Abstract
We report an approach using solid phase capturable biotinylated dideoxynucleotides (biotin-ddNTPs) in single base extension for multiplex genotyping by mass spectrometry (MS). In this method, oligonucleotide primers that have different molecular weights and that are specific to the polymorphic sites in the DNA template are extended with biotin-ddNTPs by DNA polymerase to generate 3′-biotinylated DNA products. These products are then captured by streptavidin-coated solid phase magnetic beads, while the unextended primers and other components in the reaction are washed away. The pure extension DNA products are subsequently released from the solid phase and analyzed by matrix-assisted laser desorption/ionization time-of-flight MS. The mass of the extension products is determined using a stable oligonucleotide as a common internal mass standard. Since only the pure extension DNA products are introduced to the MS for analysis, the resulting mass spectrum is free of non-extended primer peaks and their associated dimers, which increases the accuracy and scope of multiplexing in single nucleotide polymorphism (SNP) analysis. The solid phase purification approach also facilitates desalting of the captured oligonucleotides, which is essential for accurate mass measurement by MS. We selected four biotin-ddNTPs with distinct molecular weights to generate extension products that have a 2-fold increase in mass difference compared to that with conventional ddNTPs. This increase in mass difference provides improved resolution and accuracy in detecting heterozygotes in the mass spectrum. Using this method, we simultaneously distinguished six nucleotide variations on synthetic DNA templates mimicking mutations in the p53 gene and two disease-associated SNPs in the human hereditary hemochromatosis gene.
INTRODUCTION
Single nucleotide polymorphisms (SNPs), the most common genetic variations in the human genome, are important markers for identifying disease genes and for pharmacogenetic studies (1,2). SNPs appear in the human genome with an average density of one every 1000 bp (3). To perform large-scale SNP genotyping, a rapid, precise and cost-effective method is required. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (MS) (4) allows rapid and accurate sample measurements (5–7) and has been used in a variety of SNP detection methods, including hybridization (8–10), invasive cleavage (11,12) and single base extension (SBE) (5,13–17). SBE is widely used for multiplex SNP analysis. In this method, primers designed to anneal immediately adjacent to a polymorphic site are extended by a single dideoxynucleotide that is complementary to the nucleotide at the variable site. By measuring the mass of the resulting extension product, a particular SNP can be identified. Current SBE methods to perform multiplex SNP analysis using MS require unambiguous simultaneous detection of a library of primers and their extension products. However, limitations in resolution and sensitivity of MALDI-TOF MS for longer DNA molecules make it difficult to simultaneously measure DNA fragments over a large mass range (6). The requirement to measure both primers and their extension products in this range limits the scope of multiplexing.
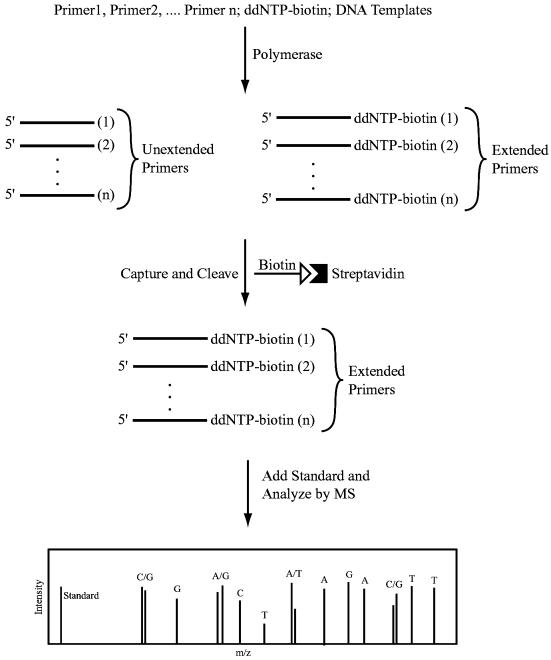
We recently developed a high fidelity DNA sequencing method using solid phase capturable biotinylated dideoxynucleotides (biotin-ddNTPs) with detection by fluorescence (18) and MS (19), eliminating the false terminations and excess primers. We also explored the use of combinatorial fluorescence energy transfer tags and biotin-ddNTPs to detect SNPs (20). We report here an approach using the solid phase capturable biotin-ddNTPs in SBE for multiplex genotyping by MALDI-TOF MS. In this method (Fig. 1), primers that have different molecular weights and that are specific to the polymorphic sites in the DNA template are extended with biotin-ddNTPs by DNA polymerase to generate 3′-biotinylated extension DNA products. The 3′-biotinylated DNAs are then captured by streptavidin-coated magnetic beads, while the unextended primers and other components in the reaction are washed away. The pure DNA extension products are subsequently released from the magnetic beads, by denaturing the biotin–streptavidin interaction with formamide, and analyzed by MALDI-TOF MS. The nucleotide at the polymorphic site is identified by analyzing the mass difference between the primer extension product and an internal mass standard added to the purified DNA products. Since the primer extension products are isolated prior to MS analysis, the resulting mass spectrum is free of non-extended primer peaks and their associated dimers, which increases the accuracy and scope of multiplexing in SNP analysis. The solid phase purification system also facilitates desalting of the captured oligonucleotides. Desalting is critical in sample preparation for MALDI-TOF MS measurements since alkaline and alkaline earth salts can form adducts with DNA fragments that interfere with accurate peak detection (21). In addition, we selected four biotin-ddNTPs with distinct molecular weights to generate extension products that have a 2-fold increase in mass difference compared to that with conventional ddNTPs. This increase in mass difference provides improved resolution and accuracy in detecting peaks in the mass spectrum. Using this method, we simultaneously distinguished six nucleotide variations in synthetic DNA templates mimicking mutations in the p53 gene. We also applied this method to PCR products to successfully detect two disease-associated SNPs in the human hereditary hemochromatosis (HHC) gene HFE (22) from multiple individuals.
Figure 1.
Scheme of single base extension for multiplex SNP analysis using biotin-ddNTPs and MALDI-TOF MS. Primers that anneal immediately next to the polymorphic sites in the DNA template are extended with a biotin-ddNTP by DNA polymerase in a sequence-specific manner. After solid phase capture and isolation of the 3′-biotinylated DNA extension fragments, MALDI-TOF MS was used to analyze these DNA products to yield a mass spectrum. From the relative mass of each primer extension product, compared to the mass of an internal standard, the nucleotide at the polymorphic site is identified.
MATERIALS AND METHODS
PCR amplification
DNA templates containing the polymorphic sites for the HHC gene HFE were amplified from genomic DNA in a total volume of 10 µl, containing 20 ng genomic DNA, 500 pmol each forward (C282Y, 5′-CTACCCCCAGAACATCACC-3′; H63D, 5′-GCACTACCTCTTCATGGGTGCC-3′) and reverse (C282Y, 5′-CATCAGTCACATACCCCA-3′; H63D, 5′-CAGTGAACATGTGATCCCACCC-3′) primer, 25 µM dNTPs (Amersham Biosciences, Piscataway, NJ), 1 U Taq polymerase (Life Technologies, Rockville, MD) and 1× PCR buffer (50 mM KCl, 1.5 mM MgCl2, 10 mM Tris–HCl). PCR amplification reactions were started at 94°C for 4 min, followed by 45 cycles of 94°C for 30 s, 59°C for 30 s and 72°C for 10 s, and finished with an additional extension step of 72°C for 6 min. Excess primers and dNTPs were degraded by adding 2 U shrimp alkaline phosphatase (Roche Diagnostics, Indianapolis, IN) and Escherichia coli exonuclease I (Boehringer Mannheim, Indianapolis, IN) in 1× shrimp alkaline phosphatase buffer. The reaction mixture was incubated at 37°C for 45 min followed by enzyme inactivation at 95°C for 15 min.
Single base extension using biotin-ddNTPs
The synthetic DNA templates containing six nucleotide variations in the p53 gene and the five primers for detecting these variations are shown in Table 1. These oligonucleotides and an internal mass standard (5′-TTTTTCTTTTTCT-3′, mol. wt 3855 Da) for MALDI-TOF MS measurement were made using an Expedite nucleic acid synthesizer (Applied Biosystems, Foster City, CA). SBE reactions contained 20 pmol primer, 10 pmol biotin-11-ddATP, 20 pmol biotin-11-ddGTP, 40 pmol biotin-11-ddCTP (NEN Life Science, Boston, MA), 80 pmol biotin-16-ddUTP (Enzo Diagnostics, Farmingdale, NY), 2 µl Thermo Sequenase reaction buffer, 1 U Thermo Sequenase in its dilution buffer (Amersham Biosciences) and 20 pmol either synthetic template or PCR product in a total reaction volume of 10 µl. For SBE using synthetic template 1, 10 pmol both wild-type and mutated templates were combined with 20 pmol primers 1 and 3 or 20 pmol primers 2 and 4. The SBE reaction with primer 5 was performed with template 2 in a separate tube. PCR products from the HFE gene were mixed with 20 pmol corresponding primers 5′-GGGGAAGAGCAGAGATATACGT-3′ (C282Y) and 5′-GACCAGCTGTTCGTGTTCTATGAT-3′ (H63D) in SBE. All extension reactions were thermal cycled for 35 cycles at 94°C for 10 s and for 30 s at the annealing temperature of 49°C for the primers with synthetic templates and at 58°C for the HFE primers.
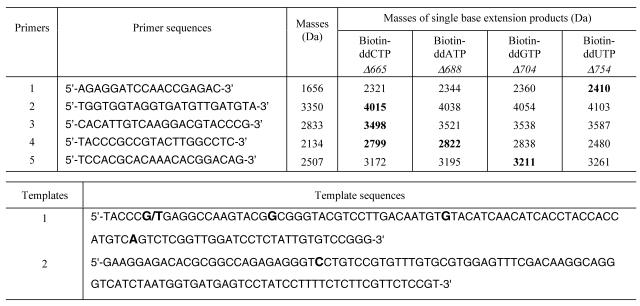
Table 1. Oligonucleotide primers and synthetic DNA templates for detecting mutations in the p53 gene.
In the top table the sequences and the calculated masses of primers and the four possible single base extension products relative to the internal mass standard are listed. The bold numbers refer to the nucleotide variations detected in the p53 gene. In the bottom table the six nucleotide variations in templates 1 and 2 are shown in bold. Template 1 contains a heterozygous genotype (G/T).
Solid phase purification
Aliquots of 20 µl of the streptavidin-coated magnetic beads (Seradyn, Ramsey, MN) were washed with modified binding and washing (B/W) buffer (0.5 mM Tris–HCl buffer, 2 M NH4Cl, 1 mM EDTA, pH 7.0) and resuspended in 20 µl of modified B/W buffer. Extension reaction mixtures of primers 1–4 with template 1 and primer 5 with template 2 were mixed in a 2:1 ratio, while extension reaction mixtures from the PCR products of the HFE gene were mixed in equal amounts. An aliquot of 20 µl of each mixed extension product was added to the suspended beads and incubated for 1 h. After capture, the beads were washed twice with modified B/W buffer, twice with 0.2 M triethyl ammonium acetate (TEAA) buffer and twice with deionized water. The primer extension products were released from the magnetic beads by treatment with 8 µl of 98% formamide solution containing 2% 0.2 M TEAA buffer at 94°C for 5 min. The released primer extension products were precipitated with 100% ethanol at 4°C for 30 min and centrifuged (Eppendorf 5417 C/R centrifuge) at 4°C and 14 000 r.p.m. for 35 min.
MALDI-TOF MS analysis
The purified primer extension products were dried and resuspended in 1 µl of deionized water and 2 µl of matrix solution. The matrix solution was made by dissolving 35 mg 3-hydroxypicolinic acid (Aldrich, Milwaukee, WI) and 6 mg ammonium citrate (Aldrich) in 0.8 ml of 50% acetonitrile. An aliquot of 10 pmol internal mass standard in 1 µl of 50% acetonitrile was then added to the sample. A sample of 0.5 µl of this mixture containing the primer extension products and internal standard was spotted on a stainless steel sample plate, air dried and analyzed using an Applied Biosystems Voyager DE MALDI-TOF mass spectrometer. All measurements were taken in linear positive ion mode with a 25 kV accelerating voltage, a 94% grid voltage and a 350 ns delay time. The obtained spectra were processed using the Voyager data analysis package.
RESULTS AND DISCUSSION
We validated the multiplex genotyping approach by detecting six nucleotide variations from synthetic DNA templates that mimic mutations in exons 7 and 8 of the p53 gene. Sequences of the templates and the corresponding primers are shown in Table 1 along with the masses of the primers and their extension products. The mass increase of the resulting single base extension products in comparison with the primers is 665 Da for addition of biotin-ddCTP, 688 Da for addition of biotin-ddATP, 704 Da for addition of biotin-ddGTP and 754 Da for addition of biotin-ddUTP. The mass data in Table 1 indicate that the smallest mass difference among any possible extensions of a primer is 16 Da (between biotin-ddATP and biotin-ddGTP). This is a substantial increase over the smallest mass difference between extension products using standard ddNTPs (9 Da between ddATP and ddTTP). This mass increase yields improved resolution of the peaks in the mass spectrum. An increased mass difference in ddNTPs fosters accurate detection of heterozygous genotypes (15), since an A/T heterozygote with a mass difference of 9 Da using conventional ddNTPs cannot be well resolved in the MALDI-TOF mass spectra. The five primers for each polymorphic site were designed to produce extension products without overlapping masses. Primers extended by biotin-ddNTPs were purified and analyzed by MALDI-TOF MS according to the scheme in Figure 1. Extension products of all five primers were well resolved in the mass spectrum free from any unextended primers (Fig. 2), allowing each of the six nucleotide variations to be unambiguously identified. Unextended primers occupy the mass range in the mass spectrum decreasing the scope of multiplexing and excess primers can dimerize to form false peaks in the mass spectrum (21). The excess primers and their associated dimers also compete for the ion current, reducing the detection sensitivity of MS for the desired DNA fragments. These complications were completely removed by carrying out SBE using biotin-ddNTPs and solid phase capture. Extension products for all four biotin-ddNTPs were clearly detected with well resolved mass values. The relative masses of the primer extension products in comparison to the internal mass standard revealed the identity of each nucleotide at the polymorphic site. In the case of heterozygous genotypes, two peaks, one corresponding to each allele (C/A), are clearly distinguishable in the mass spectrum shown in Figure 2. One advantage of MALDI-TOF MS in comparison to other detection techniques is its ability to simultaneously measure masses of DNA fragments over a certain range. In order to explore this feature to detect multiple SNPs in a single spectrum, if unextended primers are not removed, masses of all primers and their extension products must have sufficient differences to yield adequately resolved peaks in the mass spectrum. Ross et al. simultaneously detected multiple SNPs by carefully tuning the masses of all primers and extension products so that they would lie in the range 4.5–7.6 kDa without overlapping (14). Since the unextended primers occupy the mass range in the mass spectrum, by eliminating them our approach will significantly increase the scope of multiplexing in SNP analysis.
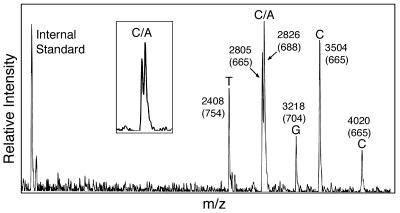
Figure 2.
Mass spectrometric detection of six nucleotide variations from synthetic DNA templates mimicking mutations in the p53 gene using biotin-ddNTPs. Masses of the extension product with reference to the internal mass standard were listed on each single base extension peak. The mass values in parentheses indicate the mass difference between the extension products and the corresponding primers whose masses are known. Four homozygous (T, G, C and C) and one heterozygous (C/A) genotypes were detected. (Inset) A magnified view of heterozygote peaks.
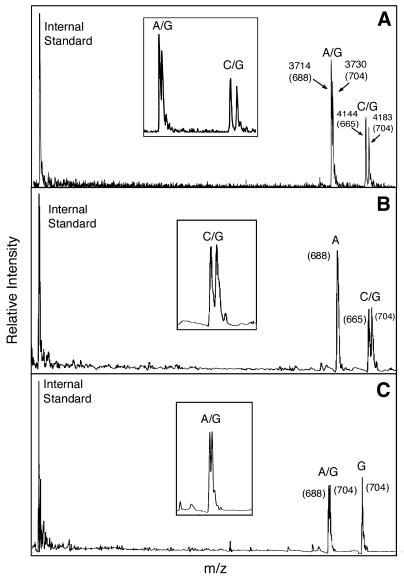
To demonstrate the ability of this method to discriminate SNPs in genomic DNA, we genotyped two disease-associated SNPs in the HHC gene (HFE) from seven individuals whose genotypes were known. HHC is a common genetic condition in Caucasians with approximately 1/400 Caucasians homozygous for the C282Y mutation, leading to iron overload and potentially liver failure, diabetes and depression (22). A subset of individuals who are compound heterozygotes for the C282Y and H63D mutations also manifest iron overload. Because of the high prevalence of these mutations and the ability to prevent disease manifestations by phlebotomy, accurate methods for genotyping these two SNPs will foster genetic screening for this condition. We generated two PCR products from each individual’s genomic DNA for the C282Y and H63D polymorphic sites of the HFE gene and then used these products for SBE with biotin-ddNTPs. After the extension reaction, the products were purified using solid phase capture according to the scheme in Figure 1 and analyzed by MALDI-TOF MS. Three mass spectra obtained from this experiment are shown in Figure 3. Figure 3A shows two heterozygous genotypes (A/G and C/G) for both loci; Figure 3B shows a homozygous genotype (A) at the first locus and a heterozygous genotype (C/G) at the second locus; Figure 3C shows a heterozygous genotype (A/G) at the first locus and a homozygous genotype (G) at the second locus. Thus, both homozygous and heterozygous genotypes were clearly determined by their distinct mass difference in the mass spectra. Homozygotes were detected as single peaks with a distinct mass, while heterozygotes appeared as two peaks which were separated by a specific mass difference (16 Da for A/G and 39 Da for C/G). All 14 genotypes from the seven individuals, including heterozygotes, were accurately determined by this method, as shown in Table 2. For this small-scale study, the genotypes were determined by eye from the distinct mass difference in the mass spectra with 100% accuracy.
Figure 3.
Multiplex SNP genotyping of the human HFE gene using mass spectrometry and biotin-ddNTPs. The mass values in parentheses indicate the mass difference between the extension products and the corresponding primers. Both homozygous and heterozygous genotypes were detected for the C282Y and H63D polymorphic sites. (Inset) A magnified view of heterozygote peaks. (A) Detection of two heterozygotes (A/G for C282Y and C/G for H63D). (B) Detection of homozygote (A) for C282Y and heterozygote (C/G) for H63D. (C) Detection of heterozygote (A/G) for C282Y and homozygote (G) for H63D.
Table 2. SNPs in the HFE gene detected in seven individuals.
| |
C282Y |
H63D |
||
|---|---|---|---|---|
| Mass difference | Genotype | Mass difference | Genotype | |
| 1 | 704 | G | 710 | G |
| 2 | 703 | G | 666/708 | C/G |
| 3 | 707 | G | 708 | G |
| 4 | 686/702 | A/G | 707 | G |
| 5 | 686/701 | A/G | 668/708 | C/G |
| 6 | 704 | G | 707 | G |
| 7 | 704 | G | 667/706 | C/G |
The mass difference between the SBE product and the corresponding primer shown in the table determines the identity of the nucleotide at the SNP site. Theoretical mass differences between a primer and the SBE product are 665, 688, 704 and 754 Da for addition of biotin-ddCTP, biotin-ddATP, biotin-ddGTP and biotin-ddUTP, respectively.
These results indicate that the use of solid phase capturable biotin-ddNTPs in SBE, coupled with MALDI-TOF MS detection, provides a rapid and accurate method for multiplex SNP detection over broad mass ranges and should greatly increase the number of SNPs that can be detected simultaneously. In multiplex SBE reactions, the oligonucleotide primers and their dideoxynucleotide extension products differ by only 1 bp, which requires analytical techniques with high resolution to resolve. In addition, a primer designed to detect one polymorphism and an extension product from another polymorphic site may have the same size, which cannot be separated by electrophoresis and other conventional chromatographic or size exclusion methods. Methods for purifying DNA samples using the strong interaction of biotin and streptavidin are widely used (23–27). By introducing the biotin moiety at the 3′ end of DNA, the solid phase-based affinity purification approach described here is a unique and effective method to remove the oligonucleotide primers from the dideoxynucleotide extension products.
To increase the stability of DNA fragments for MALDI-TOF MS measurement in multiplex SNP analysis, nucleotide analogs (28) and peptide nucleic acids (9) can be used in the construction of the oligonucleotide primers. For SNP sites of interest, a master database of primers and the resulting masses of all four possible extension products can be constructed. The experimental data from MALDI-TOF MS can then be compared with this database to precisely identify the library of SNPs automatically. This method coupled with future improvements in mass spectrometer detector sensitivity (29) will provide a platform for high throughput SNP identification unrivaled in speed and accuracy.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Anthony K. Tong and Isidore S. Edelman for many constructive comments on the manuscript. This work was supported by a Packard Fellowship for Science and Engineering (to J.J.) and the Columbia University Genomics Initiative.
REFERENCES
- 1.Kwok P.-Y. (2000) High-throughput genotyping assay approaches. Pharmacogenomics, 1, 95–100. [DOI] [PubMed] [Google Scholar]
- 2.Roses A. (2000) Pharmacogenetics and the practice of medicine. Nature, 405, 857–865. [DOI] [PubMed] [Google Scholar]
- 3.The International SNP Map Working Group (2001) A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature, 409, 928–933. [DOI] [PubMed] [Google Scholar]
- 4.Beavis R.C. and Chait,B.T. (1989) Matrix-assisted laser-desorption mass spectrometry using 355 nm radiation. Rapid Commun. Mass Spectrom., 3, 436–439. [DOI] [PubMed] [Google Scholar]
- 5.Li J., Butler,J.M., Tan,Y., Lin,H., Royer,S., Ohler,L., Shaler,T.A., Hunter,J.M., Pollart,D.J., Monforte,J.A. and Becker,C.H. (1999) Single nucleotide polymorphism determination using primer extension and time-of-flight mass spectrometry. Electrophoresis, 20, 1258–1265. [DOI] [PubMed] [Google Scholar]
- 6.Griffin T.J. and Smith,L.M. (2000) Single-nucleotide polymorphism analysis by MALDI-TOF mass spectrometry. Trends Biotechnol., 18, 77–84. [DOI] [PubMed] [Google Scholar]
- 7.Graber J.H., Smith,C.L. and Cantor,C.R. (1999) Differential sequencing with mass spectrometry. Genet. Anal. Biomol. Eng., 14, 215–219. [DOI] [PubMed] [Google Scholar]
- 8.Stoerker J., Mayo,J.D., Tetzlaff,C.N., Sarracino,D.A., Schwope,I. and Richert,C. (2000) Rapid genotyping by MALDI-monitored nuclease selection from probe libraries. Nat. Biotechnol., 18, 1213–1216. [DOI] [PubMed] [Google Scholar]
- 9.Ross P.L., Lee,K. and Belgrader,P. (1997) Discrimination of single-nucleotide polymorphisms in human DNA using peptide nucleic acid probes detected by MALDI-TOF mass spectrometry. Anal. Chem., 69, 4197–4202. [DOI] [PubMed] [Google Scholar]
- 10.Jiang-Baucom P., Girard,J.E., Butler,J. and Belgrader,P. (1997) DNA typing of human leukocyte antigen sequence polymorphisms by peptide nucleic acid probes and MALDI-TOF mass spectrometry. Anal. Chem., 69, 4894–4898. [DOI] [PubMed] [Google Scholar]
- 11.Griffin T.J., Hall,J.G., Prudent,J.R. and Smith,L.M. (1999) Direct genetic analysis by matrix-assisted laser desorption/ionization mass spectrometry. Proc. Natl Acad. Sci. USA, 96, 6301–6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyamichev V., Mast,A.L., Hall,J.G., Prudent,J.R., Kaiser,M.W., Takova,T., Kwiatkowski,R.W., Sander,T.J., de Arruda,M., Arco,D.A., Neri,B.P. and Brow,M.A.D. (1999) Polymorphism identification and quantitative detection of genomic DNA by invasive cleavage of oligonucleotide probes. Nat. Biotechnol., 17, 292–296. [DOI] [PubMed] [Google Scholar]
- 13.Haff L.A. and Smirnov,I.P. (1997) Multiplex genotyping of PCR products with mass tag-labeled primers. Nucleic Acids Res., 25, 3749–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross P., Hall,L., Smirnov,I.P. and Haff,L. (1998) High level multiplex genotyping by MALDI-TOF mass spectrometry. Nat. Biotechnol., 16, 1347–1351. [DOI] [PubMed] [Google Scholar]
- 15.Fei Z., Ono,T. and Smith,L.M. (1998) MALDI-TOF mass spectrometric typing of single nucleotide polymorphisms with mass-tagged ddNTPs. Nucleic Acids Res., 26, 2827–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang K., Fu,D.-J., Julien,D., Braun,A., Cantor,C.R. and Köster,H. (1999) Chip-based genotyping by mass spectrometry. Proc. Natl Acad. Sci. USA, 96, 10016–10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taranenko N.I., Allman,S.L., Golovlev,V.V., Taranenko,N.V., Isola,N.R. and Chen,C.H. (1998) Sequencing DNA using mass spectrometry for ladder detection. Nucleic Acids Res., 26, 2488–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ju J. (1999) US patent 5 876 936.
- 19.Edwards J.R., Itagaki,Y. and Ju,J. (2001) DNA sequencing using biotinylated dideoxynucleotides and mass spectrometry. Nucleic Acids Res., 29, e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong A.K. and Ju,J. (2001) Single-nucleotide polymorphism detection by combinatorial fluorescence energy transfer tags and biotinylated dideoxynucleotides. Nucleic Acids Res., 30, e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roskey M.T., Juhasz,P., Smirnov,I.P., Takach,E.J., Martin,S.A. and Haff,L.A. (1996) DNA sequencing by delayed extraction-matrix-assisted laser desorption/ionization time of flight mass spectrometry. Proc. Natl Acad. Sci. USA, 93, 4724–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanson E.H., Imperatore,G. and Burke,W. (2001) HFE gene and hereditary hemochromatosis: a HuGE review. Am. J. Epidemiol., 154, 193–206. [DOI] [PubMed] [Google Scholar]
- 23.Langer P.R., Waldrop,A.A. and Ward,D.C. (1981) Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc. Natl Acad. Sci. USA, 78, 6633–6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawkins T.L., O’Connor-Morin,T., Roy,A. and Santillan,C. (1994) DNA purification and isolation using a solid-phase. Nucleic Acids Res., 22, 4543–4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uhlen M. (1989) Magnetic separation of DNA. Nature, 340, 733–734. [DOI] [PubMed] [Google Scholar]
- 26.Wahlberg J., Lunderberg,J., Hultman,T. and Uhlen,M. (1990) General colorimetric method for DNA diagnostics allowing direct solid-phase genomic sequencing of the positive samples. Proc. Natl Acad. Sci. USA, 87, 6569–6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tong X. and Smith,L.M. (1992) Solid-phase method for the purification of DNA sequencing reactions. Anal. Chem., 64, 2672–2677. [DOI] [PubMed] [Google Scholar]
- 28.Schneider K. and Chait,B.T. (1995) Increased stability of nucleic acids containing 7-deaza-guanosine and 7-deaza-adenosine may enable rapid DNA sequencing by matrix-assisted laser desorption mass spectrometry. Nucleic Acids Res., 23, 1570–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hilton G.C., Martinis,J.M., Wollman,D.A., Irwin,K.D., Dulcie,L.L., Gerber,D., Gillevet,P.M. and Twerenbold,D. (1998) Impact energy measurement in time-of-flight mass spectrometry with cryogenic microcalorimeters. Nature, 391, 672–675. [DOI] [PubMed] [Google Scholar]