Abstract
The purpose of this study was to examine the dynamic stability of two groups of children with different dynamic resources in changing contexts. The stability of the lower extremity segments of preadolescent children (8–10 years old) with and without Down syndrome (DS) was evaluated as children walked on a motorized treadmill at varying speeds. Tools from nonlinear dynamics, maximum Lyapunov exponent, and approximate entropy were used to assess the behavioral stability of segmental angular displacements of the thigh, shank, and foot. Our results suggest that children with DS show decreased dynamic stability during walking in all segments and that this might be a consequence of inherently different subsystem constraints between these groups. Differences between groups also varied, though not uniformly, with speed, suggesting that inherent differences could further constrain the behavioral response to changing task demands.
Keywords: nonlinear dynamics, regularity, children, Down syndrome, gait
One of the perennial puzzles faced by behavioral scientists is how to explain the fluctuations observed in human behavioral patterns, even in well-practiced tasks. Previously, scientists described fluctuations as error, or noise, in a system. However, dynamic-systems theory has provided an alternative explanation proposing that fluctuations might be a consequence of the dynamic self-organization of complex systems. According to this approach, instability is inherently present and increases during behavioral transitions that occur as a system moves away from its preferred state. Recent attempts to understand the sources of instability have proposed that some fluctuations could actually be beneficial to a system, providing it with a flexibility to adapt to changing constraints (Buzzi, Stergiou, Kurz, Hageman, & Heidel, 2003; Mitra, Riley, & Turvey, 1997; Pool, 1989; Riley & Turvey, 2002). As such, quantifying the amount of instability present in a system as the behavior unfolds might provide useful information about the integrity of that system. In addition, differences in stability among populations, or over time, could provide insight into differences in the organizational properties of a system.
Past attempts to quantify and examine movement fluctuations focused on the use of traditional statistical methods (i.e., standard deviation, coefficient of variation). Although these measures provide some useful information with regard to variability, they are limited in their ability to quantify and provide only a discrete measure to represent all points. In addition, in cyclic movements these measures treat each individual cycle independently, ignoring initial conditions or previous states. Recently, scientists have begun to use tools from nonlinear dynamics to assess the point-to-point fluctuations continuously in movement trajectories throughout the cycle (Buzzi et al., 2003; Dingwell, Cusumano, Cavanagh, & Sternad, 2001; Mitra, Amazeen, & Turvey, 1998). Such measures provide insight into how behavior unfolds, taking into account previous states of a system (e.g., cycle trajectory in its work space). These tools have been used to assess a variety of motor tasks in a number of different populations, ranging from adult neuropathic walking to independent sitting in infants.
Dingwell and colleagues have published a number of articles that illustrate the use of nonlinear tools to reveal differences in the stability patterns of neuropathic and normal human gait during over-ground and treadmill walking (Dingwell et al., 2001; Dingwell & Cusumano, 2000; Dingwell, Cusumano, Sternad, & Cavanagh, 2000). In one of their first studies, Dingwell et al. (2000) used nonlinear measures to compare local dynamic stability, which is the sensitivity of the system to infinitesimal perturbations, of the kinematics of gait patterns between adults suffering from diabetic neuropathy and healthy adults with no neurological impairment. They found that adults with diabetic neuropathy demonstrate greater local dynamic stability than those without impairment. They hypothesized that the increased local stability could be a result of their strategy to decrease walking speed to compensate for the sensory loss associated with their neuropathy. Dingwell and colleagues added that attempts to characterize the stability of a system using traditional measures of variability are inadequate because they equate stability, the continuous behavior of a system in the presence of local infinitesimal perturbations, with variability, the average difference among cycles.
In a more recent study, Dingwell et al. (2001) examined changes in local dynamic stability associated with over-ground and treadmill walking in healthy adults. Local dynamic stability assessed via nonlinear measures and discrete-point variability (standard deviation of mean knee-joint displacement and maximum knee extension) were calculated and compared for both modes of walking. Their results showed a poor correlation between nonlinear and traditional measures for both over-ground and treadmill conditions, suggesting that nonlinear measures quantified different aspects of the behavior than traditional measures did. They argued that traditional discrete measures of variability do not provide an accurate means for assessing stability of the locomotor system, because they essentially masked the stride-to-stride fluctuations, and that nonlinear measures could, in fact, be more appropriate for addressing questions related to control of locomotor stability and balance.
Harbourne and Stergiou (2003) recently examined the dynamic stability of the developing infant’s postural control system using tools from nonlinear dynamics. By examining the center-of-pressure tracings of infants with typical development (TD) at three different skill stages, they found that with practice and experience the developing neuromotor system became more stable, as evidenced by decreased divergence and increased regularity of center-of-pressure trajectories. In addition, they found that the underlying structure of the postural control system of infants with TD might be deterministic in nature, relying on previous states of the system for subsequent control strategies. They concluded that the development of postural control is a dynamic and deterministic process, with fluctuations in stability as infants select a successful control strategy for independent and efficient sitting. This series of studies and others provide evidence that nonlinear tools might be valid and useful tools to assess the dynamic processes that underlie human behavior and could provide insights to underlying control mechanisms that would not be evident using traditional discrete measures of variability. In this study we chose to examine the gait patterns of a population traditionally viewed as being highly unstable, yet poorly understood: preadolescent children with Down syndrome (DS).
Individuals with DS have a number of cognitive and anatomical deficiencies associated with their syndrome. These include decreased cognitive abilities, delayed cognitive and motor development, increased visual abnormalities, increased joint laxity, decreased muscle tone, decreased kinesthetic perception, and deficits in “coincidence timing” (Block, 1991). In addition, individuals with DS have been anecdotally described as having qualitative differences in their gait patterns, often described as having a “waddling” walk. Nonetheless, their sagittal-plane kinematics strongly resemble those of an individual with typical development (Ulrich, Haehl, Buzzi, Kubo, & Holt, submitted for publication). Individuals with DS have also been characterized by an increase in variability of outcome measures of performance across multiple measures of a movement task (Block & Clark, 1988; Parker & Bronks, 1982; Parker, Bronks, & Snyder, 1986; Selby-Silverstein, Hillstrom, Palisano, Hice, & Kugler, 1992).
Therefore, the purposes of this study were
To use the nonlinear measure maximum Lyapunov exponent to examine changes in dynamic stability of gait patterns in two groups of children with distinct dynamic resources: preadolescent children with and without DS.
To use the nonlinear approximate entropy to examine changes in complexity of gait patterns in preadolescent children with and without DS.
To examine how changes in context (treadmill speed) affect dynamic stability and complexity.
Methods
Participants
Eight children with DS and 8 age-matched children with TD between 8 and 10 years old (mean age = 8.75 ± 0.86 years) participated in the study. This age was chosen because it represents an age at which these children have had significant practice (6–9 years) on this complex task and at which growth (increases in segment length and mass) has been fairly steady for several years. Participants were recruited via parent support groups, local school systems, and ads placed on a campus e-mail list server. The institutional review board at the University of Michigan approved all procedures. Participants and their parents both signed informed-consent forms as required by the board. Participants received a small amount of money and a gift for their participation.
Procedures
Participants came to our lab for testing on three separate occasions. Visits were spaced approximately 1 week apart and lasted for approximately 1.5 hrs each. Day 1 consisted of a practice session in which we explained all procedures and children practiced all tasks. A practice session was necessary to enable the children with DS to be comfortable with the research team and protocol, thereby ensuring that we collected the best data possible.
On Day 2, unperturbed walking data were collected. On arriving at the lab, children changed into bathing suits that they brought with them (girls) or bathing suits that were provided (boys). The skin surface of the sites requiring markers were marked with a hypoallergenic eyebrow pencil, and passive reflective markers (2.54 cm) were placed bilaterally at the temporomandibular joints, shoulders, elbows, greater trochanters, lateral femoral condyles, lateral malleoli, heels, and third metacarpophalangeal joints. After a minimum of five warm-up trials, a minimum of five test trials were collected as participants walked over ground at their comfortable speed across a 4.58-m GAITRite mat (CIR Systems, Inc., Clifton, NJ) placed in the middle of a 11.5-m walkway. From the test trials we used GAITRite software to calculate average velocity across trials, which we subsequently used to set the treadmill speed to match individual performance during the next phase of testing.
Children were then provided time to warm up on the treadmill (Parker brand) under four progressive conditions: holding onto the support bar in front of them, at speeds slower than their preferred speed, at preferred speed, and at their preferred speed without holding on. We then collected five 30-s trials at their preferred speed. Preferred speed on the treadmill was operationalized as 75% of the over-ground preferred speed based on our pilot work and the work of others (Jeng, Holt, Fetters, & Certo, 1996). Preferred speeds on a treadmill are slower than over ground, and it is very difficult for children with DS to reliably report their preferred speed, because of cognitive limitations. Our pilot work confirmed that children with DS were consistent in their self-selected preferred speed over ground across multiple visits to the laboratory. Subsequent to the walking trials, anthropometric data for each child were collected (height, weight, and segment lengths). Our data were aligned with published data for children with DS, suggesting that we had a reasonable sample of the population. Children with DS had shorter arms, thighs, shanks, and feet and were significantly shorter in height than children with TD (Table 1).
Table 1.
Mean Anthropometric Data for Children With DS and TD
| DS group, M (SD) | TD group, M (SD) | |
|---|---|---|
| Head/neck length (cm) | 25.06 (4.55) | 28.43 (4.07) |
| Trunk length (cm) | 41.08 (1.90) | 40.41 (3.70) |
| Arm length (cm)* | 20.61 (1.94) | 22.80(1.88) |
| Forearm length (cm) | 18.13 (2.13) | 19.86(1.56) |
| Thigh length (cm)* | 26.58 (4.24) | 31.90(3.36) |
| Shank length (cm)* | 26.76 (3.20) | 29.46 (1.42) |
| Foot length (cm)* | 19.14(1.88) | 21.70(1.16) |
| Height (cm)* | 123.51 (9.48) | 133.68 (7.05) |
| Weight (kg) | 29.92 (7.83) | 30.73 (4.23) |
Note. DS = Down syndrome; TD = typical development.
p < .05
Day 3 included collection of unperturbed- and perturbed-walking data. On this day we repeated the over-ground walking trials as collected in Day 2. Next, children warmed up on the treadmill then were tested during trials in which we challenged their walking by adjusting the belt speed to five settings, two slower than preferred, preferred, and two faster than preferred (40%, 57%, 75%, 92%, and 110% of overground speed). Order of presentation was from slowest to fastest. Children with DS are not completely comfortable with the fastest speeds, so speeds could not be randomized without exceeding their level of tolerance. For this study, only the 40%, 75%, and 110% speeds were used for analyses because these represent the extremes around the preferred speed and were therefore adequate for addressing the research question.
Data Analyses
Kinematic data were collected for all over-ground and treadmill trials using a six-camera (60 Hz) real-time system (Peak Performance Technologies, Centennial, CO), which provided marker position data. In addition, a single video camera provided behavioral data of the sessions. Behavioral data were used to determine times on the treadmill during which the children walked as naturally as possible. When children interrupted their natural gait by raising an arm (to scratch their nose or fix then-glasses) or turning toward the video camera (which occurred more frequently in children with DS than in those with TD), we eliminated these trials, or segments of trials, from further analysis. Periods of usable data were determined and yielded an average of 1,656 and 1,796 data points for further analysis for the group with DS and the control group, respectively. This number is considered adequate for the nonlinear analyses employed in this study (Grassberger & Procaccia, 1983; Pincus, 1995; Wolf, Swift, Swinney, & Vastano, 1985). Subsequently, sagittal-plane segmental angles of the thigh, shank, and foot as children walked on the treadmill were calculated using Peak Motus software. The continuous data, or time series, for the segmental angular data for the thigh, shank, and foot collected on Day 3 were then analyzed using nonlinear techniques. We used only unfiltered data in our analyses to obtain a more accurate representation of the instability inherent hi the system (Dingwell et al., 2000; Harbourne & Stergiou, 2003). The maximum Lyapunov exponent (LyE) and approximate entropy (ApEn) were calculated for each segmental angular time series and then averaged across groups and speeds.
The LyE is a measure of the stability of a dynamical system and its dependence on initial conditions. A dynamical system, such as the human during walking, is highly dependent on the initial conditions, or constraints, that underlie its function (Longstaff & Heath, 1999). The LyE measures the rate of exponential divergence of trajectories in state space. As nearby points separate, they diverge rapidly and produce instability. The LyE estimates this instability, which is largely affected by the initial conditions of the system. Periodic systems will result in LyEs that are negative or zero, whereas nonperiodic or random systems will result in a positive LyE value. Systems that are more dynamically stable will result in lower LyE values, whereas systems that are less dynamically stable will result in higher LyE values.
In this study, the maximum LyE was calculated using a time-series-analysis program written by Sprott and Rowlands (1992). Accurate calculations of these measures require determination of the number of embedded dimensions. The embedded dimension represents the number of dimensions needed to unfold the structure of a dynamical system. A dynamical system can be extracted from a single time series, and the topological structure of the system can be created (Mitra, Amazeen, & Turvey, 1998). Accuracy in identifying the number of embedded dimensions is necessary to ensure that the dynamical properties of the system remain unchanged when deriving multidimensional dynamic information from a unidimensional time series (Dingwell, Cusumano, Sternad, & Cavanagh, 1998). We identified five embedded dimensions for our data set. The number of embedded dimensions was calculated using a global-false-nearest-neighbor analysis (Abarbanel, 1996), which describes the minimum number of variables required to form a valid state space from a given time series. Previous work using these nonlinear tools with lower extremity gait data has also determined that five embedded dimensions are the minimum required to reconstruct a valid state space (Buzzi et al., 2003; Dingwell et al., 2000).
The ApEn of each time series was also calculated to assess the complexity of each time series. Complexity is a measure of the irregularity or unpredictability of a system. The more complex a system, the less regular it is (Pincus & Goldberger, 1994; Ryan, Goldberger, Pincus, Mietus, & Lipsitz, 1994). ApEn is a “regularity statistic” that quantifies the predictability of fluctuations in a time series (Pincus, 1991). ApEn measures the logarithmic probability that a set of data points of m length will remain r distance apart on subsequent comparisons of a data set of N length (Ryan et al., 1994). The lower the ApEn value, the greater the likelihood of the points remaining the same distance apart and, consequently, the lower the complexity of the system. Higher ApEn values coincide with a less regular, more complex system. ApEn values typically range between 0 and 2 (Vaillancourt & Newell, 2000), with values closer to 2 indicating greater complexity and those closer to 0 indicating more predictability. We computed ApEn following the procedure outlined by Pincus and Goldberger (1994) using programs written hi Matlab software (The Mathworks, Natick, MA). Two and 20% of the standard deviation of the respective time series were used for m and r, respectively. These values have been previously reported in the movement literature as providing good statistical validity for the measurement of ApEn (Vaillancourt & Newell, 2000). For N, all time series were reduced to 1,000 data points because this represented the shortest available time series and, thus, became the limiting factor. Mean ApEn values were calculated for each group.
Statistical Analysis
Statistical analysis was performed using SPSS® statistical software (SPSS, Inc., Chicago). Differences between groups, segments, and speeds for the LyE and ApEn were tested using a 2 × 3 × 3 (Group × Segment × Speed) analysis of variance (ANOVA) with repeated measures (speed). Anthropometric data were tested for group differences using one-way ANOVAs.
Results
LyE
Analysis of the LyE resulted in statistical significance for all three main effects: group F(1, 42) = 60.96, p ≤ .001; segment F(2,42) = 10.01, p ≤ .001; speed F(2, 84) = 9.68, p ≤ .001. Only one interaction effect reached significance, Speed × Segment, F(4,84) = 6.07, p ≤ .001. Figures 1(a)–1(c) illustrate that LyEs were higher for children with DS than for those with TD across all three segments and speeds. The interaction (Figure 1[d]), however, shows that the tendency was for LyE values to decrease as speed increased for both groups, with the exception that for the thigh, children with DS showed an increase, rather than decrease, and greater divergence from children with TD than they did at the slower speeds.
Figure 1.
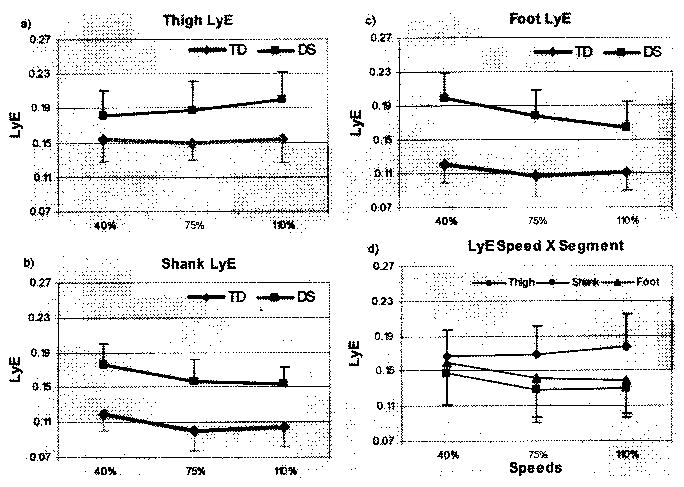
Group means and standard deviations of Lyapunov exponent (LyE) of (a) thigh, (b) shank, and (c) foot for children with Down syndrome (DS) and typical development (TD) across speeds, (d) Speed × Segment interaction.
ApEn
ApEn values differed significantly for all three main effects: group F(1, 42) = 82.59, p ≤.001; segment F(2,42) = 54.94, p ≤ .001; speed F(2,84) = 95.13,p ≤ .001. In addition, the Group × Segment two-way interaction was statistically significant, F(2,42) = 12.25, p ≤ .001, as was the three-way Speed × Group × Segment interaction, F(4,84) = 6.99, p ≤ .001. The three-way interaction is caused primarily by large differences in the thigh and foot between groups. Figure 2 shows that both groups and all segments tended to decrease ApEn as speed increased. Children with DS, however, demonstrated higher values than those with TD for the thigh and foot segments in particular, and the difference between groups increased as speed increased for the thigh and decreased as speed increased for the foot. Conversely, mean values for these two groups were more similar at the shank.
Figure 2.
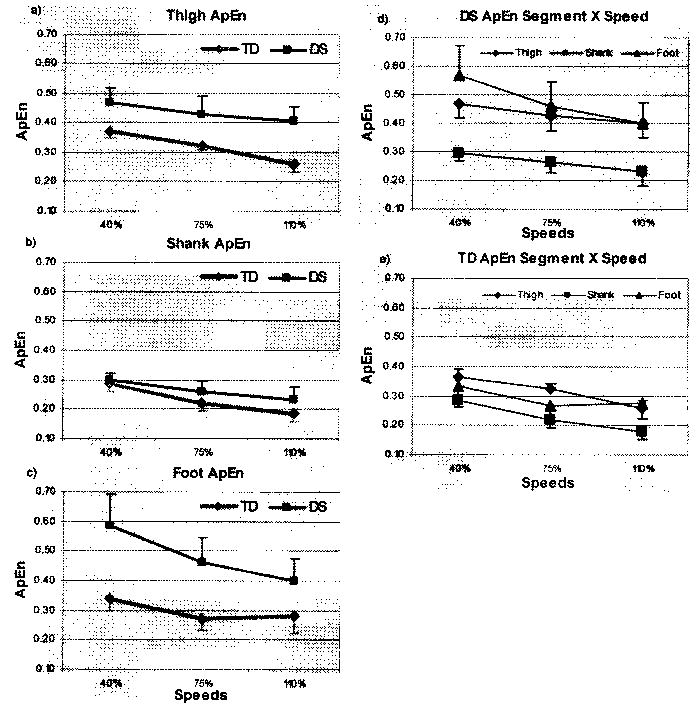
Group means and standard deviations of approximate entropy (ApEn) of (a) thigh, (b) shank, and (c) foot for children with Down syndrome (DS) and typical development (TD) across speeds. Segment × Speed interaction for (d) children with DS and (e) children with TD.
Because people with DS walk more slowly than those with TD we believe it is important to present stability data as a function of speed. One might propose, for example, that decreased stability of behavior is caused simply by walking more slowly. Figure 3 shows the LyE and ApEn data plotted for individual participants as a function of treadmill speed. These data show that with the exception of ApEn for the shank, at all speeds, values for children with DS were higher (i.e., less stable) than for those with TD, including at comparable speeds.
Figure 3.
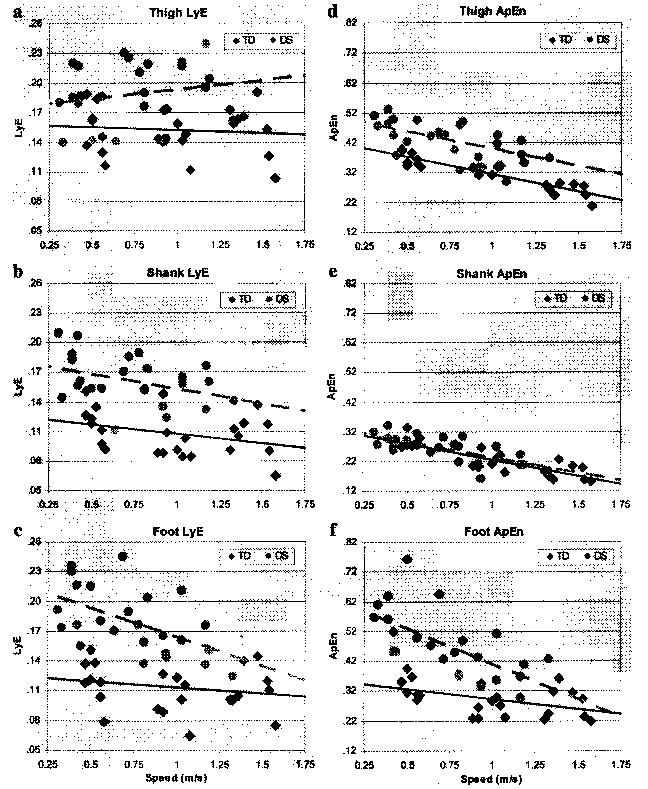
Scatterplots of (left column) Lyapunov exponent (LyE) and (right column) approximate entropy (ApEn) for all values for all segments for children with Down syndrome (DS) and typical development (TD) at each treadmill walking speed. Dashed (—) and solid lines (—) represent linearly regressed line of best fit for children with DS and TD, respectively (y = mx + b).
Discussion
The results suggest that across several aspects of their behavior, children with DS display significantly greater dynamic instability than children with TD. Previous research has documented increased variability in this population, based on traditional measures of means and standard deviations. Our results showed that underlying these variability markers is a system whose movement patterns unfold, as well, over time in a less stable manner. We used the dynamic tools of LyE and ApEn to examine this possibility, and across both variables we found significant group differences and, further, that these stability characteristics were context (speed) and body-segment sensitive.
Overall, LyE of the group with DS was significantly higher for all segments and across all speeds. This indicates that the rate at which the trajectories of cyclic patterns diverged from cycle-to-cycle during walking was greater for children with DS than for those with TD. ApEn values were also generally higher for the children with DS, particularly at the thigh and foot. This indicates that fluctuations within each cycle were more unpredictable than for the children with TD. When analyzed in this way, we think that the data suggest that even the most stable attractor for the children with DS possesses greater instability from moment to moment than that of children with TD. In the population with DS this increased instability might result from their inherently different neuromuscular and joint systems. The behavior of the limb segments self-organizes and evolves within and between cycles based on the constraints on the system. Previous research has demonstrated that children with DS have different physical and physiological constraints on their system that might predispose them to inherent lower extremity instability. These constraints include increased joint laxity, particularly at the hip; decreased strength; relatively small feet; and hypotonia (Block, 1991; Ulrich et al., submitted for publication). These constraints, although affording effective locomotion, could result in a behavioral attractor that is inherently prone to wander. The increased divergence in the segmental trajectories of this group indicates that from one gait cycle to the next, the segment’s behavior changes continuously as a result of these fluctuations in relevant constraints, marking the increased instability of the group. In addition, the increased ApEn values for the group with DS indicate a greater irregularity in the behavior of their lower extremity segments. Increased irregularity, signifying less predictability of the fluctuations in behavior, has been argued to reflect a reduction in the adaptability of a system (Riley & Turvey, 2002). Future studies need to test directly the rate at which children with DS adapt to point perturbations during walking to support this argument.
Greater unpredictability in a system’s fluctuations might be an indicator of reduced adaptability, but it is difficult to ascertain how much regularity is actually desirable, or beneficial, to the system. At which point do fluctuations become detrimental, reducing overall control? To determine whether these instabilities are problematic, it is necessary to perturb the system and observe its response. In this study, we did not provide a point perturbation but we did manipulate the contexts that we think affect or increase the challenge to the motor system. We required children to walk at their self-selected comfortable speed plus a much slower (40% of self-selected) and faster (110%) speed. At comparable relative speeds, the group with DS showed a greater change in stability characteristics with an increase in speed. That is, they exhibited a change (increase for some segments, decrease for others) of greater magnitude than that of the children with TD (e.g., see Figures 1[c] and 2[c]). Specifically, at the shank and, especially, the foot, the rate of cycle-to-cycle trajectory change (LyE) decreased and the complexity of fluctuations decreased (ApEn) with an increase in speed. This suggests that being encouraged to walk faster than preferred might have pushed the system into more consistent behavior at these levels. At the thigh, however, the increase could have posed a greater challenge to their particularly lax hips, serving instead to further reduce the unstable behavior of that segment.
In general, the stability curves for the children with TD tended to show a slightly parabolic shape across speeds, consistent with data from previous studies of gait in which variability is lowest at self-selected speeds, attributed to optimization of energy efficiency (Deidrich & Warren, 1995; Hoyt & Taylor, 1981). In contrast, a linearly increasing or decreasing slope was evident in the children with DS. That is, the children with DS tended to show increased instability when pushed to slow down their gait but to improve stability when forced to walk faster than preferred. This might be a result of, as Latash and Anson (1996) argued, the unique constraints of individuals with disabilities that could require optimization on one or more different variables than for those with typical physical and cognitive constraints. Speed faster than self-selected might be, mechanically, more stable by these measures, but individuals with disabilities could perceive their capacity to control their movements in the real world to be reduced at this faster pace. Thus, their optimality criteria might take into account cognitive and experiential concerns in addition to the biomechanical efficiency of the system. In future studies, we plan to test stability of the system more directly by perturbing the system during walking. This will allow us to use these tools to look at local dynamic stability as a function of adaptability to point perturbations to the system. In this study we have established a baseline of stability measures when the context itself is highly predictable and quite steady.
In addition to the speed effects on all stability measures, segmental effects were evident in both groups. This was particularly true for the ApEn measure, with lower values in the shank than in the thigh and foot. This suggests that the shank could be less complex (more predictable) than the other lower extremity segments and might be explained by the location of the shank as the anatomical link between the thigh and the foot in the lower extremity segmental chain. Because of its location, it remains more constant, whereas the proximal (thigh) and distal (foot) segments are more free to vary.
The results of this study demonstrate significant differences in the local dynamic stability of walking patterns of children with DS and TD as a function of speed and system constraints. Nonetheless, this study focused on the measurement of dynamic stability of peripheral-joint kinematics and thus only describes changes in the behavior of the periphery with changing system and context constraints. In future studies we plan to compare the stability of segmental components of the system or endpoint effector (foot) with variables representing the global behavior of the system, such as the center of mass. Efforts to compare the relationship between global and segmental measures will enhance our understanding of how the effects of local stability variations affect global behavioral patterns. Although our study provides evidence that changing constraints do, in fact, affect the stability of behavioral patterns, further evidence is needed to tease apart potential mechanisms responsible for maintaining stability in the face of changing constraints. Although we think that the group differences in stability derive primarily from the increased joint laxity and decreased tone of the children with DS, we acknowledge that without further analysis we cannot eliminate the possibility that anthropometric differences (see Table 1) could have influenced these measures.
In conclusion, the children with DS generally showed greater behavioral instability than did the children with TD across segments and speeds during walking. It is likely that, based on the constraints of their system resulting from their syndrome, the children with DS possess an inherently more unstable biomechanical system. That instability manifests in the behavioral trajectories of the lower extremity segments during walking. Nonetheless, stability increased as speed increased, even beyond their self-selected speed, suggesting a stabilizing role on the legs within the range of speeds and in this context.
Acknowledgments
We thank the children and families who participated in this study; the Families Exploring Down Syndrome (FEDS) group of Detroit, and the Down Syndrome Association of Greater Toledo for their support. We also thank the anonymous reviewers for their helpful suggestions, David Vaillancourt for sharing his approximate-entropy program with us, and Nicholas Stergiou for his helpful comments. This research was supported by the National Institutes of Health (NIH) via a grant awarded to Beverly Ulrich (R01-HD42728-01).
References
- Abarbanel, H.D.I. (1996). Analysis of observed chaotic data. New York: Springer-Verlag.
- Block ME. Motor development in children with Down syndrome: A review of the literature. Adapted Physical Activity Quarterly. 1991;8:179–209. [Google Scholar]
- Block, M.E., & Clark, J.E. (1988). Interlimb coordination in the walking gait of children with Down syndrome. Abstracts of the Annual Meeting of the North American Society for the Psychology of Sport and Physical Activity (p. 38).
- Buzzi UH, Stergiou N, Kurz MJ, Hageman PA, Heidel J. Nonlinear dynamics indicates aging affects variability during gait. Clinical Biomechanics. 2003;18:435–443. doi: 10.1016/s0268-0033(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Deidrich FJ, Warren WH., Jr Why change gaits? Dynamics of the walk-run transition. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:183–202. doi: 10.1037//0096-1523.21.1.183. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Cusumano JP. Nonlinear time series analysis of normal and pathological human walking. Chaos. 2000;10:848–863. doi: 10.1063/1.1324008. [DOI] [PubMed] [Google Scholar]
- Dingwell, J.B., Cusumano, J.P., Stemad, D., & Cavanagh, PR. (1998). Using Lyapunov exponents to quantify dynamic stability during continuous overground locomotion. Proceedings of the Third North American Congress on Biomechanics (pp. 125–126).
- Dingwell JB, Cusumano JP, Cavanagh PR, Sternad D. Local dynamic stability versus kinematic variability of continuous overground and treadmill walking. Journal of Biomechanical Engineering. 2001;123:27–32. doi: 10.1115/1.1336798. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Cusumano JP, Sternad D, Cavanagh PR. Slower speeds in patients with diabetic neuropathy lead to improved local dynamic stability of continuous overground walking. Journal of Biomechanics. 2000;33:1269–1277. doi: 10.1016/s0021-9290(00)00092-0. [DOI] [PubMed] [Google Scholar]
- Grassberger P, Procaccia I. Measuring the strangeness of strange attractors. Physica D. 1983;9:189–208. [Google Scholar]
- Harbourne RT, Stergiou N. Nonlinear analysis of the development of sitting postural control. Developmental Pyschobiology. 2003;42:368–377. doi: 10.1002/dev.10110. [DOI] [PubMed] [Google Scholar]
- Hoyt DF, Taylor CR. Gait and energetics of locomotion in horses. Nature. 1981;292:239–240. [Google Scholar]
- Jeng SF, Holt KG, Fetters L, Certo C. Self-optimization of walking in non-disabled children and children with spastic hemiplegic cerebral palsy. Journal of Motor Behavior. 1996;28:15–27. doi: 10.1080/00222895.1996.9941729. [DOI] [PubMed] [Google Scholar]
- Latash ML, Anson JG. What are the “normal movements” in atypical populations? Behavioral and Brain Sciences. 1996;19:55–68. [Google Scholar]
- Longstaff MG, Heath RA. A non-linear analysis of the temporal characteristics of handwriting. Human Movement Science. 1999;18:485–524. [Google Scholar]
- Mitra S, Amazeen PG, Turvey MT. Intermediate motor learning as decreasing active (dynamical) degrees of freedom. Human Movement Science. 1998;17:17–65. [Google Scholar]
- Mitra S, Riley MA, Turvey MT. Chaos in human rhythmic movement. Journal of Motor Behavior. 1997;29:195–198. doi: 10.1080/00222899709600834. [DOI] [PubMed] [Google Scholar]
- Parker AW, Bronks R. Gait of children with Down syndrome. Archives of Physical Medicine. 1982;61:345–351. [PubMed] [Google Scholar]
- Parker AW, Bronks R, Snyder CW. Walking patterns in Down’s syndrome. Journal of Mental Deficiency Research. 1986;30:317–330. doi: 10.1111/j.1365-2788.1986.tb01327.x. [DOI] [PubMed] [Google Scholar]
- Pincus S. Approximate entropy as a measure of system complexity. Proceedings of the National Academy of Science USA. 1991;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus S. Approximate entropy (ApEn) as a complexity measure. Chaos. 1995;5:110–117. doi: 10.1063/1.166092. [DOI] [PubMed] [Google Scholar]
- Pincus SM, Goldberger AL. Physiological time-series analysis: What does regularity quantify? American Journal of Physiology. 1994;266 (Heart Circulatory Physiology 35):H1643–H1656. doi: 10.1152/ajpheart.1994.266.4.H1643. [DOI] [PubMed] [Google Scholar]
- Pool R. Is it healthy to be chaotic? Science. 1989;243:604–607. doi: 10.1126/science.2916117. [DOI] [PubMed] [Google Scholar]
- Riley MA, Turvey MT. Variability and determinism in motor behavior. Journal of Motor Behavior. 2002;34:99–125. doi: 10.1080/00222890209601934. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Golberger AL, Pincus SM, Mietus J, Lipsitz LA. Gender- and age-related differences in heart rate dynamics: Are women more complex than men? Journal of the American College of Cardiology. 1994;24:1700–1707. doi: 10.1016/0735-1097(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Selby-Silverstein, L., Hillstrom, H., Palisano, R., Hice, G., & Kugler, F. (1992). Aberrant gait of children with Down syndrome and the efficacy of treatment with foot orthoses. In M. Woollacott & F. Horak (Eds.), Posture and gait: Control mechanisms 1992, Vol. 2. Xlth International Symposium of the Society for Postural and Gait Research (pp. 427–430).Portland: University of Oregon Press.
- Sprott, J.C., & Rowlands, G. (1992). Chaos data analyzer. New York: American Institute of Physics.
- Ulrich, B.D., Haehl, V., Buzzi, U.K., Kubo, M., & Holt, K.G. Modeling control strategies in populations with unique dynamic resources: preadolescents with and without Down syndrome (submitted for publication). [DOI] [PubMed]
- Vaillancourt DE, Newell KM. The dynamics of resting postural tremor in Parkinson’s disease. Clinical Neurophysiology. 2000;111:2046–2056. doi: 10.1016/s1388-2457(00)00467-3. [DOI] [PubMed] [Google Scholar]
- Wolf A, Swift JB, Swinney HL, Vastano JA. Determining Lyapunov exponents from a time series. Physica D. 1985;16:285–317. [Google Scholar]


