Abstract
Objective
Low-dose methotrexate (MTX), a mainstay in the therapy of rheumatoid arthritis, is effective in only 60–70% of patients, a finding mirrored by poor anti-inflammatory efficacy in some animal models, most notably collagen-induced arthritis. To determine whether genetic factors or the model itself were responsible for the poor response to MTX we directly compared the responses of four inbred mouse strains to MTX in the air pouch model of acute inflammation.
Methods
Exudate leukocyte count and adenosine concentration were determined in inbred mice treated with methotrexate (0.75 mg/kg intraperitoneally [IP] every week for 4 weeks) or vehicle 4 hours after injection of carrageenan into the air pouch using previously described methods(1). Quantitative trait locus mapping was performed using an in silico method (2, 3) to identify loci potentially associated with each phenotype.
Results
MTX significantly reduced the exudate leukocyte count in C57BL/6 and BALB/c, but not DBA/1 (the strain used in the collagen arthritis model) or DBA/2 mice. In a parallel fashion MTX increased adenosine concentration in inflammatory exudates of C57BL/6 and BALB/c, but not DBA/1 or DBA/2 mice. Anti-inflammatory and adenosine responses to MTX in DBA1xC57BL/6 F1 and F2 offspring were most consistent with single genetic loci being responsible for each phenotype. In silico mapping identified partially overlapping loci containing candidate genes involved in both responses.
Conclusion
Genetic factors contribute to the anti-inflammatory efficacy of methotrexate and a single locus involved in methotrexate-induced adenosine upregulation is likely responsible for the observed resistance to MTX in DBA/1 mice.
INTRODUCTION
Low-dose methotrexate, the most commonly administered disease-modifying therapy for rheumatoid arthritis, is effective in only 60–75% of patients (4). While much effort has been placed into identifying risk factors for poor and/or toxic response, (5–7), the majority of methotrexate failures remain unexplained. A number of recent studies have addressed the potential role of genetic background in determining response to methotrexate (6, 8–10), focusing on the impact of single nucleotide polymorphisms (SNPs) in genes of the folate uptake and metabolism pathways on the propensity to methotrexate toxicity and/or efficacy. While these data have offered some evidence for the association of SNPs with toxicity and, to a lesser degree, efficacy, no clear demonstration of genetically-based resistance to the anti-inflammatory properties of methotrexate has been offered to date.
The demonstration of inbred murine strain-specific resistance to the anti-inflammatory effects of methotrexate would establish the principle that genetic factors affect methotrexate efficacy. Methotrexate, at pharmacologically relevant doses (<1mg/kg/wk), suppresses inflammation in the murine air pouch model of inflammation and the adjuvant arthritis model of Rheumatoid Arthritis by increasing extracellular concentrations of the anti-inflammatory autocoid adenosine (11–16). In contrast, we and others (((17–19), Cronstein et al unpublished data) have observed that pharmacologically relevant doses of methotrexate do not suppress collagen-induced arthritis in the inbred mouse strain DBA/1. To determine whether the anti-inflammatory response to methotrexate has a genetic basis we directly compared the capacity of low-dose methotrexate to suppress air pouch inflammation in several inbred mouse strains. We report here that a pharmacologically relevant dose of methotrexate fails to suppress air pouch inflammation or upregulate extracellular adenosine in DBA/1J , but not C57BL/6J mice. Moreover, we report evidence that both phenotypes are due to genetic differences at one or a small number of loci. Finally, we utilized single nucleotide polymorphism (SNP) allele data for four inbred strains, including DBA/1J and C57BL/6J, to apply a recently-developed in silico mapping method and identify quantitative trait loci likely to be responsible for the observed phenotype differences.
MATERIALS AND METHODS
Materials
Carrageenan (type I) was obtained from Sigma (St. Louis, MO). Methotrexate was purchased from Immunex (San Juan, PR). HPLC grade MeOH was purchased from Fisher Scientific (Hampton, NH). All other materials were of the highest quality that could be obtained.
Induction of air pouches and carrageenan-induced inflammation
C57BL/6J, DBA/1J and DBA/2J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Mice were housed in the New York University (NYU) animal facility, fed regular mouse chow and given access to drinking water ad libitum. Mice in each experiment were matched in age, weight and gender. All procedures described below were reviewed and approved by the Institutional Animal Care and Use Committee of NYU Medical Center and carried out under the supervision of the facility veterinary staff. To induce air pouches, 10–15-week-old mice were injected subcutaneously on the back with 3 ml of air. After 2 days, the pouches were reinflated with 1.5 ml of air. On day 6, inflammation was induced by injection of 1 ml of a suspension of carrageenan (2% weight/volume in calcium- and magnesium-free phosphate buffered saline solution [PBS]) into the air pouch, as we have previously described (12). After 4 hours, the mice were killed by CO2 narcosis, the pouches were flushed with 2 ml of PBS, and exudates were harvested. Aliquots were diluted 1:1 with methylene blue (0.01% w/v in PBS), and cells were counted in a standard hemocytometer chamber (American Optical, Buffalo, NY).
Treatment with methotrexate or vehicle
Animals were given weekly intraperitoneal (IP) injections of either methotrexate (0.75 mg/kg) or vehicle (0.9% saline) for 4 weeks, and experiments were carried out within 3 days of the last dose of methotrexate.
Quantitation of adenosine in inflammatory exudates
Aliquots of inflammatory exudates were added to an equal volume of 10% (w/v) trichloroacetic acid and kept on ice, followed by extraction of the organic phase with freon/trioctylamine (31/9). The aqueous phase was applied to a C-18 Sep-Pak cartridge (Waters, Milford, MA) and eluted off with methanol. After evaporation of the methanol, the samples were reconstituted in water, and the adenosine concentration was determined by reverse-phase high-performance liquid chromatography, as previously described (5). Samples were applied to a Bondapack C-18 column (Waters, Milford, MA) and eluted with a linear 0–40% gradient of 0.01M ammonium phosphate (pH 5.5) and methanol formed over 70 minutes with a flow rate of 1.5 ml/minute. Adenosine was identified by retention time and by the characteristic ultraviolet absorption spectrum, and the concentration was calculated by comparison with standards, as previously described.
Generation and phenotyping of C57BL/6J x DBA/1J F1 and F2 progeny
In order to further examine the potential genetic component to the methotrexate resistance observed in the DBA/1J strain, we utilized an intercross strategy in the manner described by Reeves et al (20), phenotyped both F1 and F2 progeny for both leukocyte count and exudate adenosine concentration with and without methotrexate treatment using the methods described, and then analyzed the phenotypic data using methods described by Lander and Botstein(21). Methotrexate-responsive (C57BL/6J) and methotrexate-resistant (DBA/1J) mice were grouped in mating trios, consisting of either two C57BL/6J females and one DBA/1J male or vice-versa. The parental mating cage of each F1 mouse was recorded in order to allow for detection of any X chromosome-linked effect. Gender-matched F1 progeny from each mating cage were phenotyped starting at approximately 6 weeks after birth using the methods described above. Male and female F1 progeny were then intercrossed and F2 progeny phenotyped as described above.
Estimation of number of loci responsible for methotrexate response phenotypes
Two methods were utilized to estimate the number of quantitative trait loci responsible for conferring the observed phenotypes. Genetic variance, or the difference between the variances (squares of standard deviations) of the means of the genetically-unique F2 and the genetically identical F1 mouse phenotypes, represents the portion of the variance that is due to genetic factors in difference to environmental variance and experimental error (21). This value was determined for both the methotrexate-induced anti-inflammatory response and adenosine upregulation phenotypes. The Castle-Wright formula, which estimates the number of segregating loci contributing to a trait based on the genetic variance and degree of phenotypic difference between strains, was then applied as described by Lander and Botstein (21). Second, we determined the number of F2 progeny exhibiting parental phenotypes. The fraction of F2s exhibiting a parental phenotype is an indicator of the number of quantitative trait loci responsible for the trait, since this fraction reaches a maximum if only one locus is responsible and decreases as the number of QTL increases. Because for both of the phenotypes tested in the current study the ranges of parental phenotypes overlapped and thus the total number of F2 mice exhibiting parental phenotypes could not be directly assessed, the fraction of the F2 population falling two or more standard deviations below or above the mean parental values (ie. for leukocyte count, below the responsive (C57BL/6J) parental mean and above the resistant (DBA/1J) parental mean, opposite for adenosine upregulation phenotype) was determined. The number of F2s meeting these criteria was compared to the number expected for the given sample size assuming two loci (i.e. more than one locus) were responsible for conferring the observed phenotypic difference.
In silico quantitative trait locus mapping
In separate analysis, we screened for quantitative trait loci associated with methotrexate response (percent change in leukocyte count) and with methotrexate-induced increase in extracellular adenosine using an in silico method based on that previously described by Grupe et al and modified by Smith et al (2, 3). The screen was performed on the C57BL/6J and DBA/1J strains as well as DBA/2J and BALB/cJ, for which the phenotypes of interest had previously been measured in our laboratory. First, a series of 5268 SNPs for which genotype information was known for at least 2 out of the 4 strains was partitioned into blocks based on genetic distance. Because the SNPs were originally annotated by physical and not genetic position, markers at the desired genetic distance intervals were identified on the Roche Mouse Database, and the physical positions of these markers was determined using the Ensemble Mouse Database by entering either marker names or flanking sequence as query terms. A set of 131 blocks covering the genome resulted, averaging 12cM (largest 39.7cM) each. The SNPs were then sorted based on their physical positions relative to the marker locations, and adjacent blocks were combined to form 110 overlapping bins consisting of an average of 71 SNPS per bin with a low of 17 and a high of 146. For each bin and for each strain pair, all SNPs for which genotype information was available were classified as either synonymous or polymorphic. The fraction of all SNPs within each overlapping bin that were polymorphic between each strain pair was determined for each of the six pairs, resulting in a set of six values representing the relative genetic divergences among each of six strain combinations for each of 110 overlapping bin sets.
In order to compare fold differences in methotrexate response, the values for percent change in leukocyte count (described earlier) were converted to log values, and the difference between logs was calculated for each of the six strain comparisons(3). For the adenosine upregulation phenotype, log values of percent increase in adenosine concentration could not be determined since the change was slightly, though not significantly negative for two strains. Thus, this phenotype was classified based on whether or not a significant change in adenosine concentration was observed in methotrexate-treated relative to untreated mice. Strains for which a change resulted were assigned a value of 1, while those for which there was no change were set at 0, such that a comparison between a strain in which a change was observed and one in which one was not would result in a phenotypic difference of 1 – 0, or 1, while a comparison between two strains in which methotrexate had a similar effect would result in a net difference of 0. The resulting set of six values of 1 or 0 was then correlated to the proportional genetic divergence values as described above.
A correlation coefficient was calculated for the resulting sets of six phenotypic difference values, one for each phenotype, against each of the 110 sets of six proportional genetic divergence values described above. The values were scaled and R values determined as described by Smith et al (3). Based on the findings of Grupe et al, QTL identification was based on the R value cutoff that eliminated the highest percentage of the genome without surpassing 90%.
Statistical analysis
Overall differences between groups were analyzed by Mann-Whitney Rank Sum tests. Determination of the number of F2 mice categorized as having parental phenotypes was performed using chi-squared tests. All statistical analyses were performed using Microsoft Excel and SigmaStat software (SPSS, Chicago, IL).
RESULTS
Methotrexate suppresses inflammation in air pouches formed on C57Bl/6J mice but not DBA/1J mice
The degree of inflammation induced by carrageenan, as measured by leukocyte count in the air pouch exudates, was determined in methotrexate-treated and untreated mice (Table 1). In C57BL/6J mice methotrexate treatment reduced air pouch exudate leukocyte levels by 50% compared to untreated mice. In contrast, exudate leukocyte levels did not fall significantly in methotrexate-treated DBA/1J mice relative to control.
Table 1.
Methotrexate-induced reduction in exudate white blood cell count in inbred mouse strains
| Strain | Untreated (×106 cells/ml) | Treated (×106 cells/ml) | % Decrease | Log change leukocyte count |
|---|---|---|---|---|
| C57Bl/6 | 3.4 +/− 0.4 (n=23) | 1.7 +/− 0.2* (n=25) | 50% | 1.70 |
| DBA/1 | 2.0 +/− 0.1 (n=12) | 1.8 +/− 0.2 (n=14) | 8% | 0.90 |
| DBA/2 | 2.7 +/− 0.2 (n=9) | 1.9 +/− 0.3 (n=9) | 29% | 1.46 |
| BALB/c | 4.0 +/0 0.4 (n=18) | 1.5 +/− 0.1* (n=18) | 62% | 1.79 |
| C57 x DBA/1 F1 | 2.0 +/− 0.4 (n=13) | 1.7 +/− 0.2 (n=18) | 16% | N/A |
P < 0.001 versus untreated, Mann-Whitney Rank Sum Test
Leukocyte count in inflammatory air pouch exudates of methotrexate treated or untreated mice were determined as described in Materials and Methods. Four inbred strains plus F1 progeny from C57LB/6J x DBA/1J crosses were tested. For the in silico analysis, values for percent reduction in exudate leukocyte concentration induced by methotrexate were converted to log change in order to express relative changes as fold differences.
To determine whether the observed differences in anti-inflammatory efficacy across strains were specific to methotrexate and not simply due to strain-specific variation in the severity of inflammation induced, we tested those strains resistant to methotrexate for response to dexamethasone (1mg/kg). Dexamethasone significantly reduced air pouch leukocyte count in DBA/1 (42% reduction relative to control, p<0.05, n=11) mice, a response similar to those previously reported in methotrexate-responsive strains (12, 22). Dexamethasone also induced a significant reduction in leukocyte count in DBA/2 mice (46% reduction relative to control, p<0.05, n=8, a strain closely related to DBA/1 that is also resistant to methotrexate (see below).
Methotrexate increases the adenosine concentration in air pouch exudates in C57BL/6J but not DBA/1J mice
Because we have previously demonstrated that methotrexate-mediated suppression of leukocyte accumulation in the air pouch is due to increased adenosine concentrations in the air pouch exudates, we determined adenosine concentrations in the air pouch exudates of control and methotrexate-treated mice in both the C57BL/6J and DBA/1J strains (Table 2). In methotrexate-treated C57Bl/6J mice, adenosine levels in the exudates increased by 75% over the levels in vehicle-treated controls. In contrast, methotrexate treatment did not affect the air pouch exudate levels in DBA/1J mice.
Table 2.
Methotrexate-induced increase in exudate adenosine concentration in inbred mouse strains
| Strain | Untreated (nM) | Treated (nM) | Percent Change | Increase |
|---|---|---|---|---|
| C57/Bl | 124 +/− 27 (n=11) | 216 +/− 25* (n=15) | 75% | + |
| DBA/1 | 174 +/− 29 (n=8) | 156 +/− 19 (n=6) | −11% | − |
| DBA/2 | 158 +/− 50 (n=5) | 145 +/− 31 (n=7) | −8% | − |
| BALB/c | 570 +/− 90 (n=16) | 1110 +/− 190* (n=16) | 95% | + |
| C57 x DBA/1 F1 | 230 +−/ 45 (n=7) | 258 +/− 26 (n=12) | 13% | N/A |
P < 0.05 versus untreated, Mann-Whitney Rank Sum Test
Adenosine concentration in inflammatory air pouch exudates of methotrexate treated or untreated mice were determined as described in Materials and Methods. Four inbred strains plus F1 progeny from C57LB/6J x DBA/1J crosses were tested. Because log values could not be determined for methotrexate-induced adenosine upregulation in all tested strains, each strain was categorized based on the presence or absence of a statistically significant change (see Materials and Methods).
Distributions of both methotrexate-response-related phenotypes in F2 progeny from a C57BL/6J x DBA/1J cross suggest a single locus is responsible for each
In C57BL/6J x DBA/1J F1 mice methotrexate treatment induced a slight but not significant reduction in leukocyte accumulation in air pouches. There was no significant difference between male F1 mice derived from mating trios with DBA/1J male relative to C57BL6J male parents, suggesting no X-linked effect. The variance (square of standard deviation) of the F1 progeny was 194, while that of the F2s was greater at 2461, resulting in a genetic variance (σG) of 2267. The Castle-Wright estimate (D = (Distance between means)2 / 8 σG ) based on the mean leukocyte counts in the C57BL/6J versus DBA/1J mice was (27)2 / (8)(2267) = 0.04. Among the F2 progeny, the number of individuals exhibiting leukocyte counts two standard deviations below the mean value for C57BL/6 mice and above the mean for DBA/1 mice was significantly greater than would be expected if more than one locus were responsible for conferring the observed inter-strain difference. (p<0.05, data not shown,).
The exudate adenosine concentration was not significantly higher in methotrexate-treated F1 mice than saline-treated controls (Table 2). The variance of the F1 progeny was 8259, while that of the F2s was greater at 9763, resulting in a genetic variance of 1504. The Castle-Wright estimate based on the mean phenotypic adenosine concentrations in C57BL/6 versus DBA/1 methotrexate-treated mice was therefore (93)2 / 8 (1504) = 0.72. The number of F2 progeny with exudate adenosine concentrations two standard deviations below and above the DBA/1 and C57BL/6 means, respectively, was significantly greater than would be expected if more than one locus were responsible for the observed inter-strain difference. (p<0.05, data not shown)
Efficacy of methotrexate correlates with the increase in exudate adenosine concentration in the air pouch model of acute inflammation
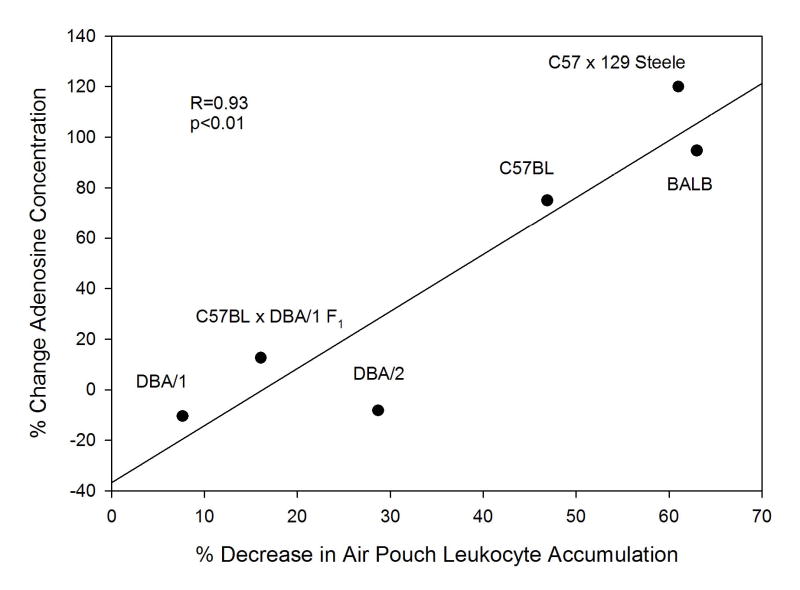
Because both the suppression of leukocyte accumulation and the increase in adenosine concentration induced by methotrexate varied in two strains of mice and given the previously-demonstrated role for adenosine in modulating the anti-inflammatory properties of methotrexate in this model, we asked whether the two phenotypes were correlated. In order to perform this analysis data from additional inbred strains were required. We examined the effect of methotrexate treatment on the adenosine concentrations and leukocyte counts in the air pouch exudates of DBA/2J mice, a strain closely related to DBA/1J. While there appeared to be a slight anti-inflammatory response to methotrexate in DBA/2J mice (29% reduction in leukocyte count relative to untreated controls), the change did not reach significance (Table 1). Moreover there was no methotrexate-induced upregulation of adenosine (Table 2). In addition, results were added from previously published studies in another strain of inbred mice, BALB/cJ, performed under identical experimental conditions which revealed a marked methotrexate-induced decrease in exudate leukocyte count and increase in adenosine concentration. In the tested strains, the methotrexate-induced reduction in air pouch leukocyte count was highly correlated with the increase in adenosine concentration (Figure 1, r = 0.927, p = 0.02).
Figure 1.
Degree of reduction in leukocyte count and upregulation in adenosine concentration are correlated in inflammatory air pouch exudates of methotrexate-treated mice across inbred strains. Correlation coefficient and p value were determined by Pearson product moment correlation.
In silico quantitative trait locus mapping for anti-inflammatory response to methotrexate and methotrexate-induced increases in air pouch adenosine concentrations reveals partially overlapping loci
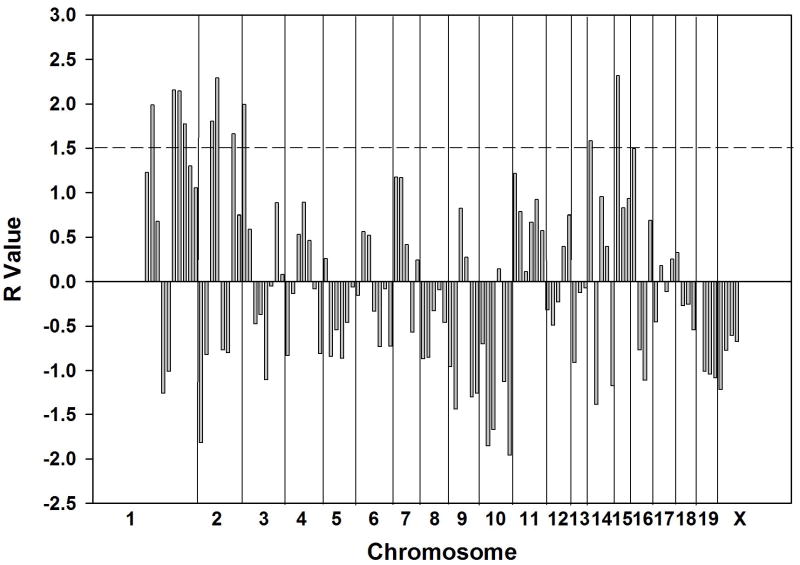
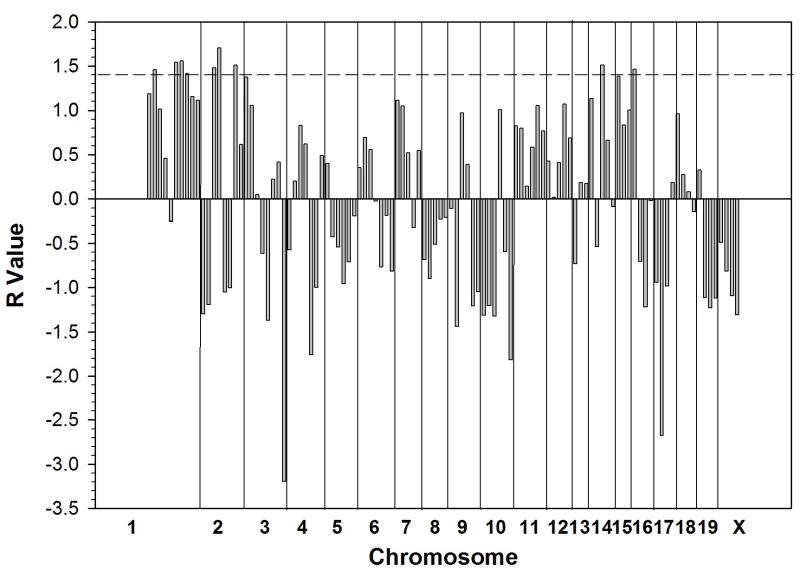
We performed QTL mapping by separate computerized analysis based on the identification of loci at which the pattern of genotypic divergence among the tested strains is most consistent with the observed differences for two phenotypes: 1) the anti-inflammatory efficacy of methotrexate as measured by reduction in exudate leukocyte count and 2) the degree of upregulation in exudate adenosine concentration induced in methotrexate-treated relative to control mice. The phenotypic differences between strains (difference in log values for leukocyte count reduction and presence or absence of adenosine upregulation, respectively) were calculated (Tables 1, 2). The analysis for anti-inflammatory efficacy identified 10 bins, grouped into 7 loci, meeting the required threshold (R value greater than 1.5)(Figure 2): 8.7 – 21.1cM (R = 1.99) and 40 – 87cM on chromosome 1 (3 bins: R = 2.16, 2.15, 1.77), 12.9 – 49.8cM (2 bins: R = 1.80, 2.29) and 60.1 – 94cM of chromosome 2 (R = 1.66), prox – 15.3cM on chromosome 3 (R = 1.99), 0 – 21cM on chromosome 14 (R = 1.58) and 0 – 17cM on chromosome 15 (R = 2.32). The adenosine upregulation analysis identified 9 bins, grouped into 6 loci, meeting the required threshold (R > 1.4) (Figure 3). These loci were located at 8.7 – 21.1 (R = 1.46) and 40 – 87cM on chromosome 1 (3 bins: R = 1.54, 1.56, 1.42), 12.9 – 49.8cM (2 bins: R = 1.48, 1.70) and 60.1 – 94 on chromosome 2 (R = 1.51), 21 – 53cM on chromosome 14 (R = 1.52) and 0 – 25cM on chromosome 16 (R = 1.47). Thus, at least 4 of the same and/or overlapping loci were identified in the two screens.
Figure 2.
Quantitative trait loci potentially associated with the degree of reduction in leukocyte concentration in inflammatory exudates of methotrexate-treated mice. Each of 110 vertical bars represents a genetic “bin” of an average of 17cM of genetic distance (see Materials and Methods). Divisions between bins from different chromosomes are indicated. R value is equal to the number of standard deviations above the normalized mean correlation value of genetic divergence and phenotypic divergence across the genome for the tested strains (2, 3). Horizontal bar at R of 1.5 corresponds to the cutoff value that eliminated the highest percentage of the genome without surpassing 90%. (2).
Figure 3.
Quantitative trait loci potentially associated with the degree of increase in adenosine concentration in inflammatory exudates of methotrexate-treated mice. Each of 110 vertical bars represents a genetic “bin” of an average of 17cM of genetic distance (see Materials and Methods). Divisions between bins from different chromosomes are indicated. R value is equal to the number of standard deviations above the normalized mean correlation value of genetic divergence and phenotypic divergence across the genome for the tested strains (2, 3). Horizontal bar at R of 1.4 corresponds to the cutoff value that eliminated the highest percentage of the genome without surpassing 90%. (1).
DISCUSSION
In the current study, we report that DBA/1J mice, in contrast to C57BL/6J mice, are resistant to the anti-inflammatory effects of methotrexate in the air pouch model of acute inflammation. We further report that, in DBA/1J but not C57BL/6J mice, methotrexate fails to induce an increase in exudate concentrations of adenosine, a potent endogenous anti-inflammatory agent which has previously been shown to mediate the anti-inflammatory effects of methotrexate in this model (1, 12, 13, 23). Genetic analysis based on the phenotypes of parental, F1 and F2 mice from a C57BL/6J x DBA/1J cross suggest that single loci are responsible for both the differences in methotrexate-induced reduction of exudate leukocyte count and upregulation of adenosine concentration observed between the two strains, and are together consistent with the hypothesis that these traits are conferred by the same locus.
The methotrexate resistance observed in DBA/1J mice is strain-specific and, therefore, genetically based, so phenotypic analysis of F1 and F2 intercross progeny was performed to further elucidate the nature of the effect. The low Castle-Wright estimates for the two phenotypes studied indicate that genetic variations at single loci best explain each of the observed differences in the DBA1 mice. However, the Castle-Wright value is only an estimate and is based on certain assumptions including: 1. the QTLs are unlinked; 2. the genes from both strains have effects of equal magnitude, and; 3. that all alleles in the “high” and “low” strains increase and decrease the phenotype, respectively(21). Partly because of these limitations the calculated value tends to underestimate the actual number of responsible loci (21). However, the fraction of F2 progeny exhibiting values within the ranges of the parental phenotypes is in both cases consistent with the Castle-Wright estimates, and these data are together most consistent with a single locus being responsible for each of the observed phenotypes.
The results reported here strongly support the hypothesis that, in the air pouch model of inflammation, genetic factors influence methotrexate efficacy, a phenomenon not clearly established in the clinic. Recently, much attention has been paid to the importance of genetic background, particularly the utility of single nucleotide polymorphisms (SNPs), in predicting the susceptibility of individuals to heritable disease as well as their response to drug therapies (6, 24–26) . The potential role for pharmacogenetics in identifying determinants of poor methotrexate response and/or toxicity was recently reviewed by McLeod et al (6) and Krajinovic et al (27). A number of small studies have suggested that single nucleotide polymorphisms in genes involved in folate metabolism are associated with methotrexate toxicity and/or efficacy (8–10, 28). These studies have however been limited by small patient populations and inconsistent definitions of methotrexate efficacy in Rheumatoid Arthritis. Moreover, the poor efficacy of methotrexate in any given individual with Rheumatoid Arthritis may result from such non-drug-related factors as increased disease activity, caffeine consumption or commencement of methotrexate therapy late in the course of the disease when Rheumatoid Arthritis is less amenable to any therapy. Studies in large patient cohorts with well-defined criteria for methotrexate response will therefore be required to identify any genetic factors associated with resistance to methotrexate in the clinic. In contrast, the current study employs a murine model in order to establish evidence for the principle of genetically-based resistance to the anti-inflammatory properties of methotrexate in the absence of such variables. While no true animal model for RA is available the air pouch model was chosen for this study due to its inducibility in a number of inbred strains as well as its established utility as a means of screening agents for potential efficacy in acute and chronic inflammation (29–33).
The correlation between the quality of response to methotrexate and its ability to upregulate exudate adenosine concentration is consistent with previous reports from this laboratory. Through the use of models of both acute and chronic inflammation, a role for adenosine, acting through its cell surface receptors, has been demonstrated as a primary mediator of methotrexate’s anti-inflammatory effects (1, 12, 13, 34). The most recent evidence supporting this hypothesis includes the observations that methotrexate failed to suppress air pouch inflammation in adenosine A2A receptor knockout mice (1), that adenosine deaminase or adenosine A2A receptor antagonist added to inflamed air-pouches attenuated the anti-inflammatory effect (12) of methotrexate treatment and that methotrexate does not suppress inflammation in animals lacking ecto-5′nucleotidase, an enzyme responsible for generation of extracellular adenosine from AMP (MC Montesinos, BN Cronstein and L Thompson, unpublished). In contrast, Andersson et al reported that methotrexate’s ability to suppress antigen-induced arthritis in rats was not inhibited by adenosine antagonists. In this study, however, the methotrexate dose (0.2 –0.3 mg/kg/day) was substantially higher than the range that is commonly used to treat RA and, unlike in RA patients, the anti-inflammatory effect was completely reversed by folic acid (35). Importantly, evidence for the role of adenosine in the anti-inflammatory properties of methotrexate has been reported in more than one animal model (1, 13) as well as in RA patients (36), and is thus not an air pouch model-specific phenomenon. Interestingly, there was no observed correlation between the exudate adenosine concentrations reached in methotrexate-untreated mice after carrageenan administration and the degree of inflammation reached across strains, a finding which suggests that relative changes, rather than absolute concentrations of adenosine may influence methotrexate efficacy. The current data thus lend further support to the hypothesis that the anti-inflammatory efficacy of methotrexate depends at least in part on its ability to upregulate extracellular levels of adenosine.
Taken together, the strong correlation across inbred strains between the anti-inflammatory efficacy of methotrexate and the methotrexate-induced increase in air pouch exudate adenosine concentration, the prior demonstration that adenosine is an important mediator of the anti-inflammatory properties of methotrexate, the similarity of the calculated estimates for the number of loci responsible for resistance to both effects in DBA/1J mice and the overlapping loci identified by independent in silico analysis for each phenotype are consistent with the hypothesis that the two DBA/1J-specific methotrexate resistance phenotypes share a genetic basis. It is likely, based on this and prior studies, that in such a case the gene responsible for both phenotypes would be involved in methotrexate metabolism, methotrexate-induced adenosine upregulation (modulation, catabolism or uptake) or adenosine response. Overlapping loci identified by in silico quantitative trait locus screens based on both methotrexate-induced decrease in leukocyte count and methotrexate-induced increase in adenosine concentration include CD-26 (DPP4), or adenosine deaminase complexing protein, adenosine deaminase (ADA) and folylpolyglutamate synthetase (FPGS) (Table 3). ADA and DPP4 are directly involved in adenosine metabolism(37–40), while FPGS is known to add glutamate residues to methotrexate thereby increasing its intracellular retention and, in turn, ability to upregulate adenosine (41, 42). Thus, the in silico screens identified loci containing candidate genes for which alleles altering regulation or function could directly explain both of the observed phenotypes.
Table 3.
Genes within identified loci relevant to MTX-induced adenosine upregulation and anti-inflammatory efficacy
| Chromosome | Position(cM) | R Value (Response, Adenosine Release) | Putative Candidate Gene |
|---|---|---|---|
| 2 | 12.9–34.5, 23.5–49.8 | 1.80, 1.48 2.29, 1.70 | DPP4 (CD26 –adenosine deaminase complexing protein 2), folylpolyglutamate synthetase (FPGS) |
| 2 | 60.1–94 | 1.66, 1.53 | adenosine deaminase (ADA) |
Position (cM) indicates the genetic bins selected by each analysis in which the indicated candidate genes are located. Neighboring, overlapping bins that were both selected are grouped as a single locus. R values for each analysis (Response, or reduction in exudate leukocyte concentration, and adenosine release due to methotrexate treatment) and each selected bin are given. Identification of candidate genes mapping within selected loci was performed using the Mouse Genome Informatics database available on the Jackson Laboratory website.
Interestingly, the loci identified by this analysis did not include the folate metabolism-related genes on which patient-based association studies have focused, but the genetic basis for methotrexate resistance in DBA/1J is likely to represent only one of a number of mechanisms by which methotrexate resistance could occur in patients. In the DBA/2 mice the marginal methotrexate-induced reduction in exudate leukocyte count in the absence of an increase in exudate adenosine concentration in DBA/2 mice seems inconsistent with an adenosine-dependent anti-inflammatory mechanism for methotrexate. However, the change observed in the methotrexate-treated DBA-2 mice did not achieve statistical significance and the magnitude of the change was less than that observed in the strains of mice in which upregulation of exudate adenosine levels accompanied the anti-inflammatory effects of methotrexate.
The in silico method applied here has been demonstrated to be effective in identifying the regions of the genome most likely to be responsible for observed phenotypic differences without missing loci previously identified by traditional quantitative trait locus screens, while requiring far less time and the sacrifice of fewer animals (2, 3). This method, like traditional QTL mapping, cannot by itself establish associations between particular genes and phenotypes. However, because the mechanisms of methotrexate-induced adenosine upregulation, adenosine metabolism and adenosine reuptake are well-elucidated and the involved genes have been identified and characterized, we have simply identified which of these genes fall within the identified loci. These candidates warrant particular attention in the context of future experiments using methods commonly employed to build upon QTL data, such as comparative, microarray-based expression assays(43–45).
The current data demonstrate that genetic factors influence the efficacy of low-dose methotrexate in the air pouch model of inflammation, and are consistent with the role of adenosine as an important mediator in its anti-inflammatory mechanism of action in this model. Moreover, the data reinforce the potential for identifying genetic risk factors that contribute to methotrexate failure in patients.
Footnotes
This work was supported by grants from the National Institutes of Health (AR41911, AA13336 and GM56268), King Pharmaceuticals, the General Clinical Research Center (M01RR00096) and by the Kaplan Cancer Center of New York University School of Medicine.
References
- 1.Montesinos MC, Desai A, Delano D, Chen JF, Fink JS, Jacobson MA, Cronstein BN. Adenosine A2A or A3 receptors are required for inhibition of inflammation by methotrexate and its analog MX-68. Arthritis Rheum. 2003;48:240–247. doi: 10.1002/art.10712. [DOI] [PubMed] [Google Scholar]
- 2.Grupe A, Germer S, Usuka J, Aud D, Belknap JK, Klein RF, Ahluwalia MK, Higuchi R, Peltz G. In silico mapping of complex disease-related traits in mice. Science. 2001;292:1915–1918. doi: 10.1126/science.1058889. [DOI] [PubMed] [Google Scholar]
- 3.Smith JD, James D, Dansky HM, Wittkowski KM, Moore KJ, Breslow JL. In silico quantitative trait locus map for atherosclerosis susceptibility in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:117–122. doi: 10.1161/01.atv.0000047461.18902.80. [DOI] [PubMed] [Google Scholar]
- 4.Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, Genovese MC, Wasko MC, Moreland LW, Weaver AL, Markenson J, Finck BK. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–1593. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- 5.van Ede AE, Laan RF, Blom HJ, De Abreu RA, van de Putte LB. Methotrexate in rheumatoid arthritis: an update with focus on mechanisms involved in toxicity. Semin Arthritis Rheum. 1998;27:277–292. doi: 10.1016/s0049-0172(98)80049-8. [DOI] [PubMed] [Google Scholar]
- 6.Ranganathan P, Eisen S, Yokoyama WM, McLeod HL. Will pharmacogenetics allow better prediction of methotrexate toxicity and efficacy in patients with rheumatoid arthritis? Ann Rheum Dis. 2003;62:4–9. doi: 10.1136/ard.62.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohosone Y, Okano Y, Kameda H, Hama N, Matsumura M, Nojima T, Nakamura K, Kuwana M, Ogasawara T, Hirakata M, Yoshida T, Mimori T, Akizuki M, Ikeda Y. [Toxicity of low-dose methotrexate in rheumatoid arthritis--clinical characteristics in patients with MTX-induced pancytopenia and interstitial pneumonitis] Ryumachi. 1997;37:16–23. [PubMed] [Google Scholar]
- 8.Urano W, Taniguchi A, Yamanaka H, Tanaka E, Nakajima H, Matsuda Y, Akama H, Kitamura Y, Kamatani N. Polymorphisms in the methylenetetrahydrofolate reductase gene were associated with both the efficacy and the toxicity of methotrexate used for the treatment of rheumatoid arthritis, as evidenced by single locus and haplotype analyses. Pharmacogenetics. 2002;12:183–190. doi: 10.1097/00008571-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Kumagai K, Hiyama K, Oyama T, Maeda H, Kohno N. Polymorphisms in the thymidylate synthase and methylenetetrahydrofolate reductase genes and sensitivity to the low-dose methotrexate therapy in patients with rheumatoid arthritis. Int J Mol Med. 2003;11:593–600. [PubMed] [Google Scholar]
- 10.Berkun Y, Levartovsky D, Rubinow A, Orbach H, Aamar S, Grenader T, Abou Atta I, Mevorach D, Friedman G, Ben-Yehuda A. Methotrexate related adverse effects in patients with rheumatoid arthritis are associated with the A1298C polymorphism of the MTHFR gene. Ann Rheum Dis. 2004;63:1227–1231. doi: 10.1136/ard.2003.016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seitz M. Molecular and cellular effects of methotrexate. Curr Opin Rheumatol. 1999;11:226–232. doi: 10.1097/00002281-199905000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Cronstein BN, Naime D, Ostad E. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest. 1993;92:2675–2682. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montesinos MC, Yap JS, Desai A, Posadas I, McCrary CT, Cronstein BN. Reversal of the antiinflammatory effects of methotrexate by the nonselective adenosine receptor antagonists theophylline and caffeine: evidence that the antiinflammatory effects of methotrexate are mediated via multiple adenosine receptors in rat adjuvant arthritis. Arthritis Rheum. 2000;43:656–663. doi: 10.1002/1529-0131(200003)43:3<656::AID-ANR23>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 14.Morabito L, Montesinos MC, Schreibman DM, Balter L, Thompson LF, Resta R, Carlin G, Huie MA, Cronstein BN. Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5′-nucleotidase-mediated conversion of adenine nucleotides. J Clin Invest. 1998;101:295–300. doi: 10.1172/JCI1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan ES, Cronstein BN. Molecular action of methotrexate in inflammatory diseases. Arthritis Res. 2002;4:266–273. doi: 10.1186/ar419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cutolo M, Sulli A, Pizzorni C, Seriolo B, Straub RH. Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann Rheum Dis. 2001;60:729–735. doi: 10.1136/ard.60.8.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piper JR, DeGraw JI, Colwell WT, Johnson CA, Smith RL, Waud WR, Sirotnak FM. Analogues of methotrexate in rheumatoid arthritis. 2. Effects of 5-deazaaminopterin, 5,10-dideazaaminopterin, and analogues on type II collagen-induced arthritis in mice. J Med Chem. 1997;40:377–384. doi: 10.1021/jm950553y. [DOI] [PubMed] [Google Scholar]
- 18.Wunder A, Muller-Ladner U, Stelzer EH, Funk J, Neumann E, Stehle G, Pap T, Sinn H, Gay S, Fiehn C. Albumin-based drug delivery as novel therapeutic approach for rheumatoid arthritis. J Immunol. 2003;170:4793–4801. doi: 10.4049/jimmunol.170.9.4793. [DOI] [PubMed] [Google Scholar]
- 19.Neurath MF, Hildner K, Becker C, Schlaak JF, Barbulescu K, Germann T, Schmitt E, Schirmacher P, Haralambous S, Pasparakis M, Meyer Zum Buschenfelde KH, Kollias G, Marker-Hermann E. Methotrexate specifically modulates cytokine production by T cells and macrophages in murine collagen-induced arthritis (CIA): a mechanism for methotrexate-mediated immunosuppression. Clin Exp Immunol. 1999;115:42–55. doi: 10.1046/j.1365-2249.1999.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Eustachio, R.H.R.a.P. 1997–1999. Genetic and Comparative Mapping in Mice. In Genome Analysis: A Laboratory Manual. E.D. Green, editor. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 71–131.
- 21.Lander ES, Botstein D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics. 1989;121:185–199. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cronstein BN, Montesinos MC, Weissmann G. Salicylates and sulfasalazine, but not glucocorticoids, inhibit leukocyte accumulation by an adenosine-dependent mechanism that is independent of inhibition of prostaglandin synthesis and p105 of NFkappaB. Proc Natl Acad Sci U S A. 1999;96:6377–6381. doi: 10.1073/pnas.96.11.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cronstein BN, Naime D, Ostad E. The antiinflammatory effects of methotrexate are mediated by adenosine. Adv Exp Med Biol. 1994;370:411–416. doi: 10.1007/978-1-4615-2584-4_89. [DOI] [PubMed] [Google Scholar]
- 24.Schmith VD, Campbell DA, Sehgal S, Anderson WH, Burns DK, Middleton LT, Roses AD. Pharmacogenetics and disease genetics of complex diseases. Cell Mol Life Sci. 2003;60:1636–1646. doi: 10.1007/s00018-003-2369-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson JA. Pharmacogenetics: potential for individualized drug therapy through genetics. Trends Genet. 2003;19:660–666. doi: 10.1016/j.tig.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Roses AD. Pharmacogenetics place in modern medical science and practice. Life Sci. 2002;70:1471–1480. doi: 10.1016/s0024-3205(01)01532-6. [DOI] [PubMed] [Google Scholar]
- 27.Krajinovic M, Moghrabi A. Pharmacogenetics of methotrexate. Pharmacogenomics. 2004;5:819–834. doi: 10.1517/14622416.5.7.819. [DOI] [PubMed] [Google Scholar]
- 28.Dervieux T, Furst D, Lein DO, Capps R, Smith K, Walsh M, Kremer J. Polyglutamation of methotrexate with common polymorphisms in reduced folate carrier, aminoimidazole carboxamide ribonucleotide transformylase, and thymidylate synthase are associated with methotrexate effects in rheumatoid arthritis. Arthritis Rheum. 2004;50:2766–2774. doi: 10.1002/art.20460. [DOI] [PubMed] [Google Scholar]
- 29.Wisniewski HG, Hua JC, Poppers DM, Naime D, Vilcek J, Cronstein BN. TNF/IL-1-inducible protein TSG-6 potentiates plasmin inhibition by inter-alpha-inhibitor and exerts a strong anti-inflammatory effect in vivo. J Immunol. 1996;156:1609–1615. [PubMed] [Google Scholar]
- 30.Sud S, Yang SY, Evans CH, Robbins PD, Wooley PH. Effects of cytokine gene therapy on particulate-induced inflammation in the murine air pouch. Inflammation. 2001;25:361–372. doi: 10.1023/a:1012898513512. [DOI] [PubMed] [Google Scholar]
- 31.De Leon EJ, Alcaraz MJ, Dominguez JN, Charris J, Terencio MC. A new chloroquinolinyl chalcone derivative as inhibitor of inflammatory and immune response in mice and rats. J Pharm Pharmacol. 2003;55:1313–1321. doi: 10.1211/0022357021747. [DOI] [PubMed] [Google Scholar]
- 32.Ospina LF, Calle J, Arteaga L, Pinzon R, Alcaraz MJ, Paya M. Inhibition of acute and chronic inflammatory responses by the hydroxybenzoquinonic derivative rapanone. Planta Med. 2001;67:791–795. doi: 10.1055/s-2001-18839. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Pastor P, Randazzo A, Gomez-Paloma L, Alcaraz MJ, Paya M. Effects of petrosaspongiolide M, a novel phospholipase A2 inhibitor, on acute and chronic inflammation. J Pharmacol Exp Ther. 1999;289:166–172. [PubMed] [Google Scholar]
- 34.Cronstein BN. The mechanism of action of methotrexate. Rheum Dis Clin North Am. 1997;23:739–755. doi: 10.1016/s0889-857x(05)70358-6. [DOI] [PubMed] [Google Scholar]
- 35.Andersson SE, Johansson LH, Lexmuller K, Ekstrom GM. Anti-arthritic effect of methotrexate: is it really mediated by adenosine? Eur J Pharm Sci. 2000;9:333–343. doi: 10.1016/s0928-0987(99)00073-1. [DOI] [PubMed] [Google Scholar]
- 36.Nesher G, Mates M, Zevin S. Effect of caffeine consumption on efficacy of methotrexate in rheumatoid arthritis. Arthritis Rheum. 2003;48:571–572. doi: 10.1002/art.10766. [DOI] [PubMed] [Google Scholar]
- 37.Franco R, Casado V, Ciruela F, Saura C, Mallol J, Canela EI, Lluis C. Cell surface adenosine deaminase: much more than an ectoenzyme. Prog Neurobiol. 1997;52:283–294. doi: 10.1016/s0301-0082(97)00013-0. [DOI] [PubMed] [Google Scholar]
- 38.Gorrell MD, Gysbers V, McCaughan GW. CD26: a multifunctional integral membrane and secreted protein of activated lymphocytes. Scand J Immunol. 2001;54:249–264. doi: 10.1046/j.1365-3083.2001.00984.x. [DOI] [PubMed] [Google Scholar]
- 39.Lambeir AM, Durinx C, Scharpe S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40:209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- 40.Hirschhorn R, Ratech H. Isozymes of adenosine deaminase. Isozymes Curr Top Biol Med Res. 1980;4:131–157. [PubMed] [Google Scholar]
- 41.Liani E, Rothem L, Bunni MA, Smith CA, Jansen G, Assaraf YG. Loss of folylpoly-gamma-glutamate synthetase activity is a dominant mechanism of resistance to polyglutamylation-dependent novel antifolates in multiple human leukemia sublines. Int J Cancer. 2003;103:587–599. doi: 10.1002/ijc.10829. [DOI] [PubMed] [Google Scholar]
- 42.Rots MG, Pieters R, Peters GJ, Noordhuis P, van Zantwijk CH, Kaspers GJ, Hahlen K, Creutzig U, Veerman AJ, Jansen G. Role of folylpolyglutamate synthetase and folylpolyglutamate hydrolase in methotrexate accumulation and polyglutamylation in childhood leukemia. Blood. 1999;93:1677–1683. [PubMed] [Google Scholar]
- 43.Lyons MA, Wittenburg H, Li R, Walsh KA, Leonard MR, Churchill GA, Carey MC, Paigen B. New quantitative trait loci that contribute to cholesterol gallstone formation detected in an intercross of CAST/Ei and 129S1/SvImJ inbred mice. Physiol Genomics. 2003;14:225–239. doi: 10.1152/physiolgenomics.00073.2003. [DOI] [PubMed] [Google Scholar]
- 44.Pitman WA, Korstanje R, Churchill GA, Nicodeme E, Albers JJ, Cheung MC, Staton MA, Sampson SS, Harris S, Paigen B. Quantitative trait locus mapping of genes that regulate HDL cholesterol in SM/J and NZB/B1NJ inbred mice. Physiol Genomics. 2002;9:93–102. doi: 10.1152/physiolgenomics.00107.2001. [DOI] [PubMed] [Google Scholar]
- 45.Tabakoff B, Bhave SV, Hoffman PL. Selective breeding, quantitative trait locus analysis, and gene arrays identify candidate genes for complex drug-related behaviors. J Neurosci. 2003;23:4491–4498. doi: 10.1523/JNEUROSCI.23-11-04491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]