Abstract
Numerous studies of cluster formation and dissociation have been conducted to determine properties of matter in the transition from the condensed phase to the gas phase using materials as diverse as atomic nuclei, noble gasses, metal clusters, and amino acids. Here, electrospray ionization is used to extend the study of cluster dissociation to peptides including leucine enkephalin with 7–19 monomer units and 2–5 protons, and somatostatin with 5 monomer units and 4 protons under conditions where its intramolecular disulfide bond is either oxidized or reduced. Evaporation of neutral monomers and charge separation by cluster fission are the competing dissociation pathways of both peptides. The dominant fission product for all leucine enkephalin clusters studied is a proton-bound dimer, presumably due to the high gas-phase stability of this species. The branching ratio of the fission and evaporation processes for leucine enkephalin clusters appears to be determined by the value of z2/n for the cluster where z is the charge and n the number of monomer units in the cluster. Clusters with low and high values of z2/n dissociate primarily by evaporation and cluster fission respectively, with a sharp transition between dissociation primarily by evaporation and primarily by fission measured at a z2/n value of ~0.5. The dependence of the dissociation pathway of a cluster on z2/n is similar to the dissociation of atomic nuclei and multiply charged metal clusters indicating that leucine enkephalin peptide clusters exist in a state that is more disordered, and possibly fluid, rather than highly structured in the dissociative transition state. The branching ratio, but not the dissociation pathway of [somatostatin5 + 4H]4+ is altered by the reduction of its internal disulfide bond indicating that monomer conformational flexibility plays a role in peptide cluster dissociation.
Bulk properties of matter and the properties of isolated gas-phase atoms and molecules can have radically different characteristics in terms of structure, work function, ion solvation, etc. (for a review see reference [1]). One motivation behind cluster research is that the transitional characteristics between bulk and molecular properties can be discovered by studying intermediate states of matter. Investigators have explored a wide variety of materials, including charged droplets of various liquids [2, 3], clusters of noble metals [4–8], metal-ligand clusters [1, 9–11], highly charged clusters of noble gasses [12, 13], small molecules [14, 15], and ultra-cold clusters of transition metals [16]. Recently, investigators have formed noncovalently bound clusters of biologically important molecules, including clusters of amino acids and small peptides [17–28], and have studied their dissociation processes [18, 20, 26–28]. Multiply charged “clusters” of proteins consisting of multimeric, noncovalently associated protein complexes with a high degree of solution-phase structural specificity have been liberated into the gas phase [29–35]. From a simple mass measurement, the stoichiometry of the complex can be determined. There has also been much interest in determining if structural information can be inferred from gas-phase dissociation of the complexes [30, 33, 35, 36].
Gas-phase dissociation of a wide variety of clusters have been investigated [1–3, 5–8, 10–16, 18–20, 24, 26, 27, 30, 35–40]. In the case of multiply charged clusters, the two competing processes for dissociation are the evaporation of a neutral atom or molecule and the ejection of charged subunits. The latter occurs through a fission process in which the cluster dissociates into two or three charged fragments that then separate due to coulombic repulsion [41]. The competing processes of evaporation and fission have been described by various liquid-drop models for clusters as diverse as atomic nuclei [42], multiply charged metal clusters [5, 6], and highly charged solvent droplets [2, 3]. The recent development of femtosecond lasers capable of rapidly ionizing many molecules in atomic or molecular clusters has permitted access to another dissociation process whereby a cluster undergoes a coulombic explosion, resulting in the isotropic ejection of many charged fragments [12–15].
General characteristics of cluster dissociation are well illustrated by multiply charged metal clusters. Evidence for their dissociation was first observed by Sattler et al. who, by measuring the mass and charge of Pb, Xe, and NaI clusters, observed half-integer cluster numbers that were interpreted to be doubly charged clusters [4]. It was found that the cluster size necessary to support two charges is much higher for Xe than for Pb or NaI due to the much weaker intermolecular attraction in Xe. Doubly charged Au clusters containing 9 –17 atoms were isolated and collisionally activated by Saunders [5, 6]. These clusters were found to dissociate by competing mechanisms of neutral Au atom evaporation, and fission by the ejection of Au3+ and other products. Following collisional activation, larger Au clusters dissociate primarily by successive evaporation of neutral Au atoms while the smaller clusters, such as Au102+ and Au92+, dissociate primarily through fission. Interestingly, the log of the ratio of the rates of fission to evaporation was found to vary linearly with z2/n, where z is the cluster charge and n is the number of gold atoms in the cluster. This behavior corresponds closely to nuclear fission [43] and led Saunders to suggest that a similar liquid-drop model used to describe nuclear fission would also describe metal cluster dissociation [5, 6]. Recent work on the dissociation of noble metal clusters has shown that neutral evaporation and trimer fission are also the primary dissociation pathways of larger Au clusters and other metals [7, 8].
In contrast to the many cluster dissociation experiments that have been done with atoms and small molecules, only a few studies have been conducted with clusters of large, biologically relevant molecules. These have included studies of the dissociation and charge partitioning among the fragments of both homogeneous protein complexes that are biologically active in solution [29 –35, 44, 45] and proteins that are nonspecifically aggregated [36, 44]. Studies have also been conducted on clusters of smaller, biologically relevant molecules, and these have tended to focus on amino acids and small peptides. Amino acid and peptide clusters can be readily formed by electrospray ionization (ESI) and initial reports were concerned with clusters of monomers (M) of the form [Mn + nH]n+ that appear at the same nominal m/z as the singly protonated monomer. These ions are indistinguishable in low resolution mass spectra from the monomer and larger clusters and could potentially complicate the interpretation of dissociation spectra [17, 19, 21, 23, 46]. While the formation of such highly charged molecular clusters by ESI is interesting in its own right and indicative of the gentle nature of the ESI process, the elucidation of dissociation pathways for clusters of the type [Mn + nH]n+ is more complicated as the fragments are likely to be at the same m/z as the parent ion.
Some observations have been made of amino acid and peptide clusters formed by ESI of the type [Mm + nH]n+ where m > n [18, 20, 22–28, 40, 47]. Much of this work has focused on octamers of the amino acid serine that have been found to form with a preference for homochirality [20, 22, 25–27, 48]). Clusters of serine as large as [44Ser + 4H]4+ were isolated by Beauchamp and coworkers and collisionally activated in an ion trap mass spectrometer [20]. The cluster dissociation revealed that the large serine clusters dissociate by a combination of fission and evaporation, but the specific dissociation pathways are difficult to determine because the primary fragment ions of noncovalently bound complexes are often sufficiently activated to undergo subsequent dissociation. For example, although the activation of [30Ser + 3H]3+ was shown to produce a dissociation spectrum with fragments ranging in size from [23Ser + 3H]3+ to [29Ser + 3H]3+ [20], it is not known whether the fragments are a result of sequential evaporation of single amino acids or if several dissociation pathways exist whereby different sizes of neutral serine clusters evaporate from the original cluster. Because the primary activation products are more likely to provide the most information about the structure of the initial cluster, it is important to distinguish between primary and secondary fragments. A useful technique for distinguishing between primary and secondary dissociation products is double resonance (DR).
DR was initially developed to identify chemically coupled processes in equilibrium experiments conducted in ion cyclotron resonance mass spectrometers [49] and was later adapted for Fourier-transform mass spectrometry [50] and other kinds of mass spectrometry [51]. DR experiments are done by applying an on-resonance ejection waveform simultaneous with the dissociation event, with the aim of ejecting primary fragment ions before they undergo subsequent dissociation. If the ejection of an ion perturbs the abundance of another ion, it indicates that the second ion is formed by subsequent dissociation of the ejected ion and not directly from the activated precursor ion.
In this work, the dissociation pathways of multiply charged peptide clusters of leucine enkephalin and both oxidized and reduced somatostatin are elucidated using collisionally activated dissociation and DR. The effects of cluster size and charge on the dissociation pathways of leucine enkephalin clusters are compared with the dissociation of multiply charged metal clusters. To the authors’ knowledge, this is the first application of DR to multiply charged cluster dissociation.
Experimental
Mass Spectrometry
The experiments were conducted on a 110 mm bore 9.4 T Fourier-transform mass spectrometer that was constructed in collaboration with Bruker Daltonics (Billerica, MA) (Figure 1). This instrument has been described elsewhere [36]. The ion optics and introduction system are a standard Bruker design. Ions are generated by nanoelectrospray using borosilicate capillaries that are pulled to a ~4 μm tip with a model P-87 capillary puller (Sutter Instruments, Novato, CA). A small volume of sample solution (4-10 μL) is loaded into the borosilicate capillary and a platinum wire is inserted into the solution at the end of the capillary. The capillary is positioned ~2 mm from the source inlet and a potential of ~900 V is applied to the platinum wire.
Figure 1.
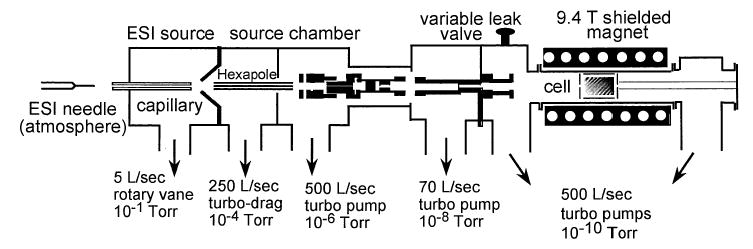
Diagram of the 9.4 tesla Berkeley-Bruker Fourier-transform mass spectrometer.
The electrospray generated ions are accumulated in a storage hexapole prior to injection through multiple ion lenses and three stages of differential pumping into the ultra high vacuum chamber (3 × 10−9 torr) of the instrument where the ions are trapped in a cylindrical Bruker Infinity cell. Each ion injection is accompanied by a pulse of nitrogen gas introduced into the vacuum chamber at a pressure of ~2 × 10−6 torr to enhance trapping and to damp the motion of the ions in the cell.
Chemicals
Leucine enkephalin (Tyr-Gly-Gly-Phe-Leu), somatosta-tin (Ala-Gly-Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-Ser-Cys [Disulfide bridge: 3–14]) and dithiolthreitol (DTT) were purchased from Sigma-Aldrich C. (St. Louis, MO) and were used without further treatment except for the disulfide reduction described below. Methanol and acetic acid (99.9%) were purchased from EM Science (Gibbstown, NJ) and from Fisher Scientific (Pittsburgh, PA) respectively.
Disulfide Bond Reduction
For each day of experiments, a fresh solution with a concentration of 5 mM somatostatin (ST) and 50 mM DTT was made in Millipore 18.2 MΩ H2O. The solution was placed in a sealed eppendorf tube covered with parafilm, and heated in a water bath at 80 −95 °C for ~50 min. Disulfide reduction was nearly complete as verified by an average increase of 2 Da in the peptide mass following the reduction procedure. ST remained reduced for several hours following the reduction procedure. Fresh, reduced solutions of ST were prepared for each day of experiments.
Cluster Formation
Solutions of leucine enkephalin (LE) and oxidized ST for ESI were prepared at concentrations of ~2.5 and ~1.3 mM, respectively, in 1:1 water:methanol + 2 % acetic acid by volume. The relatively high peptide concentrations were found to enhance cluster formation. Solutions of reduced ST for ESI were prepared by diluting stock solutions of 5 mM somatostatin and 50 mM DTT to final concentrations of 1.3 mM and 13 mM, respectively, in 1:1 water:methanol + 2 % acetic acid. In order to stabilize the nanoelectrospray emission, ammonium acetate was added to the reduced ST solution containing 13 mM DTT until the final concentration was 100 mM. This technique is often useful for obtaining nanoelectrospray signal from solutions containing a high concentration of small molecules, such as DTT, which often result in unsteady nanoelectrospray currents.
The signal intensity of peptide clusters is enhanced by tuning for selective accumulation in the external hexapole of the instrument. This is done by accumulating the ions in the hexapole for 3 s (versus ≤ 1.0 s typically), increasing the hexapole dc offset from ~2.7 to 3.5–4.5 V and injecting multiple hexapole accumulations into the ion cell prior to detection. The particular clusters measured in the mass spectra using a given set of tuning conditions are also sensitive to the voltage applied to the nanoelectrospray needle. In general, slowly decreasing the applied nanoelectrospray voltage after initiating ion current favors the formation of larger clusters. By tuning the hexapole offset and the second skimmer potentials and adjusting the electrospray voltage, it is possible to dramatically change the appearance of the mass spectra so that the abundance of a cluster of interest is greatly enhanced.
Double Resonance and Collisional Activation
Modifications to the electronics of the APEX II mass spectrometer were implemented in order to do DR experiments. An additional transmitter board and a HI-222 analog switch from Bruker Daltonics were installed in the high frequency unit of the APEX II mass spectrometer. Modifications to the multiplexer board of the mass spectrometer were made in-house according to specifications provided by Scott Daniels (Bruker Daltonics, Billerica MA) that permit two excite frequencies to be applied simultaneously to ions in the cell of the mass spectrometer. The software to implement DR experiments is a modification of code written by Dr. Christian Berg (Bruker Daltonics, Billerica MA).
Peptide clusters of interest were isolated using two identical correlated sweeps with a pulse of nitrogen gas introduced into the ion cell between the sweeps. Following the double isolation, the clusters were dissociated by sustained off-resonance irradiation collisionally activated dissociation (SORI-CAD) [52] in which an excitation waveform was applied +600 Hz off-resonance for two seconds with an applied peak-to-peak potential of 6 –10 V. A pulse of nitrogen gas was introduced for 90 ms, briefly raising the pressure to ~2 × 10−6 torr. During DR experiments, a second excitation waveform is applied on-resonance to the fragment ion under investigation for the duration of the SORI excitation and for an additional 1.0 s to eject fragment ions that continue to dissociate after the SORI waveform is turned off. The amplitude of the DR waveform was adjusted to eject the fragment ions as rapidly as possible while avoiding additional excitation of the precursor ion or any of the other fragment ions. The maximum voltage that could be applied without additionally exciting the precursor or other fragment ions varied from 4 –32 V pk–pk depending on the m/z and the proximity of other fragment ions of the DR waveform.
Results
Multiply charged, gas-phase noncovalent clusters of leucine enkephalin (LE) and somatostatin (ST) can be readily formed from concentrated solutions using nanoelectrospray ionization. The gas-phase dissociation pathways of these clusters were investigated using collisional activation and double resonance (DR) ejection. Many of the primary fragment ions produced from the dissociation of these clusters have sufficient internal energies to undergo further fragmentation before ion detection making DR experiments useful for determining dissociation pathways. In DR experiments, one of the fragment ions resulting from dissociation of a precursor cluster ion is continuously ejected from the ion cell while the precursor ion is activated. If subsequent dissociation of the fragment ion requires more time than ion ejection (~3–24 ms for these experiments depending on the amplitude of the DR ejection pulse), the products of this ion will not appear in the DR spectrum. Thus, secondary dissociation products are identified by their decrease in abundance when a DR waveform is applied to eject their parent ion. Dissociation pathways for several different clusters of LE and one cluster of ST with its individual peptides containing either oxidized or reduced disulfide bonds are described in the following sections.
It should be noted that each cluster ion is isolated by two correlated sweeps with a pulse of gas between the sweeps. After an initial correlated sweep, the isolated cluster undergoes some dissociation after a pulse of nitrogen collision gas is introduced. When the cluster is re-isolated by a second, identical correlated sweep, the remaining ions do not dissociate as readily. This result suggests that either the nonspecific peptide clusters examined in this study exist in more than one gas-phase configuration that have different stabilities or that the ions are kinetically excited after trapping. The clusters investigated in this study are those that survive the double isolation.
Leucine Enkephalin 7-Mer2+
Doubly protonated LE clusters consisting of seven monomers (7LE2+) are readily formed by nanoelectrospray (Figure 2a). Dissociation of 7LE2+ (Figure 3a) results in abundant LE+ and 2LE+ ions along with lower abundance 3LE+, 4LE+, and 5LE+ ions. Interestingly, the abundance of 2LE+ greatly exceeds that of its complimentary ion, 5LE+, and the complimentary ion to LE+, 6LE+, is absent from the dissociation spectrum. Although mass discrimination may account for a very small fraction of the asymmetry observed, secondary fragmentation accounts for the majority. Application of a DR ejection pulse to the m/z corresponding to 6LE+ causes no change in the appearance of the mass spectrum indicating that 6LE+ is not a significant dissociation product of 7LE2+. When a DR ejection pulse is applied to the 5LE+ peak (Figure 3b), the abundance of the other fragmentation peaks change considerably. The abundance of 4LE+ and 3LE+ are attenuated, suggesting that a substantial fraction of these ions are formed by subsequent dissociation of the primary 5LE+ fragment. Application of a DR waveform at the frequency of the 4LE+ ion (Figure 3c) results in a further attenuation of 3LE+ and 2LE+ ions. The isotopic distribution of the 3LE+ peak shows that 6LE2+ is present in the dissociation spectra with an intensity as high as 10% of the 3LE+ peak indicating that the 7LE2+ → 6LE2+ + LE0 process does occur. The abundance of 6LE2+ varies considerably from scan to scan and is often not detectable within the signal to noise of the experiment. Based on the observed abundance of the 6LE2+ peak, the 7LE2+ → 6LE2+ + LE0 process does not constitute more than ~ 0.005% of the total dissociation.
Figure 2.
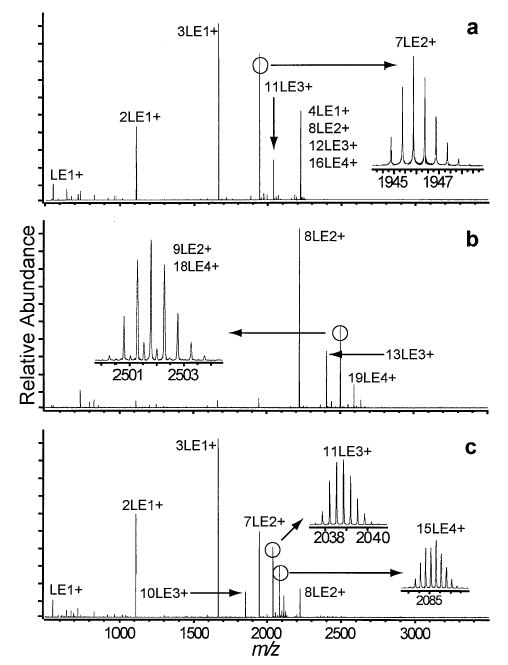
Nanoelectrospray mass spectra of 2.5 mM LE in 1:1 water:methanol + 2% acetic acid with electrospray voltage and hexapole accumulation parameters optimized for maximal ion intensity of the (a) 7LE2+, (b) 9LE2+, and (c) 11LE3+ and 15LE4+ clusters.
Figure 3.
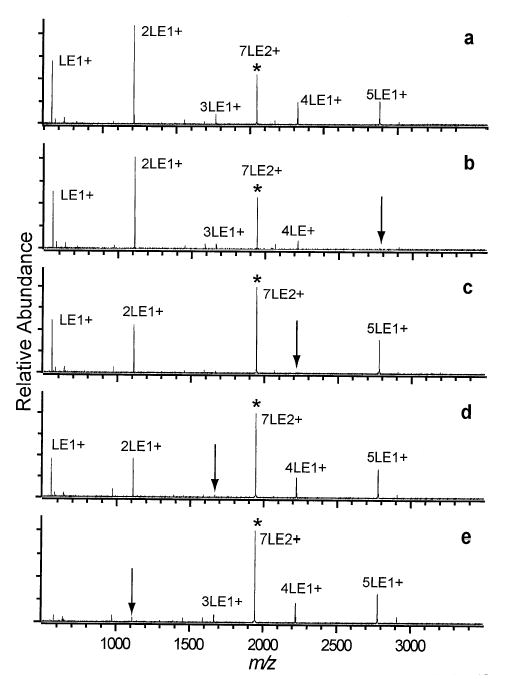
Sustained off-resonance irradiation collisionally activated dissociation (SORI-CAD) spectra of the 7LE2+ cluster (a) without double resonance (DR) and with DR ejection applied at the m/z corresponding to the (b) 5LE+, (c) 4LE+, (d) 3LE+, and (e) 2LE+ clusters.
The 4LE+ and 3LE+ peaks can be formed by two possible dissociation processes. Either the 3LE+ peak is formed by evaporation of neutral LE from larger, singly charged clusters, or it is formed by symmetric fission of 7LE2+ to form complimentary 3LE+ and 4LE+. The results of the DR experiments indicate that most, if not all, of the 4LE+ and 3LE+ are formed by subsequent LE evaporation from 5LE+. Ejection of 5LE+ (Figure 3b) greatly attenuates the 4LE+ and 3LE+ abundance, indicating that the 5LE+ → 4LE+ → 3LE+ pathway does occur. DR ejection of the 4LE+ (Figure 3c) virtually eliminates the 3LE+, and the 2LE+ and LE+ peaks are attenuated. DR ejection of the 3LE+ (Figure 3d) leads to the same attenuation of 2LE+ and LE+, as DR ejection of 4LE+ (Figure 3c), strongly suggesting that 7LE2+ → 4LE+ + 3LE+ does not occur. If 7LE2+ → 4LE+ + 3LE+ did occur, DR ejection of the 3LE+ would be expected to cause additional attenuation of 2LE+ and LE+. Thus, the majority of 4LE+ and 3LE+ in Figure 3b occurs due to 5LE+ undergoing rapid dissociation during the ~3 ms required to eject the protonated 5LE+ from the ion cell. Ejection of 2LE+ virtually eliminates the peak corresponding to LE+ indicating that LE+ is not a significant primary fragment ion. A summary of the dissociation pathways of 7LE2+ deduced from these DR experiments is depicted in Scheme 1.
Scheme 1.
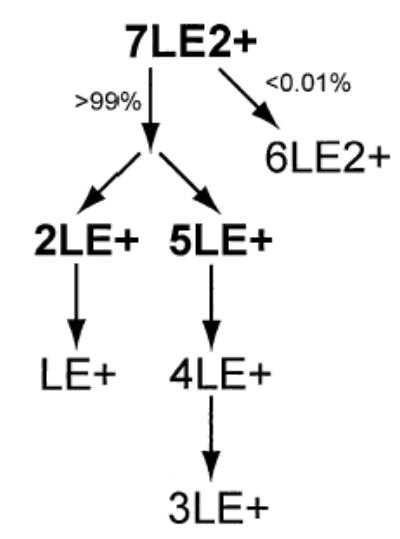
Leucine Enkephalin 9-Mer2+
A slight adjustment of the hexapole accumulation parameters and the voltage applied to the nanoelectrospray needle causes a dramatic shift in the relative peak intensities of the LE cluster ions, such that the abundance of 9LE2+ greatly exceeds that of 7LE2+ (Figure 2b). The isotopic distribution of 9LE2+ indicates the presence of 18LE4+ (Figure 2b inset). Both 9LE2+ and 18LE4+ are activated during SORI excitation and some products from dissociation of 18LE4+ are apparent as indicated by the appearance of 16LE3+ formed by the pathway 18LE4+ → 16LE3+ + 2LE+. Unlike 9LE2+, which dissociates into many fragments by multiple pathways, 18LE4+ appears to dissociate only by the ejection of 2LE+. The 16LE3+ does not appear to undergo further dissociation as DR ejection changes the appearance of other peaks in the spectrum very little (data not shown). It should be noted that the ratio of 9LE2+ and 18LE4+ varied during the DR experiment, resulting in a slight variation in the relative abundance of 16LE3+ to the other fragment clusters.
Unlike the 7LE2+, which dissociates almost entirely by fission, 9LE2+ dissociates by a combination of evaporation and fission processes. The dissociation spectrum of 9LE2+ (Figure 4a) shows that a large number of cluster fragments are formed. The most abundant of these is the 8LE2+/4LE+ peak. Although 8LE2+ and 4LE+ have the same nominal m/z, the isotopic distribution indicates that while both clusters are present, approximately 70% of the peak is 8LE2+, produced directly from 9LE2+ by the evaporation of neutral LE. A comparison of Figure 4a and e shows that double resonance ejection of 8LE2+ results in the elimination of 7LE2+ and 3LE+ and a decrease in the relative abundance, compared to the parent ion, of all other fragment clusters except for 16LE3+ and 7LE+. The abundance of 7LE2+ and 3LE+ during DR ejection of 8LE2+ indicates that 7LE2+ is formed by two consecutive losses of neutral LE from 9LE2+ and that 3LE+ is formed by neutral loss from 4LE+. The decrease in abundance of 5LE+ and 6LE+ during DR ejection of 8LE2+ indicates that after dissociation by the 8LE2+ → 2LE+ + 6LE+ pathway, 6LE+ can undergo neutral loss to form 5LE+. Note that the abundance of 6LE+ normalized to 9LE2+ is significantly attenuated by a DR pulse on 8LE2+ (Figure 4e) from the abundance of 6LE+ normalized to 9LE2+ in the regular activation spectrum (Figure 4a). It is evident that very little LE+ is formed directly from the 8LE2+ as the ejection of 2LE+ virtually eliminates LE+ (Figure 4h). DR ejection of 7LE+, 6LE+ and 5LE+ all cause decrease in signal and a change in the isotopic distribution for 8LE2+/4LE+ indicating that the 4LE+ in the 8LE2+/4LE+ peak is primarily produced by evaporation of neutral LE from larger singly charged clusters. DR ejection of 7LE2+ (Figure 4f) alters the relative abundance of the peaks in the spectra very little except for a small decrease in 5LE+ and 2LE+ suggesting that the 9LE2+ → 8LE2+ → 7LE2+ → (fragments) pathway is not a dominant process under the dissociation conditions used.
Figure 4.
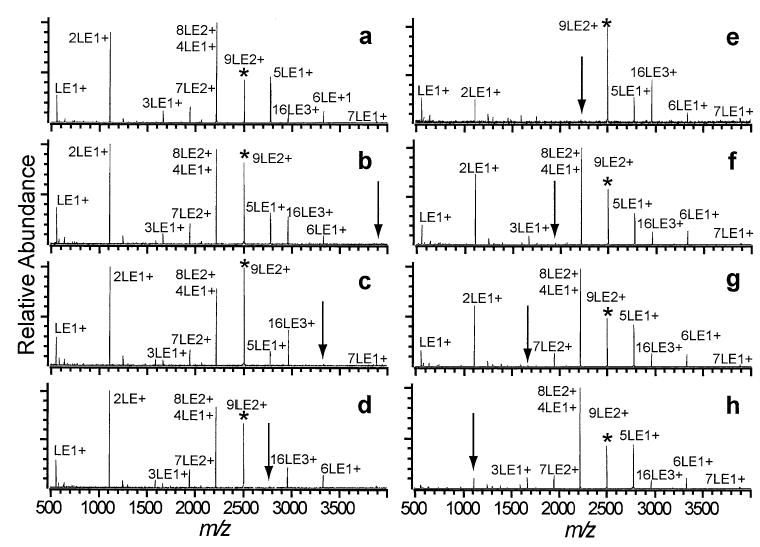
SORI-CAD spectra of the 9LE2+ cluster (a) without DR and with DR ejection applied at the m/z corresponding to the (b) 7LE+, (c) 6LE+, (d) 5LE+, (e) 8LE2+/4LE+, (f) 7LE2+, (g) 3LE+, and (h) 2LE+ clusters.
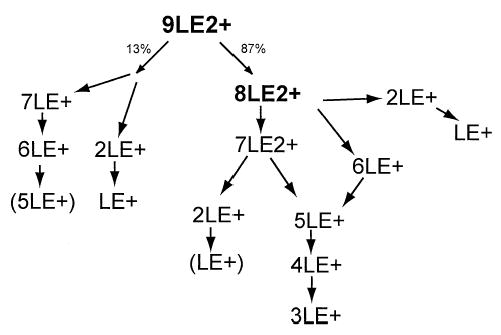
The relative branching ratios for the evaporation and fission dissociation processes were approximated by comparing the spectra for activation of 9LE2+ and DR ejection of 8LE2+ (Figure 4a, e). Specifically, the intensities of the fragment ions in Figure 4e were normalized to the parent ion and summed to represent the total fission process as any ions formed by LE evaporation from 9LE2+ would have been ejected by the DR waveform. The approximate percentage of fission was then determined by dividing the normalized intensity of all peaks resulting exclusively from fission of the parent ion (Figure 4e) by the total normalized intensity of all the fragment peaks in the initial activation spectrum (Figure 4a). The resulting quotient indicates ~13% dissociation by fission. This method slightly underestimates fission dissociation as a portion of the 4LE+ ion ejected during the DR pulse is formed by sequential evaporation of neutral LE from the primary fragment ion 7LE+. The 7LE+ ion is formed by the 9LE2+ → 2LE+ + 7LE+ process as indicated by the decrease in the 8LE2+/4LE+ peak when a DR waveform is applied to 7LE+ (Figure 4b). A summary of the dissociation pathways of 9LE2+ deduced from these DR experiments is presented in Scheme 2.
Scheme 2.
Leucine Enkephalin 11-Mer3+
The hexapole accumulation and nanoelectrospray voltage can be tuned to produce a high abundance of 11LE3+ and 15LE4+ (Figure 2c). The activation spectrum of 11LE3+ is much simpler than that for 9LE2+ (Figure 5a). As in the case of 7LE2+, the primary dissociation pathway is asymmetric fission, specifically the ejection of a proton-bound dimer, 2LE+, and complimentary 9LE2+. The subsequent dissociation of 9LE2+ by the evaporation of a neutral to form the 8LE2+ and by fission and subsequent evaporation to form 5LE+ and 4LE+ is consistent with the dissociation pathways of the 9LE2+ cluster discussed above. The peak labeled 10LE2+/5LE+ is identified as a combination of the two clusters by the isotopic distribution. The formation of 10LE2+ from 11LE3+ indicates that the 11LE3+ cluster also dissociates by a highly asymmetric fission process ejecting LE+. This is clearly a minor pathway with a branching ratio of no more than 2%, and is difficult to measure in spectra with poor signal to noise (as in Figure 5c, d). It should be noted that ideally, the relative peak intensity for Fourier-transform mass spectrometry is proportional to the charge of the ions under investigation. For example, an equal number of 9LE2+ and 2LE+ ions would produce a relative peak intensity ratio of 2:1 for 9LE2+ and 2LE+. Even considering the effect of charge, the sum of the intensities for the 2LE+ and LE+ is more than half of the sum of the intensities of the 9LE2+, 5LE+/10LE2+, and 8LE2+ peaks (Figure 5a). This indicates that there is a slight mass bias towards lower m/z ions.
Figure 5.
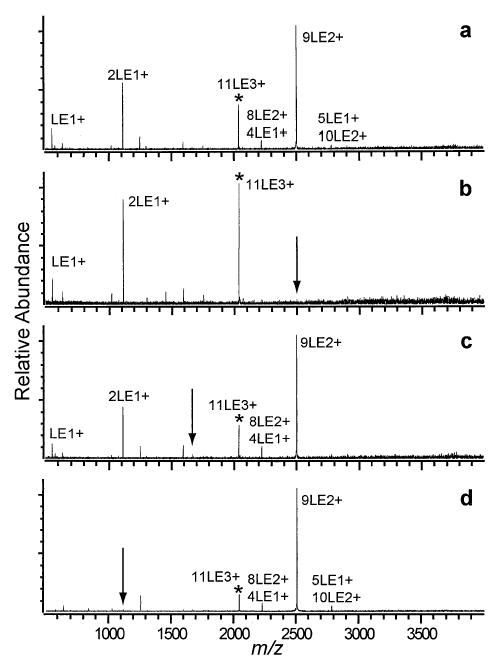
SORI-CAD spectra of the 11LE3+ cluster (a) without DR and with DR ejection applied at the m/z corresponding to the (b) 9LE2+, (c) 3LE+, and (d) 2LE+ clusters.
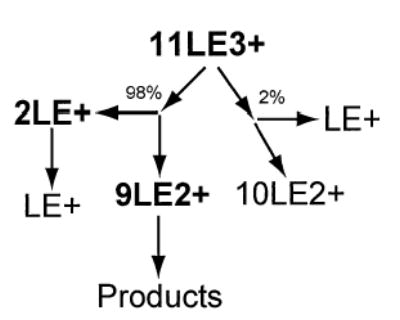
DR ejection of 9LE2+ eliminates 8LE2+ indicating that the same 9LE2+ → 8LE2+ + LE0 dissociation pathway measured for the 9LE2+ cluster occurs. Ejection of 9LE2+ may also decrease the relative abundance of 2LE+ slightly, suggesting that 2LE+ is formed from either 9LE2+ or 8LE2+, which is consistent with the DR experiments mentioned above (Figure 5b). As observed in the case of both 7LE2+ and 9LE2+, protonated LE is formed primarily from 2LE+ (Figure 5d). A summary of the dissociation pathways of 11LE3+ deduced from these DR experiments is presented in Scheme 3.
Scheme 3.
Leucine Enkephalin 15-Mer4+
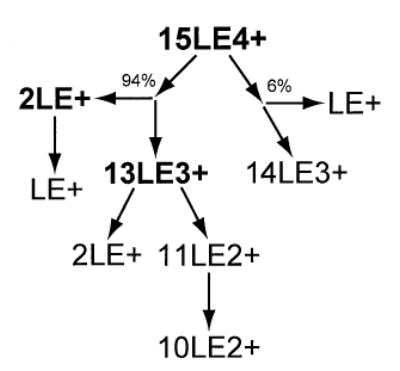
The 15LE4+ cluster is highly fissionable and difficult to isolate, as evidenced by a small amount of 13LE3+ formed by collisional excitation during the isolation procedure (Figure 6a). Activation of 15LE4+ results in a number of dissociation products from both fission and evaporation processes (Figure 6b). The primary dissociation process of 15LE4+ is the ejection of 2LE+ to form 13LE3+ which undergoes further dissociation by the ejection of 2LE+ to form 11LE2+ (Figure 6c). Formation of 10LE2+ occurs by the evaporation of a neutral LE from 11LE2+. This was confirmed by another isolation experiment showing that 11LE2+ dissociates by the evaporation of neutral LE to form 10LE2+ and also by a minor fission process ejecting 2LE+ (data not shown). The 15LE4+ precursor dissociates by another competing fission process as well, ejecting LE+ to form 14LE3+. This asymmetric dissociation process is also measured for 11LE3+ but has a higher branching ratio for 15LE4+ contributing ~6% to the total dissociation (Scheme 4).
Figure 6.
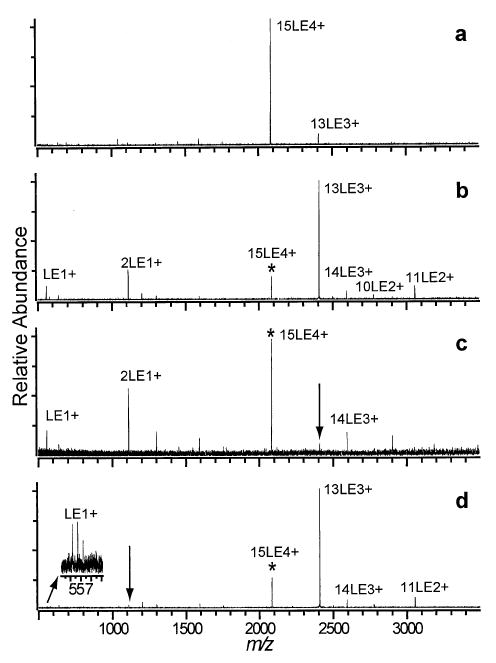
SORI-CAD spectra of the 15LE4+ cluster (a) isolation spectrum and activation spectra (b) without DR and with DR ejection applied at the m/z corresponding to the (c) 13LE3+ and (d) 2LE+ clusters.
Scheme 4.
Leucine Enkephalin 19-Mer5+
After further hexapole accumulation and nanoelectrospray voltage adjustment, it is possible to form large clusters, such as 19LE5+, with high abundance (Figure 7a). There is considerable variation in the abundance of 19LE5+ in the mass spectra with different pulled nanoelectrospray capillaries, and the relative abundance of 19LE5+ varied with time. Additional noise in the spectra is due to the installation of a resistance temperature device to measure the temperature of the vacuum chamber in the vicinity of the ion cell for another series of experiments (Figure 7b).
Figure 7.
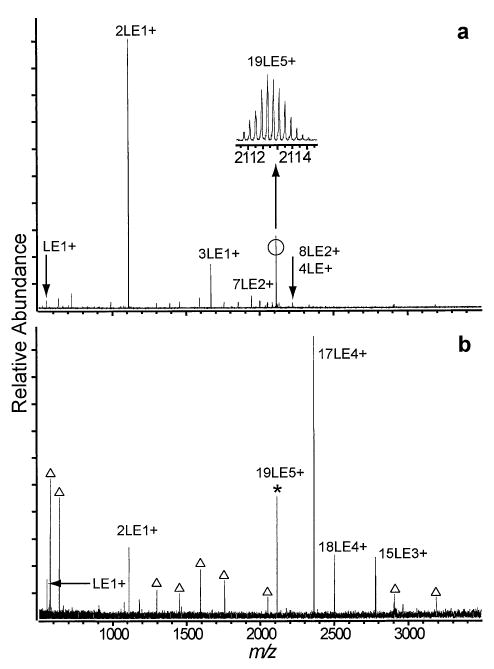
Nanoelectrospray mass spectra of 2.5 mM LE in 1:1 water:methanol + 2% acetic acid with electrospray voltage and hexapole accumulation parameters optimized for maximal ion intensity of the (a) 19LE5+ cluster and (b) a SORI-CAD spectrum of the isolated 19LE5+ cluster.
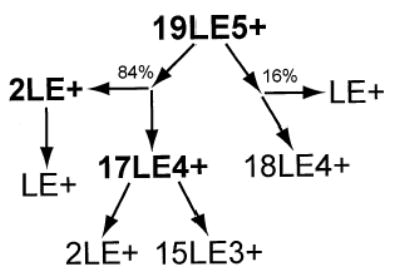
Although DR ejection was not done for 19LE5+, the dissociation pathways are easily interpreted and are consistent with those of the smaller LE clusters. The primary dissociation pathway for 19LE5+ is the ejection of 2LE+ to form 17LE4+. The 17LE4+ cluster undergoes fission by the ejection of another 2LE+ to form 15LE3+. Similar to the 15LE4+ and 11LE3+ cluster dissociation, ejection of LE+ from 19LE5+ competes with ejection of 2LE+. This highly asymmetric process is increasingly dominant with 19LE5+, and constitutes approximately 16% of the total dissociation. The proposed dissociation pathways are presented in Scheme 5.
Scheme 5.
Somatostatin 5-Mer4+ Clusters
To determine the effect of monomer flexibility on the peptide dissociation process, peptide clusters were formed with the intramolecular disulfide bond of ST either oxidized or reduced. This also provided an opportunity to examine the dissociation pathways of a peptide with a higher charge-to-subunit ratio. A smaller range of cluster sizes are formed for ST than for LE, and these experiments focus exclusively on the [somatostatin5 + 4H]4+ (5ST4+) cluster (Figure 8a, b). The native form of the peptide has a disulfide bond between residues 3 and 14. Intramolecular disulfide bonds are important in determining both the gas-phase structure of somatostatin [53] and gas-phase conformational flexibility [36]. The addition of DTT into ST solutions resulted in a reduction of an average of 4 out of 5 disulfide bonds present in each cluster as indicated by the shift in mass of two Da for each disulfide bond reduced (data not shown).
Figure 8.
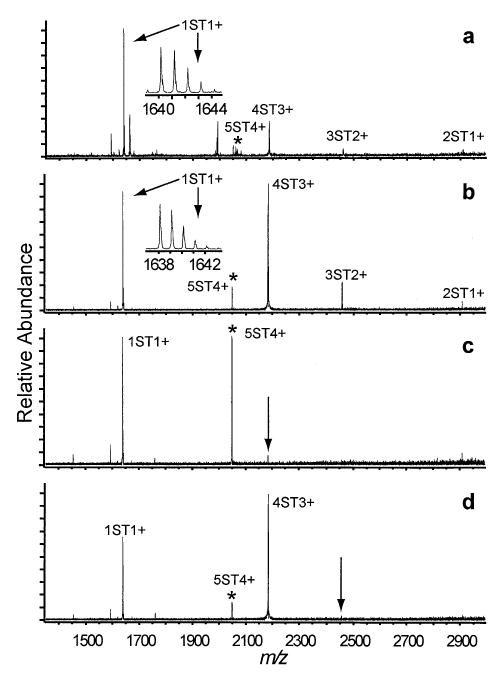
SORI-CAD spectra of the 5ST4+ with its intramolecular disulfide bonds (a) reduced and (b) oxidized and with DR ejection applied during activation of oxidized 5ST4+ at the m/z corresponding to (c) 4ST3+ and (d) 3ST2+. Inset spectra in (a) and (b) are transformed from apodized data to show the absence of higher order/charged clusters at these m/z values.
Upon SORI activation, oxidized 5ST4+ clusters dissociate by competing processes of neutral monomer evaporation and fission by the ejection of a protonated monomer (Figure 8b). Following disulfide bond reduction, identical dissociation pathways are measured (Figure 8a), but a different branching ratio is observed. The 4ST3+ fission product undergoes secondary dissociation by competing fission and evaporation processes. Subsequent sequential fission by the ejection of protonated monomers form 3ST2+ (Figure 8c) and 2ST+ (Figure 8d). The intensity of the ST+ peak is much higher than would be expected if its formation was due exclusively to fission of 5ST4+ as the charge-normalized ST+ peak would be expected to be roughly 1/3 the intensity of 4ST3+. The high abundance of ST+ indicates that a fraction of these ions must be formed by other dissociation pathways. The DR experiments show that the following processes occur: 5ST4+ → 4ST4+ + ST0, 4ST3+ → 3ST3+ + ST0, and 3ST2+ → 2ST2+ + ST0. The product cluster ions formed by these reactions, 4ST4+, 3ST3+, and 2ST2+, must completely dissociate into singly charged monomers. An analysis of the isotopic distribution of ST+ formed by the dissociation of 5ST4+ shows that higher order aggregates of ST+ are not detectable, so they constitute no more than 0.5% of the peak intensity of ST+. DR experiments demonstrate that the dissociation pathways of reduced 5ST4+ are the same as those of the corresponding oxidized cluster (data not shown).
Discussion
Structure of Peptide Clusters
An important question in cluster experiments with biological molecules is whether structure can be deduced from dissociation experiments. While it does not seem possible to extract a detailed analysis of the structure of leucine enkephalin (LE) and somatostatin (ST) clusters from the MS/MS and DR results mentioned above, it seems reasonable to suggest some general characteristics of the clusters in question based on the dissociation products and pathways of LE and ST.
It seems that the stability of the proton-bound dimer (2LE+) is the driving force behind the dissociation processes for the LE clusters studied. Ejection of 2LE+ is by far the most dominant fission product for all multiply charged LE clusters investigated, and for larger clusters, such as the 15LE4+ and 19LE5+, the ejection of multiple, consecutive proton-bound dimers is measured. Exceptional stability of LE proton-bound dimers has previously been observed by Schnier et al. who measured the activation energy for dissociation of LE proton-bound dimers to be 1.6 ± 0.1 eV [39].
The ejection of proton-bound dimers and protonated monomers, and the absence of protonated trimers, seems to indicate that, unlike certain helical peptides [47, 54], a maximum of two LE monomers are able to solvate a proton. A possible test to confirm this would be to measure the activation energy of neutral loss from protonated LE trimers and tetramers and compare the values with the activation energy of a proton bound dimer. If the activation energy of protonated trimers and tetramers is substantially less than 1.6 eV, the third LE peptide in the cluster is probably not interacting directly with the proton. This value would also provide an estimate of the activation energy for evaporation of a LE monomer from a cluster.
It was shown previously that conformational flexibility plays a major role in the dissociation of protein homodimers [36]. The dissociation pathways of oxidized and reduced 5ST4+ are identical although the branching ratios between fission and evaporation are different, with the reduced 5ST4+ having a higher probability of neutral ST evaporation. This indicates the conformational flexibility of subunits in the cluster plays a role in ST cluster dissociation.
Cluster Dissociation and the Liquid-Drop Model
Probably the most widely used model to explain the dissociation of multiply charged clusters is the liquid-drop model originally formulated by Lord Rayleigh [55] and later refined and extended for nuclear fission [42]. In the liquid-drop model, a cluster is treated as an incompressible fluid in which individual monomer units are bound together by surface tension arising from short-range, and even nearest-neighbor, attractive interactions. Long-range coulombic repulsion between like charges within the cluster cause surface distortions that can lead to spontaneous fission, provided the cluster has sufficient charge or temperature [55]. In the liquid-drop model, large clusters of low charge have a high barrier to spontaneous fission and tend to dissociate by the evaporation of neutral components. As a cluster with constant charge decreases in size, the barrier towards spontaneous fission tends to decrease, eventually becoming competitive with the evaporation energy, and the cluster undergoes charge separation by fission [6].
The liquid-drop model has been adapted by various researchers to explain the dissociation processes of multiply charged systems as diverse as atomic nuclei [42], van der Waals clusters [56], and alkali [37, 57, 58] and noble metal clusters [5, 6]. The analogy between these clusters and liquid drops is justified by the cluster’s approximately constant densities and fixed boundaries [6]. Clusters of the amino acids arginine [18] and serine [20, 27] have also been measured to undergo dissociation by neutral evaporation and charge separation by fission, although the liquid-drop model was not applied to these clusters.
The dissociation processes of multiply charged noble metal clusters [5–8, 59] is empirically similar to the dissociation of LE. In noble metal cluster dissociation, evaporation of a neutral metal atom competes with asymmetric fission, which is typically the ejection of a positively charged trimer or other small species. The observed fission products are known to have enhanced stability due to electronic shell effects [60, 61]. In metal cluster dissociation, the dominant dissociation process is determined by the charge of the cluster and the number of monomer units composing the cluster. Saunders showed that the log of the fission and evaporation ratio varies linearly with z2/n, where z is the charge of the cluster and n is the number of monomer units in the cluster [5, 6]. This relation is also found for nuclear decay [43], leading Saunders to suggest that similar physical principles govern the behavior of multiply charged metal clusters and atomic nuclei [6].
The results of LE cluster dissociation presented in this study qualitatively resemble the dissociation of metal clusters and atomic nuclei. Instead of the evaporation of a neutral metal ion or a neutron, LE clusters evaporate a neutral peptide, and instead of undergoing charge separation by nuclear fission or the ejection of a metal trimer, LE clusters eject a proton-bound dimer or protonated monomer. The variation of the branching ratios of the various dissociation pathways of LE clusters with z2/n is presented in Figure 9a. The primary dissociation pathway of LE clusters is neutral evaporation at low values of z2/n. For example, 9LE2+, which has a z2/n value of 0.44, dissociates by ~87% evaporation and ~13% fission. In contrast, 8LE2+, which has a z2/n value of 0.5 dissociates by ~6% evaporation and ~94% fission. At the highest values of z2/n in Figure 9a, corresponding to 15LE4+ and 19LE5+ with z2/n values of 1.07 and 1.32, respectively, an additional fission pathway consisting of the ejection of protonated LE monomer occurs with a branching ratio as high as 16% for 19LE5+.
Figure 9.
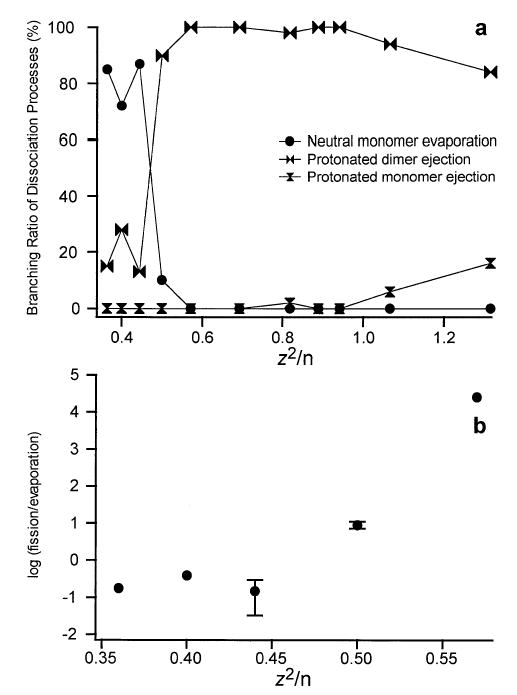
The dissociation pathways of LE clusters as a function of z2/n where z is the charge and n is the cluster number for (a) the percentage of evaporation, fission by the ejection of 2LE+ and fission by the ejection of LE+ produced by all clusters studied and (b) the log of the fission to evaporation ratio for the doubly protonated clusters studied. Error bars representing ± 1 σ are included in (b) for two data points.
The most striking feature of Figure 9a is the sharp transition that occurs between dissociation primarily by evaporation of neutral LE to dissociation primarily by the ejection of a proton-bound dimer at a z2/n value ~0.47. Similar sharp transitions between dissociation primarily by evaporation to dissociation primarily by fission have been measured for clusters of other materials. For doubly charged sodium clusters with thirty atoms, the transition occurs at a z2/n ~0.13 [37], doubly charged gold clusters with fourteen atoms at a z2/n ~0.29 [5, 6], doubly protonated arginine clusters with 12 amino acids [25] which corresponds to a z2/n value of ~0.33, and uranium nuclei with 234 nucleons at a z2/n of 36.2 [43]. For doubly charged metal clusters the z2/n value corresponding to the crossover value between dissociation primarily by evaporation and primarily by fission, is expected to correlate with the surface tension of the metal, where higher z2/n values correspond to higher surface tensions [6]. Correlating trends in the crossover between dissociation by evaporation and fission to other materials, such as peptides or amino acids, is problematic due to the significant difference in both the size and charge density of the clusters. An interesting test of the application of the liquid drop model to amino acid and peptide clusters would be to measure the z2/n values for the crossover in dissociation from primarily evaporation to fission for a series of clusters with the same charge and similar cross sections. A correlation between the z2/n value of the crossover between evaporation and fission to the activation energy of dissociation would provide supporting evidence for the utility of applying the liquid-drop model to amino acid and peptide clusters.
Although the application of the liquid-drop model to qualitatively describe the dissociation processes of LE clusters is intriguing, some significant deviations from the model occur. According to the liquid-drop model of cluster dissociation, the barrier for cluster fission decreases rapidly, while the evaporation energy for neutral loss remains relatively constant with changing z2/n. For a series of doubly charged LE clusters with different numbers of monomers and z2/n values ranging from 0.36 to 0.57, the fission barrier decreases as the value of z2/n increases. At z2/n values were the fission barrier is comparable to the evaporation energy, fission is competitive with evaporation. This is well illustrated for doubly charged gold clusters [5, 6] and isotopes of uranium nuclei [43] by linear increases in plots of the log(fission/evaporation) with increasing z2/n for these clusters. A similar plot for doubly charged LE clusters is presented in Figure 9b. For z2/n values of 0.44 to 0.57, the increase in log(fission/evaporation) is very rapid, while at lower z2/n values, the fission/evaporation ratio tends to level off to a value of 10 –20% evaporation by fission. A similar leveling off of the fission/evaporation ratio to a value of 10 –20% fission was measured for doubly charged clusters of antimony [38]. Brechignac et al. explained this result by suggesting that instead of continuing to increase with increasing cluster size, the fission barrier height evolves in such a way as to stay competitive with evaporation as the cluster size increases [38]. The interpretation of the LE cluster dissociation results would be greatly facilitated by the measurement of the activation energies of evaporation and fission for these clusters as has been reported for multiply charged silver clusters [62].
The liquid-drop model has been used previously to describe the asymmetric dissociation and charge partitioning of tetrameric streptavidin [30]. Homogeneous protein complexes of 2–6 monomer units have been shown to dissociate primarily by the ejection of a single subunit that removes 30 –50% of the total charge of the complex [29 –33, 35]. Schwartz et al. suggested that treating the protein complex as a liquid drop would explain some of the preferential charging for the ejected subunit as the surface-area-to-mass of the ejected subunit would be greater than that of the remaining cluster [30]. In the same paper, Schwartz et al. noted that the liquid-drop model could not account quantitatively for the magnitude of the charge enrichment of the leaving subunit. Subsequent work with protein homodimers has shown that other factors, such as protein conformational flexibility, the charge state of the protein complex, and the initial conformation of the protein complex play a dominant role in the dissociation pathways of protein homodimers [36]. These properties are not represented in the standard liquid-drop model and lead to significant differences between the dissociation of protein complexes and gold clusters.
Some of these same factors, such as conformational flexibility and multiple charging, of peptides within the cluster may be expected to cause deviations in cluster dissociation from the predictions of the liquid-drop model. Deviations from the liquid-drop model due to conformational flexibility are indicated by differences in the branching ratios for evaporation and fission in the dissociation of oxidized and reduced 5ST4+ (Figure 8a, b). It is interesting that the application of a model as simple as the liquid-drop model to clusters of molecules as complex as amino acids and peptides yields any useful predictions or trends. As it is, results of the DR experiments of these peptide clusters suggest that the dissociation pathways of LE clusters, and to a lesser extent ST clusters, can be described reasonably well by a simple model where dissociation is determined primarily by the collective properties of cluster size and charge.
The Use of Double Resonance in Dissociation Experiments
Without the use of DR, the interpretation of dissociation spectra can be quite challenging. The dissociation spectrum of 9LE2+ (Figure 4a) has a large number of dissociation products with very different peak intensities for complimentary fragment ions. DR revealed that there are actually only two significant dissociation pathways and that the additional fragment ion peaks and seemingly uncorrelated peak intensities arise from subsequent dissociation. Determining dissociation pathways without the use of DR is challenging, but has been done nicely in some instances. For example, Cooks and coworkers studied the dissociation of serine octamers as a function of collision energy and observed that at gentle dissociation energies, protonated serine hexamer is the first fragment ion to appear with protonated pentamers and tetramers appearing at successively higher collision energies [27]. These results strongly suggest that protonated serine pentamers and hexamers are formed by sequential evaporation of neutral serine. In many other instances, it is extremely difficult to distinguish between primary and secondary fragment ions in dissociation spectra. These results clearly show the advantages of using DR wherever feasible in the study of cluster dissociation.
Conclusions
Cluster dissociation has been studied for a variety of atomic and molecular species, such as atomic nuclei, solvent microdroplets, metal clusters and amino acids, with an aim to both understand fundamental cluster properties and to “bridge-the-gap” between bulk and molecular properties. In this study, we extend the field of cluster dissociation to peptides by elucidating the dissociation pathways of leucine enkephalin (LE) clusters with 2–5 charges and multiply protonated somatostatin (ST) clusters formed with the peptide disulfide bonds either broken or intact. The peptide clusters were dissociated by collisional activation and the dissociation pathways were elucidated by double resonance experiments.
Clusters of both LE and ST dissociate by competing pathways of the evaporation of neutral peptides and coulombically driven fission. In the case of doubly protonated LE clusters, the significant parameter that determines the branching ratio of evaporation and fission is z2/n, where z is the cluster charge and n is the number of monomer units in the cluster. Low values of z2/n correlate to dissociation primarily by neutral evaporation while high values correlate to dissociation primarily by a fission process in which a LE proton-bound dimer is ejected. A sharp transition between dissociation primarily by evaporation and primarily by fission occurs at a z2/n value of ~0.47 for doubly protonated LE clusters. LE clusters with more than two protons dissociate by fission for the z2/n values examined in these experiments. The dominant cluster fission process is the consecutive ejection of proton-bound dimers although significant amounts of protonated monomers are also observed in the fission processes of clusters with the highest z2/n values studied. Fission results in the ejection of either protonated monomers or dimers indicating that no more than two LE peptides solvate a proton in these clusters. For ST pentamers with four protons, similar competing pathways for evaporation and fission by the ejection of protonated monomer are observed. Breaking the intermolecular disulfide bond of each peptide in the cluster changes the relative contributions of the two pathways, but no new pathways are observed.
The observation of two competing dissociation processes, with their relative abundances correlated to their z2/n values, shows that the dissociation of LE clusters bears a qualitative similarity with nuclear and metal cluster dissociation. This similarity strongly suggests that the dissociation process of the LE clusters is determined by bulk properties of the cluster, namely the cluster charge state and size. Molecular properties do play at least some roll in the dissociation of peptide clusters as indicated by an increase in the branching ratio for ST cluster dissociation by evaporation when the molecular gas-phase conformational flexibility is increased.
The LE and ST clusters examined in this study are nonspecific clusters, meaning that they do not have a physiologically relevant cluster structure in solution and show no evidence of magic number clustering in the gas phase. The branching ratios for the dissociation pathways of these clusters seem to be determined by the cluster size and charge as well as the product ion stability. These are the same factors that influence the dissociation of multiply charged clusters of gold atoms. It would be interesting to compare these results to those obtained for conformationally specific clusters where the specific conformations of these clusters may play a very significant role in determining their dissociation pathways.
Acknowledgments
A transmitter board and HI-222 chip were generously provided to the authors by Bruker Daltonics (Billerica, MA). The authors would like to thank Dr. Michael Leavell (Sandia National Laboratory, Livermore, CA), Dr. Julie Leary (University of California, Berkeley), and Mr. Scott Daniels and Dr. Christian Berg (Bruker Daltonics, Billerica, MA) for their assistance in modifying the mass spectrometer for double resonance experiments. Funding for this work was provided by the National Institutes of Heath (grant R01-GM64712-01).
References
- 1.Stace AJ. Metal Ion Solvation in the Gas Phase: The Quest for Higher Oxidation States. J Phys Chem A. 2002;106:7993–8005. [Google Scholar]
- 2.Taflin DC, Ward TL, Davis EJ. Electrified Droplet Fission and the Rayleigh Limit. Langmuir. 1989;5:376 –384. [Google Scholar]
- 3.Grimm RL, Beauchamp JL. Evaporation and Discharge Dynamics of Highly Charged Droplets of Heptane, Octane, and p-Xylene Generated by Electrospray Ionization. Anal Chem. 2002;74:6291–6297. doi: 10.1021/ac025889b. [DOI] [PubMed] [Google Scholar]
- 4.Sattler K, Muhlbach J, Echt O, Pfau P, Recknagel E. Evidence for Coulomb Explosion of Doubly Charged Micro-clusters. Phys Rev Lett. 1981;47:160 –163. [Google Scholar]
- 5.Saunders WA. Fission and Liquid-Drop Behavior of Charged Gold Clusters. Phys Rev Lett. 1990;64:3046 –3049. doi: 10.1103/PhysRevLett.64.3046. [DOI] [PubMed] [Google Scholar]
- 6.Saunders WA. Metal-Cluster Fission and the Liquid-Drop Model. Phys Rev A. 1992;46:7028 –7041. doi: 10.1103/physreva.46.7028. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler J, Dietrich G, Kruckeberg S, Lutzenkirchen K, Schweikhard L, Walther C. Multicollision-Induced Dissociation of Multiply Charged Gold Clusters, Au-n(2+), n = 7–35, and Au-n(3+), n = 19 –35. Int J Mass Spectrom. 2000;202:47–54. [Google Scholar]
- 8.Kruckeberg S, Schweikhard L, Dietrich G, Lutzenkirchen K, Walther C, Ziegler J. Decay Pathway Determination of Even-Size Dicationic Silver Clusters: Ag-16(2+) and Ag-18(2+) Revisited by Pre-Precursor Selection and Sequential Decay. Chem Phys. 2000;262:105–113. [Google Scholar]
- 9.Blades AT, Jayaweera P, Ikonomou MG, Kebarle P. Ion-Molecule Clusters Involving Doubly Charged Metal-Ions (M2+) Int J Mass Spectrom Ion Processes. 1990;102:251–267. [Google Scholar]
- 10.Rodriguez-Cruz SE, Jockusch RA, Williams ER. Hydration Energies of Divalent Metal Ions, Ca2 + (H2O)(n) (n = 5–7) and Ni2 + (H2O)(n) (n = 6 –8), obtained by blackbody infrared radiative dissociation. J Am Chem Soc. 1998;120:5842–5843. doi: 10.1021/ja980716i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker NR, Wright RR, Barran PE, Cox H, Stace AJ. Unexpected stability of Cu·Ar (2+), Ag·Ar (2+), Au·Ar (2+), and their larger clusters. J Chem Phys. 2001;114:5562–5567. [Google Scholar]
- 12.Ditmire T, Tisch JWG, Springate E, Mason MB, Hay N, Marangos JP, Hutchinson MHR. High Energy Ion Explosion of Atomic Clusters: Transition from Molecular to Plasma Behavior. Phys Rev Lett. 1997;78:2732–2735. [Google Scholar]
- 13.Kumarappan V, Krishnamurthy M, Mathur D. Asymmetric High-Energy Ion Emission from Argon Clusters in Intense Laser Fields. Phys Rev Lett. 2001;87 doi: 10.1103/PhysRevLett.87.085005. article no. 085005. [DOI] [PubMed] [Google Scholar]
- 14.Snyder EM, Wei S, Purnell J, Buzza SA, Castleman AW. Femtosecond Laser-Induced Coulomb Explosion of Ammonia Clusters. Chem Phys Lett. 1996;248:1–7. [Google Scholar]
- 15.Card DA, Wisniewski ES, Folmer DE, Castleman AW. Dynamics of Coulomb Explosion and Kinetic Energy Release in Clusters of Heterocyclic Compounds. J Chem Phys. 2002;116:3554 –3567. [Google Scholar]
- 16.Pruvost L, Serre I, Duong HT, Jortner J. Expansion and Cooling of a Bright Rubidium Three-Dimensional Optical Molasses. Phys Rev A. 2000;6105:053408. [Google Scholar]
- 17.Zhan DL, Rosell J, Fenn JB. Solvation Studies of Electrospray Ions—Method and Early Results. J Am Soc Mass Spectrom. 1998;9:1241–1247. [Google Scholar]
- 18.Zhang DX, Wu LM, Koch KJ, Cooks RG. Arginine Clusters Generated by Electrospray Ionization and Identified by Tandem Mass Spectrometry. Eur Mass Spectrom. 1999;5:353–361. [Google Scholar]
- 19.Lee SW, Beauchamp JL. Fourier Transform Ion Cyclotron Resonance Study of Multiply Charged Aggregates of Small Singly Charged Peptides Formed by Electrospray Ionization. J Am Soc Mass Spectrom. 1999;10:347–351. [Google Scholar]
- 20.Julian RR, Hodyss R, Kinnear B, Jarrold MF, Beauchamp JL. Nanocrystalline Aggregation of Serine Detected by Electrospray Ionization Mass Spectrometry: Origin of the Stable Homochiral Gas-Phase Serine Octamer. J Phys Chem B. 2002;106:1219 –1228. [Google Scholar]
- 21.Counterman AE, Valentine SJ, Srebalus CA, Henderson SC, Hoaglund CS, Clemmer DE. High-Order Structure and Dissociation of Gaseous Peptide Aggregates that are Hidden in Mass Spectra. J Am Soc Mass Spectrom. 1998;9:743–759. doi: 10.1016/S1044-0305(98)00052-X. [DOI] [PubMed] [Google Scholar]
- 22.Counterman AE, Clemmer DE. Magic Number Clusters of Serine in the Gas Phase. J Phys Chem B. 2001;105:8092–8096. [Google Scholar]
- 23.Counterman AE, Hilderbrand AE, Barnes CAS, Clemmer DE. Formation of Peptide Aggregates During ESI: Size, Charge, Composition, and Contributions to Noise. J Am Soc Mass Spectrom. 2001;12:1020 –1035. [Google Scholar]
- 24.Guevremont R, Purves RW. High Field Asymmetric Waveform Ion Mobility Spectrometry-Mass Spectrometry: An Investigation of Leucine Enkephalin Ions Produced by Electrospray Ionization. J Am Soc Mass Spectrom. 1999;10:492–501. doi: 10.1016/S1044-0305(99)00016-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhang, D. X.; Koch, K. J.; Tao, A.; Cooks, R. G. Clustering of Amino Acids in the Gas Phase by Electrospray Ionization Mass Spectrometry. Proceedings of the 48th ASMS Conference on MS and Allied Topics; Long Beach, CA, June, 2000.
- 26.Koch KJ, Gozzo FC, Zhang DX, Eberlin MN, Cooks RG. Serine Octamer Metaclusters: Formation, Structure Elucidation, and Implications for Homochiral Polymerization. Chem Commun. 2001:1854 –1855. doi: 10.1039/b107148n. [DOI] [PubMed] [Google Scholar]
- 27.Cooks RG, Zhang DX, Koch KJ, Gozzo FC, Eberlin MN. Chiroselective Self-Directed Octamerization of Serine: Implications for Homochirogenesis. Anal Chem. 2001;73:3646 –3655. doi: 10.1021/ac010284l. [DOI] [PubMed] [Google Scholar]
- 28.Koch KJ, Gozzo FC, Nanita SC, Takats Z, Eberlin MN, Cooks RG. Chiral Transmission Between Amino Acids: Chirally Selective Amino Acid Substitution in the Serine Octamer as a Possible Step in Homochirogenesis. Angew Chem Int Ed. 2002;41:1721–1724. doi: 10.1002/1521-3773(20020517)41:10<1721::aid-anie1721>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Light-Wahl KJ, Schwartz BL, Smith RD. Observation of the Noncovalent Quaternary Associations of Proteins By Electrospray Ionization Mass Spectrometry. J Am Chem Soc. 1994;116:5271–5278. doi: 10.1016/1044-0305(94)85034-8. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz BL, Bruce JE, Anderson GA, Hofstadler SA, Rockwood AL, Smith RD, Chilkoti A, Stayton PS. Dissociation of Tetrameric Ions of Noncovalent Streptavidin Complexes Formed by Electrospray Ionization. J Am Soc Mass Spectrom. 1995;6:459 –465. doi: 10.1016/1044-0305(95)00191-F. [DOI] [PubMed] [Google Scholar]
- 31.Fitzgerald MC, Chernushevich I, Standing KG, Whitman CP, Kent SBH. Probing the Oligomeric Structure of an Enzyme by Electrospray Ionization Time-of-Flight Mass Spectrometry. Proc Natl Acad Sci USA. 1996;93:6851–6856. doi: 10.1073/pnas.93.14.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang ZG, Krutchinsky A, Endicott S, Realini C, Rechsteiner M, Standing KG. Proteasome Activator 11S REG or PA28: Recombinant REG αREG β Hetero-Oligomers are Heptamers. Biochemistry USA. 1999;38:5651–5658. doi: 10.1021/bi990056+. [DOI] [PubMed] [Google Scholar]
- 33.Felitsyn N, Kitova EN, Klassen JS. Thermal Decomposition of a Gaseous Multiprotein Complex Studied by Black-body Infrared Radiative Dissociation. Investigating the Origin of the Asymmetric Dissociation Behavior. Anal Chem. 2001;73:4647–4661. doi: 10.1021/ac0103975. [DOI] [PubMed] [Google Scholar]
- 34.Kitova EN, Bundle DR, Klassen JS. Thermal Dissociation of Protein–Oligosaccharide Complexes in the Gas Phase: Mapping the Intrinsic Intermolecular Interactions. J Am Chem Soc. 2002;124:5902–5913. doi: 10.1021/ja017213o. [DOI] [PubMed] [Google Scholar]
- 35.Hanson CL, Fucini P, Ilag LL, Nierhaus KH, Robinson CV. Dissociation of Intact Escherichia coli Ribosomes in a Mass Spectrometer—Evidence for Conformational Change in a Ribosome Elongation Factor g Complex. J Biol Chem. 2003;278:1259 –1267. doi: 10.1074/jbc.M208966200. [DOI] [PubMed] [Google Scholar]
- 36.Jurchen JC, Williams ER. Origin of Asymmetric Charge Partitioning in the Dissociation of Gas-Phase Protein Homodimers. J Am Chem Soc. 2003;125:2817–2826. doi: 10.1021/ja0211508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brechignac C, Cahuzac P, Carlier F, Defrutos M. Asymmetric Fission of Nan++ Around the Critical Size of Stability. Phys Rev Lett. 1990;64:2893–2896. doi: 10.1103/PhysRevLett.64.2893. [DOI] [PubMed] [Google Scholar]
- 38.Brechignac C, Cahuzac P, Carlier F, Defrutos M, Leygnier J, Roux JP. Coulombic Fission and Evaporation of Antimony Cluster Ions. J Chem Phys. 1995;102:763–769. [Google Scholar]
- 39.Schnier PD, Price WD, Strittmatter EF, Williams ER. Dissociation Energetics and Mechanisms of Leucine Enkephalin (M + H)(+) and (2M + X)(+) Ions (X = H, Li, Na, K, and Rb) Measured by Blackbody Infrared Radiative Dissociation. J Am Soc Mass Spectrom. 1997;8:771–780. doi: 10.1016/S1044-0305(97)84129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunimura M, Sakamoto S, Yamaguchi K. Alkali Metal-Mediated Proline Aggregation in Solution Observed by Cold-spray Ionization Mass Spectrometry. Org Lett. 2002;4:347–350. doi: 10.1021/ol017028f. [DOI] [PubMed] [Google Scholar]
- 41.Last I, Levy Y, Jortner J. Beyond the Rayleigh Instability Limit for Multicharged Finite Systems: From Fission to Coulomb Explosion. Proc Natl Acad Sci USA. 2002;99:9107–9112. doi: 10.1073/pnas.142253999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bohr N, Wheeler JA. The Mechanism of Nuclear Fission. Phys Rev. 1939;56:426 –450. [Google Scholar]
- 43.Halpern I. Nuclear Fission. Annu Rev Nucl Sci. 1959;9:245–341. [Google Scholar]
- 44.Versluis C, van der Staaij A, Stokvis E, Heck AJR, de Craene B. Metastable Ion Formation and Disparate Charge Separation in the Gas-Phase Dissection of Protein Assemblies Studied by Orthogonal Time-of-Flight Mass Spectrometry. J Am Soc Mass Spectrom. 2001;12:329 –336. doi: 10.1016/S1044-0305(00)00227-0. [DOI] [PubMed] [Google Scholar]
- 45.Rostom AA, Sunde M, Richardson SJ, Schreiber G, Jarvis S, Bateman R, Dobson CM, Robinson CV. Dissection of Multi-Protein Complexes Using Mass Spectrometry: Subunit Interactions in Transthyretin and Retinol-Binding Protein Complexes. Proteins. 1998;(Suppl 2):3–11. doi: 10.1002/(sici)1097-0134(1998)33:2+<3::aid-prot2>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 46.Kogan A, Ustyuzhanin P, Reuben BG, Lifshitz C. Hydtrogen/Deuterium Exchange of Monomers and Dimers of Leucine Enkephalin. Int J Mass Spectrom. 2002;213:1–4. [Google Scholar]
- 47.Kaleta DT, Jarrold MF. Noncovalent Interactions Between Unsolvated Peptides. J Phys Chem A. 2002;106:9655–9664. [Google Scholar]
- 48.Schalley CA, Weis P. Unusually Stable Magic Number Clusters of Serine with a Surprising Preference for Homochirality. Int J Mass Spectrom. 2002;221:9 –19. [Google Scholar]
- 49.Anders LR, Beauchamp JL, Dunbar RC, Baldeschwieler JD. Ion-Cyclotron Double Resonance. J Chem Phys. 1966;45:1062–1063. [Google Scholar]
- 50.Comisarow MB, Grassi V, Parisod G. Fourier-Transform Ion-Cyclotron Double-Resonance. Chem Phys Lett. 1978;57:413–416. [Google Scholar]
- 51.Asam MR, Glish GL. Tandem Mass Spectrometry of Alkali Cationized Polysaccharides in a Quadrupole Ion Trap. J Am Soc Mass Spectrom. 1997;8:987–995. [Google Scholar]
- 52.Gauthier JW, Trautman TR, Jacobson DB. Sustained Off-Resonance Irradiation for Collision-Activated Dissociation Involving Fourier-Transform Mass-Spectrometry—Collision-Activated Dissociation Technique that Emulates Infrared Multiphoton Dissociation. Anal Chim Acta. 1991;246:211–225. [Google Scholar]
- 53.Wang J, Cassady CJ. Effects of Disulfide Linkages on Gas-Phase Reactions of Small Multiply Charged Peptide Ions. Int J Mass Spectrom. 1999;182/183:233–241. [Google Scholar]
- 54.Kaleta DT, Jarrold MF. Peptide Pinwheels. J Am Chem Soc. 2002;124:1154 –1155. doi: 10.1021/ja012399c. [DOI] [PubMed] [Google Scholar]
- 55.Lord Rayleigh L. On the Equilibrium of Liquid Conducting Masses Charged with Electricity. Phil Mag. 1882;14:184 –186. [Google Scholar]
- 56.Echt O, Kreisle D, Recknagel E, Saenz JJ, Casero R, Soler JM. Dissociation Channels of Multiply Charged Vanderwaals Clusters. Phys Rev A. 1988;38:3236 –3248. doi: 10.1103/physreva.38.3236. [DOI] [PubMed] [Google Scholar]
- 57.Yannouleas C, Landman U, Brechignac C, Cahuzac P, Concina B, Leygnier J. Thermal Quenching of Electronic Shells and Channel Competition in Cluster Fission. Phys Rev Lett. 2002;89 doi: 10.1103/PhysRevLett.89.173403. article no. 173403. [DOI] [PubMed] [Google Scholar]
- 58.Saunders WA. Asymmetric Fission of Nan++ around the Critical Size of Stability—Comment. Phys Rev Lett. 1991;66:840 –840. doi: 10.1103/PhysRevLett.66.840. [DOI] [PubMed] [Google Scholar]
- 59.Naher U, Bjornholm S, Frauendorf S, Garcias F, Guet C. Fission of Metal Clusters. Phys Rep Rev Sec Phys Lett. 1997;285:245–320. [Google Scholar]
- 60.Knight WD, Clemenger K, Deheer WA, Saunders WA, Chou MY, Cohen ML. Electronic Shell Structure and Abundances of Sodium Clusters. Phys Rev Lett. 1984;52:2141–2143. [Google Scholar]
- 61.Nakamura M, Ishii Y, Tamura A, Sugano S. Shell Effects on Symmetrical Fragmentations of Alkali-Metal Clusters. Phys Rev A. 1990;42:2267–2278. doi: 10.1103/physreva.42.2267. [DOI] [PubMed] [Google Scholar]
- 62.Kruckeberg S, Dietrich C, Lutzenkirchen K, Schweikhard L, Walther C, Ziegler J. Fission Barriers of Doubly Charged Silver Clusters. Eur Phys J D. 1999;9:145–148. [Google Scholar]


