Abstract
Melanoma is a highly metastatic cancer that accounts for the majority of skin cancer deaths. Unfortunately, very few improvements have been made during the last 20 years in the management of melanoma metastases, which is the major cause of melanoma deaths. Therefore, identification of molecular targets that can be exploited in the clinic to treat metastatic disease is desperately needed. The KISS1 metastasis suppressor gene has emerged as a promising molecular target for the management of metastatic disease. This review compiles data regarding the molecular and biochemical properties of KISS1 and its cognate receptor, focusing on the properties believed to be most pertinent to the use of KISS1 in the clinical setting. In addition, clinical data that supports KISS1 as having a dual role as a prognostic indicator and a therapeutic target for the management of metastatic disease will be highlighted.
Keywords: KISS1, Metastin, Kisspeptin, GPR54, Axor12, hOT7T175, Ligand, Receptor, G -Protein Coupled Receptor, Metastasis, Metastasis Suppressor Genes, Review
2. INTRODUCTION
Skin cancer is the most common of all cancers in the United States representing nearly 45% of all newly diagnosed invasive cancers (1). While melanoma only accounts for about 6% of all invasive skin cancer cases, it is the most lethal (1). Melanoma is responsible for 73% of all skin cancer deaths and the incidence of melanoma is increasing at a rate of 3% per year since 1981 (1). In 2005, 59,580 new cases of invasive melanoma are expected (1). Although there is an overall 90% five-year survival rate for melanoma patients, survival drops precipitously if there is evidence of metastatic disease (2). Most patients with metastatic disease confined to the subcutis and lymph nodes will survive for 12 months, while patients with visceral involvement have a median survival of 4–6 months (3). These grim statistics reflect the highly metastatic nature of melanoma and its ability to colonize multiple organs and tissues. This makes therapeutic intervention very challenging in melanoma patients with disseminated disease.
Despite all the recent advances in post-operative adjuvant therapies targeting metastatic disease, “there is no single treatment that significantly improves overall survival rates in melanoma patients (3).” As a result, there has not been a significant improvement in survival rates over the last 20 years (2). The lack of reduction of melanoma mortality rates strongly indicates that there is a dire need for the identification of novel targets that can prevent or inhibit metastatic disease.
One set of promising targets for the prevention of metastatic disease are metastasis suppressors, which inhibit metastases while having little effect on primary tumor formation. Some of these proteins, most of which were discovered within the last 5–10 years, have also been shown to have an inverse correlation with tumor grade and the likelihood of metastatic disease in patients (reviewed in (4,5)). Unfortunately, little is known about their mechanism(s) of action or which step(s) in the metastatic cascade where these proteins exert their suppressive effects. In order to fully exploit metastasis suppressors as clinical targets for managing metastatic disease, more research is necessary. KISS1 is one of these promising metastasis suppressors. We will review what is known about the KISS1 melanoma metastasis suppressor, giving emphasis toward its potential as a clinical target for managing metastatic disease.
3. DISCOVERY OF KISS1 AS A MELANOMA METASTASIS SUPPRESSOR
Translocations and/or deletions involving the long arm of human chromosome 6 (6q) have been found in greater than 80% of tumors representing late-stage, metastatic melanoma (6). Based upon this observation and in order to determine if a gene(s) on chromosome 6 is(are) capable of inhibiting metastases, an intact copy of human chromosome 6 was introduced into the metastatic C8161 human melanoma cell line (neo6/C8161) using microcell-mediated chromosomal transfer (7).
The introduction of chromosome 6 suppressed metastases to lung and lymph nodes (>95%), with no discernable effect on primary tumor formation. Subtractive hybridization was carried out on metastatic C8161 and neo6/C8161 cells and a novel cDNA transcript termed KISS1 was isolated (8,9). KISS1 expression was observed in primary melanocytes while its expression was inversely correlated with metastatic potential in a panel of melanoma cell lines (8,9). When KISS1 expression was restored in C8161 cells, pulmonary metastases were inhibited by >95% following intravenous injection. Growth of orthotopic tumors was not blocked. These experiments identified KISS1 as a potent melanoma metastasis suppressor gene with metastatic suppression similar to the levels observed with the introduction of chromosome 6. While KISS1 was first suspected to be the metastasis suppressor encoded on chromosome 6, radiation hybrid mapping and fluorescence in situ hybridization showed that the KISS1 gene mapped to the long arm of chromosome 1 existing as a single locus on 1q32. This result suggested that KISS1 may be regulated by a gene on chromosome 6 (10).
4. REGULATION OF KISS1
Since an intact copy of KISS1 (i.e., wild-type, not mutated) can be isolated from C8161 cells, it is likely that KISS1 is regulated by a gene on chromosome 6 that is defective or lost in C8161 cells. Loss of heterozygosity (LOH) of 6q16.3-q23 in human melanoma metastases has been shown to correlate with the loss of KISS1 expression (11). Miele et al. confirmed that this 40 cM region on chromosome 6 was important for metastasis suppression in C8161 cells (12).
Using microarrays comparing neo6/C8161, C8161 with chromosome 6 containing a deletion of 6q16.3-q23 (neo6qdel/C8161) and parental C8161, Goldberg et al. identified TXNIP (thioredoxin interacting protein, also known as VDUP1, vitamin D up-regulated protein, TBP2, thioredoxin binding protein 2) to be consistently elevated in neo6/C8161 cells. Interestingly, like KISS1, VDUP1/TXNIP mapped to chromosome 1 and is capable of suppressing metastases in C8161. However, the regulatory gene on chromosome 6 was not identified until PCR-based karyotyping was performed on overlapping deletions of chromosome 6 in conjunction with their ability to suppress C8161 cells. Loss of the D6S457 marker on chromosome 6 was shown to correlate with a gain of metastatic competence. CRSP3 (co-factor required for SP1 activity, also known as DRIP130, vitamin D receptor interacting protein) was the closest gene to D6S457. Like KISS1, CRSP3 expression was inversely correlated with melanoma progression as modeled by a panel of cell lines. Restoration of CRSP3 expression in C8161 cells inhibited metastases. In support of CRSP3 as a KISS1 regulatory gene, cells overexpressing CRSP3 have elevated KISS1 levels. Additionally, 20 patients undergoing melanoma surgery showed a 62.9% correlation between CRSP3 expression and KISS1 expression (13). Therefore, CRSP3 is a strong candidate gene involved in the regulation of KISS1 expression. Combined with data showing that TXNIP re-expression could increase KISS1, Goldberg hypothesized that there is a metastasis regulatory axis CRSP3 -> TXNIP -> KISS1 (13).
5. KISS1 PROTEIN CHARACTERIZATION
5.1. KISS1 sequence analysis and processing
In 2001, three labs independently identified sites in the amino acid sequence of the KISS1 protein that suggests it is processed and secreted (14–16). Careful examination of the sequence revealed that KISS1 has characteristics in common with neuropeptides which include a secretion signal, several dibasic cleavage sites and a cleavage amidation site. The first 19 amino acids of KISS1 comprise a secretion signal sequence which our laboratory has recently confirmed (unpublished). The remaining sequence of KISS1 has two dibasic cleavage sites (R56-K, R66-R) followed by a cleavage amidation site (K123-R) (Figure 1). These canonical cleavage sequences are purportedly recognized by furin or other prohormone convertases. Although, most precursors processed by furin in the constitutive pathway have the canonical sequence R-X-K/R-R or R-X-X-R, R-K and R-R are the predominant cleavage sites found in prohormones and proneuropeptides (17). Cleavage at dibasic sites R66-R and K123-R result in a 54-amino acid product termed metastin/kisspeptin-54 (KP54). Cleavage at R56-K dibasic motifs occur more rarely and is consistent with the inability to identify kisspeptin-64 (KP64) (14–18). Despite the likelihood that KISS1 is processed by prohormone convertases, it is still unknown which convertases(s) is(are) responsible for processing KISS1. In addition, the location in the cell where KISS1 processing occurs has not yet been elucidated.
Figure 1.
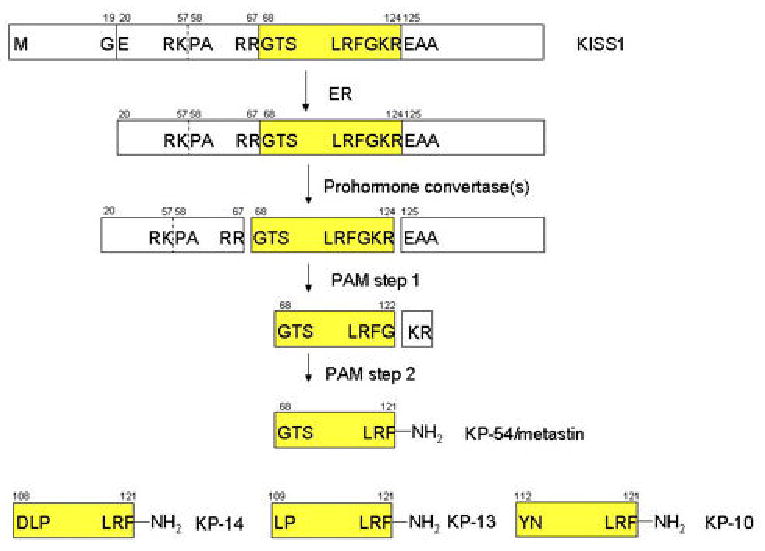
Proposed model for KISS1 protein processing by prohormone convertases. KISS1 is initially targeted to the ER by the secretion signal sequence. Once KISS1 leaves the trans Golgi, the R66-R and GK123-R cleavage sites are likely to be recognized and processed in acidic secretory vesicles by furin (family) or other prohormone convertases. K56-R is not likely to be recognized and processed because cleavage at K-R dibasic motifs rarely occurs. Peptidyl-glycine-α-amidating monooxygenase (PAM) removes the newly exposed C-terminal basic residues K-R124 and converts the C-terminal G122 to an amide producing metastin/KP54 (KP54). The production of kisspeptins 14 (KP14), 13 (KP13), and 10 (KP10) do not have any known processing sites that would lead to their creation. Whether these smaller kisspeptins occur from processing by an unknown enzyme or are a byproduct of spontaneous degradation remains to be seen.
Takino et al. identified that KISS1 as well as kisspeptins can be cleaved by matrix metalloproteinases (MMP) at G118-L119 (19). Interestingly, processing at this site abolishes kisspeptin-10’s (KP10) ability to induce focal adhesions suggesting that MMP’s may serve as a negative regulator of KISS1. In support of this, MMP expression has been shown to increase in tumors and has been shown to correlate with invasion and metastasis (20–22).
5.1.1. Proposed model for KISS1 processing
There are three major steps envisioned for the processing of KISS1 protein by prohormone convertases (Figure 1). First, endoproteolytic processing occurs at dibasic cleavage sites R-R and K-R and the newly exposed C-terminal basic residues arginine and lysine are removed by a carboxypeptidase that has a high specificity for basic residues. Finally, after the removal of the C-terminal basic residues, glycine undergoes conversion to an amide by a peptidyl-glycine-α-amidating monooxygenase (PAM) (17). Interestingly, amidation is important in enhancing the binding of neuroendocrine peptides to their cognate receptors and may play a role in inhibiting their degradation (17). The significance of amidation in mediating KISS1’s activity will be discussed below.
At this point it is worth mentioning that the term metastin is misused frequently in the literature to represent both unprocessed KISS1 as well as the processed kisspeptins. Therefore, we strongly caution readers to be wary when reading the literature. We prefer the term kisspeptins to refer to the processed forms of KISS1, mostly because it retains reference to the original name. We will refer to processed forms of KISS1 as kisspeptin (KP) followed by the size of the peptide (Figure 1).
5.2. KISS1 secretion
Horikoshi et al. identified KP54 in the plasma of men and women using a two-site enzyme immunoassay that recognizes both ends of the peptide (23). The levels of KP54 identified in both sexes were similar. However, when plasma levels were examined in pregnant women, KP54 was shown to increase in conjunction with prolactin, estrogen, and progesterone which indicates placental KISS1 expression is not inhibited by increasing hormone levels. In the third trimester, KP54 levels in the plasma were ~7,000 times higher than in non-pregnant women and returned to baseline 5 days post-partum.
Immunohistochemical localization of KP54 to the outer syncytiotrophoblast compartment of the placenta suggests that KISS1 may function as a placental derived hormone (18,23). It is plausible that KISS1 may play a role in the regulation of placental invasion based on its localization in the syncytiotrophoblast compartment of the placenta. However, it is still unclear what KISS1’s function(s) is during pregnancy and what the source of KP-54 is in males.
Bilban et al. took this one step further by examining KISS1 secretion in isolated primary human trophoblast cells (18). They were able to identify full-length KISS1 in lysate but were unable to detect any processed forms of KISS1. This result is consistent with the proposed processing of KISS1 which may occur late in the secretory pathway or even at the cell surface. Interestingly, detection of KP54 in the media of primary trophoblast cells could only be accomplished after reverse-phase HPLC and MALDI-TOF. The inability to detect KP54 in the media of cells in culture by western blot indicates that KISS1 secretion is likely to occur at low levels. Rapid degradation of KP54 is an unlikely explanation for low media levels since it could be readily detected in the serum of men and women albeit in the fmol/ml range. Although Bilban et al. also detected smaller peptides that have been termed kisspeptins-14, -13 and -10 in the media, there are no known cleavage sites in the KISS1 sequence that would suggest that these peptides could be formed. Spontaneous degradation or an artifact of sample preparation cannot be ruled out as a potential source of these peptides (14).
Despite strong evidence for KISS1 as a secreted protein, it has not been determined if KISS1 secretion occurs in metastatic human cancer cell lines that are suppressed by KISS1 (e.g. C8161.9, MelJuSo and MDA-MB-435). More importantly, it is also unclear if KISS1 secretion is required for metastasis suppression. These are important questions to address in the future in order to understand how to exploit metastasis suppression by KISS1 in the clinic. If secretion is required for metastasis suppression, exogenous administration of a naturally occurring peptide will reduce the possibility of side effects and avoid the need for gene therapy.
6. MECHANISM OF KISS1 METASTASIS SUPPRESSION
6.1. GPR54: a receptor for KISS1
GPR54 (also known as AXOR12 or hOT7T175) is an orphan G-protein coupled receptor that was identified in 1999 by Lee et al.(24). GPR54 is coupled to G proteins of the Gq/11 subfamily which, upon activation, result in the release of intracellular calcium stores and activation of phospholipase C-β (14,15,25). Interestingly, Gq/11 proteins have been implicated in regulating a diverse number of cellular functions which include secretory machinery (25). GPR54 bears structural similarities with other neuropeptide receptors and shares a 38% sequence similarity with galanin receptors. However, galanin is unable to bind or activate GPR54. Many neurotransmitters and neuromodulators terminate with the sequence Arg-Phe-NH2 (RF-NH2) but have variable amino-terminal sequences (26). KP54 has an RF-NH2 C-terminus but it was not discovered as a potential ligand to GPR54 until 2001 when three laboratories independently identified KP54 as a potent agonist to GPR54 (14–16). Stimulation of cells overexpressing human GPR54 with exogenous kisspeptins results in a robust intracellular Ca2+ release. The affinity of KP54 for GPR54 is low nanomolar which is well within any presumed physiological range. Taken together, these results support the notion that KP54 is a cognate ligand for GPR54. Ohtaki\pard fs18 et al. also showed that amidation at the C-terminus of KP54 is essential for binding and activation of GPR54. Smaller amidated kisspeptins-10, 13 and 14 which are N-terminal truncations of KP54 are also capable of binding and activating GPR54. The last 10 amino acids of KP54 appear to be the most important for binding and activating GPR54 and are highly conserved among species (27,28). However, peptides representing regions N-terminal and C-terminal to KP54 were not able to bind or stimulate GPR54 (15). Therefore, only C-terminal amidated kisspeptins are capable of binding and signaling through GPR54. What role these peptides play in KISS1’s function is still a matter of speculation because it is still unclear if the interaction between GPR54 and kisspeptins is required for metastasis suppression. Since all of the studies mentioned above were performed in cells that had experimentally elevated GPR54 that were exposed to exogenous kisspeptins (at doses which could be either pharmacologic or physiologic), the relevance should not be misinterpreted.
6.2. GPR54/kisspeptin signaling
In addition to the release of intracellular calcium stores, kisspeptin signaling through GPR54 has been shown to activate the MAP kinase pathway. Several laboratories have consistently shown phosphorylation of MAP kinases ERK1 and ERK2 after exposure to kisspeptins. This occurs regardless of the cell line examined and is irrespective of whether GPR54 levels are endogenously high or artificially elevated (14,16,29,30).
Phosphorylation of p38 has been, however, less straightforward. p38 phosphorylation was observed after kisspeptin exposure in CHO cells overexpressing GPR54 as well as in PANC-1 pancreatic carcinoma cells with endogenously high levels of GPR54 (14,29,30). p38 phosphorylation was not observed in ARO81 cells, which have high endogenous levels of GPR54 (30). Therefore, p38 phosphorylation may be cell type specific or is dependent on the levels of GPR54 expressed. ERK1/2 signaling is likely to be the predominant GPR54 pathway activated by kisspeptins because of the consistency in its activation between cell lines. However, it is still unclear if GPR54 plays a role in metastasis suppression and how ERK1/2 signaling could accomplish this.
Although the MAPK pathway appears to be the predominant pathway activated by GPR54 following exogenous kisspeptin exposure, KISS1 over-expression can activate pathways independent of MAPK. Boyd et al. found a decrease in MMP9 activity in HT-1080 cells over-expressing KISS1 that is not mediated by the MAPK pathway (31). This effect was attributed to a reduction in NFκB binding to the MMP9 promoter. The MAPK independent suppression of MMP9 may suggest alternative signaling pathways to GPR54. However, it is unclear if a decrease in MMP9 can also be observed after GPR54/kisspeptin signaling.
6.3. In vitro implications for GPR54/kisspeptin and metastasis
Several in vitro phenotypes have been observed in cells after the exposure of exogenous kisspeptins which support GPR54 as a potential regulator of metastasis (Table 1). The most extensive analysis of in vitro metastatic characteristics has been performed using CHO cells overexpressing GPR54 (CHO/GPR54). It is important to emphasize that CHO cells are non-metastatic. And while not ideal for functional assessment, they are extremely useful for biochemical characterization. When these cells are exposed to kisspeptins, a decrease in proliferation, motility, invasion, and soft agar colony formation have been observed with concomitant increases in focal adhesions and stress fiber formation (14,16). These characteristics are generally consistent with changes expected in cells which would have lower metastatic potential. Kisspeptin exposure has also consistently shown effects on migration in other cell lines with high endogenous or experimentally elevated levels of GPR54 (18,27,29,30). However, a decrease in proliferation or invasiveness has not been seen in cells with high endogenous levels of GPR54 (18,29). Recently, Becker et al. found an induction of proapoptotic genes in MDA-MB-435S cells after prolonged exposure to micromolar levels of exogenous KP10 (32). Despite consistent findings for GPR54/kisspeptin signaling in the regulation of motility, the regulation of proliferation and invasion has been less straightforward. Interestingly, over-expression of KISS1 in MDA-MB-435 human metastatic breast carcinoma cells had no effect on adhesion, motility, or invasion (33). This is in stark contrast with what was seen in CHO/GPR54 cells after exogenous exposure to kisspeptins. However, there was a significant decrease in soft agar colony formation as observed in CHO/GPR54 cells (33,34). The inability of KISS1 overexpression to inhibit motility of MDA-MB-435 cells may be due to suboptimal levels of kisspeptin secretion and/or inadequate levels of GPR54 at the cell surface. Of note, MDA-MB-435 metastases can still be suppressed by KISS1 overexpression despite low levels of GPR54 mRNA (unpublished). Therefore, inhibition of in vitro motility may not be a relevant marker for examining the potential of KISS1 metastatic suppression.
6.4. In vivo implications for GPR54/kisspeptin and metastasis
To the best of our knowledge, Ohtaki et al. is the only group so far to examine and publish the relationship between GPR54 and kisspeptin signaling in in vivo metastasis assays. B16-BL6 murine melanoma cells over-expressing human GPR54 (B16-BL6/GPR54) were injected for both experimental (intravenous injection) and spontaneous metastasis assays (subcutaneous injection). Exogenous KP10 was delivered through an osmotic pump and lung metastases were examined at the completion of the study. Interestingly, exogenous KP10 was only capable of suppressing B16-BL6/GPR54 pulmonary metastases arising from a subcutaneous injection (i.e., lung colonization was not inhibited if the tumor cells were inoculated directly into the venous circulation). This was an unexpected result because it had been previously shown that KISS1 overexpression inhibits pulmonary metastases in both spontaneous and experimental assays (8,9,33). This may suggest that GPR54/kisspeptin signaling does not play a role in KISS1 pulmonary metastasis suppression following intravenous injection and may point to the involvement of additional mechanisms by which KISS1 can inhibit metastasis. However, it is unclear if the inability of exogenous administration of KP10 to inhibit pulmonary metastases after intravenous injection is due to ‘trivial’ experimental conditions such as pharmacokinetics (e.g., inadequate local concentrations of the peptide in the lungs). Interestingly, our laboratory has detected very low levels of GPR54 mRNA in C8161.9 cells despite a potent suppression of pulmonary metastases after intravenous injection when KISS1 expression is restored (unpublished). Either low levels of GPR54 are sufficient for KISS1 metastasis suppression or KISS1 may mediate suppressive effects by interactions with other proteins within the cell or perhaps even signaling through an additional receptor(s).
7. WHICH STEP(S) OF THE METASTATIC CASCADE DOES KISS1 SUPPRESS?
Most of the in vitro data to date implicate that KISS1 metastasis suppression is mediated, in part, through its effects on growth, motility, and invasion of cancer cells. Most of these effects would suggest that the step in the metastatic cascade where KISS1 is likely to exert its suppressive effects would be at the primary site where inhibition of growth, motility and invasion would be most effective. However, in vitro assays have provided limited mechanistic insight with regard to KISS1 metastasis suppression. Therefore, the most unassailable way to determine the step in the metastatic cascade where KISS1 suppression occurs is through in vivo experimentation.
Since metastatic dissemination often occurs before clinical detection of the primary tumor, identification of molecular targets that block antecedent steps in the process will be less useful clinically. Molecular targets that prevent the proliferation of metastases once they have already disseminated will be most advantageous in the treatment of patients with metastatic disease. Goldberg, Harms et al. indicated that an intact copy of chromosome 6 suppressed pulmonary metastases of C8161 cells (neo6/C8161) at the final step of the metastatic cascade - colonization of the secondary site. Persistent single dormant neo6/C8161 cells were found in the lungs beyond 5 weeks while parental C8161 cells formed macroscopic metastases by 2–3 weeks and killed the host by ~4 wk (35). Since neo6/C8161 cells have elevated levels of KISS1, we asked whether KISS1 expression is similarly capable of maintaining metastatic dormancy in the lung. Recently, our laboratory has addressed this question by restoring KISS1 expression in green fluorescent protein-tagged C8161 cells. We found that KISS1 expression does not affect initial seeding of metastatic cells to the lung, but prevents tumor cell proliferation after arrival (K. T. Nash and D. R. Welch, manuscript in preparation). Thus, we believe that KISS1 is a promising molecular target for inhibiting growth of pre-symptomatic metastatic lesions. How KISS1 maintains dormancy of disseminated cells at the secondary site is still unclear. In addition, it is still unknown whether KISS1 will show efficacy in treating larger established metastatic lesions (masses >100–1000 cells). Recently, Palmieri and colleagues showed that restoration of Nm23 metastasis suppressor expression by treatment with methoxyprogesterone acetate (to induce a glucocorticoid transcription factor element in the Nm23 promoter) successfully reduced progression of established microscopic metastases (36). Their proof-of-principle studies are encouraging while answers to both questions will be important to resolve in order to understand the impact that KISS1 may have in the clinic.
8. EVIDENCE FOR KISS1 AS POTENTIAL CLINICAL TARGET
Progress toward understanding the mechanism of action of KISS1 and relevant clinical correlates has been significantly hampered by the unavailability of reliable, validated antibodies to detect KISS1 protein. All of the experimental evidence and clinical evidence highlighted below measured mRNA expression levels and not protein. Although this data does not detract from the potential of KISS1’s significance, development of reliable antibodies for determining protein levels in patient biopsy specimens in the future will be more pertinent. This issue is especially relevant since nascent KISS1 would not appear to be the active form.
We were the first to show that the introduction of KISS1 into highly metastatic human melanoma cell lines C8161 and MelJuSo suppressed metastases to the lung by >95% following intravenous or orthotopic injection (8,9,33). Interestingly, introduction of KISS1 into a metastatic breast cancer cell line MDA-MB-435 also showed a >95% suppression of metastases to the lung following orthotopic injection (33). Those data strongly suggested KISS1 metastasis suppression may be pertinent in tumors of widely different origins, a conclusion borne out in subsequent studies (11,30,37–40), albeit of varying quality and significance. In general, loss or reduction of KISS1 expression in several different tumor types inversely correlates with tumor progression, metastatic potential and survival. The data summarized below highlights the potential value of KISS1 as an important clinical target for the prognostication and treatment of metastatic disease.
8.1. Melanoma
Melanoma is the most lethal form of skin cancer that can affect adults of all ages including teenagers. Most melanomas have a period of superficial growth referred to as the radial growth phase in which the lesion increases in size but does not invade beyond the superficial dermal layers. Melanomas caught at this stage are not metastatic and are associated with a good prognosis. Over time, growth of the melanoma continues until it enters the vertical growth phase in which cells invade into deeper dermal layers. In general, depth of invasion correlates with metastatic competence and poor prognosis (especially >0.75 mm). Fortunately, the majority of melanomas are caught early in the radial growth phase and full-thickness excisional biopsy is curative. If the biopsy reveals invasion, staging is required for determining treatment and prognosis. If metastatic disease is revealed, treatment is usually palliative in nature because metastatic melanoma is generally incurable.
The holy grail for pathologists is to unequivocally identify patients with tumors likely to metastasize so that aggressive adjuvant therapies can be administered. If accomplished, one can avoid unnecessary treatments for patients with tumors unlikely to have spread. Unfortunately, the subjectivity of pathologic prognosis has not reached that level of sophistication. Even when the world’s most highly respected dermatopathologists read the same slides, there was >50% discordance amongst the group (41). Therefore, less subjective measures are needed. And it is in this situation where metastasis suppressors could provide enormous assistance (42).
KISS1 mRNA expression has been evaluated in human melanoma specimens from various stages of progression. Shirasaki et al. showed that KISS1 expression is reduced by 50% when primary melanomas exceed > 4mm (11). Interestingly, a dramatic drop in KISS1 expression coincides with the rapid drop in 5-year survival of patients with tumors of this size (43). In addition, Shirasaki et al. showed that there was no difference in KISS1 expression between melanoma tumors > 4 mm deep and the metastatic lesions that were examined. This result was attributed to the evidence that deeply invasive vertical growth phase melanoma cells already contain numerous cytogenetic abnormalities and are metastasis competent (44). Therefore, like Herlyn and colleagues (45), Shirasaki et al. argued that no additional genetic alterations are necessary for metastatic progression. Collectively, the data indicate that there is a strong inverse correlation between loss of KISS1 expression with gain of metastatic potential, suggesting that KISS1 may serve to enhance the staging of melanoma. Coupled with the experimental evidence showing KISS1 as a metastasis suppressor, the data suggests that KISS1 shows promise as a target for the treatment of metastatic melanoma as well.
8.2. Thyroid cancer
Thyroid cancer is the most common malignancy of the endocrine system and shows a predisposition in females over males (~2:1). Although, thyroid neoplasms can arise from every cell type that populates the gland, papillary thyroid cancer accounts for the majority of slow growing, well-differentiated thyroid malignancies. While papillary tumors tend to be locally invasive and are associated with a good prognosis, follicular thyroid cancers are associated with a higher prevalence of metastatic disease and a poor prognosis (46).
KISS1 mRNA expression was evaluated in clinical samples of follicular carcinoma and papillary carcinoma. Consistent with a suppressor of metastasis (although not measuring it directly), Ringel et al. found that papillary carcinomas were more likely to express KISS1 than follicular carcinoma (69% vs. 20%, p<0.05) (30). Since information pertaining to the clinical stage of the specimens used was not provided, an evaluation of KISS1 expression as a prognostic indicator in patients with well differentiated thyroid neoplasms cannot be inferred with certainty.
8.3. Bladder cancer
Bladder cancer is the fourth most common cancer in men and the tenth most common in women. Of the >60,000 new cases diagnosed in 2004, the vast majority (95%) were of transitional cell origin. The single most relevant prognostic indicator is colonization of lymph nodes, in which 5-year survival drops to 10–20%. Further precipitous decreases in survival occur if tumor cells colonize bone or other viscera.
KISS1 mRNA expression was evaluated in a cohort of superficial and invasive bladder neoplasms. Sanchez-Carbayo et al. found that KISS1 expression was inversely correlated with tumor stage and overall survival (39). Interestingly, every bladder tumor that had developed distant metastases showed a complete loss of KISS1 expression. Again, the results are consistent with a role of KISS1 as a metastasis suppressor.
8.4. Esophageal squamous cell carcinoma
Squamous cell carcinoma and adenocarcinoma of the esophagus is extremely lethal and kills almost as many people a year as are diagnosed. Currently, esophagoscopy is required for staging and prognosis, while CT and endoscopic ultrasound are used to detect metastatic disease. Most current therapies are primarily palliative in nature, with fewer than 5% of patients alive after 5 years. Recent utilization of surgery followed by radiation and combination chemotherapy has shown some benefit, however.
KISS1 mRNA expression was evaluated in esophageal squamous cell carcinoma (ESCC). Ikeguchi et al. found that KISS1 expression was lost in 38% of ESCC tumors and was not correlated with tumor size or degree of tumor invasion (38). Like melanoma and bladder cancer, KISS1 expression was lost late in the progression of ESCC and was directly correlated with the likelihood of lymph node metastases and a subsequent poor prognosis. Loss of KISS1 expression in ESCC may serve to augment current staging as well as improve current prognostication.
8.5. Gastric cancer
Despite a slow, steady decline in incidence, gastric cancer in the United States still accounted for 11,000 deaths in 2004. The disease is significantly more common in Asia. The majority of gastric cancers are adenocarcinomas and endoscopic biopsies are required for staging and prognosis. Five-year survival rates are 90% when the cancer is limited to the mucosa but survival drops to 3% once metastatic disease has been detected. Surgical resection is possible in only a third of patients but, in those patients, a prolongation of survival and a reduction in the recurrence rates can be observed. However, in patients with advanced gastric carcinoma, partial responses have only been observed in 30–50% of cases after the use of combination chemotherapy while radiation therapy is predominantly palliative in nature.
Dhar et al. found that gastric cancers with low KISS1 mRNA had frequent venous invasion, distant metastases, tumor recurrence and a significantly worse overall and disease-free survival (37). To date, KISS1 appears to be the strongest independent prognostic indicator for gastric cancer, suggesting that determining KISS1 expression in surgical biopsies should be considered. Although the effect of KISS1 restoration in gastric cancer metastases has not been examined, its potential as a molecular target for the treatment of metastatic disease in patients with gastric cancer should not be overlooked.
8.6. Hepatocellular carcinoma
Globally, hepatocellular carcinoma (HCC) is one of the most common tumors and is the third most common cause of cancer deaths. Staging and clinical course is based on the Okuda system which is determined by tumor size, presence of ascites, bilirubin levels, and albumin levels. Surgery is the best form of treatment before symptoms appear. But after symptoms are apparent, survival drops precipitously and current treatments offer little improvement.
KISS1 mRNA expression was evaluated in HCC. Ikeguchi et al. did not find any significant changes in KISS1 expression between normal liver and HCC samples (47). The lack of correlation with disease cannot be determined because the authors did not include the stage of the clinical specimens examined nor did they report on liver metastases.
8.7. Breast Cancer
Breast cancer is the second most common cause of cancer death in women. Tumors arise primarily from the epithelial cells lining the ducts or lobules. The most important prognostic indicator is lymph node involvement after biopsy. Once metastatic disease is detected, 5-year survival rates drop from greater than 90% to 14%. Nearly half of all breast cancer patients treated for localized disease develop metastatic disease and combinations of local therapy and systemic therapy are not curative.
In a single small study, KISS1 mRNA expression was significantly reduced in all breast cancer brain metastases examined when compared to primary tumor expression levels (40). The results, while promising, are inconclusive with regard to overall prognostic utility of KISS1.
9. GPR54/KISSPEPTIN IN NORMAL PHYSIOLOGY
Although the above studies suggest the possibility that GPR54/kisspeptin signaling could be important in metastasis suppression in human cancers, nothing about their function points to a mechanism a priori. Some recent data showing a role for both molecules in normal physiology may provide important insights into the mechanism of KISS1 in cancer metastasis. However, questions still persist regarding how this receptor-ligand pair, whose physiologic function is to control the hypothalamic-pituitary axis in puberty and pregnancy, regulates metastasis.
Co-expression of human KISS1 and GPR54 mRNAs is greatest in the placenta with a wide distribution throughout the brain including the hypothalamus and basal ganglia (15). Co-expression was also seen, but at lower levels, in the pancreas, kidney, liver, lung, prostate and small intestine (8,15,16). The predilection for co-expression of KISS1 and GPR54 mRNA in neuroendocrine tissues such as placenta, pancreas, and hypothalamus is consistent with sequence similarities between KISS1 and other neuropeptides. These similarities to neuropeptides, as well as its tissue localization, may prove to be invaluable in determining the mechanism of KISS1 metastasis suppression. We will review what is known about the physiological relationship of KISS1 and GPR54 signaling in the placenta and brain and how this may pertain to metastasis suppression.
Since the identification of high GPR54 and KISS1 expression in the placenta, several papers implied that GPR54/kisspeptin signaling may regulate placental invasion. Horikoshi et al. identified elevated levels of KP54 in the serum of pregnant women suggesting that KP54 may be a placental derived hormone (23). Janneau et al. identified that KISS1 and GPR54 expression levels can be detected in trophoblast cells, increase in early pregnancy and are lost in choriocarcinoma (48). These data were the first to implicate KISS1/kisspeptins and GPR54 in the potential regulation of the invasive properties of trophoblast cells. Bilban et al. identified higher KISS1 and GPR54 expression in first trimester (high invasiveness) trophoblasts than in term placentae (no/low invasiveness). Both KISS1 and GPR54 mRNA and protein were found in the non-invasive syncytiotrophoblast compartment of the placenta; whereas, GPR54 was also found in the invasive extravillous cytotrophoblast (18). The tissue compartmentalization in placenta could suggest autocrine, paracrine or endocrine interactions. Although, this data does not directly address GPR54/kisspeptin signaling in placental invasion, the proximity and compartmentalization is alluring. Since placental invasion is often considered to parallel metastasis it is plausible that GPR54/KISS1 signaling may be an important regulator of metastasis through its anti-invasive effects. However, the data are confusing with regard to a role in tumor cell invasion. In experimental systems, expression of KISS1 did not suppress invasion, while metastasis was suppressed (8,16,33,38).
While the data strongly support that KISS1 is a ligand for GPR54, the mechanism(s) by which KISS1 suppresses metastasis may vary depending upon which cell expresses either (or both) molecule. It is unclear whether low levels of GPR54 are sufficient to support KISS1 metastasis suppression via autocrine or intracrine signaling (Figure 2). Both C8161 and MDA-MB-435 express extremely low levels of GPR54 yet are suppressed by KISS1 in an expression-dependent manner. This concern is mitigated by the relative potency of other G-protein coupled receptors despite their modest expression.
Figure 2.
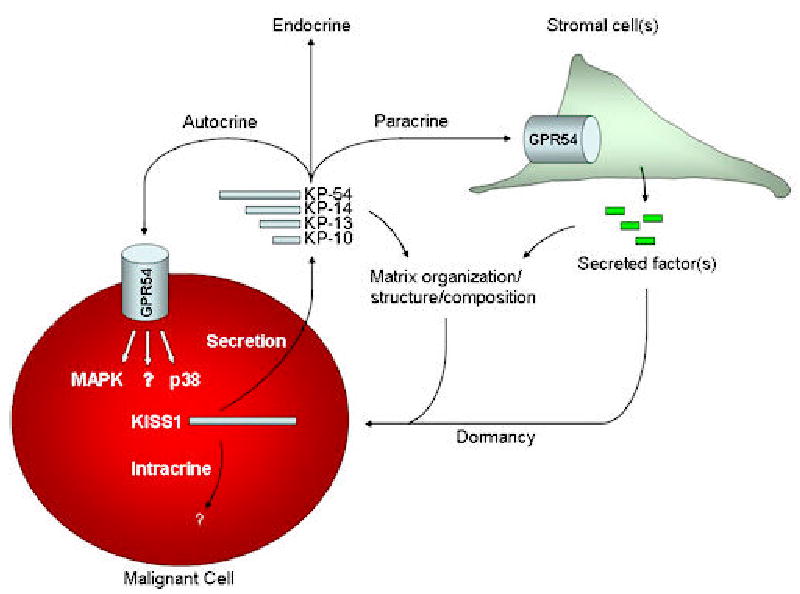
Schematic representation of the potential mechanism(s) that KISS1 may employ to suppress metastatic proliferation at the secondary site. Experimental evidence to date suggests that KISS1 may accomplish this in several ways. First, unprocessed KISS1 may interact with other proteins in the cell using an intracrine mechanism. Second, secreted kisspeptins could signal back to the metastatic cell through its interaction with GPR54 in an autocrine loop. Third, secreted kisspeptins could interact with other unknown cells and organs distant from the metastatic cells in an endocrine fashion that in turn may inhibit metastatic proliferation. Fourth, secreted kisspeptins could interact with GPR54 on the surface of stromal cells (e.g. fibroblasts, macrophages, dendritic cells, epithelial cells, etc.) regulating the secretion of peptides that have an anti-proliferative effect on metastatic cells. Finally, secreted kisspeptins or stromal peptides induced by kisspeptins may deposit in the matrix altering its organization, structure, or composition in such a way that provides an anti-proliferative signal to metastatic cells in the vicinity.
One cannot rule out the possibility that KISS1 suppression can also be mediated by paracrine or endocrine interactions with GPR54 on the surface of stromal cells (Figure 2). It is also plausible that KISS1 is capable of inhibiting metastases through receptor interactions other than GPR54 since RF-NH2 amides are common peptide motifs found ubiquitously throughout the animal kingdom (49).
Since GPR54 and KISS1 expression overlap in many areas throughout the brain it was only a matter of time before these two genes were implicated in a physiological function. In 2003, GPR54 was implicated as an important regulator of puberty in both mice and humans (50–52). Mutations in GPR54 led to isolated idiopathic hypogonadism in which patients lack pubertal development. These same effects were observed in GPR54 knockout mice (51,52). Over the last two years, a surge of papers have emerged suggesting that the interaction of GPR54 and KISS1 play a pivotal role in the onset of puberty. Kisspeptin administration has been shown to induce LH and FSH surges in rodents and primates (53–61). These effects are seen despite the mode of injection (intravenous, intraperitoneal, intracerebroventricular). The LH and FSH surge following kisspeptin exposure can be abolished with a GnRH antagonist, suggesting that kisspeptin’s activity is mediated by GnRH neurons. GnRH neurons have been shown to express the GPR54 receptor and kisspeptin administration induces c-fos activity (54,55). Interestingly, KISS1 expression is localized in areas of the hypothalamus that play a role in the regulation of neuroendocrine secretion (62). Lessons learned from kisspeptin/GPR54 signaling in the brain would support the possibility that kisspeptins binding to receptors on stromal cells in the microenvironment may regulate the secretion of peptides in a similar fashion as is seen in GnRH neurons (Figure 2). These stromal peptides could then exert an anti-proliferative effect on metastatic cells rendering them in a state of dormancy. Secreted kisspeptins or stromal peptides could also be deposited in the matrix of the microenvironment. These peptides could provide cues to metastatic cells in the vicinity rendering them in a dormant state (Figure 2). It is also possible that restoration of KISS1 expression in metastatic cells may blunt the response to growth factors released in the microenvironment rendering them incapable of proliferating to form metastatic lesions. Whatever the mechanism(s) is(are) required for KISS1 metastasis suppression, the interplay between KISS1 and the microenvironment is likely to be important.
10. PERSPECTIVE
Despite the advances that have been made since KISS1 was discovered 9 years ago, a mechanistic understanding of KISS1 metastasis suppression is still elusive. Several important questions remain to be addressed in order to determine how KISS1 metastasis suppression may be exploited in the clinic and the types of patients that may receive a benefit.
10.1. Is KISS1 secretion required for metastasis suppression?
If secretion is required, exogenous administration of naturally occurring kisspeptins could be envisioned as a potential therapy for the treatment of metastatic disease avoiding the complications associated with gene therapy. Additionally, research can be focused on evaluating kisspeptin signaling through GPR54 as well as identify other novel receptors that may play a role in the prevention and treatment of metastatic disease. This research will provide a greater mechanistic understanding of KISS1 metastasis suppression that may lead to the identification of additional downstream targets that can be exploited for metastatic therapy.
10.2. Is KISS1 metastasis suppression organ and tissue specific?
Currently, KISS1 has only been investigated as a lung and lymph node metastasis suppressor for melanoma and breast cancer. It will be interesting to determine if KISS1 is a universal metastasis suppressor capable of inhibiting metastases to multiple organs throughout the body regardless of the cancer origin. Since metastatic disease can be observed in multiple organs throughout the body, an ideal molecular target will prevent metastatic growth in any location regardless of the cancer origin. The clinical utility of KISS1 will depend on the number of organs sites where metastases can be suppressed as well as the number of cancer types this suppression is limited to.
10.3. Will KISS1 suppress progression of already disseminated cells?
If KISS1 can inhibit the proliferation of larger established metastatic lesions as well as occult metastases (less than a few millimeters), its clinical potential will be more substantial. Since restoration of KISS1 expression can maintain single disseminated metastatic cells in a state of dormancy, it is possible that adjuvant KISS1 treatment in patients with a high likelihood of occult metastatic disease may prove beneficial. The effects of restoring KISS1 expression in larger metastatic lesions has not been addressed experimentally. Therefore, the possible benefit of using KISS1 treatment in patients with large clinically detectable metastases is uncertain.
Despite many of the unresolved questions, KISS1 remains a promising molecular target for the treatment of metastatic disease and has shown great promise as a prognostic indicator for several cancers. However, greater efforts need to be made in the characterization of KISS1 metastasis suppression before its clinical value can be determined.
Acknowledgments
We are grateful for support from the U.S. Public Health Service (CA87728, CA62168, CA89019) and the National Foundation for Cancer Research Center for Metastasis Research. KTN is a trainee in the Medical Scientist Training Program at the University of Alabama at Birmingham (T32-GM08361). We also appreciate the members of the Welch lab for their helpful comments and suggestions.
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.L. A. G. Ries, M. P. Eisner, C. L. Kosary, B. F. Hankey, B. A. Miller, L. Clegg, A. Mariotto, E. J. Feuer, and B. K. Edwards, SEER cancer statistics review, National Cancer Institute, Bethesda, MD, (2004)
- 3.Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet. 2005;365:687–701. doi: 10.1016/S0140-6736(05)17951-3. [DOI] [PubMed] [Google Scholar]
- 4.Shevde LA, Welch DR. Metastasis suppressor pathways - an evolving paradigm. Cancer Lett. 2003;198:1–20. doi: 10.1016/s0304-3835(03)00304-5. [DOI] [PubMed] [Google Scholar]
- 5.Steeg PS. Metastasis suppressors alter the signal transduction of cancer cells. Nature Rev Cancer. 2003;3:55–63. doi: 10.1038/nrc967. [DOI] [PubMed] [Google Scholar]
- 6.Welch DR, Goldberg SF. Molecular mechanisms controlling human melanoma progression and metastasis. Pathobiol. 1997;65:311–330. doi: 10.1159/000164143. [DOI] [PubMed] [Google Scholar]
- 7.Welch DR, Chen P, Miele ME, McGary CT, Bower JM, Weissman BE, Stanbridge EJ. Microcell-mediated transfer of chromosome 6 into metastatic human C8161 melanoma cells suppresses metastasis but does not inhibit tumorigenicity. Oncogene. 1994;9:255–262. [PubMed] [Google Scholar]
- 8.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–1737. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene [erratum] J Natl Cancer Inst. 1997;89:1549. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 10.West A, Vojta PJ, Welch DR, Weissman BE. Chromosome localization and genomic structure of the KiSS-1 metastasis suppressor gene (KISS1) Genomics. 1998;54:145–148. doi: 10.1006/geno.1998.5566. [DOI] [PubMed] [Google Scholar]
- 11.Shirasaki F, Takata M, Hatta N, Takehara K. Loss of expression of the metastasis suppressor gene KiSS1 during melanoma progression and its association with LOH of chromosome 6q16.3-q23. Cancer Res. 2001;61:7422–7425. [PubMed] [Google Scholar]
- 12.Miele ME, Jewett MD, Goldberg SF, Hyatt DL, Morelli C, Gualandi F, Rimessi P, Hicks DJ, Weissman BE, Barbanti-Brodano G, Welch DR. A human melanoma metastasis-suppressor locus maps to 6q16.3-q23. Int J Cancer. 2000;86:524–528. doi: 10.1002/(sici)1097-0215(20000515)86:4<524::aid-ijc13>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg SF, Miele ME, Hatta N, Takata M, Paquette-Straub CA, Freedman LP, Welch DR. Melanoma metastasis suppression by chromosome 6: Evidence for a pathway regulated by CRSP3 and TXNIP. Cancer Res. 2003;63 [PubMed] [Google Scholar]
- 14.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 15.Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CGC, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. AXOR12: A novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- 16.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Torao Y, Kumano S, Takatsu Y, Matsuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 17.Rouille Y, Duguay SJ, Lund K, Furuta M, Gong Q, Lipkind G, Oliva AA, Jr, Chan SJ, Steiner DF. Proteolytic processing mechanisms in the biosynthesis of neuroendocrine peptides: the subtilisin-like proprotein convertases. Front Neuroendocrinol. 1995;16:322–361. doi: 10.1006/frne.1995.1012. [DOI] [PubMed] [Google Scholar]
- 18.Bilban M, Ghaffari-Tabrizi N, Hintermann E, Bauer S, Molzer S, Zoratti C, Malli R, Sharabi A, Hiden U, Graier W, Knofler M, Andreae F, Wagner O, Quaranta V, Desoye G. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci. 2004;117:1319–1328. doi: 10.1242/jcs.00971. [DOI] [PubMed] [Google Scholar]
- 19.Takino T, Koshikawa N, Miyamori H, Tanaka M, Sasaki T, Okada Y, Seiki M, Sato H. Cleavage of metastasis suppressor gene product KiSS-1 protein/metastin by matrix metalloproteinases. Oncogene. 2003;22:4617–4626. doi: 10.1038/sj.onc.1206542. [DOI] [PubMed] [Google Scholar]
- 20.Nomura H, Sato H, Seiki M, Mai M, Okada Y. Expression of membrane-type matrix metalloproteinase in human gastric carcinomas. Cancer Res. 1995;55:3263–3266. [PubMed] [Google Scholar]
- 21.Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Ann Rev Cell Biol. 1993;4:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 22.Ueno H, Nakamura H, Inoue M, Imai K, Noguchi M, Sato H, Seiki M, Okada Y. Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in human invasive breast carcinomas. Cancer Res. 1997;57:2055–2060. [PubMed] [Google Scholar]
- 23.Horikoshi Y, Matsumoto H, Takatsu Y, Ohtaki T, Kitada C, Usuki S, Fujino M. Dramatic elevation of plasma metastin concentrations in human pregnancy: Metastin as a novel placenta-derived hormone in humans. J Clin Endocrin Metab. 2003;88:914–919. doi: 10.1210/jc.2002-021235. [DOI] [PubMed] [Google Scholar]
- 24.Lee DK, Nguyen T, O’Neill GP, Cheng R, Liu Y, Howard AD, Coulombe N, Tan CP, Tang-Nguyen AT, George SR, O’Dowd BF. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;446:103–107. doi: 10.1016/s0014-5793(99)00009-5. [DOI] [PubMed] [Google Scholar]
- 25.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 26.Clements MK, McDonald TP, Wang R, Xie G, O’Dowd BF, George SR, Austin CP, Liu Q. FMRFamide-related neuropeptides are agonists of the orphan G-protein-coupled receptor GPR54. Biochem Biophys Res Comm. 2001;284:1189–1193. doi: 10.1006/bbrc.2001.5098. [DOI] [PubMed] [Google Scholar]
- 27.Stafford LJ, Xia CZ, Ma WB, Cai Y, Liu MY. Identification and characterization of mouse metastasis-suppressor KiSS1 and its G-protein-coupled receptor. Cancer Res. 2002;62:5399–5404. [PubMed] [Google Scholar]
- 28.Terao Y, Kumano S, Takatsu Y, Hattori M, Nishimura A, Ohtaki T, Shintani Y. Expression of KiSS-1, a metastasis suppressor gene, in trophoblast giant cells of the rat placenta. Biochim Biophys Acta. 2004;1678:102–110. doi: 10.1016/j.bbaexp.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Masui T, Doi R, Mori T, Toyoda E, Koizumi M, Kami K, Ito D, Peiper SC, Broach JR, Oishi S, Niida A, Fujii N, Imamura M. Metastin and its variant forms suppress migration of pancreatic cancer cells. Biochem Biophys Res Comm. 2004;315:85–92. doi: 10.1016/j.bbrc.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Ringel MD, Hardy E, Bernet VJ, Burch HB, Schuppert F, Burman KD, Saji M. Metastin receptor ls overexpressed in papillary thyroid cancer and activates MAP Kinase in thyroid cancer cells. J Clin Endocrin Metab. 2002;87:2399. doi: 10.1210/jcem.87.5.8626. [DOI] [PubMed] [Google Scholar]
- 31.Yan CH, Wang H, Boyd DD. KiSS-1 represses 92-kDa type IV collagenase expression by downregulating NF B binding to the promoter as a consequence of I B -induced block of p65/p50 nuclear translocation. J Biol Chem. 2001;276:1164–1172. doi: 10.1074/jbc.M008681200. [DOI] [PubMed] [Google Scholar]
- 32.Becker JA, Mirjolet JF, Bernard J, Burgeon E, Simons MJ, Vassart G, Parmentier M, Libert F. Activation of GPR54 promotes cell cycle arrest and apoptosis of human tumor cells through a specific transcriptional program not shared by other G(q)-coupled receptors. Biochem Biophys Res Comm. 2005;326:677–686. doi: 10.1016/j.bbrc.2004.11.094. [DOI] [PubMed] [Google Scholar]
- 33.Lee J-H, Welch DR. Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res. 1997;57:2384–2387. [PubMed] [Google Scholar]
- 34.Hori A, Honda S, Asada M, Ohtaki T, Oda K, Watanabe T, Shintani Y, Yamada T, Suenaga M, Kitada C, Onda H, Kurokawa T, Nishimura O, Fujino M. Metastin suppresses the motility and growth of CHO cells transfected with its receptor. Biochem Biophys Res Comm. 2001;286:958–963. doi: 10.1006/bbrc.2001.5470. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg SF, Harms JF, Quon K, Welch DR. Metastasis-suppressed C8161 melanoma cells arrest in lung but fail to proliferate. Clin Exptl Metastasis. 1999;17:601–607. doi: 10.1023/a:1006718800891. [DOI] [PubMed] [Google Scholar]
- 36.Palmieri D, Halverson DO, Ouatas T, Horak CE, Salerno M, Johnson J, Figg WD, Hollingshead M, Hursting S, Berrigan D, Steinberg SM, Merino MJ, Steeg PS. Medroxyprogesterone acetate elevation of Nm23-H1 metastasis suppressor expression in hormone receptor-negative breast cancer. J Natl Cancer Inst. 2005;97:632–642. doi: 10.1093/jnci/dji111. [DOI] [PubMed] [Google Scholar]
- 37.Dhar DK, Naora H, Kubota H, Maruyama R, Yoshimura H, Tonomoto Y, Tachibana M, Ono T, Otani H, Nagasue N. Downregulation of KiSS-1 expression is responsible for tumor invasion and worse prognosis in gastric carcinoma. Int J Cancer. 2004;111:868–872. doi: 10.1002/ijc.20357. [DOI] [PubMed] [Google Scholar]
- 38.Ikeguchi M, Yamaguchi K, Kaibara N. Clinical significance of the loss of KiSS-1 and orphan G-protein-coupled receptor (hOT7T175) gene expression in esophageal squamous cell carcinoma. Clin Cancer Res. 2004;10:1379–1383. doi: 10.1158/1078-0432.ccr-1519-02. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Carbayo M, Capodieci P, Cordon-Cardo C. Tumor suppressor role of KiSS-1 in bladder cancer - Loss of KiSS-1 expression is associated with bladder cancer progression and clinical outcome. Am J Pathol. 2003;162:609–617. doi: 10.1016/S0002-9440(10)63854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stark AM, Tongers K, Maass N, Mehdorn HM, Held-Feindt J. Reduced metastasis-suppressor gene mRNA-expression in breast cancer brain metastases. J Cancer Res Clin Oncol. 2004;131:191–198. doi: 10.1007/s00432-004-0629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farmer ER, Gonin R, Hanna NP. Discordance in the histopathologic diagnosis of melanoma and melanocytic nevi between expert pathologists. Hum Pathol. 1996;27:528–531. doi: 10.1016/s0046-8177(96)90157-4. [DOI] [PubMed] [Google Scholar]
- 42.Welch DR, Rinker-Schaeffer CW. What defines a useful marker of metastasis in human cancer? J Natl Cancer Inst. 1999;91:1351–1353. doi: 10.1093/jnci/91.16.1351. [DOI] [PubMed] [Google Scholar]
- 43.Morton DL, Davtyan DG, Wanek LA, Foshag LJ, Cochran AJ. Multivariate analysis of the relationship between survival and the microstage of primary melanoma by Clark level and Breslow thickness. Cancer. 1993;71:3737–3743. doi: 10.1002/1097-0142(19930601)71:11<3737::aid-cncr2820711143>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 44.Herlyn M, Balaban G, Bennicelli J, Guerry D, Halaban R, Herlyn D, Elder DE, Maul GG, Steplewski Z, Nowell PC. Primary melanoma cells of the vertical growth phase: similarities to metastatic cells. J Natl Cancer Inst. 1985;74:283–289. [PubMed] [Google Scholar]
- 45.Meier F, Satyamoorthy K, Nesbit M, Hsu MY, Schittek B, Garbe C, Herlyn M. Molecular events in melanoma development and progression. Front Biosci. 1998;3:D1005–D1010. doi: 10.2741/a341. [DOI] [PubMed] [Google Scholar]
- 46.Singer PA, Cooper DS, Daniels GH, Ladenson PW, Greenspan FS, Levy EG, Braverman LE, Clark OH, McDougall IR, Ain KV, Dorfman SG. Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. American Thyroid Association. Arch Intern Med. 1996;156:2165–2172. [PubMed] [Google Scholar]
- 47.Ikeguchi M, Hirooka Y, Kaibara N. Quantitative reverse transcriptase polymerase chain reaction analysis for KiSS-1 and orphan G-protein-coupled receptor (hOT7T175) gene expression in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2003;129:531–535. doi: 10.1007/s00432-003-0469-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janneau JL, Maldonado-Estrada J, Tachdjian G, Miran I, Motte N, Saulnier P, Sabourin JC, Cote JF, Simon B, Frydman R, Chaouat G, Bellet D. Transcriptional expression of genes involved in cell invasion and migration by normal and tumoral trophoblast cells. J Clin Endocrin Metab. 2002;87:5336–5339. doi: 10.1210/jc.2002-021093. [DOI] [PubMed] [Google Scholar]
- 49.Nichols R. Signaling pathways and physiological functions of Drosophila melanogaster FMRFamide-related peptides. Annu Rev Entomol. 2003;48:485–503. doi: 10.1146/annurev.ento.48.091801.112525. [DOI] [PubMed] [Google Scholar]
- 50.deRoux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang SJ, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Comm. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 52.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MBL, Crowley WF, Aparicio SAJR, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 53.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinol. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 54.Irwig MS, Fraleyb GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 55.Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. BRRC. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- 56.Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561:379–386. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Navarro VM, Castellano JM, Fernández-Fernández R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinol. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 58.Navarro VM, Castellano JM, Fernández-Fernández R, Tovar S, Roa J, Mayen A, Barreiro ML, Casanueva FF, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinol. 2005;146:1689–1697. doi: 10.1210/en.2004-1353. [DOI] [PubMed] [Google Scholar]
- 59.Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinol. 2005;146:156–163. doi: 10.1210/en.2004-0836. [DOI] [PubMed] [Google Scholar]
- 60.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- 62.Seminara SB, Kaiser UB. New gatekeepers of reproduction: GPR54 and its cognate ligand, KiSS-1. Endocrinol. 2005;146:1686–1688. doi: 10.1210/en.2005-0070. [DOI] [PubMed] [Google Scholar]


