Abstract
Previously, we found that Rad26, the yeast Cockayne syndrome B homolog and the transcription elongation factor Spt4 mediate transcription-coupled repair of UV-induced DNA damage. Here we studied the effect of DNA damage on transcription by directly analyzing the RNA polymerase II localization at active genes in vivo. A rad26 defect leads to loss of Ser5 phosphorylated RNA polymerase II localization to active genes, while localization is only transiently diminished in wild type cells. In contrast, loss of Ser5-P RNAP II localization is suppressed in spt4 cells. Interestingly, even when DNA damage is persistent the absence of Spt4 leads to a delayed loss of transcription suggesting that Spt4 is directly involved in mediating transcription shutdown. Comparative analysis of phosphorylated and non-phosphorylated RNA polymerase II localization revealed that Ser5-P RNAP II is preferentially lost in the presence of DNA damage. In addition, we found evidence for a transient Rad26 localization to active genes in response to DNA damage. These findings provide insight into the transcriptional response to DNA damage and the factors involved in communicating this response, which has direct implications for our understanding of transcription-repair coupling.
INTRODUCTION
Nucleotide excision repair (NER) is an extensively studied DNA repair mechanism capable of removing a wide variety of DNA lesions. Its conserved cut and paste mechanism removes a single stranded stretch of DNA containing the damage (1,2). The rate of repair in vivo is heterogeneous resulting predominantly from preferential repair of the transcribed strand of active genes (3). This observation directly implicates transcription in this mode of repair, which is therefore referred to as transcription-coupled repair (TCR). The fact that TCR is strand specific and that RNA polymerase II (RNAP II) is blocked by DNA damage in vitro led to the assumption that somehow the stalled RNAP II creates a favorable environment for the repair of the damage by NER (4,5).
Cells derived from patients suffering from Cockayne syndrome often carry a mutation in the Cockayne syndrome B gene. Rad26, the yeast sequence homolog of the CSB protein, has been shown to behave analogously to the CSB protein in TCR. Cells deprived of Rad26 display a lack of preferential repair of active genes (6,7). We identified previously the transcription elongation factor SPT4 as a modulator of TCR (8). We showed by direct repair analysis in vivo that abrogation of Spt4 activity leads to reactivation of TCR in rad26 cells while this mode of repair is normally dependent on Rad26. Work both in yeast as well as in human cells has shown that Spt4 acts in a complex with Spt5 in the regulation of the transition from early transcription elongation to processive elongation by monitoring the C-terminal domain (CTD) phosphorylation status (9–11). Apparently TCR can be uncoupled from Rad26 function by alterations in regulation of transcription elongation. How does Spt4 affect transcription and how does it control TCR? To gain insight into these issues it is important to have a better understanding of the direct transcriptional response to DNA damage. Previous studies in mammalian cells have shown that DNA damage is capable of inhibiting mRNA synthesis (12). However, little is known about the composition of actual transcription complexes which encounter DNA damage.
The recently developed chromatin immunoprecipitation (ChIP) procedure allows detailed analysis of yeast protein– DNA complexes in vivo (13–15). Using this assay we determined the fate of the RNAP II, Rad26 and the transcription-repair factor TFIIH in response to DNA damage.
RNAP II is differentially phosphorylated at serine residues of the CTD of the Rpb1 subunit at the onset of transcription elongation (15–17). We found that persistent DNA damage results in loss of RNAP II localization to active genes in vivo, which is counteracted by repair. Strikingly, Ser5 phosphorylated RNAP II (Ser5-P RNAP II) tends to be lost faster compared with non-phosphorylated RNAP II. In addition, loss of RNAP II localization is suppressed in spt4 mutants, which indicates that this protein is somehow involved in regulating the transcriptional response to DNA damage. Importantly, the defect in transcription shutdown after UV in spt4 cells coincides with a suppression of the rad26 TCR defect as we have shown previously (8). These novel findings suggest that the TCR defect in rad26 cells may be caused in part by a direct defect in transcription rather than an inability to alleviate RNAP II blockage at the site of the lesion. Moreover, we found that the loss of Ser5-P RNAP II at the level of the active gene coincides with a transient localization of the Rad26 protein. Taken together, our data suggest a coordinated response of the transcriptional machinery to UV-induced DNA damage.
MATERIALS AND METHODS
Strains and media
Experiments were conducted in the Saccharomyces cerevisiae W303-1B background. The strains used are listed in Table 1. All strains were kept on selective medium: 0.67% yeast nitrogen base, 2% bacto agar, 2% glucose or 2% galactose supplemented with appropriate markers.
Table 1. Yeast strains.
| Strain | Genotype | Reference/source |
|---|---|---|
| W303-1B | MATα can1-100 ade2-1 trp1-1 leu2-3,112 his3-11,15 ura3-1 | R. Rothstein |
| MGSC102 | rad26Δ::HIS3a | This laboratoryb |
| MGSC107 | rad16Δ::LEU2 rad26Δ::HIS3a | This laboratoryc |
| MGSC139 | rad14Δ::LEU2a | This laboratoryc |
| MGSC339 | spt4Δ::URA3a | This laboratoryd |
| MGSC343 | rad16Δ::hisG rad26Δ::HIS3 spt4Δ::URA3a | This laboratoryd |
| MGSC345 | rad14Δ::LEU2 spt4Δ::URA3a | This laboratoryd |
| MGSC439 | rad16Δ::hisG rad26Δ::URA3 pRAD26NHA3/HIS3a | This study |
| MGSC440 | rad3Δ::lox pRAD3NHA3/HIS3a | This study |
| MGSC444 | spt4Δ::URA3 rad3Δ::lox pRAD3NHA3/HIS3a | This study |
| MGSC445 | rad26Δ::URA3 rad3Δ::lox pRAD3NHA3/HIS3a | This study |
| MGSC482 | spt4Δ::URA3 rad14Δ::loxLEU2lox rad3Δ::lox pRAD3NHA3/HIS3a | This study |
Plasmids and gene disruptions
Plasmid pRAD26NHA3 was constructed by amplification of the complete RAD26 ORF using primers Rad26 5′ N-HA, 5′-gac tat gca gga tcc acg ata atg gaa gat aaa gag cag caa, and Rad26 3′ N-HA, 5′-ctc gag gaa ttc ctg cag gat cat gaa gca ttg tta ttc cta a. Underlined sequences are complementary to RAD26. Bold sequences are complementary to target plasmid pZM253 (GAL1-HA3,HIS3, CEN; a gift from Steven Buratowski, Harvard Medical School, Boston, MA) (18). PCR product was recombined into pZM253 at position 906 just adjacent to a unique EcoRV site at position 903 by co-transformation of the PCR product and linearized vector into yeast (GAP repair). The vector is constructed in such a way that three copies of the influenza hemagglutinin epitope are placed N-terminally in frame with the RAD26 ORF. pZM253 was digested with EcoRV and co-transformed with the RAD26 PCR product into yeast cells. Proper recombination was monitored, and functionality was tested by complementation of the rad26 phenotype (not shown). pRAD3NHA3 was constructed analogous to pRAD26NHA3. Primers used were Rad3 5′ N-HA, 5′-gac tat gca gga tcc acg ata atg aag ttt tat ata gat gat tta cca, and Rad3 3′ N-HA, 5′-ctc gag gaa ttc ctg cag gat cac tgc att tct ata tct tca tc. Underlined are RAD3 sequences, bold are sequences homologous to plasmid integration site.
Strain MGSC426 (rad16Δ::hisG rad26Δ::URA3) was constructed by disruption of RAD26 in MGSC268 [rad16Δ::hisG, as described (8)]. The rad26Δ::URA3 geneblaster was constructed by replacement of an internal BglII/XhoI fragment from the RAD26 sequence on pTZSHE6Sc (6) with an URA3 cassette. MGSC426 is used as a background for tagged Rad26 expression by transforming it with the pRAD26NHA3 plasmid resulting in strain MGSC439 (see Table 1). Tagged Rad26 corrects the rad26 UV sensitivity of this strain.
The rad3Δ::lox pRAD3NHA3/HIS3 strain was constructed by deleting one RAD3 allele from W303 diploids using a rad3Δ::loxURA3lox geneblaster. RAD3 sequences were deleted from –31 to +2372 bp relative to the 2337 bp ORF and substituted with an URA3 cassette flanked by loxP sequences. pRAD3NHA3/HIS3 was transformed into the RAD3+/– heterozygous diploids and haploids carrying a URA+ HIS+ (rad3Δ pRAD3NHA3) phenotype were isolated by sporulation and tetrad formation. Epitope-tagged Rad3 fully complements the transcription and repair defects of the rad3 mutation (not shown). The URA3 cassette was excised by shuttling in and out of a Cre expression vector, which resulted in strain MGSC440 (Table 1). MGSC440 was subsequently rendered spt4 or rad26, using the spt4Δ::URA3 geneblaster as described (8) and the rad26Δ::URA3 geneblaster described above resulting in MGSC444 and MGSC445, respectively. MGSC444 was subsequently rendered rad14 by transformation with a rad14Δ::loxLEU2lox construct (strain MGSC482). Effectively a SpeI/EcoRV RAD14 fragment was deleted (+25 in exon 1 to +1273 in the last exon relative to the 1200 bp ORF) and substituted with a LEU2 cassette flanked by loxP sequences. All gene deletions were confirmed by Southern blotting.
Chromatin immunoprecipitation
The ChIP procedure was adapted from Strahl-Bolsinger et al. (14). Cells were grown in 0.67% yeast nitrogen base, 2% galactose supplemented with appropriate markers.
The following modifications to the study by Strahl-Bolsinger et al. (14) have been made. Cells were irradiated with UV-C at a rate of 3.75 J/m2/s. UV dose was 140 J/m2 unless stated otherwise. Cells were irradiated and incubated at a cell density of 1.4 OD600 units. Cells were cross-linked in 1% formaldehyde for 10 min at 30°C. All steps were in growth medium. RIPA buffer (50 mM Tris–HCl pH 8.0, 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% sodium-deoxycholate + protease inhibitors, 1 mM PMSF, 0.3 mg/ml benzamidine, 1 µg/ml leupeptin, 1 µg/ml antipain, 1 µg/ml pepstatin, 1 µg/ml chymostatin) was used as lysis buffer. Chromatin was sonicated with 400 J to an average DNA fragment length of 0.2–0.5 kb. One hundred microliters of whole cell extract (WCE) was immunoprecipitated by incubating with either 16 µg 12CA5 (αHA), 20 µg 8WG16 (αCTD) antibody or 20 µg H14 (anti CTD-Ser5-P) (Covance). A 30 µl bed volume of protein A–Sepharose (Amersham Pharmacia) was used to immobilized antibodies. In the case of H14, IP beads were pre-coated with 30 µg of goat IgG α-mouse-IgM (Sigma). Immunoprecipitates were washed for 5 min with 1 ml RIPA followed by washes, elution, de-cross-linking and DNA isolation as described (14).
DNA was amplified in a 25 µl reaction using 0.5 U Amplitaq gold (PE Applied Biosystems) under buffer conditions as provided by the manufacturer, in the presence of 1.5 mM MgCl2, 50 µM dGTP, dTTP and dATP, 10 µM dCTP, 3.2 nM [α-32P]dCTP (6000 Ci/mmol) and 5 pmol of each primer. Cycling was 27 cycles of 30 s at 94°C, 30 s at 55°C, 1 min at 72°C, except for the first cycle 94°C step for 90 s and the last cycle 72°C step for 10 min. PCR products were resolved on 10% polyacrylamide gels. Gels were dried and signals were visualized by a Bio-Rad molecular imager.
Primers used: for GAL7 promoter, GAL7-1 5′-ggtccaaaaagcgctcgg, GAL7-2 5′-tgggaatggctagaaaaatca. Amplification results in a 244 bp fragment from –236 to +38. For GAL7 coding sequence, GAL7-3 5′-ggctcctttgaatgcgac, GAL7-4 5′-actccgttcaagtcgacaacc. Amplification results in a 299 bp fragment from +894 to +1193. For ACT1 promoter, ACT1-1 5′-ttgtattcttccttccccttt, ACT1-2 5′-gggatggtgcaagcgc. Ampli fication results in a 187 bp fragment from –151 to +36. For ACT1 coding sequence, ACT1-3 5′-tcccaggtattgccgaaag, ACT1-4 5′-aacatacgcgcacaaaagca. Amplification results in a 235 bp fragment from +2225 to +2460. Primers for the PMA1 gene were as described (15). For PMA1 promoter, PMA1–370 and PMA1–70. For PMA1 coding sequence, PMA12018 and PMA12290. Primers for the silent HML locus were 5′-ccccatcgtcttgctcttg (hybridizes to both MAT and HML locus in α-cells) and 5′-ggaacacagaaaagagcagtgaaag (HML specific). Amplification results in a 165 bp fragment. All base pair positions are relative to the translational start site of indicated gene.
RESULTS
Loss of RNAP II localization after DNA damage involves Spt4
We used ChIP to study protein localization at active genes in response to DNA damage. Cells were UV irradiated and allowed to recover for various lengths of time. Protein–DNA contacts were fixed by formaldehyde cross-linking after which cells were lysed and chromatin was sheared by sonication.
Ser5-P RNAP II and associated DNA were pulled down with the H14 monoclonal antibody from cross-linked extracts. Co-immunoprecipitated DNA was analyzed by semi-quantitative PCR. The transcriptionally silent mating type locus, HML, was used as reference during each PCR reaction. We used DNA isolated from non-precipitated WCE as a control in the PCR reaction (Fig. 1A, lanes 1 and 8).
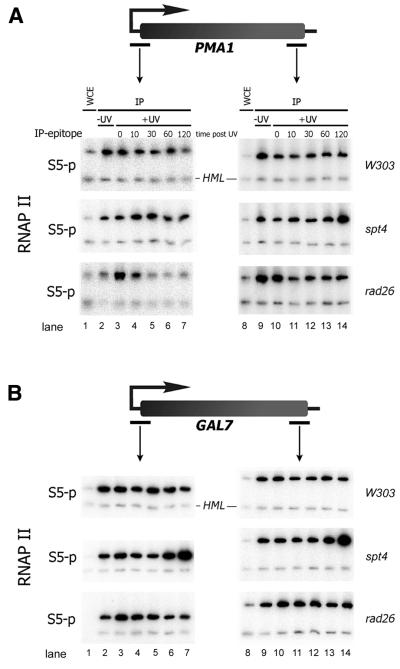
Figure 1.
Spt4 and Rad26 affect Ser5-P RNAP II localization after UV irradiation. ChIP experiment: chromatin extracts were prepared from cells either unirradiated or from irradiated cells at indicated time points post UV (in minutes). Relevant genotype of strains analyzed is indicated on the right. RNAP II was pulled down with H14 (S5-P). DNA extracted from non- precipitated WCE was included as control for difference in PCR efficiency for different loci. (A) Amplification of PMA1 promoter and coding sequences. (B) GAL7 promoter and coding sequences. HML locus was used as a negative control locus (lower bands in each panel).
We found that in unirradiated wild type cells Ser5-P RNAP II localized at both the promoter and the coding sequence of the PMA1 gene while only a background signal was observed at the HML locus (Fig. 1A, lanes 2 and 9). Ser5-P RNAP II has been reported to localize to the promoter region, which is in contrast with our findings here (15). However, in this previous report it is further mentioned that the level of Ser5 phosphorylation detected on the coding sequence may vary depending on the antibody batch used. Furthermore, the extent of phosphorylation may depend on the specific growth conditions employed as has been reported for Ser2 phosphorylation (17).
UV irradiation leads to a slight decrease in Ser5-P RNAP II localization throughout the PMA1 gene. This decrease in localization is observed almost immediately after irradiation and persists at the PMA1 promoter region.
We reported previously that the transcription elongation factor Spt4 modulates TCR activity in rad26 mutants (8). Spt4 may directly affect the transcriptional response to DNA damage. Indeed we found, in contrast to wild type cells, that in spt4 mutants Ser5-P RNAP II localization at the PMA1 promoter increases initially before localization is diminished ∼1 h post-irradiation. At the coding sequence of the PMA1 gene RNAP II localization accumulates in time after UV due to the absence of Spt4. Spt4 appears to counteract the loss of transcription at the PMA1 gene (Fig. 1A).
Rad26 is involved in TCR. Lack of Rad26 activity presumably leads to increased transcriptional blockage due to unrepaired DNA damage. In rad26 mutants RNAP II localization at the PMA1 promoter is low in unirradiated cells. Upon UV irradiation, localization of RNAP II is transiently increased followed by a rapid loss of localization 10 min after irradiation (Fig. 1A). In contrast to wild type cells, RNAP II localization is lost completely. Loss is observed predominantly at the PMA1 promoter. Strikingly, Ser5-P RNAP II remains localized at the PMA1 coding sequence. It is possible that Ser5-P RNAP II localization at this site is due to blockage and accumulation of RNAP II.
In parallel with the PMA1 gene we analyzed the GAL7 gene, which is highly transcribed in the presence of galactose. RNAP II localization at GAL7 is less prone to down regulation after UV (Fig. 1B). However, also at this locus we observe some loss of Ser5-P RNAP II at the promoter in rad26 cells and a reciprocal effect of a spt4 mutation reinforcing our observation on PMA1 that absence of Spt4 suppresses transcriptional loss after UV irradiation.
Suppression of RNAP II loss after UV in spt4 cells does not require repair of DNA damage
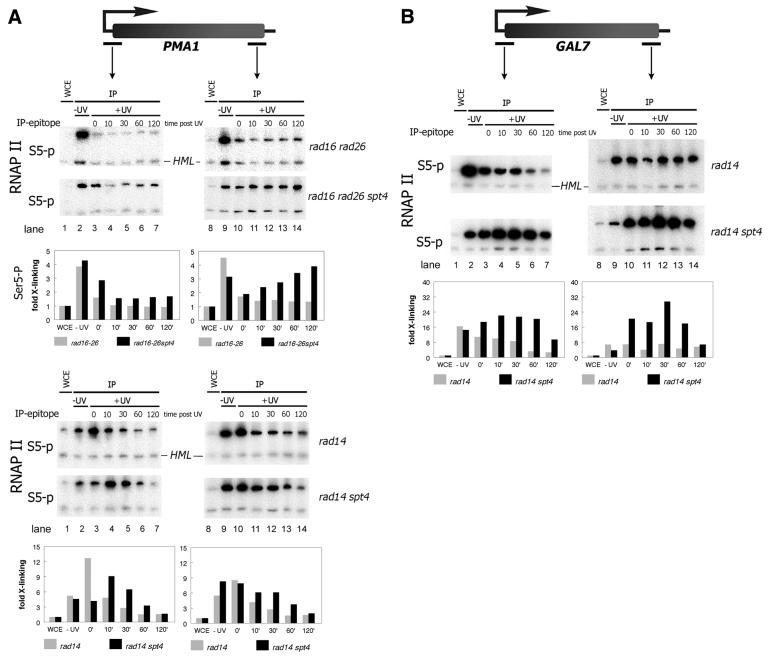
Cells lacking both the RAD16 and RAD26 genes have a defect in repair of the genome overall and a partial defect in TCR resulting in considerable UV sensitivity (7). Deletion of SPT4 results in a reduction of UV sensitivity in this background due to reactivation of TCR (8). Here we analyzed the effect of Spt4 on the fate of RNAP II localization in the rad16rad26 background where TCR is completely independent of Rad26 and modulated by Spt4 (8).
Like in rad26 cells, UV irradiation of rad16rad26 cells results in a strong reduction of Ser5-P RNAP II on the PMA1 promoter and to a lesser extent also the coding sequence (Fig. 2A). However, in rad16rad26spt4 triple mutants loss of Ser5-P RNAP II is suppressed compared with rad16rad26 cells. In the absence of Spt4, Ser5-RNAP II localization is less strongly diminished post UV as compared with rad16rad26 cells in which Spt4 is active. The effect of Spt4 is most apparent on the PMA1 coding sequence. In rad16rad26spt4 cells, like in spt4 single mutants, Ser5-P RNAP II increases in time post UV rather than decreases as is observed in the rad16rad26 cells. Strikingly, the suppression of Ser5-P RNAP II loss in rad16rad26spt4 cells correlates with the reduced UV sensitivity and activated TCR in these cells (8). It could therefore be argued that the absence of transcription down regulation in spt4 cells as shown here is merely the result of fast repair of transcription blocking lesions. To test this possibility we have analyzed the contribution of Spt4 to transcription loss after UV irradiation in a NER-deficient background in which DNA damage is persistent.
Figure 2.
Absence of Spt4 suppresses loss of RNAP II localization independent of repair. ChIP experiment as in Figure 1. (A) Amplification of PMA1 promoter and coding sequences. (B) GAL7 promoter and coding sequences. Quantification of the PCR product signals are displayed in histograms below corresponding PCR series. Fold cross-linking of is Ser5-P RNAP II to the gene is indicated as determined by the ratio of PCR product signals from the genes compared with the inactive HML locus.
NER is completely abrogated in cells lacking RAD14. In these cells, like in rad26 or rad16rad26 mutants Ser5-P RNAP II is progressively lost at the PMA1 gene after UV irradiation due to persistent DNA damage (Fig. 2A, lower panel). Strikingly, also in a NER-deficient background a spt4 defect leads to a delayed loss of Ser5-P RNAP II at the PMA1 gene. At the GAL7 gene essentially the same observation is made; however, like in rad26 cells loss is observed predominantly at the promoter (Fig. 2B). Both at PMA1 and GAL7 loss of Spt4 has a pronounced effect on Ser5-P RNAP II localization following UV. Localization is markedly increased in rad14spt4 cells up to 30 min post UV before RNAP II localization eventually levels off (Fig. 2A and B).
The fact that transcription loss after UV irradiation is suppressed in spt4 cells even in the absence of NER directly shows that the rescue of transcription is not the result of better repair. Instead the data suggest that Spt4 regulates the response to DNA damage directly at the transcriptional level.
DNA damage predominantly inhibits Ser5-P RNAP II transcription
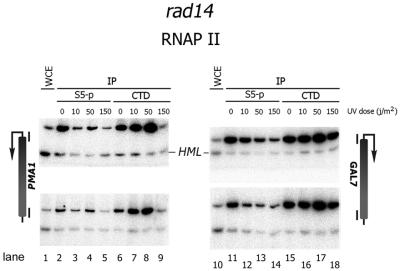
As the loss of RNAP II localization after UV appears to be regulated we decided to analyze the UV response in some more detail. We determined whether loss of localization of the H14 epitope (Ser5-P RNAP II) is due to complete loss of RNAP II or whether only the Ser5 phospho-specific epitope is lost. Therefore, we immunoprecipitated both Ser5-P RNAP II with H14 and non-Ser5-phosphorylated RNAP II with 8WG16 antibodies from rad14 cells.
We assayed the dose dependence of the loss of both forms of RNAP II (Fig. 3). Interestingly, at low UV doses a variable but clear reduction in Ser5-P RNAP II is observed while non-phosphorylated RNAP II remains localized to both the PMA1 and GAL7 genes (Fig. 3). In fact at low doses up to 50 J/m2, non-phosphorylated RNAP II appears to increase rather than to diminish. Only at high levels of UV-induced DNA damage is the 8WG16 epitope lost at the PMA1 gene while only reduced at the GAL7 gene (Fig. 3). These results indicate that UV-induced DNA damage results in differential loss of Ser5 phosphorylated and non-phosphorylated RNAP II, which is suggestive of a regulated response to DNA damage rather than a general transcription blockage by damaged DNA.
Figure 3.
Dose-dependent differential loss of Ser5-P RNAP II or non-phosphorylated RNAP II. ChIP experiment. Chromatin extracts were prepared from rad14 cells 30 min after irradiation with indicated UV doses. RNAP II was pulled down using H14 antibody (S5-P) or 8WG16 antibody (CTD). Top bands in each gel correspond to the promoter region or coding sequence of genes indicated flanking the gel. Lower bands are amplified from inactive HML locus.
TFIIH localization is not altered by damage induction or by Spt4
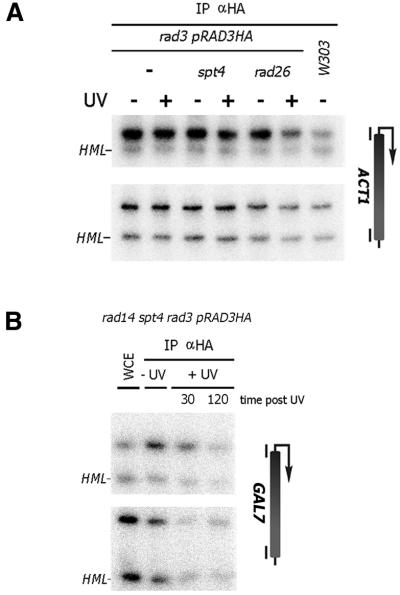
The transcription-repair factor TFIIH is associated with transcription initiation and with NER (19,20). We have reported previously that TCR during early elongation is Rad26 independent which correlates with the presence of TFIIH (21,22). As TFIIH is an essential component of the NER machinery it is conceivable that the presence of TFIIH in the transcription complex leads directly to repair without the need for Rad26. In spt4 cells TCR is completely Rad26 independent (8). Possibly, TFIIH remains localized during transcription elongation in spt4 cells, which would directly lead to transcription-repair coupling. We have tested this hypothesis by analyzing the localization of the TFIIH subunit Rad3 in spt4 cells after irradiation. Analysis of the ACT1 locus showed that Rad3 localizes predominantly to the promoter region of this gene (Fig. 4A). This is consistent with its role in the basal transcription initiation factor TFIIH. The localization is unaffected by deletion of the SPT4 or RAD26 gene. Upon irradiation with 70 J/m2 no relocalization could be observed except a reduced promoter localization in rad26 cells, which is likely to be due to transcription inhibition. We found no evidence of TFIIH relocalization to the coding sequence of the ACT1 gene (Fig. 4A). Also, at a high UV dose in the absence of Spt4 and in a NER-deficient background, which results in strong Ser5-P RNAP II localization at the GAL7 coding sequence (see Fig. 2B), no increase in Rad3 localization could be detected specifically at the coding sequence of the same gene (Fig. 4B). These results suggest that Rad3 and presumably TFIIH as a whole is not a specific determinant in the altered transcription and Rad26 independent TCR in spt4 cells.
Figure 4.
Rad3 promoter localization is not altered upon UV irradiation. ChIP experiment. Triple HA-tagged Rad3 was immunoprecipitated from indicated strains using 12CA5 antibodies. (A) Rad3 localizes to the ACT1 promoter. Chromatin extracts were prepared from cells either unirradiated or from cells irradiated with 70 J/m2 10 min after irradiation. DNA was amplified from precipitates using primers corresponding to the promoter and CDS of the ACT1. (B) Rad3 localization is not altered in the absence of NER and Spt4 after a high UV dose. Rad3 was immunoprecipitated from chromatin extracts prepared from indicated strain either unirradiated or irradiated with 140 J/m2 after indicated time points (minutes). Promoter and coding sequences were amplified from the GAL7 gene.
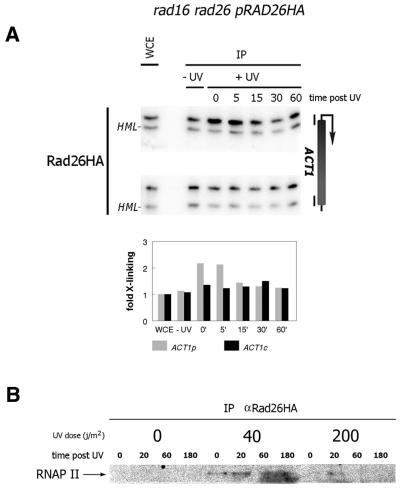
Rad26 transiently co-localizes with transcription after DNA damage induction
We have shown previously that Rad26 is specifically involved in TCR (6,7). Here we have tested whether Rad26 associates with the transcriptional machinery in vivo. Surprisingly, analysis of Rad26 localization at the ACT1 gene shows that under non-damaged conditions little or no Rad26 is localized at this gene, which suggests that Rad26 is not an integral component of the transcription machinery (Fig. 5A). Upon irradiation a low level of Rad26 is localized to the promoter of the ACT1 gene. Localization occurs transiently within minutes after irradiation and is lost 15–30 min after irradiation. The cross-linking of Rad26 to the active ACT1 locus suggests a direct co-localization of Rad26 and RNAP II. We therefore further analyzed Rad26 precipitates from cross-linked cells which were either non-irradiated or irradiated with UV. Western blot analysis of the precipitates shows a damage-dependent co-localization of Rad26 and RNAP II. At 40 J/m2 a weak but significant amount of Ser5-P RNAP II is found in the Rad26 immunoprecipitate (Fig. 5B). At the high 200 J/m2 dose some RNAP II could be detected although to a lesser extent compared with low UV dose. Possibly a high UV dose results in strong transcriptional inhibition leading to lower levels of RNAP II.
Figure 5.
Rad26 co-localizes with transcription upon UV irradiation. ChIP experiment. Triple HA-tagged Rad26 was immunoprecipitated from indicated strain using 12CA5 antibodies. (A) Rad26 localizes to the ACT1 promoter after UV. Chromatin extracts were prepared from cells either unirradiated or from irradiated cells at indicated time points (min) post 70 J/m2 of UV. DNA was amplified from precipitates using primers corresponding to the promoter and CDS of the ACT1 gene. Quantification of the PCR product signals are displayed in histograms below corresponding PCR series as in Figure 2. (B) Rad26 cross-links to RNAP II after UV. Rad26 was immunoprecipitated from chromatin extract prepared from cross-linked cells either unirradiated or from cells irradiated with indicated doses (in J/m2) at indicated time points (minutes) post UV. Precipitates were run across a SDS–PA gel blotted to a PVDF membrane and RNAP II was detected using H14 antibody.
DISCUSSION
Using ChIP we have the unique opportunity to assess the location of the transcriptional machinery in the chromatin of living cells. We show for the first time that this methodology can be used to study the localization of transcription and repair proteins in response to DNA damage.
It has not escaped our attention that UV-induced DNA damage can block DNA polymerase during PCR amplification and thereby frustrate the analysis of protein–DNA contacts. However, based on previous measurements on the induction frequency of UV-induced DNA damage (8,23) we estimate that due to the small template size for the PCR reaction <20% of target molecules carry DNA damage. In addition, comparisons between Ser5-P RNAP II and non-phosphorylated RNAP II localization are made from the same precipitate ruling out differences in damage load. Moreover, in experiments in NER-deficient cells the damage load remains constant throughout the time course of the experiment, which makes it unlikely in these cases that our observations are occluded by PCR artifacts due to the presence of damage in the template.
The involvement of Spt4 in transcript elongation and TCR prompted us to study the role of this protein in the transcriptional response to DNA damage. Our results show that (i) the loss of Ser5-P RNAP II localization is mediated in part by Spt4, which is independent of removal of DNA damage, (ii) persistent DNA damage results in the differential loss of Ser5-P RNAP II and non-phosphorylated RNAP II, and (iii) Rad26 transiently co-localizes with RNAP II after UV.
These findings argue in favor of a dynamic response to DNA damage rather than direct blockage of RNAP II at the site of the lesion as a sole transcriptional response to DNA damage. Stalled RNAP II at DNA damage may trigger a more widespread response, which is transmitted to other transcription elements. A recent report claims that if such signaling exists it is not likely to carry across the nucleus but will act at a more local level (24). It follows from our experiments that transcription is not simply blocked but may in fact continue in a different mode. While Ser5-P RNAP II is lost after low doses of UV, the localization of a RNAP II species, which we detect using 8WG16 antibodies, is affected only at higher doses. These antibodies are thought to detect predominantly unphosphorylated RNAP II but we cannot exclude that the RNAP II species we observe are modified on other residues than Ser5.
In addition to the differential response of phosphorylated and non-phosphorylated RNAP II to DNA damage, we identified Spt4 as a modulator of this response. The fact that the loss of Ser5-P RNAP II transcription due to unrepairable DNA damage can be delayed by abrogating Spt4 activity strongly suggest that the down regulation of transcription is a regulated event and is not an inevitable result of persistent DNA damage per se.
Ultimately, however, RNAP II is lost altogether. Possibly two separate mechanisms are responsible for these effects. First, rapid loss of Ser5-P RNAP II through Spt4-dependent down regulation and secondly at high levels of DNA damage, or when damages are persistent, all transcription is eventually lost which is likely to be mediated by the recently identified Rad26 associated factor, Def1 (degradation elongation factor 1) (23).
The concept of a controlled transcription response to DNA damage was also recently put forward for RNAP III transcription. Ghavidel and Schultz (25) elegantly showed that RNAP III transcription is globally down regulated through CK2-dependent phosphorylation of the TATA binding protein subunit of TFIIIB in response to DNA damage.
The Spt4-mediated down regulation of Ser5-P RNAP II transcription may well be the result of a general stress response to UV. It is interesting to note in this respect that the Drosophila Spt5 and Spt6 homologs that act in association with Spt4 have been shown to relocalize to the heat shock genes in response to stress signals (26,27). Our results indicate that in view of these earlier findings Spt4 may, possibly in concert with Spt5 and Spt6, mediate the transcriptional changes in response to stress including DNA damage.
Based on our findings reported here we propose that transcription is actively down regulated and thereby contributes to a defect in TCR. Spt4 stimulates down regulation of transcription while a defect in this transcription down regulation correlates with activation of TCR. This suggests that the TCR defect in rad26 mutants may be caused in part by a lack of transcription instead of a defect in coupling repair to stalled RNA polymerases. It is conceivable that a defect in RAD26 leads to loss of a specific subset of RNAP II complexes that correlate with Ser5 phosphorylation of RNAP II. The TFIIH subunit Kin28 is involved in Ser5 phosphorylation of the RNAP II CTD (16). Indeed, a partial TCR defect is observed in conditional kin28 mutants lacking kinase activity (28).
Furthermore, we have found a transient localization of Rad26 protein to the ACT1 gene in response to DNA damage. Possibly Rad26 may directly counteract the Spt4-mediated transcription inhibition in response to DNA damage thereby facilitating TCR.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Steve Buratowski and Zarmik Moqtaderi for the pZM253 plasmid, Maria Fousteri and Leon Mullenders for the 8WG16 antibody, Yde Steensma for help with tetrad dissections and Jesper Svejstrup for discussions and fruitful collaboration. This work was supported in part by the Institute for radiopathology and radiation protection (IRS).
REFERENCES
- 1.Prakash S. and Prakash,L. (2000) Nucleotide excision repair in yeast. Mutat. Res., 451, 13–24. [DOI] [PubMed] [Google Scholar]
- 2.de Laat W.L., Jaspers,N.G. and Hoeijmakers,J.H. (1999) Molecular mechanism of nucleotide excision repair. Genes Dev., 13, 768–785. [DOI] [PubMed] [Google Scholar]
- 3.Mellon I., Spivak,G. and Hanawalt,P.C. (1987) Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell, 51, 241–249. [DOI] [PubMed] [Google Scholar]
- 4.Selby C.P. and Sancar,A. (1990) Transcription preferentially inhibits nucleotide excision repair of the template DNA strand in vitro. J. Biol. Chem., 265, 21330–21336. [PubMed] [Google Scholar]
- 5.Selby C.P. and Sancar,A. (1993) Molecular mechanism of transcription-repair coupling. Science, 260, 53–58. [DOI] [PubMed] [Google Scholar]
- 6.van Gool A.J., Verhage,R., Swagemakers,S.M., van de Putte,P., Brouwer,J., Troelstra,C., Bootsma,D. and Hoeijmakers,J.H. (1994) RAD26, the functional S. cerevisiae homolog of the Cockayne syndrome B gene ERCC6. EMBO J., 13, 5361–5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verhage R.A., van Gool,A.J., de Groot,N., Hoeijmakers,J.H., van de Putte,P. and Brouwer,J. (1996) Double mutants of Saccharomyces cerevisiae with alterations in global genome and transcription-coupled repair. Mol. Cell. Biol., 16, 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen L.E., den Dulk,H., Brouns,R.M., de Ruijter,M., Brandsma,J.A. and Brouwer,J. (2000) Spt4 modulates Rad26 requirement in transcription-coupled nucleotide excision repair. EMBO J., 19, 6498–6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartzog G.A., Wada,T., Handa,H. and Winston,F. (1998) Evidence that Spt4, Spt5 and Spt6 control transcription elongation by RNA polymerase II in Saccharomyces cerevisiae. Genes Dev., 12, 357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wada T., Takagi,T., Yamaguchi,Y., Ferdous,A., Imai,T., Hirose,S., Sugimoto,S., Yano,K., Hartzog,G.A., Winston,F., Buratowski,S. and Handa,H. (1998) DSIF, a novel transcription elongation factor that regulates RNA polymerase II processivity, is composed of human Spt4 and Spt5 homologs. Genes Dev., 12, 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaguchi Y., Takagi,T., Wada,T., Yano,K., Furuya,A., Sugimoto,S., Hasegawa,J. and Handa,H. (1999) NELF, a multisubunit complex containing RD, cooperates with DSIF to repress RNA polymerase II elongation. Cell, 97, 41–51. [DOI] [PubMed] [Google Scholar]
- 12.Mullenders L.H. (1998) Transcription response and nucleotide excision repair. Mutat. Res., 409, 59–64. [DOI] [PubMed] [Google Scholar]
- 13.Hecht A., Strahl-Bolsinger,S. and Grunstein,M. (1999) Mapping DNA interaction sites of chromosomal proteins. Crosslinking studies in yeast. Methods Mol. Biol., 119, 469–479. [DOI] [PubMed] [Google Scholar]
- 14.Strahl-Bolsinger S., Hecht,A., Luo,K. and Grunstein,M. (1997) SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes Dev., 11, 83–93. [DOI] [PubMed] [Google Scholar]
- 15.Komarnitsky P., Cho,E.J. and Buratowski,S. (2000) Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev., 14, 2452–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho E.J., Kobor,M.S., Kim,M., Greenblatt,J. and Buratowski,S. (2001) Opposing effects of Ctk1 kinase and Fcp1 phosphatase at Ser 2 of the RNA polymerase II C-terminal domain. Genes Dev., 15, 3319–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patturajan M., Schulte,R.J., Sefton,B.M., Berezney,R., Vincent,M., Bensaude,O., Warren,S.L. and Corden,J.L. (1998) Growth-related changes in phosphorylation of yeast RNA polymerase II. J. Biol. Chem., 273, 4689–4694. [DOI] [PubMed] [Google Scholar]
- 18.Moqtaderi Z., Yale,J.D., Struhl,K. and Buratowski,S. (1996) Yeast homologues of higher eukaryotic TFIID subunits. Proc. Natl Acad. Sci. USA, 93, 14654–14658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feaver W.J., Svejstrup,J.Q., Bardwell,L., Bardwell,A.J., Buratowski,S., Gulyas,K.D., Donahue,T.F., Friedberg,E.C. and Kornberg,R.D. (1993) Dual roles of a multiprotein complex from S. cerevisiae in transcription and DNA repair. Cell, 75, 1379–1387. [DOI] [PubMed] [Google Scholar]
- 20.Drapkin R., Reardon,J.T., Ansari,A., Huang,J.C., Zawel,L., Ahn,K., Sancar,A. and Reinberg,D. (1994) Dual role of TFIIH in DNA excision repair and in transcription by RNA polymerase II. Nature, 368, 769–772. [DOI] [PubMed] [Google Scholar]
- 21.Tijsterman M., Verhage,R.A., van de Putte,P., Tasseron-de Jong,J.G. and Brouwer,J. (1997) Transitions in the coupling of transcription and nucleotide excision repair within RNA polymerase II-transcribed genes of Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA, 94, 8027–8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zawel L., Kumar,K.P. and Reinberg,D. (1995) Recycling of the general transcription factors during RNA polymerase II transcription. Genes Dev., 9, 1479–1490. [DOI] [PubMed] [Google Scholar]
- 23.Woudstra E.C., Gilbert,C., Fellows,J., Jansen,L., Brouwer,J., Erdjument-Bromage,H., Tempst,P. and Svejstrup,J.Q. (2002) A Rad26-Def1 complex coordinates repair and RNA pol II proteolysis in response to DNA damage. Nature, 415, 929–933. [DOI] [PubMed] [Google Scholar]
- 24.Mone M.J., Volker,M., Nikaido,O., Mullenders,L.H., van Zeeland,A.A., Verschure,P.J., Manders,E.M. and van Driel,R. (2001) Local UV-induced DNA damage in cell nuclei results in local transcription inhibition. EMBO Rep., 2, 1013–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghavidel A. and Schultz,M.C. (2001) TATA binding protein-associated CK2 transduces DNA damage signals to the RNA polymerase III transcriptional machinery. Cell, 106, 575–584. [DOI] [PubMed] [Google Scholar]
- 26.Andrulis E.D., Guzman,E., Doring,P., Werner,J. and Lis,J.T. (2000) High-resolution localization of Drosophila Spt5 and Spt6 at heat shock genes in vivo: roles in promoter proximal pausing and transcription elongation. Genes Dev., 14, 2635–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan C.D., Morris,J.R., Wu,C. and Winston,F. (2000) Spt5 and spt6 are associated with active transcription and have characteristics of general elongation factors in D.melanogaster. Genes Dev., 14, 2623–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tijsterman M., Tasseron-de Jong,J.G., Verhage,R.A. and Brouwer,J. (1998) Defective Kin28, a subunit of yeast TFIIH, impairs transcription-coupled but not global genome nucleotide excision repair. Mutat. Res., 409, 181–188. [DOI] [PubMed] [Google Scholar]