Abstract
Airway inflammation is thought to play a major role in the pathogenesis of bronchial asthma. The precise role of individual inflammatory cells, mediator and asthma related genes in allergic lung diseases is not completely understood. The uteroglobin-related protein (UGRP) 1 was proposed to be an asthma candidate gene and play a role in regulating lung inflammation, however its precise function in the airways remains obscure. In this investigation, we used a mouse model of allergic airway inflammation to establish a relationship between UGRP 1 and IL-5 in airway inflammation. Ovalbumin (OVA) challenged mice demonstrate eosinophilia in airway tissues and high levels of IL-5 in bronchoalveolar lavage (BAL) fluid analogous to that found in bronchial asthma. Interestingly, these “OVA-challenged” mice show down-regulation of Ugrp1 expression as compared with the control group. Regression analysis further demonstrates a significant negative correlation between Ugrp1 mRNA expression in the lung and IL-5 levels in BAL fluid with r = 0.948 and P < 0.0001 when IL-5 levels were normalized by log transformation. Intranasal instillation of IL-5 to mice revealed an inhibitory effect of IL-5 on the expression of Ugrp1 mRNA. Together, these results indicate an involvement of IL-5 in the down-regulation of Ugrp1 expression in airway inflammation such as allergic asthma disease.
Keywords: Uteroglobin-related protein 1 (UGRP 1), Interleukin-5 (IL-5), Airway inflammation, Allergic asthma
Abbreviations: UGRP, Uteroglobin-related protein; CCSP, Clara cell secretory protein; OVA, ovalbumin; BAL, bronchoalveolar lavage; IL, Interleukin; BHR, bronchial hyperresponsiveness; SCGB, secretoglobin; MARCO, macrophage scavenger receptor with collagenous structure
1. Introduction
The underlying pathogenesis of bronchial asthma is airway inflammation, which involves several types of inflammatory cells and mediators that are responsible for a cascade of signal transduction linking the initial stimulus to the abnormality in airway inflammation [1]. Expression of localized cytokine and chemokines, secretion of pro-inflammatory mediators, and the recruitment of lymphocytes and specific leukocyte cell types such as eosinophils have been shown to be critical events in the pathogenesis of asthma and its clinical symptoms [2,3]. Additionally, complex patterns of genetic predispositions were found to be involved in the pathogenesis of the disease. Both genome-wide and candidate gene approaches have been used in genetic studies of asthma in an attempt to reveal the molecular mechanisms. The most notable asthma candidate susceptibility genes are located within the human chromosome region 5q31–34, in which many genes involved in inflammation reside including IL-4, IL-5, IL-9, IL-13, and β2-adrenergic receptor [4–9].
Interleukin-5 (IL-5) is produced by a number of cell types, including lymphocytes, mast cells, eosinophils, and airway smooth muscle and epithelial cells [10–12]. It is responsible for orchestrating eosinophilic inflammation; differentiation and activation of eosinophil precursors to mature eosinophils and prolongation of their survival in the allergic site of inflammation in the airways [13–16]. IL-5 has an effect on enhancing eosinophil degranulation to release several proteins such as major basic protein (MBP), eosinophil cationic protein (ECP) and eosinophil peroxidase, and activating adhesion molecules on the vascular endothelium, leading to tissue damages in airway epithelial cells [17–21]. IL-5 was shown to be important in the pathogenesis of allergic inflammation in the lung. Thus, mice injected together with Nippostrongylus organisms and anti-IL-5 antibodies failed to develop eosinophilia [22], and the administration of monoclonal anti-IL-5 antibodies to an animal model of asthma abrogated airway eosinophilic response and bronchial hyperresponsiveness (BHR) associated with antigen challenge [23]. Overproduction of IL-5 in transgenic mice led to persistent eosinophila and airway inflammation [24], whereas IL-5 knockout mice failed to develop pulmonary eosinophilia and airway hyperresponsiveness after antigen challenge [25]. In human asthmatics, IL-5 administrated in lung airways acted directly as a chemoattractant for eosinophils recruitment and as an activator of infiltrating eosinophils [26].
Uterogloblin-related protein 1 (UGRP 1), also called SCGB3A2 [27], is a small homodimeric secretory protein (~10 kDa), constitutively highly expressed in the lung, particularly in the epithelial cells of trachea, bronchus and bronchioles [28]. UGRP 1 possesses significant amino acid sequence similarity to the Uteroglobin/Clara cell secretory protein (UG/CCSP) [28] that exhibits several immunomudulatory and anti-inflammatory effects in the lung [27,29,30]. Mouse and human UGRP 1 share 81% amino acid sequence identity [28]. By using fluorescence in situ hybridization (FISH), the human UGRP1 gene was assigned to chromosome 5q31–32 [31], one of the most extensively investigated chromosomal regions in the pathogenesis of asthma, and an area that contains a cluster of genes encoding numerous T helper type (Th) 2 cytokines [32]. The mRNA level of Ugrp1 is down-regulated in inflamed mouse lungs, whereas the expression level returned to normal following dexamethasone treatment [28]. Further, a polymorphism (G/A) was identified at −112 bp of the human UGRP1 gene promoter that was associated with an increased risk of bronchial asthma in a Japanese population of adult asthmatic patients [31]. Recently, a macrophage scavenger receptor with collagenous structure (MARCO) was identified as a receptor for UGRP 1, which is expressed in lung alveolar macrophages and is involved in pulmonary inflammation [33]. These results suggest that UGRP 1 may play a role in regulating the local immune response in the lung. However, the precise functional role(s) of UGRP 1 in lung airway inflammation, particularly with respect to cytokine regulation remains obscure.
In the current study, we demonstrate that mouse challenged with ovalbumin (OVA) show high levels of IL-5 in bronchoalveolar lavage (BAL) fluid and these levels are inversely correlated with the levels of Ugrp1 expression in lung. Furthermore, lung Ugrp1 expression decreased following intranasal instillation of IL-5 to naïve mouse. These studies suggest an involvement of IL-5 in decreased expression of Ugrp1 gene in inflamed mouse airways.
2. Materials and methods
2.1. Animals
Female 129Sv mice were used in the present study. All animals were housed in rooms with a 12 h day/12 h night diurnal cycle and were given food and water ad libitum. The animal studies were carried out in accordance with the Using Animals in Intramural Research Guidelines (NIH Animal Research Advisory Committee, NIH, Bethesda, MD) and approved by the Institutional Animal Care and Use Committee.
2.2. Aeroallergen treatment of mice
Six-week-old mice were sensitized by i.p. injection of mixture of 10 μg ovalbumin (OVA; Sigma Chemical Co., St. Louis, MO) and aluminum hydroxide gel (ImjectAlum, Pierce, Rockford, IL; 2.25 mg/mouse) on days 0 and 5. On day 12, the “OVA-challenged” or “sensitized control” mice were exposed to an aerosol of OVA (5 mg/ml) in saline or saline alone, respectively under conscious state for 30 min. The aerosol was generated by a jet nebulizer (PARI LC Plus; PARI Respiratory Equipment, Inc., Monterey, CA) driven by an air compressor (PRONEB Ultra; PARI Respiratory Equipment, Inc.) in a plexiglass chamber (220 × 230 mm, height: 140 mm). Twenty-four hours later, mice were euthanized and bronchoalveolar lavage fluid was collected for determination of IL-5 levels and whole lungs were processed to determine Ugrp1 expression.
2.3. Collection of bronchoalveolar lavage (BAL) fluid and quantitation of IL-5 level
The chest of each animal was opened and a 20-gauge blunt needle was introduced into the upper trachea. The lung was lavaged with 1 ml of phosphate-buffered saline (PBS; pH 7.5, room temperature) and bronchoalveolar lavage (BAL) fluid was collected. The lavage obtained from each animal was pooled after re-infusing PBS twice before final collection. The collected BAL fluid was centrifuged at 500 × g for 5 min at 4 °C, and aliquots of the supernatant were frozen on dry ice and stored at −80 °C for cytokine analysis. IL-5 levels were measured using murine cytokine ELISA system (ENDOGEN, EM-IL5) according to the manufacturer’s instructions.
2.4. Histological analysis and immunohistochemistry
Lung tissues below the main bronchi were inflated with an intratracheal injection of 10% formaldehyde solution (Fisher Scientific, Pittsburgh, PA) to preserve pulmonary architecture. After dehydration, the fixed lung samples were embedded in Paraplast Plus paraffin (Fisher HealthCare, Houston, TX) and serial sections of 5 μm thickness were prepared. Both hematoxylineosin (HE) and Diff-Quik (DQ; Baxter Healthcare, Miami, FL) staining were performed for the identification of eosinophils based on the manufacturer’s descriptions with slight modification. For immunohistochemical staining, sections were treated with 0.3% H2O2 in methanol to block endogenous peroxidase activity and then 5% skim milk in PBS. Sections were incubated with a rabbit polyclonal antibody to mouse UGRP 1 [28] (1:500 dilution) for 1 h at room temperature, followed by biotinylated goat anti-rabbit IgG (1:50 dilution) for 30 min at room temperature. Bound antibody was visualized with the Vectastain ABC staining kit (Vector Laboratory Inc., Burlingame, CA) using 3,3′-diaminobenzidine as a peroxidase substrate. Sections were rinsed with PBS, counterstained with Hematoxylin QS (Vector Laboratories Inc., Burlingame, CA) and mounted in permanent mounting medium.
2.5. Intranasal instillation of IL-5
Seven-week-old mice (n = 3–5 in each group) were anesthetized with i.p. administration of 2.5% Avertin and allowed to breathe spontaneously. Sterile PBS (20 μl, control) or recombinant mouse IL-5 (20 and 60 ng in 20 μl PBS; R&D systems, Minneapolis, MN) was intranasally instilled into the trachea of each animal. After 24 h, each mouse was euthanized, and lung tissues were harvested and immediately frozen in liquid nitrogen for later mRNA analysis.
2.6. RNA isolation and Northern blot analysis
Frozen tissues were transferred to a mortar containing liquid nitrogen and were pulverized using pestle to disrupt tissues. The powdered tissues (100 mg) were transferred to a homogenizer containing 1 ml TRIzol®reagent (Invitrogen Life Technologies, Carlsbad, CA) and total RNAs were prepared according to the manufacturer’s instruction. The quality and quantity of purified RNAs were spectrophotometrically evaluated.
Total RNAs were electrophoresed on a 1% agarose gel containing 0.22 M formaldehyde and blotted onto Gene Screen Plus nylon membranes (Perkin-Elmer Life Sciences, Boston, MA). Filters were hybridized in PerfectHyb Plus hybridization buffer (Sigma, St. Louis, MO) with a mouse Ugrp1 full-length cDNA as a probe, that was labeled with [α-32P]dCTP (3000 Ci/mmol; Perkin-Elmer Life Sciences Inc., Boston, MA) using Ready-To-Go DNA labeling beads (Amersham Biosciences, Piscataway, NJ). Hybridization was performed at 65 °C overnight. The filter was washed twice with 2× SSC containing 0.5% SDS at 65 °C for 20 min and was re-hybridized with mouse β-actin or Gapdh cDNA as a loading control. Gene expression was determined using a Storm phosphorimager (Molecular Dynamics, Sunnyvale, CA) after overnight exposure to an intensifying screen and the intensity of the Ugrp1 and β-actin or Gapdh mRNA bands was determined with ImageQuant software (Amersham Biosciences, Piscataway, NJ).
2.7. Data analysis
All group data are represented as the means ± S.E. The difference between control and treated groups was tested by unpaired Student’s t-test. Differences were considered statistically significant at P < 0.05. Correlation between the levels of Ugrp1 mRNA expression in airways and IL-5 in BAL fluids was analyzed by Fisher’s regression analysis.
3. Results
3.1. Characterization of allergic airways inflammation in mice
Aerosol challenge of mice with OVA induced a significant increase in eosinophils in the lung, in comparison to mice given aerosol saline alone. Histological examination of lung sections stained with hematoxylin and eosin demonstrated that “OVA-challenged” mouse lung tissues have characteristic eosinophilic inflammation in widespread areas of airway walls of bronchi and broncheoli (Fig. 1A, left panels). The structural integrity determined by Diff-Quik staining further indicated that eosinophils are the most infiltrated cells (Fig. 1A, right panels). Airway epithelial cells were further examined for the expression of UGRP1 by immunostaining sections with anti-UGRP 1 antibody. Ugrp1 expression was rarely detected in airway epithelial cells of “OVA-challenged” mice whereas “sensitized control” mice showed high levels of UGRP1 expression in the airways (Fig. 1B), demonstrating that antigen challenge has caused down-regulation of UGRP 1 in mouse airways.
Fig. 1.
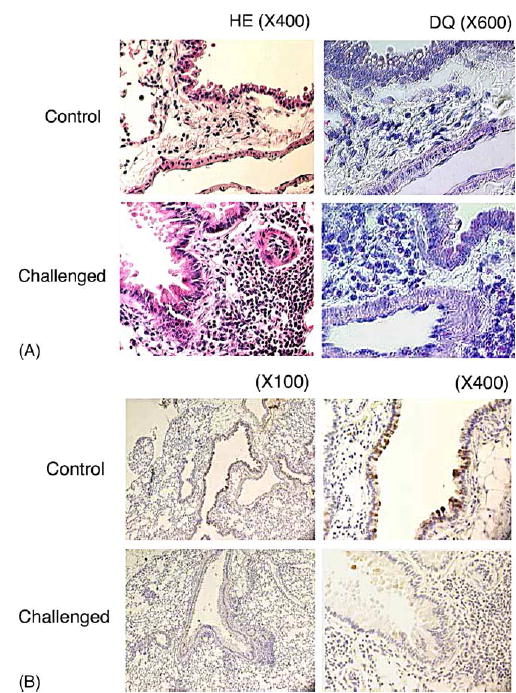
Histological analysis of eosinophils and UGRP1 expression in lung tissues. (A) Histopathological pulmonary changes on lung tissues after saline (Control; upper panels) or ovalbumin (OVA) inhalation (Challenged; lower panels) in mice. Sections of formalin-fixed lung tissues were stained with hematoxylin and eosin (HE; left panels) or Diff-Quik (DQ; right panels) before examination by light microscopy. Magnification: left panels, ×400; right panels, ×600. Marked eosinophils infiltration was observed in “OVA-challenged” mice. (B) Immunohistochemical staining for UGRP 1 using rabbit anti-mouse UGRP 1 antibody in lungs obtained from sensitized control (Control; upper panels) or OVA-challenged (Challenged; lower panels) mice. Magnifications: ×100 (left panels) and ×400 (right panels). The UGRP 1-positive staining in the epithelial cells is observed in Control mice, but only faint staining is found in Challenged group.
3.2. Relationship between IL-5 and the expression of Ugrp1 mRNA in antigen challenged mice
IL-5 is a pro-inflammatory cytokine involved in inflammatory processes, and in particular, it is known to be responsible for eosinophilic inflammation [13–16]. Thus, the level of IL-5 in BAL fluids obtained from control and “OVA-challenged” mice was assessed by using ELISA. The IL-5 levels in BAL fluids of “OVA-challenged” mice were dramatically elevated as compared with “sensitized control” mice (Fig. 2). The level of Ugrp1 expression was next examined by Northern blot analysis using lung mRNAs prepared from “OVA-challenged” and “sensitized” mice, The expression of Ugrp1 mRNA in the airways of “OVA-challenged” mice was significantly reduced as compared with that of “sensitized control” mice (P < 0.05) (Fig. 3). The correlation between IL-5 levels in BAL fluids and the levels of Ugrp1 mRNA expression in the lungs was further analyzed by regression analysis. A significant inverse correlation between the levels of IL-5 and Ugrp1 mRNA expression was obtained with a correlation coefficient (r) of 0.948 with P < 0.0001 when IL-5 levels were normalized by log transformation (Fig. 4). These results indicate the involvement of IL-5 in the inhibitory effects of Ugrp1 expression in inflamed mouse airways.
Fig. 2.
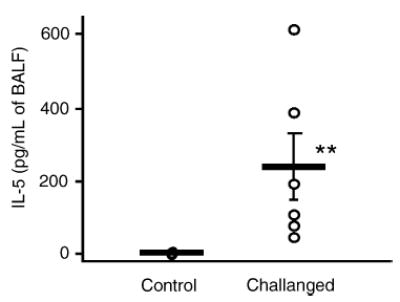
IL-5 levels in bronchoalveolar lavage fluids (BALF) obtained from sensitized control (Control) and OVA-challenged (Challenged) mice. The levels of IL-5 were determined by ELISA. A dot represents each value obtained from Control (n = 7) and Challenged (n = 6) mice, respectively, and a bar represents the mean ± S.E. Significantly elevated levels of IL-5 in BAL fluids were observed in the Challenged group (**P < 0.01).
Fig. 3.
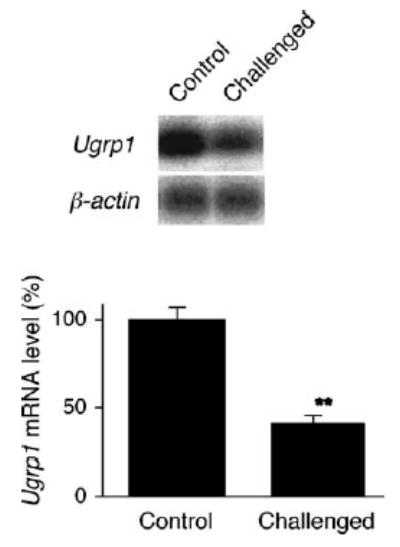
Levels of Ugrp1 mRNA expression in the lungs of sensitized control (Control) and OVA-challenged (Challenged) mice. (A) Northern blot analysis using RNAs extracted from individual whole lungs of Control and Challenged mice. The membrane was serially hybridized with radiolabeled mouse Ugrp1 and β-actin (loading control) probes. The representative result from three separate experiments are shown. (B) Bar graph showing the relative Ugrp1 mRNA levels. Data represent the mean ± S.E from Control (n = 7) and Challenged (n = 6) mice, respectively. The Ugrp1 mRNA expression is significantly decreased in lungs of Challenged group as compared with Control mice (**P < 0.01).
Fig. 4.
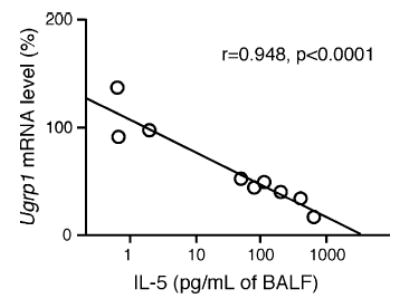
Correlation between the levels of Ugrp1 mRNA expression in lungs and IL-5 levels in bronchoalveolar lavage fluids (BALF). Relative airway Ugrp1 mRNA levels after normalization to β-actin were regressed against IL-5 levels in BALF after normalization by log transformation. A significant correlation between the two parameters was observed (r = 0.948, P < 0.0001 by Fisher’s regression analysis).
3.3. Effect of intranasal administration of IL-5 on lung Ugrp1 mRNA expression
In order to confirm the direct correlation between the levels of IL-5 and the expression of Ugrp1 in lung, intranasal instillation of IL-5 was performed into naïve mice. Northern blot analysis demonstrated that lung tissues obtained from mice intranasally treated with IL-5 had lower levels of Ugrp1 mRNA expression as compared with control mice, with a dose of 60 ng reaching statistical significance (Fig. 5). These results suggest that IL-5 may, at least in part, directly suppress Ugrp1 expression in mouse lungs.
Fig. 5.
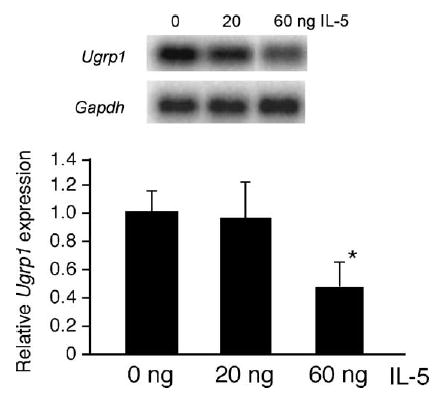
Effect of intranasally administered IL-5 on the Ugrp1 mRNA levels in lungs. (A) A representative autoradiograph from Northern blot analysis using lung RNAs isolated from mice that received intranasal instillation of 20 and 60 ng of recombinant mouse IL-5. The membrane was serially hybridized with radiolabeled Ugrp1 and Gapdh probes. (B) Bar graph showing the relative Ugrp1 mRNA levels that is plotted against the amount of IL-5 administered. Quantitation of Ugrp1 or Gapdh mRNA bands was performed using a Storm phosphoimager and ImageQuant software. The value of each group is the mean of those obtained from 3–5 mice ± S.E. A statistically significant difference was observed between 0 and 60 ng IL-5 (*P < 0.05).
4. Discussion
In the present study, by using mouse model we demonstrated that IL-5 is involved in reduced expression of Ugrp1 in allergic airway inflammation. In this animal model, lung eosinophilic inflammation and high levels of IL-5 in bronchoalveolar lavage fluids (BALF) were observed after ovalbumin (OVA) challenge. Interestingly, lung obtained from “OVA-challenged” mice showed significantly lower Ugrp1 expression at both the protein and mRNA levels than those from control mice when examined using immunohistochemistry and northern blot analysis, respectively. Moreover, down-regulation of Ugrp1 mRNA expression occurred in mice intranasally instilled with IL-5. Since, UGRP1 is expressed only in the epithelial cells of the airways and probably due to quality of the antibody used, western blotting using a whole lung did not produce a clear band that can be quantitated for protein expression levels. Further, although northern blotting is not quantitative, it clearly demonstrated that the level of Ugrp1 mRNA was reduced by approximately a half of the control levels in the lungs of “OVA-challenged” mice and after 60 ng intranasal administration of IL-5. Taken together, these results support the inhibitory effect of IL-5 on the expression of Ugrp1 in allergic airway inflammation in mice.
The importance of IL-5 in airway inflammation and the pathogenesis of bronchial asthma have been well documented [22–26]. Shi et al. showed that IL-5 challenge of allergic asthmatics significantly induced airway hyperactivity as well as airway eosinophilia [26]. The involvement of IL-5 in airway inflammation was further suggested by studies using animal models; airway eosinophilia and hyperresponsiveness were induced by i.p. injection of IL-5-producing CV-1 cells to guinea pigs, and the allergic response, that is also induced by OVA challenge, was inhibited by the treatment with anti-IL-5 antibody [34,35]. Similarly, several studies showed an inhibitory effect of anti-IL-5 antibodies on the development of eosinophilia [22,23] and bronchial hyperresponsiveness (BHR) associated with antigen challenge [23]. On the other hand, overproduction of IL-5 in transgenic mice led to persistent eosinophila and airway inflammation [24], whereas IL-5 knockout mice failed to develop pulmonary eosinophilia and airway hyperresponsiveness after antigen challenge [25]. A mouse deficient in the IL-5 receptor α -subunit was shown to be resistant to airway eosinophilic inflammation and bronchial hyperresponsiveness after OVA sensitization and repeated inhalation of antigen even though IL-5 levels in BALF of this mouse was increased to similar levels found in wild-type mice [36]. These results indicate that IL-5Rα plays an important role in IL-5 mediated airway eosinophia and hyperresponsiveness. In this respect, it is interesting to note that an increase in number of IL-5Rα -subunits was observed in endobronchial biopsies of asthmatic subjects as compared to control subjects [37].
UGRP 1 may have an anti-inflammatory function in the airways with respect to bronchial asthma based on several lines of evidence. Firstly, human UGRP1 gene was localized on human chromosome 5q31–32, where one of the asthma susceptibility genes has been assigned [31,32]. Secondly, amino acid sequence of UGRP 1 is similar to the Uteroglobin/Clara cell secretory protein (UG/CCSP) [28] that is believed to play a role in immunomodulatory and anti-inflammatory activities in the lung [27,29]. In this regard, a recent paper elegantly demonstrated that when treated with recombinant UG before challenge, UG inhibited Th2 cytokine expression and eosinophil infiltration in lungs of OVA-sensitized and challenged UG knockout mice that otherwise manifest exaggerated allergic response, through blockage of prostaglandin D2 receptor-mediated nuclear factor-κB activation and suppression of cyclooxygenase-2 expression [30]. The third evidence is that reduced Ugrp1 expression in inflamed mouse lungs returned to normal level after dexamethasone treatment [28]. In forth, a G/A polymorphism was identified in the human UGRP1 gene promoter that is associated with an increased risk of bronchial asthma in a Japanese population of adult asthmatic patients [31]. The presence of a G/A polymorphism in human UGRP1 gene promoter, however, is currently controversial due to other papers that report no association between a G/A polymorphism and an increased risk of bronchial asthma [38,39]. And lastly, MARCO was identified as a possible UGRP 1 receptor that is involved in pulmonary inflammation [33]. In spite of these suggestive evidences, the exact function of UGRP 1 in airway inflammation is still unknown, and thus further studies are required to address the question.
In the current work, Ugrp1 expression was significantly reduced by intranasal administration of 60 ng IL-5 to naïve mice. The result suggests that IL-5 may act, at least in part, directly on lung epithelial cells to suppress Ugrp1 gene expression. The intranasal administration of 60 ng IL-5 may be sufficient to reduce Ugrp1 mRNA since no further reduction of the Ugrp1 expression was observed when 600 ng of IL-5 was used (data not shown). However, the inhibition of Ugrp1 gene expression by OVA challenge appeared to be more efficient. Thus, other inflammatory mediators may also be involved in the reduction of Ugrp1 expression induced by antigen inhalation. In fact, we have found the higher levels of IL-9 in BAL fluids of an allergic inflammation model mouse and that intranasal administration of IL-9 to naïve mice reduces Ugrp1 gene expression, similar to that obtained with IL-5 in the current study [40]. This suggests that the level of Ugrp1 expression may be reduced as a result of increased levels of any Th2 cytokines, alone or in combination, as seen in inflamed lungs. However, the effects of Th2 cytokines other than IL-5 and IL-9 on Ugrp1 expression levels and whether UGRP 1 levels return to baseline levels as inflammation resolves and IL-5/9 levels decline needs to be examined.
In summary, we demonstrated that expression of Ugrp1 is reduced in antigen induced airway inflammation in mice. The increased IL-5 level in airways may be, at least in part, involved in the decreased expression of Ugrp1 in airway epithelial cells.
Acknowledgments
We would like to thank Jorge Paiz for his technical assistance and Dr. Frank Gonzalez for his critical review of the manuscript. AS was supported by the National Cancer Institute and by a Royal Golden Jubilee Ph.D. Scholarship from the Thailand Research Fund. This study was carried out in partial fulfillment of the requirement for the Ph.D. degree for AS from the Department of Pharmacology, Faculty of Science, Mahidol University, Bangkok, Thailand.
References
- 1.Djukanovic R. Airway inflammation in asthma and its consequences: implications for treatment in children and adults. J Allerg Clin Immunol. 2002;109:539–48. doi: 10.1067/mai.2002.124568. [DOI] [PubMed] [Google Scholar]
- 2.Empey DW, Laitinen LA, Jacob WM, Nadel JA. Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory tract infection. Am Rev Respir Dis. 1976;113:131–9. doi: 10.1164/arrd.1976.113.2.131. [DOI] [PubMed] [Google Scholar]
- 3.O’Byrne PM. Allergen-induced airway hyperresponsiveness. J Allergy Clin Immunol. 1988;81:119–27. doi: 10.1016/0091-6749(88)90230-8. [DOI] [PubMed] [Google Scholar]
- 4.Cookson WO, Moffatt MF. Genetics of asthma and allergic disease. Hum Mol Genet. 2000;9:2359–64. doi: 10.1093/hmg/9.16.2359. [DOI] [PubMed] [Google Scholar]
- 5.Marsh DG, Neely JD, Breazeale DR, Ghosh B, Freidhoff LR, Ehrlich-Kautzky E, Schou C, Krishnaswamy G, Beaty TH. Linkage analysis of IL4 and other chromosome 5q31. 1 markers and total serum immunoglobulin E concentrations. Science. 1994;264:1152–6. doi: 10.1126/science.8178175. [DOI] [PubMed] [Google Scholar]
- 6.Marsh DG, Neely JD, Breazeale DR, Ghosh B, Freidhoff LR, Schou C, Beaty TH. Genetic basis of IgE responsiveness: relevance to the atopic diseases. Int Arch Allerg Immunol. 1995;107:25–8. doi: 10.1159/000236920. [DOI] [PubMed] [Google Scholar]
- 7.Levitt RC, Eleff SM, Zhang LY, Kleeberger SR, Ewart SL. Linkage homology for bronchial hyperresponsiveness between DNA markers on human chromosome 5q31–q33 and mouse chromosome 13. Clin Exp Allergy. 1995;25(Suppl 2):61–3. doi: 10.1111/j.1365-2222.1995.tb00424.x. [DOI] [PubMed] [Google Scholar]
- 8.Postma DS, Bleecker ER, Amelung PJ, Holroyd KJ, Xu J, Panhuysen CI, Meyers DA, Levitt RC. Genetic susceptibility to asthma–bronchial hyperresponsiveness coinherited with a major gene for atopy. N Engl J Med. 1995;333:894–900. doi: 10.1056/NEJM199510053331402. [DOI] [PubMed] [Google Scholar]
- 9.Renauld JC. New insights into the role of cytokines in asthma. J Clin Pathol. 2001;54:577–89. doi: 10.1136/jcp.54.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bressler RB, Lesko J, Jones ML, Wasserman M, Dickason RR, Huston MM, Cook SW, Huston DP. Production of IL-5 and granulocyte-macrophage colony-stimulating factor by naive human mast cells activated by high-affinity IgE receptor ligation. J Allerg Clin Immunol. 1997;99:508–14. doi: 10.1016/s0091-6749(97)70078-2. [DOI] [PubMed] [Google Scholar]
- 11.Takatsu K. Interleukin-5. Curr Opin Immunol. 1992;4:299–306. doi: 10.1016/0952-7915(92)90080-x. [DOI] [PubMed] [Google Scholar]
- 12.Yokota T, Coffman RL, Hagiwara H, Rennick DM, Takebe Y, Yokota K, Gemmell L, Shrader B, Yang G, Meyerson P, et al. Isolation and characterization of lymphokine cDNA clones encoding mouse and human IgA-enhancing factor and eosinophil colony-stimulating factor activities: relationship to interleukin 5. Proc Natl Acad Sci U S A. 1987;84:7388–92. doi: 10.1073/pnas.84.21.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clutterbuck EJ, Hirst EM, Sanderson CJ. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: comparison and interaction with IL-1, IL-3, IL-6, and GMCSF. Blood. 1989;73:1504–12. [PubMed] [Google Scholar]
- 14.Yamaguchi Y, Suda T, Suda J, Eguchi M, Miura Y, Harada N, Tominaga A, Takatsu K. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med. 1988;167:43–56. doi: 10.1084/jem.167.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaguchi Y, Hayashi Y, Sugama Y, Miura Y, Kasahara T, Kitamura S, Torisu M, Mita S, Tominaga A, Takatsu K. Highly purified murine interleukin 5 (IL-5) stimulates eosinophil function and prolongs in vitro survival IL-5 as an eosinophil chemotactic factor. J Exp Med. 1988;167:1737–42. doi: 10.1084/jem.167.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez AF, Sanderson CJ, Gamble JR, Campbell HD, Young IG, Vadas MA. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988;167:219–24. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh GM, Hartnell A, Wardlaw AJ, Kurihara K, Sanderson CJ, Kay AB. IL-5 enhances the in vitro adhesion of human eosinophils, but not neutrophils, in a leucocyte integrin (CD11/18)-dependent manner. Immunology. 1990;71:258–65. [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh GM, Hartnell A, Moqbel R, Cromwell O, Nagy L, Bradley B, Furitsu T, Ishizaka T, Kay AB. Receptor expression and functional status of cultured human eosinophils derived from umbilical cord blood mononuclear cells. Blood. 1990;76:105–11. [PubMed] [Google Scholar]
- 19.Walsh GM, Moqbel R, Nagakura T, Iikura Y, Kay AB. Enhancement of the expression of eosinophil IgE receptor (Fc epsilon R2) and its function by platelet-activating factor. J Lipid Mediat. 1990;2(Suppl):S177–86. [PubMed] [Google Scholar]
- 20.Fujisawa T, Kephart GM, Gray BH, Gleich GJ. The neutrophil and chronic allergic inflammation Immunochemical localization of neutrophil elastase. Am Rev Respir Dis. 1990;141:689–97. doi: 10.1164/ajrccm/141.3.689. [DOI] [PubMed] [Google Scholar]
- 21.Gleich GJ. The eosinophil and bronchial asthma: current understanding. J Allergy Clin Immunol. 1990;85:422–36. doi: 10.1016/0091-6749(90)90151-s. [DOI] [PubMed] [Google Scholar]
- 22.Rennick DM, Thompson-Snipes L, Coffman RL, Seymour BW, Jackson JD, Hudak S. In vivo administration of antibody to interleukin-5 inhibits increased generation of eosinophils and their progenitors in bone marrow of parasitized mice. Blood. 1990;76:312–6. [PubMed] [Google Scholar]
- 23.Coffman RL, Seymour BW, Hudak S, Jackson J, Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1989;245:308–10. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- 24.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, Brenneise IE, Horton MA, Haczku A, Gelfand EW, Leikauf GD, Lee NA. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–56. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi H, Qin S, Huang G, Chen Y, Xiao C, Xu H, Liang G, Xie Z, Qin X, Wu J, Li G, Zhang C. Infiltration of eosinophils into the asthmatic airways caused by interleukin 5. Am J Respir Cell Mol Biol. 1997;16:220–4. doi: 10.1165/ajrcmb.16.3.9070605. [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee AB, Chilton BS. The uteroglobin/Clara cell protein family. Ann NY Acad Sci. 2000;923:1–356. [Google Scholar]
- 28.Niimi T, Keck-Waggoner CL, Popescu NC, Zhou Y, Levitt RC, Kimura S. UGRP1, a uteroglobin/CCSP-related protein, is a novel lung-enriched downstream target gene for the T/EBP/NKX2. 1 homeodomain transcription factor. Mol Endocrinol. 2001;15:2021–36. doi: 10.1210/mend.15.11.0728. [DOI] [PubMed] [Google Scholar]
- 29.Singh G, Katyal SL. Clara cells and Clara cell 10 kD protein (CC10) Am J Respir Cell Mol Biol. 1997;17:141–3. doi: 10.1165/ajrcmb.17.2.f138. [DOI] [PubMed] [Google Scholar]
- 30.Mandal AK, Zhang Z, Ray R, Choi MS, Chowdhury B, Pattabiraman N, Mukherjee AB. Uteroglobin represses allergen-induced inflammatory response by blocking PGD2 receptor-mediated functions. J Exp Med. 2004;199:1317–30. doi: 10.1084/jem.20031666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niimi T, Munakata M, Keck-Waggoner CL, Popescu NC, Levitt RC, Hisada M, Kimura S. A polymorphism in the human UGRP1 gene promoter that regulates transcription is associated with an increased risk of asthma. Am J Hum Genet. 2002;70:718–25. doi: 10.1086/339272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niimi T, Copeland NG, Gilbert DJ, Jenkins NA, Srisodsai A, Zimonjic DB, Keck-Waggoner CL, Popescu NC, Kimura S. Cloning, expression, and chromosomal localization of the mouse Ugrp2 gene that encodes a member of the novel uteroglobin-related protein gene family. Cytogenet Genome Res. 2002;97:120–7. doi: 10.1159/000064067. [DOI] [PubMed] [Google Scholar]
- 33.Bin LH, Nielson LD, Liu X, Mason RJ, Shu HB. Identification of uteroglobin-related protein 1 and macrophage scavenger receptor with collagenous structure as a lung-specific ligand-receptor pair. J Immunol. 2003;171:924–30. doi: 10.4049/jimmunol.171.2.924. [DOI] [PubMed] [Google Scholar]
- 34.Van Oosterhout AJ, Ladenius AR, Savelkoul HF, Van Ark I, Delsman KC, Nijkamp FP. Effect of anti-IL-5 and IL-5 on airway hyperreactivity and eosinophils in guinea pigs. Am Rev Respir Dis. 1993;147:548–52. doi: 10.1164/ajrccm/147.3.548. [DOI] [PubMed] [Google Scholar]
- 35.Van Oosterhout AJ, Van Ark I, Hofman G, Savelkoul HF, Nijkamp FP. Recombinant interleukin-5 induces in vivo airway hyperresponsiveness to histamine in guinea pigs. Eur J Pharmacol. 1993;236:379–83. doi: 10.1016/0014-2999(93)90475-w. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka H, Kawada N, Yamada T, Kawada K, Takatsu K, Nagai H. Allergen-induced airway inflammation and bronchial responsiveness in interleukin-5 receptor alpha chain-deficient mice. Clin Exp Allerg. 2000;30:874–81. doi: 10.1046/j.1365-2222.2000.00828.x. [DOI] [PubMed] [Google Scholar]
- 37.Yasruel Z, Humbert M, Kotsimbos TC, Ploysongsang Y, Minshall E, Durham SR, Pfister R, Menz G, Tavernier J, Kay AB, Hamid Q. Membrane-bound and soluble alpha IL-5 receptor mRNA in the bronchial mucosa of atopic and nonatopic asthmatics. Am J Respir Crit Care Med. 1997;155:1413–8. doi: 10.1164/ajrccm.155.4.9105087. [DOI] [PubMed] [Google Scholar]
- 38.Jian Z, Nakayama J, Noguchi E, Shibasaki M, Arinami T. No evidence for association between the −112G/A polymorphism of UGRP1 and childhood atopic asthma. Clin Exp Allerg. 2003;33:902–4. doi: 10.1046/j.1365-2222.2003.01703.x. [DOI] [PubMed] [Google Scholar]
- 39.Heinzmann A, Dietrich H, Deichmann KA. Association of uteroglobulin-related protein 1 with bronchial asthma. Int Arch Allerg Immunol. 2003;131:291–5. doi: 10.1159/000072141. [DOI] [PubMed] [Google Scholar]
- 40.Chiba Y, Kusakabe T, Kimura S. Decreased expression of uteroglobin-related protein 1 in inflamed mouse airways is mediated by IL-9. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1193–8. doi: 10.1152/ajplung.00263.2004. [DOI] [PubMed] [Google Scholar]


