Abstract
The effects of shock as a result of Escherichia coli endotoxin on certain hemodynamic and biochemical parameters and mortality were studied in the baboon. The ability of methylprednisolone succinate (MPS) in massive doses and/or rapid infusion of a buffered electrolyte solution to influence the results was then examined. Shock secondary to Escherichia coli endotoxin was associated with a decrease in RBC 2,3-DPG. This fall in RBC 2,3-DPG was not significantly influenced by MPS. Likewise, MPS did not prevent the changes in oxygen metabolism or systemic hemodynamics, nor was it associated with any decrease in mortality associated with endotoxic shock. Buffered electrolyte solution therapy improved both hemodynamics and mortality, but did not significantly protect against changes in RBC 2,3-DPG.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
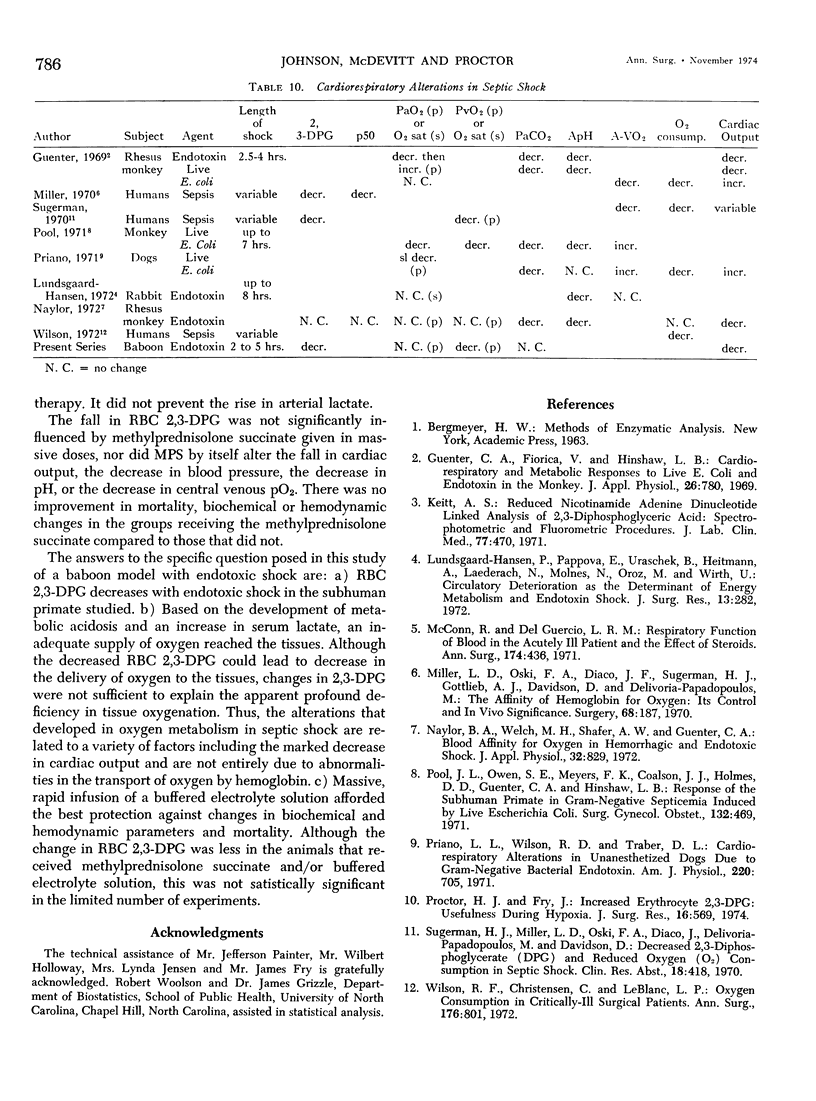
- Guenter C. A., Fiorica V., Hinshaw L. B. Cardiorespiratory and metabolic responses to liver E. coli and endotoxin in the monkey. J Appl Physiol. 1969 Jun;26(6):780–786. doi: 10.1152/jappl.1969.26.6.780. [DOI] [PubMed] [Google Scholar]
- Keitt A. S. Reduced nicotinamide adenine dinucleotide-linked analysis of 2,3-diphosphoglyceric acid: spectrophotometric and fluorometric procedures. J Lab Clin Med. 1971 Mar;77(3):470–475. [PubMed] [Google Scholar]
- Lundsgaard-Hansen P., Pappova E., Urbaschek B., Heitmann L., Laederach A., Molnes N., Oroz M., Wirth U. Circulatory deterioration as the determinant of energy metabolism in endotoxin shock. J Surg Res. 1972 Dec;13(6):282–288. doi: 10.1016/0022-4804(72)90078-9. [DOI] [PubMed] [Google Scholar]
- McConn R., Del Guercio L. R. Respiratory function of blood in the acutely ill patient and the effect of steroids. Ann Surg. 1971 Sep;174(3):436–450. doi: 10.1097/00000658-197109000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. D., Oski F. A., Diaco J. F., Sugerman H. J., Gottlieb A. J., Davidson D., Delivoria-Papadopoulos M. The affinity of hemoglobin for oxygen: its control and in vivo significance. Surgery. 1970 Jul;68(1):187–195. [PubMed] [Google Scholar]
- Naylor B. A., Welch M. H., Shafer A. W., Guenter C. A. Blood affinity for oxygen in hemorrhagic and endotoxic shock. J Appl Physiol. 1972 Jun;32(6):829–833. doi: 10.1152/jappl.1972.32.6.829. [DOI] [PubMed] [Google Scholar]
- Pool J. L., Owen S. E., Meyers F. K., Coalson J. J., Holmes D. D., Guenter C. A., Hinshaw L. B. Response of the subhuman primate in gram-negative septicemia induced by live Escherichia coli. Surg Gynecol Obstet. 1971 Mar;132(3):469–477. [PubMed] [Google Scholar]
- Priano L. L., Wilson R. D., Traber D. L. Cardiorespiratory alterations in unanesthetized dogs due to gram-negative bacterial endotoxin. Am J Physiol. 1971 Mar;220(3):705–711. doi: 10.1152/ajplegacy.1971.220.3.705. [DOI] [PubMed] [Google Scholar]
- Proctor H. J., Fry J. Increased erythrocyte 2,3-DPG: usefulness during hypoxia. J Surg Res. 1974 Jun;16(6):569–574. doi: 10.1016/0022-4804(74)90087-0. [DOI] [PubMed] [Google Scholar]
- Wilson R. F., Christensen C., LeBlanc L. P. Oxygen consumption in critically-ill surgical patients. Ann Surg. 1972 Dec;176(6):801–804. doi: 10.1097/00000658-197212000-00025. [DOI] [PMC free article] [PubMed] [Google Scholar]


