Abstract
Streptococcus macedonicus ACA-DC 198, a strain isolated from Greek Kasseri cheese, produces a food-grade lantibiotic named macedocin. Macedocin has a molecular mass of 2,794.76 ± 0.42 Da, as determined by electrospray mass spectrometry. Partial N-terminal sequence analysis revealed 22 amino acid residues that correspond with the amino acid sequence of the lantibiotics SA-FF22 and SA-M49, both of which were isolated from the pathogen Streptococcus pyogenes. Macedocin inhibits a broad spectrum of lactic acid bacteria, as well as several food spoilage and pathogenic bacteria, including Clostridium tyrobutyricum. It displays a bactericidal effect towards the most sensitive indicator strain, Lactobacillus sakei subsp. sakei LMG 13558T, while the producer strain itself displays autoinhibition when it is grown under conditions that do not favor bacteriocin production. Macedocin is active at pHs between 4.0 and 9.0, and it retains activity even after incubation for 20 min at 121°C with 1 atm of overpressure. Inhibition of macedocin by proteolytic enzymes is variable.
In recent years, numerous food-poisoning outbreaks involving various pathogens, along with increasing concern about the preservation of minimally processed foods, have spurred a growing awareness in the importance of food safety. This has prompted new approaches for inhibiting food-borne pathogens and for prolonging the shelf life of food products. In particular, there has been increasing interest in the antimicrobial activity of lactic acid bacteria (LAB). LAB have been used for centuries in the fermentation of foods, not only for flavor and texture development but also because of their ability to produce antimicrobial compounds which prevent the growth of spoilage and pathogenic microorganisms (9).
Some of these antimicrobial compounds, bacteriocins, are defined as proteinaceous compounds which kill closely related bacteria. In particular, the lantibiotics have attracted much attention in the last decade because of the success of the well-characterized lantibiotic nisin as a food preservative (3). Indeed, the commercial exploitation of bacteriocins to date has been restricted mainly to the food applications of nisin, the prototype LAB bacteriocin first discovered in 1928 by Rogers (51). Numerous lantibiotics have been identified and extensively characterized, resulting in increased understanding at both the structural and mechanistic levels (24, 32, 39, 53). So far, streptococcal lantibiotics have been isolated mainly from oral streptococci. These bacteria are commonly found in the oral cavities and upper respiratory tracts of humans and animals, although they may be isolated from almost any type of clinical specimen as well (18). Some examples include salivaricin A produced by Streptococcus salivarius (52), mutacin MT6223 produced by Streptococcus sobrinus (31), mutacin B-Ny266 (37), mutacin 1140 (19), mutacin I (48), mutacin II (40), and mutacin III (47) produced by Streptococcus mutans, and, finally, streptococcins SA-FF22 (20, 22, 23, 57, 58, 59) and SA-M49 (21) produced by Streptococcus pyogenes. Because the lantibiotic-producing oral streptococci cross the border of pathogenicity, it is obvious that there is no chance that these organisms can be used in food applications.
Although several problems concerning bacteriocin production in food environments still need to be addressed, the use of bacteriocin-producing cultures in food has considerable advantages over the use of purified bacteriocin preparations (7). Recently, some lactic streptococci isolated from Greek Kasseri cheese were assigned to a newly described Streptococcus species designated Streptococcus macedonicus (60). Several S. macedonicus strains were found to display antimicrobial activity against Clostridium tyrobutyricum (16). The spores from anaerobic, spore-forming, butyric acid bacteria, such as C. tyrobutyricum and Clostridium butyricum, appear quite frequently in milk. These bacteria convert lactic acid into butyric acid, which causes off-flavors in cheese. Furthermore, the carbon dioxide and hydrogen gas produced are responsible for the so-called late blowing, which is a serious defect, especially in hard and semihard cheeses, like Kasseri, Emmental, Gruyère, Grana, Edam, and Gouda, in which Clostridium growth is favored by the rather high pH of the cheeses (55, 62).
In this context, the possibility of using a food-grade microorganism such as S. macedonicus as a culture that provides protection against clostridia in cheese was challenging. The aims of the present work were to describe the structure and the biochemical properties of the bacteriocin produced by S. macedonicus ACA-DC 198 and to determine its antimicrobial spectrum and mode of action in view of its applicability as a starter culture in cheese production.
MATERIALS AND METHODS
Strain and growth conditions.
S. macedonicus ACA-DC 198, isolated from Greek Kasseri cheese (ACA-DC Collection of the Laboratory of Dairy Research, Agricultural University of Athens, Athens, Greece), was used throughout this study. It was stored at −30°C in sterile skim milk (10%, wt/vol; Irish Dairy Board, Dublin, Ireland). Before experimental use, the strain was subcultured twice (inoculum, 1% [vol/vol]) in sterile milk containing yeast extract (0.3%, wt/vol; Merck, Darmstadt, Germany) at 37°C for 24 h unless otherwise stated. Final growth was performed in the latter medium under the same conditions. Growth was assessed by measurement of the pH and the optical density at 480 nm (OD480) (26).
Quantitative determination of bacteriocin activity.
The well diffusion assay (56) was used in the macedocin purification and biochemical characterization procedure (Lactococcus lactis subsp. lactis LMG 6890T was used as the indicator strain), as well as for testing bacteriocin activity against Clostridium strains. The wells were filled with 50-μl portions of serial twofold dilutions (in 50 mM sodium phosphate buffer, pH 6.5) of a bacteriocin sample, and inhibition zones were measured after 12 h of incubation at 30°C. The activity was expressed in arbitrary units (AU) per milliliter, corresponding to 50 μl of the highest dilution that caused a clear zone of inhibition.
The soft agar assay (10) was used to determine the antimicrobial spectrum of the bacteriocin (apart from the antimicrobial spectrum against Clostridium strains). Briefly, 10-μl portions of serial twofold dilutions (in 50 mM sodium phosphate buffer, pH 6.5) of the S. macedonicus ACA-DC 198 cell-free culture supernatant or the culture supernatant after ammonium sulfate precipitation (50% saturation) were spotted onto lawns of an indicator organism. The overlaid agar plates were incubated for at least 12 h and examined for inhibition zones. The activity was expressed in AU per milliliter, corresponding to 10 μl of the highest dilution that caused a clear zone of inhibition.
Inhibitory spectrum of the bacteriocin.
The bacterial strains used as indicator organisms and the growth conditions used for them are listed in Table 1. The antagonistic activity of S. macedonicus ACA-DC 198 was tested by the soft agar assay or the well diffusion assay (Clostridium strains), except that only cell-free culture supernatant and the fraction obtained by ammonium sulfate precipitation (50% saturation) were tested (no dilutions were prepared). For each indicator strain the appropriate solid medium was used. Solid media were prepared by adding 1.5% (wt/vol) granulated agar (Oxoid) to the broth media. Overlay agar was prepared by adding 0.75% (wt/vol) granulated agar (Oxoid).
TABLE 1.
Inhibitory spectrum of macedocin
| Indicator organism | Cultivation
|
Sensitivity to macedocinb
|
|||
|---|---|---|---|---|---|
| Mediuma | Incubation temp (°C) | Conditions | SUPc | ASPd | |
| Bacillus cereus LMG 660e | BHI | 30 | Aerobic | − | +/− |
| Bacillus cereus LMG 6923T | BHI | 30 | Aerobic | − | − |
| Bacillus cereus LMG 13569 | BHI | 30 | Aerobic | − | + |
| Bacillus subtilis LMG 813 | BHI | 30 | Aerobic | − | + |
| Bacillus subtilis LMG 7135T | BHI | 30 | Aerobic | − | + |
| Bacillus subtilis LMG 17721 | BHI | 30 | Aerobic | + | + |
| Bacillus subtilis LMG 17722 | BHI | 30 | Aerobic | − | + |
| Bacillus subtilis LMG 17727 | BHI | 30 | Aerobic | +/− | + |
| Bacillus subtilis LMG 17728 | BHI | 30 | Aerobic | + | + |
| Bifidobacterium angulatum LMG 10503T | CAB | 37 | Anaerobic | − | + |
| Bifidobacterium bifidum LMG 13195 | CAB | 37 | Anaerobic | − | + |
| Bifidobacterium breve LMG 11042T | CAB | 37 | Anaerobic | +/− | + |
| Bifidobacterium breve LMG 13208 | CAB | 37 | Anaerobic | − | + |
| Bifidobacterium catenulatum LMG 11043T | CAB | 37 | Anaerobic | + | + |
| Bifidobacterium infantis LMG 8811T | CAB | 37 | Anaerobic | +/− | + |
| Bifidobacterium infantis LMG 10499 | CAB | 37 | Anaerobic | − | + |
| Bifidobacterium longum LMG 13197T | CAB | 37 | Anaerobic | +/− | + |
| Bifidobacterium pseudolongum subsp. pseudolongum LMG 11571T | CAB | 37 | Anaerobic | + | + |
| Clostridium sporogenes LMG 13570 | RCM | 37 | Anaerobic | − | + |
| Clostridium tyrobutyricum LMG 1285T | RCM | 37 | Anaerobic | + | + |
| Clostridium tyrobutyricum LMG 13571 | RCM | 37 | Anaerobic | − | + |
| Enterococcus casseliflavus EC 24 | MRS | 37 | Microaerophilic | − | − |
| Enterococcus casseliflavus LMG 10745T | MRS | 37 | Microaerophilic | +/− | + |
| Enterococcus durans LMG 10746T | MRS | 37 | Microaerophilic | − | + |
| Enterococcus faecalis LMG 7937T | MRS | 37 | Microaerophilic | − | − |
| Enterococcus faecalis LMG 16337 | MRS | 37 | Microaerophilic | − | − |
| Enterococcus faecalis Y | MRS | 37 | Microaerophilic | − | +/− |
| Enterococcus faecium CTC 492 | MRS | 37 | Microaerophilic | − | +/− |
| Enterococcus faecium LMG 11423T | MRS | 37 | Microaerophilic | +/− | +/− |
| Enterococcus gallinarum LMG 13129T | MRS | 37 | Microaerophilic | − | − |
| Enterococcus hirae LMG 6399T | MRS | 37 | Microaerophilic | − | − |
| Lactobacillus acidophilus ACA-DC 106 | MRS | 37 | Microaerophilic | − | − |
| Lactobacillus acidophilus LA1 | MRS | 37 | Microaerophilic | − | + |
| Lactobacillus acidophilus LMG 13550T | MRS | 37 | Microaerophilic | − | + |
| Lactobacillus brevis ACA-DC 3407 | MRS | 37 | Microaerophilic | +/− | + |
| Lactobacillus curvatus subsp. curvatus LMG 13553T | MRS | 30 | Microaerophilic | + | + |
| Lactobacillus delbrueckii subsp. bulgaricus LMG 6901T | MRS | 37 | Microaerophilic | + | + |
| Lactobacillus delbrueckii subsp. delbrueckii ACA-DC 81 | MRS | 37 | Microaerophilic | + | + |
| Lactobacillus fermentum ACA-DC 114 | MRS | 37 | Microaerophilic | +/− | + |
| Lactobacillus fermentum LMG 6902T | MRS | 37 | Microaerophilic | + | + |
| Lactobacillus fermentum LMG 8896 | MRS | 37 | Microaerophilic | + | + |
| Lactobacillus gasseri ACA-DC 85a | MRS | 37 | Microaerophilic | + | + |
| Lactobacillus helveticus LMG 13555T | MRS | 42 | Microaerophilic | + | + |
| Lactobacillus johnsonii ATCC 33200 | MRS | 37 | Microaerophilic | +/− | + |
| Lactobacillus paracasei subsp. paracasei ACA-DC 116 | MRS | 37 | Microaerophilic | − | − |
| Lactobacillus paracasei subsp. paracasei LMG 13552 | MRS | 37 | Microaerophilic | − | − |
| Lactobacillus paracasei subsp. tolerans ACA-DC 177 | MRS | 37 | Microaerophilic | − | − |
| Lactobacillus pentosus ACA-DC 3356T | MRS | 37 | Microaerophilic | +/− | + |
| Lactobacillus plantarum ACA-DC 113 | MRS | 37 | Microaerophilic | + | + |
| Lactobacillus plantarum ACA-DC 142 | MRS | 37 | Microaerophilic | +/− | + |
| Lactobacillus plantarum ATCC 14917T | MRS | 37 | Microaerophilic | +/− | + |
| Lactobacillus plantarum LMG 13556 | MRS | 37 | Microaerophilic | − | + |
| Lactobacillus rahmnosus ACA-DC 112 | MRS | 37 | Microaerophilic | − | − |
| Lactobacillus reuteri ATCC 53608 | MRS | 37 | Microaerophilic | + | + |
| Lactobacillus reuteri LMG 13557T | MRS | 37 | Microaerophilic | + | + |
| Lactobacillus sakei subsp. sakei LMG 13558T | MRS | 30 | Microaerophilic | + | + |
| Lactococcus garviae ACA-DC 219 | MRS | 30 | Microaerophilic | +/− | + |
| Lactococcus lactis subsp. cremoris LMG 6897T | MRS | 30 | Microaerophilic | + | + |
| Lactococcus lactis subsp. lactis ACA-DC 46 | MRS | 30 | Microaerophilic | − | − |
| Lactococcus lactis subsp. lactis ACA-DC49 | MRS | 30 | Microaerophilic | − | − |
| Lactococcus lactis subsp. lactis ACA-DC 73 | MRS | 30 | Microaerophilic | − | − |
| Lactococcus lactis subsp. lactis LMG 6890T | MRS | 30 | Microaerophilic | + | + |
| Lactococcus lactis subsp. lactis LMG 8522 | MRS | 30 | Microaerophilic | + | + |
| Leuconostoc mesenteroides subsp. cremoris LMG 13562 | MRS | 25 | Microaerophilic | + | + |
| Leuconostoc mesenteroides subsp. dextranicum ACA-DC 135 | MRS | 25 | Microaerophilic | + | + |
| Listeria innocua CTC 1014 | BHI | 30 | Microaerophilic | − | +/PICK> |
| Listeria innocua LMG 11387T | BHI | 30 | Microaerophilic | − | + |
| Listeria innocua LMG 13568 | BHI | 30 | Microaerophilic | − | − |
| Listeria innocua RZS | BHI | 30 | Microaerophilic | − | + |
| Listeria ivanovii LTH 3097 | BHI | 30 | Microaerophilic | − | + |
| Listeria monocytogenes Scott A | TSB | 30 | Microaerophilic | − | − |
| Micrococcus luteus LMG 4050T | NB | 30 | Aerobic | + | + |
| Pediococcus pentosaceus LMG 13560 | MRS | 30 | Microaerophilic | + | + |
| Pediococcus pentosaceus LMG 13561 | MRS | 30 | Microaerophilic | − | + |
| Propionibacterium acidipropionici LMG 13572 | MRS | 30 | Anaerobic | + | + |
| Propionibacterium freudenreichii subsp. shermanii LMG 16424T | MRS | 30 | Anaerobic | − | − |
| Staphylococcus carnosus LMG 13567 | BHI | 37 | Microaerophilic | +/− | + |
| Streptococcus macedonicus ACA-DC 198 | LAPTg | 37 | Microaerophilic | − | + |
| Streptococcus macedonicus ACA-DC 206T | MRS | 37 | Microaerophilic | − | − |
| Streptococcus thermophilus ACA-DC 4 | MRS | 42 | Microaerophilic | + | + |
| Streptococcus thermophilus ACA-DC 9 | MRS | 42 | Microaerophilic | + | + |
| Streptococcus thermophilus ACA-DC 79 | MRS | 42 | Microaerophilic | − | − |
| Streptococcus thermophilus LMG 13564 | MRS | 42 | Microaerophilic | + | + |
| Streptococcus thermophilus LMG 13565 | MRS | 42 | Microaerophilic | − | +/− |
BHI, brain heart infusion broth (Oxoid); MRS, de Man-Rogosa-Sharpe broth (Oxoid); NB, nutrient broth (Oxoid); RCM, reinforced clostridial medium (Oxoid); TSB, tryptone soy broth (Oxoid); CAB, Columbia agar base containing special peptone (23 g/liter; Oxoid), glucose (20 g/liter; Merck), and sodium chloride (5 g/liter) and adjusted to pH 7 before autoclaving; LAPTg, see Materials and Methods.
−, not sensitive (no inhibition zone), +/−, weakly sensitive (hazy inhibition zone), +, sensitive (clear inhibition zone).
SUP, S. macedonicus culture supernatant.
ASP, 50% ammonium sulfate precipitate of the S. macedonicus culture supernatant.
LMG, Laboratorium Microbiologie Gent, Ghent, Belgium; CTC, Centre de Tecnologia de la Carn, Monells, Spain; ACA-DC, Collection of the Agricultural University of Athens, Athens, Greece; ATCC, American Type Culture Collection, Manassas, Va.; RZS, Rijks Zuivel Station, Melle, Belgium; LTH, Lebensmitteln Technologie Hohenheim, Hohenheim, Germany.
Mode of inhibition.
To investigate the mode of inhibition, the bacteriocin was obtained by the two-step procedure of De Vuyst et al. (10), except that 500 ml of skim milk (10%, wt/vol) containing yeast extract (0.3%, wt/vol; Merck), inoculated with S. macedonicus ACA-DC 198 (inoculum, 1% [vol/vol]) and incubated at 37°C for 12 h, was used. Also, isopropanol was used instead of a mixture of chloroform and methanol. The inhibition assay was performed as follows. Two- or four-milliliter portions of a bacteriocin solution containing 3,200 AU/ml were added to 9-ml portions of exponentially growing cultures of Lactobacillus sakei subsp. sakei LMG 13558T (the most sensitive strain), Lactobacillus paracasei subsp. paracasei LMG 13552 (a nonsensitive strain), and S. macedonicus ACA-DC 198 (the producer strain) in de Man-Rogosa-Sharpe (MRS) medium (4) (Oxoid). The preparations were incubated at 30, 30, and 37°C, respectively. Growth was monitored by measuring the OD600 and by plate counting on LAPTg agar (S. macedonicus) (49) and MRS agar (lactobacilli; Oxoid). LAPTg agar was composed of yeast extract (10 g/liter; Merck), bacteriological peptone (15 g/liter; Oxoid), tryptone (10 g/liter; Oxoid), Tween 80 (1 ml/liter; Merck), glucose (10 g/liter; Merck), and granulated agar (15 g/liter; Oxoid). All media were sterilized in an autoclave at 121°C and 2.1 × 105 Pa for 20 min; glucose was sterilized separately.
For the producer strain S. macedonicus ACA-DC 198, the inhibition assay was also performed in a medium that favors bacteriocin production, namely, skim milk supplemented with yeast extract (0.3%, wt/vol). Two milliliters of a bacteriocin solution containing 1,600 AU/ml was added to 5 ml of an exponentially growing culture. The culture was incubated at 37°C, and growth was monitored by measuring the OD480 (26) and by plate counting on LAPTg agar (49). For the control strain, 2 ml of a milk protein solution was added instead of 2 ml of the bacteriocin solution. The milk protein solution was prepared by using the two-step isolation method (10) with 500 ml of sterilized skim milk supplemented with yeast extract (0.3%, wt/vol). Briefly, the caseins were removed by adjusting the pH of the milk to 4.0 with lactic acid (90%) at 55°C, followed by centrifugation (20,000 × g, 30 min, 4°C). After salting out with ammonium sulfate (50% saturation), the whey proteins were collected by centrifugation (20,000 × g, 30 min, 4°C) and dissolved in sodium phosphate buffer (pH 6.5). Finally, the whey fraction was subjected to isopropanol extraction, the sediment was dissolved in ultrapure water, and 2 ml was added to the control culture.
Effects of growth media on bacteriocin production.
The effects of different growth media, listed in Table 2, on both the growth and the bacteriocin production of S. macedonicus ACA-DC 198 were tested. Subculturing and final growth were performed in the appropriate media at 37°C. Bacteriocin activity in the cell-free culture supernatant was determined at the end of the log phase by using the soft agar assay as described above with L. sakei subsp. sakei LMG 13558T as the indicator strain. To determine cell-associated bacteriocin activity, the pH-dependent methods described by Tagg and Wannamaker (59) and Yang et al. (63) were used during growth of S. macedonicus ACA-DC 198 in MRS broth.
TABLE 2.
Bacteriocin production by S. macedonicus ACA-DC 198 in various growth media
| Growth mediuma | Bacteriocin production (AU/ml)b |
|---|---|
| Skim milk (10%, wt/vol) | − |
| Skim milk (10%, wt/vol) supplemented with yeast extract (0.3%, wt/vol) | 200 |
| Skim milk (10%, wt/vol) supplemented with yeast extract (1.5%, wt/vol) | 300 |
| Skim milk (10%, wt/vol) supplemented with tryptone (0.3%, wt/vol) | 400 |
| Skim milk (10%, wt/vol) supplemented with tryptone (1.5%, wt/vol) | 400 |
| Skim milk (10%, wt/vol) supplemented with lactalbumin hydrolysate (0.3%, wt/vol) | 400 |
| Skim milk (10%, wt/vol) supplemented with lactalbumin hydrolysate (1.5%, wt/vol) | 400 |
| Skim milk (10%, wt/vol) supplemented with Casamino Acids (0.3%, wt/vol) | 150 |
| Skim milk (10%, wt/vol) supplemented with Casamino Acids (1.5%, wt/vol) | − |
| Skim milk (10%, wt/vol) supplemented with casein hydrolysate (0.3%, wt/vol) | 50 |
| Skim milk (10%, wt/vol) supplemented with casein hydrolysate (1.5%, wt/vol) | − |
| MRS broth | +/− |
| MRS broth containing pepticase (2%, wt/vol) instead of Polypeptone and meat extract | − |
| MRS broth containing casein hydrolysate (2%, wt/vol) instead of Polypeptone and meat extract | − |
| MRS broth containing bacteriological peptone (0.25%), Lab Lemco (0.2%), and yeast extract (0.1%, wt/vol) | − |
| MRS broth containing bacteriological peptone (0.50%), Lab Lemco (0.4%), and yeast extract (0.2%, wt/vol) | − |
| MRS broth containing bacteriological peptone (0.75%), Lab Lemco (0.6%), and yeast extract (0.3%, wt/vol) | − |
| MRS broth supplemented with lactose (2%) | − |
| MRS broth supplemented with whey protein concentrate (1 or 2%, wt/vol) | − |
| MRS broth supplemented with lactose (2%) and whey protein concentrate (1 or 2%, wt/vol) | − |
| MRS broth supplemented with lactose (2%) and lactalbumin hydrolysate (2%, wt/vol) | − |
| M17 broth | − |
| Elliker broth | − |
| Nutrient broth | − |
| LAPTg | − |
| LAPTg supplemented with yeast extract (0.3%, wt/vol) | − |
Media and medium components were obtained from the following sources: skim milk, Belgomilk (Kallo, Belgium); yeast extract, Merck (Darmstadt, Germany); Casamino Acids, Bio-Trading (Bierbeek, Belgium); lactalbumin hydrolysate, casein hydrolysate, tryptone, bacteriological peptone, and Lab Lemco, Oxoid (Basingstoke, Hampshire, England); MRS broth, Oxoid, Merck, Biokar Diagnostics (Beauvais, France), and LabM (Bury, England); pepticase, Sigma (Steinheim, Germany); lactose, Merck (Darmstadt, Germany); whey protein concentrate, Danmark Protein (Videbæk, Denmark); and M17 broth, Elliker broth, and nutrient broth, Biokar Diagnostics.
−, no bacteriocin production, +/−, occasional and weak bacteriocin production. L. sakei subsp. sakei LMG 13558T was used as the indicator strain.
During growth in skim milk supplemented with yeast extract (0.3%, wt/vol), bacteriocin production in the cell-free culture supernatant was determined at various times (3, 6, 9, 12, and 24 h) by using the well diffusion assay described above with L. lactis subsp. lactis LMG 6890T as the indicator strain.
Purification of the bacteriocin.
All the purification steps were performed at room temperature by using a fast protein liquid chromatography system (Waters 650E advanced protein purification system). All of the columns used were purchased from Amersham-Pharmacia (Uppsala, Sweden).
Skim milk (500 ml) supplemented with yeast extract (0.3%, wt/vol) was inoculated with S. macedonicus ACA-DC 198 (inoculum, 1% [vol/vol]) and incubated at 37°C for 24 h. The cells were removed by centrifugation at 15,000 × g and 4°C for 30 min. The pH of the supernatant was adjusted to 6.5 with 5 N NaOH, and the supernatant was saturated up to 50% with ammonium sulfate. After overnight stirring at 4°C, the bacteriocin was pelleted by centrifugation at 15,000 × g and 4°C for 30 min and dissolved in 20 mM Tris buffer (pH 7.5).
The sample was then applied to a Resource Q column (inside diameter, 16 mm; length, 30 mm) equilibrated with 20 mM Tris-HCl buffer (pH 7.5). Elution was performed at a flow rate of 2 ml/min with a linear 0 to 1.0 M NaCl gradient in the same buffer. The nonretained, bacteriocin-containing fraction was properly diluted in 20 mM phosphate buffer (pH 5.5) and applied to a Resource S column (inside diameter, 16 mm; length, 30 mm) equilibrated with the same buffer. Elution was performed at a flow rate of 2 ml/min with a linear 0 to 1.5 M NaCl gradient in the same buffer. The bacteriocin-containing fractions were pooled, properly diluted in double-distilled water containing 0.1% (vol/vol) trifluoroacetic acid, and applied to a Resource RPC column (inside diameter, 6.4 mm; length, 100 mm) equilibrated with the same solvent. Elution was performed at a flow rate of 2 ml/min with a linear 0 to 100% acetonitrile gradient containing 0.1% (vol/vol) trifluoroacetic acid. The bacteriocin-containing fractions were pooled, lyophilized, and dissolved in 20 mM phosphate buffer (pH 6.0) containing 0.25 M NaCl. The sample was then filtered on a Superdex peptide HR 10/30 column equilibrated with the same buffer. Elution was performed at a flow rate of 0.5 ml/min. The bacteriocin-containing fractions were pooled and used for characterization of the bacteriocin.
PAGE.
Two electrophoretic systems were used to control the purification steps and to determine the molecular weight of the bacteriocin. Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (10% acrylamide separating gel) was performed by the method of Laemmli (28). Myosin (molecular weight, 205,000), β-galactosidase (116,000), phosphorylase b (97,400), bovine serum albumin (66,000), egg albumin (45,000), and carbonic anhydrase (29,000) were used as marker proteins (Sigma, Steinheim, Germany). Discontinuous Tricine-SDS-PAGE (with 4, 10, and 20% acrylamide used for the stacking, spacer, and separating gels, respectively) was performed by the procedure of Schägger and Von Jagow (54). Cytochrome c (molecular weight, 12,500), aprotinin (6,500), and substance P (1,348) were used as marker proteins (Sigma).
The latter procedure was used for postelectrophoretic detection of bacteriocin activity, and it was performed as described by De Vuyst et al. (10). The gel assayed for antimicrobial activity was overlaid with soft MRS agar, inoculated with the indicator strain L. sakei subsp. sakei LMG 13558T (inoculum, 4% [vol/vol]), incubated at 30°C for 24 h, and observed for the formation of inhibition zones.
Protein concentration determination.
Protein concentrations were determined by the method of Lowry et al. (30) by using bovine serum albumin as the standard.
Effect of pH on bacteriocin activity.
Purified bacteriocin (pH 6.0; 50 μl) was mixed with 100 μl of buffer (50 mM acetate, pH 4.0 or 5.0; 50 mM potassium phosphate, pH 6.0 or 7.0; or 50 mM Tris-HCl, pH 8.0 or 9.0) and incubated for 4 and 24 h and for 1 week at 30°C. The bacteriocin activity was then determined by using the well diffusion assay with L. lactis subsp. lactis LMG 6890T as described above.
Effect of temperature on bacteriocin activity.
Purified bacteriocin (pH 6.0) was heated for 1 h at 97°C (with a heat block) and for 5, 10, and 20 min at 121°C with 1 atm of overpressure (with an autoclave). The bacteriocin activity was then determined as described above. Furthermore, purified bacteriocin was stored for 4 weeks at −70, −20, 4, and 30°C. The bacteriocin activity was determined at 1-week intervals by using the well diffusion assay with L. lactis subsp. lactis LMG 6890T as described above.
Effects of enzymes on bacteriocin activity.
Purified bacteriocin was incubated at 30°C for 1, 4, and 24 h in the presence of ficin and trypsin (0.05 M sodium phosphate buffer, pH 7.0); α-chymotrypsin, protease, and proteinase K (0.05 M sodium phosphate buffer, pH 7.5); renin (0.05 M sodium phosphate buffer, pH 6.0); and pepsin (0.2 M citric acid buffer, pH 2.0). All enzymes were used at a final concentration of 2 mg/ml and were obtained from Sigma. Purified bacteriocin in buffer without enzyme, enzyme-buffer solutions, and buffers alone were used as controls. The bacteriocin activity was determined by using the well diffusion assay with L. lactis subsp. lactis LMG 6890T as described above.
N-terminal amino acid sequencing.
A purified sample of the bacteriocin was applied to a 476 A protein sequencer (Applied Biosystems, Foster City, Calif.). Homology searches were carried out with the National Center for Biotechnology Information and Swiss-Prot protein databases (BLAST search [1]).
MS.
Mass spectra were recorded with a Q-TOF mass spectrometer (Micromass, Manchester, United Kingdom) equipped with a nanoelectrospray source. Three microliters of a high-performance liquid chromatography fraction was mixed with a similar amount of 50% acetonitrile-0.1% formic acid in water, and this mixture was loaded onto a nanospray capillary (Protana, Odense, Denmark). Generally, 1,250 V was applied to the spraying capillary, which was gently broken to initiate the spray formation. Argon was used as the collisional induced dissociation gas for mass spectrometry (MS)-MS experiments at a pressure of 105 Pa. The collision energy was set at 40 V.
RESULTS
Effects of growth media on bacteriocin production.
The composition of the growth medium was critical for bacteriocin production by S. macedonicus ACA-DC 198. Indeed, S. macedonicus ACA-DC 198 grew well in all media tested. However, bacteriocin activity was detected only when the strain was grown in skim milk supplemented with nitrogen sources such as yeast extract, tryptone, lactalbumin hydrolysate, Casamino Acids, and casein hydrolysate at a concentration of 0.3% (wt/vol) (Table 2). Increasing the concentration of tryptone or lactalbumin hydrolysate to 1.5% (wt/vol) did not influence the bacteriocin production, while a slight increase in production was observed with 1.5% (wt/vol) yeast extract. On the other hand, increasing the concentration of Casamino Acids or casein hydrolysate inhibited bacteriocin production by S. macedonicus ACA-DC 198.
Occasionally, activity was detected when growth took place in MRS broth. Moreover, no cell-associated bacteriocin activity was observed after pH-dependent extraction methods were used. Also, heating of the cell suspension to promote desorption of cell-associated material did not result in any inhibitory activity. Finally, no bacteriocin production was observed in the other growth media tested.
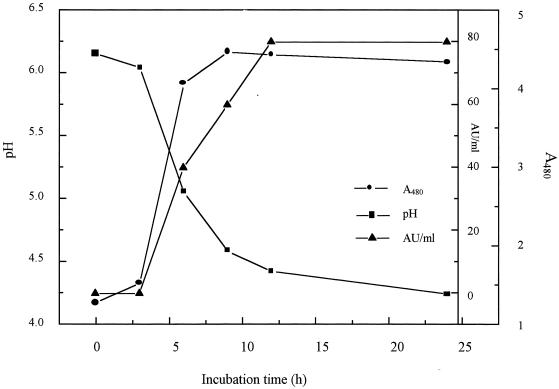
Bacteriocin production in skim milk supplemented with yeast extract (0.3%, wt/vol) started in the early exponential growth phase, reached a maximum in the early stationary phase, and then remained constant, reflecting primary metabolite kinetics (Fig. 1).
FIG. 1.
Growth of S. macedonicus ACA-DC 198 and bacteriocin production in skim milk (10%, wt/vol) containing 0.3% yeast extract at 37°C.
Spectrum of inhibition of the bacteriocin.
Macedocin exhibited a broad spectrum of inhibition (Table 1). When the cell-free culture supernatant of S. macedonicus ACA-DC 198 was tested against the 84 indicator strains used, 41 of them were found to be sensitive. The activity spectrum included several LAB, as well as gram-positive spoilage and pathogenic bacteria, such as Bacillus subtilis and C. tyrobutyricum. Apart from Enterococcus casseliflavus LMG 10745T and Enterococcus faecium LMG 11423T, none of the enterococcal strains tested was affected. This was also the case with the Listeria monocytogenes, Listeria innocua, and Listeria ivanovii strains used.
When the S. macedonicus ACA-DC 198 cell-free culture supernatant was concentrated by ammonium sulfate precipitation and then tested against the same set of indicator strains, more strains (64) were found to be sensitive. Moreover, with this preparation activity was detected against Bacillus cereus, Clostridium sporogenes, L. innocua, and L. ivanovii. Even the producer strain itself was inhibited.
A quantitative screening analysis was performed with strains clearly inhibited by the cell-free culture supernatant (data not shown). The greatest inhibition was observed with L. lactis subsp. lactis LMG 6890T (160 AU/ml), L. sakei subsp. sakei LMG 13558T (225 AU/ml, as determined by the spot-on-lawn method), Micrococcus luteus LMG 4050T (200 AU/ml), C. tyrobutyricum LMG 1285T (120 AU/ml), and Propionibacterium acidipropionici LMG 13572 (250 AU/ml). When the well diffusion method was used, C. tyrobutyricum LMG 1285T and L. sakei subsp. sakei LMG 13558T showed the same sensitivity (120 and 160 AU/ml, respectively). All values represent the averages of two experiments.
Mode of inhibition.
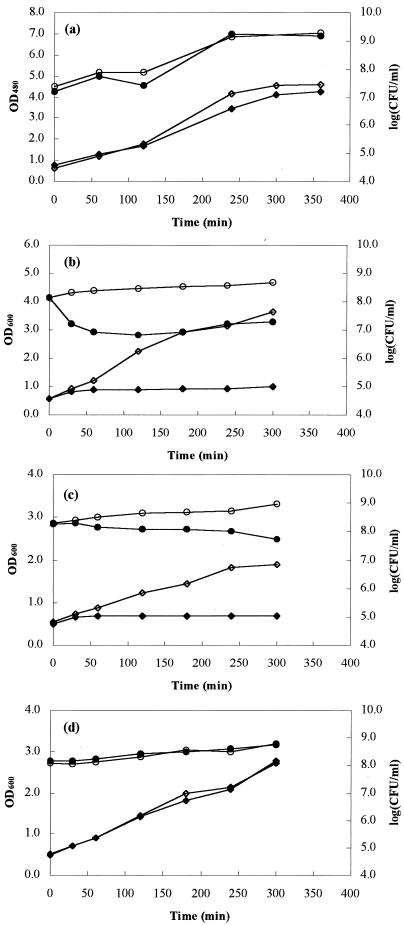
Exposure of the indicator strain L. sakei subsp. sakei LMG 13558T to partially purified bacteriocin at a concentration of 400 AU/ml resulted in a small reduction in the viable cell count (from 1.9 × 108 to 5.5 × 107 CFU/ml) after 5 h of incubation at 30°C (Fig. 2). Increasing the bacteriocin activity (to 800 AU/ml) did not result in a greater reduction in the indicator cell count. The initial population, 2.3 × 108 CFU/ml (OD600, 0.71), was reduced to 1.3 × 108 CFU/ml (OD600, 0.77) after 1 h, while a population containing 4.2 × 107 CFU/ml (OD600, 0.86) was present at the end of the incubation period (data not shown). Under these experimental conditions, the bacteriocin exhibited a bactericidal mode of action.
FIG. 2.
Mode of action of macedocin against S. macedonicus ACA-DC 198 grown in skim milk supplemented with yeast extract (0.3%, wt/vol) (a), S. macedonicus ACA-DC 198 grown in MRS broth (b), L. sakei subsp. sakei LMG 13558T (c), and L. paracasei subsp. paracasei LMG 13552 (d). Symbols: ○ and •, log CFU per milliliter; ◊ and ⧫, OD600 (MRS broth) or OD480 (milk medium); • and ⧫, medium supplemented with bacteriocin (400 AU/ml in MRS broth or 600 AU/ml in milk medium); ○ and ◊, medium without bacteriocin.
Addition of macedocin did not influence the growth of L. paracasei subsp. paracasei LMG 13552 (Fig. 2). After 5 h of incubation the viable cell count had increased from 1.2 × 108 to 6.1 × 108 CFU/ml for the control strain, while in the presence of the bacteriocin the cell count increased from 1.4 × 108 to 5.7 × 108 CFU/ml. The OD600 of the cell suspensions increased by 2.2 and 2.3 absorbance units for the control and the treated cell suspension, respectively.
When the producer strain was grown in MRS medium in the presence of partially purified bacteriocin (400 AU/ml), a 10-fold reduction in the viable cell count, from 1.4 × 108 to 8.4 × 106 CFU/ml, was observed after 1 h of incubation at 37°C (Fig. 2). Incubation for more than 1 h did not significantly change the number of viable cells of S. macedonicus ACA-DC 198. After 5 h of incubation the population level reached 1.9 × 107 CFU/ml, indicating that there was slight regrowth of the producer strain. A similar effect was obtained with a bacteriocin activity of 800 AU/ml, except that no regrowth phase was observed (data not shown). The S. macedonicus population was reduced from 1.2 × 107 to 7.5 × 105 CFU/ml (OD600, 0.86) in 1 h, remained almost constant during further incubation, and reached a level of 4.0 × 105 CFU/ml (OD600, 0.85) after 5 h. Under these conditions, the producer strain clearly displayed autoinhibition. This cannot be attributed to the presence of other end products, such as lactic acid. At the beginning and at the end of the incubation period, the cell-free culture supernatant of the control strain was assayed for antimicrobial activity by using S. macedonicus ACA-DC 198 as the indicator strain. No inhibition zones were observed, indicating that the growth inhibition was due to the presence of macedocin. The absorbance values for all treated cell suspensions remained constant during incubation in the presence of bacteriocin, indicating that there was no cell lysis.
When S. macedonicus ACA-DC 198 was grown in the medium that was favorable for bacteriocin production (skim milk supplemented with yeast extract) and in the presence of partially purified bacteriocin (600 AU/ml), no autoinhibition was observed. The control strain as well as the strain in the presence of the macedocin reached the stationary phase after 6 h of incubation (Fig. 2). At that time, the optical densities of the control suspension and the experimental suspension were 4.6 and 4.3 absorbance units, respectively, while the viable cell counts were 1.9 × 109 and 1.5 × 109 CFU/ml, respectively. In the inhibition assay, the bacteriocin activity (600 AU/ml) remained constant during the incubation period. For the control strain, no bacteriocin activity was observed at the beginning of the experiment, while an activity of 200 AU/ml was observed after 12 h of incubation.
Purification of the bacteriocin.
A combination of ammonium sulfate precipitation, anion- and cation-exchange chromatography, reverse-phase chromatography, and gel filtration was used for purification of the bacteriocin to homogeneity. The activity was not retained on the anion-exchange column at pH 7.5, while 0.48 M NaCl and 42.2% (vol/vol) acetonitrile were necessary for elution of the bacteriocin from the cation-exchange column (pH 5.5) and the reverse-phase column, respectively. Finally, on the gel filtration column the bacteriocin eluted as a symmetrical peak. Purification was followed by SDS-PAGE for both high- and low-molecular weight proteins. The data for recovery and degree of purification are summarized in Table 3.
TABLE 3.
Purification of S. macedonicus ACA-DC 198 bacteriocin
| Purification step | Vol (ml) | Activity (AU/ml) | Protein concn (mg/ml) | Total activity (AU) | Sp act (AU/mg) | Fold purification | Yield (%) |
|---|---|---|---|---|---|---|---|
| Crude | 455 | 160 | 2.1 | 72,800 | 76.2 | 1 | 100.0 |
| (NH4)2SO4 precipitation | 120 | 320 | 2.0 | 38,400 | 160.0 | 2.1 | 52.7 |
| Resource Q, pH 7.5 | 186 | 160 | 1.3 | 29,760 | 123.1 | 1.6 | 40.9 |
| Resource S, pH 5.5 | 40.5 | 320 | 0.4 | 12,960 | 800.0 | 10.5 | 17.8 |
| Resource RPC | 5.4 | 2,560 | 1.4 | 13,824 | 1,828.6 | 24.0 | 19.0 |
| Superdex peptide | 7.5 | 152 | 0.06 | 1,140 | 2,533.3 | 33.2 | 1.6 |
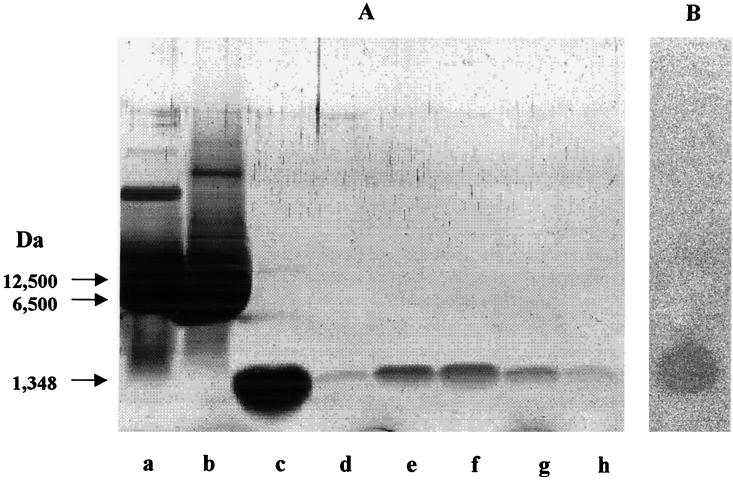
Molecular weight determination.
In the discontinuous Tricine-SDS-PAGE analysis the purified bacteriocin gave only one band, which corresponded to a molecular weight of approximately 2,500 (Fig. 3A). In the postelectrophoretic detection analysis, this band was active against the indicator strain L. sakei subsp. sakei LMG 13558T (Fig. 3B). By using gel filtration chromatography on a Superdex peptide HR 10/30 column that was previously standardized with cytochrome c (molecular weight, 12,500), aprotinin (6,500), and substance P (1,348), a molecular weight of 550 was calculated.
FIG. 3.
(A) Tricine SDS-PAGE. Lanes a, b and c, marker proteins (cytochrome c, aprotinin, and substance P, respectively); lanes d, e, f, g, and h, fractions from the Superdex peptide HR 10/30 column (fractions 34, 35, 36, 37, and 38, respectively). (B) Postelectrophoretic detection of the antimicrobial activity with L. sakei subsp. sakei LMG 13558T as the indicator strain.
Effects of pH, temperature, and enzymes on bacteriocin activity.
Even after 1 week of incubation at 30°C at pH values ranging from 4 to 9, the purified bacteriocin from S. macedonicus ACA-DC 198 retained full activity. Additionally, the bacteriocin was found to be very stable to heat, as no loss of activity was observed after incubation for 1 h at 97°C or even after incubation for 20 min at 121°C with 1 atm of overpressure. Finally, storage for 4 weeks at −70, −20, 4, and 30°C did not affect bacteriocin activity.
Proteolytic enzymes, such as α-chymotrypsin, proteinase K, and protease (type VIII from Bacillus licheniformis), inactivated the purified bacteriocin after 1 h of incubation, while ficin inactivated the purified bacteriocin after 4 h. Treatment with renin and trypsin resulted in full inactivation only after 24 h. Of the proteolytic enzymes tested, only pepsin did not affect bacteriocin activity after 24 h of treatment.
N-terminal amino acid sequence determination and MS analysis.
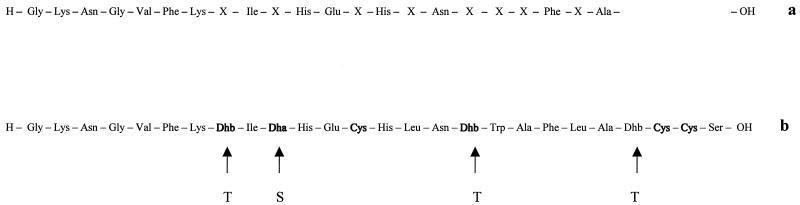
Edman degradation revealed an amino acid sequence stretch of 22 residues (Fig. 4). Eight of these residues could not be identified (residues X). The reaction probably stopped after the 22nd cycle, because of the occurrence of modified amino acids, possibly dehydroalanine and dehydrobutyrine, which cannot be recognized by Edman sequencing. Subsequent protein homology searches showed that the sequenced residues are identical to the corresponding amino acids of the lantibiotics SA-FF22 (Fig. 4) and SA-M49, both of which were isolated from S. pyogenes strains (20, 21, 23).
FIG. 4.
Amino acid sequences of the S. macedonicus ACA-DC 198 bacteriocin (a) and of SA-FF22 from S. pyogenes FF22 (b). Residues in boldface type are cross-linked through thioether bonds to form lanthionine (didehydroalanine and Cys) and methyllanthionine (didehydrobutyrine and Cys). Dha, didehydroalanine; Dhb, didehydrobutyrine; T, threonine; S, serine.
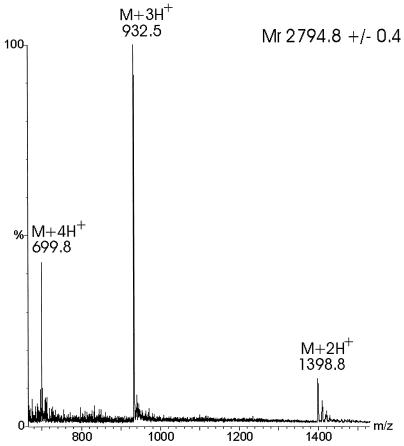
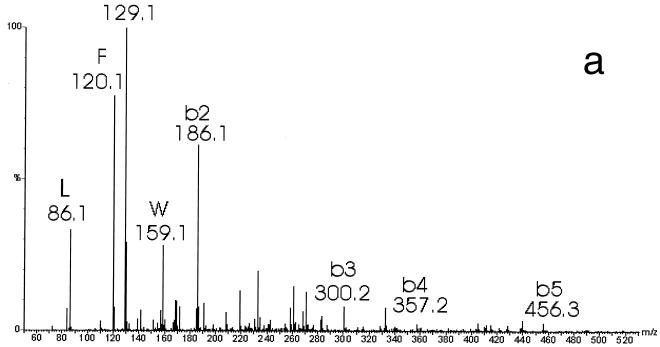
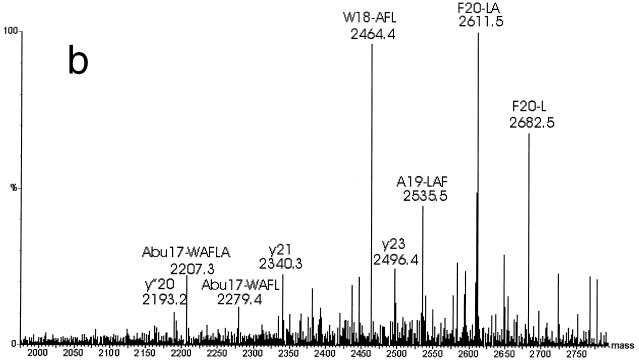
When the purified bacteriocin was analyzed by electrospray ionization MS in the positive mode, an average molecular mass of 2,794.76 ± 0.42 Da was found (Fig. 5). Mainly triple (m/z 932.8) and quadruple (m/z 699.8) protonated molecular ions were observed. The S. macedonicus ACA-DC 198 bacteriocin was further subjected to MS-MS analysis. Fragmentation of the triply charged ion (m/z 932.8) yielded relatively poor spectra with limited information. The lower-mass parts of the spectra showed mainly b series ions. This series corresponds to successive cleavage of the sequence from the N-terminal part (Fig. 6a). The singly charged product ions, m/z 186.1, 300.2, 357.2, and 455, correspond to the sequence GKNGV, which is consistent with the bacteriocin N-terminal sequence obtained by the Edman degradation (Fig. 4). Furthermore, high-mass fragments (Fig. 6b) indicate that there are some y fragments, which are cleavage products from the C-terminal part. Moreover, several peaks that are consistent with internal fragments due to double fragmentations were identified. These cleavages occurred after the Abu17, Trp18, Ala19, and Phe20 residues, combined with consecutive fragmentation of the newly appearing N termini after this fragmentation site (Abu is aminobutyric acid), which indicates the cross-link between Abu17 and Cys25 in the SA-FF22 molecule (cleavage after F20 [-L or -LA], A19 [-FL], W18 [- AFL or -AFLA], and Abu17 [-WAFL or -WAFLA]). Overall, weak fragmentation was observed, probably due to the presence of strong intrachain linkages similar to those seen for peptides involving disulfide bridges.
FIG. 5.
Electrospray ionization MS analysis of S. macedonicus ACA-DC 198 bacteriocin. The recorded multiple charged ions are consistent with a molecular mass estimate of 2,794.76 ± 0.42 Da.
FIG. 6.
MS-MS analysis of S. macedonicus ACA-DC 198 bacteriocin. (a) b series ions obtained by fragmentation of the triply charged ion (m/z 932.8) and corresponding to successive cleavage of the sequence from the N-terminal part; (b) fragments in the high-mass area of the MS-MS spectrum showing specific internal fragments. Fragments are designated on the basis of the amino acid where the peptide bond is broken, together with the sequence stretch that is cleaved from the newly appearing N terminus. Some y ions, resulting from fragmentations at the C terminus, are present as well.
DISCUSSION
When grown in milk supplemented with nitrogen sources, S. macedonicus ACA-DC 198 produced an antimicrobial compound with a rather broad antibacterial spectrum. Furthermore, the sensitivity of this compound to various proteolytic enzymes confirmed its proteinaceous nature. The data from the purification procedure suggest that the S. macedonicus ACA-DC 198 bacteriocin is a cationic and highly hydrophobic peptide. These are all common characteristics of many bacteriocins produced by LAB, particularly lantibiotics and class II bacteriocins (14, 27, 32).
Initially, SDS-PAGE of the purified bacteriocin revealed a molecular weight of about 2,500. This was later confirmed by electrospray mass spectroscopy (2,794.76). Therefore, the much lower molecular weight estimated by gel filtration (550) was attributed to erratic chromatographic migration of the molecule. A similar discrepancy has been observed in the case of lacticin 481, which was explained by the hydrophobicity of the molecule and the presence of intramolecular thioether cross-linkages (46). The low molecular weight of the S. macedonicus ACA-DC 198 bacteriocin strengthened the hypothesis that it is a lantibiotic. Similar molecular weights have been reported for various lantibiotics isolated from streptococci; these molecular weights include 2,315 for salivaricin A (52), 2,795 for streptococcin SA-FF22 (23), and 2,263 to 3,245 for the mutacins (19, 37, 40, 47, 48).
The S. macedonicus ACA-DC 198 bacteriocin retains activity at a broad range of pHs and is very heat stable, since no activity was lost even under sterilization conditions. These findings correspond well with a lantibiotic structure, as it is well known that good pH and thermal stability go along with low-molecular-weight proteins containing thioether bridges. Indeed, other lantibiotics isolated from various streptococci have similar features. Early studies of streptococcin SA-FF22 indicated that this lantibiotic is stable at acidic pHs but not at alkaline pHs (57). Mutacin MT6223 is also stable at a pH range from 2 to 7 and is resistant to treatment at 100°C for 20 min; although it retains activity even after 3 months at 4°C or in a frozen state, it looses 50% of its activity after 4 weeks of storage at room temperature (31). Finally, mutacin II was found to be stable and biologically active over a wide range of pH values (pH 4 to 10) or after heat treatment at 100°C for 30 min (40).
The partial N-terminal amino acid sequence is identical to that of the lantibiotics SA-FF22 (Fig. 4) and SA-M49, both of which were isolated from pathogenic S. pyogenes strains (20, 21, 23). The streptococcins SA-FF22 and SA-M49 both have a molecular weight of 2,794, and their amino acid sequences were deduced from the nucleotide sequences of the corresponding genes. For streptococcin SA-FF22, it was established that positions 8, 10, 13, and 17, which failed to yield amino acid signals during Edman sequencing, correspond to the positions of threonine, serine, cysteine, and threonine, respectively, suggesting that streptococcin SA-FF22 contains one lanthionine and two β-methyllanthionine residues. This was confirmed by chiral-phase gas chromatography (23). The fact that Edman sequencing of the S. macedonicus bacteriocin gave no results for the same positions, while the rest of the amino acid sequence exhibited 100% homology with streptococcin SA-FF22, along with the MS data obtained in the present work, contributes to our final conclusion that the two molecules are identical.
Although S. macedonicus ACA-DC 198 grew well in all media tested, bacteriocin was produced only when the strain was grown in skim milk supplemented with nitrogen sources. The failure to recover active bacteriocin in some liquid media may have been due to specific deficiencies in the growth medium, to the concomitant synthesis of bacteriocin-inactivating substances, such as proteolytic enzymes, or to the cell-associated location of the bacteriocin under particular growth conditions. However, S. macedonicus ACA-DC 198 was previously found to be nonproteolytic (16). Furthermore, in the present study no cell-associated bacteriocin activity was observed when the strain was grown in MRS medium, indicating that there was complete release of the bacteriocin into the extracellular environment. It is generally accepted that environmental conditions, such as growth medium, pH, and temperature, not only affect the amount of cells produced but also determine the bacteriocin production (29, 58, 64). It has been reported that complex media, such as MRS medium, SM8, and TGE broth, are most effective for production of various bacteriocins (2, 6, 61). However, it has been observed that some bacteriocin-producing LAB strains have a bacteriocin inhibitory effect on bacteria in milk as well (13, 17, 41, 50). The enhanced bacteriocin production when S. macedonicus ACA-DC 198 is grown in a natural food such as milk makes the potential use of this strain as a protective culture in dairy products more challenging.
Like the production of many other bacteriocins, bacteriocin production by S. macedonicus ACA-DC 198 displays primary metabolite kinetics (8, 11, 43). Since bacteriocin production is a growth-related process, good cell growth frequently goes hand in hand with production of high bacteriocin levels. Bacteriocin activity reaches a maximum level at the end of the logarithmic phase, while no loss of activity was observed during the stationary phase. Indeed, in general, the bacteriocin activity of LAB is maximal at the end of the exponential growth phase (8, 25, 35, 44). On the other hand, a decrease in bacteriocin activity during the exponential and/or stationary growth phase of the producing microorganism has been reported as well. This has been attributed to several mechanisms, such as protein aggregation, proteolytic degradation by specific or nonspecific enzymes, and bacteriocin adsorption to the producer cells (8, 43, 44). As mentioned above, this is not the case for the bacteriocin of S. macedonicus ACA-DC 198. Nevertheless, the stability of this compound in a real cheese environment still needs to be studied. However, results obtained in this study encourage the use of S. macedonicus ACA-DC 198 as protective starter culture in dairy fermentations.
Macedocin inhibits a broad spectrum of LAB, as well as several food spoilage and pathogenic bacteria. Its inhibitory effect seems to be species and/or strain dependent. It is generally accepted that bacteriocins produced by LAB kill phylogenetically closely related bacteria, even though many characterized bacteriocins concur with this definition. For instance, it has become apparent that some bacteriocins have a broad range, inhibiting many different species and/or genera (32). Due to its broad inhibitory spectrum and its unique properties, nisin is the only bacteriocin used and approved for use in several foods worldwide (3). Remarkably, besides the extreme pH and heat stability of the S. macedonicus ACA-DC 198 bacteriocin and its high activity levels in milk through fermentation, another very interesting finding of this study is that C. tyrobutyricum is inhibited by this compound. As mentioned above, C. tyrobutyricum is responsible for so-called late blowing, which is a serious defect, especially in hard and semihard cheeses, like Kasseri cheese. On the other hand, S. macedonicus itself has been isolated from traditional Kasseri cheese, as part of the secondary cheese microflora (60). Therefore, S. macedonicus can be considered an organism that is well adapted to the environment of this type of cheese. In this case, the use of S. macedonicus as an anticlostridium starter culture in Kasseri cheese seems to be a straightforward option. In the initial studies of SA-FF22 it was demonstrated that this bacteriocin is active against many strains of related streptococci and only certain gram-positive bacteria (57). Bacteriocins of members of the mutans streptococcal group have been found to be active against other members of the mutans group, several gram-positive and gram-negative bacterial pathogens, including representative strains of Staphylococcus spp., Actinomyces spp., Clostridium spp., Neisseria spp., L. monocytogenes, and Helicobacter pylori (19, 31, 34, 38, 40), and strains that are resistant to nisin and antibiotics such as vancomycin (37). Finally, salivaricin A was also found to be active against M. luteus T-18 (52).
Investigation of the effect of macedocin on the optical density of actively metabolizing cells of the susceptible indicator strain L. sakei subsp. sakei LMG 13558T at 30°C indicated that in spite a slight decrease in the number of CFU, there was no apparent change in the optical density of the culture, and hence, the lethal effect was not accompanied by cell lysis. In this context, the activity of macedocin against the indicator strain L. sakei subsp. sakei LMG 13558T can be considered bactericidal. Tagg et al. (58) observed a similar reduction in viable cell counts when streptocin A was added to resting cell suspensions of group A streptococcus strain PF 1643 incubated at 25 and 37°C, while the cell death induced was not associated with cell lysis. In that case, the temperature of incubation had a marked effect on the bactericidal action, with less killing occurring at a lower temperature. It is generally accepted that bacteriocin-induced cell death occurs in a concentration- and time-dependent manner (14). Not only the inhibitor concentration but also factors related to the target cell and the cell environment influence the effectiveness of a bacteriocin (14, 32). Mørtvedt-Abildgaard et al. (36) reported that the action of lactocin S, a lantibiotic produced by L. sakei L45, is strongly pH dependent and affects the target organism only at pH values below 6. Nisin delays the growth of L. monocytogenes at 8°C but has bacteriostatic activity at 4°C (12).
Although specific immunity proteins and a secondary ABC transporter system are assumed to protect the producer strain against the inhibitory action of its own product (32), autoinhibition of S. macedonicus ACA-DC 198 was noticed when the strain was grown in MRS broth, in which S. macedonicus ACA-DC 198 is actually not able to produce macedocin. In contrast, when the producer strain was grown in milk supplemented with yeast extract, a medium that favors macedocin production, no autoinhibition was observed. Autoantagonism was also described for strains of the Bacteroides fragilis group (15) and Fusobacterium spp. (42). In particular, incomplete immunity to homologous bacteriocin has been reported previously for the streptococcin producer strain S. pyogenes FF22 (57, 58). According to McLaughlin et al. (33), the multimeric ABC transporter complex could not keep the lantibiotic concentration below the critical level, which is necessary to form pores in the cytoplasmic membrane. Furthermore, these authors demonstrated that expression of the immunity genes requires the presence of a two-component regulatory system, which is in turn activated by an environmental signal factor (33). In the case of S. macedonicus ACA-DC 198, the lack of autoinhibition when the producer strain was grown in a medium suitable for bacteriocin production may indicate that expression of the immunity genes takes place during transcription of the biosynthetic genes. The latter is probably activated by a regulatory system, as described above. The nitrogen supplement may contain the signal factor for activation of the regulatory system.
In conclusion, S. macedonicus ACA-DC 198 produces a lantibiotic which was characterized in this study. The molecule was found to be identical to the streptococcins SA-FF22 and SA-M49 produced by S. pyogenes. S. pyogenes belongs to the group A streptococci, which are important human pathogens that produce numerous virulence factors, such as hemolysins (streptolysins O and S), erythrogenic toxins, and pyrogenic exotoxins (45). On the other hand, S. macedonicus is a food-grade microorganism, isolated from cheese, which does not exhibit potential pathogenicity traits. Therefore, we suggest that the bacteriocin from S. macedonicus ACA-DC 198 should be designated macedocin, according to the rule proposed by de Vos et al. (5) (i.e., a bacteriocin should be named after the species name of its producer and not after the genus name since several species belonging to the same genus often produce different bacteriocins). Moreover, the proposed name is necessary to clearly distinguish the food-grade S. macedonicus bacteriocin from the S. pyogenes bacteriocins that are not food grade. This is considered necessary to avoid further confusion regarding food legislation and consumer awareness when food applications of macedocin come up. The need for expanded and legal use of bacteriocins in foods is obvious, especially in light of consumers' demands for safe and minimally processed foods that have adequate shelf life and are convenient and the global need for increasing the supply of healthy and safe foods. On the other hand, problems concerning low production levels and the instability of bacteriocins in certain food environments still need to be addressed. However, it is generally accepted that using bacteriocin-producing cultures in food has considerable advantages over using purified bacteriocin preparations. The use of purified bacteriocin preparations requires extensive and costly purification schemes and toxicology tests and may project an image of nonnatural additives (for instance, with respect to the concentrations used). Thus, the use of S. macedonicus as a protective starter culture in food fermentations seems to be a straightforward option.
Acknowledgments
Marina D. Georgalaki thanks the Idrima Kratikon Ypotrofion (State Scholarship Foundation of Greece) for financial support. Luc De Vuyst acknowledges financial support from the Research Council of the Vrije Universiteit Brussel (OZR-VUB). The Fund for Scientific Research Flanders (FWO-Vlaanderen) is acknowledged for research grants to Luc De Vuyst and Jozef Van Beeumen and for a postdoctoral fellowship awarded to Bart Devreese.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Biswas, S. R., P. Ray, M. C. Johnson, and B. Ray. 1991. Influence of growth conditions on the production of a bacteriocin, pediocin AcH, by Pediococcus acidilactici H. Appl. Environ. Microbiol. 57:1265-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delves-Broughton, J., P. Blackburn, R. J. Evans, and J. Hugenholtz. 1996. Applications of the bacteriocin, nisin. Antonie Leeuwenhoek 69:193-202. [DOI] [PubMed] [Google Scholar]
- 4.de Man, J. C., M. Rogosa, and M. E. Sharpe. 1960. A medium for the cultivation of lactobacilli. J. Appl. Ind. Bacteriol. 23:130-135. [Google Scholar]
- 5.de Vos, W. M., G. Jung, and H.-G. Sahl. 1991. Appendix: definitions and nomenclature of lantibiotics, p. 457-463. In G. Jung and H.-G. Sahl (ed.), Nisin and novel lantibiotics. ESCOM Science Publishers, Leiden, The Netherlands.
- 6.De Vuyst, L. 1995. Nutritional factors affecting nisin production by Lactococcus lactis subsp. lactis NIZO 22186 in a synthetic medium. J. Appl. Bacteriol. 78:28-33. [Google Scholar]
- 7.De Vuyst, L. 2000. Technology aspects related to the application of functional starter cultures. Food Technol. Biotechnol. 38:105-112. [Google Scholar]
- 8.De Vuyst, L., and E. J. Vandamme. 1992. Influence of the carbon source on nisin production in Lactococcus lactis subsp. lactis batch fermentations. J. Gen. Microbiol. 138:571-578. [DOI] [PubMed] [Google Scholar]
- 9.De Vuyst, L., and E. J. Vandamme. 1994. Antimicrobial potential of lactic acid bacteria, p. 91-142. In L. De Vuyst and E. J. Vandamme (ed.), Bacteriocins of lactic acid bacteria. Blackie Academic and Professional, London, United Kingdom.
- 10.De Vuyst, L., R. Callewaert, and B. Pot. 1996. Characterization of the antagonistic activity of Lactobacillus amylovorus DCE 471 and the large-scale isolation of its bacteriocin amylovorin L471. Syst. Appl. Microbiol. 19:9-20. [Google Scholar]
- 11.De Vuyst, L., R. Callewaert, and K. Crabbe. 1996. Primary metabolite kinetics of bacteriocin biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocin production under unfavourable growth conditions. Microbiology 142:817-827. [DOI] [PubMed] [Google Scholar]
- 12.Duffes, F., C. Corre, F. Leroi, X. Dousset, and P. Boyaval. 1999. Inhibition of Listeria monocytogenes by in situ produced and semipurified bacteriocins of Carnobacterium spp. on vacuum-packed, refrigerated cold-smoked salmon. J. Food Prot. 62:1394-1403. [DOI] [PubMed] [Google Scholar]
- 13.Enan, G. 2000. Inhibition of Bacillus cereus ATCC 14579 by plantaricin UG1 in vitro and in food. Nahrung 44:364-377. [DOI] [PubMed] [Google Scholar]
- 14.Ennahar, S., T. Sashihara, K. Sonomoto, and A. Ishizaki. 2000. Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol. Rev. 24:85-106. [DOI] [PubMed] [Google Scholar]
- 15.Farias, L. M., M. A. Carvalho, C. A. Damasceno, E. O. Cisalpino, and E. C. Vieira. 1992. Bacteriocin-like activity of Bacteroides fragilis group isolated from marmosets. Res. Microbiol. 143:151-159. [DOI] [PubMed] [Google Scholar]
- 16.Georgalaki, M. D., P. Sarantinopoulos, E. S. Ferreira, L. De Vuyst, G. Kalantzopoulos, and E. Tsakalidou. 2000. Metabolic properties of Streptococcus macedonicus strains isolated from Greek Kasseri cheese. J. Appl. Microbiol. 88:817-825. [DOI] [PubMed] [Google Scholar]
- 17.Giraffa, G., D. Carminati, and T. G. Tarelli. 1995. Inhibition of Listeria innocua in milk by bacteriocin-producing Enterococcus faecium 7C5. J. Food Prot. 58:621-623. [DOI] [PubMed] [Google Scholar]
- 18.Hardie, J. M. 1986. Genus Streptococcus, p. 1043-1071. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. The Williams and Wilkins Co., Baltimore, Md.
- 19.Hillman, J. D., J. Novak, E. Sagura, J. A. Guitierrez, T. A. Brooks, P. J. Crowley, M. Hess, A. Azizi, K.-P. Leung, D. Cvitkovitch, and A. S. Bleiweis. 1998. Genetic and biochemical anlysis of mutacin 1140, a lantibiotic from Streptococcus mutans. Infect. Immun. 66:2743-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hynes, W. L., J. J. Ferretti, and J. R. Tagg. 1993. Cloning of the gene encoding streptococcin A-FF22, a lantibiotic produced by Streptococcus pyogenes, and determination of its nucleotide sequence. Appl. Environ. Microbiol. 59:1969-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hynes, W. L., V. L. Friendand, and J. J. Ferretti. 1994. Duplication of the lantibiotic structural gene in M-type 49 group A streptococcus strains producing streptococcin A-M49. Appl. Environ. Microbiol. 60:4207-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jack, R., R. Benz, J. Tagg, and H.-G. Sahl. 1994. The mode of action of SA-FF22, a lantibiotic isolated from Streptococcus pyogenes strain FF22. Eur. J. Biochem. 219:699-705. [DOI] [PubMed] [Google Scholar]
- 23.Jack, R. W., A. Carne, J. Metzger, S. Stefanovic, H.-G. Sahl, G. Jung, and J. R. Tagg. 1994. Elucidation of the structure of SA-FF22, a lanthionine-containing antibacterial peptide produced by Streptococcus pyogenes strain FF22. Eur. J. Biochem. 220:455-462. [DOI] [PubMed] [Google Scholar]
- 24.Jack, R. W., J. R. Tagg, and B. Ray. 1995. Bacteriocins of gram-positive bacteria. Microbiol. Rev. 59:171-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaiser, A. L., and T. J. Montville. 1993. The influence of pH and growth rate on production of bacteriocin, bavaricin MN, in batch and continuous culture. J. Appl. Bacteriol. 75:536-540. [Google Scholar]
- 26.Kanasaki, M., S. Breheny, A. J. Hillier, and G. R. Jago. 1975. Effect of temperature on the growth and acid production of lactic acid bacteria. 1. A rapid method for the estimation of bacterial populations in milk. Aust. J. Dairy Technol. 30:142-144. [Google Scholar]
- 27.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12:39-86. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 29.Leroy, F., and L. De Vuyst. 1999. Temperature and pH control that prevail during fermentation of sausages are optimal for the production of the antilisterial bacteriocin sacasin K. Appl. Environ. Microbiol. 65:974-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-276. [PubMed] [Google Scholar]
- 31.Loyola-Rodriguez, J. P., I. Morisaki, K. Kitamura, and S. Hamada. 1992. Purification and properties of extracellular mutacin, a bacteriocin from Streptococcus sobrinus. J. Gen. Microbiol. 138:269-274. [DOI] [PubMed] [Google Scholar]
- 32.McAuliffe, O., R. P. Ross, and C. Hill. 2001. Lantibiotics: structure, biosynthesis and mode of action. FEMS Microbiol Rev. 25:285-308. [DOI] [PubMed] [Google Scholar]
- 33.McLaughlin, R. E., J. J. Ferretti, and W. L. Hynes. 1999. Nucleotide sequence of the streptococcin A-FF22 lantibiotic regulon: model for production of the lantibiotic SA-FF22 by strains of Streptococcus pyogenes. FEMS Microbiol. Lett. 175:171-177. [DOI] [PubMed] [Google Scholar]
- 34.Morency, H., M. Mota-Meira, G. LaPointe, C. Lacroix, and M. C. Lavoie. 2001. Comparison of the activity spectra against pathogens of bacterial strains producing a mutacin or a lantibiotic. Can. J. Microbiol. 47:322-331. [PubMed] [Google Scholar]
- 35.Mørtvedt, C. I., and I. F. Nes. 1989. Bacteriocin production by a lactobacillus strain isolated from fermented meat. Eur. Food Chem. Proc. 1:336-341. [Google Scholar]
- 36.Mørtvedt-Abildgaard, C. I., J. Nissen-Meyer, B. Jell, B. Grenov, M. Skaugen, and I. F. Nes. 1995. Production and pH-dependent bactericidal activity of lactocin S, a lantibiotic from Lactobacillus sake L45. Appl. Environ. Microbiol. 61:175-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mota-Meira, M., C. Lacroix, G. LaPointe, and M. C. Lavoie. 1997. Purification and structure of mutacin B-Ny266: a new lantibiotic produced by Streptococcus mutans. FEBS Lett. 410:275-279. [DOI] [PubMed] [Google Scholar]
- 38.Mota-Meira, M., G. LaPointe, C. Lacroix, and M. C. Lavoie. 2000. MICs of mutacin B-Ny266, nisin A, vancomysin, and oxacillin against bacterial pathogens. Antimicrob. Agents Chemother. 44:24-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nes, I. F., D. B. Diep, L. S. Havarstein, M. B. Brurberg, V. Eijsink, and H. Holo. 1996. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 70:13-128. [DOI] [PubMed] [Google Scholar]
- 40.Novák, J., P. W. Caufield, and E. J. Miller. 1994. Isolation and biochemical characterization of a novel lantibiotic mutacin from Streptococcus mutans. J. Bacteriol. 176:4316-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nuñez, M., J. L. Rodriguez, E. Garcia, P. Gaya, and M. Medina. 1997. Inhibition of Listeria monocytogenes by enterocin 4 during the manufacture and ripening of Manchego cheese. J. Appl. Microbiol. 83:671-677. [DOI] [PubMed] [Google Scholar]
- 42.Oliveira, A. A., L. M. Farias, J. R. Nicoli, J. E. Costa, and M. A. Carvalho. 1998. Bacteriocin production by Fusobacterium isolates recovered from the oral cavity of human subjects with and without periodontal disease and of marmosets. Res. Microbiol. 149:585-594. [DOI] [PubMed] [Google Scholar]
- 43.Parente, E., and A. Ricciardi. 1994. Influence of pH on the production of enterocin 1146 during batch fermentation. Lett. Appl. Microbiol. 19:12-15. [DOI] [PubMed] [Google Scholar]
- 44.Parente, E., C. Brienza, A. Ricciardi, and G. Addario. 1997. Growth and bacteriocin production by Enterococcus faecium DPC1146 in batch and continuous culture. J. Ind. Microbiol. Biotechnol. 18:62-67. [DOI] [PubMed] [Google Scholar]
- 45.Parker, M. T. 1983. Streptococcus and Lactobacillus, p. 173-217. In G. S. Wilson, A. A. Miles, and M. T. Parker (ed.), Topley and Wilson's principles of bacteriology, virology and immunity, 7th ed., vol. 2. Edward Arnold, London, United Kingdom.
- 46.Piard, J.-C., P. M. Muriana, M. J. Desmazeaud, and T. R. Klaenhammer. 1992. Purification and partial characterization of lacticin 481, a lanthionine-containing bacteriocin produced by Lactococcus lactis subsp. lactis CNRZ 481. Appl. Environ. Microbiol. 58:279-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qi, F., P. Chen, and P. W. Caufield. 1999. Purification of mutacin III from group III Streptococcus mutans UA787 and genetic analyses of mutacin III biosynthesis genes. Appl. Environ. Microbiol. 65:3880-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi, F., P. Chen, and P. W. Caufield. 2000. Purification and biochemical characterization of mutacin I from the group I strain of Streptococcus mutans, CH43, and genetic analysis of mutacin I biosynthesis genes. Appl. Environ. Microbiol. 66:3221-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raibaud, P., M. Caulet, J. V. Galpin, and G. Moquot,. 1961. Studies on the bacterial flora of the alimentary tract of pigs. II. Streptococci: selective enumeration and differentiation of the dominant group. J. Appl. Bacteriol. 24:285-306. [Google Scholar]
- 50.Rodriguez, E., J. Tomillo, M. Nuñez, and M. Medina. 1997. Combined effect of bacteriocin-producing lactic acid bacteria and lactoperoxidase system activation on Listeria monocytogenes in refrigerated raw milk. J. Appl. Microbiol. 83:389-395. [DOI] [PubMed] [Google Scholar]
- 51.Rogers, L. A. 1928. The inhibiting effect of Streptococcus lactis on Lactobacillus bulgaricus. J. Bacteriol. 16:321-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ross, K. F., C. W. Ronson, and J. R. Tagg. 1993. Isolation and characterization of the lantibiotic salivaricin A and its structural gene salA from Streptococcus salivarius 20P3. Appl. Environ. Microbiol. 59:2014-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sahl, H.-G., and G. Bierbaum. 1998. Lantibiotics: biosynthesis and biological activities of uniquely modified peptides from gram-positive bacteria. Annu. Rev. Microbiol. 52:41-79. [DOI] [PubMed] [Google Scholar]
- 54.Schägger, H., and G. Von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 55.Steffen, C., P. Eberhard, J. O. Bosset, and M. Rüegg. 1993. Swiss-type varieties, p. 91. In P. F. Fox (ed.), Cheese: chemistry, physics and microbiology, 2nd ed., vol. 2. Chapman and Hall, London, United Kingdom.
- 56.Tagg, J. R., and A. R. McGiven. 1971. Assay system for bacteriocins. Appl. Microbiol. 21:943-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tagg, J. R., R. S. D. Read, and A. R. McGiven. 1973. Bacteriocin of a group A streptococcus: partial purification and properties. Antimicrob. Agents Chemother. 4:214-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tagg, J. R., A. S. Dajani, L. W. Wannamaker, L. W., and E. D. Gray. 1973. Group A streptococcal bacteriocin. J. Exp. Med. 138:1168-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tagg, J. R., and L. W. Wannamaker. 1978. Streptococcin A-FF22: nisin-like antibiotic substance produced by a group A streptococcus. Antimicrob. Agents Chemother. 14:36-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsakalidou, E., E. Zoidou, B. Pot, L. Wassil, L., W. Ludwig, L. A. Devriese, G. Kalantzopoulos, K. H. Schleifer, and K. Kersters. 1998. Identification of streptococci from Greek Kasseri cheese and description of Streptococcus macedonicus sp. nov. Int. J. Syst. Bacteriol. 48:519-527. [DOI] [PubMed] [Google Scholar]
- 61.Vignolo, G. M., M. N. de Kairuz, A. A. P. de Ruiz Holgado, and G. Oliver. 1995. Influence of growth conditions on the production of lactocin 705, a bacteriocin produced by Lactobacillus casei CRL 705. J. Appl. Bacteriol. 78:5-10. [Google Scholar]
- 62.Walstra, P., A. Noomen, and T. J. Geurts. 1993. Dutch-type varieties, p. 75. In P. F. Fox (ed.), Cheese: chemistry, physics and microbiology, 2nd ed., vol. 2. Chapman and Hall, London, United Kingdom.
- 63.Yang, R., M. C. Johnson, and B. Ray. 1992. Novel method to extract large amounts of bacteriocins from lactic acid bacteria. Appl. Environ. Microbiol. 58:3355-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang, R., and B. Ray. 1994. Factors influencing production of bacteriocins by lactic acid bacteria. Food Microbiol. 11:281-291. [Google Scholar]