Abstract
The practical application of commercial malolactic starter cultures of Oenococcus oeni surviving direct inoculation in wine requires insight into the mechanisms involved in ethanol toxicity and tolerance in this organism. Exposure to ethanol resulted in an increase in the permeability of the cytoplasmic membrane, enhancing passive proton influx and concomitant loss of intracellular material (absorbing at 260 nm). Cells grown in the presence of 8% (vol/vol) ethanol revealed adaptation to ethanol stress, since these cells showed higher retention of compounds absorbing at 260 nm. Moreover, for concentrations higher than 10% (vol/vol), lower rates of passive proton influx were observed in these ethanol-adapted cells, especially at pH 3.5. The effect of ethanol on O. oeni cells was studied as the ability to efficiently retain carboxyfluorescein (cF) as an indicator of membrane integrity and enzyme activity and the uptake of propidium iodide (PI) to assess membrane damage. Flow cytometric analysis of both ethanol-adapted and nonadapted cells with a mixture of the two fluorescent dyes, cF and PI, revealed three main subpopulations of cells: cF-stained intact cells; cF- and PI-stained permeable cells, and PI-stained damaged cells. The subpopulation of O. oeni cells that maintained their membrane integrity, i.e., cells stained only with cF, was three times larger in the population grown in the presence of ethanol, reflecting the protective effect of ethanol adaptation. This information is of major importance in studies of microbial fermentations in order to assign bulk activities measured by classical methods to the very active cells that are effectively responsible for the observations.
The control of activity of lactic acid bacteria that carry out malolactic fermentation (MLF) is an important feature of the technology of modern commercial wine production (26). Oenococcus oeni (10) is recognized as the principal microorganism responsible for MLF (22) under stress conditions such as those prevailing in wine. MLF consists of the decarboxylation of l-malic acid to l-lactic acid, which decreases the total acidity and improves the stability and quality of wine (25). The physiological benefits of MLF to the bacteria have been a matter of discussion for the last few years, but it is now well accepted that the malolactic activity generates an electrochemical gradient across the cytoplasmic membrane as a consequence of the electrogenic transport of monoprotonated malate and concomitant consumption of a proton in the cytoplasm during its decarboxylation (34, 39). The proton motive force generated is of sufficient magnitude to drive ATP synthesis by the H+-ATPase (6, 17, 40).
Inoculation of O. oeni starter cultures directly into wine leads to significant cell mortality (4) and consequently failure of MLF. Actually, one or more steps of reactivation and adaptation of starter cultures to wine conditions are required to enhance the survival of the bacteria when inoculated into wine (22). The ethanol and acid resistances of O. oeni are considered crucial for its survival in wine. Three mechanisms appear to be involved in the acid tolerance of O. oeni: (i) activation of MLF as a proton motive force-generating process (40), (ii) stress protein synthesis (14), and (iii) activation of the membrane-bound H+-ATPase (11).
The ethanol toxicity is generally attributed to the preferential partitioning of ethanol in the hydrophobic environment of lipid bilayers, resulting in a disruption of membrane structure that adversely affects many membrane-associated processes (29). It has been reported that membrane disordering resulting from ethanol exposure leads to leakage of intracellular compounds, including enzymatic cofactors and ions essential for cell growth and fermentation (33, 41), as well as dissipation of the electrochemical gradient across the cytoplasmic membrane (24, 29).
The preadaptation of starter cultures is very time-consuming and requires microbiological expertise. Therefore, the practical application of commercial malolactic starter cultures has been limited. The development of new industrial starters surviving direct inoculation represents a large benefit for wine technology (31). It is therefore essential to enhance our understanding of the mechanisms involved in ethanol toxicity and tolerance in O. oeni. It has been suggested that the cytoplasmic membrane may be the target for the physiological events inducing better survival of lactic acid bacteria in wine, e.g., by modifying their fatty acid composition (9, 13, 23).
The purpose of this work was to study the effect of ethanol on the membrane integrity of O. oeni. Ethanol-induced leakage of UV-absorbing compounds has been proven to be a valuable technique for monitoring the ethanol resistance of microorganisms e.g., Saccharomyces cerevisiae (25, 30, 41, 42). In addition, since the pH of wine is very low (e.g., lower than 3.5), the passive influx of protons is an important parameter to be taken into account. Both techniques involve bulk measurements and assume that all the cells will contribute equally to the global performance of MLF. The use of fluorescent probes in combination with flow cytometry allows the discrimination of O. oeni ethanol-stressed cells in different physiological states (7), i.e., the ethanol tolerance of different subpopulations can be readily identified. For that, we use a permeant (carboxy fluorescein [cF]) and an impermeant (propidium iodide [PI]) probe simultaneously to assess membrane permeability and integrity of stressed O. oeni.
Cells with intact membranes are impermeable to charged fluorescent dyes such as PI. However, if membrane integrity is lost, PI can enter the cell and, by binding to the nucleic acid, make the cells become fluorescent. The esterified prefluorochrome carboxy fluorescein diacetate (cFDA) is converted to cF by functional cytoplasmic enzymes, i.e., esterases. cF is negatively charged at physiological pH and consequently will accumulate inside cells with an intact cytoplasmic membrane. Thus, cF-stained cells have esterase activity and an intact membrane (16, 35). These measurements can often be complicated by extrusion pumps, which at the same time are powerful indicators of functioning cell metabolism (1, 2).
In our studies we use deenergized cells to exclude the interference of metabolic activity, allowing the assessment of membrane integrity by dye retention (cF) and/or dye exclusion (PI). Moreover, monitoring the leakage from cells loaded with a foreign molecule (cF) allows permeability to be studied without the superimposed effect of the molecule size. Multiparameter flow cytometry analysis of both ethanol-adapted and nonadapted O. oenis cells allows assessment of population heterogeneity, which may provide tools for optimization of MLF in wine.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
O. oeni GM (Microlife Technics, Sarasota, Fla.) was cultured at 30°C in FT80 medium (pH 4.5) (5) modified by the omission of Tween 80 and containing 10 g of dl-malic acid per liter. Glucose and fructose were autoclaved separately and added to the medium just before inoculation, at final concentrations of 2 and 8 g per liter, respectively. Early-stationary-phase cultures were diluted 100-fold in fresh medium, incubated for 24 h, and then used to obtain 1% inoculated cultures. In the adaptation experiments, the final culture medium was supplemented with 8% (vol/vol) ethanol.
Leakage of compounds absorbing at 260 nm.
The method adopted was described by Salgueiro et al. (41), with some modifications. Cells were harvested at the end of the growth phase (OD600 = 0.4) by centrifugation at 6,160 × g at 4°C for 10 min and washed twice in potassium phosphate buffer (50 mM, pH 5.2). The cell pellet and cell suspension prepared in the same buffer at a concentration of 50 mg ml−1 (dry weight) were kept on ice. Cell suspension (80 μl) was placed in an Eppendorf tube and incubated at 30°C for 30 s, and 20 μl of ethanol solution at 30°C was added. Ethanol solutions were prepared from 99% (vol/vol) ethanol in phosphate buffer in order to obtain final concentrations of 0, 8, 12, and 16% (vol/vol). Immediately after ethanol addition, the reaction was stopped by dilution with 1.4 ml of the same buffer at room temperature, and samples were centrifuged for 6 min at 8,500 × g. The supernatants were removed and filtered through cellulose filters (Nucleopor) with a pore size of 0.22 μm. The total amount of compounds absorbing at 260 nm was measured in a spectrophotometer (Beckman DU-70) and expressed as nanomoles of NAD+ (25). Similar experiments were performed with cells precultured in the same medium with 8% (vol/vol) ethanol.
Proton influx.
Cells were harvested at the end of the exponential growth phase (OD600 = 0.4) by centrifugation at 6,160 × g at 4°C for 10 min and washed twice in phosphate buffer (2 mM, pH 7.0). The cell suspension was prepared in the same buffer at a concentration of 30 mg/ml and kept on ice. Proton movements were measured at 30°C with a standard pH meter, Radiometer PHM62, connected to a recorder. In a water-jacketed cell with a volume of 10 ml, 0.3 ml of a cell suspension, distilled water, and ethanol were mixed to a final volume of 3 ml. The pH was rapidly adjusted to 3.5 or 4.5 by addition of HCl (100 mM). Subsequent pH changes were registered over a time interval in which a linear pH variation was observed. At the end of each experiment, the signal was calibrated by using a solution of HCl (10 mM). The rate of proton influx was expressed as the rate of decrease of the concentration of extracellular protons, according to Leão and van Uden (24). Similar experiments were performed with cells precultured in the presence of 8% (vol/vol) ethanol.
Loading of cells with cF.
Cells were harvested at the end of the exponential growth phase (OD600 of approximately 0.4) by centrifugation, washed twice with 50 mM potassium phosphate buffer (pH 7.0), and concentrated in the same buffer to an OD600 of 20. The cells were deenergized with 2-deoxyglucose (at a final concentration of 2 mM) by incubation at room temperature for 30 min. The cells were washed and resuspended in 50 mM potassium phosphate buffer (pH 7.0) to an OD600 of 20 for fluorimetric analyses or to an OD600 of 5 for flow cytometric analyses. A stock solution of 2.3 mg of 5(6)-carboxy fluorescein diacetate (cFDA) (Molecular Probes, Eugene, Oreg.) per ml was prepared in acetone and stored at −20°C in the dark. cFDA was added to a concentration of 50 μM to the cell suspension, and the mixture was incubated at 30°C for 15 min (or 60 min for cells pregrown in presence of 8% [vol/vol] ethanol). Immediately after labeling, the cells were spun down, washed once, and resuspended in 50 mM potassium phosphate buffer (pH 7.0) to an OD600 of 2.0 for fluorimetric analyses.
Fluorescent labeling with PI.
PI, a positively charged fluorescent nucleic acid dye, was used to stain cells with compromised membranes. Stock solutions of 1.0 mg of PI (Molecular Probes) per ml were prepared in distilled water, stored in the refrigerator, and kept in the dark. Cell suspensions at an OD600 of 5 were diluted 1,000-fold in 50 mM potassium phosphate buffer (pH 7.0), and PI was added to a final concentration of 7.5 μM. Cells were incubated at 30°C for 10 min. For double staining assays, suspensions (OD600 of 0.005) of cF-stained cells were used.
Esterase activity.
Cell extracts were prepared by disrupting 600-μl portions of cell suspension (OD600 = 40) by bead beating (six times for 30 s with 45-s intervals to cool the samples). The cell debris was removed by centrifugation at 6,160 × g at 4°C for 2 min. The cFDA hydrolysis activity of cell extracts was determined by incubation of 100 μl of 1.0 mM cFDA and 250 μl of the cell extract in 50 mM potassium phosphate buffer (pH 7.0) in a total volume of 1.0 ml at 30°C. The increase in cF concentration over time was monitored by measuring the A490 at 5-min intervals for 20 min. The values were corrected for the chemical hydrolysis of cFDA.
Measurement of cF efflux.
cF-loaded cells were washed twice and resuspended in 50 mM potassium phosphate buffer (pH 7.0) to a final OD600 of 2.0. At time zero, cell suspensions were placed in a water bath at 30°C and incubated without and with ethanol (8, 12, and 16% [vol/vol]). Samples (200 μl) were withdrawn at specific time points and immediately centrifuged to remove the cells. To measure the cF labeling capacity, labeled cells were lysed by incubation at 70°C for 15 min, and the debris was removed by centrifugation. The fluorescence of the supernatant was measured fluorimetrically (excitation at 490 ± 5 nm and emission at 515 ± 5 nm) with a Perkin-Elmer LS 50B luminescence spectrometer. From the fluorescence of the supernatants and the total labeling capacity, the intracellular concentrations of cF at the sampling points were calculated. For flow cytometry assays, a suspension of labeled O. oeni cells (OD600 = 5) was diluted 1,000-fold in 50 mM potassium phosphate buffer (pH 7.0) to a final concentration of approximately 106 cells per ml in the absence and presence of ethanol. At time zero, the cells were placed in a water bath at 30°C. Time series were made by taking 100-μl aliquots diluted with 50 mM potassium phosphate buffer (pH 7.0) to a final volume of 1 ml and analyzed immediately.
Flow cytometric analysis.
The FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, Calif.) was used for single-cell light scattering and fluorescence measurements. The samples were illuminated with a 15-mW, 488-nm, air-cooled argon-ion laser, and fluorescence emission was detected at 530 nm for cF and at >670 nm for PI. List mode data from approximately 5,000 cells were collected and processed by using the CELLQuest program (version 3.1f; Becton Dickinson). Photomultiplier amplifier gains were set in the logarithmic mode for both light scattering and fluorescence. A combination of forward scatter (FSC) and side scatter (SSC) was used to discriminate bacteria from background. Light scattering and fluorescence were triggered by side angle light scattering, with the threshold limit set to 200 channels, in order to reduce background noise. Data were analyzed with the WinMDI program (version 2.8: Joseph Trotter, John Curtin School of Medical Research, Canberra, Australia [http://jcsmr.anu.edu.au]).
Additional analytical methods.
Cellular dry weight was determined by filtering 80 ml of the cell suspensions through preweighed polyethylene filters, with a pore size of 0.22 μm, and dried at 100°C in an oven until a constant weight was reached. As a control, the dry weight of the same volume of phosphate buffer was also determined. Protein was assayed by the method of Lowry et al. (27).
RESULTS
Passive proton influx.
In order to ensure that passive proton movements were not concealed by proton extrusion through the membrane ATPase, two controls were used. Assays were performed in the presence of either 2-deoxy-d-glucose (a glucose analogue, the phosphorylation of which results in depletion of ATP) or DCCD (N,N-dicyclohexylcarbodiimide), an F0F1-ATPase inhibitor. The presence of these compounds in the assays did not affect the measurements of H+ influx, indicating that under the conditions used, there was no significant active proton movement. The passive proton influx was evaluated by extracellular alkalinization, measured by an acid-pulse titration technique as described in Materials and Methods. After rapid adjustment of the initial pH to 3.5 (pH of wine) or 4.5 (pH of growth), we observed that extracellular pH increased with time in the cell suspensions.
Ethanol induced an increase in the passive proton influx, and the rates were higher at low extracellular pH (Fig. 1). The exponential enhancement constant of proton influx induced by ethanol was not significantly affected by the initial extracellular pH and was 0.8 liters mol−1 at pH 3.5 and 4.5. Cells grown in the presence of 8% ethanol (vol/vol) (Fig. 1b) showed a higher tolerance to ethanol, since lower passive proton influx rates were observed for concentrations up to 10% (vol/vol), especially at pH 3.5.
FIG. 1.
Effect of ethanol on passive proton influx in cell suspensions of O. oeni at pH 3.5 (•) and pH 4.5 (⧫). Cells were grown at pH 4.5 and 30°C in the absence (a) or in the presence (b) of 8% (vol/vol) ethanol. The results are the means of four different assays and were performed at the growth temperature.
Leakage of intracellular compounds.
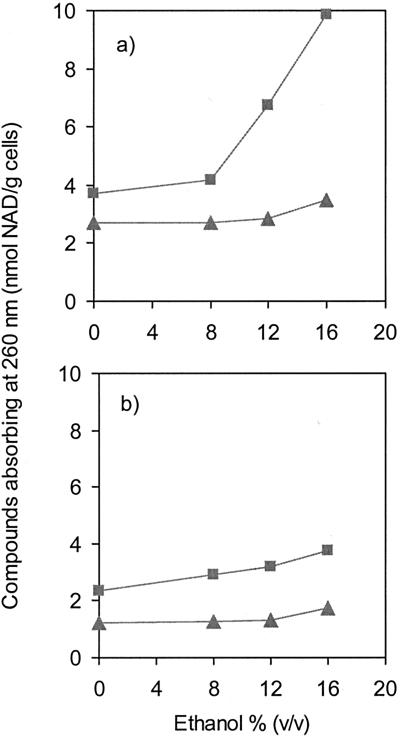
Leakage of intracellular compounds was evaluated immediately after ethanol addition in order to evaluate the instantaneous effect of ethanol shock and also after 5 min of incubation with ethanol. In O. oeni cells exposed for 5 min to increasing concentrations of ethanol, the loss of compounds absorbing at 260 nm was stimulated only by concentrations of ethanol over 8% (vol/vol) (Fig. 2a). For these concentrations, the extracellular amount of compounds absorbing at 260 nm was clearly correlated with increasing ethanol concentrations. At 16% (vol/vol) ethanol, an immediate loss of compounds absorbing at 260 nm was observed, pointing to an instantaneous disorder of the membrane (Fig. 2a). Figure 2b shows the effect of the adaptation on the ethanol-induced membrane disordering, since leakage of compounds was much lower in cells grown in the presence of 8% (vol/vol) ethanol. Comparison of Fig. 2a and 2b shows that the extracellular concentrations of compounds absorbing at 260 nm released from adapted cells after 5 min of incubation with ethanol were on the same order as the values monitored immediately after ethanol addition in nonadapted cells.
FIG. 2.
Ethanol-induced efflux of compounds absorbing at 260 nm from cells of O. oeni grown at 30°C in the absence (a) or in the presence (b) of 8% (vol/vol) ethanol. The efflux values were measured immediately after the ethanol shock (▴) and after 5 min of exposure to ethanol (▪).
Accumulation of cF.
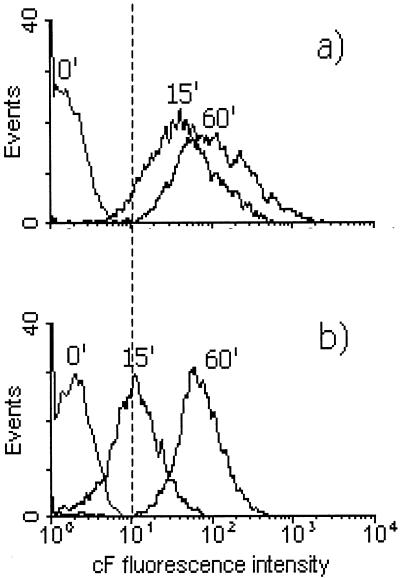
Cells grown with and without ethanol were incubated in the presence of cFDA, and the accumulation of cF was followed over time. In the flow cytometry analysis of O. oeni cells, the labeled population gave a peak in the green fluorescence histogram, which was resolved from the signal of nonlabeled cells. cFDA was efficiently taken up and hydrolyzed by O. oeni. Nearly all O. oeni cells of a nonadapted cell suspension were labeled with cF within a few minutes of incubation with cFDA, while the cells grown in the presence of 8% (vol/vol) ethanol took much longer to become fluorescent (Fig. 3). Analysis of cF staining of ethanol-grown cells after 15 min resulted in overlapping peaks of labeled and nonlabeled cells. Only after 60 min of incubation with cFDA did the labeled population give a peak in the flow cytometry histogram distinct from the nonlabeled cells (Fig. 3b). This indicates that cFDA uptake is reduced, presumably because it is less soluble in the cytoplasmic membrane of ethanol-adapted cells, since cells grown both with and without ethanol had similar esterase activity, 0.067 and 0.066 μmol of cF/min/mg of protein, respectively. This implies a change in composition and/or organization of the cytoplasmic membranes of cells grown in the presence of ethanol.
FIG. 3.
Single-parameter histograms of cF uptake in O. oeni cells grown without ethanol (a) and in presence of 8% (vol/vol) ethanol (b). Cells were incubated with 50 μM cFDA in potassium phosphate buffer (pH 7) at 30°C. Subsequently, the increase in fluorescence after 15, 30, and 60 min was measured by flow cytometry.
Ethanol-induced cF leakage.
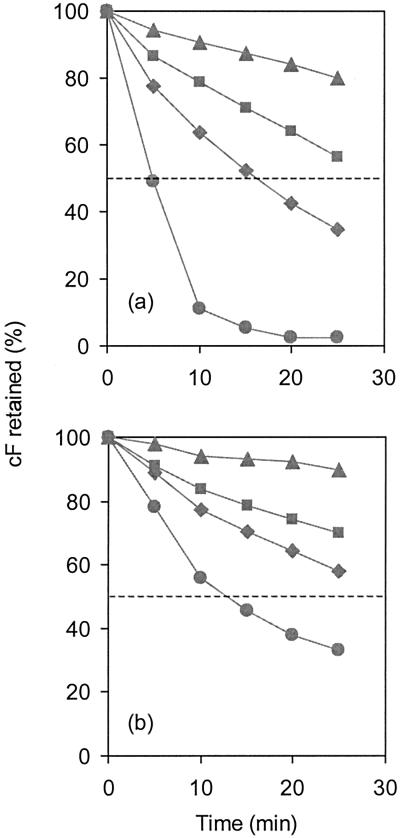
O. oeni cells retain cF when not energized and extrude the probe rapidly by an energy-dependent efflux system (data not shown). Deenergized cells of O. oeni were loaded with cF, and subsequently cF retention was studied in the presence of increasing concentrations of ethanol by spectrofluorimetrically measuring the amount of probe released in the supernatant of the cell suspension. In the absence of ethanol, a gradual release of cF into the supernatant was observed at a rate of 0.8% per min. In cells incubated with 8% and 12% (vol/vol) ethanol, the rates of cF efflux increased to approximately 1.7% and 2.6% per min, respectively. The permeabilization of cells with 16% ethanol resulted in rapid loss of cF, i.e., 50% in 5 min (Fig. 4a). O. oeni cells grown in the presence of 8% (vol/vol) ethanol were able to retain cF more efficiently (Fig. 4b), even in the absence of ethanol (rate, 0.4% per min). When these cells were exposed to 8% and 12% (vol/vol) ethanol, the rate values of cF efflux were approximately 1.2% and 1.7% per min, respectively. Notably, challenging preadapted cells with 16% (vol/vol) ethanol more than doubled the time needed to release 50% of accumulated cF (t1/2) compared to that in nonadapted cells (Fig. 4b).
FIG. 4.
Ethanol-induced cF efflux in deenergized O. oeni cells. Cells were loaded with cF by incubation at 30°C with 50 μM cFDA. The efflux of cF was measured at 30°C in 50 mM potassium buffer (pH 7.0) in nonadapted (a) and ethanol-adapted (b) cells. Concentrations of ethanol in the assay were (▴) 0, (▪) 8, (⧫) 12, and (•) 16% (vol/vol).
When the total cF retained in a suspension of O. oeni declines by 50%, this could be because all of the cells released half of the cF accumulated or because half of the cells have lost all of the probe. Thus, flow cytometric analysis that enables measurements to be made on individual cells may provide additional information. The points marked in Fig. 4, corresponding to extreme situations, were selected for more detailed analysis by flow cytometry. In the flow cytometry analyses, the O. oeni cells were easily detected by their light scatter. In the dotplot of the FSC and SSC, a region was created that comprised the cell population. Interfering particles that also had an SSC above the threshold but were not in the delineated region were thus disregarded.
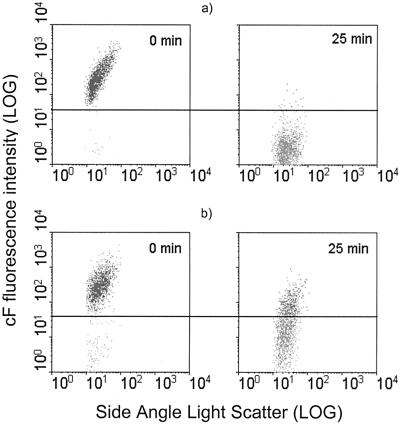
In cell suspensions of O. oeni grown without ethanol, 98% of the population could be labeled with cF. When these cells were incubated without ethanol, they efficiently retained the probe (data not shown). However, when cF-loaded cells were exposed to 16% (vol/vol) ethanol for 25 min, the probe was lost by passive diffusion, as determined from the decrease in the fluorescence intensity in the 515-nm fluorescence/side scatter dot plots (Fig. 5a). Less than 1% of the cells were considered cF fluorescent. In cells grown in the presence of 8% (vol/vol) ethanol, 95% of the population was cF labeled, suggesting that adaptation to ethanol resulted in a small additional loss of the labeling capacity of 3% of the population. After incubation of these cells with 16% (vol/vol) ethanol for 25 min, two subpopulations could be distinguished, one was able to maintain the cF and the other of which was unable to retain the probe efficiently (27.6%). Flow cytometry analysis thus revealed that the population of O. oeni is heterogeneous with regard to ethanol resistance based on the cF retention capacity.
FIG. 5.
Flow cytometric analysis of ethanol-induced cF efflux in O. oeni cells. Two-parameter dot plots are shown of cF-loaded cells previously deenergized and incubated with 16% ethanol (vol/vol) at 30°C in potassium phosphate buffer (pH 7). Samples were withdrawn immediately after ethanol addition and after 25 min of incubation. cF retention was analyzed by flow cytometry in nonadapted (a) and ethanol-adapted (b) cells.
These ethanol-stressed cells were also tested for their labeling capacity, and it was found that stressing the cells with 16% ethanol for 25 min resulted in a subpopulation that was not labeled with cF in either adapted (16%) or nonadapted (25%) cells (Fig. 6). This means that stressing O. oeni cells with 16% (vol/vol) ethanol for 15 min resulted in a loss of cF-labeling capacity of 23% and 10% of the population of nonadapted and ethanol-adapted cells, respectively.
FIG. 6.
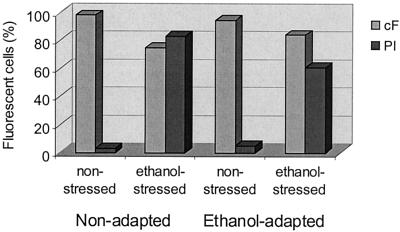
Effect of ethanol on fluorescence labeling of O. oeni with fluorescent probes. Cells were stressed with 16% (vol/vol) ethanol for 25 min, washed with potassium phosphate buffer (pH 7), and labeled in the same buffer at 30°C. PI exclusion and cF retention were analyzed by flow cytometry in nonadapted and 8% (vol/vol) ethanol-adapted cells.
PI staining.
Flow cytometric analysis of O. oeni cells revealed two subpopulations corresponding to damaged cells, i.e., cells that were PI stained, and to cells with an intact cytoplasmic membrane, which were not stained. By growing cells in the presence of 8% (vol/vol) ethanol, 5% of the population lost their membrane integrity, compared with 3% of the nonadapted cells. These results were confirmed by the results of cF labeling capacity (Fig. 6) and signify that the adaptation of O. oeni cells to ethanol is associated with increased membrane damage of a small part of the population. Furthermore, the percentage of cells stained with PI remained constant during the treatments performed for flow cytometric analysis (data not shown).
Ethanol-stressed O. oeni cells showed a strong increase in permeability to PI (Fig. 6). The PI-stained subpopulation after exposure to 16% (vol/vol) ethanol for 25 min was 83% in nonadapted cells and 61% in ethanol-adapted cells. The positive effect of adaptation was then confirmed by the fact that in ethanol-adapted cells, only 56% of the population lost the capacity to exclude PI, compared to 80% of the population of nonadapted cells.
Double staining with cFDA and PI.
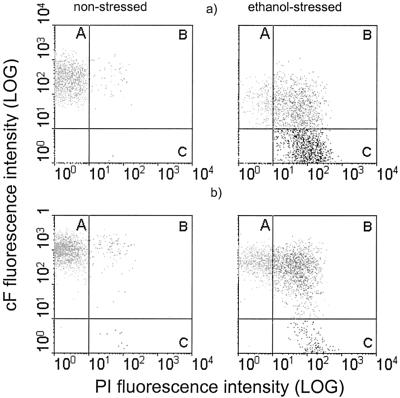
Although we observed an increase in PI staining in these ethanol-stressed cells, which points to complete damage of that subpopulation, the fraction of cF-labeled cells remained high (Fig. 6). These results suggest that ethanol stress induces a transient influx of PI into O. oeni cells. Therefore, both probes were used in combination to gain additional information about the physiological state of these ethanol-stressed cells. Staining the cells with a mixture of the two fluorescent dyes (cF and PI) revealed three main subpopulations corresponding to intact cF stained cells (A), permeable cF-PI double-stained cells (B), and damaged PI-stained cells (C) (Fig. 7). It is evident that only a minority of the cells (12%) maintained their membrane integrity (A), i.e., stained only with cF (Fig. 7a). Approximately 35% of the PI-stained cells also accumulated cF (B), indicating a progressive change in the physiological status of O. oeni, as has become evident in this subpopulation with intermediate membrane permeability. In cells grown in the presence of 8% (vol/vol) ethanol and challenged for 25 min with 16% (vol/vol) ethanol, the subpopulation that stained with both cF and PI (B) represents 47% of the total population (Fig. 7b). However, the PI-stained subpopulation was significantly smaller, i.e., 14%, compared with cells grown without ethanol (53%), while the subpopulation corresponding to intact cells was much larger (39%).
FIG. 7.
Flow cytometry analysis of double-stained (PI and cF) O. oeni cells stressed with 16% (vol/vol) ethanol for 25 min. Three main subpopulations of cells were identified in both nonadapted (a) and ethanol-adapted (b) cells, which correspond to cF-stained intact cells (A), cF- and PI-stained cells in an intermediate state of permeability (B), and PI-stained damaged cells (C).
DISCUSSION
This report clearly shows that ethanol disords the O. oeni cytoplasmic membrane, leading to leakage of intracellular material absorbing at 260 nm and promoting the passive influx of protons. The inability of ethanol-stressed cells to retain cF efficiently also indicates that ethanol promotes an increase in the permeability of the O. oeni cytoplasmic membrane. Uptake of PI by O. oeni cells after exposure to ethanol further points to the ethanol-induced membrane damage. The observed effects suggest that failure of MLF after direct inoculation of O. oeni in wine may be explained by the deleterious effects of ethanol in combination with low pH of the wine.
First-order kinetics is commonly used to estimate permeability. However, the kinetics of passive efflux of intracellular compounds was complex, and the rate constant could not be easily derived (data not shown). Therefore, we used the leakage of the fluorescent probe cF to monitor the permeability of ethanol-stressed cells. Ethanol-induced membrane damage assessed by fluorimetric assays did not reflect the effect of ethanol on the permeability of the cytoplasmic membrane as monitored by leakage of compounds absorbing at 260 nm. The leakage of these intracellular compounds was only stimulated at ethanol concentrations above 8% (vol/vol). Typical compounds absorbing at 260 nm include NAD+, NADH, and AMP (25, 30). These molecules have significantly higher molecular mass than cF; NAD+ is 663 g per mol and that of cF is 376 g per mol. Consequently, a higher membrane disorganization is probably necessary to promote the leakage of intracellular compounds absorbing at 260 nm. This hypothesis is supported by the fact that cF leakage followed the global trend of ethanol concentration dependence that paralleled the increase in passive proton influx rates at increasing ethanol concentrations.
NAD+ is an important cofactor of the malolactic enzyme, and it has been reported that the delicate balance between NAD+ and NADH affects the activity of the enzyme (28). Conceivably, nonspecific leakage of intracellular material will significantly disturb this balance, and consequently it is expected that cell metabolism will be negatively affected. Moreover, it is obvious that ethanol-induced proton influx will affect physiological processes in O. oeni that are dependent on a pH gradient, such as ATP synthesis, the transport of l-leucine (39), and l-malate uptake (40, 32). Notably, Capucho and San Romão (3) observed that at pH 3.0, ethanol inhibits the malolactic activity at concentrations above 12% (vol/vol) ethanol, while at pH 5.0 no effect was detected at concentrations up to 20% (vol/vol) ethanol. This supports the idea that ethanol inhibits MLF by enhancing passive proton influx at low extracellular pH, decreasing the intracellular pH to values not suitable for malolactic enzyme activity, for which the optimum pH is 5.5 (3). MLF failure after direct inoculation of O. oeni in wine can be caused by the cytoplasmic membrane disorder induced by ethanol in combination with the low pH of the wine.
We also analyzed the effect of preadaptation to ethanol followed by exposure to high concentrations of this compound. Although permeabilization was induced by ethanol, cells grown in the presence of this compound were more resistant. Such cells showed lower passive proton influx rates at ethanol concentrations higher than 10% (vol/vol), especially at pH 3.5. O. oeni cells grown in the presence of 8% (vol/vol) ethanol also presented higher intracellular retention of compounds absorbing at 260 nm.
It is now well established that the survival of bacteria in a variety of potentially lethal conditions can be enhanced by preexposure to sublethal stress conditions of the same kind (11, 12, 18). In this work, we demonstrate that this acquisition of tolerance is a phenomenon observed not at the population level but at the level of single cells. Flow cytometric analysis of ethanol-adapted cells showed that the population is heterogeneous with respect to adaptation to ethanol. Thus, not all the cells developed a mechanism that partially increased the efficiency of their cytoplasmic membrane as a barrier, i.e., one subpopulation became completely efficient in retaining cF, while another was completely ethanol sensitive.
Our labeling experiments showed that ethanol-adapted cells of O. oeni take much longer to become cF fluorescent than nonadapted cells. This phenomenon can be explained by the difference in membrane permeability characteristics affecting the diffusion of cFDA. This suggests that cFDA is less soluble in the cytoplasmic membrane of cells grown in the presence of 8% (vol/vol) ethanol, since both cell types presented the same esterase activity. This result confirms the hypothesis that the mechanisms involved in ethanol adaptation are associated with modification of membrane composition, aiming to maintain the optimal activity of several biological processes (11).
In O. oeni, different stresses dramatically induce the expression of an 18-kDa small heat shock protein (Lo18) that was found to be peripherally associated with the membrane (15). The authors observed the synthesis of Lo18 after incubation of cells in the presence of 12% ethanol, but no induction was found at 10% (vol/vol) ethanol (14). This strongly supports the idea that the ability of this bacterium to adapt during growth at 8% (vol/vol) ethanol is related to the capacity to regulate the membrane composition. Actually, it has been firmly established that the fatty acid composition of microbial cells is often modified in response to environmental changes (19, 20, 21, 36). Garbay et al. (13) observed that the fatty acid composition of the O. oeni membrane varied when cells were grown in the presence of wine. These conditions induced a twofold increase in the ratio of unsaturated to saturated fatty acids.
The double staining of ethanol-stressed cells with cFDA and PI revealed an interesting population heterogeneity. It is generally assumed that bacterial permeability to nucleic acid-binding dyes such as PI is associated with the presence of irreparable breaches in the membrane (38), making it at the same time impossible for the cells to retain cF (8, 37). However, in this work we found large subpopulations of ethanol-stressed cells stained with both cF and PI, showing that such assumptions are too simplistic and indicating that the mechanisms of action of ethanol may be more complex than commonly perceived. PI-cF double staining has also been observed in bile salt-stressed bifidobacteria cells (Ben-amor et al., unpublished data). These results support the idea that dye exclusion assays should be designed to reflect the complexity of the process being investigated rather than to estimate cell death (43), especially if stress factors or treatments that target the cell membrane are involved.
The size of the subpopulation of cells in an intermediate state of membrane damage (double-stained cells) was similar for both adapted and nonadapted cells. However, the positive effect of ethanol adaptation was demonstrated by the fact that the subpopulation of O. oeni cells that maintained their membrane integrity, i.e., cells stained only with cF, was three times larger for cells grown in the presence of ethanol. Moreover, the subpopulation corresponding to damaged cells, i.e., stained only with PI, was almost four times smaller in these ethanol-adapted cells. Our results show that simultaneous assessment of changes in two physiological characteristics, esterase activity and membrane composition, by multiparametric flow cytometry allows distinction between different levels of ethanol damage in ethanol-adapted populations. This information is of major importance in the study of MLF in wine in order to assign bulk activities measured by classical methods to the very active cells of the starter culture that are effectively responsible for the observations. Sorting of these subpopulations and further physiological analysis represents a powerful strategy for understanding of the mechanisms involved in ethanol stress response and tolerance.
REFERENCES
- 1.Breeuwer, P., J. L. Drocourt, F. M. Rombouts, and T. Abee. 1994. Energy-dependent, carrier-mediated extrusion of carboxyfluorescein from Saccharomyces cerevisiae allows rapid assessment of cell viability by flow cytometry. Appl. Environ. Microbiol. 60:1467-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breeuwer, P., J. L. Drocourt, N. Bunschoten, M. H. Zwietering, F. M. Rombouts, and T. Abee. 1995. Characterization of uptake and hydrolysis of fluorescein diacetate and carboxyfluorescein diacetate by intracellular esterases in Saccharomyces cerevisiae, which result in accumulation of fluorescent products. Appl. Environ. Microbiol. 61:1614-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capucho, I., and M. V. San Romão. 1994. Effect of ethanol and fatty acids on malolactic activity of Oenococcus oeni. J. Appl. Microbiol. Biotechnol. 42:391-395. [Google Scholar]
- 4.Carre, E. 1982. Recherches sur la croissance des bacteries lactiques en vinification. Désacidification biologique des vins. Ph.D. thesis. Université de Bordeaux II, Bordeaux, France.
- 5.Cavin, J. F., P. Schmitt, A. Arias, J. Lin, and C. Divies. 1988. Plasmid profiles in Leuconostoc species. Microbiol. Aliment. Nutr. 6:55-62. [Google Scholar]
- 6.Cox, D. J., and T. Henick-Kling. 1989. Malolactic fermentation, a chemiosmotic energy-yielding (ATP) decarboxylation reaction. J. Bacteriol. 171:5750-5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davey, H., and D. Kell. 1996. Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single-cell analysis. Microbiol. Rev. 60:641-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deere, D., J. Shen, G. Vesey, P. Bell, P. Bissinger, and D. Veal. 1998. Flow cytometry and cell sorting for yeast viability assessment and cell selection. Yeast 14:147-160. [DOI] [PubMed] [Google Scholar]
- 9.Desens, C., and A. Lonvaud-Funel. 1988. Étude de la constitution lipidique de membranes de bactéries lactiques utilisées en vinification. Conn. Vigne Vin. 22:25-32. [Google Scholar]
- 10.Dicks, L. M. T., F. Dellaglio, and M. D. Collins. 1995. Proposal to reclassify Leuconostoc oenos as Oenococcus oeni [corrig.] gen. nov., comb. nov. Int. J. Syst. Bacteriol. 45:395-397. [DOI] [PubMed] [Google Scholar]
- 11.Drici-Cachon, Z., J. Guzzo, J.-F. Cavin, and C. Diviès. 1996. Acid tolerance in Leuconostoc oenos. Isolation and characterisation of an acid-resistant mutant. Appl. Microbiol. Biotechnol. 44:785-789. [Google Scholar]
- 12.Foster, J. W. 1991. Salmonella acid shock proteins are required for the adaptive acid response. J. Bacteriol. 173:6896-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garbay, S., N. Rozes, and A. Lonvaud-Funel. 1995. Fatty acid composition of Leuconostoc oenos, incidence of growth conditions and relationship with malolactic efficiency. Food Microbiol. 12:387-395. [Google Scholar]
- 14.Guzzo, J., J. F. Cavin, and C. Diviès. 1994. Induction of stress proteins in Leuconostoc oenos to perform direct inoculation of wine. Biotechnol. Lett. 16:1189-1194. [Google Scholar]
- 15.Guzzo, J., F. Delmas, F. Pierre, M. P. Jobin, J. Van Beeumen, J. F. Cavin, and C. Diviès. 1997. A small heat shock protein from Leuconostoc oenos induced by multiple stresses and during stationary growth phase. Lett. Appl. Bacteriol. 24:393-396. [DOI] [PubMed] [Google Scholar]
- 16.Haugland, R. P. 1996. Handbook of fluorescent probes and research chemicals. Molecular Probes Inc., Eugene, Oreg.
- 17.Henick-Kling, T., D. J. Cox, and E. B. Olsen. 1991. Production de l'énergie durant la fermentation malolactique. Rev. [/oe]nol. 132:63-66. [Google Scholar]
- 18.Heyde, M., and R. Portalier. 1990. Acid shock proteins of Escherichia coli. FEMS Microbiol. Lett. 69:19-26. [DOI] [PubMed] [Google Scholar]
- 19.Kamimura, K., F. Hiroyuki, T. Osamu, and Y. Yukiho. 1993. Effects of growth pressure and temperature on fatty acid composition of barotolerant deep sea bacterium. Appl. Environ. Microbiol. 59:924-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaneda, T. 1991. Iso and anteiso acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol. Rev. 55:288-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kieft, T. L., D. B. Ringelberg, and D. L. White. 1994. Changes in ester-linked phospholipid fatty acid profiles of subsurface bacteria during starvation and desiccation in a porous medium. Appl. Environ. Microbiol. 60:3292-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lafon-Lafourcade, S., E. Carre, and P. Ribéreau-Gayon. 1983. Occurrence of lactic acid bacteria during the different stages of vinification and conservation of wines. Appl. Environ. Microbiol. 46:874-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanéelle, G., and J. Asselineau. 1990. Biochimie bactérienne. Structures, biosynthèses et fonctions des constituants des enveloppes. Ministère de la Recherche et de l’Ensaignement Supérieure, Paris, France.
- 24.Leão, C., and N. van Uden. 1984. Effects of ethanol and other alkanols on passive proton influx in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 774:43-48. [DOI] [PubMed] [Google Scholar]
- 25.Lee, T. C., and M. J. Lewis. 1968. Identifying nucleotidic material released by fermenting brewer's yeast. J. Food Sci. 33:119-123. [Google Scholar]
- 26.Lonvaud-Funel, A. 1999. Lactic acid bacteria in the quality improvement and depreciation of wine. Antonie van Leeuwenhoek 76:317-331. [PubMed] [Google Scholar]
- 27.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 28.Miranda, M., A. Ramos, M. Veiga-da-Cunha, M. C. Loureiro-Dias, and H. Santos. 1997. Biochemical basis for glucose-induced inhibition of malolactic fermentation in Leuconostoc oenos. J. Bacteriol. 179:5347-5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra, P. 1993. Tolerance of fungi to ethanol, p. 189-208. In D. H. Jennings (ed.), Stress tolerance of fungi.. Marcel Dekker, New York, N.Y.
- 30.Nash, C. H., and N. A. Sinclair. 1968. Thermal injury and death in an obligatory psycrophilic yeast, Candida nivalis. Can. J. Microbiol. 14:691-697. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen, J. C., C. Prahl, and A. Lonvaud-Funel. 1996. Malolactic fermentation in wine by direct inoculation with freeze-dried Leuconostoc oenos cultures. Am. J. Enol. Vitic. 47:42-48. [Google Scholar]
- 32.Olsen, E. B., J. B. Russell, and T. Henick-Kling. 1991. Electrogenic l-malate transport by Lactobacillus plantarum: a basis for energy derivation from malolactate fermentation. J. Bacteriol. 173:6199-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osman, Y. A., and L. O. Ingram. 1985. Mechanism of ethanol inhibition of fermentation in Zymomonas mobilis CP4. J. Bacteriol. 164:173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poolman, B., D. Molenaar, E. J. Smid, T. Ubbink, T. Abee, P. P. Renault, and W. N. Konings. 1991. Malolactic fermentation: electrogenic malate uptake and malate/lactate antiport generate metabolic energy. J. Bacteriol. 176:6030-6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson, J. P., Z. Darzynkiewicz, P. N. Dean, A. Orfao, P. S. Ribinovitch, C. C. Stewart, H. J. Tanke, L. L. Wheeless, and L. G. Dressler (ed.). 1997. Current protocols in cytometry, vol. 1. Wiley, New York, N.Y.
- 36.Rose, A. H. 1989. Influence of environment on microbial lipid composition, p. 255-275. In C. Ratledge and S. G. Wilkinson (ed.), Microbial lipids, vol. 2. Academic Press, New York, N.Y.
- 37.Ross, D. D., C. C. Joneckis, J. V. Ordonez, A. M. Sisk, R. K. Wu, A. W. Hamburger, and R. E. Nora. 1989. Estimation of cell survival by flow cytometric quantification of fluorescein diacetate/propidium iodide viable cell number. Cancer Res. 49:3776-3782. [PubMed] [Google Scholar]
- 38.Roth, B., M. Poot, S. Yue, and P. J. Millard. 1997. Bacterial viability and antibiotic susceptibility testing with Sytox Green nucleic acid stain. Appl. Environ. Microbiol. 63:2421-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salema, M., B. Poolman, J. S. Lolkema, M. C. Loureiro Dias, and W. N. Konings. 1994. Uniport of monoanionic l-malate in membrane vesicles from Leuconostoc oenos. Eur. J. Biochem. 225:289-295. [DOI] [PubMed] [Google Scholar]
- 40.Salema, M., J. S. Lolkema, M. V. San Romão, and M. C. Loureiro Dias. 1996. The proton motive force generated in Leuconostoc oenos by l-malate fermentation. J. Bacteriol. 178:3127-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salgueiro, S. P., I. Sá-Correia, and J. M. Novais. 1988. Ethanol-induced leakage in Saccharomyces cerevisiae: kinetics and relationship to yeast ethanol tolerance and alcohol fermentation productivity. Appl. Environ. Microbiol. 54:903-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma, S. C., D. Raj, M. Forouzandeh, and M. P. Bansal. 1996. Salt-induced changes in lipid composition and ethanol tolerance in Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 56:189-195. [DOI] [PubMed] [Google Scholar]
- 43.Weisenthal, L. M., and M. E. Lippman. 1985. Clonogenic and nonclonogenic in vitro chemosensitivity assays. Cancer Treat. Rep. 69:1985.. [PubMed] [Google Scholar]