Abstract
The decrease in the sensitivity of electrospray ionization mass spectrometry caused by the presence of metal salts, such as sodium chloride, in the sample matrix is well known and is particularly problematic for biological samples. We report here that addition of high levels of ammonium acetate can improve analyte signal in aqueous electrospray solutions and counteracts the signal suppression caused by sodium chloride. A ~3-fold improvement in S/N is obtained by adding 8 M ammonium acetate to aqueous solutions of cytochrome c without added sodium chloride. No organic solvents or acids are added into the electrospray solutions. The signal-to-noise ratios of cytochrome c and ubiquitin (10−5 M) ions formed from aqueous solutions containing 2.0 × 10−2 M sodium chloride are improved by factors of ~7 and 11, respectively, by adding 7 M ammonium acetate to the solution. We propose that this effect is a result of the precipitation of Na+ and Cl− from solution within the evaporating electrospray droplets prior to the formation of gas-phase protein ions. This method is potentially useful for improving the abundance of protein ions formed from solutions in which the molecules have a nativelike conformation and is particularly advantageous for such solutions that have high levels of sodium.
Electrospray ionization (ESI)1 is invaluable for generating multiply charged molecular ions of large molecules, such as proteins and oligonucleotides, for molecular weight measurement by mass spectrometry (MS)2,3 and identification or structural characterization by tandem mass spectrometry (MS/MS).4–11 The multiple charging of analyte ions increases the effective mass range of mass spectrometers and promotes the formation of structurallyinformativefragmentionsduringMS/MSexperiments.4–11 Molecular weight measurements of proteins have been made using as little as ~10−18 mol of sample.12 However, the sensitivity of ESI-MS is typically lowered by the presence of salts, detergents, acids, and other species in the sample matrix.13–21 For example, Wang and Cole reported that the addition of 10−2 M cesium chloride to electrospray solutions of lysozyme (10−6 M) resulted in a 330-fold reduction in analyte ion abundance.15 Addition of 10−2 M ammonium acetate produced a 30-fold reduction in analyte ion abundance.15 The signal suppression caused by salts is particularly problematic for the analysis of biological samples where the physiological ionic strength is ~0.15 M.22
Several methods have been developed to counteract matrix effects in ESI-MS. The offending species can be removed from solution prior to ESI-MS using a variety of techniques, including liquid chromatography,23,24 ion exchange,25,26 solid-phase extraction,27,28 and dialysis.23,29 These sample cleanup methods can be automated and performed on-line, reducing the labor and time required for an analysis.23–29 The use of nanoelectrospray (na-noES)30 provides a substantial improvement in sensitivity for salt-containing solutions over that obtained with conventional ESI. For example, Karas and co-workers reported that the signal-to-noise ratios (S/Ns) of insulin (10−5 M) ions formed by nanoES from solutions containing 10−2 M sodium chloride were comparable to those of insulin ions formed by conventional ESI from solutions containing 10−3 M sodium chloride.31 Thus, nanoES improves the tolerance for salt-containing samples by 1 order of magnitude.31
It also has been demonstrated that signal suppression can be counteracted by the addition of certain organic solvents to electrospray solutions. For example, Apffel et al. reported that a 10–50-fold improvement in the abundance of peptide ions formed from solutions containing trifluoroacetic acid (TFA) is obtained by addition of a 3:1 mixture of propionic acid and 2-propanol.32 The authors proposed that “the process primarily responsible for signal suppression by strong acids such as TFA” is “ion-pair formation between analyte ions...and TFA anions,” and that propionic acid counteracts signal suppression by driving the equilibrium in solution toward neutral TFA, which then evaporates to some extent from the droplets prior to the formation of gas-phase analyte ions.32 The role of 2-propanol is as a “carrier solvent” that may also aid in generating a stable signal (the addition of 2-propanol alone and 3:1 propionic acid/2-propanol produced a 1.8- and 30-fold improvement in signal, respectively).32 Similarly, Yamaguchi et al. reported a 100-fold improvement in sensitivity for ions formed from solutions containing ammonium acetate and acetic acid, by addition of the low vapor pressure solvent 2-(2-methoxyethoxy)ethanol (2-MEE; bp 193 °C).33 The authors speculated that “the ion-suppression effect of acetate ion” is responsible in part for the poor ESI response obtained from ammonium acetate/acetic acid-containing solutions and that 2-MEE counteracts signal suppression by allowing acetic acid and water to preferentially evaporate from the electrospray droplets prior to ion formation.33
Here, we report that both the abundance and the reproducibility of ion signal of cytochrome c and ubiquitin ions formed by nanoES from aqueous solutions containing 0 or 2.0 × 10−2 M sodium chloride are significantly improved by adding 7 or 8 M ammonium acetate to electrospray solutions. We propose that this effect is due to the removal of Na+ and Cl− from solution by precipitation within the evaporating electrospray droplets prior to the formation of gas-phase protein ions. To the best of our knowledge, this is the first demonstration of an additive for counteracting signal suppression in ESI that is compatible with aqueous, nativelike electrospray solutions, i.e., solutions without added organic solvents or acids.
EXPERIMENTAL SECTION
Experiments are performed on a Fourier transform mass spectrometer equipped with a 9.4-T superconducting magnet and an external ESI source. This instrument is described elsewhere.34 Ions are generated by nanoelectrospray30 using needles that are made from 1.0-mm-o.d., 0.78-mm-i.d. borosilicate capillaries. These capillaries are pulled to a tip with an inner diameter of ~4 μm using a Flaming/Brown micropipet puller (model P-87, Sutter Instruments, Novato, CA). The electrospray is initiated by applying a potential of ~800–1400 V to a Pt wire (0.127-mm diameter, Aldrich, Milwaukee, WI) inserted into the nanoelectrospray needle to within ~2 mm of the tip. The wire and nanoelectrospray needle are held in place with a patch clamp holder (WPI Instruments, Sarasota, FL). The flow rates are between 20 and 100 nL/min. No pneumatic assistance or back pressure is used. A new nanoelectrospray tip is used for each measurement to eliminate the possibility of cross-contamination. The electrospray voltage is carefully adjusted to optimize the abundance of analyte ions prior to recording a mass spectrum. The electrospray voltages for solutions containing 10−5 M cytochrome c and 0, 1.0 × 10−3, 1.0 × 10−1, or 8 M ammonium acetate are 1460 ± 70, 1300 ± 100, 920 ± 30, and 880 ± 60 V, respectively. The electrospray voltages for solutions containing 10−5 M cytochrome c, 2.0 × 10−2 M sodium chloride, and 0 or 7 M ammonium acetate are 1040 ± 180 and 860 ± 80 V, respectively. The electrospray voltages for solutions containing 10−5 M ubiquitin, 2.0 × 10−2 M sodium chloride, and 0, 1.0 × 10−1, or 7 M ammonium acetate are 1120± 120, 1060 ± 200, and 750 ± 70 V, respectively. The ions and droplets generated by nanoelectrospray are sampled from atmospheric pressure through a glass capillary. A countercurrent flow of heated nitrogen (120 °C) is used to promote evaporation of the electrospray droplets. Ions are accumulated in an external hexapole ion trap and gated into the cell. Ions are dynamically trapped in the cell using pulses of nitrogen (peak pressure ~(1–5) × 10−7 Torr). Cell pressures are monitored using an uncalibrated ion gauge located above one of the turbomolecular pumps.
Ammonium acetate (98.5%), sodium chloride (99.9%), and sodium acetate (99.5%) were obtained from Fisher Scientific (Fair Lawn, NJ). Ammonium chloride (99.5+%) was obtained from Aldrich. Equine cytochrome c (96%; 12 kDa) and bovine ubiquitin (>90%; 8.6 kDa) were obtained from Sigma (St. Louis, MO) and used without further purification. Eight replicate measurements were taken of aqueous electrospray solutions containing 10−5 M cytochrome c, 2.0 × 10−2 M sodium chloride, and 0 or 7 M ammonium acetate. Five replicate measurements were taken of aqueous electrospray solutions containing 10−5 M ubiquitin, 2.0 × 10−2 M sodium chloride, and 0, 1.0 × 10−1, or 7 M ammonium acetate. Three replicate measurements were taken of aqueous electrospray solutions containing 10−5 M cytochrome c, 0 M sodium chloride, and 0, 1.0 × 10−3, 1.0 × 10−1, or 8 M ammonium acetate. The pH of the 8 M ammonium acetate stock solution is ~7. The densities of saturated aqueous solutions of ammonium acetate, sodium acetate, and ammonium chloride were each measured in triplicate by weighing aliquots dispensed from a 2-mL volumetric pipet on an analytical balance. Water was used to calibrate the pipet. Experimental errors are reported as ± one standard deviation from the mean.
RESULTS AND DISCUSSION
Cytochrome c without Added Sodium Chloride.
The S/Ns of the most intense charge states of cytochrome c (10−5 M) formed from aqueous solutions containing 0, 1.0 × 10−3, and 1.0 × 10−1 M ammonium acetate (NH4Ac), the 8+, 8+, and 7+, respectively, do not significantly differ at the 95% confidence level (CL; Table 1). However, the S/N of the most intense charge state formed from 8 M NH4Ac, the 7+, is 2.7 times greater than that of the most intense charge state, the 8+, formed from 0 M NH4Ac. Thus, addition of 8 M NH4Ac results in a significant improvement in the sensitivity for cytochrome c ions formed from aqueous solutions. The increased analyte S/N reported here appears to be contrary to the results reported by Wang and Cole, who reported a 10–30-fold decrease in protein ion signal with the addition of up to 10−1 M NH4Ac.15 This discrepancy presumably stems from the use of nanoelectrospray in our experiments (flow rates of (2–10) × 10−8 L/min) versus the use of conventional electrospray (1.6 × 10−6 L/min)15 in the experiments of Wang and Cole (nanoelectrospray has a superior tolerance for solutions of high ionic strength).31
Table 1.
Average Signal-to-Noise Ratios of Charge States of Cytochrome c (10−5 M) Formed from Aqueous Solutions Containing 0, 1.0 × 10−3, 1.0 × 10−1, and 8 M Ammonium Acetatea
| [NH4Ac] (M) | 9+ | 8+ | 7+ | 6+ |
|---|---|---|---|---|
| 0 | 71 ± 25 | 446 ± 59 | 88 ± 7 | < 2 |
| 1.0 × 10−3 | 51 ± 7 | 427 ± 82 | 71 ± 25 | 4 ± 6 |
| 1.0 × 10−1 | < 2 | 155 ± 58 | 336 ± 33 | 58 ± 21 |
| 8 | < 2 | 379 ± 88 | 1228 ± 272 | 50 ± 30 |
For each solution, the signal-to-noise ratio of the charge state of highest abundance is in boldface type. Reported errors are ±1 standard deviation from the mean for three replicate measurements.
Representative mass spectra are shown in Figure 1. In the spectrum formed from 0 M NH4Ac, the maximum charge state, zMax, the charge state of highest abundance, zMain, and the minimum charge state, zMin, are 9+, 8+, and 7+, respectively (Figure 1a). Up to three adducted Na+ are observed for both the 9+ and 8+, and up to ~six adducted Na+ are observed for the 7+ (insets, Figure 1a). Sodium adducts account for 35 ± 5% of the total cytochrome c ion abundance, and the most intense peak in each charge state cluster is the nonadducted, protonated molecular ion, i.e., the (M + nH)n+. The spectrum obtained from 1.0 × 10−3 M NH4Ac exhibits the same zMax, zMain, and zMin (Figure 1b). With 1.0 × 10−1 M NH4Ac, the charge distribution is shifted to lower charge. The zMax, zMain, and zMin are 8+, 7+, and 6+, respectively, and the most intense peaks in the 6+ and 7+ peak groups correspond to adduction of one Na+ (Figure 1c). The lower charge could be due to competition for charge between the protein and ammonium/acetate or due to conformational effects at high ionic strength. Sodiated ions account for 71 ± 12% of the total analyte ion abundance in the spectrum, although the maximum extents of sodiation of the 8+ and 7+ charge states are essentially the same as those observed from 0 M NH4Ac. The higher abundance of sodium adducts observed with 1.0 × 10−1 M NH4-Ac presumably results from sodium impurities in the NH4Ac used in these experiments (the purity stated by the manufacturer is 98.5%). With 8 M NH4Ac, zMax, zMain, and zMin are the same as those obtained from 1.0 × 10−1 M NH4Ac; however, the abundance and maximum extent of adduction observed for all charge states are lower than those observed from the other solutions (Figure 1d). The most intense peak in each charge-state cluster is the protonated, nonadducted molecular ion. Remarkably, the abundances of sodium adducted ions are dramatically reduced. Some adducts of ammonia (17 Da) and acetic acid (60 Da) are observed, and these adducts account for 23 ± 4% of the analyte ion abundance (insets, Figure 1d), a percentage even lower than that of the sodium adducts formed from 0 M NH4Ac (35%; Figure 1a). Spectra obtained from 1 M NH4Ac are virtually identical to those obtained from 8 M NH4Ac, except that the former exhibit less acetic acid adduction than the latter (data not shown).
Figure 1.
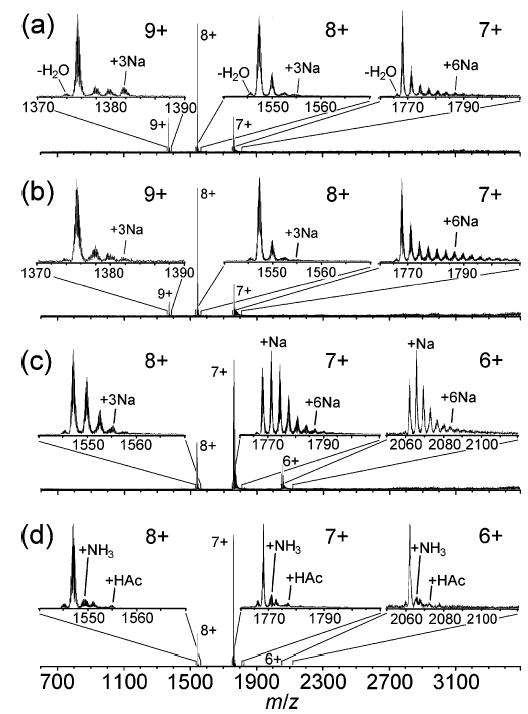
ESI mass spectra of cytochrome c (10−5 M) ions formed from aqueous solutions containing (a) 0, (b) 1.0 × 10−3, (c) 1.0 × 10−1, and (d) 8 M ammonium acetate. In the analyte ions that have sodium adducts, each sodium cation replaces a proton; i.e., the net increase in mass is 22 Da per sodium ion adducted.
Interestingly, sodium adduction often increases with decreasing charge state (insets, Figure 1a–c). For example, the proportion of analyte ion abundance due to sodium adducts of the 7+ exceeds that of the 8+ for ions formed from 0, 1.0 × 10−3, and 1.0 × 10−1 M NH4Ac by 83, 148, and 58% (relative), respectively (Table 2). For 0 M NH4Ac, the relative sodium adduct abundance for the 9+ is slightly higher than that of the 8+ (36 vs 29%); however; these values are not significantly different at the 95% confidence level. Karas and co-workers also noted a trend of increasing sodiation with decreasing charge state of protein ions formed by ESI, which they attributed to the different charge states being formed from droplets that have undergone different extents of evaporation.31 According to this mechanism, the observed degree of sodium adduction correlates with the sodium concentration within the droplets at the time of ion formation. For example, high charge states originate from the early stages of the chain of droplet evaporation/fission and thus are formed from droplets with sodium concentrations similar to that of the bulk electrospray solution. Low charge states are formed from droplets that have undergone more extensive evaporation and, consequently, have an increased sodium concentration.31
Table 2.
Percentages of Analyte Ion Abundance Due to Adduction for Charge States of Cytochrome c (10−5 M) Formed from Aqueous Solutions Containing Different Concentrations of Ammonium Acetatea
| [NH4Ac] (M) | 9+ | 8+ | 7+ | 6+ |
|---|---|---|---|---|
| 0 | 36 ± 8 | 29 ± 3 | 53 ± 8 | <DL |
| 1.0 × 10−3 | 25 ± 2 | 29 ± 6 | 72 ± 10 | <DL |
| 1.0 × 10−1 | <DL | 48 ± 15 | 76 ± 10 | 82 ± 5 |
| 8 | <DL | 20 ± 2 | 23 ± 5 | 26 ± 4 |
For 0, 1.0 × 10−3, and 1.0 × 10−1 M NH4Ac, the adducts are due to sodium (+22 Da; Na+ displaces H+). For 8 M NH4Ac, the adducts are due to ammonia (17 Da) and acetic acid (60 Da). Reported errors are ±1 standard deviation from the mean for three replicate measurements. <DL indicates that a given charge state is below detection limits.
Cytochrome c with Added Sodium Chloride.
The S/Ns of charge states of cytochrome c formed from aqueous solutions containing 2.0 × 10−2 M NaCl and 0 or 7 M NH4Ac are listed in Table 3. The S/N of the most intense charge state formed from 7 M NH4Ac, the 7+, is greater than that of the most intense charge state formed from 0 M NH4Ac, the 8+, by a factor of 6.7. Cytochrome c ions are below detection limits in three of the eight spectra collected from 0 M NH4Ac; however, these ions are present at S/N > 48 in all eight of the spectra obtained from 7 M NH4Ac (data not shown). Thus, addition of very high concentrations of NH4Ac to solutions containing NaCl can improve both the abundance and the reproducibility of analyte signal.
Table 3.
Signal-to-Noise Ratios of Charge States of Cytochrome c (10−5 M) Formed from Aqueous Solutions Containing 2.0 × 10−2 M Sodium Chloride and 0 or 7 M Ammonium Acetatea
| [NH4Ac] (M) | 8+ | 7+ | 6+ |
|---|---|---|---|
| 0 | 22 ± 21 | 12 ± 10 | < 2 |
| 7 | 24 ± 10 | 147 ± 84 | 5 ± 4 |
For each solution, the signal-to-noise ratio of the charge state of highest abundance is in boldface type. Reported errors are ±1 standard deviation from the mean for eight replicate measurements.
Representative mass spectra are shown in Figure 2. The spectrum obtained from 0 M NH4Ac (Figure 2a) is dominated by a series of cluster ions, Na(NaCl)n+ (n = 3–28), with an average mass, weighted by ion abundance, of 827 ± 94 Da. The peaks at m/z 257, 783, and 1309, which are markedly more intense than their immediate neighbors, correspond to magic numbers n = 4, 13, and 22, respectively, of Na(NaCl)n+. Magic numbers of sodium chloride cluster ions have been observed previously and correspond to clusters with special structural stability.35–38 Other series of cluster ions are interposed among Na(NaCl)n+ at lower abundance, including Na2(NaCl)m2+. In addition to sodium chloride cluster ions, cytochrome c charge states 8+ and 7+ are observed in five of the eight spectra obtained from this solution, at <3% of the total ion abundance (TIA). Peaks corresponding to the adduction of up to ~9 and up to ~14 Na+ to the 8+ and 7+ charge states of cytochrome c, respectively, are observed (Figure 2a). These extents of adduction are 3 and 2.3 times, respectively, as high as those observed from the corresponding solution without added NaCl (insets, Figure 1a). Interestingly, the maximum number of adducted Na+ observed for the 7+ is approximately equal to the number of acidic groups in the protein (13). In addition to the series of peaks corresponding to sodiated molecular ions, the 7+ also has a series of approximately five “haystacks” spaced 210–350 Da apart (7+ inset, Figure 2a). The spacing between the haystacks roughly corresponds to the mass of the magic number cluster Na(NaCl)4+ (257 Da), indicating that these haystacks represent cytochrome c ions with different numbers of attached sodium chloride clusters. These protein/sodium chloride clusters may be a result of sodium chloride crystallizing on the protein during desolvation.
Figure 2.
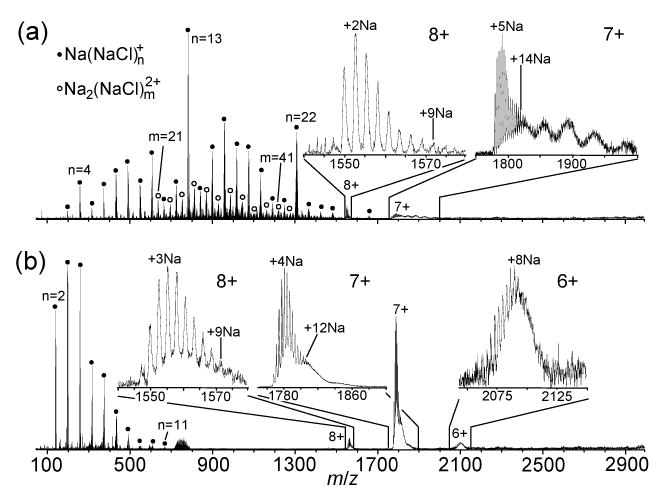
ESI mass spectra of cytochrome c (10−5 M) ions formed from aqueous solutions containing 2.0 × 10−2 M sodium chloride and (a) 0 or (b) 7 M ammonium acetate. Cluster ions Na(NaCl)n+ and Na2(NaCl)m2+ are indicated by closed circles and open circles, respectively.
With 7 M NH4Ac (Figure 2b), the distribution of Na(NaCl)n+ (n = 2–11) cluster ions is observed at a dramatically lower average mass, 272 ± 13 Da, compared to that observed from 0 M NH4Ac. Cytochrome c charge states are observed at ~10–50% TIA in all of the eight spectra taken from this solution (data not shown). The maximum observed extents of Na+ adduction of the 8+ and 7+ charge states of cytochrome c are approximately the same as those obtained from 0 M NH4Ac (insets, Figure 2a, b); however, the 7+ peak group does not contain a series of haystacks extending to m/z 2000. Instead, it has a shoulder that falls to the baseline by m/z ~1860 (7+ inset, Figure 2b), indicating a decreased extent of sodium chloride clustering on the protein relative to that observed from 0 M NH4Ac. The observed decrease in the mass and abundance of sodium chloride cluster ions and cytochrome c/sodium chloride cluster ions observed with the addition of 7 M NH4Ac could be due to a reduction of clustering in solution due to the high ionic strength or it could be due to incorporation of NH4+ and Ac− into the NaCl clusters at their early stages of formation. This could inhibit their subsequent growth or may significantly reduce their stability due to the very high volatility of NH4Ac. This result is also consistent with the partial removal of Na+ and Cl− by precipitation from solution prior to ion formation. The mechanism for this process is discussed in a subsequent section.
Ubiquitin with Added Sodium Chloride.
The S/Ns of ubiquitin ions formed from aqueous solutions containing 2.0 × 10−2 M NaCl and 0, 1.0 × 10−1, or 7 M NH4Ac are listed in Table 4. The most intense ubiquitin charge state formed from each solution is the 5+. The S/Ns of the 5+ formed from 0 and 1.0 × 10−1 M NH4Ac are not significantly different (95% CL); however, the S/N of the 5+ formed from 7 M NH4Ac is 11 times greater than that formed from 0 M NH4Ac (Table 4). Thus, as also demonstrated with cytochrome c, adding 7 M NH4Ac significantly improves the abundance of ubiquitin ions formed from aqueous solutions with 2.0 × 10−2 M NaCl.
Table 4.
Signal-to-Noise Ratios of Charge States of Ubiquitin (10−5 M) Formed from Aqueous Solutions Containing 2.0 × 10−2 M Sodium Chloride and 0, 1.0 × 10−1, or 7 M Ammonium Acetatea
| [NH4Ac] (M) | 6+ | 5+ |
|---|---|---|
| 0 | < 2 | 68 ± 131 |
| 1.0 × 10−1 | 3 ± 5 | 31 ± 50 |
| 7 | 28 ± 10 | 751 ± 167 |
For each solution, the signal-to-noise ratio of the charge state of highest abundance is in boldface type. Reported errors are ±1 standard deviation from the mean for five replicate measurements.
Representative mass spectra are shown in Figure 3. The spectrum obtained from 0 M NH4Ac exhibits the 5+ charge state of ubiquitin with varying extents of adduction and cluster ions Na(NaCl)n+ and Na2(NaCl)m2+ (Figure 3a). The most intense peak in the 5+ peak group corresponds to an adduction of +1200 Da, and a maximum extent of adduction of ~+4030 Da is observed (Figure 3a). The latter corresponds to ions that contain 32% adducting species by mass. (In one of the five spectra taken from this solution, the most intense peak in the 5+ peak group corresponds to the protein with five adducted Na+, and the abundances of the higher mass adducts rapidly fall off to a maximum degree of adduction of +2400 Da; data not shown.) A series of approximately five haystacks is observed in the 5+ peak cluster (Figure 3a), as also observed with cytochrome c 7+ ions (inset, Figure 2a) and presumably represents NaCl crystallites on the protein ions. With 1.0 × 10−1 M NH4Ac, the peak of highest abundance for ubiquitin 5+ corresponds to the protein with eight adducted Na+ (+176 Da; adducting Na+ displaces constituent protons in the protein), and the maximum extent of adduction is roughly +1350 Da (Figure 3b). With 7 M NH4Ac, the peak of highest abundance in the 5+ peak group corresponds to an adduction of two Na+ (+44 Da), the maximum extent of adduction is ~+380 Da, and the nonadducted, protonated molecular ion is observed at 47% relative abundance (Figure 3c). In addition to sodium adducts, the 5+ also exhibits mixed adducts that contain Na+ and NCl (inset, Figure 3c). In the 6+ peak group of Figure 3c, the peak of highest abundance corresponds to one adducted Na+, the protonated molecular ion is observed at 61% relative abundance, and up to six adducted Na+ are observed (Figure 3c).
Figure 3.
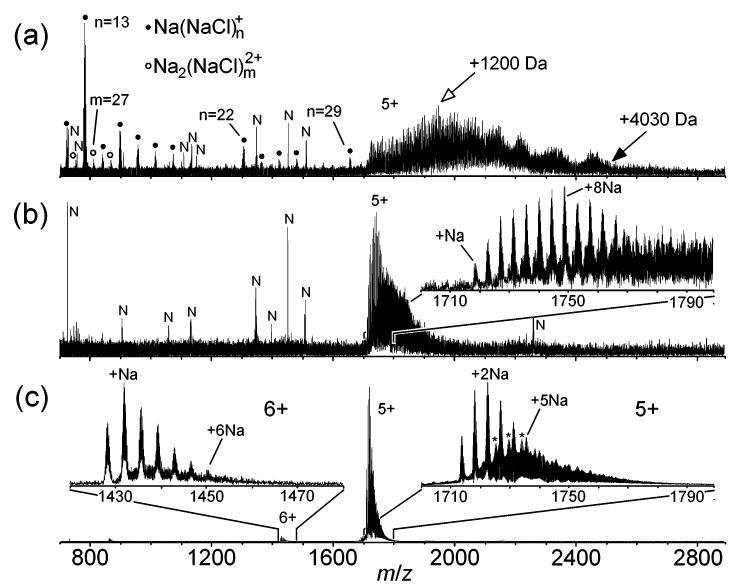
ESI mass spectra of ubiquitin (10−5 M) ions formed from aqueous solutions containing 2.0 × 10−2 M sodium chloride and (a) 0, (b) 1.0 × 10−1, or (c) 7 M ammonium acetate. Cluster ions Na(NaCl)n+ and Na2(NaCl)m2+ are indicated by closed circles and open circles, respectively. In (a), the open and closed arrows denote ubiquitin 5+ ions with an adduction of +1200 and 4030 Da, respectively. In the insets of (c), the asterisks denote peaks that are +36 Da relative to sodium adduct peaks and presumably represent sodium adducts that contain a single adducted NCl. Noise peaks are denoted by the letter “N”.
Precipitation.
An ion in solution can be precipitated by addition of a counterion with which the ion forms an insoluble compound. Whether or not a precipitate forms depends on the concentrations of the different ions in solution and the solubilities of the different compounds that may be formed between the cations and anions.39 The molar solubility of NH4Ac, 8.3 M (4 °C), is greater than those of NaAc, NH4Cl, and NaCl, 5.9 (20 °C), 4.2 (15 °C), and 5.3 M (20 °C), respectively, indicating that NH4 Ac can precipitate Na+ and Cl−.40 No precipitates form upon preparing an aqueous solution containing 2.0 × 10−2 M NaCl and 7 M NH4-Ac. However, the solution becomes saturated in NH4Ac after 15.9% of the solvent has evaporated under equilibrium conditions (Figure 4). This corresponds to a spherical droplet that has evaporated to 94% of its original diameter. At this stage of evaporation and beyond, [NH4+] and [Ac−] are both 8.3 M, and further evaporation results in the precipitation of NH4Ac. NH4Cl begins to precipitate after 99.0% of the solvent has evaporated, when [Cl−] reaches 2.1 M. NaAc then begins to precipitate after 99.5% of the solvent has evaporated, when [Na+] reaches 4.2 M (Figure 4). For comparison, a solution containing 2.0 × 10−2 M NaCl and 0 M NH4Ac becomes saturated in NaCl once 99.6% of the solvent has evaporated, when [Na+] and [Cl−] reach 5.3 M. Thus, the evaporation of >99.6% of the solvent from the solution containing 7 M NH4Ac results in concentrations of Na+ and Cl− that are 79 and 40%, respectively, of those of the corresponding solution with 0 M NH4Ac that has evaporated to the same extent. The greater reduction in [Cl−] than in [Na+] is a consequence of NH4Cl having a lower solubility than NaAc.40
Figure 4.
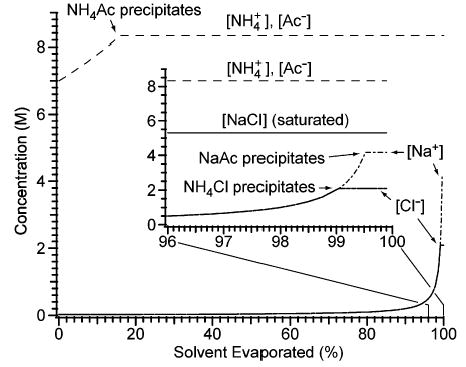
Evolution of the solution-phase concentrations of NH4+, Na+, acetate (Ac−), and Cl− during the evaporation of an aqueous solution that initially contains 2.0 × 10−2 M NaCl and 7 M NH4Ac. The inset shows detail for 96–100% of solvent evaporated. For comparison, the solubility of sodium chloride, 5.3 M, is represented by a solid line, labeled “[NaCl] (saturated)”, in the inset. The plot is based on equilibrium data from refs 40 and 41.
The precipitations described above are for a system at equilibrium; however, the evaporation of electrospray droplets may be under kinetic control instead of thermodynamic control (the time scale from droplet generation to ion formation is ~10−4–10−3 s).42 One possible consequence of this is [NH4Ac] in the droplets may exceed 8.3 M due to supersaturation, which in turn may decrease the extent of solvent evaporation required to precipitate NH4Cl and NaAc, compared to that of a system at equilibrium.43 In addition, high concentrations of NH4Ac and short evaporation times promote the inclusion of impurity ions, such as Na+ and Cl−, within precipitating NH4Ac. As indicated by the calculations described in the preceding paragraph, the coprecipitation of Na+ and Cl− with NH4Ac is expected to require less solvent evaporation than precipitation of pure NH4Cl and NaAc due to the high concentration (84% of saturation) of NH4Ac in the bulk electrospray solution.
CONCLUSIONS
The sensitivity and reproducibility of ESI-MS is significantly compromised by the presence of metal salts in electrospray solutions. Until now, no means to counteract this problem has been available for use with aqueous, nativelike electrospray solutions except to remove the offending species from solution prior to ESI-MS, using dialysis, ion exchange, or other sample cleanup methods. We have demonstrated a convenient, inexpensive method to improve the ESI-MS abundance of protein ions formed from aqueous solutions, namely, by addition of 7–8 M ammonium acetate. Addition of 8 M ammonium acetate to cytochrome c solutions that do not contain added sodium chloride produces a ~3-fold improvement in S/N and dramatically reduces the extent of sodiated adduct ions observed in the spectra. The addition of 7 M ammonium acetate to aqueous cytochrome c and ubiquitin solutions containing 2.0 × 10−2 M sodium chloride results in a ~7- and 11-fold improvement, respectively, in analyte S/N and a decrease in the mass and relative abundance of sodium chloride and protein/sodium chloride cluster ions. We propose that this effect is due to the precipitation of Na+ and Cl− within the evaporating electrospray droplets, as sodium acetate and ammonium chloride and/or as a coprecipitate with ammonium acetate prior to the formation of gas-phase analyte ions. This process essentially amounts to performing preparative wet chemistry in electrospray droplets instead of at the laboratory bench. This method appears to be a simple solution to overcoming one of the major limitations of electrospray ionization and should be particularly advantageous for the analysis of cell extracts or other biological samples that have high levels of salt.
Acknowledgments
Generous financial support for this research was provided by the National Institutes of Health (Grant R01-GM64712-01).
References
- 1.Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Science. 1989;246:64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- 2.Kelleher NL, Senko MW, Siegel MM, McLafferty FW. J Am Soc Mass Spectrom. 1997;8:380–383. [Google Scholar]
- 3.Lipton MS, Pasa-Tolic L, Anderson GA, Anderson DJ, Auberry DL, Battista JR, Daly MJ, Fredrickson J, Hixson KK, Kostandarithes H, Masselon C, Markillie LM, Moore RJ, Romine MF, Shen Y, Strittmatter E, Tolic N, Udseth HR, Venkateswaran A, Wong KK, Zhao R, Smith RD. Proc Natl Acad Sci USA. 2002;99:11049–11054. doi: 10.1073/pnas.172170199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLafferty FW. Acc Chem Res. 1994;27:379–386. [Google Scholar]
- 5.Williams ER. Anal Chem. 1998;70:179–185A. doi: 10.1021/ac981773x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Connor PB, Speir JP, Senko MW, Little DP, McLafferty FW. J Mass Spectrom. 1995;30:88–93. [Google Scholar]
- 7.Mørtz E, O’Connor PB, Roepstorff P, Kelleher NL, Wood TD, McLafferty FW, Mann M. Proc Natl Acad Sci USA. 1996;93:8264–8267. doi: 10.1073/pnas.93.16.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jockusch RA, Schnier PD, Price WD, Strittmatter EF, Demirev PA, Williams ER. Anal Chem. 1997;69:1119–1126. doi: 10.1021/ac960804q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zubarev RA, Horn DM, Fridriksson EK, Kelleher NL, Kruger NA, Lewis MA, Carpenter BK, McLafferty FW. Anal Chem. 2000;72:563–573. doi: 10.1021/ac990811p. [DOI] [PubMed] [Google Scholar]
- 10.Iavarone AT, Williams ER. Anal Chem. 2003;75:4525–4533. doi: 10.1021/ac034144i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iavarone AT, Paech K, Williams ER. Anal Chem. 2004;76:2231–2238. doi: 10.1021/ac035431p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valaskovic GA, Kelleher NL, McLafferty FW. Science. 1996;273:1199–1202. doi: 10.1126/science.273.5279.1199. [DOI] [PubMed] [Google Scholar]
- 13.Ikonomou MG, Blades AT, Kebarle P. Anal Chem. 1990;62:957–967. [Google Scholar]
- 14.Tang L, Kebarle P. Anal Chem. 1993;65:3654–3668. [Google Scholar]
- 15.Wang G, Cole RB. Anal Chem. 1994;66:3702–3708. [Google Scholar]
- 16.Constantopoulos TL, Jackson GS, Enke CG. J Am Soc Mass Spectrom. 1999;10:625–634. doi: 10.1016/S1044-0305(99)00031-8. [DOI] [PubMed] [Google Scholar]
- 17.Pan P, McLuckey SA. Anal Chem. 2003;75:5468–5474. doi: 10.1021/ac034344u. [DOI] [PubMed] [Google Scholar]
- 18.Mirza UA, Chait BT. Anal Chem. 1994;66:2898–2904. doi: 10.1021/ac00090a017. [DOI] [PubMed] [Google Scholar]
- 19.Varghese J, Cole RB. J Chromatogr, A. 1993;652:369–376. doi: 10.1016/0021-9673(93)83255-Q. [DOI] [PubMed] [Google Scholar]
- 20.Ogorzalek Loo RR, Dales N, Andrews PC. Protein Sci. 1994;3:1975–1983. doi: 10.1002/pro.5560031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rundlett KL, Armstrong DW. Anal Chem. 1996;68:3493–3497. doi: 10.1021/ac960472p. [DOI] [PubMed] [Google Scholar]
- 22.Creighton, T. E. Proteins: Structures and Molecular Properties, 2nd ed.; W. H. Freeman and Co.: New York, 1993.
- 23.Dalluge JJ, Fresenius J Anal Chem. 2000;366:701–711. doi: 10.1007/s002160051564. and references therein. [DOI] [PubMed] [Google Scholar]
- 24.Pascoe R, Foley JP, Gusev AI. Anal Chem. 2001;73:6014–6023. doi: 10.1021/ac0106694. [DOI] [PubMed] [Google Scholar]
- 25.Huber CG, Buchmeiser MR. Anal Chem. 1998;70:5288–5295. doi: 10.1021/ac980791b. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Y, Hofstadler SA. Anal Biochem. 2003;316:50–57. doi: 10.1016/s0003-2697(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 27.Cai JY, Henion J. Anal Chem. 1996;68:72–78. doi: 10.1021/ac950763i. [DOI] [PubMed] [Google Scholar]
- 28.Shen YF, Moore RJ, Zhao R, Blonder J, Auberry DL, Masselon C, Pasa-Tolic L, Hixson KK, Auberry KJ, Smith RD. Anal Chem. 2003;75:3596–3605. doi: 10.1021/ac0300690. [DOI] [PubMed] [Google Scholar]
- 29.Liu C, Wu Q, Harms AC, Smith RD. Anal Chem. 1996;68:3295–3299. doi: 10.1021/ac960286j. [DOI] [PubMed] [Google Scholar]
- 30.Wilm M, Mann M. Anal Chem. 1996;68:1–8. doi: 10.1021/ac9509519. [DOI] [PubMed] [Google Scholar]
- 31.Juraschek R, Dülcks T, Karas M. J Am Soc Mass Spectrom. 1999;10:300–308. doi: 10.1016/S1044-0305(98)00157-3. [DOI] [PubMed] [Google Scholar]
- 32.Apffel A, Fischer S, Goldberg G, Goodley PC, Kuhlmann FE. J Chromatogr, A. 1995;712:177–190. doi: 10.1016/0021-9673(95)00175-m. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi J, Ohmichi M, Jingu S, Ogawa N, Higuchi S. Anal Chem. 1999;71:5386–5390. doi: 10.1021/ac990664v. [DOI] [PubMed] [Google Scholar]
- 34.Jurchen JC, Williams ER. J Am Chem Soc. 2003;125:2817–2826. doi: 10.1021/ja0211508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bariak TM, Campana JE, Wyatt JR, Colton RJ. J Phys Chem. 1983;87:3441–3445. [Google Scholar]
- 36.Zhang D, Cooks RG. Int J Mass Spectrom. 2000;195/196:667–684. [Google Scholar]
- 37.Hao C, March RE, Croley TR, Smith JC, Rafferty SP. J Mass Spectrom. 2001;36:79–96. doi: 10.1002/jms.107. [DOI] [PubMed] [Google Scholar]
- 38.Zhou SL, Hamburger M. Rapid Commun Mass Spectrom. 1996;10:797–800. [Google Scholar]
- 39.Brady, J. E.; Holum, J. R. Chemistry: The Study of Matter and its Changes; John Wiley and Sons: New York, 1993.
- 40.Molar solubilities are calculated from the molal (mol of solute/kg of solvent) solubilities given in Dean, J. A. Lange’s Handbook of Chemistry, 15th ed.; McGraw-Hill: New York, 1999, and the densities of saturated solutions of ammonium acetate, ammonium chloride, sodium acetate, and sodium chloride, which are 1.073 ± 0.006, 1.078 ± 0.008, 1.120 ± 0.001, and 1.17 g/mL, respectively, at 20 °C. The densities were measured in our laboratory, except for that of the sodium chloride solution, which is from ref 41.
- 41.Lide, D. R., Ed. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, 1996.
- 42.Kebarle P, Tang L. Anal Chem. 1993;65:972–986A. [Google Scholar]
- 43.Harris, D. C. Quantitative Chemical Analysis, 4th ed.; W. H. Freeman and Co.: New York, 1995.


