Abstract
Purpose:
Phosphodiesterase (PDE) inhibitors are important therapeutic agents, but their effects on photoreceptor PDE (PDE6) and photoreceptor cells are poorly understood. We characterized the potency and selectivity of various classes of PDE inhibitors on purified rod and cone PDE6 and on intact rod outer segments (ROS).
Methods:
The inhibition constant (KI) of isozyme-selective PDE inhibitors was determined for purified rod and cone PDE6. Perturbations of cGMP levels in isolated ROS suspensions by PDE inhibitors were quantitated by a cGMP enzyme-linked immunoassay.
Results:
Most PDE5-selective inhibitors are excellent PDE6 inhibitors. Vardenafil, a potent PDE5 inhibitor (KI = 0.2 nM), is the most potent PDE6 inhibitor tested (KI = 0.7 nM). Zaprinast is the only drug that inhibits PDE6 more potently than PDE5. PDE1-selective inhibitors were equally effective in inhibiting PDE6. In intact ROS, PDE inhibitors elevated cGMP levels but none fully inhibited PDE6. Their potency to elevate cGMP levels in ROS was much lower than their ability to inhibit the purified enzyme. Competition between PDE5/6-selective drugs and the inhibitory γ subunit for the active site of PDE6 is proposed to reduce the effectiveness of drugs at the enzyme active site.
Conclusions:
Several classes of PDE inhibitors equally well inhibit PDE6 as the PDE family to which they are targeted. In intact ROS, high PDE6 concentrations, binding of the γ subunit to the active site, and calcium feedback mechanisms attenuate the effectiveness of PDE inhibitors to inhibit PDE6 and disrupt the cGMP signaling pathway during visual transduction.
Introduction
Visual transduction is mediated by complex biochemical pathways that precisely regulate cGMP levels in the signal transducing outer segment portion of vertebrate retinal photoreceptors for reviews, see 1–3]. Disruptions of cGMP metabolism in retinal photoreceptors have serious consequences for visual functioning. Most genetic mutations correlated with retinitis pigmentosa and related diseases are found in genes coding for proteins of the phototransduction cascade, including photoreceptor phosphodiesterase (PDE6), guanylate cyclase (GC), and their associated regulatory subunits 4, 5. In many such cases, persistent elevation of cGMP concentration in retinal photoreceptors results in disruption of retinal development and/or photoreceptor apoptosis.
The rod and cone photoreceptor PDE6 belong to a superfamily of eleven distinct cyclic nucleotide PDEs 6. Rod and cone PDE6 are most closely related to PDE5—a cGMP-specific, cGMP binding PDE—in structural, biochemical and pharmacological properties 7. Drugs that selectively and potently target PDE5 such as sildenafil (Viagra®), vardenafil (Levitra®), and tadalafil (Cialis®) have recently been approved for treatment of male erection dysfunction. These drugs represent the first major successful application of PDE inhibitor therapy to an individual family of PDEs, supplanting nonspecific methylxanthine PDE inhibitors (e.g., theophylline, caffeine) used in the past 8–11.
Remarkably little is known of the effects of PDE5-selective and other family-specific drugs on PDE6 and on cGMP metabolism in photoreceptors. Pre-clinical and clinical data on the effects of sildenafil reveal significant but transitory effects on visual function, presumably through inhibition of photoreceptor PDE6 12. Tadalafil and vardenafil, two other approved drugs, show lesser effects on visual function 13. No systematic research of purified rod and cone PDE6 inhibition by various classes of PDE-selective inhibitors has been published in the literature, and only isolated data exist on potential differences between inhibition of the rod and cone PDE6 isozymes 14, 15. Electrophysiological measurements of individual rod photoreceptors exposed to IBMX 16, 17 or zaprinast 18 demonstrate direct effects of PDE6 inhibition on the light responsiveness of rod photoreceptors, consistent with a drug-induced elevation in cGMP content 19. Effects of PDE inhibitors on the electroretinogram or on human psychophysical measurements of visual function [reviewed in 12] are also consistent with direct inhibition of PDE6 in rods and cones.
In this paper, we survey the potency and selectivity of PDE inhibitors targeted to PDE1 through PDE5 to inhibit purified rod and cone PDE6. We also examine the effects of nonselective and PDE5/6-selective inhibitors on the cGMP concentration in the signal-transducing outer segment of rod photoreceptors. We show a general lack of discrimination of rod and cone photoreceptor PDE6 with respect to most drugs that have been designed to target a specific PDE family. However, in terms of the effectiveness of PDE inhibitors to elevate cGMP levels in intact ROS, we have identified several mechanisms that oppose and minimize the ability of PDE inhibitors to disrupt cGMP metabolism in photoreceptor cells.
Methods
Materials.
Frogs (Rana catesbeiana) were obtained from Charles Sullivan, Inc., and kept in controlled lighting conditions (12 h dark, 12 h light) for 2 weeks before use. Animals were treated in accordance with ARVO guidelines, and protocols were approved by the institutional animal care and use committee. Bovine retinas were purchased from W.L. Lawson, Inc. E4021 was a gift from Eisai Co., Ltd, vardenafil was provided by Bayer and sildenafil and tadalafil were synthesized. All other reagents were from Sigma. All PDE inhibitors were prepared as stock solutions in DMSO, and diluted in buffer before use so that the final concentration of DMSO was always less than 1%. Ringer’s solution consisted of (in mM): 105 NaCl, 2 KCl, 2 MgCl2, 1 CaCl2, 5 glucose, 10 HEPES, pH 7.5. The ROS homogenization buffer contained (in mM): 100 Tris, pH 7.5, 10 MgCl2, 0.5 EDTA, 1 dithiothreitol, 0.5 mg/ml BSA, and mammalian protease inhibitor cocktail (Sigma). PDE assay buffer contained 20 mM Tris, pH 7.5, 10 mM MgCl2, and 0.5 mg/ml BSA.
PDE6 purification and PDE activity assay.
Membrane-associated bovine rod and soluble cone PDE6 was purified from frozen bovine retinas exactly as described recently 20. Activation of rod and cone PDE6 was carried out by limited trypsin proteolysis of the inhibitory γ subunit 20. Rod or cone PDE6 was incubated with each drug for 15 min at room temperature prior to substrate addition. PDE activity was measured by either a phosphate release microplate assay (2 mM cGMP, 0.2 nM PDE6) or by a radiotracer assay (1.0 μM cGMP, 2.0 pM PDE6) 21; similar KI values were obtained with both assays.
Purification of intact ROS and preparation of ROS homogenates.
Intact frog ROS were purified on a discontinuous Percoll density gradient as described previously 21, 22. In brief, ROS were detached from dark-adapted retinas by gentle shaking in Ringer’s supplemented with 5% Percoll. The ROS were then purified by centrifugation in a discontinuous Percoll gradient consisting of 5, 30, 44 and 60% Percoll. Intact ROS were recovered from the 44/60 % Percoll interface, and were judged to be >90% osmotically sealed as determined by exclusion of the dye, didansylcysteine 23. The total cGMP levels in these ROS (0.008 ± 0.001 mol cGMP per mol rhodopsin; n = 16) are similar to previous measurements of isolated photoreceptors 22 and photoreceptors attached to the retina 24.
The concentration of rhodopsin in ROS suspensions was determined spectrophotometrically 25. To buffer the intracellular free calcium concentration of ROS at their dark-adapted level [~500 nM for amphibian ROS; 26], intact ROS were incubated in Ringer’s supplemented with 1.09 mM EGTA for 10 minutes before addition of a PDE inhibitor.
Homogenized ROS were prepared by pooling intact ROS with disrupted ROS (found at the 30/44% Percoll interface). Percoll was removed by dilution with Ringer’s and subsequent centrifugation (1 min at 3000 × g). The ROS pellet was resuspended in homogenization buffer and homogenized until no organelle structures were visible by phase-contrast microscopy 27. ROS nucleotides (particularly cGMP) were depleted (>95% loss) by incubating homogenized ROS at 22°C for 1 h 28.
cGMP concentration measurement.
cGMP was extracted by quenching with 50% HCl/ethanol, followed by centrifugation. The acidic supernatant containing cGMP was dried down in a vacuum concentrator. The cGMP concentration was determined by cGMP enzyme-linked immunoassay (Amersham) with reference to standards treated identically to the experimental samples.
Data analysis.
The inhibition constant (KI) was calculated from the sigmoidal concentration dependence curve using the following equation 29: KI = IC50/(1+[S]/KM), where IC50 is the concentration of inhibitor that reduces catalytic activity in vitro by 50%, [S] is the substrate concentration, and KM is the Michaelis constant. The following values for KM were used: 14 μM for purified bovine rod PDE6 30; 7 μM for purified bovine cone PDE6 (B.A. Valeriani and R.H. Cote, unpublished data); 20 and 60 μM for activated or non-activated frog PDE6, respectively 31. All experiments were repeated at least three times and average values are reported as the mean ± S.D. Curve fitting was performed using Sigmaplot (SPSS, Inc).
Results
Certain classes of PDE inhibitors fail to discriminate photoreceptor PDE6.
We first tested a set of non-specific and family-specific PDE inhibitors for their ability to inhibit purified PDE6. Both rod and cone PDE6 were tested in their activated state in which the inhibitory γ subunit is absent. Dose-response curves were generated for each inhibitor, and the drug inhibition constant (KI) was calculated based on the IC50 and knowledge of the KM for each enzyme (see Methods).
Table 1 summarizes the results of testing 15 PDE inhibitors that represent both non-specific inhibitors (e.g., IBMX) as well as class-specific inhibitors of PDE1 through PDE5. [Family-specific inhibitors of PDE7-PDE11 are not currently available.] We found that inhibitors of PDE3 and PDE4 were much less potent in inhibiting rod or cone PDE6 than their own PDE family. The selectivity (defined as the ratio of inhibition constants for PDE6 versus PDE-X) ranged from 40–50 (for the PDE4 inhibitor rolipram) to ~300 (for the PDE3 inhibitor cilostamide) and up to 5700 for the PDE4 inhibitor YM976. The PDE2 inhibitor, EHNA, showed low potency and a modest ability to discriminate PDE2 from PDE6. In contrast, both PDE1 inhibitors tested (8-methoxymethyl-IBMX and vinpocetine) are unable to discriminate PDE1 from rod PDE6. Interestingly, 8-methoxymethyl-IBMX shows 10-fold selectivity for cone PDE6 compared to PDE1, although in neither case is the drug very potent (cone PDE6 KI = 0.4 μM). We conclude that vinpocetine and 8-methoxymethyl-IBMX are more accurately defined as PDE1/6-specific inhibitors.
Table 1.
The efficacy and selectivity of PDE inhibitors to inhibit purified, activated bovine rod and cone PDE6.
| PDE6 KI (nM)b | Selectivityc | ||||||
|---|---|---|---|---|---|---|---|
| Class | Inhibitor | PDE(X)KI (nM)a | Rod | Cone | 6R/6C | 6R/X | 6C/X |
| 1 | vinpocetine | 11500 | 21000 ± 6100 | 13000 ± 1200 | 1.6 | 1.8 | 1.1 |
| 8-Me-IBMX | 3300 | 1600 ± 100 | 430 ± 30 | 3.7 | 0.5 | 0.1 | |
| 2 | EHNA | 2500 | 28000 ± 2400 | 13000 ± 1600 | 2.2 | 11 | 5 |
| 3 | cilostamide | 10 | 2800 ± 170 | 3400 ± 660 | 0.8 | 280 | 340 |
| 4 | rolipram | 490 | 28000 ± 620 | 22000 ± 4000 | 1.3 | 57 | 45 |
| YM 976 | 3.3 | 19000 ± 1500 | 5200 ± 630 | 3.7 | 5760 | 1580 | |
| 5 | tadalafil | 3.3 | 2100 ± 150 | 700 ± 60 | 3.0 | 640 | 210 |
| dipyridamole | 580 | 480 ± 30 | 190 ± 14 | 2.5 | 0.8 | 0.3 | |
| T-1032 | 1.2 | 75 ± 12 | 26 ± 7 | 2.9 | 63 | 22 | |
| T-0156 | 23d | 51 ± 4.0 | 61 ± 5 | 0.8 | 2.2 | 2.6 | |
| zaprinast | 325 | 30 ± 3.0 | 32 ± 6 | 0.9 | 0.1 | 0.1 | |
| sildenafil | 4.4 | 11 ± 1.0 | 4.7 ± 0.5 | 2.3 | 2.5 | 1.1 | |
| E4021 | 2.9 | 2.9 ± 0.5 | 2.9 ± 0.5 | 1.0 | 1.0 | 1.0 | |
| vardenafil | 0.25 | 0.71 ± 0.06 | 0.3 ± 0.03 | 2.4 | 2.8 | 1.2 | |
| NS | IBMX | -- | 4490 ± 804 | 1410 ± 453 | 3.2 | -- | -- |
Average of KI values for each family of PDE obtained from the literature. IBMX is non-selective (NS).
The KI was determined from the equation: KI = IC50 (1+ [cGMP]/KM), where the IC50 was obtained from the dose-response curve for each inhibitor for rod and cone PDE6 and the KM values for bovine rod and cone PDE6 are 14 μM or 7.0 μM, respectively (see Methods).
Selectivity is defined as the ratio of the KI values for the two indicated PDEs: 6R = rod PDE6; 6C = cone PDE6, and; X = PDE family 1 through 5.
Reported value is the IC50, because the KI value could not be calculated.
Most of the so-called PDE5-selective inhibitors tested in this study should be considered PDE5/6 inhibitors (Table 1). While vardenafil (KI < 1 nM) is the most potent inhibitor of PDE6, it shows only ≤ 3-fold selectivity for PDE5 over PDE6. Both E4021 and sildenafil are potent PDE6 inhibitors (KI ≤ 10 nM), but also lack discrimination of PDE5 versus PDE6. Only tadalafil [selectivity ratio of 210 (cone PDE6) or 640 (rod PDE6)] and T-1032 (selectivity ratio of 20–60) represent authentic PDE5-selective inhibitors. Interestingly, zaprinast, a first generation PDE5-targeted inhibitor (which also weakly inhibits PDE1 with a 10-fold higher KI value), has 10-fold higher potency for PDE6 than for PDE5 (Table 1), and should be considered a “PDE6-selective” inhibitor.
When examining pharmacological differences between rod and cone PDE6, we found that 8-methoxymethyl-IBMX and its parent compound, IBMX, showed 3–4-fold preference for inhibiting cone PDE6 compared to the rod isozyme. None of the inhibitors tested in Table 1 preferred binding to rod PDE6 compared to cone PDE6.
PDE inhibitors significantly elevate cGMP levels in intact ROS only when guanylate cyclase activity is controlled.
We next assessed the effects of PDE inhibitors on cGMP levels in metabolically active, isolated rod photoreceptor suspensions. When 400 μM IBMX was incubated with dark-adapted frog ROS under standard isolation conditions, a 20% elevation of cGMP levels was observed compared to control ROS suspensions (Fig. 1). [A species difference in the potency of IBMX to inhibit PDE6 cannot account for this result, because the KI of frog PDE6 (Table 2) is virtually identical to the value for bovine PDE6 (Table 1).]
Fig. 1. Synergistic effect of inhibiting PDE6 and buffering intracellular Ca2+ on cGMP levels in intact ROS.
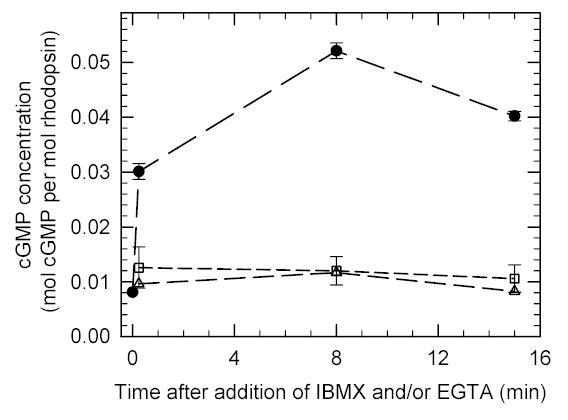
Purified ROS suspensions ([rhodopsin] = 4.8 μM) were prepared in complete darkness as described in Methods. Two portions were incubated with either 1.09 mM EGTA ([Ca2+]free = 500 nM; squares) or 400 μM IBMX (triangles). A third sample was first pre-incubated for 10 min with EGTA followed by IBMX addition (circles). Portions were quenched at the indicated times with 50% HCl/ethanol. The cGMP concentration was determined by an enzyme-linked immunoadsorbant assay, and reported relative to the rhodopsin content of the sample. Data points at the mean ± S.D. of three experiments.
Table 2.
Comparison of the effects of IBMX and vardenafil on frog rod PDE6 catalytic activity and on cGMP levels of intact frog ROS.
| frog PDE6 KI (μM)a | Intact ROS (μM)b | |||
|---|---|---|---|---|
| Inhibitor | activated | nonactivated | observed EC50 | predicted IC50 |
| IBMX | 4.3 ± 0.5 | 14 ± 1.3 | 1000 | 73 |
| vardenafil | 0.0019 ± 0.0004 | 0.022 ± 0.004 | 50 | 0.14 |
The KI values were determined as described in Table 1, using nucleotide-depleted frog ROS homogenates to measure nonactivated (10 μM cGMP, 4.0 nM PDE6) or activated (1.0 μM cGMP, 20 pM PDE6) PDE6 (see Methods).
Intact frog ROS (4.8 μM rhodopsin) were prepared as described in Methods. The observed EC50 is an estimate from the results of Fig. 3. The predicted IC50 was calculated with the equation: IC50 = KI*(1 + [cGMP]/KM) where the nonactivated KI value is used, the cGMP concentration is the dark-adapted value for intact ROS (50 μM), and the KM (60 μM) refers to the value for nonactivated frog PDE6 with cGMP as the substrate 31.
Based on a similar report on the effects of IBMX on the cGMP content of rabbit retina where almost equal reductions in the rates of cGMP hydrolysis and synthesis were observed 32, it seemed likely that inhibition of guanylate cyclase might compensate for the inhibitory effects of IBMX on PDE6 in frog ROS in Fig. 1. Because IBMX is known to elevate internal calcium concentrations in ROS 33 which would then inhibit the calcium-sensitive guanylate cyclase, we decided to “clamp” the free calcium concentration of ROS at its dark-adapted value of ~500 nM (see Methods). By itself, buffering the calcium concentration to 500 nM in the Ringer’s solution elevated cGMP levels by ~50% (Fig. 1), consistent with an earlier study 34. However, when pre-incubation with a calcium-buffered Ringer’s was combined with treatment with 400 μM IBMX, we observed a 5-fold elevation of cGMP concentration in frog ROS (Fig. 1). Thus, IBMX inhibition of PDE6 in intact, dark-adapted ROS results in elevation of cellular cGMP concentration when guanylate cyclase activity is held constant by buffering free calcium. In order to isolate the effect of PDE5/6 inhibitors on PDE6 in intact ROS, the remaining experiments were performed under buffered calcium conditions.
PDE inhibitors fail to completely block PDE6 in intact ROS.
To see whether high-potency PDE5/6 inhibitors behaved differently from IBMX, we compared the time course of cGMP elevation when sildenafil, vardenafil, or IBMX were incubated with ROS maintained in a 500 nM calcium-Ringer’s solution. Fig. 2 shows that cGMP levels in ROS reached an elevated, stable plateau within 2 min after exposure to the PDE inhibitor. The relatively slow action of PDE inhibitors on cGMP metabolism in intact ROS contrasts with the instantaneous inhibition of purified, activated PDE6 by these drugs in vitro (data not shown), and the rapid (< 1 sec) inactivation of PDE6 inferred from electrophysiological recordings 35. The new steady-state level of cGMP following drug exposure suggests that PDE6 is not completely inhibited at the inhibitor concentrations tested in Fig. 2.
Fig. 2. Time course of drug-induced increases in cGMP concentration in intact ROS.
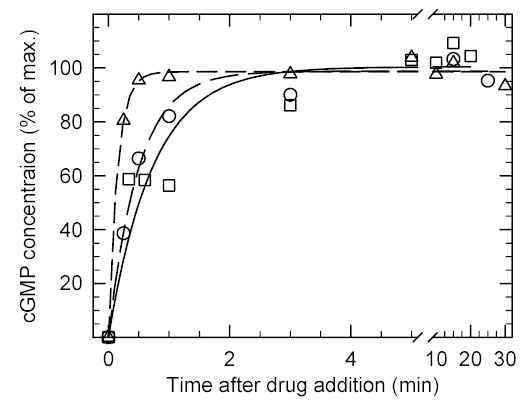
Purified ROS (0.008 mol cGMP/mol rhodopsin) were pre-incubated with 1.09 mM EGTA for 10 min to buffer the free calcium concentration. At time zero, 400 μM IBMX (circles), 50 μM sildenafil (triangles) or 100 μM vardenafil (squares) were added, and samples quenched in 50% HCl/ethanol for cGMP determinations. The results were normalized to the average maximum stimulation for each drug (units: mol cGMP per mol rhodopsin): IBMX, 0.040; vardenafil, 0.035, and; sildenafil, 0.014. Data points are the mean of three experiments in which the standard deviation was ≤ 13% of the mean value.
We next determined the dose-response relationship of IBMX, sildenafil, and vardenafil on dark-adapted ROS maintained in a calcium-buffered Ringer’s solution. For all three inhibitors, the aqueous solubility limit of each drug was reached before a plateau in the cGMP stimulation could be observed (Fig. 3). For 2.0 mM IBMX or 125 μM vardenafil, a 5-fold elevation of cGMP levels was observed (Fig. 3A), whereas 110 μM sildenafil induced a < 2-fold increase in cGMP content in ROS (Fig. 3B). Because no evidence of saturation was observed for these drugs, these concentrations represent a minimum estimate of the effective dose needed to produce a half-maximal change (EC50) in ROS cGMP levels.
Fig 3. PDE inhibitors induce dose-dependent elevations of ROS cGMP levels that do not saturate.
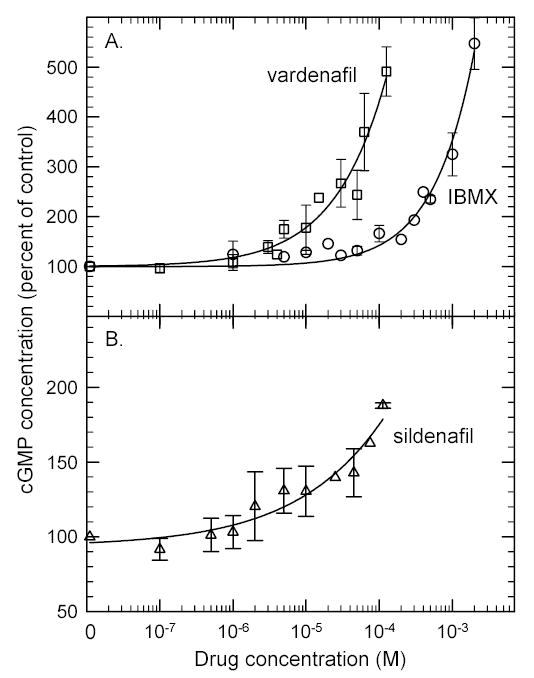
Ca2+-bufferedROS suspensions (10 min pre-incubation with 1.09 mM EGTA) were treated with the indicated concentration of IBMX (circles), sildenafil (triangles) or vardenafil (squares) for 10 min, and then acid-quenched for cGMP extraction and quantitation. The data points are the mean ± S.D. of three experiments.
The relative potency with which PDE inhibitors affect purified PDE6 catalysis in vitro (i.e., KI) compared with perturbing cGMP levels in intact ROS (i.e., EC50) is difficult to quantify. However, to a first approximation we can calculate the predicted IC50 for an inhibitor to block PDE6 activity in the presence of the cGMP concentration in dark-adapted ROS (50 μM), and assuming no diffusional barriers. As shown in Table 2, the predicted IC50 value for IBMX (73 μM) is at least 14-fold lower than the minimum EC50 estimated from Fig. 3. In contrast, the predicted IC50 for vardenafil (140 nM) is at least 350-fold lower than the minimum EC50 for intact ROS.
Competition between the inhibitory γ subunit and PDE inhibitors reduces their effectiveness in inhibiting PDE6.
In dark-adapted ROS studied above, rod PDE6 exists in its nonactivated state in which the catalytic dimer (αβ) is inhibited by binding of 2 inhibitory γ subunits [for review, see 36]. Fig. 4 demonstrates that vardenafil’s inhibitory potency for the PDE6 αβγ2 holoenzyme is reduced 12-fold compared to the activated PDE6 αβ catalytic dimer. In contrast, IBMX inhibition of PDE6 holoenzyme is reduced only 3-fold relative to activated PDE6. This discrepancy may be accounted for by competition between the γ subunit and PDE inhibitor for a common binding site in the catalytic pocket (see Discussion).
Fig 4. PDE6 holoenzyme is less susceptible to inhibition by PDE inhibitors.

ROS suspensions were homogenized, and soluble proteins and metabolites were separated from PDE6-containing ROS membranes by centrifugation (see Methods). The ROS membranes were further depleted of endogenous nucleoside triphosphates by incubation at 22ºC for 30 min. Nonactivated PDE6 holoenzyme (4 nM; open symbols) was incubated with vardenafil (panel A) or IBMX (panel B) for 15 min before addition of 10 μM [3H]cGMP to assay catalytic activity. Activated PDE6 (20 pM; filled symbols) was incubated with vardenafil (panel A) or IBMX (panel B) for 15 min before adding 1 μM [3H]cGMP. The results shown here are typical of at least three other experiments.
To test this idea further, we examined the ability of IBMX, sildenafil, and vardenafil to compete at the active site not only with cGMP but also with the γ subunit. Fig. 5 shows that at low drug concentrations both vardenafil and sildenafil—but not IBMX—can stimulate the PDE6 holoenzyme 2–3-fold when 2 mM cGMP is used as the substrate. [This paradoxical stimulation of PDE6 αβγ2 activity at low drug concentrations was first observed with zaprinast 14 and E4021 37.] At higher drug concentrations, the expected inhibitory action of all three inhibitors is seen. Under identical conditions, activated PDE6 catalytic dimers (αβ) do not show stimulation of catalysis at low drug concentrations (data not shown). These results are consistent with high-affinity, bulkier PDE5/6 inhibitors competing with γ binding in the catalytic pocket, whereas the smaller IBMX molecule inhibits catalysis without greatly affecting γ binding (see Discussion).
Fig 5. PDE5/6-selective inhibitors—but not IBMX—can stimulate catalysis at high cGMP concentrations.
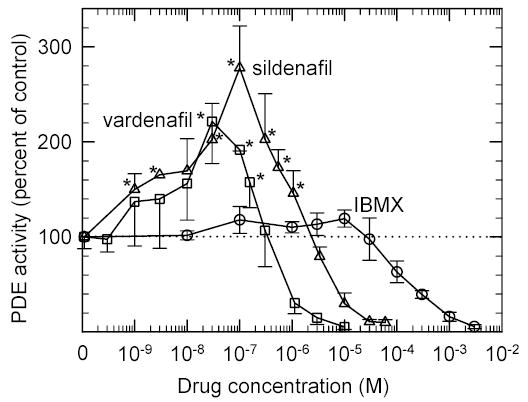
ROS membranes (2.0 nM PDE6 concentration) depleted of soluble proteins and nucleotides were incubated with IBMX (circles), sildenafil (triangles) or vardenafil (squares) for 15 min. Catalytic activity was determined by a colorimetric assay with 2 mM cGMP. The data was normalized to the basal PDE6 activity for plotting. The data represent the mean ± S.D. (n = 3). * Statistically significant, p<0.05.
Discussion
Photoreceptor PDE6 is susceptible to inhibition by PDE1-, PDE2- and most PDE5-directed drugs.
A central issue in the clinical development of PDE inhibitor therapy is the specificity of the drug for the targeted PDE. Table 1 is the first systematic analysis of the inhibition of rod and cone PDE6 by inhibitors that were designed to target PDE families 1 through 5. With the exception of PDE3- and PDE4-selective inhibitors and a few PDE5-selective inhibitors, most of the tested compounds in Table 1 lack selectivity (KI ratio less than 10) for the PDE family they are intended to target. The 10-fold selectivity of zaprinast for PDE6 compared to PDE5 makes this compound the only “PDE6-selective” inhibitor that we tested. The high selectivity of of the PDE5 inhibitor, tadalafil, for PDE5 over PDE6 bodes well for future drug development in which adverse side effects on vision resulting from PDE6 inhibition may be minimized further.
None of the compounds we tested showed major differences in their inhibition of rod and cone PDE6 isozymes, although two xanthine analogs (IBMX and 8-methoxymethyl-IBMX) showed 3–4-fold selectivity for cone PDE6. Differences in amino acid residues contacting these inhibitors and/or differences in conformation of the active site 38 may explain this preference of cone PDE6 for xanthine-based inhibitors.
The ability of PDE6 to bind to several different classes of PDE inhibitors may reflect the unique catalytic properties of the photoreceptor enzyme. Unlike the other ten PDE families, PDE6 operates with very high catalytic efficiency for cGMP [kcat/KM = 4 × 108 M−1s−1; ref. 36]. While the low affinity of substrate (KM = 20 μM for cGMP) and the high catalytic constant (up to 8000 cGMP hydrolyzed per sec) of PDE6 are ideally suited for the millisecond time-scale activation of PDE6 required for visual transduction 2, these same properties may allow a wide variety of inhibitor compounds to enter the catalytic pocket and inhibit catalysis.
The effect of PDE inhibitors on cGMP levels in photoreceptors is attenuated by calcium feedback regulation of guanylate cyclase and by high PDE6 concentrations in the outer segment.
The observation that PDE inhibitors only modestly elevate cGMP levels in intact ROS unless the free calcium concentration is buffered (Fig. 1) can be explained as follows: Inhibition of PDE6 by drug entry into the outer segment will transiently elevate cGMP levels. This causes cGMP-gated ion channels to open, allowing entry of sodium and calcium into the ROS. Elevation of intracellular calcium inhibits guanylate cyclase and offsets the reduced hydrolysis of cGMP by the inhibited PDE6, resulting in little or no increase in cGMP concentration 32. Upon buffering free calcium in the medium, this calcium feedback mechanism is blocked, allowing continued synthesis of cGMP in conjunction with reduced cGMP hydrolysis, and a net increase in intracellular cGMP concentration.
The plateau in steady state cGMP levels following incubation of IBMX or PDE5/6-selective inhibitors with ROS suspensions (Fig. 2) was unexpected, since PDE6 inhibition in conjunction with continued cGMP synthesis by guanylate cyclase in the calcium-buffered Ringer’s should lead to continual increases in intracellular cGMP concentration. Also unexpected was the failure of all tested PDE inhibitors to show saturation behavior when cGMP levels in ROS were assayed as a function of inhibitor concentration in Fig. 3. In contrast, both nonactivated and activated PDE6 in vitro display stereotypical dose-response behavior with IBMX or vardenafil (Fig. 4 ). Assaying PDE6 inhibition in situ in intact ROS (Fig. 3) must take into consideration the very high PDE6 concentration in the outer segment of rod photoreceptors [40 μM catalytic subunit concentration in frog rods; 39]. While the high PDE6 concentration associated with the outer segment membranes facilitates the single-photon sensitivity of photoreceptors 40, it also requires very high drug concentrations to stoichiometrically inhibit PDE6 in ROS. [For example, in Fig. 3 the highest tested concentrations of sildenafil and vardenafil are only ~3-fold greater than the PDE6 subunit concentration in ROS.] In summary, the unexpected behavior in Figs. 2 and 3 can be accounted for if some of the PDE6 in intact ROS cannot be inhibited under our experimental conditions.
The state of activation of PDE6 determines the potency of PDE inhibitors.
For the case of the closely related PDE5 family of phosphodiesterases, allosteric activation of PDE5 by cGMP binding to the GAF domain enhances the binding affinity of sildenafil, tadalafil and vardenafil 41,42. Fig. 4 demonstrates a parallel phenomenon for PDE6, whereby activated PDE6 catalytic dimer (αβ) is more potently inhibited by PDE inhibitors than the nonactivated holoenzyme (αβγ2). This effect is more pronounced for vardenafil (Fig. 4A) than for IBMX (Fig. 4B). Furthermore, low concentrations of sildenafil or vardenafil—but not IBMX—can cause an apparent stimulation of nonactivated PDE6 when the cGMP substrate concentrations are very high (Fig. 5). This latter effect is likely due to mutually exclusive competition between the γ subunit, drug, and substrate for a common binding site in the catalytic pocket, in conjunction with an ~1000-fold greater affinity of γ [KD ~ pM; 43, 44] than drug (KI ~ nM; Tables 1 and 2) for the active site.
These results have important implications for the architecture of the catalytic site of PDE6. IBMX, a xanthine derivative, has been shown to occupy a subpocket within the active site of PDE5 normally occupied by the guanine ring of cGMP 38, and makes many of the same interactions observed for the authentic substrate. While IBMX, sildenafil, and vardenafil all share contact points in this region, the two PDE5/6-selective inhibitors also contact the catalytic pocket of PDE5 at additional sites that account for the 1000-fold higher affinity of these drugs {Sung, 2003 6552 /id}. Interestingly, many of these PDE5 contacts interacting with the ethoxyphenyl and methoxypiperazine groups of sildenafil are close to amino acids that in PDE6 have been implicated in γ binding to the catalytic domain 46, 47. If the bulkier PDE5/6-selective inhibitors overlap in their binding sites with the γ interaction sites in the catalytic pocket of PDE6, this would explain the mutually exclusive competition of γ and drug that weakens the affinity of these drugs for nonactivated PDE6 (Fig. 4) and leads to the paradoxical activation of PDE6 holoenzyme at low drug concentrations (Fig. 5).
Conclusions.
In summary, when assessing the clinical efficacy of PDE inhibitor therapy to treat human diseases, the following effects on photoreceptor PDE6 must be considered: the ability of systemically administered PDE inhibitors to cross the blood-retinal barrier to reach the photoreceptors (not addressed in this study); the intrinsic pharmacological selectivity of the drug for PDE6 inhibition; the unique cellular context of the rod outer segment in which PDE6 resides; the state of activation of PDE6 (and hence its competition with the γ subunit), and; the inter-relatedness of cGMP and calcium metabolism in visual signaling. With regards to calcium, the fact that elevation of cGMP levels by PDE inhibitors will increase intracellular calcium concentration in photoreceptors may have serious consequences, considering that photoreceptor apoptosis and retinal degeneration are believed to result from sustained cGMP elevation and/or calcium overload 48, 49. For these reasons, administration of PDE inhibitors for novel therapeutic uses must be evaluated for potential adverse effects on photoreceptor viability as well as visual function.
Acknowledgments
We thank Bev Valeriani for providing purified cone PDE6 used in some of these experiments.
Footnotes
This work was supported by National Institutes of Health grant EY-05798, by Bayer Healthcare AG, and by the New Hampshire Agricultural Experiment Station (Scientific Contribution #2259).
References
- 1.Pugh EN, Lamb TD. Phototransduction in vertebrate rods and cones: molecular mechanisms of amplification, recovery and light adaptation. In: Stavenga DG, DeGrip WJ, Pugh EN, editors. Molecular Mechanisms in Visual Transduction. New York: Elsevier Science B.V.; 2000:183–255.
- 2.Arshavsky VY, Lamb TD, Pugh EN., Jr G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–187. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Cote RH. cGMP signaling in vertebrate retinal photoreceptor cells. Front Biosci. 2005;10:1191–1204. doi: 10.2741/1612. [DOI] [PubMed] [Google Scholar]
- 4.Farber DB. From mice to men: The cyclic GMP phosphodiesterase gene in vision and disease: The Proctor Lecture. Invest Ophthalmol Vis Sci. 1995;36:263–275. [PubMed] [Google Scholar]
- 5.Duda T, Koch KW. Retinal diseases linked with photoreceptor guanylate cyclase. Mol Cell Biochem. 2002;230:129–138. [PubMed] [Google Scholar]
- 6.Francis SH, Turko IV, Corbin JD. Cyclic nucleotide phosphodiesterases: relating structure and function. Prog Nucleic Acid Res Mol Biol. 2001;65:1–52. doi: 10.1016/s0079-6603(00)65001-8. [DOI] [PubMed] [Google Scholar]
- 7.Cote RH. Characteristics of photoreceptor PDE (PDE6): similarities and differences to PDE5. Int J Impot Res. 2004;16:S28–S33. doi: 10.1038/sj.ijir.3901212. [DOI] [PubMed] [Google Scholar]
- 8.Schudt C, Dent G, Rabe KF. Phosphodiesterase Inhibitors New York: Academic Press; 1996.
- 9.Manganiello V. Cyclic nucleotide phosphodiesterase 5 and sildenafil: promises realized. Mol Pharmacol. 2003;63:1209–1211. doi: 10.1124/mol.63.6.1209. [DOI] [PubMed] [Google Scholar]
- 10.Lin CS, Xin ZC, Lin GT, Lue TF. Phosphodiesterases as therapeutic targets. Urology. 2003;61:685–691. doi: 10.1016/s0090-4295(02)02439-1. [DOI] [PubMed] [Google Scholar]
- 11.Lincoln TM. Cyclic GMP and phosphodiesterase 5 inhibitor therapies: what’s on the horizon? . Mol Pharmacol. 2004;66:11–13. doi: 10.1124/mol.104.001388. [DOI] [PubMed] [Google Scholar]
- 12.Laties AM, Zrenner E. Viagra (sildenafil citrate) and ophthalmology. Prog Retin Eye Res. 2002;21:485–506. doi: 10.1016/s1350-9462(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 13.Uckert S, Stief CG, Jonas U. Current and future trends in the oral pharmacotherapy of male erectile dysfunction. Expert Opin Investig Drugs. 2003;12:1521–1533. doi: 10.1517/13543784.12.9.1521. [DOI] [PubMed] [Google Scholar]
- 14.Gillespie PG, Beavo JA. Inhibition and stimulation of photoreceptor phosphodiesterases by dipyridamole and M&B 22,948. Mol Pharmacol. 1989;36:773–781. [PubMed] [Google Scholar]
- 15.Zhang J, Kuvelkar R, Wu P, Egan RW, Billah MM, Wang P. Differential inhibitor sensitivity between human recombinant and native photoreceptor cGMP-phosphodiesterases (PDE6s) Biochem Pharmacol. 2004;68:867–873. doi: 10.1016/j.bcp.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Lipton SA, Rasmussen H, Dowling JE. Electrical and adaptive properties of rod photoreceptors in Bufo marinus. J Gen Physiol. 1977;70:771–791. doi: 10.1085/jgp.70.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capovilla M, Cervetto L, Torre V. Antagonism between steady light and phosphodiesterase inhibitors on the kinetics of the rod photoresponses. Proc Natl Acad Sci U S A. 1982;79:6698–6702. doi: 10.1073/pnas.79.21.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rispoli G, Gillespie PG, Detwiler PB. Comparative effects of phosphodiesterase inhibitors on detached rod outer segment function. In: Borsellino A, Cervetto L, Torre V, editors. Sensory Transduction. New York: Plenum Press; 1990:157–167.
- 19.Lolley RN, Farber DB, Rayborn ME, Hollyfield JG. Cyclic GMP accumulation causes degeneration of photoreceptor cells: simulation of an inherited disease. Science. 1977;196:664–666. doi: 10.1126/science.193183. [DOI] [PubMed] [Google Scholar]
- 20.Pentia DC, Hosier S, Collupy RA, Valeriani BA, Cote RH. Purification of PDE6 isozymes from mammalian retina. Methods in Molecular Biology. 2005;in press [DOI] [PubMed]
- 21.Cote RH. Kinetics and regulation of cGMP binding to noncatalytic binding sites on photoreceptor phosphodiesterase. Methods Enzymol. 2000;315:646–672. doi: 10.1016/s0076-6879(00)15873-2. [DOI] [PubMed] [Google Scholar]
- 22.Cote RH, Biernbaum MS, Nicol GD, Bownds MD. Light-induced decreases in cGMP concentration precede changes in membrane permeability in frog rod photoreceptors. J Biol Chem. 1984;259:9635–9641. [PubMed] [Google Scholar]
- 23.Yoshikami S, Robinson WE, Hagins WA. Topology of the outer segment membranes of retinal rods and cones revealed by a fluorescent probe. Science. 1974;185:1176–1179. doi: 10.1126/science.185.4157.1176. [DOI] [PubMed] [Google Scholar]
- 24.Blazynski C, Cohen AI. Rapid declines in cyclic GMP of rod outer segments of intact frog photoreceptors after illumination. J Biol Chem. 1986;261:14142–14147. [PubMed] [Google Scholar]
- 25.Bownds D, Gordon-Walker A, Gaide Huguenin AC, Robinson W. Characterization and analysis of frog photoreceptor membranes. J Gen Physiol. 1971;58:225–237. doi: 10.1085/jgp.58.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pugh EN, Duda T, Sitaramayya A, Sharma RK. Photoreceptor guanylate cyclases: a review. Biosci Rep. 1997;17:429–473. doi: 10.1023/a:1027365520442. [DOI] [PubMed] [Google Scholar]
- 27.Dumke CL, Arshavsky VY, Calvert PD, Bownds MD, Pugh EN. Rod outer segment structure influences the apparent kinetic parameters of cyclic GMP phosphodiesterase. J Gen Physiol. 1994;103:1071–1098. doi: 10.1085/jgp.103.6.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cote RH, Brunnock MA. Intracellular cGMP concentration in rod photoreceptors is regulated by binding to high and moderate affinity cGMP binding sites. J Biol Chem. 1993;268:17190–17198. [PubMed] [Google Scholar]
- 29.Cheng Y-C, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 30.Mou H, Cote RH. The catalytic and GAF domains of the rod cGMP phosphodiesterase (PDE6) heterodimer are regulated by distinct regions of its inhibitory γ subunit. J Biol Chem. 2001;276:27527–27534. doi: 10.1074/jbc.M103316200. [DOI] [PubMed] [Google Scholar]
- 31.D’Amours MR, Cote RH. Regulation of photoreceptor phosphodiesterase catalysis by its noncatalytic cGMP binding sites. Biochem J. 1999;340:863–869. [PMC free article] [PubMed] [Google Scholar]
- 32.Ames A, III, Barad M. Metabolic flux of cyclic GMP and phototransduction in rabbit retina. J Physiol (Lond ) 1988;406:163–179. doi: 10.1113/jphysiol.1988.sp017374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cervetto L, McNaughton PA. The effects of phosphodiesterase inhibitors and lanthanum ions on the light-sensitive current of toad retinal rods. J Physiol (Lond) 1986;370:91–100. doi: 10.1113/jphysiol.1986.sp015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polans AS, Kawamura S, Bownds MD. Influence of calcium on guanosine 3′,5′-cyclic monophosphate levels in frog rod outer segments. J Gen Physiol. 1981;77:41–48. doi: 10.1085/jgp.77.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodgkin AL, Nunn BJ. Control of light-sensitive current in salamander rods. J Physiol (Lond) 1988;403:439–471. doi: 10.1113/jphysiol.1988.sp017258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cote RH. Structure, function, and regulation of photoreceptor phosphodiesterase (PDE6). In: Bradshaw RA, Dennis EA, editors. Handbook of Cell Signaling. San Diego: Academic Press; 2003:453–457.
- 37.D’Amours MR, Granovsky AE, Artemyev NO, Cote RH. The potency and mechanism of action of E4021, a PDE5-selective inhibitor, on the photoreceptor phosphodiesterase depends on its state of activation. Mol Pharmacol. 1999;55:508–514. [PubMed] [Google Scholar]
- 38.Huai Q, Liu Y, Francis SH, Corbin JD, Ke H. Crystal structures of phosphodiesterases 4 and 5 in complex with inhibitor 3-isobutyl-1-methylxanthine suggest a conformation determinant of inhibitor selectivity. J Biol Chem. 2004;279:13095–13101. doi: 10.1074/jbc.M311556200. [DOI] [PubMed] [Google Scholar]
- 39.Cote RH, Bownds MD, Arshavsky VY. cGMP binding sites on photoreceptor phosphodiesterase: Role in feedback regulation of visual transduction. Proc Natl Acad Sci U S A. 1994;91:4845–4849. doi: 10.1073/pnas.91.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pugh EN, Jr, Lamb TD. Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta. 1993;1141:111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- 41.Corbin JD, Blount MA, Weeks JL, Beasley A, Kuhn KP, Ho YS, Saidi LF, Hurley JH, Kotera J, Francis SH. [3H]sildenafil binding to phosphodiesterase-5 is specific, kinetically heterogeneous, and stimulated by cGMP. Mol Pharmacol. 2003;63:1364–1372. doi: 10.1124/mol.63.6.1364. [DOI] [PubMed] [Google Scholar]
- 42.Blount MA, Beasley A, Zoraghi R, Sekhar KR, Bessay EP, Francis SH, Corbin JD. Binding of tritiated sildenafil, tadalafil, or vardenafil to the phosphodiesterase-5 catalytic site displays potency, specificity, heterogeneity, and cGMP stimulation. Mol Pharmacol. 2004;66:144–152. doi: 10.1124/mol.66.1.144. [DOI] [PubMed] [Google Scholar]
- 43.Wensel TG, Stryer L. Reciprocal control of retinal rod cyclic GMP phosphodiesterase by its gamma subunit and transducin. Prot Struct Funct Genet. 1986;1:90–99. doi: 10.1002/prot.340010114. [DOI] [PubMed] [Google Scholar]
- 44.Paglia MJ, Mou H, Cote RH. Regulation of photoreceptor phosphodiesterase (PDE6) by phosphorylation of its inhibitory γ subunit re-evaluated. J Biol Chem. 2002;277:5017–5023. doi: 10.1074/jbc.M106328200. [DOI] [PubMed] [Google Scholar]
- 45.Sung BJ, Hwang KY, Jeon YO, Lee JI, Heo YS, Kim JH, Moon J, Yoon JM, Hyun YL, Kim E, Eum SJ, Park SY, Lee JO, Lee TG, Ro S, Cho JM. Structure of the catalytic domain of human phosphodiesterase 5 with bound drug molecules. Nature. 2003;425:98–102. doi: 10.1038/nature01914. [DOI] [PubMed] [Google Scholar]
- 46.Artemyev NO, Natochin M, Busman M, Schey KL, Hamm HE. Mechanism of photoreceptor cGMP phosphodiesterase inhibition by its gamma-subunits. Proc Natl Acad Sci U S A. 1996;93:5407–5412. doi: 10.1073/pnas.93.11.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Granovsky AE, Artemyev NO. Identification of the γ-subunit interacting residues on photoreceptor cGMP phosphodiesterase, PDE6α′. J Biol Chem. 2000;275:41258–41262. doi: 10.1074/jbc.M008094200. [DOI] [PubMed] [Google Scholar]
- 48.Farber DB, Lolley RN. Cyclic guanosine monophosphate elevation in degenerating photoreceptor cells of the C3H mouse retina. Science. 1974;186:449–451. doi: 10.1126/science.186.4162.449. [DOI] [PubMed] [Google Scholar]
- 49.Fox DA, Poblenz AT, He L. Calcium overload triggers rod photoreceptor apoptotic cell death in chemical-induced and inherited retinal degenerations. Ann N Y Acad Sci. 1999;893:282–285. doi: 10.1111/j.1749-6632.1999.tb07837.x. [DOI] [PubMed] [Google Scholar]


