Abstract
The lactococcal abortive infection mechanisms AbiA and AbiG were introduced into Streptococcus thermophilus 4035, and a range of phages capable of infecting this host were examined for sensitivity to these mechanisms. AbiA proved effective against six phages when examined at a growth temperature of 30°C but had no effect on any of the phages when tested at 37 or 42°C. AbiG failed to affect any of the S. thermophilus phages at 30, 37, or 42°C.
Bacteriophage problems encountered in high-temperature dairy fermentations such as those used to make mozzarella cheese and yogurt are due mainly to Streptococcus thermophilus phages (8). Very few phage defense mechanisms have been described for S. thermophilus. This could be due to the scarcity of plasmids in this species and/or to the fact that it has only been in recent years that any progress has been made in the genetic analysis of this microorganism. In contrast, over 40 phage resistance systems in Lactococcus spp. have been identified, the majority being plasmid encoded, and they can be grouped into four mechanisms on the basis of their mode of action: blocking of phage adsorption, blocking of phage DNA injection, restriction-modification (R-M), and abortive infection (1, 4). A single report on a possible Abi mechanism in an S. thermophilus strain has been made (8). It is of value to apply some of the wealth of knowledge associated with lactococcal phage resistance to other bacteria and possibly use these systems as alternatives or adjuncts to innate S. thermophilus defense systems. The lactococcal R-M system LlaDCHI was successfully expressed in a food-grade S. thermophilus strain conferring resistance to phages isolated from yogurt and mozzarella whey (9). In this study, AbiA and AbiG were selected for introduction into S. thermophilus since these systems have displayed activity against lactococcal P335 phages (12); this is the group with which S. thermophilus phages show some homology, and thus, it may also be a target for these Abi systems (2).
The bacterial strains, bacteriophages, and plasmids used in this study are listed in Table 1. A 2,191-bp PCR fragment corresponding to abiA was amplified from pCI829 (3) and cloned into pMG36CT, a vector utilizing rolling-circle-type replication capable of replicating in S. thermophilus (11, 13) to create the plasmid pMGA. A 2,016-bp PCR fragment corresponding to abiG, amplified from pCI750 (10), was also cloned into PstI/SalI-digested pMG36CT to create plasmid pMGG. pMGA and pMGG, as well as pMGCT, were subsequently introduced into S. thermophilus 4035, a host sensitive to multiple phages (Table 1), via electroporation, at frequencies of 5 × 102, 3 × 102, and 5 × 102 transformants per μg of DNA, respectively.
TABLE 1.
Bacterial strains, plasmids, and phages
| Strain, phage, or plasmid | Relevant characteristic(s) | Original source or referencea |
|---|---|---|
| Strains | ||
| Lactococcus lactis subsp. cremoris | ||
| AC8147 | AbiA+ | 3 |
| LOC735 | AbiG+ | 9 |
| S. thermophilus 4035 | Plasmid-free industrial strain used in yogurt manufacture | Quest International |
| Phages | ||
| Q1 | Lytic phage for host S. thermophilus 4035 | 8 |
| Q8 | Lytic phage for host S. thermophilus 4035 | Quest International |
| Q9 | Lytic phage for host S. thermophilus 4035 | Quest International |
| 1FN | Lytic phage for host S. thermophilus 4035 | NIZO collection |
| 2FN | Lytic phage for host S. thermophilus 4035 | NIZO collection |
| 7202 | Lytic phage for host S. thermophilus 4035 | NIZO collection |
| Plasmids | ||
| pMG36CT | Escherichia coli-lactococcal shuttle vector; Cmr | 12 |
| pMGA | 2.2-kb abiA PCR fragment cloned in the BamHI/HindIII sites of pMG36CT | This study |
| pMGG | 2-kb abiG PCR fragment cloned in the SalI/PstI sites of pMG36CT | This study |
Quest International, Bioproducts-Cultures, Bussum, The Netherlands; NIZO collection, Ede, The Netherlands.
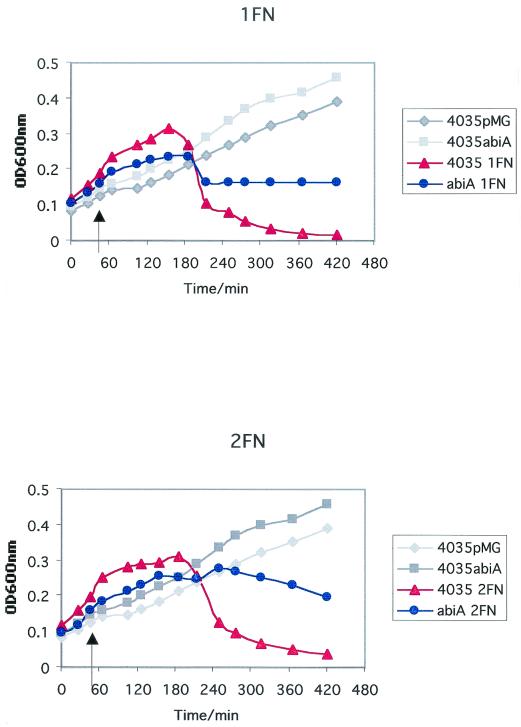
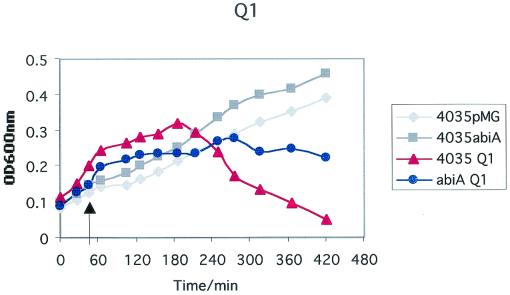
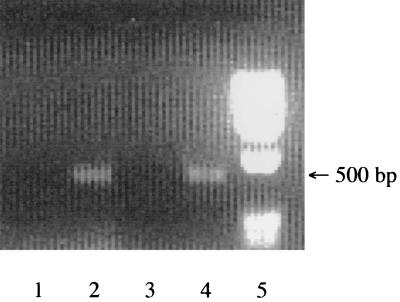
It was found that the constructs 4035/pMGCT, 4035/pMGA, and 4035/pMGG could not form turbid lawns when sloppy agar assays were attempted. The reason for this is unknown, although growth curves indicated that S. thermophilus 4035 strains containing pMG36CT grew less efficiently than did the plasmid-free wild type (data not shown). Thus, the ability of phages to lyse cultures in broth was utilized to assess phage resistance phenotypes of the S. thermophilus transformants. Comparison of the optical densities at 600 nm of samples taken from sensitive and test strains at time intervals after addition of phages indicated bacteriophage sensitivity. 4035/pMGA was lysed at the same rate as was 4035/pMGCT when they were infected with phages which are lytic for the parental strain (Table 1), when incubated at 37 and 42°C, but it proved to exhibit some resistance to all six phages when tested at 30°C. Growth curves for these strains in the presence of phages 1FN, 2FN, and Q1 are presented in Fig. 1. Phages Q8 and Q9 displayed similar degrees of sensitivity to AbiA, while φ7202 was affected to a lesser extent (data not shown). AbiG failed to affect any of the S. thermophilus phages at 30, 37, or 42°C (data not shown). AbiA has previously been demonstrated to be heat sensitive in several strain backgrounds (6, 7). The nature of the heat-sensitive phenotype is unknown, although the involvement of a heat-labile protein is possible, as reverse transcription-PCR analysis indicated that abiA, as well as abiG, was transcribed at 42°C in S. thermophilus 4035 (Fig. 2).
FIG. 1.
Growth of S. thermophilus 4035/pMGCT and S. thermophilus 4035/pMGA in the presence or absence of phage φ1FN, φ2FN, or φQ1. Arrows indicate time of addition of phages.
FIG. 2.
Results of reverse transcription-PCRs to detect products specific for abi transcripts with total cellular RNA from S. thermophilus 4035/pMGA or S. thermophilus 4035/pMGG grown at 42°C. Lanes: 1, S. thermophilus 4035/AbiA RNA; 2, S. thermophilus 4035/AbiA cDNA; 3, S. thermophilus 4035/AbiG RNA; 4, S. thermophilus 4035/AbiG cDNA; 5, molecular weight marker IX.
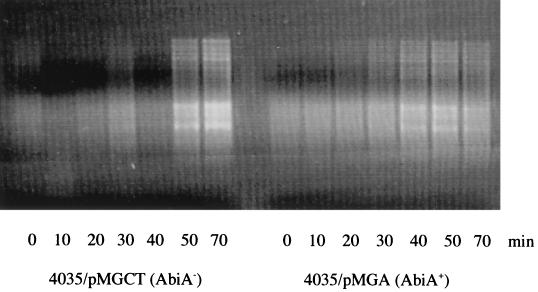
Phage DNA replication has previously been shown to be affected, be it directly or indirectly, by the presence of AbiA (5, 12). The replication of phage DNA in S. thermophilus 4035 hosts incubated at 30°C in the presence or absence of AbiA was assessed at time intervals following phage 1FN infection by the method described by Hill et al. (5). Phages were used to infect cells at a multiplicity of infection greater than 1, and samples were taken at specific time intervals after infection until the sensitive host had been lysed. Extracted DNA samples were digested with EcoRV and electrophoresed on 0.7% agarose gels (Fig. 3). Normal phage DNA replication could be seen in the sensitive 4035 cells, where the level of phage DNA increased steadily up to 70 min postinfection with phage, with eventual lysis of the culture. In cells containing AbiA, the quantity of phage DNA was also seen to increase over time but at a much lower level than in the sensitive host, indicating a significant reduction in replication activity. No lysis of this culture was observed. Therefore, AbiA activity against S. thermophilus phages appears to be similar to that operating against lactococcal phages, with a reduction in phage DNA accumulation resulting in both cases.
FIG. 3.
φ1FN DNA replication in S. thermophilus 4035/pMGCT and S. thermophilus 4035/pMGA at 0, 10, 20, 30, 40, 50, and 70 min postinfection with φ1FN.
The broad activity of AbiA and its efficacy against P335 phages made this system a likely candidate for successful improvement of phage resistance in a heterologous S. thermophilus strain. The heat-sensitive phenotype associated with this mechanism, however, makes it unsuitable for application under standard fermentations involving S. thermophilus. Ideal phage defense mechanisms need to be efficient at higher temperatures since fermentations using S. thermophilus are generally conducted at 40 to 45°C. Nevertheless, the finding that an abortive infection mechanism is functional in a genus other than its native host is novel. Furthermore, it was ascertained that its activity against S. thermophilus phages appears similar to that operating against lactococcal phages, with phage DNA replication being affected in both cases (5, 12). At the research level, the similarities between the observed sensitive S. thermophilus phages and corresponding lactococcal phages may provide valuable information as to the nature and activity of the particular mechanism under study, while the transfer of beneficial trait-bestowing genes between host species has obvious value at the industrial level.
REFERENCES
- 1.Allison, G. E., and T. R. Klaenhammer. 1998. Phage resistance mechanisms in lactic acid bacteria. Int. Dairy J. 8:207-226. [Google Scholar]
- 2.Chopin, A., A. Bolotin, A. Sorokin, S. D. Ehrlich, and M.-C. Chopin. 2001. Analysis of six prophages in Lactococcus lactis IL1403: different genetic structure of temperate and virulent phage populations. Nucleic Acids Res. 29:644-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coffey, A. G., G. F. Fitzgerald, and C. Daly. 1991. Cloning and characterisation of the determinant for abortive infection from the lactococcal plasmid pCI829. J. Gen. Microbiol. 143:1355-1362. [DOI] [PubMed] [Google Scholar]
- 4.Forde, A., and G. F. Fitzgerald. 1999. Bacteriophage defence systems in lactic acid bacteria. Antonie Leeuwenhoek 76:89-113. [PubMed] [Google Scholar]
- 5.Hill, C., I. J. Massey, and T. R. Klaenhammer. 1991. Rapid method to characterize lactococcal bacteriophage genomes. Appl. Environ. Microbiol. 57:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill, C., K. Pierce, and T. R. Klaenhammer. 1989. The conjugative plasmid pTR2030 encodes two bacteriophage defense mechanisms in lactococci, restriction modification (R+/M+) and abortive infection (Hsp+). Appl. Environ. Microbiol. 55:2416-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klaenhammer, T. R., and R. B. Sanozky. 1985. Conjugal transfer from Streptococcus lactis ME2 of plasmids encoding phage resistance, nisin resistance and lactose-fermenting ability: evidence for a high-frequency conjugative plasmid responsible for abortive infection of virulent bacteriophage. J. Gen. Microbiol. 131:1531-1541. [DOI] [PubMed] [Google Scholar]
- 8.Larbi, D., B. Decaris, and J.-M. Simonet. 1992. Different bacteriophage resistance mechanisms in Streptococcus salivarius ssp. thermophilus. J. Dairy Res. 59:349-357. [DOI] [PubMed] [Google Scholar]
- 9.Moineau, S., S. A. Walker, B. J. Holler, E. R. Vedamuthu, and P. A. Vandenbergh. 1995. Expression of a Lactococcus lactis phage resistance mechanism by Streptococcus thermophilus. Appl. Environ. Microbiol. 61:2461-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Connor, L., A. Coffey, C. Daly, and G. F. Fitzgerald. 1996. AbiG, a genotypically novel abortive infection mechanism encoded by plasmid pCI750 of Lactococcus lactis subsp. cremoris UC653. Appl. Environ. Microbiol. 62:3075-3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Sullivan, T., and G. F. Fitzgerald. 1999. Electrotransformation of industrial strains of Streptococcus thermophilus. J. Appl. Microbiol. 86:275-283. [DOI] [PubMed] [Google Scholar]
- 12.Tangney, M., and G. F. Fitzgerald. 2002. Effectiveness of the lactococcal abortive infection systems AbiA, AbiE, AbiF and AbiG against P335 type phages. FEMS Microbiol. Lett. 210:67-72. [DOI] [PubMed] [Google Scholar]
- 13.van de Guchte, M., J. M. van der Vossen, J. Kok, and G. Venema. 1989. Construction of a lactococcal expression vector: expression of hen egg white lysozyme in Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 55:224-228. [DOI] [PMC free article] [PubMed] [Google Scholar]