Abstract
The phenotypes and genotypes of 22 VanA-type vancomycin-resistant enterococci that had been isolated in Japan were examined. The VanA resistance determinant was plasmid mediated in each of the 22 strains. Of the 22 strains, 8 were isolated from different patients and 11 and 3 were obtained from different samples of chickens imported from Thailand and France, respectively. Three of the strains that were isolated from patients and the 11 strains isolated from the Thai chickens showed high-level vancomycin resistance (MICs, 512 to 1,024 μg/ml) and low-level teicoplanin resistance (MICs, 0.5 to 4 μg/ml). Each of these strains had three amino acid substitutions in the N-terminal region of the deduced VanS sequence. L50 was converted to V, E54 was converted to Q, and Q69 was converted to H compared to the vanS gene sequence of Tn1546.
Since the first reports of infections and colonizations by vancomycin-resistant enterococci (VRE) in France (10) and the United Kingdom (15), there have been reports of VRE in many locations in the United States and the European Union. The major factors contributing to the dissemination of VRE in the United States and Europe are now evident. In the United States, the major factor is likely the excessive use of glycopeptide antibiotics in the health care environment, resulting in a selective increase in VRE in human intestines (8, 11). In Europe, it is strongly suggested that the use of avoparcin as a growth promoter in animal feed has resulted in the selective increase in VRE in animal intestines, and the VRE subsequently appear in the human community (9, 13, 16, 17). In Japan, vancomycin injections have been used for the treatment of methicillin-resistant Staphylococcus aureus infections since November 1991 and infection or colonization due to VRE is rare (6, 7, 9). Until 2000, a total of eight patients with VRE were detected in four hospitals. We have conducted an investigation of VRE in imported and domestic chickens every year since 1998 with the support of the Japanese Ministry of Health, Labor, and Welfare (9). VRE have never been isolated from domestic chickens. However, VRE are isolated at a high frequency in samples from imported chickens. VRE isolation frequencies of about 20% and 30 to 50% have been observed in Thailand and France, respectively, where avoparcin has been used in animal feed (9). We compared the phenotypes and genotypes of VRE isolates from humans with those of isolates from imported chickens in Japan.
Glycopeptide resistance and pulsed-field gel electrophoresis (PFGE) analysis of VRE strains.
Twenty-two VRE strains were examined in this study. Of these strains, eight strains were isolated from eight different patients at four hospitals in Japan up until 2000 and 11 and 3 strains were obtained from chickens imported from Thailand and France, respectively, in investigations carried out in 1998 and 1999 (Table 1).
TABLE 1.
Characteristics of VRE isolated from humans and imported chickens in Japan
| Class and species | Straina | Origin | Source (specimen and/or reference) | Transfer frequency of vancomycinb resistance from wild-type strain to E. faecalis FA2-2 (no. of transconjugants/donor cell) | MIC (μg/ml)c
|
Deduced amino acid sequence or amino acid substitution(s) of VanS of VanA-type determinant | |
|---|---|---|---|---|---|---|---|
| Vancomycin | Teicoplanin | ||||||
| High teicoplanin resistance | |||||||
| E. faecium | BM4147 | France | Human (1) | <10−8 | 1,024 | 256 | Prototype |
| E. faecium | FN1 | Japan | Human (urine; 6) | <10−8 | 256 | 128 | Prototype |
| E. faecium | EF1 | Japan | Human (feces) | <10−8 | 512 | 128 | Prototype |
| E. faecium | EF2 | Japan | Human (feces) | <10−8 | 512 | 64 | Prototype |
| E. faecium | EF3 | Japan | Human (drainage) | <10−8 | 512 | 64 | Prototype |
| E. faecium | EF4 | Japan | Human (feces) | <10−8 | 512 | 64 | Prototype |
| E. faecium | FV1 | France | Chicken | <10−8 | 512 | 64 | Prototype |
| E. faecium | FV2 | France | Chicken | 10−5 | 512 | 64 | Prototype |
| E. faecalis | FV3 | France | Chicken | <10−8 | 512 | 128 | D58L |
| Low teicoplanin resistance | |||||||
| E. faecium | KV12 | Japan | Human (feces) | 10−4 | 1,024 | 4 | L50V, E54Q, Q69H |
| E. faecium | KV21 | Japan | Human (feces) | 10−4 | 512 | 2 | L50V, E54Q, Q69H |
| E. faecium | CV1 | Japan | Human (feces) | <10−8 | 512 | 0.5 | L50V, E54Q, Q69H |
| E. faecalis | TV1 | Thailand | Chicken | 10−4 | 512 | 4 | L50V, E54Q, Q69H |
| E. faecalis | TV2 | Thailand | Chicken | <10−8 | 512 | 4 | L50V, E54Q, Q69H |
| E. faecalis | TV3 | Thailand | Chicken | <10−8 | 512 | 16 | L50V, E54Q, Q69H |
| E. faecalis | TV4 | Thailand | Chicken | <10−8 | 1,024 | 16 | L50V, E54Q, Q69H |
| E. faecalis | TV5 | Thailand | Chicken | 10−5 | 1,024 | 2 | L50V, E54Q, Q69H |
| E. faecalis | TV6 | Thailand | Chicken | <10−8 | 256 | 4 | L50V, E54Q, Q69H |
| E. durans | TV7 | Thailand | Chicken | <10−8 | 512 | 2 | L50V, E54Q, Q69H |
| E. durans | TV8 | Thailand | Chicken | <10−8 | 512 | 2 | L50V, E54Q, Q69H |
| E. durans | TV9 | Thailand | Chicken | 10−5 | 512 | 4 | L50V, E54Q, Q69H |
| E. durans | TV10 | Thailand | Chicken | 10−4 | 512 | 16 | L50V, E54Q, Q69H |
| E. durans | TV11 | Thailand | Chicken | 10−5 | 1,024 | 16 | L50V, E54Q, Q69H |
| E. faecalis | GV2 | Japan | Broiler farm droppings (18) | 10−4 | 512 | 2 | L50V, E54Q, Q69H |
E. faecium BM4147 is a representative VanA-type strain, and the glycopeptide resistance determinant is encoded on transposon Tn1546. Strains TV1, TV2, TV3, TV7, and FV3 and strains TV4, TV5, TV6, TV8, TV9, TV10, TV11, FV1, and FV2 were isolated in investigations done in March 1998 and March 1999, respectively. Strains KV12 and KV21 and strain CV1 were isolated in November 1997 and July 1998, respectively.
Filter matings were performed with a donor/recipient ratio of 1:4. The recipient strain was E. faecalis FA2-2 (Rifr Fusr). The donor and recipient cells were trapped on a membrane filter (Millipore Corporation, Bedford, Mass.), incubated on a Todd-Hewitt broth (THB) agar plate at 37°C for 18 h, and suspended in 1 ml of THB. Appropriate dilutions of the mating mixture were plated onto THB agar plates containing vancomycin at 12.5 μg/ml plus rifampin at 25 μg/ml and fusidic acid at 25 μg/ml for counterselection of the donor strain, and the plates were incubated for 48 h at 37°C.
Glycopeptide resistance levels were determined by the agar dilution method with Mueller-Hinton agar in accordance with NCCLS criteria.
The levels of glycopeptide resistance were examined in the VRE strains (Table 1). Of the eight strains isolated from the patients, five strains from patients at two hospitals showed high-level vancomycin and teicoplanin resistance, with MICs of 256 to 512 μg/ml and 64 to 128 μg/ml, respectively, and the other three strains from the patients at two hospitals showed high-level vancomycin resistance, with MICs of 512 to 1,024 μg/ml, and low-level teicoplanin resistance, with MICs of 0.5 to 4 μg/ml. Of the strains isolated from the imported chickens, all four strains isolated from French chicken samples showed high-level vancomycin and teicoplanin resistance, with MICs of 512 μg/ml and 64 to 128 μg/ml, respectively. All 11 strains isolated from Thai chicken samples showed high-level vancomycin resistance, with MICs of 256 to 1,024 μg/ml, and relatively low-level teicoplanin resistance, with MICs of 2 to 8 μg/ml.
The DNA from each of the VRE strains was analyzed by PCR for the presence of vancomycin resistance gene(s) with vanA-, vanB-, and vanC-specific primers (4, 5, 12). All strains gave rise to the expected 732-bp product with the primer specific for the vanA gene, indicating that the strains were VanA-type VRE (data not shown).
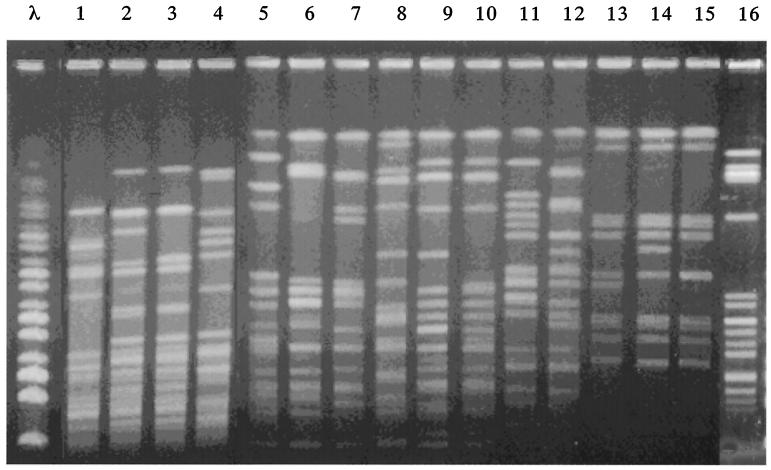
Banding patterns obtained by PFGE of SmaI-digested genomic DNA were used to compare the VRE strains. Figure 1 shows the PFGE patterns of VRE strains isolated from Thai chickens and VRE strains KV12, KV21, and CV1 isolated from patients, which showed high-level vancomycin resistance and low-level teicoplanin resistance, and also VRE strain FN1 (6) (Fig. 1; Table 1). Of these strains, two (KV12 and KV21) isolated from patients at a hospital and three (TV9, TV10, and TV11) isolated from Thai chickens, respectively, differed by one or two bands, indicating that strains of each group were related. Other strains showed different patterns, indicating that these VRE strains are different (Fig. 1)
FIG. 1.
PFGE of SmaI-digested chromosomal DNAs isolated from VRE strains. λ, a bacteriophage λ DNA ladder used as a molecular size marker. Lanes 1 to 16, VRE strains CV1, KV12, KV21, FN1, TV1, TV2, TV3, TV4, TV5, TV6, TV7, TV8, TV9, TV10, TV11, and GV2, respectively.
Conjugative transfer of vancomycin resistance.
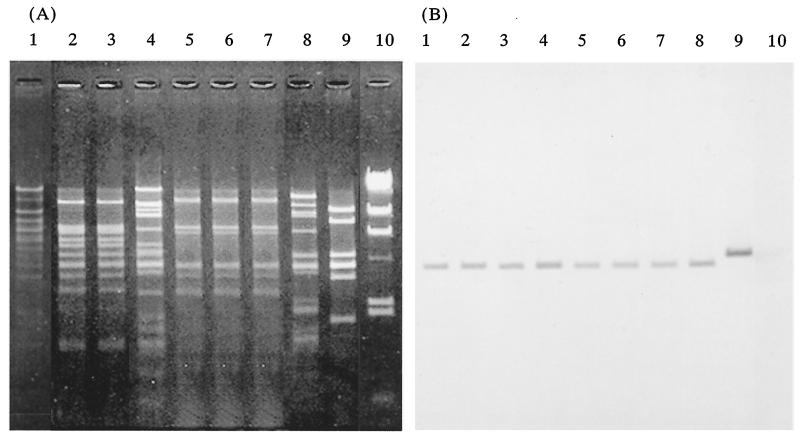
Each of the 22 VRE strains harbored several plasmids. The EcoRI restriction profiles of total plasmid DNAs isolated from the VRE strains were different (data not shown). The vanA probe hybridized to a specific EcoRI fragment (4.1 kb) of plasmid DNA in each of the strains that is specific to the prototype VanA-type gene encoded on Tn1546 (5), with the exception of the plasmid DNAs of one strain (FV2), where the probe hybridized to a 5-kbp EcoRI fragment (data not shown). The transferability of the vancomycin resistance of the VRE strains was examined between each of the strains and recipient strain Enterococcus faecalis FA2-2 (Rifr Fusr) (3) by filter mating. The vancomycin resistances of 5 of the 11 strains from Thai chickens and 2 of the 3 strains from the patients, which were high-level vancomycin resistant and low-level teicoplanin resistant, and the vancomycin resistance of one of the three strains from French chickens, which were high-level vancomycin and teicoplanin resistant, were transferred to the recipient E. faecalis FA2-2 (Rifr Fusr) strain at about 10−4 to 10−5 cells per donor cell by filter mating (Table 1). After repeated transfer experiments done by short mating between E. faecalis FA2-2 (Rifr Fusr) and E. faecalis JH2SS (Strr Spcr) (14), the transferable plasmid from each of the strains was identified from the transconjugant (Fig. 2). The plasmid DNA of the transconjugants of two strains (KV12, and KV21) isolated from patients at a hospital and three strains (TV9, TV10, and TV11) isolated from Thai chickens, respectively, exhibited the same EcoRI restriction profiles (Fig. 2). The vanA probe hybridized to a specific EcoRI fragment (4.1 kb) (5) of conjugative plasmid DNA of each the transconjugants, with the exception of the plasmid DNA isolated from strain FV2. These results indicated that the VanA determinants, which are encoded on transposons such as Tn1546, are carried on a conjugative plasmid and can transfer between enterococci and also to different replicons by transposition.
FIG. 2.
Agarose gel electrophoresis of restriction endonuclease-digested conjugative plasmid DNA and hybridization with a vanA-specific probe. (A) Agarose gel electrophoresis of EcoRI-digested plasmid DNA isolated from each the transconjugants. (B) The gel was Southern blotted and hybridized to a vanA-specific probe. Lanes 1 to 9, plasmid pMG2 and plasmid DNAs isolated from transconjugants of VRE strains KV12, KV21, TV1, TV9, TV10, TV11, TV5, and FV2, respectively. Lanes 10, HindIII-digested lambda DNA.
DNA sequence analysis of the vanS gene of the vancomycin determinants encoded on the plasmids.
A nationwide survey was conducted to investigate VRE in broiler farms in Japan in December 1996 (18). Three VanA-type VRE strains were isolated from droppings obtained from 3 of 35 broiler farms in 24 prefectures in Japan that used avoparcin (18). The three VanA-type VRE isolates showed high-level vancomycin resistance and relatively low-level teicoplanin resistance, and the VanA determinant of E. faecalis GV2, which is one of the three isolates, was analyzed in detail (7). The glycopeptide resistance of E. faecalis GV2 is mediated by conjugative plasmid pMG2 (85 kb). The nucleotide sequences of the genes for the VanA determinant encoded on pMG2 are completely identical to those of the prototype VanA-type gene encoded on Tn1546, with the exception of the gene for glycopeptide sensor protein VanS (1). There are three amino acid substitutions in the N-terminal region of the deduced sequence encoded by vanS (Table 1).
Nucleotide sequence analysis of the vanS gene of the VRE strains was performed by sequencing PCR products with vanS-specific primers (Table 2). To analyze the van determinant corresponding to vanS of the VanA-type determinant encoded on Tn1546 (2), one or two pairs of PCR primers specific for vanS were used for PCR amplifications. The pairs of PCR primers were also used to sequence the amplified genes. The nucleotide sequence and the deduced amino acid residues of the vanS gene of the VRE strains isolated from Thai chickens and from three patients at two hospitals revealed three amino acid substitutions in the N-terminal region of the deduced VanS sequence. L50 was converted to V, E54 was converted to Q, and Q69 was converted to H compared to the vanS gene sequence of Tn1546 (Table 1).
TABLE 2.
Nucleotide sequences of PCR primers for vanS of the VanA-type determinant encoded on Tn1546
| Primer no., namea | Target gene | Nucleotide sequence | Positionb | Size of PCR product (bp) | Reference |
|---|---|---|---|---|---|
| 1, VanS | vanS | 5′-AACGACTATTCCAAACTAGAAC | 4676-4697 | 1,094 | 12 |
| 2, VanS1 | vanS | 5′-GCTGGAAGCTCTACCCTAAA | 5769-5749 | 12 | |
| 3, VanS2 | vanS | 5′-CTTAAATCACCTGGACGCGATG | 4822-4843 | 7 | |
| 4, VanS3 | vanS | 5′-GCAGGATGCAAAGCTGGCCG | 5083-5102 | 7 | |
| 5, VanS4 | vanS | 5′-GAATACTAATGACAATCGCC | 4913-4894 | 7 |
Primers 1 and 2 are a primer pair for amplification of vanS. Primers 3 to 5 were used to determine the nucleotide sequence of vanS.
The positions given are from the first base of the coding sequence of the left inverted repeat of Tn1546. The nucleotide sequence of Tn1546 was obtained from the GenBank nucleotide sequence database (accession no. M97297).
The VRE strains gave rise to the expected PCR products of 1,744, 1,856, and 926 bp with the primers specific for open reading frame (ORF) 1, both ORFs 1 and 2, and ORF2, respectively, which are located on Tn1546 and encode a transposase (ORF1) and a resolvase (ORF2) (data not shown) (12). These results suggested that the VanA-type determinants of VRE strains were encoded on transposon Tn1546 or a closely related transposon.
Concluding comments.
The VRE strains isolated from French chickens exhibited high-level vancomycin and teicoplanin resistance and could not be distinguished from the representative VanA-type VRE (2, 5, 12). The VRE strains isolated from Thai chickens exhibited high-level vancomycin resistance and relatively low-level teicoplanin resistance, had three amino acid substitutions in the vanS gene of the VanA-type determinant, and were distinguished by these characters from the representative VanA-type VRE. The VRE strains isolated from three patients at the two hospitals showed the same characteristics as the VRE strains from the Thai chickens. It is very rare that identical substitutions would occur independently in each of the strains. Japan imports about 600,000 tons of chicken a year, while the nation's domestic chicken production is about 1.2 million tons a year. About 100,000 tons (16.6%) of the imported chicken come from Thailand. Although isolation of VRE is rare in enterococci from inside or outside the health care environment, these results imply that the VanA-type vancomycin resistance determinant, which resides on transposons such as Tn1546 and can be transferred between enterococci by a conjugative plasmid, can transfer from chickens to humans.
Acknowledgments
This work was supported by grants from the Ministry of Health Labor and Welfare and by grants from the Japanese Ministry of Education, Science and Culture.
REFERENCES
- 1.Arthur, M., C. Molinas, and P. Courvalin. 1992. The VanS-VanR two-component regulatory system controls synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J. Bacteriol. 174:2582-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur, M., C. Molinas, F. Depardieu, and P. Courvalin. 1993. Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J. Bacteriol. 175:117-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clewell, D. B., P. K. Tomich, M. C. Gawron-Burke, A. E. Franke, Y. Yagi, and F. Y. An. 1982. Mapping of Streptococcus faecalis plasmids pAD1 and pAD2 and studies relating to transposition of Tn917. J. Bacteriol. 152:1220-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dutka-Malen, S., S. Evers, and P. Courvalin. 1995. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 33:24-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutka-Malen, S., S. R. Leclercq, V. Coutant, J. Doval, and P. Courvalin. 1990. Phenotypic and genotypic heterogeneity of glycopeptide resistance determinants in gram-positive bacteria. Antimicrob. Agents Chemother. 34:1875-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujita, N., M. Yoshimura, T. Komori, K. Tanimoto, and Y. Ike. 1998. First report of the isolation of high-level vancomycin-resistant Enterococcus faecium from a patient in Japan. Antimicrob. Agents Chemother. 42:2150.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto, Y., K. Tanimoto, Y. Ozawa, T. Murata, and Y. Ike. 2000. Amino acid substitutions in the VanS sensor of the VanA-type vancomycin-resistant enterococcus strains result in high-level vancomycin resistance and low-level teicoplanin resistance. FEMS Microbiol. Lett. 185:247-254. [DOI] [PubMed] [Google Scholar]
- 8.Huycke, M. M., D. F. Sahm, and M. S. Gilmore. 1998. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg. Infect. Dis. 4:239-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ike, Y., K. Tanimoto, Y. Ozawa, T. Nomura, S. Fujimoto, and H. Tomita. 1999. Vancomycin-resistant enterococci in imported chickens in Japan. Lancet 353:1854.. [DOI] [PubMed] [Google Scholar]
- 10.Leclercq, R., E. Derlot, J. Duval, and P. Courvalin. 1988. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N. Engl. J. Med. 319:157-161. [DOI] [PubMed] [Google Scholar]
- 11.Martone, W. J. 1998. Spread of vancomycin resistant enterococci: why did it happen in the United States? Infect. Control Hosp. Epidemiol. 19:539-545. [DOI] [PubMed] [Google Scholar]
- 12.Miele, A., M. Bandera, and B. P. Goldstein. 1995. Use of primers selective for vancomycin resistance genes to determine van genotype in enterococci and to study gene organization in VanA isolates. Antimicrob. Agents Chemother. 39:1772-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schouten, M. A., A. Voss, and J. A. A. Hoogkamp-Korstanje. 1997. VRE and meat. Lancet 349:1258.. [DOI] [PubMed] [Google Scholar]
- 14.Tomich, P. K., F. Y. An, and D. B. Clewell. 1980. Properties of erythromycin-inducible transposon Tn917 in Streptococcus faecalis. J. Bacteriol. 141:1366-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uttley, A. H., C. H. Collins, J. Naidoo, and R. C. George. 1988. Vancomycin-resistant enterococci. Lancet i:57-58. [DOI] [PubMed]
- 16.van den Bogaard, A. E., L. B. Jensen, and E. E. Stobberingh. 1997. Vancomycin-resistant enterococci in turkeys and farmers. N. Engl. J. Med. 337:1558-1559. [DOI] [PubMed] [Google Scholar]
- 17.Witte, W. 1998. Medical consequences of antibiotic use in agriculture. Science 279:996-997. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura, H., M. Ishimaru, Y. S. Endoh, M. Suginaka, and S. Yamatani. 1998. Isolation of glycopeptide-resistant enterococci from chickens in Japan. Antimicrob. Agents Chemother. 42:3333.. [DOI] [PMC free article] [PubMed] [Google Scholar]