Abstract
The hexosamine biosynthesis pathway (HBP) is a relatively minor branch of glycolysis. Fructose-6-phosphate is converted to glucosamine-6-phosphate, catalyzed by the first and rate-limiting enzyme, glutamine:fructose-6-phosphate amidotransferase (GFAT). The major end-product is UDP-N-acetylglucosamine (UDP-GlcNAc). Along with other aminosugars generated by HBP, it provides essential building blocks for glycosyl side chains, of proteins and lipids. UDP-GlcNAc regulates flux through HBP by regulating GFAT activity and is the obligatory substrate of O-GlcNAc transferase. The latter is a cytosolic and nuclear enzyme, which catalyzes a reversible, post-translational protein modification, transferring N-acetylglucosamine in O-linkage (O-GlcNAc) to specific serine/threonine residues of proteins. The metabolic effects of increased flux through HBP are thought to be mediated by increasing O-GlcNAcylation. Several investigators proposed that HBP functions as a cellular nutrient sensor and plays a role in the development of insulin resistance and the vascular complications of diabetes. Increased flux through HBP is required and sufficient for some of the metabolic effects of sustained , increased glucose flux, which promotes the complications of diabetes, e.g. diminished expression of sarcoplasmic reticulum Ca 2+ - ATP-ase in cardiomyocytes and induction of TGF-β and plasminogen activator inhibitor –1 in vascular smooth muscle cells, mesangial cells and aortic endothelial cells. The mechanism was consistent with enhanced O-GlcNAcylation of certain transcription factors. The role of HBP in the development of insulin resistance has been controversial. There are numerous papers showing a correlation between increased flux through HBP and insulin resistance, however the causal relationship has not been established. More recent experiments in mice overexpressing GFAT in muscle and adipose tissue or exclusively in fat cells suggest that the former develop in vivo insulin resistance via cross-talk between fat cells and muscle. While the relationship between HBP and insulin resistance may be quite complex, it clearly deserves further study in concert with its role in the complications of diabetes.
Insulin resistance is a hallmark of type 2 diabetes, of uncontrolled type 1 diabetes, of obesity and the metabolic syndrome (91) and is associated with numerous other conditions, such as cystic fibrosis, uremia, septicemia, glucocorticoid excess, polycystic ovary syndrome, etc. Clinically, insulin resistance is defined as the reduced ability of insulin to lower plasma glucose, which reflects in great part impaired insulin-stimulated glucose transport into tissues, which express the insulin responsive glucose transporter, GLUT4 (skeletal and heart muscle and adipocytes). Except for a few rare conditions, the major defect(s) are down-stream of insulin’s binding to its receptors. Type 2 diabetes is a polygenic disease and several recent, excellent reviews discuss the insulin receptor signaling cascade and proposed mechanisms of impaired signal transduction in insulin resistance (57, 69, 70, 72). The propensity to insulin resistance is likely genetically determined (79), however, the expression of the phenotype is modulated by various factors including diet, exercise, and ageing.
Sustained hyperglycemia causes insulin resistance in humans (89) and in animal models (67), which lead to the concept of “glucose toxicity”. The latter accounts for the insulin resistance in uncontrolled type 1 diabetes (89). Similarly, sustained elevations of circulatory non-esterified fatty acids (NEFA) also induce insulin resistance (“lipotoxicity”). Thus, insulin resistance may represent an adaptive mechanism, which may serve to protect cells from the deleterious effects of excessive nutrient flux, such as oxidative stress. This would imply the existence of cellular biochemical sensors, which monitor the flux of nutrients. McGarry first identified malonyl-CoA as a biochemical sensor, which regulates the switch from fatty acid to glucose oxidation in the liver (47). Several laboratories have proposed that flux through the hexosamine synthesis pathway (HSP) may function as a cellular nutrient sensor and play a role in the development of insulin resistance and the vascular complications of diabetes (4, 5, 24, 44, 66). This review will address the experimental evidence, which supports and questions this hypothesis and the proposed mechanisms by which the HSP may exert these effects.
A role for excess glucose flux via HSP in insulin resistance was first proposed by Marshall et al in 1991, based on experiments using isolated rat adipocytes (43). In this system, pre-exposure of the cells to insulin and high glucose act synergistically to induce resistance of glucose transport to subsequent acute stimulation by insulin. The insulin resistance develops only if a complete amino acid mixture or glutamine is present in the medium during preincubation with high glucose and insulin. The requirement for glutamine suggested the involvement of the HSP (43).
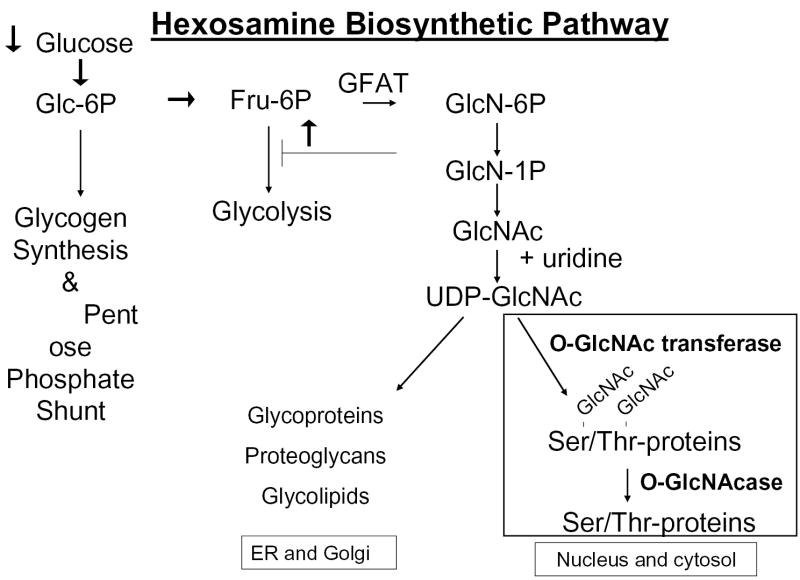
The HSP is a relatively minor branch of the glycolytic pathway, encompassing ~ 3% of total glucose utilized (43). (Fig 1). Entry into the HSP is catalyzed by the first and rate limiting enzyme glutamine: fructose-6-phosphate (F-6-P) amidotransferase (GFAT), which converts F-6-P and glutamine to glucosamine-6-phosphate (GlcN-6-P) and glutamate. Subsequent steps metabolize GlcN-6-P to UDP-N-acetylglucosamine (UDP-GlcNAc), UDP-N-acetylgalactosamine (UDP-GalNAc) and CMP-syalic acid, essential building blocks of the glycosyl side chains of glycoproteins, glycolipids, proteoglycans and gangliosides. UDP-GlcNAc is of particular interest because a) quantitatively it is the major end product of the HSP, b) it is an allosteric feedback inhibitor of GFAT, which regulates glucose entry into the pathway and c) it is the obligatory substrate of O-GlcNAc transferase (OGT). The latter is a cytosolic and nuclear enzyme, which catalyzes a reversible post-translational protein modification, whereby N-acetylglucosamine (GlcNAc) is transferred in O-linkage to specific serine/threonine residues of numerous proteins (37, 42). The sites of O-GlcNAc modification (O-GlcNAcylation) are often identical or adjacent to known phosphorylation sites, suggesting a regulatory function (14). Functional significance of O-GlcNAcylation has been reported for several proteins (85), including the transcription factors Sp1 (16, 26, 65, 78, 86), c-myc (34), CREB (40), Stat 5 (21), and PDX-1 (18), as well as cytosolic and nuclear enzymes, e.g. glycogen synthase (54, 55) and RNA polymerase II (14). Of particular interest in the context of insulin resistance is that insulin receptor substrates (IRS)-1 and 2 (1, 17, 56, 77) and probably also GLUT 4 (8) are subject to O-GlcNAcylation. While the O-GlcNAc modification of IRS-1 in the references cited was based on immunological methods, an O-GlcNAcylation site on IRS-1 was recently identified by mass spectrometry (2). The reversible, O-GlcNAc modification of proteins has been suggested by many investigators as a mechanisms by which increased HSP activity could cause insulin resistance and the complications of diabetes.
Fig.1.
A simplified schematic representation of the hexosamine biosynthetic pathway. The black arrows indicate that flux through the pathway can be increased by accelerating glucose entry or by inhibiting glycolysis distal to fructose-6-phosphate. GFAT: glucosamine-fructose-6-phosphate amidotransferase. (Adapted from Ref. 2)
There is considerable evidence indicating that increased activity of the HSP can cause insulin resistance in cell culture models and in rodents in vivo. In the model mentioned above, where sustained exposure to high glucose in the presence of insulin caused insulin resistance in adipocytes, treatment of the cells with inhibitors of GFAT activity prevented this effect. Furthermore, glucosamine, (GlcN) which enters the HSP bypassing GFAT, also caused insulin resistance but at much lower doses than glucose. The effect of GlcN infusions on the development of insulin resistance in rodents, undergoing insulin clamp studies has been extensively studied (3, 56, 68). Rossetti reported in 1995, that infusion of GlcN increased the concentrations of UDP-GlcNAc in muscle, and markedly decreased insulin stimulated total body glucose utilization in healthy control, but not in diabetic rats, which were already insulin resistant (68). Previous studies had demonstrated that in vitro treatment of isolated muscles with GlcN inhibited the insulin response of glucose transport, without affecting insulin receptor and GLUT4 expression (62). Sustained hyperglycemia, which causes insulin resistance, also increased UDP-HexNAc concentrations in muscles (63). The insulin resistance, which develops in rats infused with lipid emulsions is also associated with increased UDP-GlcNAc in muscle, presumably reflecting impaired glycolytic flux distal to fructose-6-P resulting in increased flux via HSP (28). Based on various clamp studies, Hawkins et al. proposed that the UDP-GlcNAc concentration in skeletal muscle may modulate the insulin responsiveness of glucose transport (27, 28). However, this conclusion has been questioned by Choi et al. (12), who found that increasing circulating free fatty acids induces peripheral insulin resistance without concomitant increases in the concentrations of UDP-GlcNAc or UDP-GalNAc in muscle.
Mice overexpressing GLUT1 in muscle exhibit chronically increased muscle glucose flux, increased muscle glycogen, mild fasting hypoglycemia without significant changes in circulating insulin or glucagon. Insulin fails to stimulate glucose transport in the insulin resistant muscles in vitro, although GLUT4 expression is unchanged.
Other stimuli, which normally stimulate glucose transport, e.g. IGF-1, hypoxia and contractile activity, are also ineffective in GLUT-1 overexpressing mice (22). UDP-HexNAc concentrations and GFAT activity are markedly increased in these muscles (9), as well as the O-GlcNAc modification of numerous membrane associated proteins, which may include GLUT4 and/or proteins associated with GLUT4 (8), suggesting but not proving that the HSP may be involved. Other suggestive correlations include that, GFAT activity and UDP-Hex NAc concentrations are increased in leptin deficient, insulin resistant ob/ob mice (7), while at the other end of the spectrum, UDP-HexNAc concentrations are reduced in muscles of growth hormone deficient rats (64) and in rats with chronic caloric restriction and enhanced insulin sensitivity (20).
In vitro studies from Holloszy’s laboratory make a strong argument against the role of the HSP in glucose/insulin induced insulin resistance in muscle (35). In this model, isolated epitrochlearis muscles were incubated for 5 hours with high doses of insulin and either a high concentration of glucose (36 mM) or normal glucose (5 mM), before glucose transport was measured. The latter was reduced by 50% in the high glucose treated group and this was unchanged when the high glucose-induced accumulation of UDP-HexNAc was prevented by inclusion of a GFAT-inhibitor. (Note that the same GFAT inhibitor reversed glucose-induced insulin resistance in earlier studies in adipocytes (43).) Incubation with actinomycin-D, cycloheximide or activation of AMP-activated protein kinase each reversed the high glucose-induced insulin resistance in the muscle suggesting that a rapidly turning over protein and dephosphorylation of a transcription factor may be involved (35). In a follow up study they provide evidence that exposure to GlcN or to high glucose cause insulin resistance in skeletal muscle by different mechanisms (25). We reached similar conclusions in 3T3-L1 adipocytes (49, 50).
Transgenic mice overexpressing GFAT in skeletal muscle and adipocytes develop peripheral insulin resistance as they age, as determined by euglycemic insulin clamp studies. GLUT4 expression in muscle is unchanged, and the insulin resistance likely represents defective translocation of GLUT4 to the muscle cell membrane, or a defect in docking and/or incorporation of GLUT4 into the plasmalemma of muscle in vivo (15, 30). However, upon in vitro testing, the insulin response of glucose transport of isolated muscles from GFAT overexpressing, in vivo insulin resistant mice is identical to that of wild type mice (29). When GFAT was overexpressed only in adipocytes, the mice developed fat cell hypertrophy, increased plasma leptin and decreased adiponectin, as well as total body insulin resistance by insulin clamp studies. The authors conclude that the in vivo insulin resistance which develops in mice overexpressing GFAT in adipocytes alone (29) or in adipocytes and in muscle cells, likely reflects cross talk between adipocytes and skeletal muscle and may be mediated by the decreased circulating adiponectin levels in GFAT overexpressing mice (29). Note the differences in phenotype between the transgenic mice overexpressing GLUT1 in muscle or GFAT in muscle and adipose tissue. While the mouse with chronically increased glucose flux into muscle develops profound resistance to insulin in vitro (22), the GFAT overexpressing mouse manifests insulin resistance only in vivo but not in vitro. It should be noted, however, that GFAT overexpression in these transgenic models was relatively low.
In a preliminary report, Silvana Obici presented data on transgenic mice overexpressing GFAT only in skeletal muscle, developed in collaboration with Rossetti’s laboratory (52). Contrary to their expectations, when studied by the euglycemic insulin clamp technique, there were no significant differences either in whole body glucose utilization or in hepatic glucose output between transgenic and control mice, although GFAT expression was ~ 3 fold higher in transgenic muscle, UDP.GlcNAc concentration was increased > 20%, and O-GlcNAc modification of proteins was also increased in transgenic muscles compared to controls. Circulating leptin was unchanged, but leptin expression in muscles appeared to be increased. Insulin-stimulated 2-deoxyglucose transport into muscle appeared to be slightly blunted in the transgenic mice (52). The lack of an effect on muscle insulin resistance is in marked contrast with previous observations from this laboratory where hexosamine flux in muscle was increased during relatively short (hours to days) time periods by general infusion of GlcN, glucose or other nutrients (27, 28, 53, 61, 68)
The validity of using GlcN infusions to model the role of HSP in insulin resistance has been questioned (31, 49, 50, 76). GlcN is present at very low concentrations in plasma, and its production in the cell is limited by feedback inhibition of GFAT activity by UDP-GlcNAc. When extracellular GlcN is increased, by infusions of GlcN, GlcN-6-PO4 accumulates markedly in cells, (700-500 fold in heart and skeletal muscle respectively (76), while this compound is nearly undetectable in cells exposed to glucose alone, suggesting that UDP-HexNAc generation from GlcN is limited at a step distal to hexokinase (50). The accumulation of GlcN-6-PO4 depletes intracellular ATP, which can cause spurious insulin resistance by blocking early IR signaling. This has been clearly demonstrated in 3T3-L1 adipocytes, incubated with high concentrations of insulin, and GlcN without glucose (31). However, under milder conditions ATP-depletion due to GlcN treatment is much reduced and does not account for GlcN-induced insulin resistance. Nevertheless, there are clear differences between the metabolic effects of high glucose and GlcN, in fat cells and in muscle, although both models can induce insulin resistance and promote the O-GlcNAc modification of certain proteins. (25, 49, 50). Therefore, results using GlcN to model the role of the HBP in the development of insulin resistance need to be interpreted with caution. In support of this, we have conducted gene-array analysis on 3T3-L1 adipocytes incubated under various conditions that elicit (or do not) insulin resistance. While sustained 8 hr exposure to high (25mM) glucose + low dose insulin (0.6 nM) generated similar insulin resistance to incubation with 5mM glucose + 2.5 mM GlcN + low dose insulin, GlcN exposed cells exhibited marked changes in the expression of numerous genes, which were specific to GlcN exposure (unpublished data with the Bioinformatics group at Hoffmann: La Roche, (Rosinski, J., So, W. V., and Martin, M.), supporting the concept that GlcN exerts numerous effects which are not duplicated by increased glucose flux via HSP.
Regardless of the mode of action of GlcN, increased glucose flux via the HSP is associated with insulin resistance, at least in experimental models. What is the mechanism? In transgenic mice, overexpressing GFAT in muscle and fat, in vivo insulin resistance was associated with impaired translocation of GLUT4 to the muscle plasma membrane (15). The expression of GLUT1 and GLUT4 was unchanged. Similarly, in 3T3-L1 adipocytes, insulin resistant glucose transport, which developed following pre-incubation in high glucose or low glucose + GlcN, (provided a low concentration of insulin was present) was not accompanied by changes in GLUT4 or GLUT1 expression (50). However, in one paper, studying a similar model, it was felt that the insulin resistance of glucose transport reflected the accelerated degradation of GLUT4 (73). In the 3T3-L1, adipocyte model where insulin resistance was achieved by preincubation with GlcN, a defect in GLUT4 translocation was clearly demonstrated (50). However, in the model where the same degree of insulin resistance developed following exposure to high glucose in the presence of insulin, GLUT4 translocation was less compromised (50, 51) suggesting a second defect, impaired docking or fusion of the GLUT4 carrying vesicle with the plasma membrane or decreased intrinsic activity of GLUT4. It is becoming increasingly evident that the “translocation” of GLUT4 to the plasma membrane occurs in various stages, which respond to different regulatory signals which encompass a) the movement of GLUT4 containing vesicles toward the plasma membrane, which does not require Akt activation and b) docking and fusion with the plasma membrane, which does (32, 75). Our data are consistent with the concept that the insulin resistance elicited by glucose toxicity, involves in great part a block at the docking/fusion step (51). This is supported by the observation, that the insulin regulated trafficking of Munc. 18c, a syntaxin 4-binding protein, which regulates the docking/fusion step, is disrupted in cells with high glucose or GlcN-induced insulin resistance (51). Note, that Munc-18c is subject to modification on Ser/Thr residues by O-GlcNAc, the end product of HSP (10).
Which steps of the insulin signaling cascade are compromised in insulin resistance? There is consensus that insulin resistance is a manifestation of complex alterations in signal transduction between the activated insulin receptor and its final target, (reviewed in (57, 69–72)). Most investigators have found inhibition of the proximal insulin signaling cascade to be associated with insulin resistance. In models of diabetes associated with obesity and lipotoxicity, the predominant block appears to be at the insulin stimulated activation of PI-3-kinase, due to its reduced association with IRS-1 and 2. This in turn represents the reduced insulin-stimulated activation (tyrosine phosphorylation) of IRS, which reflects IRS phosphorylation on specific Ser and Thr residues. The involvement of protein kinase C, specifically PKC θ, has been implicated in this process, although other mechanisms, e.g. cytokines, NFκβ, and stress-activated MAP-kinases (particularly p38) contribute (71). On the other hand in high glucose induced insulin resistance, in muscle and fat in vitro, insulin activation of IRS-1 associated PI-3-kinase was unaffected, and the block in signal transduction appeared to be located downstream of PI-3-kinase, at the level of Akt activation (39, 49). Alternatively, in a model of short preincubation of muscles with high glucose and insulin, no defect in the signaling cascade was detected, the participation of the HSP was excluded, and the induction of a rapidly turning over protein was postulated as the cause of insulin resistance (35). Clearly, different experimental models can block insulin signaling at different sites, with insulin resistant glucose transport being the common outcome. If the HSP contributes to insulin resistance, its role in the different models and cells will have to be defined.
How does increased flux through the HSP cause insulin resistance or participate in the development of the complications of diabetes? The accumulation of UDP-GlcNAc in tissues promotes the O-GlcNAc modification on Ser/Thr residues of selected proteins, and this process is believed to mediate the effects of HSP. Increased glucose flux into cells and via HSP does promote O-GlcNAcylation. The process is catalyzed by a cytosolic/nuclear enzyme, O-GlcNAc transferase (OGT), which is responsive to UDP-GlcNAc concentrations in the physiological range (37, 42). UDP-GlcNAc regulates OGT in part by mass action i.e. by the availability of substrate and in part allosterically in a protein substrate selective manner (38, 84). Numerous investigators have reported increased O-GlcNAc modification of proteins, (demonstrated immunologically), in tissue culture or in experimental animals, under conditions of insulin resistance where glucose flux into cells was chronically increased, or after exposure to GlcN or in cells overexpressing GFAT (8, 44, 56, 66, 90). However, the proteins were only rarely identified, and a causal connection was not established. More recently, more direct evidence has emerged. The enzyme, which removes the O-GlcNAc modification from Ser/Thr, is O-GlcNAcase, which has been cloned (19, 83). A pharmacological agent, which is a competitive inhibitor of O-GlcNAcase has been developed, (PUGNAC) (23). When 3T3-L1 adipocytes are incubated with PUGNAC, the removal of O-GlcNAc from proteins is inhibited, and the cells develop insulin resistant glucose transport, as well as inhibition of insulin stimulated Akt activation. Although IRS-1 and IRS-2 are modified by O-GlcNAc, their insulin-stimulated Tyr-phosphorylation was not affected (77). Thus, PUGNAC reproduces the same scenario as that observed in these cells after incubation in high glucose + low dose insulin (49, 50).
Transgenic mice with modest overexpression of O-GlcNAc transferase is skeletal muscle and in adipocytes develop hyperinsulinemia, insulin resistance (as assessed by euglycemic insulin clamp studies), hyperleptinemia, without changes in body weight, glycemia or GLUT4 expression in muscle (45), a phenotype which is similar to that achieved by overexpressing GFAT in muscle and fat tissue (15, 30).
A link between the O-GlcNAc modification and complications of diabetes has been demonstrated in neonatal cardiomyocytes, in studies of diabetic cardiomyopathy. When cardiomyocytes are incubated in high (25 mM) vs low (5 mM) glucose, abnormalities in Ca++ cycling develop in high glucose treated cells, which are attributable to reduced mRNA and protein expression of sarcoplasmic reticulum Ca2+ - ATP-ase (SERCA2a). CA2+ cycling and SERCA2a expression were restored toward normal by treating high glucose exposed cells with an adenovirus expressing O-GlcNAcase (13) The regulation was at the transcriptional level as demonstrated by parallel regulation of two transcription factors which regulate SERCA2a expression. Thus in the neonatal cardiomyocyte model overexpression of an enzyme, which removes O-GlcNAc from proteins reversed the deleterious effects caused by preexposure to high glucose.
We carried out similar experiments in 3T3-L1 adipocytes, where we tried to reverse or mitigate high glucose/insulin induced insulin resistance, by adenovirus mediated O-GlcNAcase overexpression (6). Preexposure to high glucose + low dose insulin decreased the maximal and half-maximal insulin response of glucose transport by 40–50% compared to cells preincubated in 5 mM glucose. This down-regulation was similar in cells infected with O-GlcNAcase adenovirus or empty virus. Impaired insulin-stimulated Akt activation in insulin resistant cells was also unaffected by O-GlcNAcase overexpression. In post-nuclear supernatants of infected cells O-GlcNAcase enzyme activity and protein expression were increased 5-fold, and O-GlcNAc modified proteins were decreased. However, in isolated nuclei no significant increase in O-GlcNAcase activity was detected, suggesting poor nuclear access of the recombinant enzyme (6), confirming similar observations in COS7 cells (19). The lack of effect of O-GlcNAcase overexpression in preventing or mitigating insulin resistance may reflect a lack of participation of this pathway in the development of insulin resistance. However, if increased protein O-GlcNAcylation plays a causative role in the development of insulin resistance, it likely involves transcriptional regulation. A factor which may affect O-GlcNAcylation, and the development of insulin resistance may be O-GlcNAcase transport into the nucleus. This is of particular interest in view of increasing evidence indicating that both OGT and O-GlcNAcase are regulated by post-transcriptional modification and by alternative splicing, and that they interact with numerous binding partners (19, 83, 84). Histones and transcription factors are regulated by a number of post-translational modifications, which in turn regulate gene expression. These modifications occur in large multi-subunit complexes. The corepressor Sim3A, known to recruit histone deacetylase, (HDAC), also recruits OGT via its TPR domain to specific genes (87). Thus OGT and HDAC may act in concert to repress transcription. On the other hand, O-GlcNAcase has been recently shown to be a bifunctional protein, with the N-terminus expressing the O-GlcNAcase activity and the C-terminus acting as a histone acetyltransferase (HAT) in vitro, with a typical HAT domain that has both active and inactive states (74). Thus O-GlcNAcase may function to reduce the state of glycosylation of transcriptional activators while increasing the acetylation of histones, to allow for the concerted activation of eukaryotic gene transcription. The factors regulating traffic of O-GlcNAcase in and out of the nucleus, and its interaction with specific genes deserve further study.
There is a remarkable, evolving consensus regarding the role of the HSP in the pathogenesis of the renal/vascular complications of diabetes. The accumulation of extracellular matrix in the glomerulus is an early and hallmark event in the development of diabetic glomerulosclerosis, and it has been long known that sustained hyperglycemia promotes its development, in patients and in experimental models of diabetes. Different mechanisms have been implicated in the hyperglycemia induced increased matrix production, including activation of the polyol pathway, increased non-enzymatic glucosylation end products and high glucose-induced stimulation of protein kinase C (36). A role for the HSP in the effects of hyperglycemia on growth factor gene expression in vascular smooth muscle cells was first proposed by McClain et al. in 1992 (46). More recently it has become evident that TGF-β synthesis is required for the effects of high glucose (92); the glucose effect was mitigated by treating cells with anti-TGFβ antibody (92) or with antisense TGF-β1 oligonucleotide (36). Furthermore, glucosamine reproduced the effect of glucose on TGF-β1 induction, albeit with greater potency, and blocking GFAT activity with antisense GFAT oligonucleotide or with a chemical inhibitor of GFAT activity inhibited high glucose/GlcN stimulation of TGF-β1 synthesis (36). Overexpressing GFAT in mesangial cells, induced TGF-β1 and fibronectin expression in cells incubated in 5 mM glucose (81). Thus TGF-β1 expression is dependent at least in part on HSP activity.
An elegant, recent paper addresses the mechanism by which increased HSP activity may stimulate TGFβ1 expression. The promoter of TGFβ1 (−1013 to −1002 region) contains a sequence which is highly homologous to glucose response elements (GIRE-s) previously identified in genes of glucose regulated proteins such as liver pyruvate kinase. GIRE-s bind to upstream stimulatory factors, USF1 and 2, which enhance TFGβ1 expression. Incubation with high glucose or increasing flux through HSP by overexpressing GFAT increased the expression of USF1 and 2, although the proteins themselves were not O-GlcNAc modified. Increased USF expression and DNA binding activity led to upregulation of TFGβ1 promoter activity (80). Note however, that the GIRE-domain is not the only site at which TGFβ1 is regulated. There are two AP-1 binding sites on the promoter (−448/−412 and −371/−363 respectively), which are regulated by PKC and MAP-kinase dependent pathways, which are also involved in glucose-mediated activation of TGF-β1 expression (80).
Hyperglycemia stimulates the expression of plasminogen activator inhibitor 1, (PAI-1) in vascular smooth muscle cells (11), aortic endothelial cells (16) and in mesangial cells (33), and this is thought to be an important factor in the development of vascular disease in diabetes. The impetus for looking for a role of the HSP in glucose stimulated expression of PAI-1 was the observation that high glucose exerted its effect by activating two adjacent Sp1 binding sites in the N-terminal flanking region of the PAI-1 (−85−42) promoter (11). Sp1 was the first transcription factor identified as an O-GlcNAc modified protein, it has multiple O-GlcNAc modification sites, and its phosphorylation on Ser/Thr is inversely proportional to its O-GlcNAc modification (26, 65, 86). Furthermore, in addition to regulating the expression of PAI-1, Sp1 has been implicated in the regulation of expression of several glucose-regulated genes, e.g. acetyl-CoA carboxylase, leptin, fatty acid synthase and ATP citrate lyase (reviewed in (19). Furthermore, the O-GlcNAc modification of Sp1 increases with increasing ambient glucose or with insulin (19). Two laboratories have now shown the involvement of the HSP in PAI-1 activation of expression. Incubation in high glucose, or with GlcN or overexpression of GFAT enhanced the activation of the PAI-1 promoter, as well as stimulating TGF-β expression in mesangial cells or aortic endothelial cells (16, 33). Du et al. suggested that the initial insult exerted by hyperglycemia is increasing mitochondrial superoxide production. Oxidative stress would inhibit glyceraldehyde-3 phosphate dehydrogenase (GAPDH) activity, which in turn would stimulate flux via HSP by blocking the flow of fructose-6-P via glycolysis. The increased UDP-GlcNAc production would cause the enhanced O-GlcNAc modification of Sp1 and the ensuing events. These hypotheses are elegantly supported by experiments wherein mitochondrial superoxide production was blunted by adding an inhibitor of complex II or an uncoupler of oxidative phosphorylation, or a superoxide dismutase mimetic, or by overexpressing UCP-1 or MnSOD. While GAPDH activity was markedly decreased after cells were incubated in 30 mM glucose compared to 5 mM glucose, the above manipulations restored the activity. An inhibitor of GFAT activity, azaserine, was ineffective. However, all of the above manipulations, including azaserine, restored the enhanced O-GlcNAc modification of Sp1 in cells incubated with high glucose toward normal, as well as correcting the enhanced TGF-β1 and PAI-1 promoter activity. However, 30 mM glucose no longer stimulated PAI-1 promoter activity, when the Sp1 sites were mutated. In subsequent articles Brownlee has extended this hypothesis by postulating that the major mechanism of glucose toxicity is increased mitochondrial superoxide production, and that this event can account for the diverse manifestations in vascular cells, i.e. increased polyol pathway flux, increased advanced glycation end product formation, activation of protein kinase C and increased hexosamine flux (4, 5).
CLINICAL STUDIES
There are a very few clinical studies examining the role of HSP in insulin resistance. Yki Jarvinen reported in 1996 that GFAT activity was increased in muscle biopsies obtained from insulin resistant patients with type 2 diabetes (88). Two papers studied the effect of GlcN infusions on insulin responsiveness in humans. In one paper, minimal effects were observed, i.e. blunted insulin secretory response in response to a glucose load and mildly increased fasting glucose levels (which could represent the known inhibitory effect of GlcN on islet glucokinase), but in neither paper were any effects noted on glucose utilization during an euglycemic insulin clamp or on hepatic glucose production. Thus, humans may be less sensitive to the insulin resistance promoting effect of GlcN than rodents (48, 58). In a prospective study the effect of strict blood glucose control with iv insulin aimed at euglycemia was examined in severely insulin resistant, uncontrolled, obese, type 2 diabetic patients, on the concentration of UDP-GlcNAc and UDP-GalNAc in muscle. Patients underwent insulin clamp studies and muscle biopsies at the beginning and at the end of a 28 day treatment period. Insulin resistance improved markedly as demonstrated by a near doubling of the glucose infusion rate during the clamp, and a marked reduction in daily insulin requirements. Concomitantly, UDP-GlcNAc and UDP-GalNAc in muscle increased by ~ 40%. This likely reflects the improvement in muscle glucose transport, however, it does not support the hypothesis of a simple, positive correlation between the products of HSP in muscle and insulin resistance in humans, (59).
In a cross-sectional study 55 patients, 20 with type 2 diabetes, and the rest non-diabetic, with or without obesity, candidates for hip-replacement, were studied. The objective was to determine whether insulin resistant patients would have increased UDP-HexNAc levels in fat and muscle, and whether there would be a correlation between UDP-HexNAc levels and metabolic parameters. The only significant finding was a positive correlation between UDP-HexNAc and circulating FFA and leptin concentrations in adipocytes, but not in muscle. The study does not provide evidence for a role for HSP in insulin resistance in humans, although a case for its proposed nutrient-sensing role in adipocytes can be made. (60).
As to genetic studies, 412 caucasian, non-diabetic metabolically characterized individuals were screened for expression of two single nucleotide polymorphisms (SNP) in the 5-prime flanking region of GFAT. One of them (−913 G/A) was associated with a significantly higher body mass index , percent body fat, and increased intramyocellular lipid content in males but not in females (82). A recent publication reports that a single nucleotide polymorphism in intron 10 of the gene expressing O-GlcNAcase is associated with type 2 diabetes in Mexican Americans (41). Intron 10 contains an alternate stop codon and may lead to decreased expression of the 130 kDa isoform, which is predicted to contain the O-GlcNAcase activity. The gene is located on chromosome 10q and overlaps a region, which has been previously shown to be associated with type two diabetes
CONCLUSIONS
There is strong evidence supporting a role for the HSP in the physiopathology of the vascular/renal complications of diabetes. The mode of action appears to be transcriptional regulation, likely modulated by O-GlcNAc modification of transcription factors. Proof of the effect has been provided by gene overexpression and deletion experiments in several tissue culture models, although its role in vivo will have to be more fully established. The role of the HSP as a determinant in the development of insulin resistance is more problematic. While it is clear that overexpression of GFAT or of O-GlcNAc transferase can produce insulin resistance in mice, the requirement for increased HSP flux for the establishment of glucose induced insulin resistance has been questioned. Nevertheless, with the rapidly increasing knowledge of the O-GlcNAc modification of selected proteins and their regulation, and the fact that uncontrolled diabetes affects this process, it seems likely that a role for the HSP in the development of the metabolic syndrome and insulin resistance will prevail.
Acknowledgments
This work was supported in part by a grant (2R01-DK02001) from the NIH. The expert assistance of Katherine A. Robinson, MS, in the preparation of this manuscript is gratefully acknowledged
References
- 1.Andreozzi F, D'Alessandris C, Federici M, Laratta E, Del Guerra S, Del Prato S, Marchetti P, Lauro R, Perticone F, Sesti G. Activation of the hexosamine pathway leads to phosphorylation of insulin receptor substrate-1 on Ser307 and Ser612 and impairs the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin insulin biosynthetic pathway in RIN pancreatic beta-cells. Endocrinology. 2004;145:2845–2857. doi: 10.1210/en.2003-0939. [DOI] [PubMed] [Google Scholar]
- 2.Ball LE, Berkaw MN, and Buse MG. Comparison of methods for the detection of O-GlcNAc modified proteins. Abstract #2602. Proceedings of the 53rd Conference of Mass Spectrometry and Allied Topics, San Antonio, TX, 2005.
- 3.Baron AD, Zhu JS, Zhu JH, Weldon H, Maianu L, Garvey WT. Glucosamine induces insulin resistance in vivo by affecting GLUT 4 translocation in skeletal muscle. Implications for glucose toxicity. J Clin Invest. 1995;96:2792–2801. doi: 10.1172/JCI118349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 5.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 6.Buse MG, Robinson KA. O-GlcNAcase overexpression does not prevent insulin resistance in 3T3-L1 adipocytes. Diabetes. 2005;54:A-324. (Abstract) [Google Scholar]
- 7.Buse MG, Robinson KA, Gettys TW, McMahon EG, Gulve EA. Increased activity of the hexosamine synthesis pathway in muscles of insulin-resistant ob/ob mice. Am J Physiol. 1997;272:E1080–1088. doi: 10.1152/ajpendo.1997.272.6.E1080. [DOI] [PubMed] [Google Scholar]
- 8.Buse MG, Robinson KA, Marshall BA, Hresko RC, Mueckler MM. Enhanced O-GlcNAc protein modification is associated with insulin resistance in GLUT1-overexpressing muscles. Am J Physiol Endocrinol Metab. 2002;283:E241–250. doi: 10.1152/ajpendo.00060.2002. [DOI] [PubMed] [Google Scholar]
- 9.Buse MG, Robinson KA, Marshall BA, Mueckler M. Differential effects of GLUT1 or GLUT4 overexpression on hexosamine biosynthesis by muscles of transgenic mice. J Biol Chem. 1996;271:23197–23202. doi: 10.1074/jbc.271.38.23197. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Liu P, Thurmond DC, Elmendorf JS. Glucosamine-induced insulin resistance is coupled to O-linked glycosylation of Munc18c. FEBS Lett. 2003;534:54–60. doi: 10.1016/s0014-5793(02)03774-2. [DOI] [PubMed] [Google Scholar]
- 11.Chen YQ, Su M, Walia RR, Hao Q, Covington JW, Vaughan DE. Sp1 sites mediate activation of the plasminogen activator inhibitor-1 promoter by glucose in vascular smooth muscle cells. J Biol Chem. 1998;273:8225–8231. doi: 10.1074/jbc.273.14.8225. [DOI] [PubMed] [Google Scholar]
- 12.Choi CS, Lee FN, Youn JH. Free fatty acids induce peripheral insulin resistance without increasing muscle hexosamine pathway product levels in rats. Diabetes. 2001;50:418–424. doi: 10.2337/diabetes.50.2.418. [DOI] [PubMed] [Google Scholar]
- 13.Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem. 2003;278:44230–44237. doi: 10.1074/jbc.M303810200. [DOI] [PubMed] [Google Scholar]
- 14.Comer FI, Hart GW. Reciprocity between O-GlcNAc and O-phosphate on the carboxyl terminal domain of RNA polymerase II. Biochemistry. 2001;40:7845–7852. doi: 10.1021/bi0027480. [DOI] [PubMed] [Google Scholar]
- 15.Cooksey RC, Hebert LF, Zhu JH, Wofford P, Garvey WT, McClain DA. Mechanism of hexosamine-induced insulin resistance in transgenic mice overexpressing glutamine:fructose-6-phosphate amidotransferase: decreased glucose transporter GLUT4 translocation and reversal by treatment with thiazolidinedione. Endocrinology. 1999;140:1151–1157. doi: 10.1210/endo.140.3.6563. [DOI] [PubMed] [Google Scholar]
- 16.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106:466–472. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- 18.Gao Y, Miyazaki J, Hart GW. The transcription factor PDX-1 is post-translationally modified by O-linked N-acetylglucosamine and this modification is correlated with its DNA binding activity and insulin secretion in min6 beta-cells. Arch Biochem Biophys. 2003;415:155–163. doi: 10.1016/s0003-9861(03)00234-0. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y, Wells L, Comer FI, Parker GJ, Hart GW. Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic beta-N-acetylglucosaminidase from human brain. J Biol Chem. 2001;276:9838–9845. doi: 10.1074/jbc.M010420200. [DOI] [PubMed] [Google Scholar]
- 20.Gazdag AC, Wetter TJ, Davidson RT, Robinson KA, Buse MG, Yee AJ, Turcotte LP, Cartee GD. Lower calorie intake enhances muscle insulin action and reduces hexosamine levels. Am J Physiol Regul Integr Comp Physiol. 2000;278:R504–512. doi: 10.1152/ajpregu.2000.278.2.R504. [DOI] [PubMed] [Google Scholar]
- 21.Gewinner C, Hart G, Zachara N, Cole R, Beisenherz-Huss C, Groner B. The coactivator of transcription CREB-binding protein interacts preferentially with the glycosylated form of Stat5. J Biol Chem. 2004;279:3563–3572. doi: 10.1074/jbc.M306449200. [DOI] [PubMed] [Google Scholar]
- 22.Gulve EA, Ren JM, Marshall BA, Gao J, Hansen PA, Holloszy JO, Mueckler M. Glucose transport activity in skeletal muscles from transgenic mice overexpressing GLUT1. Increased basal transport is associated with a defective response to diverse stimuli that activate GLUT4. J Biol Chem. 1994;269:18366–18370. [PubMed] [Google Scholar]
- 23.Haltiwanger RS, Grove K, Philipsberg GA. Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-beta-N-acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate. J Biol Chem. 1998;273:3611–3617. doi: 10.1074/jbc.273.6.3611. [DOI] [PubMed] [Google Scholar]
- 24.Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Bergfeld R, Giardino I, Brownlee M. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9:294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- 25.Han DH, Chen MM, Holloszy JO. Glucosamine and glucose induce insulin resistance by different mechanisms in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2003;285:E1267–1272. doi: 10.1152/ajpendo.00255.2003. [DOI] [PubMed] [Google Scholar]
- 26.Han I, Kudlow JE. Reduced O glycosylation of Sp1 is associated with increased proteasome susceptibility. Mol Cell Biol. 1997;17:2550–2558. doi: 10.1128/mcb.17.5.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawkins M, Angelov I, Liu R, Barzilai N, Rossetti L. The tissue concentration of UDP-N-acetylglucosamine modulates the stimulatory effect of insulin on skeletal muscle glucose uptake. J Biol Chem. 1997;272:4889–4895. doi: 10.1074/jbc.272.8.4889. [DOI] [PubMed] [Google Scholar]
- 28.Hawkins M, Barzilai N, Liu R, Hu M, Chen W, Rossetti L. Role of the glucosamine pathway in fat-induced insulin resistance. J Clin Invest. 1997;99:2173–2182. doi: 10.1172/JCI119390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hazel M, Cooksey RC, Jones D, Parker G, Neidigh JL, Witherbee B, Gulve EA, McClain DA. Activation of the hexosamine signaling pathway in adipose tissue results in decreased serum adiponectin and skeletal muscle insulin resistance. Endocrinology. 2004;145:2118–2128. doi: 10.1210/en.2003-0812. [DOI] [PubMed] [Google Scholar]
- 30.Hebert LF, Daniels MC, Zhou J, Crook ED, Turner RL, Simmons ST, Neidigh JL, Zhu JS, Baron AD, McClain DA. Overexpression of glutamine:fructose-6-phosphate amidotransferase in transgenic mice leads to insulin resistance. J Clin Invest. 1996;98:930–936. doi: 10.1172/JCI118876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hresko RC, Heimberg H, Chi MM, Mueckler M. Glucosamine-induced insulin resistance in 3T3-L1 adipocytes is caused by depletion of intracellular ATP. J Biol Chem. 1998;273:20658–20668. doi: 10.1074/jbc.273.32.20658. [DOI] [PubMed] [Google Scholar]
- 32.James DE. MUNC-ing around with insulin action. J Clin Invest. 2005;115:219–221. doi: 10.1172/JCI24158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James LR, Fantus IG, Goldberg H, Ly H, Scholey JW. Overexpression of GFAT activates PAI-1 promoter in mesangial cells. Am J Physiol Renal Physiol. 2000;279:F718–727. doi: 10.1152/ajprenal.2000.279.4.F718. [DOI] [PubMed] [Google Scholar]
- 34.Kamemura K, Hayes BK, Comer FI, Hart GW. Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: alternative glycosylation/phosphorylation of THR-58, a known mutational hot spot of c-Myc in lymphomas, is regulated by mitogens. J Biol Chem. 2002;277:19229–19235. doi: 10.1074/jbc.M201729200. [DOI] [PubMed] [Google Scholar]
- 35.Kawanaka K, Han DH, Gao J, Nolte LA, Holloszy JO. Development of glucose-induced insulin resistance in muscle requires protein synthesis. J Biol Chem. 2001;276:20101–20107. doi: 10.1074/jbc.M010599200. [DOI] [PubMed] [Google Scholar]
- 36.Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher ED. High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J Clin Invest. 1998;101:160–169. doi: 10.1172/JCI119875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreppel LK, Blomberg MA, Hart GW. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem. 1997;272:9308–9315. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- 38.Kreppel LK, Hart GW. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J Biol Chem. 1999;274:32015–32022. doi: 10.1074/jbc.274.45.32015. [DOI] [PubMed] [Google Scholar]
- 39.Kurowski TG, Lin Y, Luo Z, Tsichlis PN, Buse MG, Heydrick SJ, Ruderman NB. Hyperglycemia inhibits insulin activation of Akt/protein kinase B but not phosphatidylinositol 3-kinase in rat skeletal muscle. Diabetes. 1999;48:658–663. doi: 10.2337/diabetes.48.3.658. [DOI] [PubMed] [Google Scholar]
- 40.Lamarre-Vincent N, Hsieh-Wilson LC. Dynamic glycosylation of the transcription factor CREB: a potential role in gene regulation. J Am Chem Soc. 2003;125:6612–6613. doi: 10.1021/ja028200t. [DOI] [PubMed] [Google Scholar]
- 41.Lehman DM, Fu DJ, Freeman AB, Hunt KJ, Leach RJ, Johnson-Pais T, Hamlington J, Dyer TD, Arya R, Abboud H, Goring HH, Duggirala R, Blangero J, Konrad RJ, Stern MP. A single nucleotide polymorphism in MGEA5 encoding O-GlcNAc-selective N-acetyl-beta-D glucosaminidase is associated with type 2 diabetes in Mexican Americans. Diabetes. 2005;54:1214–1221. doi: 10.2337/diabetes.54.4.1214. [DOI] [PubMed] [Google Scholar]
- 42.Lubas WA, Frank DW, Krause M, Hanover JA. O-Linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J Biol Chem. 1997;272:9316–9324. doi: 10.1074/jbc.272.14.9316. [DOI] [PubMed] [Google Scholar]
- 43.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266:4706–4712. [PubMed] [Google Scholar]
- 44.McClain DA. Hexosamines as mediators of nutrient sensing and regulation in diabetes. J Diabetes Complications. 2002;16:72–80. doi: 10.1016/s1056-8727(01)00188-x. [DOI] [PubMed] [Google Scholar]
- 45.McClain DA, Lubas WA, Cooksey RC, Hazel M, Parker GJ, Love DC, Hanover JA. Altered glycan-dependent signaling induces insulin resistance and hyperleptinemia. Proc Natl Acad Sci U S A. 2002;99:10695–10699. doi: 10.1073/pnas.152346899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McClain DA, Paterson AJ, Roos MD, Wei X, Kudlow JE. Glucose and glucosamine regulate growth factor gene expression in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1992;89:8150–8154. doi: 10.1073/pnas.89.17.8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGarry JD. The mitochondrial carnitine palmitoyltransferase system: its broadening role in fuel homoeostasis and new insights into its molecular features. Biochem Soc Trans. 1995;23:321–324. doi: 10.1042/bst0230321. [DOI] [PubMed] [Google Scholar]
- 48.Monauni T, Zenti MG, Cretti A, Daniels MC, Targher G, Caruso B, Caputo M, McClain D, Del Prato S, Giaccari A, Muggeo M, Bonora E, Bonadonna RC. Effects of glucosamine infusion on insulin secretion and insulin action in humans. Diabetes. 2000;49:926–935. doi: 10.2337/diabetes.49.6.926. [DOI] [PubMed] [Google Scholar]
- 49.Nelson BA, Robinson KA, Buse MG. Defective Akt activation is associated with glucose- but not glucosamine-induced insulin resistance. Am J Physiol Endocrinol Metab. 2002;282:E497–506. doi: 10.1152/ajpendo.00438.2001. [DOI] [PubMed] [Google Scholar]
- 50.Nelson BA, Robinson KA, Buse MG. High glucose and glucosamine induce insulin resistance via different mechanisms in 3T3-L1 adipocytes. Diabetes. 2000;49:981–991. doi: 10.2337/diabetes.49.6.981. [DOI] [PubMed] [Google Scholar]
- 51.Nelson BA, Robinson KA, Buse MG. Insulin acutely regulates Munc18-c subcellular trafficking: altered response in insulin-resistant 3T3-L1 adipocytes. J Biol Chem. 2002;277:3809–3812. doi: 10.1074/jbc.C100645200. [DOI] [PubMed] [Google Scholar]
- 52.Obici S. Hexosamine pathway contribution to insulin resistance-New insights from muscle specific GFAT transgenic. 65th annual meeting of the American Diabetes Association, 2005.
- 53.Obici S, Wang J, Chowdury R, Feng Z, Siddhanta U, Morgan K, Rossetti L. Identification of a biochemical link between energy intake and energy expenditure. J Clin Invest. 2002;109:1599–1605. doi: 10.1172/JCI15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parker G, Taylor R, Jones D, McClain D. Hyperglycemia and inhibition of glycogen synthase in streptozotocin-treated mice: role of O-linked N-acetylglucosamine. J Biol Chem. 2004;279:20636–20642. doi: 10.1074/jbc.M312139200. [DOI] [PubMed] [Google Scholar]
- 55.Parker GJ, Lund KC, Taylor RP, McClain DA. Insulin resistance of glycogen synthase mediated by o-linked N-acetylglucosamine. J Biol Chem. 2003;278:10022–10027. doi: 10.1074/jbc.M207787200. [DOI] [PubMed] [Google Scholar]
- 56.Patti ME, Virkamaki A, Landaker EJ, Kahn CR, Yki-Jarvinen H. Activation of the hexosamine pathway by glucosamine in vivo induces insulin resistance of early postreceptor insulin signaling events in skeletal muscle. Diabetes. 1999;48:1562–1571. doi: 10.2337/diabetes.48.8.1562. [DOI] [PubMed] [Google Scholar]
- 57.Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. 2000;106:165–169. doi: 10.1172/JCI10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pouwels MJ, Jacobs JR, Span PN, Lutterman JA, Smits P, Tack CJ. Short-term glucosamine infusion does not affect insulin sensitivity in humans. J Clin Endocrinol Metab. 2001;86:2099–2103. doi: 10.1210/jcem.86.5.7470. [DOI] [PubMed] [Google Scholar]
- 59.Pouwels MJ, Span PN, Tack CJ, Olthaar AJ, Sweep CG, van Engelen BG, de Jong JG, Lutterman JA, Hermus AR. Muscle uridine diphosphate-hexosamines do not decrease despite correction of hyperglycemia-induced insulin resistance in type 2 diabetes. J Clin Endocrinol Metab. 2002;87:5179–5184. doi: 10.1210/jc.2002-020440. [DOI] [PubMed] [Google Scholar]
- 60.Pouwels MJ, Tack CJ, Span PN, Olthaar AJ, Sweep CG, Huvers FC, Lutterman JA, Hermus AR. Role of hexosamines in insulin resistance and nutrient sensing in human adipose and muscle tissue. J Clin Endocrinol Metab. 2004;89:5132–5137. doi: 10.1210/jc.2004-0291. [DOI] [PubMed] [Google Scholar]
- 61.Ravussin E. Cellular sensors of feast and famine. J Clin Invest. 2002;109:1537–1540. doi: 10.1172/JCI16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robinson KA, Sens DA, Buse MG. Pre-exposure to glucosamine induces insulin resistance of glucose transport and glycogen synthesis in isolated rat skeletal muscles. Study of mechanisms in muscle and in rat-1 fibroblasts overexpressing the human insulin receptor. Diabetes. 1993;42:1333–1346. doi: 10.2337/diab.42.9.1333. [DOI] [PubMed] [Google Scholar]
- 63.Robinson KA, Weinstein ML, Lindenmayer GE, Buse MG. Effects of diabetes and hyperglycemia on the hexosamine synthesis pathway in rat muscle and liver. Diabetes. 1995;44:1438–1446. doi: 10.2337/diab.44.12.1438. [DOI] [PubMed] [Google Scholar]
- 64.Robinson KA, Willi SM, Bingel S, Buse MG. Decreased hexosamine biosynthesis in GH-deficient dwarf rat muscle. reversal with GH, but not IGF-I, therapy. Am J Physiol. 1999;276:E435–442. doi: 10.1152/ajpendo.1999.276.3.E435. [DOI] [PubMed] [Google Scholar]
- 65.Roos MD, Su K, Baker JR, Kudlow JE. O glycosylation of an Sp1-derived peptide blocks known Sp1 protein interactions. Mol Cell Biol. 1997;17:6472–6480. doi: 10.1128/mcb.17.11.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rossetti L. Perspective: Hexosamines and nutrient sensing. Endocrinology. 2000;141:1922–1925. doi: 10.1210/endo.141.6.7566. [DOI] [PubMed] [Google Scholar]
- 67.Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990;13:610–630. doi: 10.2337/diacare.13.6.610. [DOI] [PubMed] [Google Scholar]
- 68.Rossetti L, Hawkins M, Chen W, Gindi J, Barzilai N. In vivo glucosamine infusion induces insulin resistance in normoglycemic but not in hyperglycemic conscious rats. J Clin Invest. 1995;96:132–140. doi: 10.1172/JCI118013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saltiel AR. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell. 2001;104:517–529. doi: 10.1016/s0092-8674(01)00239-2. [DOI] [PubMed] [Google Scholar]
- 70.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 71.Savage DB, Petersen KF, Shulman GI. Mechanisms of insulin resistance in humans and possible links with inflammation. Hypertension. 2005;45:828–833. doi: 10.1161/01.HYP.0000163475.04421.e4. [DOI] [PubMed] [Google Scholar]
- 72.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thomson MJ, Williams MG, Frost SC. Development of insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 1997;272:7759–7764. doi: 10.1074/jbc.272.12.7759. [DOI] [PubMed] [Google Scholar]
- 74.Toleman C, Paterson AJ, Whisenhunt TR, Kudlow JE. Characterization of the histone acetyltransferase (HAT) domain of a bifunctional protein with activable O-GlcNAcase and HAT activities. J Biol Chem. 2004;279:53665–53673. doi: 10.1074/jbc.M410406200. [DOI] [PubMed] [Google Scholar]
- 75.van Dam EM, Govers R, James DE. Akt activation is required at a late stage of insulin-induced GLUT4 translocation to the plasma membrane. Mol Endocrinol. 2005;19:1067–1077. doi: 10.1210/me.2004-0413. [DOI] [PubMed] [Google Scholar]
- 76.Virkamaki A, Yki-Jarvinen H. Allosteric regulation of glycogen synthase and hexokinase by glucosamine-6-phosphate during glucosamine-induced insulin resistance in skeletal muscle and heart. Diabetes. 1999;48:1101–1107. doi: 10.2337/diabetes.48.5.1101. [DOI] [PubMed] [Google Scholar]
- 77.Vosseller K, Wells L, Lane MD, Hart GW. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 2002;99:5313–5318. doi: 10.1073/pnas.072072399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walgren JL, Vincent TS, Schey KL, Buse MG. High glucose and insulin promote O-GlcNAc modification of proteins, including alpha-tubulin. Am J Physiol Endocrinol Metab. 2003;284:E424–434. doi: 10.1152/ajpendo.00382.2002. [DOI] [PubMed] [Google Scholar]
- 79.Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med. 1990;113:909–915. doi: 10.7326/0003-4819-113-12-909. [DOI] [PubMed] [Google Scholar]
- 80.Weigert C, Brodbeck K, Sawadogo M, Haring HU, Schleicher ED. Upstream stimulatory factor (USF) proteins induce human TGF-beta1 gene activation via the glucose-response element-1013/-1002 in mesangial cells: up-regulation of USF activity by the hexosamine biosynthetic pathway. J Biol Chem. 2004;279:15908–15915. doi: 10.1074/jbc.M313524200. [DOI] [PubMed] [Google Scholar]
- 81.Weigert C, Friess U, Brodbeck K, Haring HU, Schleicher ED. Glutamine:fructose-6-phosphate aminotransferase enzyme activity is necessary for the induction of TGF-beta1 and fibronectin expression in mesangial cells. Diabetologia. 2003;46:852–855. doi: 10.1007/s00125-003-1122-8. [DOI] [PubMed] [Google Scholar]
- 82.Weigert C, Thamer C, Brodbeck K, Guirguis A, Machicao F, Machann J, Schick F, Stumvoll M, Fritsche A, Haring HU, Schleicher ED. The -913 G/A glutamine:fructose-6-phosphate aminotransferase gene polymorphism is associated with measures of obesity and intramyocellular lipid content in nondiabetic subjects. J Clin Endocrinol Metab. 2005;90:1639–1643. doi: 10.1210/jc.2004-0058. [DOI] [PubMed] [Google Scholar]
- 83.Wells L, Gao Y, Mahoney JA, Vosseller K, Chen C, Rosen A, Hart GW. Dynamic O-glycosylation of nuclear and cytosolic proteins: further characterization of the nucleocytoplasmic beta-N-acetylglucosaminidase, O-GlcNAcase. J Biol Chem. 2002;277:1755–1761. doi: 10.1074/jbc.m109656200. [DOI] [PubMed] [Google Scholar]
- 84.Wells L, Kreppel LK, Comer FI, Wadzinski BE, Hart GW. O-GlcNAc transferase is in a functional complex with protein phosphatase 1 catalytic subunits. J Biol Chem. 2004;279:38466–38470. doi: 10.1074/jbc.M406481200. [DOI] [PubMed] [Google Scholar]
- 85.Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science. 2001;291:2376–2378. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- 86.Yang X, Su K, Roos MD, Chang Q, Paterson AJ, Kudlow JE. O-linkage of N-acetylglucosamine to Sp1 activation domain inhibits its transcriptional capability. Proc Natl Acad Sci U S A. 2001;98:6611–6616. doi: 10.1073/pnas.111099998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang X, Zhang F, Kudlow JE. Recruitment of O-GlcNAc transferase to promoters by corepressor mSin3A: coupling protein O-GlcNAcylation to transcriptional repression. Cell. 2002;110:69–80. doi: 10.1016/s0092-8674(02)00810-3. [DOI] [PubMed] [Google Scholar]
- 88.Yki-Jarvinen H, Daniels MC, Virkamaki A, Makimattila S, DeFronzo RA, McClain D. Increased glutamine:fructose-6-phosphate amidotransferase activity in skeletal muscle of patients with NIDDM. Diabetes. 1996;45:302–307. doi: 10.2337/diab.45.3.302. [DOI] [PubMed] [Google Scholar]
- 89.Yki-Jarvinen H, Helve E, Koivisto VA. Hyperglycemia decreases glucose uptake in type I diabetes. Diabetes. 1987;36:892–896. doi: 10.2337/diab.36.8.892. [DOI] [PubMed] [Google Scholar]
- 90.Yki-Jarvinen H, Virkamaki A, Daniels MC, McClain D, Gottschalk WK. Insulin and glucosamine infusions increase O-linked N-acetyl- glucosamine in skeletal muscle proteins in vivo. Metabolism. 1998;47:449–455. doi: 10.1016/s0026-0495(98)90058-0. [DOI] [PubMed] [Google Scholar]
- 91.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 92.Ziyadeh FN, Sharma K, Ericksen M, Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. J Clin Invest. 1994;93:536–542. doi: 10.1172/JCI117004. [DOI] [PMC free article] [PubMed] [Google Scholar]