Abstract
Several lines of research show that cells of the immune response are sensitive to thermal variations in their microenvironment, such as that which occurs during inflammation and fever; these data have led to the hypothesis that strategic applications of heat could assist in controlling tumor growth in animal models. The innate immune response is known to play a critical role in the development of effective anti-tumor immunity and granulocytes such as polymorphonuclear neutrophils (PMNs), as key mediators of inflammation, have been suggested to have the potential to initiate immune response cascades against tumors. Thus, we hypothesized that PMNs may play a crucial role in mediating the anti-tumor effects of a mild, fever-range whole body hyperthermia (FR-WBH) protocol, where core body temperatures are raised to 39.5–40°C for 8 hrs. Indeed, in BALB/c mice bearing the colon tumor CT26, the anti-tumor effects of WBH correlates with increased granulocytic infiltrate at the tumor site as determined using immunohistochemical analysis for Gr-1+ cells. In both BALB/c mice bearing CT26 and SCID mice bearing human colon tumors, PMN depletion in vivo using anti-Gr-1 ascites ablated the anti-tumor effect of mild WBH. Because mild thermal stress is also found to enhance the respiratory burst of granulocytes, these data collectively suggest that the thermal stimulation of granulocytes may help to prevent tumor establishment. Overall, these results may have implications for the design of thermal therapy protocols in cancer immunotherapy.
Keywords: fever, mild thermal stress, granulocytes, polymorphonuclear neutrophils, cancer
ABBREVIATIONS: Ca2+ calcium, DHR dihydrorhodamine, FR-WBH fever-range whole body hyperthermia, IL- interleukin, LPS lipopolysaccharide, PMN polymorphonuclear neutrophil, s.c. subcutaneous, SCID severe combined immunodeficient, TNF tumor necrosis factor
INTRODUCTION
The increase in body temperature that occurs during the inflammatory response to infection is an evolutionarily conserved event that has been associated with increased metabolic cost and survival benefit [1]. Indeed, studies using ectotherms, where organisms exhibit a behavioral fever (i.e. they exhibit movement to a warmer environment upon infection), as well as mammalian studies have shown that increased temperatures significantly enhance survival after potentially mortal bacterial infections [2–7]. However, despite this association with survival, the role of the thermal component of fever and/or inflammation is still one of the least understood aspects of an immune response.
The highly conserved nature of the fever response to infection in nature suggests that fever-range increases in body temperature would be most likely to have broad immunomodulatory effects on different immune effector cells, including those of the innate immune response. Indeed, there are various reports suggesting that mild, physiologically relevant increases in temperature may enhance the activity of NK cells, macrophages and granulocytes (Reviewed in [8]). Our group in particular has found that a fever-range hyperthermia of 39.5–40°C maintained for 6 to 8hrs can enhance several endpoints of innate immunity. For example, this mild thermal stress was found to enhance the activation associated migration of dendritic cells in the skin [9]. Furthermore, a fever-range whole body hyperthermia (FR-WBH) was found to enhance mouse serum levels of the inflammatory cytokines TNF-α and IL-6 in an LPS induced acute phase reaction in vivo [10]. These in vivo results were similar to that found by Jiang et al [11], but are in direct contrast to that seen when applying hyperthermia treatment in vitro. In vitro hyperthermia has been consistently found by us and others to inhibit pro-inflammatory cytokine production by peritoneal macrophages [10,12–16], thus emphasizing the importance of studying the effects of in vivo WBH treatment. Interestingly, it was also found that FR-WBH alone, without additional mitogenic stimuli, did not have any effect on serum levels of inflammatory cytokines in mice [10]. This suggests that fever-range hyperthermia may not affect immune cells that are not also in the process of responding to pathogens. Indeed, it is encouraging to believe that increased temperatures will not induce potentially damaging immune cell activity when there is no real danger to the host that needs to be overcome. Similar results were seen when investigating the effects of fever-range temperatures on the oxidative metabolism or respiratory burst that is associated with anti-microbial activity of macrophages. Specifically, fever-range hyperthermia alone did not appear to stimulate macrophage NO2-production, but it did appear to lower the threshold for macrophage responses to LPS [17]. Taken together, these results suggest that mild thermal stress in the form of in vivo FR-WBH may have the potential to enhance inflammatory immune responses against disease.
Importantly, it has been established that mild WBH can help control not only infections but also the growth of tumors in various animal models. For example, in a rat tumor model, without any other additional therapy, mild WBH treatment resulted in both a delay of tumor growth as well as a reduced incidence of lymph node metastases [18]. Using various murine tumor models, our group has established that this WBH treatment can delay the growth of murine tumors in BALB/c mice and human tumors in SCID mice [19,20]. Furthermore, the WBH induced delay in tumor growth appeared to be associated with an increased infiltration of the tumors by leukocytes, including granulocytes such as PMNs [19]. This led us to hypothesize that the anti-tumor effects of FR-WBH may be dependent on the thermal enhancement of inflammatory activity of granulocytes. Thus, here we analyze the relationship between FR-WBH and granulocytes in the control of colon tumor growth in mice.
MATERIALS AND METHODS
Mice
Female BALB/c and SCID mice (Taconic Laboratories, Germantown, PA) ranging from 8–10 weeks of age were used in these experiments, all of which were approved by the Institute Animal Care and Use Committee at Roswell Park.
Tumor Growth
Prior to administration into BALB/c or SCID mice, murine colon tumor line CT26 or human colon tumor line HT29 was maintained in RPMI media with 10% FCS, 2mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 55mM β-mercaptoethanol. Tumor cells were then washed, resuspended in sterile 0.9% saline at a final concentration of 2x107 per mL, and 50μL aliquots (106 cells) were injected subcutaneously (s.c.) into the lower abdomen of each mouse. Tumor volumes were determined using the equation (x2y)/2, where x was the shortest diameter and y was the longest diameter of each s.c. tumor.
Fever-range Whole Body Heating (WBH)
To prevent dehydration, mice were injected i.p. with 1ml sterile 0.9% saline, and then placed in pre-heated cages (5 mice/cage). Cages were placed in an environmental chamber (Memmert model BE500), and within 30 min the average core body temperatures of the mice were raised to 39 – 39.5ºC, which was then maintained for 8 hrs. Core temps were monitored using microchip transponders implanted s.c. into the dorsal thoracic area. Control mice were kept at RT and subjected to manipulations similar to that of the heated mice. WBH treatments were initiated one day after tumor cell injection and repeated once a week.
Immunohistochemistry
Zn2+ fixed, paraffin-embedded sections of tumor, lung, liver or normal colon tissue were deparaffinized and rehydrated. After the endogenous peroxidase activity was quenched for 30 min in 3% hydrogen peroxide in PBS, nonspecific binding was blocked by treatment with 0.03% casein in PBS. The primary antibodies of rat anti-Gr-1 and rat IgG2a isotype control (BD Pharmingen, Los Angeles, CA) were diluted 1:1000 and applied for 1hr at room temperature in a humidified chamber. After the sections had been washed with PBS + 0.5% Tween, a biotinylated goat anti-rat antibody (BD Pharmingen) at 1:250 dilution was applied for 30 min at room temperature in a humidified chamber. This was followed by washing in PBS three times, incubation with streptavadin, three more washes, and then DAB peroxidase substrate (Vector Laboratories, Inc., Bulingame, CA) was applied. After using hematoxylin as a counterstain, the slides were dehydrated and coverslips were mounted with Permount (Fisher Scientific, Pittsburgh, PA).
Granulocyte Depletion
Ascites, generated using the ant-Gr-1 producing RB6-8C5 hybridoma in SCID mice, or purified NA/LE anti-Gr-1 antibody (clone RB6-8C5, BD Pharmingen) was injected i.p. to systemically deplete granulocytes in mice. Treatments involved 75 μL injections of anti-Gr-1 ascites every 12 days in SCID mice or 100μL injections of pure anti-Gr-1 every 7 days in BALB/c mice, and were initiated one day prior to tumor cell injection.
Respiratory Burst Measurements
Using PerC wash (1 x PBS, 0.1% BSA, 10 U/mL heparin, 0.54 mM EDTA) as previously described [21], peritoneal cells were collected from heated or control BALB/c mice, pelleted and resuspended in 200μL of PBS before incubating with 25μL of a 30μg/mL solution of dihydrorhodamine (DHR) at 37ºC for 5 min. Cells were washed with PBS before being fixed in 2% paraformaldehyde. Flow cytometric analysis was used to determine the percent of granulocyte gated cells that expressed oxidised DHR (i.e., rhodamine+).
Statistical Analysis
Mean control values were compared to mean experimental values at each time point using unpaired Student’s t-tests. P-values less than or equal to 0.05 were considered to represent statistically significant differences.
RESULTS
Anti-Tumor Effect of FR-WBH Correlates with Increased Tumor Infiltrating Granulocytes
Previous studies have revealed that mild thermal stress applied in vivo can delay the growth of established CT26 tumors in BALB/c mice [19]. Here we have extended these studies by examining the ability of fever-range whole body hyperthermia (FR-WBH) to prevent the establishment and growth of CT26 cells inoculated subcutaneously into BALB/c mice. As expected, weekly administration of FR-WBH significantly delayed the outgrowth of CT26 (Figure 1), and the third application of FR-WBH on day 15 in particular was able to significantly inhibit the growth of established tumors in mice for 3 more days. This is consistent with what has been previously observed when FR-WBH has been administered to mice bearing established murine colon tumors [19,20].
Figure 1.
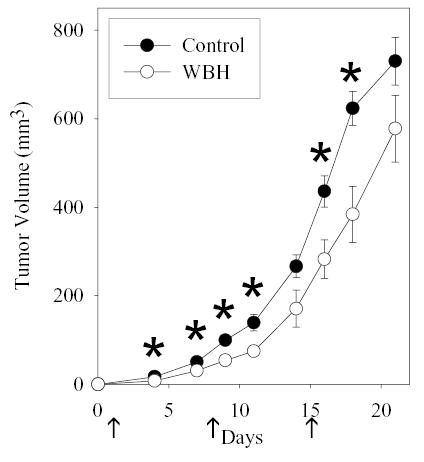
FR-WBH delays CT26 growth in BALB/c mice. Weekly WBH treatments (arrows) were initiated one day after 106 CT26 cells were injected s.c. and tumor growth was monitored in both heated (white circles) and unheated (black circles) mice. Average tumor volumes ± S.E. are depicted with an n= 5 mice per group. *, p < 0.05 when comparing values from heated and non-heated mice at each time point using the unpaired Student’s t-test.
While H&E staining had suggested that infiltration of polymorphonuclear neutrophils (PMNs) might be enhanced in tumors that underwent mild thermal stress [19], immunohistochemical staining was performed here to better define the tumor infiltrating leukocyte populations upon FR-WBH treatment of CT26 bearing mice. Using antibodies that recognize the Gr-1 antigen, numbers of granulocytes that migrated to the tumor site were found to be significantly enhanced by fever-range thermal stress (Figure 2). This increased granulocytic infiltrate was observed within 4 hrs of FR-WBH. Importantly, this effect on granulocyte homing appeared to be specific to the tumor as increased granulocyte infiltration into the mouse lung, liver, or normal colon tissue was not observed at any time during or after an 8hr FR-WBH treatment (data not shown).
Figure 2.
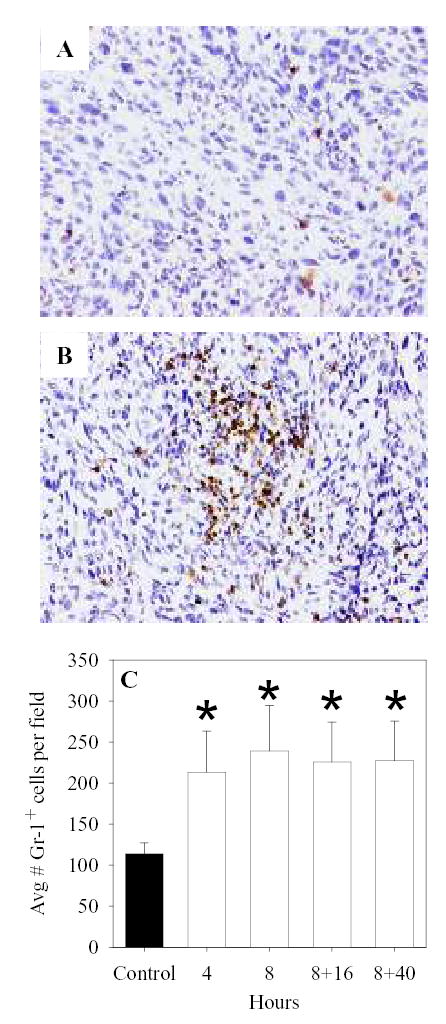
Increased numbers of Gr-1+ cells are observed in CT26 tumors of heated mice. After CT26 tumors were established s.c., BALB/c mice were heated (B, and white bars of C) or left as controls (A and black bar of C). At different times during and after and 8hr WBH, tumors were harvested, fixed, embedded in paraffin and then sectioned and stained for the granulocyte marker Gr-1. Average numbers of positively staining cells were determined per 40X field in three fields of sections made with three different tumors at each time point. *, p < 0.05 when compared to non-heated controls.
Granulocyte Depletion Ablates the Anti-Tumor Effect of FR-WBH
To determine whether or not the ability of FR-WBH to delay tumor growth is dependent on granulocytes, antibody depletion studies were performed (Figure 3). Loss of granulocytes in BALB/c mice that had been inoculated with CT26 ablated the ability of FR-WBH to inhibit the growth of these tumors. This was also examined using SCID mice inoculated with human colon tumor cells (Figure 3B). This SCID model, where T cells and B cells are absent, was of particular interest due to its potential to isolate the role of innate immune cells such as granulocytes. Not only was it found that weekly WBH administration inhibited the outgrowth of HT29 tumors, but this effect of WBH was again lost when granulocytes were depleted in vivo. Overall, these data suggest that granulocytes play an important role in FR-WBH induced tumor growth control.
Figure 3.
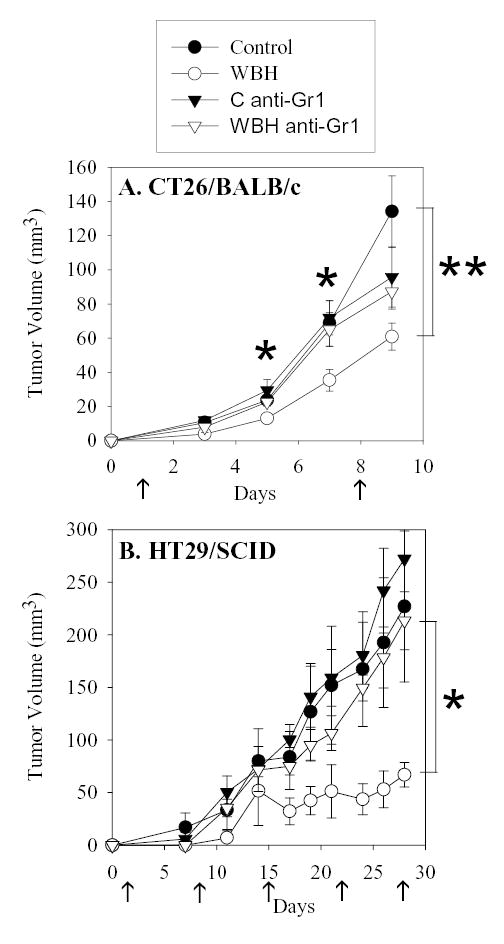
Granulocyte depletion ablates the effect of FR-WBH on colon tumor cell lines grown in mice. BALB/c mice were injected s.c. with 106 CT26 cells (A) or SCID mice were injected s.c. with 106 HT29 cells (B) one day after granulocyte depletion was initiated (anti-Gr-1). FR-WBH commenced on day 1, and was repeated every 7 days (arrows). Average tumor volumes ± S.E. are depicted with an n= 5 mice per group. **, p < 0.05 when comparing values from heated (WBH) and non-heated (Control) groups; *, p < 0.05 when comparing values from granulocyte depleted and non-depleted WBH treated groups using the unpaired Student’s t-test.
FR-WBH Upregulates Granulocyte Respiratory Burst
To begin to assess the ability of mild thermal stress to regulate granulocyte activity in vivo, dihydrorhodamine staining was used for the measurement of neutrophil respiratory burst. Because of the highly sensitive nature of these suicidal inflammatory cells, these studies were performed using granulocytes harvested from the peritoneum, which allowed the least manipulation of cells (i.e., lysing of red blood cells and homogenization of tissue was not required). Importantly, a significantly higher percent of granulocytes exhibited respiratory burst after WBH treatment compared to controls (Figure 4).
Figure 4.
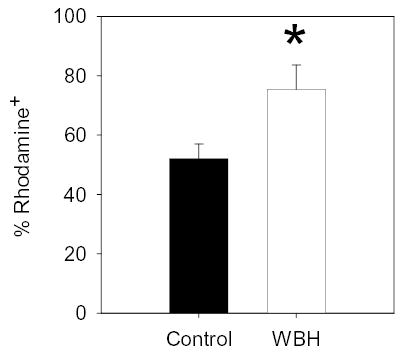
FR-WBH enhances respiratory burst of granulocytes. Directly after an 8hr FR-WBH treatment, peritoneal cells were collected from heated (white bar) or control (black bar) BALB/c mice and stained with DHR. Flow cytometric analysis was used to determine the percent of granulocyte gated cells that expressed oxidised DHR (i.e., rhodamine+). N = 3 mice per group. *, p < 0.05 when using the unpaired Student’s t-test.
DISCUSSION
Consistent with previous studies examining established tumors, these results reveal the potential for WBH to significantly slow the establishment and growth of colon tumor cells in mice in a manner that correlates with enhanced tumor-specific granulocyte infiltration. This ability of FR-WBH to enhance granulocyte homing to the tumor is consistent with other reports showing the increased accumulation of granulocytes at the site of inoculation in various infectious models upon administration of mild thermal stress in vivo [5,22–24]. Furthermore, studies examining the thermal regulation of lymphocyte migration suggest that the mechanism by which WBH induces the homing of granulocytic leukocytes to the tumor bed may be mediated by L-selectin dependent adhesion [25,26]. Thus, future studies investigating the role of chemokines and adhesion molecules such as L-selectin in the WBH mediated granulocyte homing specifically to the tumor site are currently underway.
While the increased homing of granulocytes to the tumor site may be enough to mediate the WBH-induced tumor growth control, our data (Fig. 4) suggest that mild thermal stress might also enhance the activity of the granulocytes once they reach the tumor. The ability of mild thermal stress to enhance the respiratory burst of murine granulocytes is supported by various studies performed by others. Specifically, enhanced respiratory burst of human PMNs has been found after mild thermal stress both in vivo [27] and in vitro[28]. Indeed, in contrast to temperatures of 41°C and higher, where PMN activity is generally inhibited (reviewed in [8]), temperatures ranging from 38 to 40°C appear to stimulate PMNs, including their bactericidal activity [28–31], and phagocytosis [22,32]. However, the regulation of PMN tumoricidal activity by mild thermal stress has yet to be fully examined.
The potential importance of PMNs in cancer immunotherapy may be inferred by their ability to both respond to and produce cytokines [33,34], thus making them involved in a complex cross-talk with immune and endothelial cells that bridges innate and adaptive immunity. Interestingly, circulating granulocytes in many cancer patients have been reported to be impaired in their ability to produce superoxide anion [35], to perform antibody-dependent tumor cell lysis [36], and to mobilize in vivo to skin chambers [37]. While PMNs are normally a scarce reactive component of both human and animal tumors [38], it has been suggested that these cells are active in immunosurveillance against several tumors [38–40]. During rIL-2 infusion in patients with malignant melanoma and renal cell carcinoma, there has been phenotypic and functional evidence of potent PMN activation [38]. Furthermore, in cytokine-transfected tumor models, it was observed that the pro-inflammatory cytokines and an influx of granulocytes at the tumor site results in a debulking phase of tumor rejection, which is followed by the inducement of specific anti-tumor T cell responses that complete the tumor rejection and produce long-lived anti-tumor immunity [41,42]. Overall, studies such as these support the importance of identifying therapeutic approaches, such as the FR-WBH described in this report, which are capable of stimulating the recruitment and activity of PMNs or granulocytes within the tumor microenvironment.
On the other hand, there have been immune inhibitory effects ascribed to various inflammatory mediators, including granulocytes (reviewed in [43]). Furthermore, with colon cancer in particular, progression of this disease has been associated with chronic inflammation [44–46]. That is, in contrast to acute inflammatory responses, where infection is resolved within a matter of days, chronic inflammation appears to generate a ‘potentially vicious self-sustaining loop’ which results in a microenvironment that is favorable for the survival and growth of tumors [46]. However, the FR-WBH protocol utilized in this study, where the animal experiences mild thermal stress for a total of 8hrs, might be thought of as more similar to an acute (i.e., not chronic) inflammatory episode. Thus we suggest that the roles of chronic and acute inflammatory processes should be separately investigated in the laboratory with regards to their clinical potential in the treatment of cancer. The studies presented here suggest that the inflammatory events supported by a FR-WBH treatment have the potential to help control the growth of tumors.
It is still not clear how granulocytes are able to sense changes in their thermal microenvironment. One might speculate that temperature regulates overall cellular metabolism in such a way that it affects the respiratory burst activity of all cells. Alternatively, immune cells such as granulocytes may be particularly sensitive to temperatures in a manner that has evolved in concert with the fever response to help enhance their response to infections. For example, membrane associated events such as those involving the regulation of lipid fluidity or Ca2+ channels may be involved in the ability of immune cells to sense changes in their thermal microenvironment. The expression of several genes that respond to changes in temperature appear to be influenced or controlled by the membrane’s physical state [47]. Heat-induced changes in intracellular free Ca2+, which correlated with the activation of phosphoinositide turnover, has led to the speculation that this class of lipids as well as Ca2+ homeostatis may be involved in cellular responses to heat [48]. In addition, our laboratory has most recently become interested in investigating the expression of TRPV receptors, which are Ca2+-permeant ion channels that have been identified as heat receptors on sensory neurons [49], on various immune cells types including granulocytes. Overall, further analysis of the mechanisms by which mild thermal stress regulates granulocyte mediated tumor-growth control will help clarify when thermal therapy might best be clinically administered.
Acknowledgments
The authors wish to thank Dr. Elizabeth A. Repasky for many discussions which helped direct these studies and for critical review of this manuscript. This work was supported in part by the National Institutes of Health grants R01 CA71599, P01 CA94045 and R21 CA098852, and the Roswell Park Cancer Institute core grant CA16056.
References
- 1.Kluger MJ, Kozak W, Conn CA, Leon LR, Soszynski D. The adaptive value of fever. . Infect. Dis. Clin. N. Am. 1996;10(1):1–20. doi: 10.1016/s0891-5520(05)70282-8. [DOI] [PubMed] [Google Scholar]
- 2.Vaughn LK, Bernheim HA, Kluger MJ. Fever in the lizard Dipsosaurus dorsalis. Nature. 1974;252(5483):473–474. doi: 10.1038/252473a0. [DOI] [PubMed] [Google Scholar]
- 3.Bell JF, Moore GJ. Effects of high ambient temperature on various stages of rabies virus infection in mice. . Infect. Immun. 1974;10(3):510–515. doi: 10.1128/iai.10.3.510-515.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Covert JB, Reynolds WW. Survival value of fever in fish. Nature. 1977;267(5607):43–45. doi: 10.1038/267043a0. [DOI] [PubMed] [Google Scholar]
- 5.Bernheim HA, Bodel PT, Askenase PW, Atkins E. Effects of fever on host defense mechanisms after infection in the lizard Dipsosaurus dorsalis. . Br. J. Exp. Pathol. 1978;59(1):76–84. [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang Q, Cross AS, Singh IS, Chen TT, Viscardi RM, Hasday JD. Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect. Imm. 2000;68(3):1265–1270. doi: 10.1128/iai.68.3.1265-1270.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt JR, Rasmussen AFJ. The influence of environmental temperature on the course of experimental herpes simplex infection. . J. Infect. Dis. 1960;107:356. doi: 10.1093/infdis/107.3.356. [DOI] [PubMed] [Google Scholar]
- 8.Hasday JD, Fairchild KD, Shanholtz C. The role of fever in the infected host. . Microbes, Infect. 2000;2(15):1891–1904. doi: 10.1016/s1286-4579(00)01337-x. [DOI] [PubMed] [Google Scholar]
- 9.Ostberg JR, Kabingu E, Repasky EA. Thermal regulation of dendritic cell activation and migration from skin explants. Int. J. Hyperthermia. 2003;19(5):520–533. doi: 10.1080/02656730310001607986. [DOI] [PubMed] [Google Scholar]
- 10.Ostberg JR, Taylor SL, Baumann H, Repasky EA. Regulatory effects of fever-range whole-body hyperthermia on the LPS-induced acute inflammatory response. . J. Leuk. Biol. 2000;68(6):815–820. [PubMed] [Google Scholar]
- 11.Jiang Q, Detolla L, Singh IS, et al. Exposure to febrile temperature upregulates expression of pyrogenic cytokines in endotoxin-challenged mice. Am. J. Physiol. 1999;276(6 Pt 2):R1653–1660. doi: 10.1152/ajpregu.1999.276.6.R1653. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt JA, Abdulla E. Down-regulation of IL-1 beta biosynthesis by inducers of the heat-shock response. J. Immunol. 1988;141(6):2027–2034. [PubMed] [Google Scholar]
- 13.Ensor JE, Wiener SM, McCrea KA, Viscardi RM, Crawford EK, Hasday JD. Differential effects of hyperthermia on macrophage interleukin-6 and tumor necrosis factor-alpha expression. . Am. J. Physiol. 1994;266(4 Pt 1):C967–974. doi: 10.1152/ajpcell.1994.266.4.C967. [DOI] [PubMed] [Google Scholar]
- 14.Klostergaard J, Barta M, Tomasovic SP. Hyperthermic modulation of tumor necrosis factor-dependent monocyte/macrophage tumor cytotoxicity in vitro. J. Biol. Resp. Mod. 1989;8(3):262–277. [PubMed] [Google Scholar]
- 15.Fouqueray B, Philippe C, Amrani A, Perez J, Baud L. Heat shock prevents lipopolysaccharide-induced tumor necrosis factor-alpha synthesis by rat mononuclear phagocytes. Eur. J. Immunol. 1992;22(11):2983–2987. doi: 10.1002/eji.1830221133. [DOI] [PubMed] [Google Scholar]
- 16.Snyder YM, Guthrie L, Evans GF, Zuckerman SH. Transcriptional inhibition of endotoxin-induced monokine synthesis following heat shock in murine peritoneal macrophages. . J. Leuk. Biol. 1992;51(2):181–187. doi: 10.1002/jlb.51.2.181. [DOI] [PubMed] [Google Scholar]
- 17.Pritchard, M.T., Li, Z., & Repasky, E.A. Nitric oxide production is regulated by fever-range thermal stimulation of murine macrophages. J. Leuk. Biol. 2004, Accepted pending revision. [DOI] [PubMed]
- 18.Matsuda H, Strebel FR, Kaneko T, et al. Long duration-mild whole body hyperthermia of up to 12 hours in rats: feasibility, and efficacy on primary tumour and axillary lymph node metastases of a mammary adenocarcinoma: implications for adjuvant therapy. Int. J. Hyperthermia. 1997;13(1):89–98. doi: 10.3109/02656739709056433. [DOI] [PubMed] [Google Scholar]
- 19.Burd R, Dziedzic TS, Xu Y, Caligiuri MA, Subjeck JR, Repasky EA. Tumor cell apoptosis, lymphocyte recruitment and tumor vascular changes are induced by low temperature, long duration (fever-like) whole body hyperthermia. . J. Cell. Physiol. 1998;177(1):137–147. doi: 10.1002/(SICI)1097-4652(199810)177:1<137::AID-JCP15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 20.Repasky EA, Tims E, Pritchard M, Burd R. Characterization of mild whole-body hyperthermia protocols using human breast, ovarian, and colon tumors grown in severe combined immunodeficient mice. Infect. Dis. Ob. Gyn. 1999;7(1–2):91–97. doi: 10.1155/S1064744999000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostberg JR, Repasky EA. Comparison of the effects of two different whole body hyperthermia protocols on the distribution of murine leukocyte populations. Int. J. Hyperthermia. 2000;16(1):29–43. doi: 10.1080/026567300285402. [DOI] [PubMed] [Google Scholar]
- 22.van Oss CJ, Absolom DR, Moore LL, Park BH, Humbert JR. Effect of temperature on the chemotaxis, phagocytic engulfment, digestion and O2 consumption of human polymorphonuclear leukocytes. . J. Reticuloendothel. Soc. 1980;27(6):561–565. [PubMed] [Google Scholar]
- 23.Nahas GG, Tannieres ML, Lennon JF. Direct measurement of leukocyte motility: effects of pH and temperature. Proc. Soc. Exp. Biol. Med. 1971;138(1):350–352. doi: 10.3181/00379727-138-35894. [DOI] [PubMed] [Google Scholar]
- 24.Bryant RE, DesPrez RM, VanWay MH, Rogers DE. Studies on human leukocyte motility. I. Effects of alterations in pH, electrolyte concentration, and phagocytosis on leukocyte migration, adhesiveness, and aggregation. . J. Exp. Med. 1966;124:483–499. doi: 10.1084/jem.124.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang WC, Goldman LM, Schleider DM, et al. Fever-range hyperthermia enhances L-selectin-dependent adhesion of lymphocytes to vascular endothelium. . J. Immunol. 1998;160(2):961–969. [PubMed] [Google Scholar]
- 26.Evans SS, Wang WC, Bain MD, Burd R, Ostberg JR, Repasky EA. Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood. 2001;97(9):2727–2733. doi: 10.1182/blood.v97.9.2727. [DOI] [PubMed] [Google Scholar]
- 27.Kappel M, Kharazmi A, Nielsen H, Gyhrs A, Pedersen BK. Modulation of the counts and functions of neutrophils and monocytes under in vivo hyperthermia conditions. Int. J. Hyperthermia. 1994;10(2):165–173. doi: 10.3109/02656739409009341. [DOI] [PubMed] [Google Scholar]
- 28.Johansen KS, Berger EM, Repine JE. Effect of temperature on polymorphonuclear leukocyte function. . Acta Pathol. Microbiol. Immunol. Scand. 1983;91(6):355–359. [PubMed] [Google Scholar]
- 29.Hiruma M, Kagawa S. Effects of hyperthermia on phagocytosis and intracellular killing of Sporothrix schenckii by polymorphonuclear leukocytes. . Mycopathol. 1986;95(2):93–100. doi: 10.1007/BF00437167. [DOI] [PubMed] [Google Scholar]
- 30.Roberts N. J., Jr., & Steigbigel, R.T. Hyperthermia and human leukocyte functions: effects on response of lymphocytes to mitogen and antigen and bactericidal capacity of monocytes and neutrophils. . Infect. Immun. 1977;18(3):673–679. doi: 10.1128/iai.18.3.673-679.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sebag J, Reed WP, Williams RC. Effect of temperature on bacterial killing by serum and by polymorphonuclear leukocytes. Infect. Immun. 1977;16:947–954. doi: 10.1128/iai.16.3.947-954.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellingson HV, Clark PF. The influence of artificial fever on mechanisms of resistance. J. Immunol. 1942;43:65–83. [Google Scholar]
- 33.Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol. Today. 1995;16(1):21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 34.Lloyd AR, Oppenheim JJ. Poly’s lament: the neglected role of the polymorphonuclear neutrophil in the afferent limb of the immune response. Immunol. Today. 1992;13(5):169–172. doi: 10.1016/0167-5699(92)90121-M. [DOI] [PubMed] [Google Scholar]
- 35.Hara N, chinose Y, Asoh H, Yano T, Kawasaki M, Ohta M. Superoxide anion-generating activity of polymorphonuclear leukocytes and monocytes in patients with lung cancer. Cancer. 1992;69(7):1682–1687. doi: 10.1002/1097-0142(19920401)69:7<1682::aid-cncr2820690707>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 36.Dallegri F, Ballestrero A, Ottonello L, Patrone F. Defective antibody-dependent tumour cell lysis by neutrophils from cancer patients. . Clin. Exp. Immunol. 1989;77:58–61. [PMC free article] [PubMed] [Google Scholar]
- 37.Frumento G, Bonvini E, Minervini F, Dallegri F, Patrone F, Sacchetti C. Defective neutrophil mobilization to skin chambers in cancer patients. . J. Cancer Res. Clin. Oncol. 1984;107:53–56. doi: 10.1007/BF00395491. [DOI] [PubMed] [Google Scholar]
- 38.Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97(2):339–345. doi: 10.1182/blood.v97.2.339. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto Y, Saiki I, Murata J, Okuyama H, Tamura M, Azuma I. Recombinant human granulocyte colony-stimulating factor inhibits the metastasis of hematogenous and non-hematogenous tumors in mice. Int. J. Cancer. 1991;49(3):444–449. doi: 10.1002/ijc.2910490323. [DOI] [PubMed] [Google Scholar]
- 40.Midorikawa Y, Yamashita T, Sendo F. Modulation of the immune response to transplanted tumors in rats by selective depletion of neutrophils in vivo using a monoclonal antibody: abrogation of specific transplantation resistance to chemical carcinogen-induced syngeneic tumors by selective depletion of neutrophils in vivo. Cancer Res. 1990;50(19):6243–6247. [PubMed] [Google Scholar]
- 41.Colombo MP, Forni G. Immunotherapy. I: Cytokine gene transfer strategies. . Cancer, Met. Rev. 1997;16(3–4):421–432. doi: 10.1023/a:1005980418533. [DOI] [PubMed] [Google Scholar]
- 42.Musiani P, Allione A, Modica A, et al. Role of neutrophils and lymphocytes in inhibition of a mouse mammary adenocarcinoma engineered to release IL-2, IL-4, IL-7, IL-10, IFN-alpha, IFN-gamma, and TNF-alpha. . Lab. Invest. 1996;74(1):146–157. [PubMed] [Google Scholar]
- 43.Kiessling R, Wasserman K, Horiguchi S, et al. Tumor-induced immune dysfunction.[comment]. Cancer Immunol. Immunother. 1999;48(7):353–362. doi: 10.1007/s002620050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ernst P. Review article: the role of inflammation in the pathogenesis of gastric cancer. Alimentary Pharmacol. Ther. 1999;13(Suppl 1):13–18. doi: 10.1046/j.1365-2036.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 45.Orlando RC. Mechanisms of epithelial injury and inflammation in gastrointestinal diseases. . Rev. Gastroenterol. Disord. 2002;2(Suppl 2):S2–8. [PubMed] [Google Scholar]
- 46.Schwartsburd PM. Chronic inflammation as inductor of pro-cancer microenvironment: pathogenesis of dysregulated feedback control. Cancer Met. Rev. 2003;22(1):95–102. doi: 10.1023/a:1022220219975. [DOI] [PubMed] [Google Scholar]
- 47.Vigh L, Maresca B, Harwood JL. Does the membrane’s physical state control the expression of heat shock and other genes? . Trends Biochem. Sci. 1998;23(10):369–374. doi: 10.1016/s0968-0004(98)01279-1. [DOI] [PubMed] [Google Scholar]
- 48.Calderwood SK, Stevenson MA, Hahn GM. Effects of heat on cell calcium and inositol lipid metabolism. . Rad. Res. 1988;113(3):414–425. [PubMed] [Google Scholar]
- 49.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]


