Abstract
Objective:
To determine whether body size and perceived figure, both current and historical, are associated with a diagnosis of endometriosis on laparoscopy.
Design:
Cohort study of consecutively identified patients undergoing laparoscopy for tubal sterilization or as a diagnostic procedure.
Setting:
Two university-affiliated hospitals.
Patient(s):
A cohort of 84 women ages 18–45 years. Endometriosis was visualized in 32 cases; 52 women (controls) had no visualized endometriosis, including 22 undergoing tubal sterilization and 30 with other gynecologic pathology.
Interventions:
None.
Main Outcome Measure(s):
Body mass index (kg/m2) from self-report and perception of body figure were compared for their ability to predict case status (diagnosed endometriosis), using logistic regression models. Longitudinal trends in BMI based on perceived figure at 5-year intervals from age 15 years were compared using mixed linear models.
Results:
Based on self-report, women diagnosed with endometriosis were taller, thinner, and had a significantly lower BMI. In this series, cases were more likely to be late maturers (menarche ≥ 14 y) and late to initiate sexual activity (≥ 21 y), while they were less likely to be gravid, parous, and a current smoker. Adjusting for age (in years), being tall (height ≥ 68 in), and parity (yes, no), a higher current BMI was statistically protective for a diagnosis of endometriosis, regardless of whether BMI was determined by self-report (adjusted odds ratio [AOR]=0.88, 95% confidence interval [CI] 0.79–0.99) or from perceived figure (AOR=0.86, 95% CI 0.75–0.99). For every unit increase in BMI (kg/m2), there was an approximate 12–14% decrease in the likelihood of being diagnosed with endometriosis. In an adjusted repeated measures model, BMI was 21.3 ± 0.6 kg/m2 (estimate ± SE) for women with endometriosis, compared with 23.2 ± 0.4 kg/m2 for the controls, a difference over all ages of –1.9 ± 0.8 kg/m2 (P = .045). This is a consistent difference of about 10 lb at every age, assuming an average height of about 64.5 in.
Conclusions:
In a laparoscopy cohort, women diagnosed with endometriosis were found to have a lower BMI (leaner body habitus), both at the time of diagnosis and historically. That women diagnosed with endometriosis may have a consistently lean physique during adolescence and young adulthood lends support to the suggestion of there being an in utero or early childhood origin for endometriosis.
Keywords: body figure, body mass index, endometriosis, fetal origin, silhouette
INTRODUCTION
Endometriosis is defined as the presence of functional uterine tissue and stroma external to the uterus. Lesions range from superficial deposits scattered throughout the pelvic and abdominal cavity, which may be treated expectantly with medication or simple ablative surgery, to deep and invasive endometriosis with adhesions, which requires surgical removal (1, 2). Although there are accompanying symptoms that may be indicative, including chronic pelvic pain, adnexal masses, dyspareunia, and infertility, endometriosis is always finally diagnosed by visualizing the lesions, cysts, implants and nodules by laparoscopy (3). There are virtually no incidence figures for endometriosis, although prevalence is often estimated to be 10–15% of women of reproductive age (4–7). Prevalence, a function of incidence and duration of disease, varies by characteristics of the women under surveillance (e.g., infertile, symptomatic or surgical indications for laparoscopy) and study definitions.
While a number of menstrual, reproductive, and biologic risk factors, such as a shorter menstrual cycle length, heavier menstrual volume, earlier age at menarche, greater parity, taller height, lesser weight, and greater waist-to-hip ratio, have been associated with risk of endometriosis (8–14), most remain equivocal as risk factors reflecting unstated ambiguity underlying the epidemiology of endometriosis. Lifestyle and environmental factors have also been identified as risk factors, although they have not been causally implicated. Alcohol and caffeine consumption have been associated with an increased risk, and cigarette smoking with a statistically protective effect (6, 8, 9) in some studies. However, interpretation of findings is seriously limited by inconsistent exposure windows when estimating risk (e.g., varying time periods before diagnosis for cases or a comparable referent interval for controls) or the inclusion/omission of relevant study covariates in multivariate models.
Most recently, following discovery of a dose-dependent relation between dioxin and severity of endometriosis (15), exposure to hormonally active environmental chemicals has been studied as a risk factor for endometriosis, consistent with the presumed estrogen-dependent nature of the disease. Polychlorinated biphenyls (PCBs) and dioxin have been associated with risk of confirmed endometriosis among women undergoing laparoscopy (16–18), especially PCB congeners with anti-estrogenic properties (19).
It has been difficult to develop a useful clinical profile for endometriosis because some of the risk factors identified are seemingly contradictory and little research has been done to evaluate longitudinal trends. For example, the association of endometriosis with a relatively lean physique (tall, thinner), early menarche, and a greater waist-to-hip ratio is counterintuitive, given that girls with early menarche tend instead to be heavier (20, 21). The association of a lean habitus with a diagnosis of endometriosis has been demonstrated primarily in cross-sectional studies (9, 10, 12, 13), begging the question of whether it is a characteristic of women who develop endometriosis or instead a consequence of their lifestyle and relative infertility.
The objective of these analyses was to determine whether body size by self-report and perceived figure (habitus), both current and historical, are associated with a diagnosis of endometriosis by laparoscopy. This avenue of research is also highly relevant for evaluating the natural history of endometriosis given recent suggestions of a role of the fetal environment in risk of endometriosis (22) and when considering exposure to lipophilic environmental chemicals, because of the increasing evidence of their possible role in the development of endometriosis (16–19).
MATERIALS AND METHODS
Study population and sample
A cohort of 100 consecutive women undergoing laparoscopy for the first time were identified at one of two participating University-affiliated hospitals in western New York between April, 1999 and January, 2000. Women were recruited into the study providing they met the following inclusion criteria: age 18–40 years and scheduled for laparoscopy either for tubal sterilization or as a diagnostic procedure, regardless of preoperative diagnosis. This design served two purposes critical for assessing etiology: 1) all women were at risk for endometriosis in that they were of reproductive age and currently menstruating, and 2) laparoscopic visualization of the pelvis occurred in all women. Eighty-four (84% response) women were eligible and agreed to participate by giving informed consent. Approval for the conduct of this study was obtained from the Institutional Review Boards at the affiliated University and participating hospitals.
Interview data collection
The laparoscopic surgeons who agreed to refer patients first informed women about the study and asked about their willingness to be contacted by the study’s research assistant for further details. A research assistant, who was unaware of women’s preoperative diagnoses, then contacted women who consented, and the women were interviewed in their home prior to surgery. A standardized questionnaire elicited information on sociodemographic, reproductive and medical history, including age at menarche, age at first sexual activity (intercourse), and lifestyle characteristics. Limited aspects of diet (i.e., sport fish consumption, meat, and dairy foods) were also collected for their role as a source of exposure to PCBs (19) and may used to replicate the recent findings of Parazzini et al (23) in future analyses. The questionnaire also solicited information on potential confounders, including gravidity, parity, self-reported height and weight, and current cigarette smoking, because all of these have been reported to be associated with endometriosis, either as protective or risk factors (11, 24).
The women were asked not only to report their height and current weight, but also their minimum and maximum nonpregnant weight since age 18 years, and age at those weights. Women were asked to recall their body shape and size (Figure 1), according to the graded 9-figure scale (numbered from left) developed by Stunkard et al (25), and to recall their figure in 5-year intervals up to their current age: i.e., 15–19, 20–24, 25–29, 30–34, 35–39, and 40–44 years.
FIGURE 1.
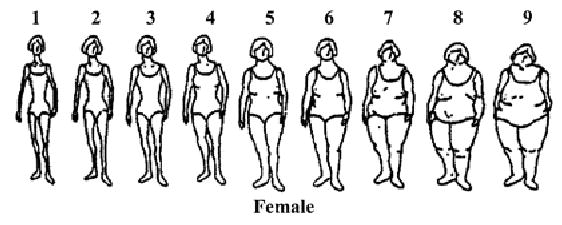
Body figures for self-report of body size among women. Figures are from Stunkard et al (25), used with permission.
Operative procedures
Laparoscopic surgeons, who were experienced with endometriosis and were expected to visualize the entire pelvis, were asked to complete standardized operative reports immediately following the surgery. The reports ascertained information on postoperative diagnosis and other pathology visualized at the time of laparoscopy, regardless of surgical indication. Women observed to have endometriosis were considered cases (n = 32) and compared with women undergoing laparoscopy who did not meet the case definition (controls, n = 52). Among the controls, 22 women had laparoscopies for tubal sterilization with no gynecologic pathology observed while 30 women had other gynecologic pathology noted (e.g., fibroids, polycystic ovaries, pelvic inflammatory disease, idiopathic infertility). The two groups of controls were combined because analysis revealed no statistically significant differences between them and there was no visualized endometriosis.
Severity of endometriosis was staged according to the American Society for Reproductive Medicine’s (formerly, the American Fertility Society) Revised Definitions (26, 27) as: Stage I (minimal), Stage II (mild), Stage III (moderate), and Stage IV (severe). The benefit to this system is that it encompasses location, number, size and depth of the lesions along with presence/absence of adhesions, although a drawback is that the scoring system can be affected by inter-rater variations and an anatomical orientation for pelvic exploration. It should also be noted that the case definition for endometriosis is controversial, especially for minimal or mild disease, because it tends to rely on the appearance of subtle and non-pigmented lesions (28), and other classificatory systems have been recently proposed (2). No formal assessment of physician inter-rater reliability with regard to severity of endometriosis was conducted. However, surgeons were unaware of women’s self-reported body size.
Derived variables for perceived body size and figure
Body mass index (BMI, kg/m2) was calculated from the self-reported heights and weights for current, adult minimum, and maximum weight. The accuracy of self-reported height and weight in women has recently been reviewed (29), with women tending to overestimate height and underestimate weight. However, self-report is still a useful technique in interview situations where direct measurement is not feasible or historical information is sought. Reliance on the self-reported data was unlikely to have biased the findings. Because heavier women, such as multiparous women undergoing laparoscopy for tubal ligation, tend to underestimate their weight, discrepancy between the groups based on faulty recall or misperception of figure should minimize, not exaggerate, the differences between them (bias toward null).
To determine weight history and longitudinal trends, the 9-figure scale (25) was assigned a centile and a BMI, using a ranking procedure similar to that developed for girls at menarche (30). For figures 1 to 9, the centiles assigned were the 5th, 10th, 25th, 50th, 75th, 90th, 95th, 97th, and 99th, respectively. A BMI (kg/m2) was assigned to each centile cutoff using the distribution for women at 20–45 years (31); for the figures 1 to 9, the BMIs assigned were 18.5, 19.3, 20.7, 22.8, 26.4, 31.6, 35.9, 40.0, 45.0 kg/m2, respectively.
The validity of this approach was tested by comparing current BMI based on self-reported height and weight with the BMI estimated from perceived size based on the figure scale, separately for cases and controls. For the cases, mean BMI based on reported height and weight (23.7 ± 3.8 kg/m2) did not differ from the mean (23.1 ± 3.7 kg/m2) based on perceived figure. For the controls, mean BMI based on reported height and weight (26.9 ± 6.2 kg/m2) was somewhat higher than the mean (25.0 ± 5.2 kg/m2) for BMI derived from the figures, but the difference was not significant. Overall, the correlation (r = 0.84, P < .0001) between the BMI from self-report and from perceived figure was highly significant, and the validity was consistent with other studies that have used this technique for remote recall of body size (32–34).
Statistical analysis
Means and standard deviations were calculated by disease status for all continuous variables of interest, such as, current age, weight, height, and recalled age at menarche, age at first sexual activity (intercourse), minimum adult weight, maximum adult weight, and onset of smoking. Student’s t-tests were used to compare the means between groups; frequencies were obtained for categorical and dichotomous variables and compared by Chi-square (χ2).
Logistic regression techniques were used to determine the extent to which current body size, either from self-report or perceived figure, was associated with a diagnosis of endometriosis on laparoscopy. Potential confounders were included based on forward selection and backward deletion. To test whether differences in body figure (as BMI) between women with and without a diagnosis of endometriosis reflected a longitudinal trend and to control for the multicolinearity of age confounders, a repeated measures (blocking for individual subject) SAS PROC MIXED model was used with a variance structure selected to provide the best fit (35). For chronological age, each BMI from perceived figure was assigned the midpoint of the corresponding age group (e.g., for the 15–20 age group, age 17 was used as the midpoint), and selection of confounders to include in the model was based on the change in the likelihood ratio.
RESULTS
Endometriosis was visualized in 32 (cases) of the 84 participating women; 52 women (controls) had no visualized endometriosis, including 22 undergoing tubal sterilization and 30 with other gynecologic pathology. The 32 women with endometriosis had visually confirmed disease irrespective of symptoms or medical history, which have both been reported to misclassify disease (1, 36). Among the cases, 20 were reported to have endometriosis Stage I or II (minimum-mild); 12 had Stage III or IV endometriosis (moderate-severe).
The majority of the study population was non-Hispanic white (89%). The cases diagnosed with endometriosis were somewhat older than the controls and had more years of completed education (Table 1). Cases were more likely to have completed college, while the controls had some college education.
TABLE 1.
Sociodemographic and reproductive characteristics of 84 women who underwent laparoscopy
| Endometriosis | No endometriosis | ||||
|---|---|---|---|---|---|
| n | n | P | |||
| Current age (y)a | 32 | 32.7 ± 4.4 | 52 | 31.6 ± 5.0 | NS |
| Age at menarche (y) | 31 | 13.1 ± 1.4 | 48 | 12.5 ± 1.7 | .082 b |
| Age at first sexual activity (y) | 30 | 19.8 ± 3.2 | 51 | 17.5 ± 3.9 | .0093 |
| Education (y) | 32 | 15.5 ± 2.2 | 52 | 13.8 ± 2.4 | .0014 |
| Non-Hispanic white (%) | 29 | 90.6 | 46 | 88.5 | NS |
| Age at menarche (%) | .018 | ||||
| Early, ≤ 11 y | 2 | 6.5 | 16 | 33.3 | |
| Average, 12–13 y | 19 | 61.3 | 23 | 47.9 | |
| Late, ≥ 14 y | 10 | 32.3 | 9 | 18.8 | |
| Age at first sexual activity (%) | .0016 | ||||
| ≤ 17 y | 6 | 20.0 | 31 | 60.8 | |
| 18–20 y | 14 | 46.7 | 13 | 25.5 | |
| ≥ 21 y | 10 | 33.3 | 7 | 13.7 | |
| Gravid (yes, %) | 11 | 34.4 | 33 | 63.5 | .0095 |
| Parous (yes, %) | 5 | 15.6 | 26 | 50.0 | .0015 |
| Employed (yes, %) | 29 | 90.6 | 43 | 84.3 | NS |
| Married (yes, %)c | 25 | 78.1 | 40 | 76.9 | NS |
| Current smoker (yes, %) | 4 | 12.5 | 23 | 45.1 | .002 |
Note: NS = not significant; SD = standard deviation.
Values are means ± SD, unless indicated.
P value determined by Student’s t-test for means and χ2 for distributions.
Currently married includes living as married.
Women diagnosed with endometriosis differed from the controls in ways relating to reproduction and lifestyle characteristics. Cases attained menarche at later ages than the controls; 32.3% of the cases, as compared with 18.8% (P < .02) of the controls, were classified as late maturers (menarche ≥ 14 y). The cases also became sexually active at significantly later ages, while controls were more likely to be gravid, parous, and current smokers. However, in this study, women diagnosed with endometriosis did not differ from the controls in terms of most of the symptoms generally associated with endometriosis. This may reflect the heterogeneity of the control women. There were no significant differences in reported length of menstrual cycle, menstrual volume, occurrence of irregular periods, recent pelvic pain (past year), or dyspareunia, although women diagnosed with endometriosis were more likely than the controls to report painful bowel movements in the past year (43.7% for cases vs 17.3% for controls, P = .008),
Based on self-report, women with endometriosis were, on average, somewhat taller, thinner, and had a significantly lower BMI at diagnosis, with the current difference in self-reported weight being just over 15 lb (Table 2). Although the means for self-reported height did not differ, women with endometriosis were significantly (P <.04) more likely to report a height of 68 in or greater. The differences in weight were consistent historically, with the difference in reported minimum weight at about age 22 being about 6 lb, and maximum weight at age 28 about 10 lb. Comparing women with endometriosis classified as Stages I and II (minimum-mild) and Stages III and IV (moderate-severe) with the controls indicated that while there was an apparent dose-response effect in BMI, the differences in BMI among stages of disease tended not to be significantly different because of the limited sample sizes. Women with moderate-severe disease reported a BMI of 22.8 ± 3.4 kg/m2, compared with 24.3 ± 3.9 kg/m2 for those with minimum-mild disease and 26.9 ± 6.2 kg/m2 for controls. Findings were similar for perceived figure.
TABLE 2.
Current and historical body size and BMI based on self-report by diagnosis for 84 women who underwent laparoscopy
| Endometriosis | No endometriosis | ||||
|---|---|---|---|---|---|
| n | mean ± SD | n | mean ± SD | P | |
| Age (y) | 32 | 32.7 ± 4.4 | 52 | 31.6 ± 5.0 | NS |
| BMI (kg/m2) | 31 | 23.7 ± 3.8 | 52 | 26.9 ± 6.2 | .006a |
| Height (in) | 32 | 65.2 ± 3.2 | 52 | 64.4 ± 2.3 | NS |
| < 63 in (%) | 7 | 21.9 | 11 | 21.2 | .034a |
| 63 – 67.9 in | 16 | 50.0 | 37 | 71.2 | |
| ≥ 68 in | 9 | 28.1 | 4 | 7.7 | |
| Weight (lb) | 31 | 143.4 ± 28.8 | 52 | 158.9 ± 38.6 | .056 |
| Age at minimum weight (y) | 32 | 23.0 ± 4.3 | 52 | 22.4 ± 4.8 | NS |
| Minimum weight (lb) | 32 | 119.9 ± 17.8 | 52 | 125.3 ± 26.3 | NS |
| Age at maximum weight (y) | 32 | 27.8 ± 6.2 | 52 | 28.9 ± 5.4 | NS |
| Maximum weight (lb) | 32 | 157.8 ± 36.8 | 52 | 167.3 ± 41.4 | NS |
Note: BMI = body mass index; NS = not significant; SD = standard deviation.
P value determined by Student’s t-test for means and χ2 for distributions.
The historical averages by 5-year age periods for body figure by disease status are given in Table 3. Women with endometriosis and controls reported weight gain through time, as indicated by a perceived change in figure. For both cases and controls, the change in BMI translates into an approximate 14–15 lb weight gain (assuming an average height of about 64.5 in) from age 15 y to their current age, but, at every age, controls without endometriosis tended to be heavier than women diagnosed with endometriosis on laparoscopy.
TABLE 3.
BMI and BMI percentile estimated from perceived body figure historically and currently by diagnosis for 84 women who underwent laparoscopy
| Endometriosis |
No endometriosis |
|||||
|---|---|---|---|---|---|---|
| Age category | n | Centilea | BMI (kg/m2) mean ± SD | n | Centile | BMI (kg/m2) mean ± SD |
| 15–19 y | 32 | 22nd | 20.6 ± 2.6 | 52 | 26th | 21.0 ± 3.5 |
| 20–24 y | 32 | 27th | 21.1 ± 2.9 | 52 | 36th | 22.1 ± 3.5 |
| 25–29 y | 31 | 35th | 21.7 ± 2.3 | 45 | 40th | 22.8 ± 4.4 |
| 30–34 y | 22 | 43rd | 22.8 ± 3.6 | 32 | 50th | 24.0 ± 4.1 |
| 35–39 y | 12 | 50th | 23.7 ± 4.1 | 12 | 51st | 24.4 ± 4.8 |
| 40–45 y | ----- | ----- | ----- | 2 | 86th | 33.2 ± 9.6 |
| Current | 32 | 45th | 23.1 ± 3.7 | 52 | 55th | 25.0 ± 5.2 |
Note: BMI = body mass index; SD = standard deviation.
Values are the average centile for the group based on the assigned centile for each figure.
Logistic regression was used to evaluate the adjusted odds of being diagnosed with endometriosis on laparoscopy (Table 4). In unadjusted models, early menarche (age ≤ 11 y) was associated with about an 85% decreased risk of endometriosis, while being tall (height ≥ 68 in) was associated with a nearly fivefold increased risk. Increasing age at first sexual activity (intercourse) increased the risk of diagnosis, but parity was statistically protective. For each unit (kg/m2) increase in BMI, either from self-report or perceived figure, there was a corresponding decrease in the likelihood of being diagnosed with endometriosis on laparoscopy. In models adjusting for confounders, including current age (in years), being tall (height ≥ 68 in), and parity (yes, no), a higher current BMI continued to be statistically protective for a diagnosis of endometriosis, regardless of whether BMI was determined by self-report (adjusted odds ratio [AOR]=0.88, 95% confidence interval [CI] 0.79–0.99) or from perceived figure (AOR=0.86, 95% CI 0.75-0.99). While early menarche and age at first sexual activity were both independently related to risk of diagnosis, neither confounded the relationship between BMI and risk of endometriosis. For every unit increase in BMI (kg/m2), there was an approximate 12–14% decrease in the likelihood of being diagnosed with endometriosis on laparoscopy. The test for fixed effects of BMI over time demonstrated that the difference in BMI from perceived figure was significant over all ages. In a repeated measures model, adjusting for age (midpoint of the age interval), age at first sexual activity, being tall, and parity, the BMI estimate was 21.3 ± 0.6 kg/m2 (estimate ± SE) for women with endometriosis, compared with 23.2 ± 0.4 kg/m2 for the controls, a difference over all ages of –1.9 ± 0.8 kg/m2 (P = .014). This is a difference of at least 10 lb at every age interval, assuming a height of about 64.5 in.
TABLE 4.
Crude and adjusted odds ratios for predicting diagnosis of endometriosis for women undergoing laparoscopy
| Crude OR (95% CI) | AOR (95% CI)a | AOR (95% CI) | |
|---|---|---|---|
| Current age (y) | 1.05 (0.96–1.16) | 1.08 (0.96–1.21) | 1.08 (0.96–1.22) |
| Early menarche (≤ 11 y)b | 0.14 (0.03–0.65) | ||
| Age at first sexual activity (y)b | 1.19 (1.03–1.37) | ||
| Tall (height ≥ 68 in) | 4.70 (1.31–16.86) | 4.79 (1.06–21.69) | 7.90 (1.56–39.89) |
| Parous (yes) | 0.19 (0.06–0.56) | 0.21 (0.07–0.69) | 0.19 (0.06–0.61) |
| BMI (kg/m2) from self-reportb | 0.89 (0.80–0.98) | 0.88 (0.79–0.99) | |
| BMI from perceived figure | 0.90 (0.81–1.01) | 0.86 (0.75–0.99) |
Note: AOR = adjusted odds ratio; BMI = body mass index; CI = confidence interval; OR = odds ratio.
Sample size for model adjusting for all covariates, n = 83.
Sample size for model with early menarche, n = 80; for age at first sexual activity, n = 81; for BMI from self-report, n = 83.
DISCUSSION
Among women undergoing laparoscopy either for tubal sterilization or as a diagnostic procedure, we found that, based on self-report, women diagnosed with endometriosis on laparoscopy were taller, thinner, and had a significantly lower BMI, with the current difference in weight being just over 15 lb. Women diagnosed with endometriosis were more likely to be late physical maturers (menarche ≥ 14 y) and late to initiate sexual activity (≥ 21 y), while they were less likely to be gravid, parous, and a current smoker than unaffected women. Adjusting for age, being tall (≥ 68 in), parity, and independent of age at menarche and initiation of sexual activity, a higher current BMI was statistically protective for a diagnosis of endometriosis, regardless of whether BMI was determined by self-report or from perceived figure. That is, for every unit increase in BMI (kg/m2), there was an approximate 12–14% decrease in the likelihood of being diagnosed with endometriosis. In a repeated measures model, adjusting for age, age at first sexual activity, being tall, and parity, the difference over all ages from 15–45 y was –1.9 ± 0.8 kg/m2 for women diagnosed with endometriosis. This is a consistent difference of women with endometriosis being thinner by about 10 lb at every age, assuming an average height of about 64.5 in.
Our findings lend support for earlier observations linking endometriosis risk to increasing height (9), a pear shape upper body (11) and a lower BMI (9–12, 37). Hemmings et al (13), in a study of 680 women who underwent diagnostic laparoscopy in Montréal area institutions, found that those diagnosed with endometriosis were more than twice (OR=2.6, 95% CI 1.1–5.0) as likely to be underweight (BMI ≤ 18.5 kg/m2) compared with controls, although at the same time it was noted that women with endometriosis were more likely to have never conceived (OR=1.5, 95% CI 1.0–2.0) and would not have been exposed to weight changes with pregnancy. An inverse relation between BMI and prevalence of minimal or mild endometriosis in a case control study of women undergoing laparoscopy has been reported (36). Currently, there are no standardized classifications for assigning pear or apple body shape on the basis of measuring subcutaneous adipose tissue despite concerns about the relationship of regional fat distribution with diabetes, heart disease or endometriosis. Computerized optical systems for measuring subcutaneous adipose tissue are reported to have utility for quantifying body shape (apple vs pear) among women (38), and may offer promise for understanding the role of adiposity, body shape (regional fat distribution) and endometriosis.
While early menarche is often cited as a risk factor for endometriosis by current authors (7, 39) the relation is far from clear. Recent research focusing on women undergoing diagnostic laparoscopy report a late onset of menarche as a risk factor for endometriosis (37) consistent with our finding. In addition, our work further adjusts for other physical and biologic determinants of puberty onset and progression, thereby increasing our confidence in this finding. Recent authors observing a relation between early menarche and endometriosis have relied on self-reported physician diagnosed endometriosis (40) or self-reported laparoscopic-confirmed incident cases of endometriosis where the distribution may have been biased by excluding the most severe cases that were diagnosed at early chronological ages (14, 41). With regard to age at first sexual intercourse, we found only one paper that addressed this factor. Similar to our finding, women with endometriosis reported an older age at first intercourse in relation to friend controls (P= .01) but not other medical controls (P = .10) (42).
Endometriosis is often described as a clinical enigma, which impacts study design and related methodologic issues. Despite the potential selection biases associated with the use of women undergoing diagnostic or therapeutic laparoscopy for ascertainment of cases and controls, laparoscopic confirmation of disease remains the gold standard for diagnosis. There are no clinical or laboratory tests with sufficient sensitivity and specificity for diagnosing endometriosis in lieu of laparoscopy or laparotomy. Attempts to diagnose women using symptoms, clinical findings or ultrasonography have produced disappointing findings except, possibly, for ovarian endometriosis (36). A recent randomized double-blind trial of magnetic resonance imaging (MRI) for the detection of biopsy-confirmed endometriosis reported a sensitivity of only 69% and a specificity of 75% (43).
Our data suggest that despite endometriosis being thought of as an estrogen-dependent disease, affected women appear not to respond in the expected manner by gaining weight and seem to remain significantly leaner almost throughout their twenties and early thirties. When stratifying by severity of disease, women with more advanced disease have an even lower BMI than control women or women with milder endometriosis. Further elucidation of the role of body figure over critical windows is needed to better understand the natural history of endometriosis.
Our findings need to be cautiously interpreted. As with all studies, choice of control group is likely to impact study findings. Our control group comprises a heterogeneous group of women, but all have been reported to be free of visualized disease. Choice of control group is a complicated issue and often relies on other women with pelvic complaints or fertile women seeking sterilization procedures. While menstruating women comprise the target population, there are few approaches for their identification. Relatively few menstruating women undergo laparoscopy even among symptomatic women. The extent to which selection factors impact etiologic findings remains unknown. Endometriosis may be present, but unrecognized, by surgeons resulting in misclassification of women on disease status, although such misclassification is unlikely to be modified with regard to BMI.
In addition, study data on body figure were retrospectively reported, although the correlation between current and historic body size offers some support for the validity of the self-report data. Further, any discrepancy between the groups based on faulty recall or misperception of figure should have minimized, not exaggerated, the differences between them (bias toward null).
Lastly, the extent to which we have adequately addressed potential confounders impacts residual confounding and the interpretation of findings. The relatively limited understanding of the epidemiology of endometriosis coupled with incomplete attention to correlated factors such as age, fertility, and body figure in past work underscores the challenges that lie ahead. We do not anticipate that a lean physique will be a sensitive indicator of disease; however, it may provide an additional piece of information helpful in developing a clinical (inclusive of anthropometric) profile of susceptible women. The consistency of a lean body over critical windows of adolescence and early adulthood suggest a potential role for peri-conceptional and in utero factors in the etiology of endometriosis, perhaps as a consequence of intrauterine growth restriction and subsequent alterations of the postnatal hormonal milieu (22) or indicative of Müllerianosis (44). However, while pursuing these early origins, attention should also be paid to the comorbidity of endometriosis (e.g., autoimmune disorders) that has recently been reported (45, 46).
Footnotes
Capsule: In a laparoscopy cohort, women ages 18-45 years diagnosed with endometriosis had consistently lower body mass index (leaner habitus) than women without endometriosis, both at time of diagnosis and historically.
Supported in part by National Institutes of Health grant NIH-ES 09045-01.
References
- 1.Duleba AJ. Diagnosis of endometriosis. Obstet Gynecol Clin North Am. 1997;24:331–46. doi: 10.1016/s0889-8545(05)70307-7. [DOI] [PubMed] [Google Scholar]
- 2.Garry R. The endometriosis syndromes: a clinical classification in the presence of aetiological confusion and therapeutic anarchy. Hum Reprod. 2004;19:760–8. doi: 10.1093/humrep/deh147. [DOI] [PubMed] [Google Scholar]
- 3.Melis GB, Ajossa S, Guerriero S, Paoletti AM, Angiolucci M, Piras B, Caffiero A, Mais V. Epidemiology and diagnosis of endometriosis. Ann NY Acad Sci. 1994;734:352–7. doi: 10.1111/j.1749-6632.1994.tb21765.x. [DOI] [PubMed] [Google Scholar]
- 4.Olive DL, Schwartz LB. Endometriosis. N Engl J Med. 1993;328:1759–69. doi: 10.1056/NEJM199306173282407. [DOI] [PubMed] [Google Scholar]
- 5.Redwine DB. Was Sampson wrong? Fertil Steril. 2002;78:686–93. doi: 10.1016/s0015-0282(02)03329-0. [DOI] [PubMed] [Google Scholar]
- 6.Cramer DW, Missmer SA. The epidemiology of endometriosis. Ann NY Acad Sci. 2002;995:11–22. doi: 10.1111/j.1749-6632.2002.tb02761.x. [DOI] [PubMed] [Google Scholar]
- 7.Viganò P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Clin Obstet Gynaecol. 2004;18:177–200. doi: 10.1016/j.bpobgyn.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Missmer SA, Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin. 2003;30:1–9. doi: 10.1016/s0889-8545(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 9.Cramer DW, Wilson E, Stillman RJ, Berger MJ, Belisle S, Schiff I, Albrecht B, Gibson M, Stadel BV, Schoenbaum SC. The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA. 1986;255:1904–8. [PubMed] [Google Scholar]
- 10.Darrow SL, Vena JE, Batt RE, Zielezny MA, Michalek AM, Selman S. Menstrual cycle characteristics and the risk of endometriosis. Epidemiology. 1993;4:135–42. doi: 10.1097/00001648-199303000-00009. [DOI] [PubMed] [Google Scholar]
- 11.McCann SE, Freudenheim JL, Darrow SL, Batt RE, Zielezny MA. Endometriosis and body fat distribution. Obstet Gynecol. 1993;82:545–9. [PubMed] [Google Scholar]
- 12.Signorello LB, Harlow BL, Cramer DW, Spiegelman D, Hill JA. Epidemiologic determinants of endometriosis: a hospital-based case-control study. Ann Epidemiol. 1997;7:267–74. doi: 10.1016/s1047-2797(97)00017-3. [DOI] [PubMed] [Google Scholar]
- 13.Hemmings R, Rivard M, Olive DL, Poliquin-Flueury J, Gagné D, Hugo P, Gosselin D. Evaluation of risk factors associated with endometriosis. Fertil Steril. 2004;81:1513–21. doi: 10.1016/j.fertnstert.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 14.Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160:784–96. doi: 10.1093/aje/kwh275. [DOI] [PubMed] [Google Scholar]
- 15.Rier SE, Martin DC, Bownam RE, Dmowski WP, Becker JL. Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam Appl Toxicol. 1993;21:433–441. doi: 10.1006/faat.1993.1119. [DOI] [PubMed] [Google Scholar]
- 16.Mayani A, Barel S, Soback S, Almagor M. Dioxin concentrations in women with endometriosis. Hum Reprod. 1997;12:373–375. doi: 10.1093/humrep/12.2.373. [DOI] [PubMed] [Google Scholar]
- 17.Pauwels A, Schepens PJ, D’Hooghe T, Delbecke L, Dhont M, Brouwer A, Weyler J. The risk of endometriosis and exposure to dioxins and polychlorinated biphenyls: a case-control study of infertile women. Hum Reprod. 2001;16:2050–5. doi: 10.1093/humrep/16.10.2050. [DOI] [PubMed] [Google Scholar]
- 18.Eskenazi B, Mocarelli P, Warner M, Chee WY, Gerthoux PM, Samuels S, Needham LL, Patterson DG., Jr Maternal serum dioxin levels and birth outcomes in women of Seveso, Italy. Environ Health Perspect. 2003;111:947–53. doi: 10.1289/ehp.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buck Louis GM, Weiner JM, Whitcomb BW, Sperrazza R, Schisterman EF, Lobdell DT, Crickard K, Greizerstein H, Kostyniak PJ. Environmental PCB exposure and risk ofendometriosis. Hum Reprod. 2005;20:279–85. doi: 10.1093/humrep/deh575. [DOI] [PubMed] [Google Scholar]
- 20.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Relation of age at menarche to race, time period, and anthropometric dimensions: The Bogalusa Heart Study. Pediatrics 2002;110(4):e43. URL: http://www.pediatrics.org/cig/content/full/110/4/e43 [DOI] [PubMed]
- 21.Wang Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics. 2002;110:903–10. doi: 10.1542/peds.110.5.903. [DOI] [PubMed] [Google Scholar]
- 22.Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Michels KB, Hunter DJ. In utero exposures and the incidence of endometriosis. Fertil Steril. 2004;82:1501–8. doi: 10.1016/j.fertnstert.2004.04.065. [DOI] [PubMed] [Google Scholar]
- 23.Parazzini F, Chiaffarino F, Surace M, Chatenoud L, Cipriani S, Chiantera V, Benzi G, Fedele L. Selected food intake and risk of endometriosis. Hum Reprod. 2004;19:1755–9. doi: 10.1093/humrep/deh395. [DOI] [PubMed] [Google Scholar]
- 24.Batt RE, Buck GM, Smith RA. Health and fertility among women surgically treated for endometriosis. J Am Assoc Gynecol Laparosc. 1997;4:435–42. doi: 10.1016/s1074-3804(05)80035-0. [DOI] [PubMed] [Google Scholar]
- 25.Stunkard AJ, Sorensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. In: Kety SS, Rowland LP, Sidman RL, Matthysse SW (eds). The genetics of neurological and psychiatric disorders. New York, NY: Raven Press, 1983:115–20. [PubMed]
- 26.American Fertility Society. Revised American Fertility Society classification of endometriosis: 1985. Fertil Steril. 1985;43:351–2. doi: 10.1016/s0015-0282(16)48430-x. [DOI] [PubMed] [Google Scholar]
- 27.American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–21. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 28.Koninckx PR. Is mild endometriosis a disease? Hum Reprod. 1994;9:2202–5. doi: 10.1093/oxfordjournals.humrep.a138419. [DOI] [PubMed] [Google Scholar]
- 29.Engstrom JL, Paterson SA, Doherty A, Trabulsi M, Speer KL. Accuracy of self-reported height and weight in women: an integrative review of the literature. J Midwifery Womens Health. 2003;48:338–45. doi: 10.1016/s1526-9523(03)00281-2. [DOI] [PubMed] [Google Scholar]
- 30.Must A, Phillips SM, Stunkard AJ, Naumova EN. Expert opinion on body mass index percentiles for figure drawings at menarche. Int J Obes. 2002;26:876–9. doi: 10.1038/sj.ijo.0801986. [DOI] [PubMed] [Google Scholar]
- 31.Frisancho AR. Anthropometric standards for the assessment of growth and nutritional status. Ann Arbor: University of Michigan Press, 1990.
- 32.Koprowski C, Coates RJ, Bernstein L. Ability of young women to recall past body size and age at menarche. Obes Res. 2001;9:478–85. doi: 10.1038/oby.2001.62. [DOI] [PubMed] [Google Scholar]
- 33.Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B, Rand WM. Recall of early menstrual history and menarcheal body size: After 30 years, how well do women remember? Am J Epidemiol. 2002;155:672–9. doi: 10.1093/aje/155.7.672. [DOI] [PubMed] [Google Scholar]
- 34.Field AE, Franko DL, Striegel-Moore RH, Schreiber GB, Crawford PB, Daniels SR. Race differences in accuracy of self-reported childhood body size among white and black women. Obes Res. 2004;12:1136–44. doi: 10.1038/oby.2004.142. [DOI] [PubMed] [Google Scholar]
- 35.SAS Online Documentation. Cary, NC: SAS Institute, Inc, 1999. URL: http://v8doc.sas.com/sashtml/
- 36.Eskenazi B, Warner M, Bonsignore L, Olive D, Samuels S, Vercellini P. Validation study of nonsurgical diagnosis of endometriosis. Fertil Steril. 2001;76:929–35. doi: 10.1016/s0015-0282(01)02736-4. [DOI] [PubMed] [Google Scholar]
- 37.Berube S, Marcoux S, Maheux R Canadian Collaborative Group on Endometriosis. Characteristics related to the prevalence of minimal or mild endometriosis in infertile women. Epidemiology. 1998;9:504–10. doi: 10.1097/00001648-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Möller R, Tafeit E, Sudi K, Reibnegger G. Quantifying the ‘appleness’ or ‘pearness’ of the human body by subcutaneous adipose tissue distribution. Ann Hum Biol. 2000;27:47–55. doi: 10.1080/030144600282370. [DOI] [PubMed] [Google Scholar]
- 39.Batt RE, Mitwally MFM. Endometriosis from thelarche to midteens: pathogenesis and prognosis, prevention and pedagogy. J Pediatr Adolesc Gynecol. 2003;16:337–47. doi: 10.1016/j.jpag.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Moen MH, Schei B. Epidemiology of endometriosis in a Norwegian county. Acta Obstet Gynecol Scand. 1997;76:559–62. doi: 10.3109/00016349709024584. [DOI] [PubMed] [Google Scholar]
- 41.Missmer SA, Hankinson SE, Spiegleman D, Barbieri RL, Malspeis S, Willett WC, Hunter DJ. Reproductive history and endometriosis among premenopausal women. Obstet Gynecol. 2004;104:965–74. doi: 10.1097/01.AOG.0000142714.54857.f8. [DOI] [PubMed] [Google Scholar]
- 42.Darrow SL, Selman S, Batt RE, Zielezny MA, Vena JE. Sexual activity, contraception, and reproductive factors in predicting endometriosis. Am J Epidemiol. 1994;140:500–9. doi: 10.1093/oxfordjournals.aje.a117276. [DOI] [PubMed] [Google Scholar]
- 43.Stratton P, Winkel C, Premkumar A, Chow C, Wilson J, Hearns-Stokes R, Heo S, Merino M, Nieman LK. Diagnostic accuracy of laparoscopy, magnetic resonance imaging, and histopathologic examination for the detection of endometriosis. Fertil Steril. 2003;79:1078–85. doi: 10.1016/s0015-0282(03)00155-9. [DOI] [PubMed] [Google Scholar]
- 44.Batt RE, Mitwally MFM. Endometriosis from thelarche to midteens: pathogenesis and prognosis, prevention and pedagogy. J Pediatr Adolesc Gynecol. 2003;16:337–47. doi: 10.1016/j.jpag.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715–24. doi: 10.1093/humrep/17.10.2715. [DOI] [PubMed] [Google Scholar]
- 46.Matarese G, De Placido G, Nikas Y, Alviggi C. Pathogenesis of endometriosis : natural immunity dysfunction or autoimmune disease? Trends Mol Med. 2003;9:223–8. doi: 10.1016/s1471-4914(03)00051-0. [DOI] [PubMed] [Google Scholar]


