Abstract
Purpose
Bone marrow (BM)–derived hematopoietic precursor cells are thought to participate in the growth of blood vessels during postnatal vasculogenesis. In this investigation, multichannel laser scanning confocal microscopy and quantitative image analysis were used to study the fate of BM-derived hematopoietic precursor cells in corneal neovascularization.
Methods
We used a bone marrow-reconstituted mouse model in which the bone marrow from enhanced green fluorescent protein (GFP)-positive mice was transplanted into C57BL/6 mice. Basic fibroblast growth factor (bFGF) was used to induce corneal neovascularization in mice. Using this mouse model we set out to test the vasculogenic potential of adult BM-derived cells and their progeny in vivo. Seventy-two histologic sections selected by systematic random sampling from 4 mice were immunostained and imaged with a confocal microscope, and analyzed with Volocity image analysis software.
Results
Our findings reveal that BM-derived endothelial cells do not contribute to bFGF-induced neovascularization in cornea. BM-derived periendothelial vascular mural cells (pericytes) were detected at sites of neovascularization, whereas endothelial cells of blood vessels originated from pre-existing blood vessels in limbal capillaries. Fifty three percent of all neovascular pericytes originated from BM, and 47% of them originated from pre-existing corneoscleral limbus capillaries. Ninety six percent and 92% of BM-derived pericytes also expressed CD 45 and CD 11b, respectively, suggesting their hematopoietic origin from the bone marrow.
Conclusions
Pericytes of new corneal vessels have a dual source; bone marrow and pre-existing limbal capillaries. These findings establish bone-marrow as a significant effector organ in corneal disorders associated with neovascularization.
INTRODUCTION
The neovascularization of tissues is accomplished by two distinct processes: vasculogenesis (V) and angiogenesis (A) (1) (2) (3) (4) (5) (6). During angiogenesis, preexisting blood vessels form neovascular sprouts. In vasculogenesis, blood vessels develop from progenitor cells that coalesce and differentiate to form vessels. The walls of neovascular blood vessel capillaries are composed of two principal cell types: vascular endothelial cells (VEC), and Rouget cells (7). Rouget cells, also known as mural cells or pericytes (peri = around, cyte = cell) form an outer sheath (8) (6) (9) surrounding the endothelial cells on the outer aspect of microvessels (10).
Reports about pericyte or endothelial differentiation from bone marrow hematopoietic cells in various neovascularization models (11) (12) (13) (14–18) are controversial: Two reports have suggested that adult bone marrow (BM)-derived precursors give rise to PC but not VEC (11) (12) , and numerous other reports suggested that bone marrow (BM)-derived precursors give rise to vascular endothelial cells (VEC) (14–18). Differences in tissues, disease models, and neovascularization models may be a reason for this controversy. Difficulty in identifying pericytes, which are in very close spatial proximity to endothelial cells, might be another factor resulting in this contraversy.
The normal mammalian cornea is one of the few tissues that is devoid of pre-existing lymphatics and blood vessels. Therefore, presence of any blood vessels and lymphatic vessels in the cornea always signifies a pathologic process. The actual in vivo differentiation capacity of adult BM-derived hematopoietic precursors cells into pericytes or endothelial cells in corneal neovascularization remains unclear as do the contribution of vasculogenesis and angiogenesis of new vessels i.e. the vessels formed by bone marrow-derived precursor cells (vasculogenesis) and the vessels formed by the cells sprouting from pre-existing vessels (angiogenesis).
Whereas endothelial cells have been studied extensively, much less is known about pericytes. As the name indicates (“peri” around and “cyto” cell) pericytes surround the endothelial cells on the abluminal aspect of microvessels (10). A recent search of the PUBMED database at http://www.ncbi.nlm.nih.gov/reveals a 109-fold difference between the numbers of papers published on these two vascular cell types. Since the cellular processes, and the role of circulating bone marrow-derived progenitor cells underlying neovascular sprout formation remain incompletely understood (11) (19) (20), increased attention to pericytes and their interaction with endothelial cells will yield a better understanding of the role of bone marrow in corneal disorders associated with neovascularization.
We used a bone marrow-reconstituted mouse model in which the bone marrow from enhanced green fluorescent protein (GFP)-positive mice was transplanted into normal C57BL/6 mice. Using this mouse model we set out to test the neovascular potential of adult BM-derived hematopoietic progenitor cells and their progeny in vivo. We used the basic fibroblast growth factor (bFGF)-induced mouse corneal neovascularization model to quantify vasculogenesis and angiogenesis. In addition, we differentiated quantitatively BM-derived pericytes from angiogenic pericytes derived from pre-existing vessels.
We show that bone marrow-derived vascular precursors contributed only to the formation of pericytes (PC) but not vascular endothelium (VEC) in corneal neovascularization. This investigation reveals that a significant fraction of neovascular pericytes in the cornea derive from BM-derived hematopoietic progenitor cells rather than pre-existing vessels suggesting an extensive role for bone marrow and vasculogenesis in corneal neovascularization. We also show that a small fraction of lymphatic endothelial cells in lymphangiogenic vessel walls in cornea (LEC) derive from BM-derived hematopoietic precursor cells, whereas the majority of lymphatic capillaries originate from pre-existing limbal lymphatics.
METHODS
All animal studies were performed in accordance with The National Institutes of Health Office of Laboratory Animal Welfare (OLAW) guidelines and ARVO Statement for the Use of Animals in Research, and were approved by the La Jolla Institute for Molecular Medicine animal research committee.
Animals and Bone Marrow Transplantations
Mice reconstituted with enhanced green fluorescent protein positive (GFP+) syngeneic bone marrow (BM) cells were created to study the behavior of BM cells in vivo. Briefly, BM cells were collected by flushing the femurs and tibias of female C57BL/6 TgN(ACTbEGFP)1Osb mice (Jackson Laboratory, Bar Harbor, ME) with ice-cold phosphate buffered saline (PBS) using a 25-gauge needle. Following the lysis of red blood cells, bone marrow progenitor cells were resuspended in phosphate buffered saline (PBS). After counting, the cells were spun down and resuspended at a concentration of 3x106 cells/100 microliters. The recipient male mice (4 mice) were irradiated by a lethal dosage of 1,000 Rad, 10Gy. Three days prior to the irradiation, the animals were housed in sterilized cages and fed standard chow that has been irradiated. The mice drank water containing the antibiotic Sulfamethoxazole/Trimethoprim to reduce the risk of opportunistic infections. The animals were housed in sterile cages and fed sterile food throughout the procedure. Three hours post-irradiation, 3x106 cells were injected retro-orbitally via the ophthalmic venous plexus in the right orbit. We found previously that this procedure is much more effective than using the tail vein injections. It takes on average less than 30 seconds to inject 100 microliters of cells versus several minutes to vasodilate the mice before trying to inject the cells via the tail vein, and many times unsuccessfully. In addition, the animals are less stressed when this ophthalmic venous plexus injection procedure is used. Fluorescence activated cell sorter (FACS) analysis showed that peripheral blood cells of the recipients were completely reconstituted with GFP+ cells 4 weeks after transplantation. Peripheral blood smears stained with May-Grunwald revealed Barr body chromatin (the inactivated X chromosome seen in females) in recipient male mice corroborating the reconstitution of bone marrow after transplantation (Figure 1A). More than 95% reconstitution of the bone marrow by GFP+ donor-derived cells is previously reported with similar bone marrow transplantation techniques (11) (21).
FIGURE.
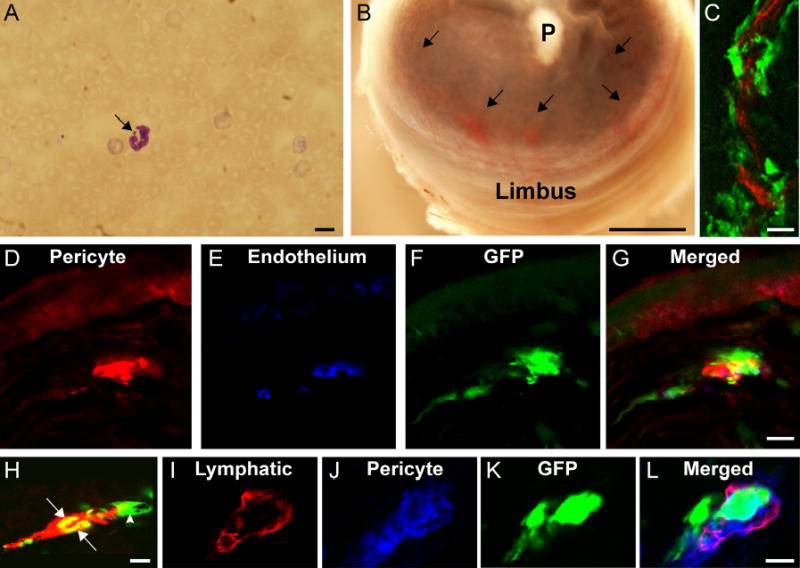
A. Peripheral blood smear stained with May-Grunwald shows a polymorphonuclear leukocyte with Barr body chromatin in the form of drumstick (arrow) in a bone marrow recipient male mouse. Scale bar indicates 10 μm.
B. Polymer (hydron) pellet (P) containing 90 ng of bFGF implanted into the corneal stroma of bone marrow recipient mouse. Arrows indicate the neovasculature penetrating the cornea from the corneoscleral limbus 7 days after pellet implantation surgery. Scale bar indicates 300 μm.
C. Frozen section of neovascularized cornea (as seen in Figure B) obtained from a C57BL/6 mouse in which the bone marrow was reconstituted with enhanced green fluorescent protein (GFP)-positive bone marrow via transplantation. Blood vessel endothelial cells (red) were identified by using confocal microscopy in conjunction with combined immunohistochemical staining for CD31, CD105, and flk-1. Donor-derived (GFP-positive) pericytes formed perivascular sleeves (green) around GFP-negative blood vessel endothelium (red). Scale bar indicates 10μm.
D-G. Frozen section of neovascularized cornea stained using immunohistochemistry to identify pericytes (NG2, red in D), and blood vessel endothelium (combined CD31 + CD105 + flk-1, blue in E). Bone marrow derived cells express GFP (green) in F and G. Merged image (G) shows both GFP-positive (bone marrow derived) and GFP-negative (limbus-derived) pericytes investing the GFP-negative blood vessel endothelium (blue). Scale bar indicates 10 μm.
H. Frozen section of neovascularized cornea stained using immunohistochemistry to identify lymphangiogenic endothelium with LYVE-1 (red). A lymphangiogenic sprout in the cornea shows both LYVE-1+ and GFP– cells (red) and LYVE-1+ and GFP+ cells (yellow) as indicated by arrows. Arrowhead: perivascular cell investing the lymphatic wall like a sleeve.
I-L. Frozen section of neovascularized cornea immunostained for LYVE-1 (red in I), and NG2 (blue in J) for identification of lymphatic endothelium and pericytes, respectively. Bone marrow-derived cells are identified by their GFP expression (green in K). Pericytes (blue) show discontinuous investment of the lymphatic capillary wall (red). Both bone marrow-derived (green) and non-bone marrow derived pericytes (blue) support the lymphatic capillary wall (merged image of I, J and K in panel L). Scale 5μm.
Basic Fibroblast Growth Factor-Induced Corneal Neovascularization Model
Basic fibroblast growth (bFGF) is known to be a potent stimulus for angiogenesis and lymphangiogenesis (22). Implantation of hydron pellets containing bFGF (90ng) induces both neovascularization and lymphangiogenesis in the mouse cornea (23) (24) (25). The mouse corneal micropocket assay, a non-inflammatory neovascularization assay, was performed as previously described (23). Previous studies using this in vivo model have shown the contribution of pericytes co-expressing NG2 proteoglycan and PDGF β-receptor to neovascular vessel walls as the new vessels spread into the cornea (26) (27). Slow-release polyhydroxyethyl methacrylate (hydron) pellets (0.4 x0.4 x 0.2 mm) were formulated to contain 90 ng recombinant basic fibroblast growth factor (bFGF) (LifeTechnologies, Grand Island, NY) and 45 μg sucrose aluminum sulfate (sucralfate) (Sigma, St. Louis, MO). The mice reconstituted with enhanced green fluorescent protein positive (GFP+) syngeneic bone marrow (BM) cells were anesthestized with avertin (0.015– 0.017 ml/g body weight), and a single hydron pellet was surgically implanted into the corneal stroma of the left eye, 0.7 mm from the corneoscleral limbus (Figure 1B). On postoperative day 7, mice were sacrificed and their eyes enucleated.
Immunohistochemistry, Confocal Microscopic Imaging, and Image Analysis
Enucleated eyes were fixed in 4% paraformaldehyde for 6 hours, cryoprotected in 20% sucrose overnight, and frozen in O.C.T. embedding compound (Miles, Inc., Elkhardt, IN). Cryostat sections (40 μm) were air-dried onto Superfrost slides (Fisher Scientific, Pittsburgh, PA). Pericytes were identified by labeling with rabbit, or rat antibodies against the NG2 proteoglycan, or rabbit PDGF β-receptor antibody (28) (26) (27, 29) (30) (11). Both NG2 and PDGF β-receptors are regarded as specific markers for pericytes (31) (32).
Lymphatic endothelium was identified by immunolabeling with rabbit anti-mouse LYVE-1 antibody as described (33) (34). Blood vessel endothelial cells were identified using a cocktail of rat antibodies against mouse endoglin (CD105), PECAM-1 (CD31), and VEGF receptor-2 (flk-1) (Pharmingen, San Diego, CA) (35) (29) (27) a strategy that was utilized previously to maximize labeling of all blood vessel endothelial cells. Immunohistochemical identification of hematopoietic cells was made using rat anti-mouse CD45 (LCA, Ly-5) (Pharmingen, San Diego, CA), and rat anti mouse CD11b (Mac-1α) antibodies (Pharmingen, San Diego, CA) as previously described (11).
Alexa-647 and alexa-594-labeled goat anti-rat secondary antibodies were obtained from Molecular Probes (Eugene, OR), and rhodamine red X (RRX)-labelled goat anti-rabbit secondary antibodies were obtained from Jackson Immuno Research Laboratories (West Grove, PA). Slides were mounted with Vectashield (Vector Laboratories, Burlingame, CA).
Following cryosectioning, sections representing the entire thickness of the cornea were selected from the numbered sections (180 sections from 4 mice) by using systematic random sampling (36). The sampled histological sections (72 sections) were analyzed with a multi-channel laser scanning confocal microscope. Briefly, optical sections were obtained from the specimens using the Fluoview 1000 laser scanning confocal microscope (Olympus USA, Melville, NY) in the three-channel sequential scanning mode. Serial optical sections (1 μm each) across the entire thickness (40 μm) of the histological specimens were overlaid (Z-stack) to provide reconstructions of entire vessels. This allowed unambiguous identification of the spatial relationship between pericytes and endothelial cells in the vessel wall.
The Volocity image analysis software (Openlab-Improvision Inc, Lexington MA) (serial # 88262001) was used for differential quantification of pericytes (PC), blood vessel endothelium (VEC), and lymphatic endothelium (LEC) for GFP expression, CD45 expression, and CD11b expression.
RESULTS
Contribution of Bone Marrow-Derived Pericyte Precursors to Neovascularization
Confocal microscopic examination of histological sections (Figure 1 C-G) revealed evidence of donor-derived (GFP-positive) and host (limbal capillary)-derived (GFP-negative) pericytes forming perivascular sleeves around GFP-negative blood vessel endothelium. We were unable to find evidence of bone marrow-derived blood vessel endothelial cells in corneal neovasculature. This suggests that neovascular blood vessel endothelium in cornea originates from corneoscleral limbal blood vessel capillaries, whereas pericytes have a dual origin: corneoscleral limbus vasculature and bone-marrow hematopoietic cells. In 22 histologic slides from 4 mice, 53% of all neovascular pericytes were GFP-positive and 47% of them were GFP-negative (standard error of mean +/- 3.7%). Quantification based on 40 histologic slides revealed that 96%(standard error of mean +/- 0.7%) of GFP-positive/NG2-positive pericytes also expressed CD 45, whereas 92% (standard error of mean +/- 1.7%) of GFP-positive/NG2-positive pericytes expressed CD 11b. This suggests a hematopoietic origin from bone marrow of these pericytes.
Contribution of Bone Marrow to Lymphangiogenic Endothelium
Lymphangiogenic sprouts in the cornea showed both LYVE-1+ and GFP–, and LYVE+ and GFP+ endothelium (Fig. 1H , arrows). Of 10 histologic slides examined, eight percent of LYVE-1+ cells were GFP+, whereas 92% of LYVE-1+ cells were GFP–, suggesting that BM-derived progenitors contribute minimally to lymphangiogenesis in cornea. Lymphangiogenic vessel walls in the cornea showed discontinuous investment of lymphatic endothelium by NG2+ and PDGFß-receptor–positive periendothelial cells (which are GFP+) in the form of incomplete sleeves (Fig. 1H 1I 1J 1K 1L) . Quiescent lymphatic capillaries in the corneoscleral limbus where no intervention was performed revealed no pericytes on the lymphatic capillary wall.
DISCUSSION
Three observations presented in our study seem especially noteworthy:
First, pericytes of the corneal new vessels have a dual source both from bone marrow (vasculogenesis) and pre-existing limbal capillaries (angiogenesis). More pericytes originate from bone marrow than pre-existing limbal capillaries by a factor of 1.12. Our results also suggest that while majority of bone marrow-derived pericytes in corneal neovascularization derive from hematopoietic precursor cells, a small fraction (4%-8 %) of bone marrow-derived pericytes in the cornea derive from non-hematopoietic bone marrow precursor cells. These non-hematopoietic progenitor cells may represent bone marrow stromal or mesenchymal stem cells (37) (38) (39) (40) (41). These findings establish bone-marrow with its hematopoietic and non-hematopoietic elements as a significant effector organ in corneal disorders associated with neovascularization.
Second, the diverse origin of pericytes and their spatial proximity to each other in the neovascular sprouts raise the possibility of a neovascularization mechanism composed of both angiogenesis and vasculogenesis. By definition angiogenesis and vasculogenesis refer to the formation blood vessels from prexisting blood vessels and stem cells, respectively. This suggests the possibility of a synergy or overlap of two mechanisms operating simultaneously in the same neovascular sprouts, rather than independent progression of angiogenesis from vasculogenesis. Further studies are warranted to elucidate the homing mechanisms of circulating bone marrow derived pericyte precursors through the preexisting endothelium and pericyte layer (barrier) at neovascular sprouts. This homing process might involve extensive pericyte-endothelium and pericyte-pericyte adhesion, and extravasation (emigration) processes along with weakening of the junctional complexes between these cell types. Our findings, coupled with our previous reports (28) (26) (29) (11), also corroborate the findings of other investigators revealing the participation of nascent pericytes during the earliest stages of neovascularization (42) (43) (9) (44) (45) (46) (31). Although pericytes are widely regarded to be the microvascular equivalent of smooth muscle cells, the origin, development, and function of these cells appear to be variable and complex (47) (48) (49). Our ability to detect the precocious contribution of pericytes to microvascular development depends heavily on the use of NG2 proteoglycan and PDGF β-receptor as markers for these cells at an early stage of their development (32) (31). NG2 proteoglycan on pericytes is a functional player in angiogenesis and its extrinsic (pharmacological) or intrinsic (genetic) inhibition is associated with significant decrease in angiogenesis and decrease pericyte/ endothelium investment ratios in neovascularization (27) (30). NG2, a membrane-spanning chondroitin sulfate proteoglycan associated with mitotically active, nascent pericytes (PC), exhibits several properties which suggest it is a functional player in neovascularization (30) (27) (29) (26) (28). NG2 appears to serve as a co-receptor for both bFGF (basic fibroblast growth factor) and PDGF (platelet-derived growth factor) (50) (51). Pericyte-NG2 chondroitin sulfate proteoglycan binds to extracellular matrix components such as type V, type VI and type II collagen, tenascin, and laminin (52) (53). Biochemical data also demonstrate the involvement of both galectin-3 and α3β1 integrin in the VEC response to pericyte-NG2, and show that NG2, galectin-3, and α3β1 form a complex on the cell surface promoting cell motility (54). Our recent findings revealed decreased neovascularization following intrinsic (NG2 knockout mice) or extrinsic (hydron polymer pellets containing NG2 neutralizing antibody) targeting of NG2 proteoglycan in ischemia, neurofibromatosis type 1 (NF1) and in the corneal bFGF-induced neovascularization model (30) (27). In the retinal vessels of NG2 knockout mice, proliferation of both pericytes and endothelial cells following ischemia is significantly reduced, and the pericyte: endothelial cell ratio falls from the wild type value of 0.86 to 0.24 (27). When pericyte-NG2 proteoglycan is targeted, a 53% reduction in tumor neovascularization is possible in NF1-derived malignant peripheral nerve sheath tumor (MPNST) (30). One of the traditional markers for pericyte identification has been the expression of alpha-smooth muscle actin (α-SMA). However, a growing body of evidence suggests that α-SMA is a late marker for differentiated pericytes and therefore may be poorly expressed in developing angiogenic microvasculature (31, 32). Since only a fraction of developing pericytes can be identified on the basis of α-SMA expression (32) (55) (9) (56) (57) (58), we used NG2 and PDGF β-receptor immunohistochemistry to identify pericytes (31, 32) (59) (29).
Third, our investigations also identified discontinuous investment of lymphangiogenic capillary walls by BM-derived periendothelial cells in lymphangiogenesis induced by bFGF. These pericytes may provide the capillary wall with some temporary physical support until the lymphatic endothelium establishes direct connection with the extracellular matrix. The same cells may also represent a transitional stage of BM-derived precursors before they transdifferentiate into lymphatic endothelium. In our studies so far, we have not identified pericytes in quiescent lymphatic capillary walls.
Recent studies suggest that dual targeting of pericytes and endothelial cells improves the efficacy of treatments aimed at inhibiting neovascular vessels (60, 61) (62) (27) (30). Clearly, the design of improved targeting strategies aimed at pericytes and lymphatic endothelial cells that derive from hematopoietic precursors will depend on further understanding of the role of pericytes in neovascularization. Our study provides evidence that bone marrow derived pericytes contribute to the early phases of neovascular sprout formation during pathological neovascularization and lymphangiogenesis. As significant players in neovascularization, bone-marrow derived precursors represent an additional target for treatments designed to down-regulate neovascularization in cornea.
Acknowledgments
This work has been supported by grants from NIH (National Institute of Child Health and Human Development) RO3 HD044783, the U.S. Department of Defense Prostate Cancer Research Program New Investigator Award PC020822, and Tobacco-Related Disease Research Program of University of California Idea Award (TRDRP 13IT-0067) to Dr. Ugur Ozerdem.
Contributor Information
Ugur Ozerdem, La Jolla Institute for Molecular Medicine.
Kari Alitalo, Biomedicum Helsinki, University of Helsinki.
Petri Salven, University of Helsinki.
Andrew Li, University of California San Diego.
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.D’Amore PA. Mechanisms of retinal and choroidal neovascularization. Invest Ophthalmol Vis Sci. 1994;35(12):3974–9. [PubMed] [Google Scholar]
- 3.Campochiaro PA. Retinal and choroidal neovascularization. J Cell Physiol. 2000;184(3):301–10. doi: 10.1002/1097-4652(200009)184:3<301::AID-JCP3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267(16):10931–4. [PubMed] [Google Scholar]
- 5.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 6.Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 7.Rouget CMB. Sur la contractilité capillaires sanguins. Comptes rendus de l’Académie des. Sciences. 1879;88:916–918. [Google Scholar]
- 8.Sims DE. Recent advances in pericyte biology--implications for health and disease. Can J Cardiol. 1991;7(10):431–43. [PubMed] [Google Scholar]
- 9.Nehls V, Denzer K, Drenckhahn D. Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res. 1992;270(3):469–74. doi: 10.1007/BF00645048. [DOI] [PubMed] [Google Scholar]
- 10.Rhodin JA. Ultrastructure of mammalian venous capillaries, venules, and small collecting veins. J Ultrastruct Res. 1968;25(5):452–500. doi: 10.1016/s0022-5320(68)80098-x. [DOI] [PubMed] [Google Scholar]
- 11.Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104(7):2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, et al. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94(2):230–8. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 13.Heil M, Ziegelhoeffer T, Mees B, Schaper W. A different outlook on the role of bone marrow stem cells in vascular growth: bone marrow delivers software not hardware. Circ Res. 2004;94(5):573–4. doi: 10.1161/01.RES.0000124603.46777.EB. [DOI] [PubMed] [Google Scholar]
- 14.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 15.Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol. 2004;287(3):C572–9. doi: 10.1152/ajpcell.00330.2003. [DOI] [PubMed] [Google Scholar]
- 16.Asahara T, Isner JM. Endothelial progenitor cells for vascular regeneration. J Hematother Stem Cell Res. 2002;11(2):171–8. doi: 10.1089/152581602753658385. [DOI] [PubMed] [Google Scholar]
- 17.Asahara T. Endothelial progenitor cells for neovascularization. Ernst Schering Res Found Workshop. 2003;(43):211–6. doi: 10.1007/978-3-662-05352-2_12. [DOI] [PubMed] [Google Scholar]
- 18.Shirakawa K, Furuhata S, Watanabe I, Hayase H, Shimizu A, Ikarashi Y, et al. Induction of vasculogenesis in breast cancer models. Br J Cancer. 2002;87(12):1454–61. doi: 10.1038/sj.bjc.6600610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3(6):401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 20.Darland DC, D’Amore PA. Blood vessel maturation: vascular development comes of age. J Clin Invest. 1999;103(2):157–8. doi: 10.1172/JCI6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galimi F, Summers RG, van Praag H, Verma IM, Gage FH. A role for bone marrow-derived cells in the vasculature of noninjured CNS. Blood. 2005;105(6):2400–2. doi: 10.1182/blood-2004-02-0612. [DOI] [PubMed] [Google Scholar]
- 22.Cao R, Eriksson A, Kubo H, Alitalo K, Cao Y, Thyberg J. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ Res. 2004;94(5):664–70. doi: 10.1161/01.RES.0000118600.91698.BB. [DOI] [PubMed] [Google Scholar]
- 23.Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D’Amato RJ. A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci. 1996;37(8):1625–32. [PubMed] [Google Scholar]
- 24.Kenyon BM, Browne F, D’Amato RJ. Effects of thalidomide and related metabolites in a mouse corneal model of neovascularization. Exp Eye Res. 1997;64(6):971–8. doi: 10.1006/exer.1997.0292. [DOI] [PubMed] [Google Scholar]
- 25.Chang LK, Garcia-Cardena G, Farnebo F, Fannon M, Chen EJ, Butterfield C, et al. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc Natl Acad Sci U S A. 2004;101(32):11658–63. doi: 10.1073/pnas.0404272101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozerdem U, Monosov E, Stallcup WB. NG2 proteoglycan expression by pericytes in pathological microvasculature. Microvasc Res. 2002;63(1):129–34. doi: 10.1006/mvre.2001.2376. [DOI] [PubMed] [Google Scholar]
- 27.Ozerdem U, Stallcup WB. Pathological angiogenesis is reduced by targeting pericytes via the NG2 proteoglycan. Angiogenesis. 2004;7(3):269–276. doi: 10.1007/s10456-004-4182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222(2):218–27. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- 29.Ozerdem U, Stallcup WB. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6(3):241–9. doi: 10.1023/B:AGEN.0000021401.58039.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozerdem U. Targeting neovascular pericytes in neurofibromatosis type 1. Angiogenesis 2005; In press. [DOI] [PMC free article] [PubMed]
- 31.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314(1):15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 32.McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med. 2003;9(6):713–25. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- 33.Witmer AN, Van Blijswijk BC, Van Noorden CJ, Vrensen GF, Schlingemann RO. In vivo angiogenic phenotype of endothelial cells and pericytes induced by vascular endothelial growth factor-a. J Histochem Cytochem. 2004;52(1):39–52. doi: 10.1177/002215540405200105. [DOI] [PubMed] [Google Scholar]
- 34.Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10(9):974–81. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- 35.Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci U S A. 2000;97(26):14608–13. doi: 10.1073/pnas.97.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dawson B, Trapp RG. Basic and clinical biostatistics. Third ed. New York: McGraw-Hill; 2001.
- 37.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 38.Pereira RF, O’Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, et al. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1998;95(3):1142–7. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 40.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura T, Ishikawa F, Sonoda KH, Hisatomi T, Qiao H, Yamada J, et al. Characterization and distribution of bone marrow-derived cells in mouse cornea. Invest Ophthalmol Vis Sci. 2005;46(2):497–503. doi: 10.1167/iovs.04-1154. [DOI] [PubMed] [Google Scholar]
- 42.Schlingemann RO, Rietveld FJ, de Waal RM, Ferrone S, Ruiter DJ. Expression of the high molecular weight melanoma-associated antigen by pericytes during angiogenesis in tumors and in healing wounds. Am J Pathol. 1990;136(6):1393–405. [PMC free article] [PubMed] [Google Scholar]
- 43.Schlingemann RO, Oosterwijk E, Wesseling P, Rietveld FJ, Ruiter DJ. Aminopeptidase a is a constituent of activated pericytes in angiogenesis. J Pathol. 1996;179(4):436–42. doi: 10.1002/(SICI)1096-9896(199608)179:4<436::AID-PATH611>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 44.Wesseling P, Schlingemann RO, Rietveld FJ, Link M, Burger PC, Ruiter DJ. Early and extensive contribution of pericytes/vascular smooth muscle cells to microvascular proliferation in glioblastoma multiforme: an immuno-light and immuno-electron microscopic study. J Neuropathol Exp Neurol. 1995;54(3):304–10. doi: 10.1097/00005072-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Amselgruber WM, Schafer M, Sinowatz F. Angiogenesis in the bovine corpus luteum: an immunocytochemical and ultrastructural study. Anat Histol Embryol. 1999;28(3):157–66. doi: 10.1046/j.1439-0264.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- 46.Redmer DA, Doraiswamy V, Bortnem BJ, Fisher K, Jablonka-Shariff A, Grazul-Bilska AT, et al. Evidence for a role of capillary pericytes in vascular growth of the developing ovine corpus luteum. Biol Reprod. 2001;65(3):879–89. doi: 10.1095/biolreprod65.3.879. [DOI] [PubMed] [Google Scholar]
- 47.Le Lievre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34(1):125–54. [PubMed] [Google Scholar]
- 48.Sims DE. The pericyte--a review. Tissue Cell. 1986;18(2):153–74. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- 49.Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169(1):1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- 50.Goretzki L, Burg MA, Grako KA, Stallcup WB. High-affinity binding of basic fibroblast growth factor and platelet- derived growth factor-AA to the core protein of the NG2 proteoglycan. J Biol Chem. 1999;274(24):16831–7. doi: 10.1074/jbc.274.24.16831. [DOI] [PubMed] [Google Scholar]
- 51.Grako KA, Stallcup WB. Participation of the NG2 proteoglycan in rat aortic smooth muscle cell responses to platelet-derived growth factor. Exp Cell Res. 1995;221(1):231–40. doi: 10.1006/excr.1995.1371. [DOI] [PubMed] [Google Scholar]
- 52.Tillet E, Ruggiero F, Nishiyama A, Stallcup WB. The membrane-spanning proteoglycan NG2 binds to collagens V and VI through the central nonglobular domain of its core protein. J Biol Chem. 1997;272(16):10769–76. doi: 10.1074/jbc.272.16.10769. [DOI] [PubMed] [Google Scholar]
- 53.Burg MA, Tillet E, Timpl R, Stallcup WB. Binding of the NG2 proteoglycan to type VI collagen and other extracellular matrix molecules. J Biol Chem. 1996;271(42):26110–6. doi: 10.1074/jbc.271.42.26110. [DOI] [PubMed] [Google Scholar]
- 54.Fukushi J, Makagiansar IT, Stallcup WB. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin. Mol Biol Cell. 2004;15(8):3580–90. doi: 10.1091/mbc.E04-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nehls V, Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha- actin. J Cell Biol. 1991;113(1):147–54. doi: 10.1083/jcb.113.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res. 1998;53(6):637–44. doi: 10.1002/(SICI)1097-4547(19980915)53:6<637::AID-JNR1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 57.Boado RJ, Pardridge WM. Differential expression of alpha-actin mRNA and immunoreactive protein in brain microvascular pericytes and smooth muscle cells. J Neurosci Res. 1994;39(4):430–5. doi: 10.1002/jnr.490390410. [DOI] [PubMed] [Google Scholar]
- 58.Alliot F, Rutin J, Leenen PJ, Pessac B. Pericytes and periendothelial cells of brain parenchyma vessels co-express aminopeptidase N, aminopeptidase A, and nestin. J Neurosci Res. 1999;58(3):367–78. [PubMed] [Google Scholar]
- 59.Sundberg C, Ljungstrom M, Lindmark G, Gerdin B, Rubin K. Microvascular pericytes express platelet-derived growth factor-beta receptors in human healing wounds and colorectal adenocarcinoma. Am J Pathol. 1993;143(5):1377–88. [PMC free article] [PubMed] [Google Scholar]
- 60.Pietras K, Hanahan D. A Multitargeted, Metronomic, and Maximum-Tolerated Dose “Chemo-Switch” Regimen is Antiangiogenic, Producing Objective Responses and Survival Benefit in a Mouse Model of Cancer. J Clin Oncol 2004. [DOI] [PubMed]
- 61.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111(9):1287–95. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saharinen P, Alitalo K. Double target for tumor mass destruction. J Clin Invest. 2003;111(9):1277–80. doi: 10.1172/JCI18539. [DOI] [PMC free article] [PubMed] [Google Scholar]


