Abstract
Prefrontal cortex (PFC) contributes to working memory functions via executive control processes that do not entail the storage, per se, of mnemonic representations. One of these control processes may be a sensory gating mechanism that facilitates retention of representations in working memory by down-regulating the gain of the sensory processing of intervening irrelevant stimuli. This idea was tested by scanning healthy young adults with functional magnetic resonance imaging while they performed a delayed face-recognition task. The 2 x 2 factorial design varied the factors of Memory (present, absent) and Distraction (present, absent). During memory-present trials, target and probe stimuli were individual gray-scale male faces. Memory-absent trials were identical, except that they employed the same recurring female faces (denoting a “no memory” trial). Distraction-present trials featured rapid serial visual presentation of bespectacled male faces during the two middle sec of the delay. The first step of the analyses identified dorsolateral PFC (dlPFC) and inferior occipitotemporal cortex (IOTC) voxels exhibiting delay-period activity in memory-present/distraction-absent trials – i.e., the “unfilled” delay. Within these voxels, distraction-evoked activity in dlPFC was markedly higher during trials that required the concurrent short-term retention of information than on those that did not, whereas the opposite effect was seen in IOTC. These results are consistent with the view that processes related to sensory gating account for a portion of the delay-period activity that is routinely observed in dorsolateral PFC.
INTRODUCTION
The prefrontal cortex (PFC) makes important contributions to the control of cognitive processes, including working memory (Monsell & Driver, 2000; Stuss & Knight, 2002). But what is the function of the delay-period activity that is observed almost universally in the PFC of monkeys and humans performing simple tasks of short-term memory that make no overt demands on executive control? By one influential view, it represents the active short-term retention (STR) of information (Courtney, 2004; Goldman-Rakic & Leung, 2002). This view, in effect, equates different regions of the PFC with the storage buffers of Baddeley and Hitch’s (1974) model of working memory; for a more detailed review, see Postle, Druzgal, & D’Esposito (2003). An alternative approach to this question, in contrast, comes from a framework that holds that working memory functions do not arise from the operation of one or more specialized systems, but rather that they arise from the recruitment, via attention, of systems that have evolved to accomplish sensory, representational, and/or motoric goals. From this perspective, the PFC is not the neural substrate for working memory storage buffers, because the STR of information is supported in a domain-specific manner in areas responsible for perception, knowledge representation, language, and motor control (e.g., Jonides, Lacey, & Nee, 2005; Pasternak & Greenlee, 2005; Postle, Awh, Jonides, Smith, & D’Esposito, 2004; Postle, Berger, & D’Esposito, 1999; Postle & D’Esposito, 1999, 2003; Postle et al., 2003; Ruchkin, Grafman, Cameron, & Berndt, 2003). This view derives support from a considerable body of neuropsychological data indicating that the STR of information (as measured by span and delay tasks) does not depend on the integrity of PFC when monkeys (e.g., Malmo, 1942; Petrides, 2000) and humans (e.g., Chao & Knight, 1998; Chao & Knight, 1995; D’Esposito & Postle, 1999; Milner, 1964) are tested under optimal conditions.
If it is not storage, what might be the function of delay-period activity in PFC? There are several possibilities, including attentional selection (Kane & Engle, 2003; Lebedev, Messinger, Kralik, & Wise, 2004; Passingham & Rowe, 2002; Rowe, Stephan, Friston, Frackowiak, & Passingham, 2005; Rowe, Toni, Josephs, Frackowiak, & Passingham, 2000), top-down control (variously described, for example, as “guided activation” (Miller & Cohen, 2001),”adaptive coding” (Duncan & Miller, 2002), and “proactive control” (Braver, Gray, & Burgess, in press)), stimulus transformation and response preparation (e.g., Bor, Duncan, Wiseman, & Owen, 2003; Curtis, Rao, & D’Esposito, 2004; D’Esposito, Ballard, Zarahn, & Aguirre, 2000; Fukushima, Hasegawa, & Miyashita, 2004; Fuster, 1995; Pochon et al., 2001; Takeda & Funahashi, 2002, 2004), and motivation and/or reward expectancy (e.g., Watanabe, 1996, 2002). For extensive reviews of this question, see Curtis and D’Esposito(2003) and Passingham and Sakai (2004). The present study was designed to test yet another candidate function of PFC delay-period activity – the mediation of interference.
One indication that the PFC plays an important role in interference mediation comes from the fact that impairments begin to emerge in the working memory performance of PFC-lesioned subjects when distraction or interference is present in the environment (Chao & Knight, 1998; Chao & Knight, 1995; D’Esposito & Postle, 1999; Malmo, 1942) or in the cognitive system (as is the case with proactive interference (PI), Milner, 1964; Thompson-Schill et al., 2002). Further evidence comes from electrophysiological and neuroimaging studies. With electrophysiology, Chao and Knight (1998) provided evidence that lateral PFC contributes to “attentional filtering” or “sensory gating” by showing that the evoked response to distracting auditory stimuli was greater for patients with PFC lesions than for control subjects. With PET and fMRI, several groups have investigated the selective sensitivity to PI of the portion of left Inferior Frontal Gyrus corresponding to Brodmann’s Area (BA) (e.g., D’Esposito, Postle, Jonides, & Smith, 1999; Jonides, Marshuetz, Smith, Reuter-Lorenz, & Koeppe, 2000; Jonides, Smith, Marshuetz, Koeppe, & Reuter-Lorenz, 1998; Postle, Berger, Goldstein, Curtis, & D’Esposito, 2001; Postle & Brush, 2004; Postle, Brush, & Nick, 2004). And two other neuroimaging studies have gone beyond demonstrating the PFC’s sensitivity to interference by showing that PFC plays an active role in its mediation. In one, Sakai, Rowe, and Passingham (2002) found that PFC activity during an unfilled delay period predicted task accuracy on trials when this unfilled period was followed by a distractor task. They attributed this to a PFC-controlled “active maintenance” process that strengthened mnemonic representations via the strengthening of the coupling of activity between superior frontal cortex (Brodmann’s Area (BA) 8) and Intraparietal Sulcus1. In another study, Gray, Chabris, and Braver (2003) found that PFC activity correlated with performance on trials of an n-back working memory task that featured a high level of PI, but only in subjects who had been independently determined to be of high general fluid intelligence. Like these two, the present study was also designed to evaluate evidence for a PFC-based control mechanism involved in the mediation of interference. It’s design would permit a two-stage process of (1) the identification of delay-period activity from unfilled delay periods, followed by (2) an assessment of whether the voxels identified in step 1 might contribute to a sensory gating function. Thus, it would explicitly evaluate one candidate explanation of the function of PFC delay-period activity.
Although there are many ways to implement a gating mechanism, the present study was designed to detect one that operates by regulating the gain of sensory processing such that the processing of potentially disrupting afferent sensory information is down-regulated when this information might interfere with the STR of other, behaviorally prioritized information. There is considerable evidence for such a mechanism from studies measuring event-related potentials. In these studies, an increase in alpha-band power over posterior areas during short-term memory task performance is interpreted as evidence for “inhibition of task irrelevant processes” (Klimesch et al. (1999) p. 403, (see also Chao & Knight, 1998; Jensen, Gelfand, Kounios, & Lisman, 2002)). The task featured in this report was delayed face recognition, implemented in a 2 x 2 design that varied the factors of memory (present, absent) and distraction (present, absent; Figure 1). The logic of the experiment depended on the assumption that the PFC delay-period activity that was expected on memory-present/distraction-absent trials would not reflect the STR of face information. This assumption was supported by two previous studies of delayed face recognition indicating that PFC delay-period activity was logically incompatible with a STR function. One of these studies employed a multistep ABBA-like design intended to winnow out delay-period activity that may be correlated with, but not necessary for, the STR of face information. Although the results from each subject revealed Delay 1-specific activity in many brain areas, including PFC, posterior Fusiform Gyrus, and posterior parietal cortex, only a subset of these voxels retained the Delay 1 signal during Delay 2, and posterior Fusiform Gyrus was the only region in which, in each subject, voxels retained the Delay 1 signal during Delay 3 (Postle et al., 2003). The second study demonstrated that varying instructions -- “remember face(s)” vs. “remember scene(s)” -- modulated the delay-period activity of the fusiform face area or the parahippocampal place area in a category-specific manner, but had no effect on PFC (i.e., PFC activity did not distinguish between STR of faces vs. STR of houses, Ranganath, DeGutis, & D’Esposito, 2004). Instead of STR, the sensory gating hypothesis posits that some portion of PFC delay-period activity on memory-present/ distraction-absent trials reflects a tonically active mechanism that monitors the environment for potentially distracting stimuli or events, and that regulates the gain of sensory processing when needed.
Figure 1.
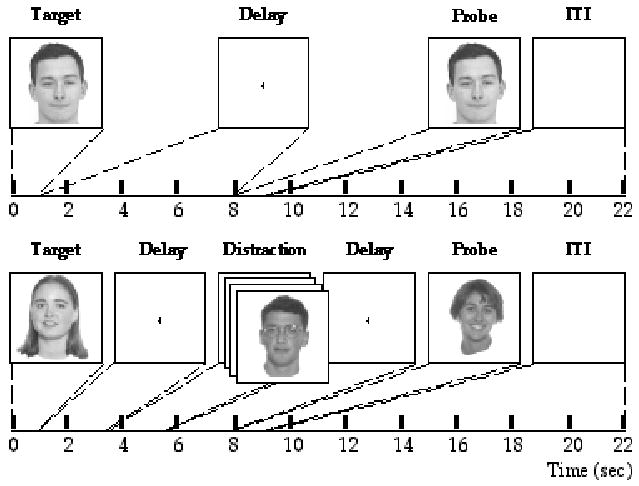
Illustration of a memory-present/distraction-absent trial and a memory-absent/ distraction-present trial.
And so the logic of the experimental approach was, first, to identify face memory-related activity in dorsolateral PFC (dlPFC) and inferior occipitotemporal cortex (IOTC) by identifying voxels in these two regions demonstrating significant delay-period activity in memory-present/distraction-absent trials (i.e., in “standard” delayed-recognition conditions), and, second, to assess the distraction-evoked activity in these voxels of interest (VOIs) by measuring delay-period activity from memory-absent/distraction-present and memory-present/distraction-present trials. We assumed that a dlPFC-based sensory gating mechanism would be engaged to a larger extent by the conjunction of the need to retain a memory with the presence of distracting noise in the environment than by the presence of distraction alone. Thus, we predicted greater delay-period activity in dlPFC VOIs – the hypothesized source of control – in memory-present/distraction-present than in memory-absent/distraction-present trials. In contrast, we predicted that the gain modulating effects of this gating process would result in decreased activity at the site of its action during the conjunction of memory and distraction -- that is, to relatively decreased delay-period activity in IOTC VOIs in memory-present/distraction-present as compared with memory-absent/distraction-present trials. Restated in terms of our experimental design, we predicted an interaction such that the manipulation of the factor of memory on distraction trials would have a greater effect on dlPFC VOIs than on IOTC VOIs. We did not know, a priori, whether or not the outcome would be a crossover interaction (i.e., whether in IOTC VOIs the delay-period effect on memory-present/ distraction-present trials would in fact be quantitatively lower than on memory-absent/ distraction-present trials) or whether the difference would be in the same direction as in dlPFC VOIs, but markedly smaller.
RESULTS
Behavior
Behavioral results, presented inTable 1, indicated that performance was high in all conditions. (Due to technical problems, accuracy data were not collected from two subjects, and RT data were not collected from four subjects.) An ANOVA assessing accuracy as a function of memory (present, absent) and distraction (present, absent) revealed a main effect of memory that approached significance (F(1,13) = 3.6; p = .08), a main effect of distraction (F(1,13) = 5.2; p < .05), and an interaction (F(1,13) = 18.1; p < .001). The analogous analysis of reaction time (RT) data revealed main effects of memory (F(1,11) = 51.2; p < .0001) and distraction (F(1,11) = 22.8; p < .001) and an interaction (F(1,11) = 21.5; p < .001). The main effect of distraction reflects the fact that RTs were faster for trials with distraction than without it. Although this effect was not expected, it may be that the rapid serial visual presentation (RSVP) distraction elicited a general increase of arousal, or a greater level of motor preparation (e.g., Connolly, Goodale, Goltz, & Munoz, in press). Together, the accuracy and RT results confirmed the intuition that of the two critical conditions in this study, memory-absent/distraction-present and memory-present/distraction-present, the latter would be the more difficult.
Table 1.
Behavioral performance: Accuracy (mean proportion correct) and RT (msec)
| Memory | |||
|---|---|---|---|
| Present | Absent | ||
| Present | |||
| Accuracy (SE) | 0.874 (0.020) | 0.931 (0.023) | |
| Distraction | RT (SE) | 766.1 (40.1) | 474.4 (36.8) |
| Absent | |||
| Accuracy (SE) | 0.945 (0.021) | 803.6 (38.1) | |
| RT (SE) | 0.934 (0.024) | 633.9 (47.8) | |
fMRI
Delay-period activity was observed on memory-present/distraction-absent trials in the dlPFC and the IOTC in each of the sixteen subjects (Figure 2). Within the functionally defined VOIs, distractor-evoked activity was markedly higher for memory-present than for memory-absent trials in dlPFC, whereas IOTC showed a trend in the opposite direction (Figures 3 and 4). ANOVA assessing raw distraction-evoked activity in VOIs in the two regions (dlPFC, IOTC) and the two critical trial types (memory-absent/ distraction-present, memory-present/distraction-present trials) revealed a main effect of region (F(1,15) = 11.97; p < .005), an effect of trial type that approached significance (F(1,15) = 3.96; p = .07), and an interaction (F(1,15) = 15.23; p = .001). These results were broadly consistent with our predictions. However, because the main effect of region indicated that the overall evoked response was greater in IOTC than in dlPFC, it was possible that the differential patterns of activity observed in these two VOIs was attributable, at least in part, to a difference in the inherent level of activity in the two regions in which they were located. To rule out this possibility, the effects from each subject’s VOIs were normalized by dividing, within VOI, the distractor-evoked effects from both trial types of interest by the memory-absent/distraction-present effect. This had the effect of transforming the memory-absent/distraction-present effect in both regions from each subject to the arbitrarily selected value of 1.0, and transforming the memory-present/distraction-present effect into a proportion of the memory-absent/ distraction-present effect. After this transformation was performed we excluded the data from one subject (#12) from further analyses, because the normalized memory-present/ distraction-present effect from the dlPFC VOIs of this subject was in excess of 3 standard deviations greater than the mean2. For the remaining 15 subjects the normalized data indicated that the mean delay-evoked response on memory-present/distraction-present trials in dlPFC VOIs was 139% greater than on memory-absent/distraction-present trials (a significant pairwise effect at t(14) = 2.1; p = .05), whereas in IOTC VOIs the mean delay-evoked response on memory-present/distraction-present trials was 7.5% smaller than it was on memory-absent/distraction-present trials (a pairwise effect that was not significant: t(14) = -1.2; n.s.). Thus, the pattern of the normalized effects retained the features of the raw data (Fig. 4.b.), as confirmed by ANOVA assessing the normalized results from the remaining 15 subjects, which produced a main effect of region (F(1,14) = 4.9; p < .05), a borderline effect of trial type (F(1,14) = 3.8; p = .07), and an interaction (F(1,14) = 4.9; p < .05).
Figure 2.
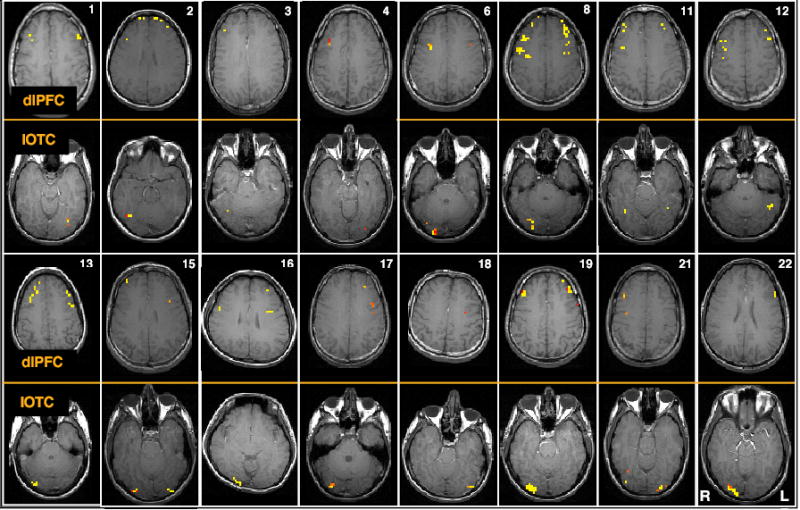
Two representative slices from each subject, illustrating dlPFC and IOTC VOIs. Memory-present/distraction-absent activity was observed, in dlPFC, bilaterally in 13 subjects, and unilaterally in right hemisphere in 2 subjects and in left hemisphere in 1 subject; in IOTC in was observed bilaterally in 4 subjects, unilaterally in right hemisphere in 9 subjects, and unilaterally in left hemisphere in 3 subjects.
Figure 3.
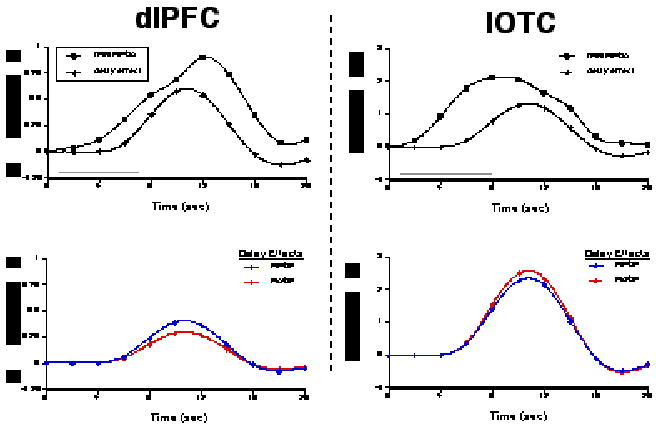
An example of results from a single subject (#21 from Fig. 2). The two upper plots illustrate the trial-averaged time series data from dlPFC and IOTC VOIs from memory-present/distraction-absent trials (“fMRI MP/DA”), and the corresponding delay-period covariates scaled by their parameter estimates (“delay effect”). The gray bars along the horizontal axes indicate the duration of the delay period. The two lower plots illustrate the delay effects from these same VOIs from memory-present/distraction-present (“MP/DP”) and memory-absent/ distraction-present (“MA/DP”) trials. Note that the signal intensity scales differ on plots for the two regions.
Figure 4.
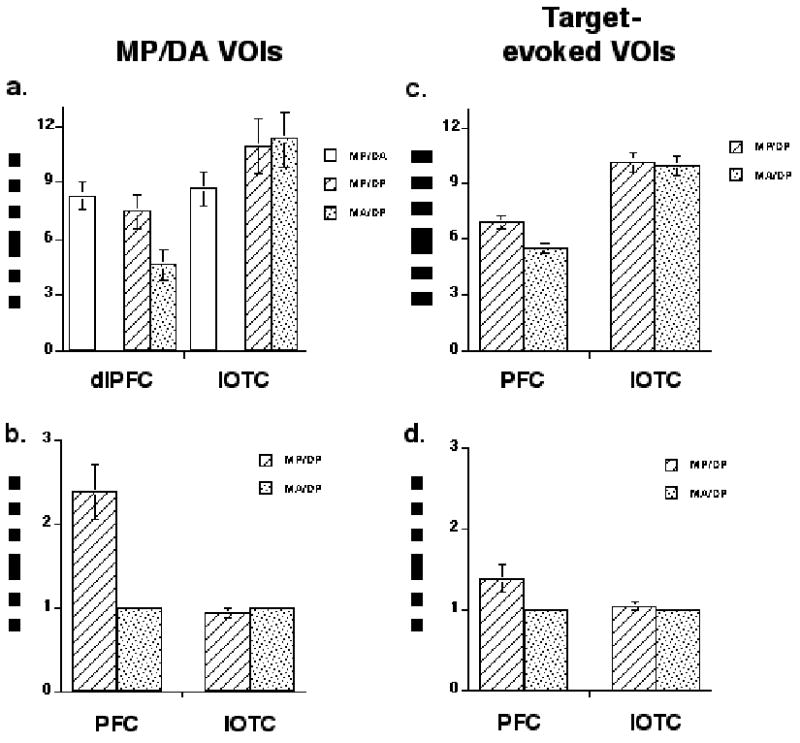
Group average effects. a. memory-present/distraction-absent delay-period VOIs with unscaled data; b. memory-present/distraction-absent delay-period VOIs with normalized data; c. target-evoked VOIs with unscaled data; d. target-evoked VOIs with normalized data. MP/DA = memory-present/distraction-absent; MP/DP = memory-present/distraction-present; MA/DP = memory-absent/ distraction/present; error bars represent SEM.
One alternative to the sensory-gating interpretation of these results might be that PFC delay-period activity on memory-present/distraction-present trials reflected dual tasking – the STR of target face information plus the incidental encoding (or some other type of processing) of the distracting faces. This seems unlikely, however, because PFC delay-period activity was numerically lower on memory-present/distraction-present trials than on memory-present/distraction-absent trials (although this effect did not achieve significance: t(15) = −1.3; n.s., Figure 4.a.).
How specific was this pattern of results? To assess this question, we applied the same contrasts to voxels in the dlPFC and IOTC that were selected for a different property -- responsivity to visually presented faces. Specifically, we identified voxels in dlPFC and IOTC that responded to the Target epoch of the task (and removed from these VOIs any voxels that were also active during the delay period of memory-present/ distraction-absent trials). In this way, we identified voxels from the same regions in which we tested the sensory gating hypothesis, but whose function was different (presumably related to visual perception and/or memory encoding, but not to retention or another delay-period process). For the raw data from these target-evoked VOIs (Figure 4.c.), as compared to the memory-evoked VOIs, there was again a strong main effect of region (F(1,14) = 33.4; p < .0001), a weaker borderline effect of trial type (F(1,14) = 3.3; p = .09), and now only a trend toward an interaction (F(1,14) = 4.0; p .07). When data from the target-evoked VOIs were normalized in the same manner as had been the data from the delay-evoked VOIs (Fig. 4.d.), their analysis with ANOVA revealed only a borderline effect of VOI (F(1,14) = 4.0; p = .06), for the first time, a reliable effect of trial type, (F(1,14) = 5.1; p < .05), and, again, a trend toward an interaction (F(1,14) = 4.0; p = .06).
The weakening of the Region x Trial type interaction in target-evoked VOIs as compared with delay-evoked VOIs could be consistent with the claim that this interaction, predicted to be the fMRI signature of a sensory gating mechanism, was selectively seen only in the delay-evoked VOIs. It could also, however, simply reflect a situation in which the interaction terms for the two types of VOI fell on either side of the .05 threshold, but did not differ from each other. To distinguish between these two possibilities, we compared the effects of the experimental manipulation on delay-period activity across the two types of VOIs with an omnibus ANOVA that directly compared the effect of memory on normalized distraction-evoked activity across target-evoked and delay-evoked VOIs. The factors for the omnibus ANOVA were region (dlPFC, IOTC), trial type (memory present, memory absent), and epoch defining the VOIs (target, delay). A three-way interaction would be necessary to support the claim that the differential effect of memory demands on distraction-evoked activity in dlPFC and IOTC was greater for delay-evoked than for target-evoked VOIs. The ANOVA revealed a main effect of region (F(1,14) = 4.9; p < .05), a borderline effect of trial type (F(1,14) = 4.3; p = .06), and no effect of epoch (F(1,14) = 2.8; p > .1), no interaction of Epoch x Trial type (F(1,14) = 2.8; p > .1) but reliable interactions of Epoch x Region (F(1,14) = 4.7; p < .05) and of Region x Trial type (F(1,14) = 4.9; p < .05), and a reliable interaction of Epoch x Region x Trial type (F(1,14) = 4.7; p < .05). Thus, it confirmed the specificity of the sensory gating effect to delay-evoked VOIs.
DISCUSSION
The results demonstrate that delay-sensitive voxels in two brain regions, the dlPFC and the IOTC, respond differentially to the RSVP of faces conditioned on the behavioral context in which these faces are presented: the dlPFC response was 139% greater during concurrent face memory, whereas the IOTC response was 7.5% lower. This pattern was also seen to be selective, because a different group of dlPFC voxels (those that are target-sensitive) only increased its activity by 38%, and the analogously different group of IOTC voxels increased their activity by 3%. These results are therefore consistent with a model in which one function of dlPFC voxels that are active during unfilled delay periods of delayed-recognition tasks is to perform a sensory gating function. By this account the detection of potentially interfering stimuli in the environment triggers activity in networks of dlPFC neurons3 which, in turn, has the effect of suppressing, perhaps via thalamic relays, the afferent flow of sensory information (Knight, Staines, Swick, & Chao, 1999). This would have beneficial effects for the signal-to-noise ratio in the networks of posterior neurons that are responsible for the STR of information. Thus, a PFC-based control process may serve to protect the contents of working and short-term memory from the deleterious effects external interference, even though it does not support STR operations themselves.
This account makes two broader claims about the working memory literature. First, it offers an explanation as to why primates with PFC lesions can perform normally on tasks of working and short-term memory when potential sources of distraction and inference are minimal, but are impaired on the same tasks when distraction and/or interference is high (Chao & Knight, 1998; Chao & Knight, 1995; D’Esposito & Postle, 1999; Malmo, 1942; Milner, 1964; Thompson-Schill et al., 2002). Second, it proposes that sensory gating may account for a portion of the unfilled delay-period activity that is routinely observed in PFC across a wide variety of neuroimaging studies, including those that do not explicitly include distraction. In relation to this second claim, however, we cannot rule out the possibility that the dlPFC activity observed during memory-present/ distraction-absent trials was due to a specific feature of our design: Because subjects could not predict which memory trials would also contain distraction, an effective strategy may have been to implement a sensory gating strategy on every memory trial. To rule this out would require a two stage process of first scanning “naïve” subjects performing several blocks of only memory-present/distraction-absent trials, then introducing the factor of delay-period distraction for a subsequent set of scans.
The present study fits within a growing body of results that document control, rather than STR, functions for the PFC. These include the monitoring and transformation of information held in working memory (Bor et al., 2003; D’Esposito, Postle, Ballard, & Lease, 1999; Petrides, 1995) and the attentional selection of information (de Fockert, Rees, Frith, & Lavie, 2001; M.A. Lebedev et al., 2004; Sakai et al., 2002). These approaches, that propose well-defined, testable mechanisms, represent an advance beyond the vague notion of “maintenance” that is often invoked in interpretations of PFC activity. The present study suggests one such alternative to “maintenance” to account for sustained PFC activity observed during the simplest tests of STR of information.
METHODS
Subjects
Sixteen healthy young adults (mean age = 22.4, 13 males) participated in this study. All provided informed consent and were paid in compensation for participating in the study.
Behavioral procedure
The 2 x 2 factorial design featured the factors of Memory (present, absent) and Distraction (present, absent). Each trial had the same structure: Target (1 sec) followed by Delay (7 sec) followed by Probe (1 sec) followed by ITI (13 sec). During memory-present trials, target and probe stimuli were individual gray-scale male faces (none wearing glasses) appearing on white backgrounds, and subjects indicated with a button press whether the latter matched the former (p = .5). Memory-absent trials were procedurally identical, except that they featured the same recurring female faces -- face “A” always the target, which denoted a “no-memory” trial, face “B” always the probe. On memory-absent trials, subjects were cued by the probe to indicate with a button press whether or not distracting faces appeared during the delay period (p = .5), thereby matching the motor response demands of memory-present trials. Distraction-present trials featured RSVP (3 Hz) of the faces of males wearing glasses (to differentiate distraction items from memoranda) during the two middle sec of the Delay (Figure 1). Subjects were instructed to look at the distracting faces during all trials on which they appeared, and to fixate centrally on distraction-absent trials. Stimuli were presented via eye pieces mounted on the head coil (Avotec Visible Eye), and fitted with an infrared-based eye tracking system (SMI Inc.). Fixation was monitored during scanning and analyzed offline with iLab software (Gitelman, 2002). Trials during which subjects failed to fixate centrally from time 3 sec to time 5 sec were discarded from subsequent analyses (Figure 5). This criterion ensured that any evidence for differences in delay-period activity between conditions couldn’t be attributed to theoretically uninteresting differences in, for example, the amount of visual stimulation produced by the distracting stimuli, or the amount of oculomotor activity. Trials of each of the four types were equiprobable and presented in a randomly determined order.
Figure 5.
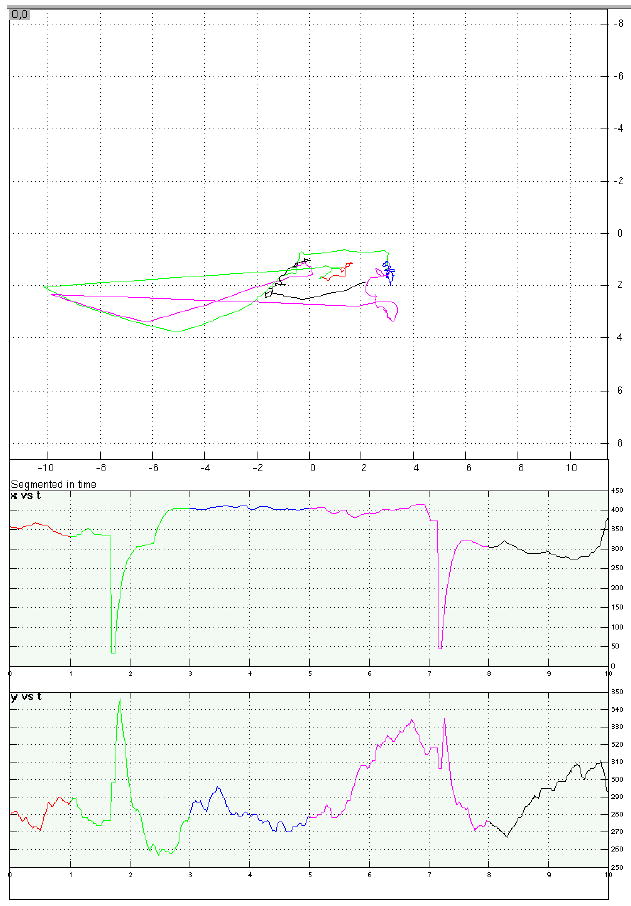
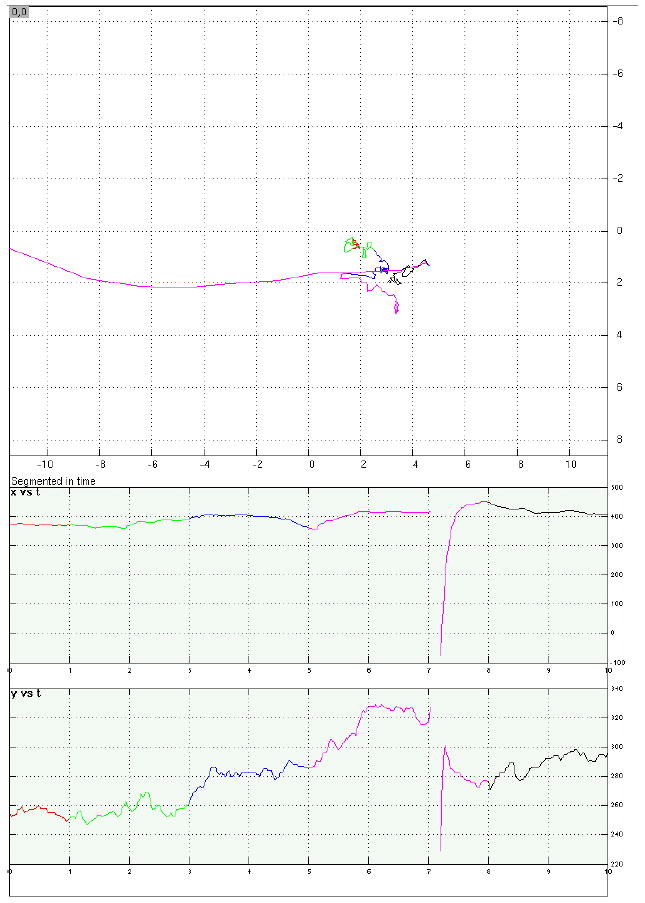
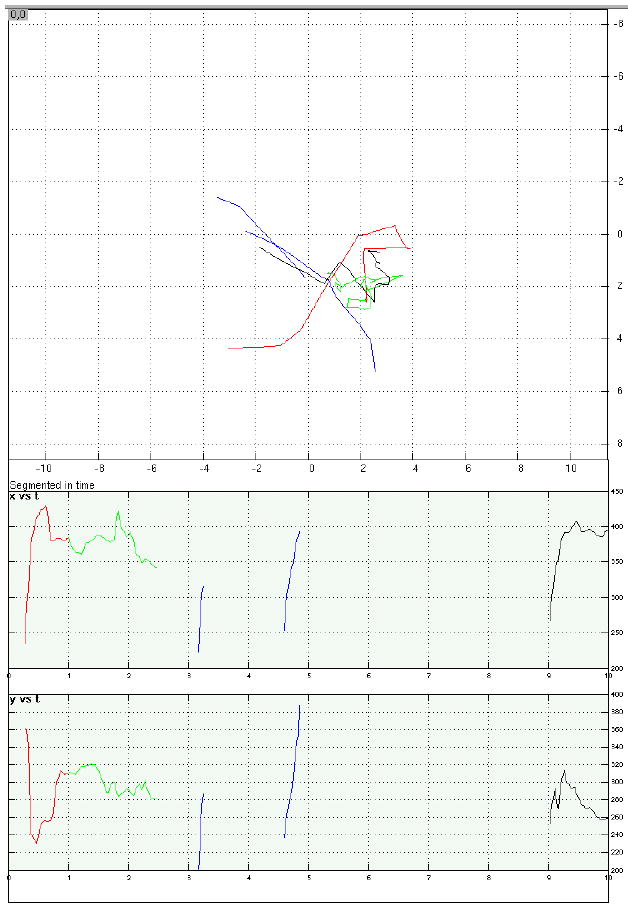
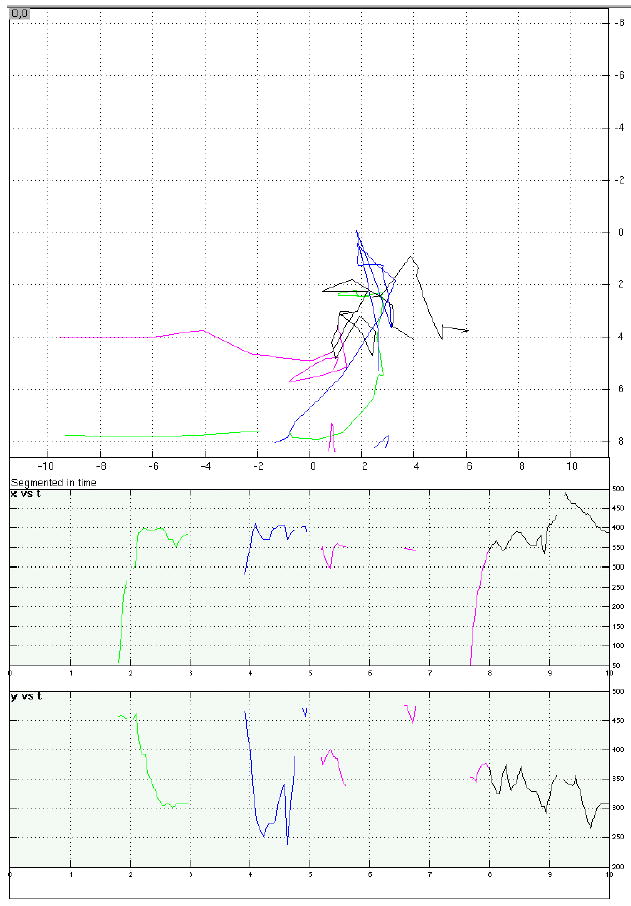
Examples of eye-position data from four representative trials.
a. A memory-present/distraction-present trial with acceptable fixation during distraction. Top panel is a 2D representation of eye position on the display “screen” over the course of the trial; axis labels correspond to degrees of visual angle. Bottom panels display horizontal (“x”) and vertical (“y”) eye position as a function of time, with time in sec on the horizontal axes and position in pixels on the vertical axes (each pixel measured .58 mm2). For all three panels, trial epoch is color coded: red = target stimulus; green = early delay; blue = distraction portion of the delay period; fuchsia = late delay; black = probe stimulus plus first sec of ITI.
b. A memory-absent/distraction-present trial with acceptable fixation during distraction. All conventions same as with Figure 5.a. Interrupted portion of late delay corresponds to a blink, when eyes were closed.
c. A memory-present/distraction-present trial that was discarded due to unacceptable fixation during distraction. All conventions same as with Figure 5.a.
d. A memory-absent/distraction-present trial that was discarded due to unacceptable fixation during distraction. All conventions same as with Figure 5.a.
fMRI data acquisition
Whole-brain images were acquired with a 3T scanner (GE Signa VH/I). High resolution T1-weighted images (32 axial slices, .9375 mm x .9375 mm x 4 mm) were obtained in every participant, and a gradient echo, echoplanar sequence (TR=2000ms, TE=50ms) was used to acquire data sensitive to the blood oxygen level dependent (BOLD) signal (Kwong et al., 1992; Ogawa et al., 1992) within a 64 x 64 matrix (32 axial slices coplanar with T1, 3.75 mm x 3.75 mm x 4 mm). Scans of the delayed-recognition task were preceded a scan in which we derived an estimate of the hemodynamic response function (HRF) for each participant. During this scan each participant performed a simple reaction-time task that required a bimanual button press once every 20 sec in response to a brief change in shape of the fixation stimulus. A partial F-test associated with a Fourier basis covariate set (Josephs, Turner, & Friston, 1997) was used to evaluate the significance of task correlated activity in each voxel of primary somatosensory and motor cortical regions of interest (Aguirre, Zarahn, & D’Esposito, 1998). An HRF estimate was extracted from the suprathreshold voxels of these ROIs by spatially averaging their time series, filtering the resultant averaged fMRI time series to remove high (>0.244 Hz) and low (<0.05 Hz) frequencies, adjusting it to remove the effects of nuisance covariates (Friston, Holmes, Poline, Heather, & Frackowiak, 1995), and trial averaging. The HRF characterizes the fMRI response resulting from a brief impulse of neural activity (Boynton, Engel, Glover, & Heeger, 1996), and can vary markedly across participants (Aguirre et al., 1998). The subject-specific HRFs were used to convolve independent variables entered into the modified general linear model (GLM, Worsley & Friston, 1995) that we used to analyze the data from the scans of the delayed-recognition task4. The eight scans of the delayed-recognition task each lasted 6 min 12 sec (5:52 of task preceded by 20 sec of dummy pulses to achieve a steady state of tissue magnetization).
fMRI data processing
The fMRI time series data were filtered and adjusted as described previously (Postle, Zarahn, & D’Esposito, 2000). The principle of the fMRI time series analysis was to model the fMRI signal changes evoked by each stimulus presentation epoch with covariates comprised of BOLD impulse response functions shifted along the timeline of the task to represent various trial epochs (Figure 1, Postle et al., 2000; Zarahn, Aguirre, & D’Esposito, 1997). The least-squares solution of the GLM of the fMRI time series data yielded parameter estimates that were associated with each covariate of interest. The smoothness of the fMRI response to neural activity allows fMRI evoked responses that arise from temporally dependent events to be resolved on the order of 4 sec. (That is, if the covariates modeling these events are spaced at 4 sec intervals, the covariate positioned at time 0 sec will account for virtually all of the variance attributable to an event starting at time 0 sec and of duration of 1 sec or less, thereby leaving the covariate positioned at time 4 sec relatively uncontaminated by variance attributable to the time 0 sec event (see Fig. 6 of Zarahn et al., 1997)). Differences in fMRI signal (either between conditions or vs. baseline) were tested by computing t-statistics from contrasts between parameter estimates associated with the covariates in question.
Our analysis strategy was to identify delay-specific activity in two regions, the dlPFC and the IOTC5 from memory-present/distraction-absent trials. This would identify delay-period activity comparable to that observed in virtually all whole-brain neuroimaging studies of similar tasks (e.g., with face stimuli, Courtney, Ungerleider, Keil, & Haxby, 1996; Courtney, Ungerleider, Keil, & Haxby, 1997; Druzgal & D’Esposito, 2001; Postle et al., 2003; Ranganath & D’Esposito, 2001). Masks corresponding to these two regions were drawn on each subject’s anatomical scans. Voxels located within these masks, which became the VOIs, were identified on t-maps of delay-period activity from memory-present/distraction-absent trials, thresholded to a regionwise ∞ of .05 (one-tailed) with Bonferroni correction for the number of voxels in the mask. Note that with this approach, with spatially unsmoothed data, individual voxels whose t-value exceeds threshold can be interpreted as “meaningful” data (Postle et al., 2000). Thus, this procedure differed somewhat from other applications of “region of interest (ROI) analyses”, in that no selection criteria were imposed with respect to cluster size or proximity of critical voxels. This was done, among other reasons, because we did not know a priori in which subregion of PFC to expect to find sensory gating-related activity. Within the VOIs, we evaluated the responses evoked by RSVP of faces during memory-present/distraction-present trials vs. memory-absent/distraction-present trials. (Memory-absent/distraction-absent trials do not figure directly into the test of the sensory gating hypothesis, but were necessary in order to prevent anticipation of distraction on memory-absent trials.)
Acknowledgments
The author thanks Olufunsho Faseyitan and Craig Rypstat for programming assistance, and O.F. and Christopher Jordan for help with data collection and analysis. This research was supported by the National Institutes of Health, RO1 MH064498.
Footnotes
This may be an empirical example of the “active maintenance” proposed by Miller and Cohen (2001).
The raw value of this subject’s memory-absent/distraction-present effect in dlPFC was .51, and so dividing the memory-present/distraction-present by this value inflated it by a factor of almost 2, the opposite direction than that effected by the normalization procedure in all other subjects except one, whose raw memory-absent/distraction-present effect in dlPFC was .98.
The fact that delay-period activity in the dlPFC VOIs was numerically lower during memory-present/ distraction-present than during memory-present/distraction-absent trials is consistent with the idea that the sensory gating mechanism, once triggered by neurons that are tonically active during unfilled delay periods, is implemented by a broader PFC network that includes regions that are not typically active during unfilled delay periods. Although the data are not shown here, it is certainly the case that, in every subject, considerably more dlPFC voxels were active during the memory-present/distraction-present delay than during the memory-present/distraction-absent delay.
The HRF also varies in shape across brain regions within an individual participant (e.g., Miezen, Maccotta, Ollinger, Petersen, & Buckner, 2000). However, recent work indicates that the magnitude of variability in the shape of the HRF is greater across individuals within a region than it is across regions within an individual (Handwerker, Ollinger, & D’Esposito, 2004). Additionally, it indicates that employing a participant-specific HRF can improve the sensitivity and magnitude estimates of the least-squares solution of the GLM over comparable analyses that employ a generic HRF model (Handwerker et al., 2004).
The broader search space of IOTC was used instead of a more circumscribed area such as the fusiform face area because, as can be seen in Figure 2, there was a considerable amount of topological variability of memory-present/distraction-absent delay-period activity in the posterior ventral stream, and no one area was activated in all subjects.
References
- Aguirre GK, Zarahn E, D’Esposito M. The variability of human, BOLD hemodynamic responses. NeuroImage. 1998;8:360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Baddeley, A. D., & Hitch, G. J. (1974). Working Memory. In G. H. Bower (Ed.), The Psychology of Learning and Motivation (Vol. 8, pp. 47–89). New York: Academic Press.
- Bor D, Duncan J, Wiseman RJ, Owen AM. Encoding strategies dissociate prefrontal activity from working memory demand. Neuron. 2003;37:361– 367. doi: 10.1016/s0896-6273(02)01171-6. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. The Journal of Neuroscience. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver, T. S., Gray, J. R., & Burgess, G. C. (in press). Explaining the Many Varieties of Working Memory Variation: Dual Mechanisms of Cognitive Control. In A. Conway, C. Jarrold, M. Kane, A. Miyake & J. Towse (Eds.), Variation in Working Memory Oxford: Oxford University Press.
- Chao L, Knight R. Contribution of human prefrontal cortex to delay performance. Journal of Cognitive Neuroscience. 1998;10:167–177. doi: 10.1162/089892998562636. [DOI] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Human prefrontal lesions increase distractibility to irrelevant sensory inputs. NeuroReport. 1995;6:1605–1610. doi: 10.1097/00001756-199508000-00005. [DOI] [PubMed] [Google Scholar]
- Connolly, J. D., Goodale, M. A., Goltz, H. C., & Munoz, D. P. (in press). fMRI activation in the human frontal eye field is correlated with saccadic reaction time. Journal of Neurophysiology [DOI] [PubMed]
- Courtney SM. Attention and cognitive control as emergent properties of information representation in working memory. Cognitive, Affective, & Behavioral Neuroscience. 2004;4:501–516. doi: 10.3758/cabn.4.4.501. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby J. Object and spatial visual working memory activate separate neural systems in human cortex. Cerebral Cortex. 1996;6:39–49. doi: 10.1093/cercor/6.1.39. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Transient and sustained activity in a distributed neural system for human working memory. Nature. 1997;386:608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Rao VY, D’Esposito M. Maintenance of spatial and motor codes during oculomotor delayed response tasks. The Journal of Neuroscience. 2004;24:3944–3952. doi: 10.1523/JNEUROSCI.5640-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Ballard D, Zarahn E, Aguirre GK. The role of prefrontal cortex in sensory memory and motor preparation: an event-related fMRI study. NeuroImage. 2000;11:400–408. doi: 10.1006/nimg.2000.0571. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR. The dependence of span and delayed-response performance on prefrontal cortex. Neuropsychologia. 1999;37:1303–1315. doi: 10.1016/s0028-3932(99)00021-4. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain & Cognition. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Jonides J, Smith EE. The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related functional MRI. Proceedings of the National Academy of Sciences, USA. 1999;96:7514–7519. doi: 10.1073/pnas.96.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual attention. Science. 2001;291:1803–1806. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- Druzgal TJ, D’Esposito M. A neural network reflecting decisions about human faces. Neuron. 2001;32:947–955. doi: 10.1016/s0896-6273(01)00519-0. [DOI] [PubMed] [Google Scholar]
- Duncan, J., & Miller, E. K. (2002). Cognitive focus through adaptive neural coding in the primate prefrontal cortex. In D. Stuss & R. Knight (Eds.), Principles of Frontal Lobe Function (pp. 278–291). Oxford: Oxford University Press.
- Friston KJ, Holmes AP, Poline JB, Heather JD, Frackowiak RSJ. Analysis of fMRI time-series revisited. NeuroImage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Hasegawa I, Miyashita Y. Prefrontal neuronal activity encodes spatial target representations sequentially updated after nonspatial target-shift cues. Journal of Neurophysiology. 2004;91:1367–1380. doi: 10.1152/jn.00306.2003. [DOI] [PubMed] [Google Scholar]
- Fuster, J. M. (1995). Memory in the Cerebral Cortex Cambridge, MA: MIT Press.
- Gitelman DR. ILAB: a program for post experimental eye movement analysis. Behavioral Research Methods, Instruments, and Computers. 2002;34:605–612. doi: 10.3758/bf03195488. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic, P. S., & Leung, H.-C. (2002). Functional architecture of the dorsolateral prefrontal cortex in monkeys and humans. In D. T. Stuss & R. T. Knight (Eds.), Principles of Frontal Lobe Function (pp. 85–95). Oxford, U.K.: Oxford University Press.
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nature Neuroscience. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Handwerker DA, Ollinger JM, D’Esposito M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. NeuroImage. 2004;21:1639–1651. doi: 10.1016/j.neuroimage.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the Alpha Band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cerebral Cortex. 2002;12:877–882. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- Jonides J, Lacey SC, Nee DE. Processes of working memory in mind and brain. Current Directions in Psychological Science. 2005;14:2–5. [Google Scholar]
- Jonides J, Marshuetz C, Smith EE, Reuter-Lorenz PA, Koeppe RA. Age differences in behavior and PET activation reveal differences in interference resolution in verbal working memory. Journal of Cognitive Neuroscience. 2000;12:188–196. doi: 10.1162/089892900561823. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter-Lorenz PA. Inhibition of verbal working memory revealed by brain activation. Proceedings of the National Academy of Sciences. 1998;95:8410–8413. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs O, Turner R, Friston K. Event-related fMRI. Human Brain Mapping. 1997;5:243–248. doi: 10.1002/(SICI)1097-0193(1997)5:4<243::AID-HBM7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychonomic Bulletin & Review. 2003;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schwaiger J, Auinger P, Winker T. ‘Paradoxical’ alpha synchronization in a memory task. Cognitive Brain Research. 1999;7:493–501. doi: 10.1016/s0926-6410(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Knight RT, Staines WR, Swick D, Chao LL. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychologica. 1999;101:159–178. doi: 10.1016/s0001-6918(99)00004-9. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proceedings of the National Academy of Science USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedev MA, Messinger A, Kralik JD, Wise SP. Representation of attended versus remembered locations in prefrontal cortex. PloS Biology. 2004;2:1919–1935. doi: 10.1371/journal.pbio.0020365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmo RB. Interference factors in delayed response in monkey after removal of the frontal lobes. Journal of Neurophysiology. 1942;5:295–308. [Google Scholar]
- Miezen FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. NeuroImage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen J. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Milner, B. (1964). Some effects of frontal lobectomy in man. In J. M. Warren & K. Akert (Eds.), The Frontal Granular Cortex and Behavior (pp. 313–334). New York: McGraw-Hill.
- Monsell, S., & Driver, J. (Eds.). (2000). Control of Cognitive Processes: Attention and Performance XVIII Cambridge, MA: MIT Press.
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, et al. Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping using MRI. Proceedings of the National Academy of Science USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham D, Sakai K. The prefrontal cortex and working memory: physiology and brain imaging. Current Opinion in Neurobiology. 2004;14:163–168. doi: 10.1016/j.conb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Passingham, R. E., & Rowe, J. B. (2002). Dorsal prefrontal cortex: maintenance in memory or attentional selection? In D. T. Stuss & R. T. Knight (Eds.), Principles of Frontal Lobe Function (pp. 221–232). Oxford: Oxford University Press.
- Pasternak, T., & Greenlee, M. W. (2005). Working memory in primate sensory systems. Nature Reviews Neuroscience, 6 [DOI] [PubMed]
- Petrides, M. (1995). Functional organization of human frontal cortex for mnemonic processing: evidence from neuroimaging studies. In Structure and Functions of the Human Prefrontal Cortex (pp. 85–96). New York: Annals of the New York Academy of Sciences, vol. 769. [DOI] [PubMed]
- Petrides M. Dissociable roles of mid-dorsolateral prefrontal and anterior inferotemporal cortex in visual working memory. Journal of Neuroscience. 2000;20:7496–7503. doi: 10.1523/JNEUROSCI.20-19-07496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Poline JB, Crozier S, Lehericy S, Pillon B, et al. The role of dorsolateral prefrontal cortex in the preparation of forthcoming actions: an fMRI study. Cerebral Cortex. 2001;11:260–266. doi: 10.1093/cercor/11.3.260. [DOI] [PubMed] [Google Scholar]
- Postle BR, Awh E, Jonides J, Smith EE, D’Esposito M. The where and how of attention-based rehearsal in spatial working memory. Cognitive Brain Research. 2004;20:194–205. doi: 10.1016/j.cogbrainres.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Postle BR, Berger JS, D’Esposito M. Functional neuroanatomical double dissociation of mnemonic and executive control processes contributing to working memory performance. Proceedings of the National Academy of Sciences (USA) 1999;96:12959–12964. doi: 10.1073/pnas.96.22.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Berger JS, Goldstein JH, Curtis CE, D’Esposito M. Behavioral and neurophysiological correlates of episodic coding, proactive interference, and list length effects in a running span verbal working memory task. Cognitive, Affective, and Behavioral Neuroscience. 2001;1:10–21. doi: 10.3758/cabn.1.1.10. [DOI] [PubMed] [Google Scholar]
- Postle BR, Brush LB. The neural bases of the effects of item-nonspecific proactive interference in working memory. Cognitive, Affective, & Behavioral Neuroscience. 2004;4:379–392. doi: 10.3758/cabn.4.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Brush LB, Nick AM. Prefrontal cortex and the mediation of proactive interference in working memory. Cognitive, Affective, & Behavioral Neuroscience. 2004;4:600–608. doi: 10.3758/cabn.4.4.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, D’Esposito M. “What” - then – “where” in visual working memory: an event-related fMRI study”. Journal of Cognitive Neuroscience. 1999;11:585–597. doi: 10.1162/089892999563652. [DOI] [PubMed] [Google Scholar]
- Postle BR, D’Esposito M. Spatial working memory activity of the caudate nucleus is sensitive to frame of reference. Cognitive, Affective, and Behavioral Neuroscience. 2003;3:133–144. doi: 10.3758/cabn.3.2.133. [DOI] [PubMed] [Google Scholar]
- Postle BR, Druzgal TJ, D’Esposito M. Seeking the neural substrates of working memory storage. Cortex. 2003;39:927–946. doi: 10.1016/s0010-9452(08)70871-2. [DOI] [PubMed] [Google Scholar]
- Postle BR, Zarahn E, D’Esposito M. Using event-related fMRI to assess delay-period activity during performance of spatial and nonspatial working memory tasks. Brain Research Protocols. 2000;5:57–66. doi: 10.1016/s1385-299x(99)00053-7. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D’Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31:865–873. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Ranganath C, DeGutis J, D’Esposito M. Category-specific modulation of inferior temporal activity during working memory encoding and maintenance. Cognitive Brain Research. 2004;20:37–45. doi: 10.1016/j.cogbrainres.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Stephan KE, Friston KJ, Frackowiak RSJ, Passingham RE. The prefrontal cortex shows context-specific changes in effective connectivity to motor or visual cortex during the selection of action or colour. Cerebral Cortex. 2005;15:85–95. doi: 10.1093/cercor/bhh111. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RSJ, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Ruchkin DS, Grafman J, Cameron K, Berndt RS. Working memory retention systems: a state of activated long-term memory. Behavioral and Brain Sciences. 2003;26:709–777. doi: 10.1017/s0140525x03000165. [DOI] [PubMed] [Google Scholar]
- Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nature Neuroscience. 2002;5:479–484. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- Stuss, D. T., & Knight, R. T. (Eds.). (2002). Principles of Frontal Lobe Function New York: Oxford University Press.
- Takeda K, Funahashi S. Prefrontal task-related activity representing visual cue location or saccade direction in spatial working memory tasks. Journal of Neurophysiology. 2002;87:567–588. doi: 10.1152/jn.00249.2001. [DOI] [PubMed] [Google Scholar]
- Takeda K, Funahashi S. Population vector analysis of primate prefrontal activity during spatial working memory. Cerebral Cortex. 2004;14:1328–1339. doi: 10.1093/cercor/bhh093. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill S, Jonides J, Marshuetz C, Smith EE, D’Esposito M, Kan IP, et al. Effects of frontal lobe damage on interference effects in working memory. Cognitive, Affective, and Behavioral Neuroscience. 2002;2:109–120. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Reward expectancy in primate prefrontal neurons. Nature. 1996;382:629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- Watanabe, M. (2002). Integration across multiple cognitive and motivational domains in monkey prefrontal cortex. In D. T. Stuss & R. Knight (Eds.), Principles of Frontal Lobe Function (pp. 326–337). Oxford: Oxford University Press.
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. NeuroImage. 1995;2:173–182. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre GK, D’Esposito M. A trial-based experimental design for fMRI. NeuroImage. 1997;6:122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]


