Abstract
Stat6 and IRS-2 are two important signaling proteins that associate with the cytoplasmic tail of the interleukin 4 (IL-4) receptor. Data from numerous in vitro experiments have led to a model for IL-4 signal transduction in which the Stat6 signaling pathway is responsible for the IL-4 induced changes in gene expression and differentiation events, while the IRS-2 signaling pathway provides mitogenic and antiapoptotic signals. In order to determine the relative contributions of these signaling molecules in primary lymphocytes, we have examined IL-4 responses in T cells from mice deficient for either Stat6 or IRS-2 as well as from mice doubly deficient for both genes. Both IRS-2 and, especially, Stat6 are shown to be critically involved in IL-4-induced proliferation of T cells, presumably through the cooperative regulation of the Cdk inhibitor p27kip1. Like Stat6-deficient Th cells, IRS-2-deficient cells are also compromised in their ability to secrete Th2 cytokines, revealing a previously unrecognized role for IRS-2 in Th2 cell development. Although Stat6 and/or IRS-2 expression is required for IL-4-induced proliferative and differentiative responses, both signaling proteins are dispensable for the antiapoptotic effect of IL-4. However, treatment of lymphocytes with a protein tyrosine phosphatase inhibitor is able to block the antiapoptotic effect of IL-4 specifically in Stat6- or IRS-2-deficient cells and not in wild-type cells. Our results suggest that Stat6 and IRS-2 cooperate in promoting both IL-4-induced proliferative and differentiating responses, while an additional signaling mediator that depends on protein tyrosine phosphatase activity contributes to the antiapoptotic activities of IL-4 in primary T cells.
Interleukin 4 (IL-4) is a multifunctional cytokine made by T helper type 2 (Th2) cells, basophils, mast cells, and NK1.1 T cells (reviewed in reference 29). IL-4 influences the viability, proliferative capacity, and differentiation of B and T cells, as well as other cell types. Cellular responses to IL-4 are mediated through the IL-4 receptor alpha chain (IL-4Rα) functioning as a heterodimer with the common gamma chain (γc) (reviewed in reference 24). Ligand binding to the receptor complex results in the phosphorylation of the receptor through five conserved tyrosine residues in the cytoplasmic tail of IL-4Rα (32). These conserved tyrosine residues provide binding sites for potential downstream SH2 and phosphotyrosine binding (PTB) domain signaling proteins and have been the focus of several studies attempting to elucidate the molecular nature of IL-4 signal transduction. To date, a number of cytoplasmic signaling proteins have been shown to be phosphorylated in response to IL-4R stimulation, including Jak1, Stat6, IRS-2, FRIP, SHIP, and Shp (12, 14, 25, 34, 43).
Stat6 (for signal transducer and activator of transcription) is the primary Stat protein activated and recruited to the IL-4Rα chain, and the importance of Stat6 in IL-4R signal transduction was confirmed by the analysis of Stat6-deficient mice (17, 31, 35, 42). Stat6-deficient lymphocytes are compromised in their ability to upregulate the expression of known IL-4-responsive genes (those for CD23, IL-4Rα, and major histocompatibility complex class II) in response to IL-4 (17, 22, 31, 35). Additionally, Th cells from Stat6-deficient mice are unable to activate the IL-4-induced gene program required for the differentiation of Th2 cells (17, 31, 35). Stat6-deficient lymphocytes are also compromised in their ability to respond to IL-4 mitogenically, and this defect appears to be due to a disregulation of the Cdk inhibitor p27kip1 (16).
Insulin receptor substrate (IRS) proteins, of which there are currently four family members reported (IRS-1 to -4), are large adaptor PTB domain proteins involved in insulin and IGF-1 signaling (39). IRS-2, in particular, was also identified as one of the predominant proteins phosphorylated in response to IL-4 (34, 38, 39). IRS-2, through its PTB domain, is thought to directly interact with a sequence motif (the I4R motif) within the IL-4Rα chain that is highly homologous to sequences in the insulin and IGF-1 receptors (19, 37). Although IRS proteins do not possess intrinsic enzymatic activities, they can serve as adaptors for a number of other signaling proteins (33, 39). Recruitment of IRS-2 to the activated IL-4Rα chain results in its phosphorylation and subsequent activation of downstream signaling proteins, including phosphatidylinositol-3 (PI-3) kinase (19, 33, 34). Mice deficient in IRS-2 display insulin resistance and beta cell failure, developing a pathology similar to human type 2 diabetes, confirming the importance of IRS-2 in insulin signaling (40, 41). IL-4 responses in lymphocytes from IRS-2-deficient mice have not been reported.
Deletion and mutagenesis studies of the human IL-4Rα chain in transfected cell lines has led to the functional categorization of the phosphotyrosine residues in the IL-4Rα chain cytoplasmic tail (24). The portion of the receptor including amino acids 437 to 557 and containing one of the phosphotyrosine residues appears to be important for providing a mitogenic signal to cells after IL-4 stimulation and was termed the growth domain (19, 30). Amino acids 575 to 657, which include three of the phosphotyrosine residues, transmit signals that stimulate IL-4-induced gene expression, and this region was referred to as the differentiation domain (30). Furthermore, it was demonstrated that phosphorylation of IRS-2 occurs on a phosphotyrosine residue within the growth domain, while Stat6 activation was associated with a phosphotyrosine residue within the differentiation domain (30). Subsequent studies also revealed a role for IRS proteins in mediating the antiapoptotic activity provided by IL-4 (44). From these experiments, it was concluded that signals generated through Stat6 would activate differentiation-specific gene programs induced by IL-4 while IRS-2 would mediate mitogenic and antiapoptotic signals. The studies described above of primary lymphocytes from Stat6-deficient animals support a critical role for Stat6 in regulating IL-4-induced gene expression and differentiation but also indicate that Stat6 is involved in IL-4 mediated cell growth (16). The role of IRS-2 in IL-4 responses in primary lymphocytes has not been explored.
In order to assess the relative contributions of Stat6 and IRS-2 in IL-4R signaling, we studied IL-4 responses in primary T lymphocytes derived from Stat6-deficient and IRS-2-deficient mice as well as from mice lacking both Stat6 and IRS-2 (DKO). We found that both Stat6 and IRS-2 are required for the mitogenic response of T lymphocytes to IL-4. Additionally, we found that IRS-2 plays a previously unrecognized role in the development of Th2 cells in vitro. Finally, in the absence of both IRS-2 and Stat6, IL-4 is still a potent antiapoptotic agent even in the absence of IL-4-induced Bcl-2 expression and Akt activation. Interestingly, IL-4 was unable to rescue Stat6- or IRS-2-deficient lymphocytes from apoptosis in the absence of protein tyrosine phosphatase (PTPase) activity. These findings suggest that an additional signaling mediator is involved in the antiapoptotic response to IL-4 in T cells.
MATERIALS AND METHODS
Mice.
Stat6-deficient and IRS-2-deficient mice were generated as described previously (17, 41). As IRS-2-deficient mice are sterile, mice doubly deficient for Stat6 and IRS-2 (DKO) were generated by crossing Stat6−/− IRS-2+/− mice on a C57B1/6 background (4, 40). DKO mice were produced in Mendelian ratios. When possible, IRS-2-deficient and DKO mice were used before 8 weeks of age and the onset of diabetes. Blood glucose levels were checked at the time of experimentation, and no correlation was observed between blood glucose levels and IL-4 responses.
Lymphocyte preparation and culture.
Lymphocytes were cultured in RPMI 1640 supplemented as described previously (17). Naïve Th cells were purified from lymph nodes and spleens to 98% purity by cell sorting using anti-CD4 and anti-CD62L (Pharmingen). Enriched T-cell populations were generated by panning B lymphocytes from lymph node and spleen cell cultures using antibodies to immunoglobulin M (IgM) and IgG.
Antibodies and culture additions.
Antibodies specific for Stat6, Bcl-2, Bcl-xL, and Akt were obtained from Santa Cruz Biotechnology. Antibodies specific for phosphorylated Stat6 and phosphorylated akt were obtained from New England Biolabs. Recombinant IL-4 was obtained from Peprotech. Recombinant IL-2 was provided by Chiron. Recombinant IL-12 was provided by Hoffman-LaRoche.
PI-3 kinase assays.
Splenocytes from wild-type and knockout animals were cultured with or without IL-4 for 15 min. Homogenates from these cells were assayed for PI-3 kinase activity associated with IRS-1, IRS-2, and phosphotyrosine immunoprecipitates as described previously (28). 32P incorporation was quantified using a PhosphorImager (Molecular Dynamics).
Immunoblot analysis.
Whole-cell extracts were prepared by lysing cells in a solution of 50 mM Tris, 0.5% NP-40, 5 mM EDTA, and 50 mM NaCl and clearing the lysates by centrifugation. Protein extracts were separated on an 8 to 10% polyacrylamide gel and transferred to an Optitran membrane (Schleicher and Schuell). The immunoblots were blocked for 1 h at room temperature in 5% milk in TBST (50 mM Tris [pH 7.5], 100 mM NaCl, 0.03% Tween 20) and incubated with the appropriate antibody overnight at 4°C. In the case of the phospho-specific Stat6 antibody, the blot was blocked in 1% milk. The blots were washed with TBST and incubated with anti-rabbit horseradish peroxidase-conjugated antibody (Santa Cruz Biotechnology) at room temperature. After the blots were washed with TBST, detection was carried out using enhanced chemiluminscence (ECL; Amersham) according to the manufacturer’s instructions.
In vitro Th cell differentiation.
Naïve Th cells were plated on plates coated with anti-CD3 and anti-CD28 (1 μg/ml; Pharmingen) at 1 × 106 to 2 × 106/ml in the presence of 10 ng of IL-4 (Peprotech)/ml and 10 μg of anti-gamma interferon (IFN-γ)/ml (Th2 conditions) or 1 ng of IL-12/ml and 10 μg of anti-IL-4/ml (Th1 conditions). The cultures were expanded in IL-2 (100 U/ml) 3 days later. After 1 week in culture, the cells were stimulated with phorbol myristate acetate (PMA)-ionomycin, and cytokine production was determined by intracellular cytokine staining.
Northern blot analysis.
Total RNA was isolated using TRIZOL RNA isolation reagent (Gibco/BRL). The RNA was separated on a 1.5% agarose-6% formaldehyde gel and transferred to a GeneScreen (NEN) membrane. The membrane was hybridized with radiolabeled cDNA probes for GATA-3 and γ-actin.
Propidium iodide analysis.
Naïve Th cells were cultured as described for Fig. 5, pelleted by centrifugation, and fixed in 40% ethanol. The cells were treated with RNase A (50 μg/ml) for 45 min at 37°C and subsequently stained with 700 mM propidium iodide in 3.8 × 10−2 M sodium citrate. Analysis was performed on a FACScan flow cytometer (Becton Dickinson).
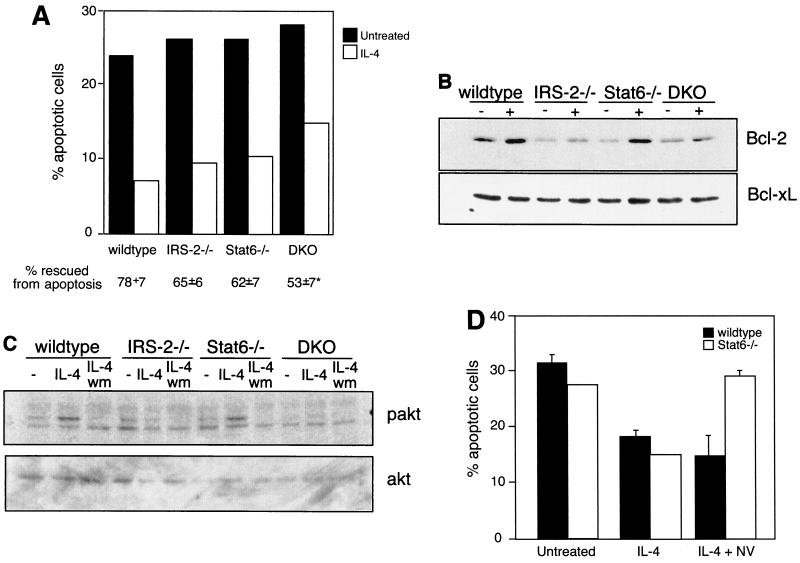
FIG. 5.
IRS-2 and Stat6 are dispensable for the antiapoptotic activities of IL-4. (A) CD4+/CD62L-high T cells were cultured for 24 to 30 h in the presence or absence of IL-4. The percentage of apoptotic cells in the resulting cultures was determined by propidium iodide staining. Percent rescued from apoptosis compares the percentage of apoptotic cells in the IL-4-treated cultures with the percentage of apoptotic cells in untreated cultures and is presented as the average of four independent experiments. *, P < 0.0001 compared to wild type. (B) Enriched T cells were cultured for 17 h in the presence (+) or absence (−) of IL-4. Whole-cell extracts were analyzed for the presence of Bcl-2 by immunoblotting. The blot was stripped and reprobed with an antibody to Bcl-xL. This result is representative of two independent experiments. (C) Enriched and cytokine-starved T cells were cultured in the presence or absence (−) of IL-4 for 3 min. Where indicated, the cells were also preincubated for 10 min with 100 nM wortmannin (wm). Whole-cell extracts were analyzed for the presence of phosphorylated Akt (pakt) by immunoblotting. The blot was stripped and reprobed with an antibody to total cellular Akt. The results are representative of four independent experiments. (D) Lymph node CD4+ T cells were cultured in the presence or absence of 10 ng of IL-4/ml and 100 μg of sodium vanadate/ml (NV) for 24 h. The percentage of apoptotic cells was determined by propidium iodide staining. The results are representative of two independent experiments. The error bars indicate standard deviations.
RESULTS
IL-4-induced PI-3 kinase activity is reduced in the absence of IRS-2.
In order to assess the relative contributions of IRS-2 and Stat6 in mediating IL-4 responses in lymphocytes, it was first necessary to determine whether the gross development of the lymphoid organs was intact in the absence of these two signaling proteins. Although IL-4 responses are compromised in Stat6-deficient animals, the overall development of the lymphocyte compartment is largely unaffected in these animals (17, 31, 35). Similarly, fluorescence-activated cell sorter (FACS) analysis of thymus, spleen, and lymph nodes from IRS-2-deficient mice, as well as mice bred to be deficient for both IRS-2 and Stat6 (DKO), revealed no gross defects in surface expression of CD3, CD4, CD8, B220, Mac-1, CD62L, or IL-4Rα (data not shown). Although we did consistently observe a slight increase in the B-cell-to-T-cell ratio in the spleen, this appeared to be due to an increase in the number of B lymphocytes, as the number of T lymphocytes was similar to that in wild-type controls (data not shown).
We have previously demonstrated that Stat6-deficient lymphocytes express and activate IRS-2 normally in response to IL-4, suggesting that IRS-2 activation is independent of Stat6 (16). Here, we also wished to determine if signaling events thought to be downstream of IRS-2 occur in the absence of Stat6. A well-established consequence of IRS-2 activation is the recruitment and activation of the PI-3 kinase (34). We assessed IL-4-induced PI-3 kinase activity associated with IRS-2, as well as with total phosphotyrosine-containing proteins, in splenocytes from wild-type, IRS-2-deficient, Stat6-deficient, and DKO animals. We found that IL-4 stimulation resulted in a large induction of PI-3 kinase activity associated with IRS-2- and phosphotyrosine-containing proteins that were immunoprecipitated from wild-type and Stat6-deficient cells (Fig. 1). As expected, PI-3 kinase activity was completely absent in IRS-2 and phosphotyrosine immunoprecipitates from IL-4-induced IRS-2-deficient and DKO cells (Fig. 1). Although PI-3 kinase activity is not detected in total phosphotyrosine immunoprecipitates from cell lysates lacking IRS-2, we do see a low level of PI-3 kinase activity associated with IRS-1 immunoprecipitates from IRS-2-deficient cells, consistent with the low level of IRS-1 expression we observe in these cells (Fig. 1) (16). Interestingly, the activity associated with IRS-1 is completely lost in DKO cells, although we are able to detect IRS-1 expression in these cells (data not shown). These data indicate that the IRS-2-dependent activation of PI-3 kinase occurs independently of Stat6 expression and, additionally, that the loss of IRS-2 expression results in a drastic loss of total PI-3 kinase activity in response to IL-4.
FIG. 1.
IL-4-induced PI-3 kinase activity is reduced in IRS-2-deficient lymphocytes. Supernatants of homogenates from IL-4-treated and untreated splenocytes were immunoprecipitated with the indicated antibodies and assayed in vitro for PI-3 kinase activity. The results are expressed as fold stimulation of activity above untreated controls and are the average (+ standard deviation) of two independent experiments.
Stat6 activation is not dependent on IRS-2 expression.
We also wished to determine if Stat6 activation occurs normally in the absence of IRS-2. To this end, we isolated T lymphocytes from wild-type and IRS-2-deficient animals and assessed the phosphorylation of Stat6 in these cells in response to IL-4. Figure 2A demonstrates that in the absence of IRS-2 expression, Stat6 is still efficiently phosphorylated and activated in response to IL-4. The IL-4-induced phosphorylation of Stat6 in IRS-2-deficient cells is functionally significant, as determined by the IL-4-induced upregulation of genes previously shown to be dependent on the expression of Stat6 in both wild-type and IRS-2-deficient lymphocytes (Fig. 2B). Based on these as well as previously published observations, we conclude that the IRS-2 and Stat6 pathways are each activated independently by IL-4.
FIG. 2.
Stat6 is activated in the absence of IRS-2. (A) Whole-cell extracts were prepared from enriched T lymphocytes cultured in the presence or absence (−) of IL-4. Activated Stat6 was detected by immunoblotting using an antibody specific for phosphorylated Stat6 (pStat6). The blot was stripped and reprobed with a total Stat6 antibody. The results are representative of three independent experiments. (B) Total splenocytes were cultured overnight in the presence (bold line) or absence (thin line) of IL-4. IL-4Rα expression on B220− cells and major histocompatibility complex class II expression on B220+ cells were determined by FACS.
Both IRS-2 and Stat6 regulate IL-4-induced proliferative responses through p27kip1.
IL-4 promotes the proliferation of lymphocytes when used as a costimulating agent. In order to determine the relative roles IRS-2 and Stat6 play in the proliferative response of T lymphocytes to IL-4, we purified naïve CD4+ T cells from wild-type, Stat6-deficient, IRS-2-deficient, and DKO mice. These cells were stimulated with a submitogenic dose of PMA (2 ng/ml) and supplemented with increasing amounts of IL-4. As shown in Fig. 3A, a dose-dependent increase in thymidine incorporation occurred in wild-type cultures in response to IL-4. As previously reported, Stat6-deficient T lymphocytes proliferated poorly in response to IL-4 (16). IRS-2-deficient T lymphocytes also responded to IL-4 at levels about 50% those of wild-type cells but still significantly better than Stat6-deficient cells. In the absence of both IRS-2 and Stat6, the cells were mitogenically unresponsive to IL-4. The proliferation defect is specific for IL-4 responses, since the cells from all four genotypes expand normally in response to anti-CD3, PMA-ionomycin, and IL-2 (Fig. 3A and data not shown). These results suggest that both IRS-2 and Stat6 pathways contribute to the proliferative response of T cells to IL-4, although Stat6 may in fact be the quantitatively important signaling component. Moreover, other potential signaling pathways emanating from the IL-4R are themselves unable to induce an IL-4-mediated proliferative response, since in the absence of both IRS-2 and Stat6, the cell population did not expand.
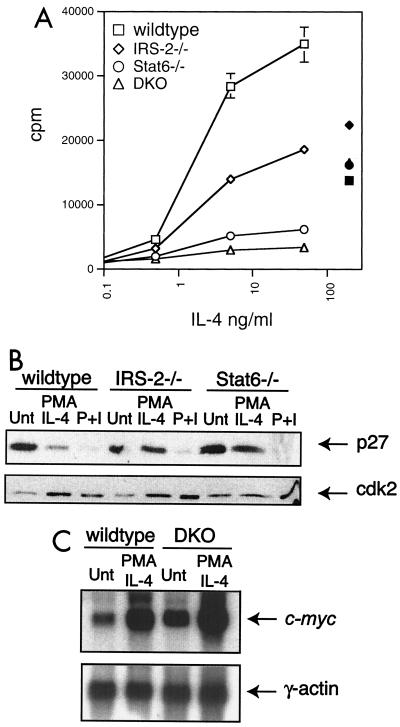
FIG. 3.
Both IRS-2 and Stat6 contribute to IL-4-induced proliferative responses. (A) FACS-purified CD4+/CD62L-high T cells were cultured in submitogenic PMA (2 ng/ml) supplemented with increasing doses of IL-4 (open symbols) or with plate-bound anti-CD3 and anti-CD28 (solid symbols). The proliferative response was determined by [3H]thymidine incorporation from a 48-h culture. The error bars indicate standard deviation. (B) Enriched T lymphocytes were cultured for 48 h in the presence of submitogenic PMA and 10 ng of IL-4/ml or 50 ng of PMA/ml and 500 ng of ionomycin/ml (P+I). p27kip1 (p27) protein levels were detected in whole-cell extracts by immunoblotting. The blot was stripped and reprobed with antibody specific for cdk2. Unt, untreated. (C) Enriched T lymphocytes were cultured in the presence or absence of submitogenic PMA (2 ng/ml) and 10 ng of IL-4/ml for 1 h. RNA was prepared, and c-myc expression was determined by Northern blot analysis. The blot was stripped and reprobed with γ-actin.
Cell cycle analysis of PMA- and IL-4-stimulated cells from IRS-2-deficient animals revealed that, as for Stat6-deficient cells, the proliferative response is blocked at the entry into S phase (reference 16 and data not shown). We had previously reported that the proliferation defect observed in Stat6-deficient cells correlated with a dysregulated expression of the Cdk inhibitor p27kip1 (16). A subsequent study of p27kip1-deficient mice demonstrated a role for p27kip1 in negatively regulating cytokine-stimulated T-cell proliferation (48). Interestingly, p27kip1 protein expression following cytokine stimulation has also been suggested to be regulated by the PI-3 kinase pathway (1). Consequently, we wished to determine whether the proliferation defect observed in IRS-2-deficient T cells also correlates with a defect in p27kip1 regulation. Protein extracts were harvested from wild-type, Stat6-deficient, and IRS-2-deficient T lymphocytes stimulated in vitro with submitogenic PMA plus IL-4 or PMA-ionomycin for 48 h, and p27kip1 protein levels were determined by immunoblotting. As shown in Fig. 3B, p27kip1 expression in wild-type cells decreases in response to mitogenic stimuli, such as IL-4 and PMA-ionomycin. As previously reported, the IL-4-induced decrease in p27kip1 expression is dependent on Stat6 signaling (16). Interestingly, p27kip1 protein levels also remain high in extracts from IL-4-treated IRS-2-deficient cells. p27kip1 expression is downregulated normally in T lymphocytes derived from all genotypes when treated with PMA-ionomycin, suggesting that the dysregulation in p27kip1 expression in the absence of IRS-2 or Stat6 is specific to compromised IL-4 signaling. On the other hand, the expression of c-myc, a proto-oncogene that is involved in cytokine-induced cell cycle progression, is induced similarly by PMA and IL-4 in wild-type and DKO cells (Fig. 3C), although this induction is clearly not sufficient to drive the DKO cells into the cell cycle (Fig. 3A).
IRS-2 signaling participates in the development of Th2 cells in vitro.
Previous studies of Stat6-deficient T cells have demonstrated the critical role Stat6 signaling plays in the development of a Th2 response (17, 31, 35). In the absence of Stat6, precursor Th cells are generally unable to induce the expression of the critical transcription factors, GATA-3 and c-maf, that drive Th2 differentiation (21, 26). This is in agreement with the notion that Stat6 is involved in directing changes in gene expression and differentiation events following IL-4 stimulation. In order to determine whether the IRS-2 pathway plays a role in Th2 cell development, we purified naïve CD4+ T cells from all four genotypes. These cells were induced to differentiate into effector Th cells in vitro by T-cell receptor stimulation and coculture with IL-12 or IL-4 to direct differentiation into the Th1 or Th2 pathway, respectively. In agreement with previous reports, in the absence of Stat6, very few IL-4- or IL-5-producing Th2 cells develop in the Th2 cultures (Fig. 4A). Instead, the majority of cytokine-producing cells express the Th1 cytokine, IFN-γ. Surprisingly, in the absence of IRS-2, IL-4-producing cells do develop in vitro under Th2 conditions, although in consistently fewer numbers (an average 30% decrease in IL-4-producing cells). Moreover, the number of IL-5-producing cells that are generated in IRS-2-deficient Th2 cultures is especially compromised (an average 45% decrease in IL-5-producing cells). These results were also confirmed by assessing cytokine levels in culture supernatants by enzyme-linked immunosorbent assay (data not shown). The defect in the production of Th2 cytokines from the IRS-2-deficient T cells is not a general defect in cytokine production, since IFN-γ and IL-2 production is normal under Th1 culture conditions (Fig. 4A and data not shown). These results suggest that IRS-2 signaling, although not absolutely required for Th2 differentiation in vitro, plays a critical role in the generation or expansion of Th2 cells and especially in the production of IL-5.
FIG. 4.
IRS-2 is required for maximal Th2 cytokine production. (A) FACS-purified CD4+/CD62L-high T cells were cultured under Th1 or Th2 skewing conditions for 1 week. Cytokine production from PMA-ionomycin-stimulated cells was assessed by intracellular cytokine staining. The results are representative of four independent experiments. (B) FACS-purified CD4+/Mel14-high T cells were cultured under Th1 (lanes 1) or Th2 (lanes 2) skewing conditions for 1 week and restimulated with anti-CD3 for 24 h. RNA was prepared from the cultured cells, and GATA-3 expression was determined by Northern blot analysis. The blot was stripped and reprobed for γ-actin.
As mentioned above, Stat6 signaling is required for the induction of GATA-3 expression in Th2 cells. GATA-3 has also been directly implicated in the transcription of the Th2 cytokine, IL-5 (46). Because we observed such a striking defect in the production of IL-5-producing cells in IRS-2-deficient cultures, we wished to determine whether IRS-2 signaling is also involved in the induction of GATA-3 expression in Th2 cells. We purified naïve CD4+ T cells from all four genotypes and cultured these cells under Th1 and Th2 skewing conditions. After 1 week in culture, RNA was harvested and analyzed for GATA-3 expression by Northern blot analysis. As shown previously, expression of GATA-3 is high in wild-type Th2 cells and barely detectable in Th1 cells (Fig. 4B). Moreover, the induction of GATA-3 expression in Stat6-deficient cells cultured under Th2 conditions is severely compromised (Fig. 4B) (26). However, GATA-3 expression is normal in IRS-2-deficient Th2 cells. We also observe normal GATA-3 induction as early as 24 h after primary stimulation in IRS-2-deficient cells cultured under Th2 conditions, suggesting that the kinetics of GATA-3 induction is also probably normal (data not shown). From these results, we conclude that although the ability to produce IL-5 is compromised in IRS-2-deficient T cells, this does not appear to be through a defect in GATA-3 expression.
Neither IRS-2 nor Stat6 signaling is required for antiapoptotic activities of IL-4.
IL-4 is capable of preventing apoptosis in B lymphocytes induced by growth factor withdrawal, Ig cross-linking, glucocorticoids, and Fas ligation (2, 6, 27). Recently, IL-4 was also shown to rescue resting T cells from death by neglect, but this response was independent of Stat6 expression and was suggested to be mediated by IRS-2 (36). In order to address whether IRS-2 signaling contributes to this activity, we purified naïve CD4 T cells from the four genotypes and cultured the cells in the presence and absence of IL-4. The number of apoptotic cells present in the cultures was determined 24 h later. As previously reported, approximately 25% of wild-type cells underwent apoptosis in the absence of cytokine, and IL-4 rescued 78% of those cells from apoptosis (Fig. 5A). Although IL-4 was slightly less potent in the rescue of Stat6-deficient cultures, the antiapoptotic activity was largely intact. Interestingly, IL-4 was also able to efficiently prevent apoptosis in IRS-2-deficient cultures, suggesting that the IRS-2 pathway is also not required for the antiapoptotic effects of IL-4. Surprisingly, even in the absence of both Stat6 and IRS-2, IL-4 was still a potent antiapoptotic cytokine, although the degree of rescue was slightly less than wild type. The antiapoptotic signal was shown to require IL-4Rα, since IL-4 failed to rescue IL-4Rα−/− T lymphocytes from apoptosis (data not shown).
In order to gain insight into the mechanism of the antiapoptotic response, we analyzed the expression of various pro- and antiapoptotic proteins in response to IL-4. The expression of the antiapoptotic regulator, Bcl-2, has been shown to be induced by IL-4 in resting T cells (36). In order to determine if Bcl-2 is still induced by IL-4 in the absence of Stat6 and/or IRS-2, we purified protein extracts from T lymphocytes cultured in the presence and absence of IL-4. IL-4 induces the expression of Bcl-2 in wild-type T cells, and the induction of Bcl-2 is not dependent on Stat6, providing an example of an IL-4-inducible gene that is not dependent on Stat6 expression (Fig. 5B). Interestingly, in cells that lack IRS-2, or both Stat6 and IRS-2, IL-4 is no longer able to upregulate Bcl-2 protein levels. Therefore, IL-4-induced Bcl-2 protein expression is dependent on the IRS-2 pathway. Since IL-4 is still capable of preventing apoptosis in the absence of IRS-2, these results imply that IL-4-induced Bcl-2 expression is dispensable for protection from apoptosis. We do not observe a modulation in the expression of several apoptotic regulators, including Bcl-xL, Mcl-1, and Bak-1, in T cells in response to IL-4 (Fig. 5B and data not shown).
The Akt pathway is activated by a number of growth factor receptors and has also been demonstrated to protect cells from apoptosis (11, 20). Akt is activated by phosphorylation in a manner dependent on PI-3 kinase (3, 7, 8). In order to determine whether the Akt pathway is activated by IL-4 in our knockout cells, purified T cells from the four genotypes were cultured in the presence or absence of IL-4. The presence of phosphorylated Akt in protein extracts from these cells was determined by immunoblotting. IL-4 was able to induce phosphorylation of Akt in cells from wild-type and Stat6-deficient animals (Fig. 5C). The observed Akt activation was dependent on PI-3 kinase, as revealed by the loss of Akt phosphorylation in cultures that include the PI-3 kinase inhibitor wortmannin (Fig. 5C). Reminiscent of what we observed for Bcl-2 expression, IL-4-induced Akt phosphorylation was found to be completely dependent on the presence of IRS-2. This finding is supported by the data in Fig. 1 showing that IL-4-induced PI-3 kinase activation is severely reduced in the absence of IRS-2 signaling. Since IL-4 is still capable of rescuing IRS-2-deficient T cells from apoptosis, these results imply that the Akt pathway, like Bcl-2 induction, is also not required for the antiapoptotic activities of IL-4.
To broaden our search for IL-4-induced changes in gene expression that could account for mediating the antiapoptotic effect of IL-4 in DKO cells, we performed gene chip microarray analysis comparing RNAs isolated from DKO T cells cultured in the presence and absence of IL-4. Surprisingly, we observed no significant changes in gene expression between IL-4-treated or untreated samples in the approximately 6,000 genes assayed, (data not shown), suggesting that in the absence of IRS-2 and Stat6 there are essentially no IL-4-induced changes in gene transcription. This also suggests that the IL-4-mediated protection from apoptosis in DKO cells is due to posttranscriptional changes and does not depend on the new synthesis of an apoptotic regulator. A previous report of IL-4R mutants transfected into myeloid cell lines indicated that IL-4-induced suppression of apoptosis was signaled through the region of the IL-4R that is associated with IRS-2 as well as a region of the IL-4R that is reminiscent of an ITIM motif (43). ITIMs are generally considered to be involved with the negative regulation of receptor signaling and are usually associated with PTPases (23). In order to address the potential role of PTPases in IL-4-induced rescue of DKO cells from apoptosis, we assessed the ability of IL-4 to rescue wild-type and DKO cells from apoptosis when cultured in the presence of sodium vanadate, a broad inhibitor of PTPase activity. We observed no significant difference in the ability of IL-4 to rescue wild-type cells from cell death when cocultured with sodium vanadate (Fig. 5D). However, we did observe a striking block in the ability of IL-4 to protect DKO cells from apoptosis in the presence of sodium vanadate. These results suggest that there is a requirement for the activity of a PTPase to mediate the protective effects of IL-4 in the absence of Stat6 and IRS-2.
DISCUSSION
In this report, we have examined the relative contributions of Stat6 and IRS-2 in a variety of IL-4 responses in primary mouse T lymphocytes. Our results suggest that both Stat6 and IRS-2 play critical roles in mediating an IL-4-induced mitogenic response. Although IL-4-induced Th2 cell differentiation is entirely dependent on Stat6 signaling, our results reveal a previously unappreciated role for IRS-2 in the maximal production of Th2 cytokines, especially IL-5. While both Stat6 and IRS-2 signaling are required for the IL-4-induced proliferative and differentiating processes described above, both are dispensable for the IL-4-dependent rescue of T lymphocytes from apoptosis induced by growth factor withdrawal. This is in spite of the fact that IL-4-induced Bcl-2 expression and Akt phosphorylation are lost in the absence of IRS-2 signaling. IL-4-dependent rescue from apoptosis in DKO cells is dependent on PTPase activity, suggesting that an additional signaling pathway that includes this enzymatic activity contributes to IL-4-induced rescue from cell death.
Previous studies of transfected cell lines have suggested that IRS-2 is the primary mediator of IL-4-induced proliferation. However, our studies of primary T cells suggest that both Stat6 and IRS-2 play important roles in the proliferative response of primary T cells to IL-4. Surprisingly, lymphocytes lacking Stat6 appear to be even more compromised than cells lacking IRS-2, suggesting that Stat6 may provide the more important mitogenic signal, at least in vitro. Interestingly, T cells lacking both Stat6 and IRS-2 are unresponsive mitogenically to IL-4, suggesting that the other signaling proteins associated with IL-4α (for example, FRIP, SHIP, Shp, and fes) are unable to compensate for the loss. Therefore, the proliferative response of primary T cells in response to IL-4 is entirely dependent on Stat6 and IRS-2 signaling, although other molecules may participate in this response.
Our studies of IL-4-induced proliferation emphasize the importance of the Cdk inhibitor p27kip1 as a central regulator of cytokine-induced proliferation in lymphocytes. Although we have demonstrated that IRS-2 and Stat6 signaling are independent, the pathways appear to converge to affect the downregulation of p27kip1 protein expression in response to IL-4 (Fig. 2B). Both Stat4 and Stat6 have been shown to be required for IL-12- and IL-4-dependent proliferation, respectively, and in the absence of these Stat proteins p27kip1 levels are dysregulated (16). A recent report demonstrated that the defect in IL-4-induced proliferation observed in Stat6-deficient lymphocytes could be corrected by the loss of p27kip1, further supporting the importance of p27kip1 in regulating cytokine-induced gene expression (48). However these Stat proteins do not appear to directly regulate p27kip1 transcription, and the mechanism by which Stat proteins modulate p27kip1 protein expression is still not understood (16). Moreover, cytokine-induced PI-3 kinase activity can also influence p27kip1 protein levels in T cells (1). We found that IL-4-induced PI-3 kinase activity is severely compromised in the absence of IRS-2, suggesting that the defect in p27kip1 expression in IRS-2-deficient cells is probably due to the failure to activate this pathway. Therefore, we propose that IL-4 regulates p27kip1 expression by two mechanisms, one that is Stat mediated and the other by IRS-2-dependent activation of PI-3 kinase or via another common target.
While Stat6 signaling is able to initiate the molecular events (GATA-3 and subsequent c-maf upregulation) involved in IL-4-induced Th2 differentiation, the role IRS-2 plays in Th2 cytokine production is unclear (21, 26). In the absence of Stat6, very few IL-4- or IL-5-producing cells are found in Th2 cultures, while in the absence of IRS-2, the number of IL-4-producing cells is modestly reduced while the number of IL-5-producing cells is especially compromised (Fig. 4A). Additionally, repeated stimulation of IRS-2-deficient cells under Th2 skewing conditions over several weeks in culture did not restore the number of IL-5-producing cells, and therefore this defect appears to be stable over time (data not shown). Several possibilities could account for these observations. The reduction of Th2 cells in IRS-deficient cultures could be due to a partial block in the production of these cells or a decrease in the transcription of Th2 cytokines. Normal induction of GATA-3 expression, a critical regulator of Th2 development and IL-5 transcription, in IRS-2-deficient Th2 cultures argues against these possibilities (Fig. 4B). Alternatively, IRS-2 signaling may be involved in the expansion or survival of committed Th2 cells in response to IL-4. These possibilities are supported by our observations that IRS-2-deficient lymphocytes fail to proliferate normally in response to IL-4 and that two survival mediators, Bcl-2 and Akt, are not induced or activated by IL-4 in the absence of IRS-2. However, a recent report of T-cell costimulation and akt activation has indicated a role for Akt activation in the production of Th1 and not Th2 cytokines (15).
IL-4-induced Stat6 activation is necessary not only to drive the development of cells expressing Th2 cytokines but also to repress the expression of the Th1 cytokine, IFN-γ (Fig. 4A) (18, 26). Although IRS-2 signaling appears to be required for maximal Th2 cytokine production, IRS-2 is not required to extinguish IFN-γ expression in Th2 cultures (Fig. 4A). GATA-3 has been shown to be able to effectively inhibit the expression of IFN-γ via the downregulation of IL-12Rβ2 expression, and the induction of GATA-3 in Th2 cells is largely dependent on Stat6 (Fig. 4A) (26). We find that GATA-3 is expressed normally in IRS-2-deficient Th2 cells (Fig. 4B), and presumably this results in the efficient inhibition of IFN-γ in IRS-2-deficient Th2 cells. We suggest that IL-4-induced Stat6 activation and subsequent GATA-3 expression are necessary to drive Th2 cytokine production and effectively inhibit IFN-γ production, while IRS-2 signaling serves to augment Th2 development by a mechanism that does not involve GATA-3 and is unknown.
As opposed to the absolute requirement for Stat6 and IRS-2 in the proliferative and differentiating functions of IL-4, Stat6 and IRS-2 are not required to protect resting T cells from apoptosis induced by growth factor withdrawal. These results suggest that another functionally important signaling molecule aside from Stat6 and IRS-2 is activated by IL-4Rα stimulation and thus is responsible for rescuing these cells from apoptosis. We cannot entirely rule out the possibility that this is simply due to the binding of another IRS family member to the I4R region of IL-4Rα, although we do not believe this to be the case. First, we are unable to detect IL-4-induced PI-3 kinase activity associated with IRS-1 or with total cellular phosphotyrosines after IL-4 stimulation in DKO cells (Fig. 1), and IL-4 is unable to activate PI-3 kinase-dependent Akt in the absence of IRS-2 (Fig. 5C). Additionally, we were unable to detect the expression of IRS-3 or IRS-4 in these cells (data not shown). However, inclusion of a PI-3 kinase inhibitor, wortmannin, results in a partial block in the ability of IL-4 to protect both wild-type and DKO cells from apoptosis (data not shown), suggesting that PI-3 kinase or a member of the PI-3 kinase superfamily may be participating in this response even in the absence of IRS-2 (5).
Interestingly, in DKO cells, we were able to reveal a dependence on a PTPase activity in IL-4-induced protection from apoptosis (Fig. 5D). We do not see a dependence on PTPase activity in wild-type cells, presumably due to the fact that sodium vanadate treatment strongly potentiates IL-4-induced Stat6 phosphorylation (13). A likely candidate PTPase to be involved in the antiapoptotic activity is Shp-1 (47). Shp-1 is preferentially expressed in hematopoietic cells and was recently reported to be constitutively associated with IL-4R in primary lymphocytes (13). Although Shp-1 has been traditionally thought to negatively regulate IL-4R signaling by influencing the phosphorylation status of Stat6, recent reports have also suggested that Shp-1, or another IL-4-induced phosphatase, can also positively influence IL-4-induced changes in proliferation and gene expression (9, 13). Additionally, receptor mutagenesis studies have suggested that the portion of the IL-4R that resembles an ITIM motif contributes to the protection of cells from apoptosis (43). We propose that in wild-type cells IRS-2 and Stat6 cooperate with a phosphatase activity to prevent the cells from undergoing apoptosis. In the absence of IRS-2 and Stat6, IL-4-induced protection from apoptosis is dependent on the tyrosine dephosphorylation of an important regulator of apoptosis. This notion is supported by numerous observations that the phosphorylation state of pro- and antiapoptotic factors (Bad and Bcl-2, for example) has been shown to affect their activity (10, 45).
The conclusions reached in this report differ from those found in previous studies using IL-4Rα mutants transfected into myeloid cell lines. These differences may reflect the use of primary cells versus cell lines in these experiments or may simply represent differences in IL-4R signaling between lymphocytes and myeloid cells. The findings presented here do, however, suggest a revision to the current model of IL-4 signal transduction. Instead of IRS-2 solely mediating IL-4-induced cell growth and Stat6 exclusively mediating the differentiating signals, we propose that in primary T lymphocytes these two independent signaling pathways collaborate to provide the maximum biological response to IL-4. Additionally, we propose that another signaling protein, presumably a PTPase, plays an important role in transmitting the antiapoptotic signal provided by IL-4 in T lymphocytes. Determining the nature of the Stat6- and IRS-2-independent antiapoptotic pathway is the focus of further investigation.
Acknowledgments
We thank Devangi Mehta, Jyothi Rengarajan, and Susanne Szabo for thoughtful review of the manuscript.
This work was supported by National Institutes of Health grant AI40171 (M.J.G.) and by the Mathers Foundation (M.J.G.). M.J.G. is a scholar of the Leukemia and Lymphoma Society. D.J.W. is a Medical Research Council Senior Clinical Fellow.
REFERENCES
- 1.Brennan, P., J. W. Babbage, B. M. Burgering, B. Groner, K. Reif, and D. A. Cantrell. 1997. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity 7:679–689. [DOI] [PubMed] [Google Scholar]
- 2.Brunetti, M., N. Martelli, A. Colasante, M. Piantelli, P. Musiani, and F. B. Aiello. 1995. Spontaneous and glucocorticoid-induced apoptosis in human mature T lymphocytes. Blood 86:4199–4205. [PubMed] [Google Scholar]
- 3.Burgering, B. M., and P. J. Coffer. 1995. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376:599–602. [DOI] [PubMed] [Google Scholar]
- 4.Burks, D. J., J. F. de Mora, M. Schubert, D. J. Withers, M. G. Myers, H. H. Towery, S. L. Altamuro, C. L. Flint, and M. F. White. 2000. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature 407:377–382. [DOI] [PubMed] [Google Scholar]
- 5.Davies, S. P., H. Reddy, M. Caivano, and P. Cohen. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foote, L. C., R. G. Howard, A. Marshak-Rothstein, and T. L. Rothstein. 1996. IL-4 induces Fas resistance in B cells. J. Immunol. 157:2749–2753. [PubMed] [Google Scholar]
- 7.Franke, T. F., D. R. Kaplan, L. C. Cantley, and A. Toker. 1997. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 275:665–668. [DOI] [PubMed] [Google Scholar]
- 8.Franke, T. F., S. I. Yang, T. O. Chan, K. Datta, A. Kazlauskas, D. K. Morrison, D. R. Kaplan, and P. N. Tsichlis. 1995. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81:727–736. [DOI] [PubMed] [Google Scholar]
- 9.Giallourakis, C., M. Kashiwada, P. Y. Pan, N. Danial, H. Jiang, J. Cambier, K. M. Coggeshall, and P. Rothman. 2000. Positive regulation of interleukin-4-mediated proliferation by the SH2-containing inositol-5′-phosphatase. J. Biol. Chem. 275:29275–29282. [DOI] [PubMed] [Google Scholar]
- 10.Haldar, S., N. Jena, and C. M. Croce. 1995. Inactivation of Bcl-2 by phosphorylation. Proc. Natl. Acad. Sci. USA 92:4507–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemmings, B. A. 1997. Akt signaling: linking membrane events to life and death decisions. Science 275:628–630. [DOI] [PubMed] [Google Scholar]
- 12.Hou, J., U. Schindler, W. J. Henzel, T. C. Ho, M. Brasseur, and S. L. McKnight. 1994. An interleukin-4-induced transcription factor: IL-4 Stat. Science 265:1701–1706. [DOI] [PubMed] [Google Scholar]
- 13.Huang, H., and W. E. Paul. 2000. Protein tyrosine phosphatase activity is required for IL-4 induction of IL-4 receptor alpha-chain. J. Immunol. 164:1211–1215. [DOI] [PubMed] [Google Scholar]
- 14.Imani, F., K. J. Rager, B. Catipovic, and D. G. Marsh. 1997. Interleukin-4 (IL-4) induces phosphatidylinositol 3-kinase (p85) dephosphorylation. Implications for the role of SHP-1 in the IL-4-induced signals in human B cells. J. Biol. Chem. 272:7927–7931. [DOI] [PubMed] [Google Scholar]
- 15.Kane, L. P., V. S. Shapiro, D. Stokoe, and A. Weiss. 1999. Induction of NF-κB by the Akt/PKB kinase. Curr. Biol. 9:601–604. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan, M. H., C. Daniel, U. Schindler, and M. J. Grusby. 1998. Stat proteins control lymphocyte proliferation by regulating p27Kip1 expression. Mol. Cell. Biol. 18:1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan, M. H., U. Schindler, S. T. Smiley, and M. J. Grusby. 1996. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity 4:313–319. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan, M. H., A. L. Wurster, and M. J. Grusby. 1998. A signal transducer and activator of transcription (Stat)4-independent pathway for the development of T helper type 1 cells. J. Exp. Med. 188:1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keegan, A. D., K. Nelms, M. White, L. M. Wang, J. H. Pierce, and W. E. Paul. 1994. An IL-4 receptor region containing an insulin receptor motif is important for IL-4-mediated IRS-1 phosphorylation and cell growth. Cell 76:811–820. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy, S. G., A. J. Wagner, S. D. Conzen, J. Jordan, A. Bellacosa, P. N. Tsichlis, and N. Hay. 1997. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 11:701–713. [DOI] [PubMed] [Google Scholar]
- 21.Kurata, H., H. J. Lee, A. O’Garra, and N. Arai. 1999. Ectopic expression of activated Stat6 induces the expression of Th2-specific cytokines and transcription factors in developing Th1 cells. Immunity 11:677–688. [DOI] [PubMed] [Google Scholar]
- 22.Linehan, L. A., W. D. Warren, P. A. Thompson, M. J. Grusby, and M. T. Berton. 1998. STAT6 is required for IL-4-induced germline Ig gene transcription and switch recombination. J. Immunol. 161:302–310. [PubMed] [Google Scholar]
- 23.Long, E. O. 1999. Regulation of immune responses through inhibitory receptors. Annu. Rev. Immunol. 17:875–904. [DOI] [PubMed] [Google Scholar]
- 24.Nelms, K., A. D. Keegan, J. Zamorano, J. J. Ryan, and W. E. Paul. 1999. The IL-4 receptor: signaling mechanisms and biologic functions. Annu. Rev. Immunol. 17:701–738. [DOI] [PubMed] [Google Scholar]
- 25.Nelms, K., A. L. Snow, J. Hu-Li, and W. E. Paul. 1998. FRIP, a hematopoietic cell-specific rasGAP-interacting protein phosphorylated in response to cytokine stimulation. Immunity 9:13–24. [DOI] [PubMed] [Google Scholar]
- 26.Ouyang, W., S. H. Ranganath, K. Weindel, D. Bhattacharya, T. L. Murphy, W. C. Sha, and K. M. Murphy. 1998. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity 9:745–755. [DOI] [PubMed] [Google Scholar]
- 27.Parry, S. L., J. Hasbold, M. Holman, and G. G. Klaus. 1994. Hypercross-linking surface IgM or IgD receptors on mature B cells induces apoptosis that is reversed by costimulation with IL-4 and anti-CD40. J. Immunol. 152:2821–2829. [PubMed] [Google Scholar]
- 28.Patti, M. E., X. J. Sun, J. C. Bruening, E. Araki, M. A. Lipes, M. F. White, and C. R. Kahn. 1995. 4PS/insulin receptor substrate (IRS)-2 is the alternative substrate of the insulin receptor in IRS-1-deficient mice. J. Biol. Chem. 270:24670–24673. [DOI] [PubMed] [Google Scholar]
- 29.Paul, W. E. 1997. Interleukin 4: signalling mechanisms and control of T cell differentiation. Ciba Found. Symp. 204:208–216. [DOI] [PubMed] [Google Scholar]
- 30.Ryan, J. J., L. J. McReynolds, A. Keegan, L. H. Wang, E. Garfein, P. Rothman, K. Nelms, and W. E. Paul. 1996. Growth and gene expression are predominantly controlled by distinct regions of the human IL-4 receptor. Immunity 4:123–132. [DOI] [PubMed] [Google Scholar]
- 31.Shimoda, K., J. van Deursen, M. Y. Sangster, S. R. Sarawar, R. T. Carson, R. A. Tripp, C. Chu, F. W. Quelle, T. Nosaka, D. A. Vignali, P. C. Doherty, G. Grosveld, W. E. Paul, and J. N. Ihle. 1996. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature 380:630–633. [DOI] [PubMed] [Google Scholar]
- 32.Smerz-Bertling, C., and A. Duschl. 1995. Both interleukin 4 and interleukin 13 induce tyrosine phosphorylation of the 140-kDa subunit of the interleukin 4 receptor. J. Biol. Chem. 270:966–970. [DOI] [PubMed] [Google Scholar]
- 33.Sun, X. J., D. L. Crimmins, M. G. Myers, M. Miralpeix, and M. F. White. 1993. Pleiotropic insulin signals are engaged by multisite phosphorylation of IRS-1. Mol. Cell. Biol. 13:7418–7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun, X. J., L. M. Wang, Y. Zhang, L. Yenush, M. G. Myers, Jr., E. Glasheen, W. S. Lane, J. H. Pierce, and M. F. White. 1995. Role of IRS-2 in insulin and cytokine signalling. Nature 377:173–177. [DOI] [PubMed] [Google Scholar]
- 35.Takeda, K., T. Tanaka, W. Shi, M. Matsumoto, M. Minami, S. Kashiwamura, K. Nakanishi, N. Yoshida, T. Kishimoto, and S. Akira. 1996. Essential role of Stat6 in IL-4 signalling. Nature 380:627–630. [DOI] [PubMed] [Google Scholar]
- 36.Vella, A., T. K. Teague, J. Ihle, J. Kappler, and P. Marrack. 1997. Interleukin 4 (IL-4) or IL-7 prevents the death of resting T cells: stat6 is probably not required for the effect of IL-4. J. Exp. Med. 186:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, H. Y., W. E. Paul, and A. D. Keegan. 1996. IL-4 function can be transferred to the IL-2 receptor by tyrosine containing sequences found in the IL-4 receptor alpha chain. Immunity 4:113–121. [DOI] [PubMed] [Google Scholar]
- 38.Wang, L. M., A. D. Keegan, W. E. Paul, M. A. Heidaran, J. S. Gutkind, and J. H. Pierce. 1992. IL-4 activates a distinct signal transduction cascade from IL-3 in factor-dependent myeloid cells. EMBO J. 11:4899–4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White, M. F., and L. Yenush. 1998. The IRS-signaling system: a network of docking proteins that mediate insulin and cytokine action. Curr. Top. Microbiol. Immunol. 228:179–208. [DOI] [PubMed] [Google Scholar]
- 40.Withers, D. J., D. J. Burks, H. H. Towery, S. L. Altamuro, C. L. Flint, and M. F. White. 1999. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat. Genet. 23:32–40. [DOI] [PubMed] [Google Scholar]
- 41.Withers, D. J., J. S. Gutierrez, H. Towery, D. J. Burks, J. M. Ren, S. Previs, Y. Zhang, D. Bernal, S. Pons, G. I. Shulman, S. Bonner-Weir, and M. F. White. 1998. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391:900–904. [DOI] [PubMed] [Google Scholar]
- 42.Wurster, A. L., T. Tanaka, and M. J. Grusby. 2000. The biology of Stat4 and Stat6. Oncogene 19:2577–2584. [DOI] [PubMed] [Google Scholar]
- 43.Zamorano, J., and A. D. Keegan. 1998. Regulation of apoptosis by tyrosine-containing domains of IL-4R alpha: Y497 and Y713, but not the STAT6-docking tyrosines, signal protection from apoptosis. J. Immunol. 161:859–867. [PubMed] [Google Scholar]
- 44.Zamorano, J., H. Y. Wang, L. M. Wang, J. H. Pierce, and A. D. Keegan. 1996. IL-4 protects cells from apoptosis via the insulin receptor substrate pathway and a second independent signaling pathway. J. Immunol. 157:4926–4934. [PubMed] [Google Scholar]
- 45.Zha, J., H. Harada, E. Yang, J. Jockel, and S. J. Korsmeyer. 1996. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14–3–3 not BCL-X(L). Cell 87:619–628. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, D. H., L. Yang, and A. Ray. 1998. Differential responsiveness of the IL-5 and IL-4 genes to transcription factor GATA-3. J Immunol. 161:3817–3821. [PubMed] [Google Scholar]
- 47.Zhang, J., A. K. Somani, and K. A. Siminovitch. 2000. Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Semin. Immunol. 12:361–378. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, S., V. A. Lawless, and M. H. Kaplan. 2000. Cytokine-stimulated T lymphocyte proliferation is regulated by p27Kip1. J. Immunol. 165:6270–6277. [DOI] [PubMed] [Google Scholar]