Abstract
THE LAUNCH OF THE CYCLOOXYGENASE-2 (COX-2) selective NSAIDs was based on 2 hypotheses: (1) the major adverse effects limiting the usefulness of nonselective NSAIDs are gastrointestinal in nature and (2) COX-2 selective NSAIDs are associated with fewer gastrointestinal adverse effects than nonselective NSAIDs. At the time of the launch, neither of these hypotheses had been proven and, as documented in this review, both remain uncertain. The increased incidence of total and nongastrointestinal serious adverse events, with the COX-2 selective NSAIDs as compared with nonselective NSAIDs, in the Celecoxib Long-term Arthritis Safety Study (CLASS) and the Vioxx Gastrointestinal Outcomes Research (VIGOR) study remains a major concern. The increased morbidity associated with the COX-2 selective NSAIDs may be a manifestation of the COX-2 selectivity of rofecoxib and celecoxib or the supramaximal doses of these drugs used in the trials. Proof that the increased harm was not caused by the COX-2 selectivity of the drugs depends on demonstration in a randomized controlled trial that COX-2 selective NSAIDs at usual doses are as effective as nonselective NSAIDs and cause fewer gastrointestinal serious adverse events without increasing the incidence of total nongastrointestinal serious adverse events.
Most new drug concepts are launched with fanfare, and it takes many years on the market for their appropriate role in practice to be established. An excellent example is the concept of cyclooxygenase-2 (COX-2) selective NSAIDs, which was launched in Canada in early 1999 with celecoxib (Celebrex). The launch of the COX-2 selective NSAIDs was based on 2 hypotheses. The first hypothesis is that the major adverse effects limiting the usefulness of nonselective NSAIDs are gastrointestinal symptoms, ulcers, ulcer complications and ulcer complications leading to death.1 The second hypothesis is that COX-2 selective NSAIDs are associated with less gastrointestinal toxicity than nonselective NSAIDs.2 At the time of the launch of COX-2 selective NSAIDs neither of these hypotheses had been proven and, as documented in this review, both remain uncertain. However, skilful marketing of these hypotheses without any published complete trial reports by the fall of 19993 resulted in celecoxib's achieving a record for the most sales in the shortest period of time. Worldwide sales of celecoxib exceeded $3.1 billion in 2001.4 In 2000, 2 large randomized controlled trials testing the second hypothesis were published. In the Celecoxib Long-term Arthritis Safety Study (CLASS)5 and the Vioxx Gastrointestinal Outcomes Research (VIGOR) study,6 celecoxib and rofecoxib respectively were compared with nonselective NSAIDs. These well-designed trials claimed to prove the safety of these agents, but the results became controversial when more complete data from the trials became available from the US Food and Drug Administration (FDA).7,8,9,10
In this review I re-examine the safety and efficacy of NSAIDs in general and include new analyses of safety data from the CLASS and VIGOR trials. I also attempt to explain the different rates of adverse events seen with the various NSAIDs used in these trials.
Pharmacology of NSAIDs
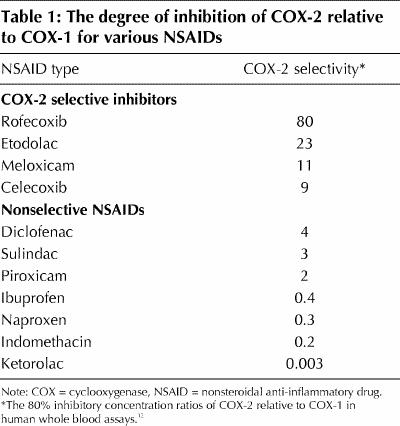
NSAIDs act by inhibiting cyclooxygenase, an enzyme involved in the formation of various prostanoids with a wide variety of pharmacologic actions.11 The COX-2 selective NSAIDs resulted from the discovery that cyclooxygenases represent at least 2 different isoenzymes, designated COX-1 and COX-2. COX-1 is mostly constitutive and is involved in such actions as platelet activation, gastrointestinal protection and kidney function. COX-2 is primarily produced in response to tissue damage and is involved in inflammatory responses to injury. The discovery of the different COX enzymes has allowed grading of the more than 20 available NSAIDs based on their ability to selectively inhibit the 2 COX enzymes. The best attempt to grade the available NSAIDs used standardized in vitro human assay systems.12 This demonstrated that ketorolac inhibits COX-1 300 times more than COX-2 at one extreme, and rofecoxib inhibits COX-2 80 times more than COX-1 at the other extreme. Four drugs marketed in Canada are claimed to be COX-2 selective. The selectivity of these drugs as well as of some nonselective NSAIDs is shown in Table 1.
Table 1
Benefits of NSAIDs
To properly put the harms of NSAIDs into perspective, it is necessary to appreciate the magnitude of the benefit derived from taking NSAIDs. The benefit of NSAIDs, based on short-term placebo-controlled trials, is a reduction in the severity of musculoskeletal pain, stiffness and swelling. Several systematic reviews of the efficacy of NSAIDs have been performed, but the reviewers have judged it impossible to systematically quantitate the magnitude of the benefit of NSAIDs because of incomplete reporting as well as biased analysis and presentation of the trial results.13,14,15,16 This is a remarkable observation for a class of drugs that is so widely used today.
Two placebo-controlled trials illustrate the magnitude of the benefit of NSAIDs as compared to placebo.17,18 In these trials the investigators used reduction in pain, as assessed with a 10-cm visual analogue scale (with 0 indicating no pain and 10 indicating maximum pain), as one of the benefit outcomes. In one trial, after 12 weeks, patients with osteoarthritis of the knee (baseline pain rating 6) had an average reduction in pain of 0.9 with 2 NSAIDs (celecoxib [100 or 200 mg twice daily] and naproxen [500 mg twice daily]) compared to placebo.17 In the second trial, rofecoxib therapy (25 mg/d for 8 weeks) in patients with rheumatoid arthritis led to a reduction in pain of 0.7 compared to placebo.18 With another outcome measure, swollen and tender joint counts (American College of Rheumatology 20% response proportion [ACR 20]), rofecoxib caused an absolute increase in ACR 20 responders of 12.2% compared to placebo.18 This means that 8 patients would have to be treated for 8 weeks with rofecoxib for 1 patient to benefit by achieving a 20% or greater reduction in tender and swollen joint counts. These modest short-term benefits in osteoarthritis and rheumatoid arthritis must be balanced against the potential harms of these drugs.
Harms of NSAIDs
Inflammation is a component of the normal healing process.19 All NSAIDs inhibit inflammation and have the potential to interfere with this healing process. Impairment of joint healing can lead to joint deterioration in the various forms of arthritis. Evidence demonstrating this potential adverse effect comes from a trial of 812 patients with osteoarthritis randomly assigned to receive indomethacin (25 mg 3 times daily), tiaprofenic acid (300 mg twice daily) or placebo.20 Patients taking either drug did not have any reduction in symptoms in comparison to placebo. Furthermore, over 1 to 2 years, those who received indomethacin and, to a lesser extent, those who received tiaprofenic acid had increased radiologic joint deterioration compared to those who received placebo. Although duplication of this trial with other NSAIDs has not been attempted, it is possible that all NSAIDs may have diminishing symptomatic benefit over time as a result of or independent of an effect leading to accelerated joint deterioration. How this potential manifests in the clinical setting may depend on NSAID potency, COX selectivity, pharmacokinetics, dosage and duration of use. Recent evidence of impaired fracture healing in rats21 supports the concept of impaired joint healing with NSAIDs and suggests that this may be worse with the COX-2 selective drugs.
Prostanoids produced by COX enzymes in the kidney are essential for the regulation of renal blood flow and other physiologic actions. Inhibition of these functions by NSAIDs has been shown in a meta-analysis to increase blood pressure by an average of 5 mm Hg (95% confidence interval [CI] 1.2–8.7 mm Hg).22 With short-term use this is probably inconsequential, but with long-term daily use the estimated risk of adverse cardiovascular events based on epidemiologic data is substantial.22 This action of NSAIDs can manifest acutely with salt and water retention and renal impairment in patients with compromised renal function or congestive heart failure.23
Potential advantages of COX-2 selective NSAIDs
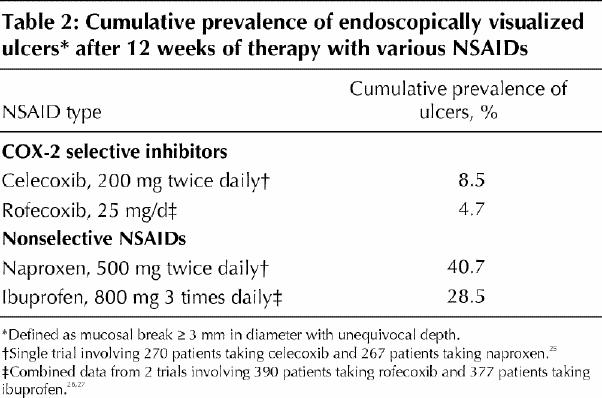
It has never been claimed that COX-2 selective NSAIDs are more beneficial in reducing the symptoms of arthritis than nonselective NSAIDs. In the CLASS and VIGOR trials, there was no significant difference in efficacy measures in the longer term between nonselective NSAIDs and COX-2 inhibitors based on the FDA data.7,8 The main potential advantage of COX-2 inhibitors is that they may have fewer toxic effects on the gastrointestinal tract as a result of having less inhibitory effect on the gastroprotective prostanoids produced by COX-1 enzymes in the gastrointestinal tract. This advantage of COX-2 selective NSAIDs has been tested in short-term trials measuring gastroduodenal ulcers and erosions by endoscopy.24,25,26 COX-2 selective NSAIDs were associated with substantially fewer endoscopically visualized ulcers (defined as a mucosal break ≥ 3 mm or more in diameter with unequivocal depth) (Table 2). However, it is not known whether such small endoscopically defined ulcers and erosions are an accurate predictor of ulcer complications, the most common presentation being gastrointestinal bleeding. Another potential advantage of COX-2 selective NSAIDs is that they may reduce the risk of gastrointestinal bleeding by not interfering with platelet aggregation, a COX-1 effect.
Table 2
Potential disadvantages of COX-2 selective NSAIDs
An understanding of the physiologic features of COX isoenzymes has led to the appreciation that drugs that preferentially inhibit COX-2 may lead theoretically to problems in thrombosis, salt and water balance, and healing. The well-known reduction of thrombotic events with low-dose ASA therapy is based on selective irreversible inhibition of COX-1-mediated platelet thromboxane production.27 In contrast, the selective inhibition of prostacyclin formation by COX-2 selective NSAIDs interferes with prostacyclin's effect of inhibiting thrombosis and permits the unopposed action of platelet thromboxane.9,10,28,29 In susceptible people this could tip the delicate balance and lead to adverse thrombotic events. Inhibiting both COX-1 and COX-2 likely retains the balance. Prostanoids (regulated by both COX-1 and COX-2) are also involved in salt, water and blood pressure regulation in the kidney via poorly understood mechanisms. Creating an imbalance of prostanoids in the kidney by selectively inhibiting the COX-2 isoenzyme may result in a greater potential for salt and water retention, hypertension and exacerbation of congestive heart failure. Furthermore, the COX-2 isoenzyme plays a critical role in fracture healing, and blocking this enzyme may inhibit healing. A study in rats showed that NSAIDs can markedly interfere with fracture healing; this effect was greater with the COX-2 selective NSAIDs rofecoxib and celecoxib than with the nonselective NSAID indomethacin.21
Benefits and harms of COX-2 selective NSAIDs from large randomized controlled trials
The CLASS and VIGOR trials were designed to test whether the potential advantages of COX-2 selective NSAIDs on the gastrointestinal tract and platelets would result in a reduced incidence of ulcer complications. The published versions of these studies5,6 focused on gastrointestinal events and provide an incomplete picture of the overall benefit and harm of celecoxib and rofecoxib30,31 (summarized recently by Wooltorton32). One striking finding from these trials is that despite the large size and duration (average 8 to 9 months), there was no decrease in the incidence of death due to gastrointestinal complications. There were no gastrointestinal-related deaths in the CLASS, and there were 4 such deaths in the VIGOR study (3 patients receiving rofecoxib and 1 receiving naproxen). Cardiovascular events were the main cause of death in both trials (69% of 36 deaths in the CLASS and 46% of 37 deaths in the VIGOR trial).
Meta-analyses of mortality and morbidity outcomes from the CLASS and VIGOR trials31 are shown in Figs. 1, 2 and 3. The incidence of total mortality was higher with COX-2 selective NSAIDs than with nonselective NSAIDs, but not significantly so (Fig. 1). The incidence of serious adverse events (SAEs), including death, admission to hospital, and any life-threatening event or event leading to serious disability, was significantly higher with COX-2 selective NSAIDs than with nonselective NSAIDs (Fig. 2). SAEs represent a combined measure of both benefit and harm; complicated ulcers account for only a small proportion of total SAEs. The incidence of complicated ulcers was significantly lower with rofecoxib than with naproxen; however, there was no difference in incidence between patients receiving celecoxib and those receiving ibuprofen or diclofenac,4 and the pooled estimate was not significant (Fig. 3).
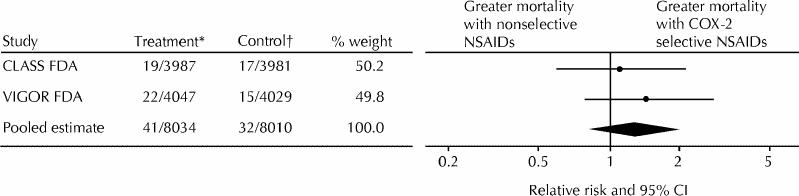
Fig. 1: Meta-analysis of relative risk of total mortality with cyclooxygenase-2 (COX-2) selective nonsteroidal anti-inflammatory drugs (NSAIDs) compared with nonselective NSAIDs in the Celecoxib Long-term Arthritis Safety Study (CLASS)5,7 and the Vioxx Gastrointestinal Outcomes Research (VIGOR) study.6,8 CI = confidence interval, FDA = US Food and Drug Administration. *Group that received COX-2 selective NSAIDs: number of participants who were affected/total number of participants. †Group that received nonselective NSAIDs: number of participants who were affected/total number of participants.

Fig. 2: Meta-analysis of relative risk of serious adverse events (SAEs) (including death, admission to hospital, and any life- threatening event or event leading to serious disability) with COX-2 selective NSAIDs compared with nonselective NSAIDs in the CLASS5,7 and the VIGOR study.6,8

Fig. 3: Meta-analysis of relative risk of complicated ulcers with COX-2 selective NSAIDs compared with nonselective NSAIDs in the CLASS5,7 and the VIGOR study.6,8
Converting the pooled increase in the relative risk (RR) of total SAEs with COX-2 selective NSAIDS to absolute terms gives an absolute risk increase of 1.3% and a number needed to harm of 78. This means that for every 78 patients treated for 9 months with a COX-2 selective NSAID instead of a nonselective NSAID, 1 patient will experience an SAE. The pooled significant increase is maintained whether or not the data are adjusted for the 5% higher duration of drug exposure for celecoxib in the CLASS trials. If complicated ulcers are subtracted from total SAEs, the absolute increase in nongastrointestinal SAEs with COX-2 selective NSAIDS is larger in magnitude and thus of greater concern.
Why do COX-2 selective NSAIDs increase nongastrointestinal serious adverse events?
The reason for the increased incidence of nongastrointestinal SAEs with COX-2 selective NSAIDs is not completely explained with the data available from the trials. In the VIGOR trial, the incidence of confirmed adjudicated (chart verified by blinded observer) thrombotic cardiovascular events was 0.6% higher with rofecoxib than with naproxen (RR 2.38 [95% CI 1.39–4.00]).8,9 This more than cancels out the reduction in absolute risk of complicated ulcers of 0.5%, but leaves an unaccounted-for increase of 1.4% in other SAEs.
It has been suggested that the increase in thrombotic cardiovascular events represents the antiplatelet effect of naproxen.9,10,29,32 However, the magnitude of the effect is too large: the overall antiplatelet benefit (mostly from ASA) from the Antithrombotic Trialists' Collaboration meta-analysis is an RR of 0.78 (95% CI 0.74–0.82).27 This does not explain the RR of 0.42 (95% CI 0.25–0.72) for thrombotic events with naproxen as compared to rofecoxib in the VIGOR trial. Moreover, the most recent cohort study showed that naproxen and other non-ASA NSAIDs do not have cardioprotective effects.33 The recent publication reanalyzing data from randomized controlled trials of rofecoxib and claiming that this observation is a reflection of the antiplatelet effect of naproxen34 is flawed, because it compares unadjudicated data with adjudicated data and bases the event rate with NSAIDS other than naproxen on short-term trials with a small number of events.
White and colleagues35 recently reanalyzed the CLASS trials for serious cardiovascular thromboembolic events and reported no significant increase with celecoxib. However, the events were not adjudicated by blinded observers, the incidence of these events is impossible to verify with the FDA data, and the authors included hemorrhagic stroke as a thromboembolic event. Moreover, careful analysis of the FDA data suggests an increase in serious cardiac events with celecoxib: the incidence of cardiac SAEs (myocardial infarction, combined anginal events and atrial arrythmias) was 0.6% higher with celecoxib than with the other NSAIDs (RR 1.55, 95% CI 1.04–2.30).7 Each of these cardiac SAEs has been reported in patients taking celecoxib and rofecoxib to the Canadian postmarketing surveillance program.36 To resolve these inconsistencies, it is essential that the full data from the VIGOR trial, the 2 separate CLASS trials and all other trials of rofecoxib and celecoxib be made available for independent, individual patient meta-analysis, as has been requested by other investigators.4
Results of the CLASS and VIGOR trials may be explained by COX-2 selectivity
If selective inhibition of COX-2 predisposes patients to SAEs (e.g., thrombosis, hypertension, congestive heart failure and impaired healing) but reduces the rate of gastrointestinal ulceration, could the results of the CLASS and VIGOR trials be predicted on the basis of the relative COX-2 selectivity of the agents used in these trials? In the VIGOR trial the most COX-2 selective NSAID, rofecoxib (Table 1), reduced the rate of complicated ulcers and increased the rates of thrombotic cardiovascular events and total SAEs as compared to naproxen, a nonselective agent. In the CLASS trials the less COX-2 selective NSAID, celecoxib, did not reduce complicated ulcer rates or increase thromboembolic event rates, and it increased the rate of total SAEs to a lesser extent as compared to the combined effects of diclofenac and ibuprofen. The COX-2 selectivity of celecoxib is not markedly different from that of diclofenac (Table 1), but ibuprofen, like naproxen, is nonselective. One would therefore predict that when comparing celecoxib to ibuprofen, the incidence of complicated ulcers should be reduced and that of nongastrointestinal SAEs increased. In contrast, when comparing celecoxib to diclofenac, one would predict little or no difference in the rates of complicated ulcers and nongastrointestinal SAEs. Data (published and unpublished) from the CLASS trials are compatible with this prediction.7,37 To properly test this hypothesis, the manufacturer would have to provide the celecoxib outcome data from the 2 separate trials for independent analysis. If this hypothesis proves to be true, it would mean that COX-2 selectivity represents a double-edged sword, with both harms and benefits. Because cardiovascular risk outweighs gastrointestinal risk in adult patients with rheumatoid arthritis and osteoarthritis,7,8 the hypothesis also predicts that the harms would outweigh the benefits in most clinical settings. This means that total SAEs would be predictably increased by COX-2 selective NSAIDs, as compared to nonselective NSAIDs, as was seen in the 2 large trials (Fig. 2).
This hypothesis predicts that other COX-2 selective NSAIDs and any new drugs with the same or greater COX-2 selectivity will share this flaw. If so, this initially appealing therapeutic concept will prove a clinical failure. The failure of selective inhibitors to live up to expectations should be quite familiar to clinicians from their experience with β1-adrenergic receptor selective and partial agonist β-blockers in the treatment of patients post myocardial infarction and patients with angina or hypertension. Two lessons we have learned from β-blockers are the following: (1) selectivity tends to be lost with higher dosages, and (2) evidence from randomized controlled trials shows a trend toward worse outcomes with β1-adrenergic receptor selective and partial agonist β-blockers as compared to nonselective β-blockers (e.g., propranolol, nadolol and timolol).38
Is the increased harm caused by COX-2 selective NSAIDs a dose-related phenomenon?
The increased incidence of nongastrointestinal SAEs in the large trials may be a manifestation of the supramaximal doses of rofecoxib and celecoxib used in the trials and of the relatively long half-lives of the 2 drugs, 14 and 11 hours respectively.11 Most drugs have some dose-related toxicity, and there is evidence of dose-related toxicity for rofecoxib.39 This hypothesis also predicts that drugs with long half-lives that inhibit COX enzymes for 24 hours a day would be associated with higher rates of toxic effects than those with shorter half-lives. The half-life of naproxen, 13 hours, is similar to that of celecoxib and rofecoxib, whereas diclofenac and ibuprofen have short half-lives, about 2 hours. If dosage and half-life are the explanation for the increased toxicity of COX-2 selective NSAIDs seen in the CLASS and VIGOR trials, the usefulness of this class of drugs may be retained. Proof that the increased harm was caused by the high doses used in the trials — and not the COX-2 selectivity of the drugs — depends on demonstration in a trial that COX-2 selective NSAIDs at lower doses are as effective as nonselective NSAIDs and cause fewer complicated ulcers without a significant increase in the incidence of nongastrointestinal SAEs.
Conclusions and suggested future trials
All NSAIDs, both COX-2 selective and nonselective, provide only a modest symptomatic benefit over placebo, and this benefit has been proven only in short-term trials. With long-term therapy, it is not known whether the benefits of this class of drugs exceed the harms. In fact, there is evidence to suggest that the opposite is true.20 Meta-analysis of FDA data from the CLASS and VIGOR trials shows, first, that COX-2 selective NSAIDs do not necessarily reduce the incidence of complicated ulcers. Second, the meta-analysis demonstrates that, rather than proving safer, COX-2 selective NSAIDs cause more morbidity (total SAEs) than nonselective NSAIDs. This increase is partly explained by increased thrombotic and cardiac adverse events, but full audit and disclosure of all data are needed to identify other causative mechanisms. Access to this information is necessary to establish whether COX-2 selective NSAIDs could have a role in the long-term treatment of patients with arthritis and to assist in designing future trials.
Long-term randomized controlled trials with appropriate hard outcomes (e.g., death, SAEs, joint surgery) are needed to answer a multitude of unanswered questions about the use of NSAIDs. Two such trials could be as follows.
A 2-year trial could test nonselective and COX-2 selective agents with 2 dosing strategies in patients with either osteoarthritis or rheumatoid arthritis. Patients would be randomly allocated to 1 of 4 arms: (1) maximum dosage of nonselective NSAIDs with long half-lives (e.g., naproxen, piroxicam) taken daily, (2) maximum dosage of COX-2 selective NSAIDs with long half-lives (e.g., meloxicam, rofecoxib) taken daily, (3) minimized dosage of nonselective NSAIDs with short half-lives (e.g., ibuprofen, immediate-release diclofenac) taken only as necessary for symptoms or (4) minimized dosage of COX-2 selective NSAIDs with shorter half-lives (e.g., etodolac, celecoxib) taken only as necessary for symptoms.
A 1-year trial could test whether the benefits exceed the harms in patients with either osteoarthritis or rheumatoid arthritis plus a history of gastrointestinal ulcer or bleeding. Patients would be randomly allocated to receive either titrated usual dosages of COX-2 selective NSAIDs or titrated usual dosages of nonselective NSAIDs.
These trials would go a long way toward helping physicians and patients know the safest NSAID and the safest approach to use for long-term therapy in various clinical settings.
β See related article page 1125
Footnotes
Competing interests: None declared.
Correspondence to: Dr. James M. Wright, Department of Pharmacology and Therapeutics, 2176 Health Sciences Mall, Vancouver BC V6T 1Z3; fax 604 822-0701; jmwright@interchange.ubc.ca
References
- 1.Lipsky PE. Progress toward a new class of therapeutics: selective COX-2 inhibition. J Rheumatol 1997;24(Suppl 49):1-8.9002001
- 2.Lane NE. Pain management in osteoarthritis: the role of COX-2 inhibitors. J Rheumatol 1997;24(Suppl 49):20-4. [PubMed]
- 3.Celecoxib (Celebrex): Is it a breakthrough drug? Ther Lett 1999;31:1-2. Available: www.ti.ubc.ca/pages/letter31.htm (accessed 2002 Sept 12)
- 4.Juni P, Rutjes AWS, Dieppe PA. Are selective COX-2 inhibitors superior to traditional non-steroidal anti-inflammatory drugs? Adequate analysis of the CLASS trial indicates that this may not be the case. BMJ 2002;324:1287-8. [DOI] [PMC free article] [PubMed]
- 5.Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. JAMA 2000;284:1247-55. [DOI] [PubMed]
- 6.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, et al, for the VIGOR Study Group. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med 2000;343:1520-8. [DOI] [PubMed]
- 7.US Food and Drug Administration. Celebrex capsules (celecoxib). NDA 20-998/S-009. 2000. Available: www.fda.gov/ohrms/dockets/ac/01/briefing/3677b1_03_med.pdf (accessed 2002 Sept 12).
- 8.US Food and Drug Administration. Vioxx gastrointestinal safety. NDA 21-042/S-007. 2000. Available: www.fda.gov/ohrms/dockets/ac/01/briefing/3677b2_03_med.doc (accessed 2002 Sept 12).
- 9.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001;286:954-9. [DOI] [PubMed]
- 10.FitzGerald GA, Cheng Y, Austin S. COX-2 inhibitors and the cardiovascular system. Clin Exp Rheumatol 2001;19(Suppl 25):S31-6. [PubMed]
- 11.FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med 2001;345:433-42. [DOI] [PubMed]
- 12.Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci U S A 1999;96:7563-8. [DOI] [PMC free article] [PubMed]
- 13.Gotzsche PC. Methodology and overt and hidden bias in reports of 196 double-blind trials of nonsteroidal anti-inflammatory drugs in rheumatoid arthritis. Controlled Clin Trials 1989;10:31-56. [DOI] [PubMed]
- 14.Rochon PA, Gurwitz JH, Simms RW, Fortin PR, Felson DT, Minaker KL. A study of manufacturer-supported trials of nonsteroidal anti-inflammatory drugs in the treatment of arthritis. Arch Intern Med 1994;154:157-63. [PubMed]
- 15.Towheed TE, Hochberg MC. A systematic review of randomized controlled trials of pharmacological therapy in osteoarthritis of the hip. J Rheumatol 1997;24:349-57. [PubMed]
- 16.Towheed TE, Hochberg MC. A systematic review of randomized controlled trials of pharmacological therapy in osteoarthritis of the knee, with an emphasis on trial methodology. Semin Arthritis Rheum 1997;26:755-70. [DOI] [PubMed]
- 17.Bensen WG, Fiechtner JJ, McMillen JI, Zhao WW, Yu SS, Woods EM, et al. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomized controlled trial. Mayo Clin Proc 1999;74:1095-105. [DOI] [PubMed]
- 18.Schnitzer TJ, Truitt K, Fleischmann R, Dalgin P, Block J, Zeng Q, et al. The safety profile, tolerability, and effective dose range of rofecoxib in the treatment of rheumatoid arthritis. Clin Ther 1999;21:1688-702. [DOI] [PubMed]
- 19.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med 1999;5:698-701. [DOI] [PubMed]
- 20.Huskisson EC, Berry H, Gishen P, Jubb RW, Whitehead J. Effects of anti-inflammatory drugs on the progression of osteoarthritis of the knee. J Rheumatol 1995;22:1941-6. [PubMed]
- 21.Simon AM, Manigrasso MB, O'Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res 2002;17:963-76. [DOI] [PubMed]
- 22.Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Intern Med 1994;121:289-300. [DOI] [PubMed]
- 23.Chen BH. COX-2 inhibitors and renal function in elderly people. CMAJ 2000;163(5):604. [PMC free article] [PubMed]
- 24.Goldstein JL, Correa P, Zhao WW, Burr AM, Hubbard RC, Verbury KM, et al. Reduced incidence of gastroduodenal ulcers with celecoxib, a novel cyclooxygenase-2 inhibitor, compared to naproxen in patients with arthritis. Am J Gastroenterol 2001;96:1019-27. [DOI] [PubMed]
- 25.Laine L, Harper S, Simon T, Bath R, Johanson J, Schwartz H, et al. A randomized trial comparing the effect of rofecoxib, a cyclooxygenase 2-specific inhibitor, with that of ibuprofen on the gastroduodenal mucosa of patients with osteoarthritis. Gastroenterology 1999;117:776-83. [DOI] [PubMed]
- 26.Hawkey C, Laine L, Simon T, Beaulieu A, Maldonado-Cocco J, Acevedo E, et al. Comparison of the effect of rofecoxib (a cyclooxygenase 2 inhibitor), ibuprofen, and placebo on the gastroduodenal mucosa of patients with osteoarthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2000;43:370-7. [DOI] [PubMed]
- 27.Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71-86. [DOI] [PMC free article] [PubMed]
- 28.McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, FitzGerald GA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci U S A 1999;96:272-7. [DOI] [PMC free article] [PubMed]
- 29.Cheng Y, Austin SC, Rocca B, Koller BH, Coffman TM, Grosser T, et al. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science 2002;296:539-41. [DOI] [PubMed]
- 30.Wright JM, Perry TL, Bassett KL, Chambers GK. Reporting of 6-month vs 12-month data in a clinical trial of celecoxib. JAMA 2001;286:2398-400. [PubMed]
- 31.Bassett K, Wright JM, Perry TL Jr, Heran B, Cole C. COX-2 inhibitors update: journal publications fail to tell the full story. Can Fam Physician 2002;48:1455-60.12371304
- 32.Wooltorton E. What's all the fuss? Safety concerns about COX-2 inhibitors rofecoxib (Vioxx) and celecoxib (Celebrex). CMAJ 2002;166(13):1692-3. [PMC free article] [PubMed]
- 33.Konstam MA, Weir MR, Reicin A, Shapiro D, Sperling RS, Barr E, et al. Cardiovascular thrombotic events in controlled, clinical trials of rofecoxib. Circulation 2001;104:2280-8. [DOI] [PubMed]
- 34.Ray WA, Stein CM, Hall K, Daugherty JR, Griffin MR. Non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease: an observational cohort study. Lancet 2002;359:118-23. [DOI] [PubMed]
- 35.White WB, Faich G, Whelton A, Maurath C, Ridge NJ, Verburg KM, et al. Comparison of thromboembolic events in patients treated with celecoxib, a cyclooxygenase-2 specific inhibitor, versus ibuprofen or diclofenac. Am J Cardiol 2002;89:425-30. [DOI] [PubMed]
- 36.Vu D, Murty M, McMorran M. Selective COX-2 inhibitors: suspected cardiovascular/cerebrovascular adverse reactions. CARN 2002;12(2):1-3.
- 37.McCormack JP, Rangno R. Digging for data from the COX-2 trials. CMAJ 2002;166(13):1649-50. [PMC free article] [PubMed]
- 38.Wright JM. Choosing a first-line drug in the management of elevated blood pressure: What is the evidence? 2: Beta-blockers. CMAJ 2000;163(2):188-92. [PMC free article] [PubMed]
- 39.Ehrich EW, Schnitzer TJ, McIlwain H, Levy R, Wolfe F, Weisman M, et al. Effect of specific COX-2 inhibition in osteoarthritis of the knee: a 6 week double blind, placebo controlled pilot study of rofecoxib. J Rheumatol 1999;26:2438-47. [PubMed]


