Case
Mrs. B, a 59-year-old woman, asks her family physician about her risk of osteoporosis and fractures. She experienced a radial fracture from a fall earlier this year. She is a nonsmoker and does not drink alcohol regularly. There is no family history of fractures. Her body mass index is 25 kg/m2. The findings of a physical examination, including examination of the spine, are unremarkable. The results of baseline blood tests are also unremarkable. Should Mrs. B undergo a bone mineral density test? How will this assessment help in determining her risk of fractures? How often should the test be repeated, and how will it guide therapy?
Earlier this year the Canadian Panel of the International Society for Clinical Densitometry (whose members are listed at the end of this article) issued standards and guidelines for the practice of densitometry in Canada.1 The guidelines were based on a review of the literature and reflected the consensus of the panel. This article summarizes the key messages from those guidelines. It focuses on the use of bone densitometry by means of central dual-energy x-ray absorptiometry (DXA) in adults. It does not address densitometry in the pediatric population, nor does it cover technologies that evaluate bone mineral density (BMD) at peripheral skeletal sites such as heel, tibia or phalanx.
Bone densitometry is invaluable for the diagnosis of osteoporosis. Osteoporosis is associated with increases in the risk of fractures, morbidity and mortality.2 It is important to identify people with osteoporosis before the onset of fractures, because the occurrence of both vertebral and appendicular fractures is associated with greater risk of subsequent fractures.3 Bone densitometry allows for accurate and precise skeletal assessment and enables the detection of osteoporosis before the development of clinical fractures.
How does bone densitometry help in defining risk of fracture?
Several technologies are available for the measurement of BMD. Central DXA is currently the technology of choice.1 It measures BMD at the lumbar spine and the hip, and, with the appropriate software, it can also be used to measure BMD at other sites.
The relation between BMD and fracture risk in untreated patients has been evaluated prospectively in a number of large, well-designed studies.4,5,6,7,8,9,10 A meta-analysis of these studies confirmed that a decrease in BMD is associated with an increased risk of fracture.5 The predictive power of BMD for hip fracture is similar to the predictive power of blood pressure for stroke and better than the predictive power of serum cholesterol level for cardiovascular disease.5 In postmenopausal women the risk for hip fracture increases by a factor of 2.6 for each age-adjusted standard deviation (SD) decline in the BMD of the femoral neck.4,5 BMD at other sites (radius, calcaneus, hip and spine) has also been shown to correlate with the fracture risk at all sites.5 The best validation of diagnostic thresholds determined by DXA has been obtained for the hip.11
A working group of the World Health Organization (WHO) defined osteoporosis on the basis of the relation between BMD and fracture risk in postmenopausal white women.12 According to the WHO definitions, people with BMD more than 2.5 SDs below the mean for young adult women have osteoporosis.12 The T-score is the number of SDs that the patient's BMD is above or below the mean reference value for young adults (the age of peak bone mass). The T-score is thus a comparison of the patient's BMD with mean BMD for the young adult population. The Z-score is the number of SDs that the patient's BMD is above or below the mean reference value for people of the same age. The Z-score is thus a comparison of the patient's BMD with that of people of the same age.12 There are currently insufficient data regarding the relation between BMD and fracture risk to allow specific definitions of osteoporosis in premenopausal women, non-white women or men.
For example, the relation between BMD and fracture risk may be different in men and women.13 Current data are contradictory, and large prospective studies are needed to clarify the relation. Racial differences in skeletal size, skeletal geometry and hip axis length may contribute to racial differences in hip fracture rates.14 Therefore, it may be inappropriate to apply the WHO criteria to other groups without modification.
In evaluating fracture risk, BMD should be considered in conjunction with other clinical risk factors for fracture.15,16 Important independent risk factors include age 65 years or older, history of fracture as an adult, family history of osteoporotic fracture (especially of the hip) and poor neuromuscular function.6 Whether and how to intervene should be decided on the basis of a combined assessment of BMD and clinical risk factors for fracture.
What are the indications and contraindications for bone densitometry?
National guidelines16,17 suggest that BMD testing be targeted at people who have clinical risk factors for osteoporosis and those with conditions or disorders associated with bone loss (Tables 1 and 2). The Study of Osteoporotic Fractures provided a detailed analysis of risk factors for hip fractures.6 The same risk factors have been shown to predict fractures at other sites, including the spine.18
Table 1
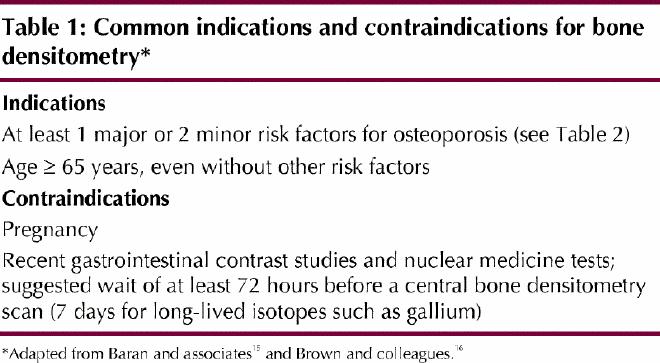
Table 2

BMD testing should be completed only if the results of the test will affect patient management. For example, a woman experiencing menopause without risk factors for osteoporosis (Table 2) does not require routine densitometry, whereas a woman with a personal history of fragility fracture after age 40 does require such testing. Fragility fractures are those that occur spontaneously or after minor trauma, such as a fall from standing height (e.g., from roller skates or ice skates), a fall from the sitting position or the lying position (e.g., from a chair or bed less than 1 m high), a fall after having missed 1 to 3 steps in a staircase or a fall while coughing.
Can I trust a change in the bone density measurement?
Current methods for measuring BMD typically demonstrate precision errors of the same order as natural short-term changes in BMD, which underscores the need for careful quality control of the instrument, the scanning technique and the analysis. The site measured is also important. In early postmenopausal women, bone loss from the spine exceeds that from the hip because of the more rapid turnover of trabecular bone, which predominates in the vertebral bodies.19 Similarly, an increase in skeletal BMD related to antiresorptive treatment (bisphosphonates, calcitonin, hormone replacement therapy or raloxifene) is usually most evident in the spine.20 For older subjects who are not receiving treatment, the decline in BMD in the hip generally exceeds that in the spine because of age-related degenerative sclerosis in the spine, which increases the BMD measurement but does not contribute to bone strength.21
The precision error provides a measure of the reproducibility of the result in a repeated measurement. It is influenced by the instrument used, as well as by technologist- and patient-dependent factors (the last 2 of these factors tending to be more important than the first one). Therefore, it is strongly recommended that, whenever possible, follow-up measurements for a given patient be obtained by the same technologist using the same scanning procedure and the same instrument as for the original measurement. In addition, each laboratory should determine the precision of its measurements by evaluating a variety of clinical subjects under conditions that reflect “real-life” situations22 and should use this information in judging the significance of any change observed in a single patient. In routine clinical settings the following reproducibilities have been reported: for lumbar spine 1.8% to 2.3%, for femoral neck 2.3% to 3.6% and for total hip 1.7% to 2.5%.23,24,25 Each laboratory should include on the BMD report its own DXA reproducibilities for each measurement site. If the clinician is to conclude (with 95% confidence) that a change is not related to measurement error, the change must be at least 2.77 times the site-specific precision error at that centre. For example, a BMD of 2% at the lumbar spine would not be statistically significant at the 95% confidence level if the precision error at the lumbar spine is 1% (since 2.77 х 1% = 2.77%, which is greater than the observed 2% change). In summary, it is important not to over-interpret small changes.
When should I repeat the bone density measurement?
Average rates of bone loss are variable. The rate of loss is greater in untreated early postmenopausal women (approximately 1% to 2% per year) than in older women (less than 1% per year).19,21,26 Follow-up BMD measurements in patients who are not receiving active treatment can help in identifying the subset of patients with rapid bone loss (“fast losers”). Repeat testing may also be useful in confirming a positive response to treatment. However, interpretation of results from testing undertaken to confirm a positive treatment effect is not completely straightforward. Although large increases in BMD are associated with large decreases in fracture risk,20 even small increases in BMD can dramatically reduce the risk of fractures.27,28 For example, raloxifene therapy has been associated with minimal increases of BMD at the lumbar spine and hip but significant decreases in the incidence of vertebral fractures.28 This pattern may indicate that some of the benefit from current antiresorptive therapies is mediated through mechanisms other than an increase in BMD.29,30 Conversely, absence of measurable change in BMD does not necessarily imply therapeutic failure and lack of antifracture benefit. In general, repeat BMD measurement can be considered 1 year after the original measurement if there is concern about rapid progressive bone loss (e.g., in glucocorticoid-induced osteoporosis, immobilization, acute gonadal insufficiency or primary hyperparathyroidism) or if the patient has started a new intervention (e.g., bisphosphonates). Less frequent repeat scanning (every 2 to 3 years) is appropriate in patients who have started therapies that increase BMD only minimally, such as nasal calcitonin and raloxifene, and those whose condition is already known to be stable or improving with drug therapy.
How significant is exposure to radiation with DXA?
Radiation safety practice requires that all radiation doses be considered harmful. However, the dose delivered by DXA is extremely small, of the order of everyday doses from background radiation (the environmental radiation to which everyone is exposed). Studies have not shown any ill effects (either long- or short-term) from such small doses.31,32
In explaining radiation risk to the patient, the clinician can relate the dose from the procedure (an effective dose equivalent between 0.0005 and 0.0060 mSv [millisievert, the unit for dose equivalence]) to the dose from background radiation (effective dose equivalent 2.5 mSv/yr, although this value varies with altitude and geologic substrate) or the dose from everyday occurrences, such as flying from coast to coast (effective dose equivalent 0.5 mSv) or undergoing chest radiography (effective dose equivalent 0.1 mSv). For example, the radiation dose from DXA is 1/1000th the dose received when a person flies from coast to coast and 1/2000th that received from chest radiography. Because radiation risk relates not only to dose, but also to dose rate, such analogies are qualitative, not quantitative.
It should be noted that a fetus is more susceptible than an adult to radiation damage. Thus, for patients who may be pregnant, the indications and benefits of the assessment should be reviewed and the BMD measurement deferred until immediately after the patient's next menstrual period, which might not be until after delivery (if the patient is actually pregnant). However, if the measurement has already been performed in a woman who might be pregnant, the additional risk associated with the radiation dose is so small that therapeutic abortion cannot be justified.
What information does the bone density laboratory need?
Clinical information is important in the interpretation of bone densitometry results. Referral information or the patient's responses to a questionnaire completed at the time of the procedure are used as background information. The pertinent information includes age, menopausal status, prior history of atraumatic fractures, loss of height and any specific treatment for osteoporosis. Other medications such as thyroid replacement therapy, anticonvulsants and corticosteroids should be mentioned. Also important are smoking history, intake of vitamin D and calcium, family history of osteoporosis, coexisting illnesses and conditions, and history of alcohol use.
Recent barium studies (within the previous week) may leave residues that will attenuate the densitometry x-ray beam and falsely elevate BMD measurements. Ingestion of radiopaque medications may have the same affect. Recently injected radioisotopes for nuclear medicine procedures may emit radiation, which will falsely reduce measured BMD. This effect may be significant for approximately 72 hours after the radioisotope has been given, longer (up to a week) for long-lived radioisotopes such as gallium.
What information should I expect to find in the bone density report?
The T-score and the WHO Working Group definitions12 of normal (T-score greater than or equal to –1), osteopenia (T-score less than –1 but greater than –2.5) and osteoporosis (T-score equal to or below –2.5) will be given. When relevant, the Z-score will also be reported. A Z-score below –2 may indicate a need for detailed evaluation of the patient for secondary causes of osteoporosis (e.g., multiple myeloma, a malabsorption syndrome or glucocorticoid-induced osteoporosis). The report should outline any technical problems that might compromise the validity of the examination, such as degenerative changes (e.g., facet joint sclerosis or osteophytes), anatomic abnormalities (e.g., severe scoliosis) or compression fractures. The report should also include a qualitative assessment of fracture risk, as well as comments on serial BMD changes for patients who have previously undergone densitometry (which should take into account the precision error of the testing densitometer in assessing whether any change occurring since the previous study is in fact significant).
The results of the BMD assessment should be considered in conjunction with other clinical risk factors for fracture and are helpful in determining the patient's future risk of fragility fractures. Table 4 of the Canadian osteoporosis guidelines16 provides information about assessing the patient's risk in light of the BMD results and factors such as sex and age.
The case revisited
Mrs. B's personal history of radial fracture after age 40 may indicate that she has osteoporosis, and further evaluation is appropriate. A BMD assessment will be helpful in assessing fracture risk. Because of the radial fracture, her risk of future fracture is greater than it would be otherwise.
On densitometry, the patient's BMD for the lumbar spine (L1 to L4) was 1.04 g/cm2, which corresponds to a T-score of –1.3. Total hip BMD was within the normal range (T-score –0.6). The patient is reassured by these results. Her current risk of fracture can be considered low, but it will increase as she ages. Some of the age-related increase in fracture susceptibility relates to bone loss, but much of it reflects other factors such as an increase in the risk of falling. This patient would benefit from appropriate calcium intake (1500 mg/day from all sources) and vitamin D supplementation (20 μg or 800 IU/day), as well as regular weight-bearing exercise.16 The interpretation of repeat assessments should take into consideration the precision error at the testing centre. Serial assessments are of value in ensuring that rapid progressive bone loss does not occur and in monitoring the effectiveness of therapy.
Conclusions
Central DXA, a proven technology for the diagnosis and management of bone mineral loss, is now widely used across Canada. Optimum benefit from the technology requires maintenance of high standards in technical application, medical supervision and interpretation of results.
Acknowledgments
We gratefully acknowledge David Hanley, Anthony Hodsman, Robert Josse, Timothy Murray and Sol Stern for their review of an early draft of the manuscript.
Footnotes
Contributors: All authors made equal contributions to the development, writing, reviewing and editing of the manuscript. All approved of the final version to be published.
Competing interests: Drs. Khan, Brown and Watts have had paid consultancies with pharmaceutical companies manufacturing products pertinent to the topic of this article during the past 2 years. Drs. Khan, Brown, Leslie, Lewiecki and Watts have received speaker fees (honoraria) or educational grants (or both) from various pharmaceutical companies. Drs. Leslie and Lewiecki have received support from pharmaceutical companies for research related to the content of this article. Drs. Leslie and Lewiecki have received travel assistance from pharmaceutical companies to attend meetings within the past 2 years. Dr. Watts receives support (through his academic institution) from several pharmaceutical companies for research related to the content of this article.
Correspondence to: Dr. Aliya A. Khan, McMaster University, 331-209 Sheddon Ave., Oakville ON L6J 1X8; fax 905 844-8966; Avkhan@aol.com
References
- 1.Khan AA, Brown J, Faulkner K, Kendler D, Lentle B, Leslie W, et al. Standards and guidelines for performing central dual x-ray densitometry from the Canadian Panel of International Society for Clinical Densitometry. J Clin Densitom 2002;5:247-57. [DOI] [PubMed]
- 2.Cooper C, Atkinson EJ, Jacobsen SJ, O'Fallon WM. Population-based study of survival after osteoporotic fractures. Am J Epidemiol 1993;137:1001-5. [DOI] [PubMed]
- 3.Ross P, Davis J, Epstein R, Wasnich R. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med 1991;114:919-23. [DOI] [PubMed]
- 4.De Laet CE, Van Hout BA, Burger H, Weel AE, Hofman A, Pols HA. Hip fracture prediction in elderly men and women: validation in the Rotterdam study. J Bone Miner Res 1998;13:1587-93. [DOI] [PubMed]
- 5.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996;312:1254-9. [DOI] [PMC free article] [PubMed]
- 6.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med 1995;332:767-73. [DOI] [PubMed]
- 7.Hans D, Dargent-Molina P, Schott AM, Sebert JL, Cormier C, Kotzki PO, et al. Ultrasonographic heel measurements to predict hip fractures in the elderly: the EPIDOS prospective study. Lancet 1996;348:511-4. [DOI] [PubMed]
- 8.Ross PD, Huang C, Davis JW, Wasnich RD. Vertebral dimension measurements improve prediction of vertebral fracture incidence. Bone 1995;16(Suppl):S257-62. [DOI] [PubMed]
- 9.Melton LJ 3rd, Atkinson EJ, O'Fallon WM, Wahner HW, Riggs BL. Long-term fracture prediction by bone mineral assessed at different skeletal sites.J Bone Miner Res 1993;8:1227-33. [DOI] [PubMed]
- 10.Black DM, Cummings ST, Melton LJ 3rd. Appendicular bone mineral and women's lifetime risk of hip fracture. J Bone Miner Res 1992;7:639-45. [DOI] [PubMed]
- 11.Kanis JA, Gluer CC. An update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors, International Osteoporosis Foundation. Osteoporos Int 2000;11:192-202. [DOI] [PubMed]
- 12.Kanis JA. Diagnosis of osteoporosis. Osteoporos Int 1997;7 Suppl 3:S108-16. [DOI] [PubMed]
- 13.Orwoll E. Assessing bone density in men. J Bone Miner Res 2000;15:1867-70. [DOI] [PubMed]
- 14.Cummings SR, Cauley JA, Palermo L, Ross PD, Wasnich RD, Black D, et al. Racial differences in hip axis lengths might explain racial differences in rates of hip fracture. Study of Osteoporotic Fractures Research Group. Osteoporos Int 1994;4:226-9. [DOI] [PubMed]
- 15.Baran DT, Faulkner KG, Genant HK, Miller PD, Pacifici R. Diagnosis and management of osteoporosis: guidelines for the utilization of bone densitometry. Calcif Tissue Int 1997;61:433-40. [DOI] [PubMed]
- 16.Brown JP, Josse RJ, for the Scientific Advisory Council, Osteoporosis Society of Canada. 2002 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. CMAJ 2002;167(10 Suppl):S1-S34. [PMC free article] [PubMed]
- 17.National Osteoporosis Foundation. Osteoporosis: review of the evidence for prevention, diagnosis, and treatment and cost-effectiveness analysis. Osteoporos Int 1998;8 Suppl 4:S7-80. [PubMed]
- 18.Black DM, Steinbuch M, Palermo L, Dargent-Molina P, Lindsay R, Hoseyni MS, et al. An assessment tool for predicting fracture risk in postmenopausal women. Osteoporos Int 2001;12:519-28. [DOI] [PubMed]
- 19.World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group. Geneva: The Organization; 1994. Tech Rep Ser 843. [PubMed]
- 20.Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 1995;333:1437-43. [DOI] [PubMed]
- 21.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997;337:670-6. [DOI] [PubMed]
- 22.Gluer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int 1995;5:262-70. [DOI] [PubMed]
- 23.Williams-Russo P, Healey JH, Szatrowski TP, Schneider R, Paget S, Ales K, et al. Clinical reproducibility of dual energy x-ray absorptiometry.J Orthop Res 1995;13:250-7. [DOI] [PubMed]
- 24.Wahner HW, Looker A, Dunn WL, Walters LC, Hauser MF, Novak C. Quality control of bone densitometry in a national health survey (NHANES III) using three mobile examination centers. J Bone Miner Res 1994;9:951-60. [DOI] [PubMed]
- 25.Phillipov G, Seaborn CJ, Phillips PJ. Reproducibility of DEXA: potential impact on serial measurements and misclassification of osteoporosis. Osteoporos Int 2001;12:49-54. [DOI] [PubMed]
- 26.Genant HK, Lucas J, Weiss S, Akin M, Emkey R, McNaney-Flint H. Low-dose esterified estrogen therapy. Arch Intern Med 1997;157:2609-15. [DOI] [PubMed]
- 27.Chesnut CH 3rd, Silverman S, Andriano K, Genant HK, Gimona A, Harris S, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the Prevent Recurrence of Osteoporotic Fractures Study. PROOF Study Group. Am J Med 2000; 109:267-76. [DOI] [PubMed]
- 28.Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA 1999;282:637-45. [DOI] [PubMed]
- 29.Riggs BL, Melton LJ 3rd, Fallon WM. Drug therapy for vertebral fractures in osteoporosis: evidence that decreases in bone turnover and increases in bone mass both determine antifracture efficacy. Bone 1996;18(3 Suppl):197S-201S. [DOI] [PubMed]
- 30.Cummings SR, Black DM, Vogt TM. Changes in BMD substantially underestimate the antifracture effects of alendronate and other antiresorptive drugs. J Bone Metab Res 1996;11(Suppl):S102.
- 31.Shapiro J. Radiation protection: a guide for scientists and physicians. 3rd ed. Cambridge (MA): Harvard University Press; 1990.
- 32.Atomic Energy Control Board (AECB). Canada: living with radiation. Ottawa: AECB; 1995.


