinases. Science 275:400–402. [DOI] [PubMed] [Google Scholar]
- 36.Nishitani, J., T. Nishinaka, C. H. Cheng, W. Rong, K. K. Yokoyama, and R. Chiu. 1999. Recruitment of the retinoblastoma protein to c-Jun enhances transcription activity mediated through the AP-1 binding site. J. Biol. Chem. 274:5454–5461. [DOI] [PubMed] [Google Scholar]
- 37.Norris, M. L., and D. E. Millhorn. 1995. Hypoxia-induced protein binding to O2-responsive sequences on the tyrosine hydroxylase gene. J. Biol. Chem. 270:23774–23779. [DOI] [PubMed] [Google Scholar]
- 38.Ravi, R., B. Mookerjee, Z. M. Bhujwalla, C. H. Sutter, D. Artemov, Q. Zeng, L. E. Dillehay, A. Madan, G. L. Semenza, and A. Bedi. 2000. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 14:34–44. [PMC free article] [PubMed] [Google Scholar]
- 39.Richard, D. E., E. Berra, E. Gothie, D. Roux, and J. Pouyssegur. 1999. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J. Biol. Chem. 274:32631–32637. [DOI] [PubMed] [Google Scholar]
- 40.Richard, D. E., E. Berra, and J. Pouyssegur. 2000. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1alpha in vascular smooth muscle cells. J. Biol. Chem. 275:26765–26771. [DOI] [PubMed] [Google Scholar]
- 41.Ryan, H. E., J. Lo, and R. S. Johnson. 1998. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 17:3005–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salceda, S., and J. Caro. 1997. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J. Biol. Chem. 272:22642–22647. [DOI] [PubMed] [Google Scholar]
- 43.Schreiber, M., A. Kolbus, F. Piu, A. Szabowski, U. Mohle-Steinlein, J. Tian, M. Karin, P. Angel, and E. F. Wagner. 1999. Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev. 13:607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott, P. H., A. Paul, C. M. Belham, A. J. Peacock, R. M. Wadsworth, G. W. Gould, D. Welsh, and R. Plevin. 1998. Hypoxic stimulation of the stress-activated protein kinases in pulmonary artery fibroblasts. Am. J. Respir. Crit. Care Med. 158:958–962. [DOI] [PubMed] [Google Scholar]
- 45.Semenza, G. L. 1994. Regulation of erythropoietin production. New insights into molecular mechanisms of oxygen homeostasis. Hematol. Oncol. Clin. N. Am. 8:863–884. [PubMed] [Google Scholar]
- 46.Semenza, G. L., P. H. Roth, H. M. Fang, and G. L. Wang. 1994. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 269:23757–23763. [PubMed] [Google Scholar]
- 47.Shweiki, D., A. Itin, D. Soffer, and E. Keshet. 1992. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359:843–848. [DOI] [PubMed] [Google Scholar]
- 48.Srinivas, V., I. Leshchinsky, N. Sang, M. P. King, A. Minchenko, and J. Caro. 2001. Oxygen sensing and HIF-1 activation does not require an active mitochondrial respiratory chain electron-transfer pathway. J. Biol. Chem. 276:21995–21998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stein, B., A. S. Baldwin, W. B. Dean, W. C. Greene, P. Angel, and P. Herrlich. 1993. Cross-coupling of the NF-KB p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J. 12:3879–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tacchini, L., L. Bianchi, A. Bernelli-Zazzera, and G. Cairo. 1999. Transferrin receptor induction by hypoxia. HIF-1-mediated transcriptional activation and cell-specific post-transcriptional regulation. J. Biol. Chem. 274:24142–24146. [DOI] [PubMed] [Google Scholar]
- 51.Tendler, D. S., C. Bao, T. Wang, E. L. Huang, E. A. Ratovitsky, D. A. Pardoll, and C. J. Lowenstein. 2001. Intersection of interferon and hypoxia signal transduction pathways in nitric oxide-induced tumor apoptosis. Cancer Res. 61:3682–3688. [PubMed] [Google Scholar]
- 52.Ubeda, M., M. Vallejo, and J. F. Habener. 1999. CHOP enhancement of gene transcription by interactions with Jun/Fos AP-1 complex proteins. Mol. Cell. Biol 19:7589–7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Dam, H., S. Huguier, K. Kooistra, J. Baguet, E. Vial, A. Van der Erb, P. Herrlich, P. Angel, and M. Castellazi. 1998. Autocrine growth and anchorage independence: two complementing Jun-controlled genetic programs of cellular transformation. Genes Dev. 12:1227–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vaux, E. C., E. Metzen, K. M. Yeates, and P. J. Ratcliffe. 2001. Regulation of hypoxia-inducible factor is preserved in the absence of a functioning mitochondrial respiratory chain. Blood 98:296–302. [DOI] [PubMed] [Google Scholar]
- 55.Whitelaw, M., I. Pongratz, A. Wilhelmsson, J.-A. Gustafsson, and L. Poellinger. 1993. Ligand-dependent recruitment of the Arnt coregulator determines DNA recognition by the dioxin receptor. Mol. Cell. Biol. 13:2504–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiesener, M. S., H. Turley, W. E. Allen, C. Willam, K. U. Eckardt, K. L. Talks, S. M. Wood, K. C. Gatter, A. L. Harris, C. W. Pugh, P. J. Ratcliffe, and P. H. Maxwell. 1998. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1alpha. Blood 92:2260–2268. [PubMed] [Google Scholar]
- 57.Wisdom, R., R. S. Johnson, and C. Moore. 1999. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J. 18:188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamashita, K., D. J. Discher, J. Hu, N. H. Bishopric, and K. A. Webster. 2001. Molecular regulation of the endothelin-1 gene by hypoxia. J. Biol. Chem. 276:12645–12653. [DOI] [PubMed] [Google Scholar]
- 59.Yao, K. S., S. Xanthoudakis, T. Curran, and P. J. O’Dwyer. 1994. Activation of AP-1 and of a nuclear redox factor, Ref-1, in the response of HT29 colon cancer cells to hypoxia. Mol. Cell. Biol 14:5997–6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zagariya, A., M. Shubangee, M. Birrer, S. Ness, B. Thimmapaya, and R. Pope. 1998. Tumor necrosis factor alpha gene regulation: enhancement of C/EBPβ-induced activation by c-Jun. Mol. Cell. Biol 18:2815–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zelzer, E., Y. Levy, C. Kahana, B. Z. Shilo, M. Rubinstein, and B. Cohen. 1998. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J. 17:5085–5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, Y., X. H. Feng, and R. Derynck. 1998. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature 394:909–913. [DOI] [PubMed] [Google Scholar]
- 63.Zundel, W., C. Schindler, D. Haas-Kogan, A. Koong, F. Kaper, E. Chen, A. R. Gottschalk, H. E. Ryan, R. S. Johnson, A. B. Jefferson, D. Stokoe, and A. J. Giaccia. 2000. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 14:391–396. [PMC free article] [PubMed] [Google Scholar]