Abstract
Integrated fish farming combines livestock production with fish farming. Animal manure is shed directly into a fish pond as fertilizer and supports the growth of photosynthetic organisms. The livestock, mainly chickens and pigs, is often fed feed containing growth promoters. In this study we investigated the impact of integrated fish farming on the levels of antimicrobial-resistant bacteria in a pond environment. One integrated broiler chicken-fish farm was studied for 2 months immediately after the start of a new fish production cycle. A significant increase over time in the resistance to six different antimicrobials was found for the indicator organism Acinetobacter spp. isolated from composite water-sediment samples. The initial resistance levels prior to the new production cycle were 1 to 5%. After 2 months the levels of resistance to oxytetracycline and sulfamethoxazole reached 100%, and the levels of resistance to ciprofloxacin were more than 80%. The long-term effects of resistance on integrated farming were studied on seven additional farms. The resistance levels were particularly high among Enterococcus spp. and were also high among Acinetobacter spp. isolated from water-sediment samples compared to the resistance levels at four control farms. In conclusion, integrated fish farming seems to favor antimicrobial-resistant bacteria in the pond environment. This could be attributed to the selective pressure of antimicrobials in the pond environment and/or to the introduction of antimicrobial-resistant bacteria from animal manure. Potential risks to human health were not addressed in this study and remain to be elucidated.
Integrated fish farming is practiced throughout southeast Asia. The farming systems are relatively confined units with little exchange of water. Manure from livestock production is administered to fish ponds; the manure is directly consumed by fish, and the release of nutrients supports the growth of mainly photosynthetic organisms (21). This integrated fish farming system produces high yields with low input, with the fish receiving limited, if any, supplementary feed. In contrast, the livestock on the integrated farms, which includes chickens and pigs, is reared intensively, and antimicrobial agents are used as growth promoters and for prophylactic and therapeutic treatment. Within integrated fish farming systems, antimicrobials, their residues, and antimicrobial-resistant bacteria may enter the fish ponds through animal manure and/or excess feeding and are potential sources of antimicrobial-resistant bacteria. However, the impact of the use of animal manure in integrated fish farm environments on the occurrence of antimicrobial-resistant bacteria has to our knowledge not been investigated previously.
Antimicrobial resistance in traditional fish farming systems in temperate waters has been intensively studied (3). A high incidence of bacteria resistant to the antimicrobials used in aquaculture, including multiply resistant bacteria, has been found in fish farms and the surrounding aquatic environments (11, 16, 24, 30-32, 34). Furthermore, residues of antimicrobials have been found in the sediments of marine fish farms (7, 17). Overfeeding and water currents around marine fish farms, particularly on the seafloor, have been shown to significantly influence the buildup of antimicrobials in sediment (10). Microbial degradation, diffusion (30), and light and temperature conditions (22, 29) have also been shown to be factors that influence the turnover of antimicrobials in sediment. Accumulation of surplus antimicrobials and antimicrobial residues may occur in integrated fish farms when the ponds are only rarely emptied at the time of fish harvest. Such a buildup could establish selective pressure favoring selection and growth of antimicrobial-resistant bacteria. Although increased levels of bacterial antimicrobial resistance in and around fish farms may only occur transiently, there is a potential risk that antimicrobial resistance genes could be disseminated into a wide range of aquatic environmental bacteria. Antimicrobials approved for use as animal growth promoters are not associated with antimicrobial therapy in humans to avoid selection of bacteria resistant to important drugs. Nevertheless, resistance to one antimicrobial within a class of antimicrobials often confers resistance to other members of the same group (cross-resistance). The use of antimicrobials as growth promoters in animal husbandry has been linked to certain antimicrobial resistance patterns among human bacterial pathogens (5, 38), suggesting that there is a possible flow of antimicrobial resistance genes between animal and human pathogens. Potential transfer of resistant bacteria and resistance genes from aquaculture environments to humans may occur through direct consumption of antimicrobial-resistant bacteria present in fish and associated products.
Two types of indicator organisms for surveillance of antimicrobial resistance were used in this study. Acinetobacter spp., which are gram-negative coccobacilli that are nonmotile, nonfermentative, and easily isolated from aquatic environments (35), have previously been used as indicators of antimicrobial resistance in aquatic environments (13-15). Due to their ubiquitous distribution in the aquatic environment and their ability to develop antimicrobial resistance under selective conditions, these organisms are suitable indicators of antimicrobial resistance in such environments. In addition, Acinetobacter spp. have increasing significance as opportunistic pathogens in clinical settings (36). Enterococcus spp. are gram-positive cocci that are mainly associated with human and animal intestines and have become increasingly important in human medicine as causes of nosocomial infections. Furthermore, clinical enterococcal isolates have acquired resistance to a wide range of antimicrobials, making the infections difficult to treat (26). Due to the ability of enterococci to transfer transposons (including conjugative transposons), resistance plasmids, and sex pheromone plasmids to a broad range of recipients, they may act as a reservoir of resistance genes for gram-positive bacteria, including human pathogens (25). Enterococci have been isolated from different aquatic habitats, such as wastewater (20, 23, 28, 37), pristine water (23, 28), and aquaculture ponds (8). Enterococcus spp. of human, veterinary, and food origin have also been used as indicators of the occurrence and transfer of antimicrobial resistance (1, 2, 19).
The objective of this study was to determine whether integrated fish farming affects the levels of antimicrobial-resistant bacteria in the aquatic environments of fish ponds. In particular, the impact on antimicrobial resistance in bacteria from integrated chicken-fish farms was assessed and compared to the impact at fish farms with no deliberate input of animal waste or antimicrobials. Acinetobacter spp. and Enterococcus spp. were used as indicator organisms to determine possible effects on antimicrobial resistance in gram-negative and gram-positive bacteria, respectively. In addition, samples from integrated duck-fish and integrated pig-fish farms were studied to assess if the level of antimicrobial resistance may be dependent on the type of integrated farming system.
MATERIALS AND METHODS
Fish farms and sample types.
Samples were taken from four integrated chicken-fish farms, including two broiler chicken-fish farms (farms B1 and B2) and two layer chicken-fish farms (farms L1 and L2), two integrated duck-fish farms (farms D1 and D2), and two integrated pig-fish farms (farms P1 and P2). A schematic representation of an integrated fish farm is shown in Fig. 1. Samples from four fish farms with no deliberate input of animal waste or antimicrobials were used as controls (farms C1, C2, C3, and C4). Like the integrated farms, the control fish farms raised different fish species in polyculture systems; the species included Isok barb (Probarbus jullieni), Java barb (Barbodes gonionotus), Nile tilapia (Oreochromis niloticus), and common carp (Cyprinus carpio). The sizes of the ponds varied between 0.8 and 1.2 ha. The farms were located in Suphanburi and Nakhon Pathom provinces, which are approximately 80 km northwest of Bangkok, Thailand.
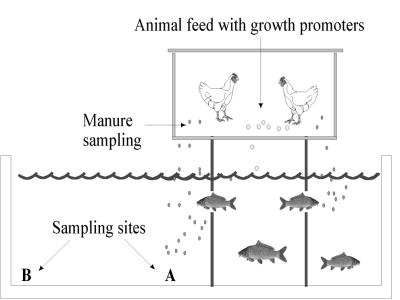
FIG. 1.
Schematic representation of an integrated chicken-fish farm. Sampling sites A and B show the relative positions of the two sites from which water-sediment samples were collected on each integrated fish farm.
Integrated broiler-fish farm B1 was studied for a 60-day period, and sampling began shortly after initiation of a new fish production cycle. The water was removed from the fish pond, and the upper 40 cm of sediment was removed before the pond was refilled with river water and stocked with fingerlings. Thus, in this pond it was possible to investigate the impact of chicken manure input on the development of bacterial antimicrobial resistance in an approximately pristine fish pond environment. During each visit to an integrated farm, two composite water-sediment samples were aspirated from the water-sediment interface at the bottom of the fish pond by using sterile plastic syringes and tubes. One sample was collected directly under the animal confinement area (designated site A), whereas the other sample was collected at the opposite end of the pond away from where the animals were kept, at site B (Fig. 1). Depending on the size of the pond, sites A and B were approximately 50 to 100 m apart. Manure samples were obtained from the animal cages by using sterile plastic bags for collection. Only one composite water-sediment sample was collected from each control fish farm during each visit. Samples were transported on ice to the laboratory and processed within 4 h after collection. Each farm was visited and sampled two to four times, either from October 1999 to January 2000 or from April to June 2000.
Isolation of Acinetobacter spp. and Enterococcus spp.
Acinetobacter spp. and Enterococcus spp. were isolated on Baumann medium (6) and Slanetz-Bartley medium (Oxoid Ltd., Basingstoke, England), respectively. Only the integrated broiler and layer chicken farms and the control fish farms were sampled for Enterococcus spp. Manure samples were diluted in 0.85% NaCl before they were inoculated onto the agar, while 0.1-ml water-sediment samples were directly inoculated onto agar plates. The plates were incubated at 30°C for 18 to 24 h (Acinetobacter spp.) or at 37°C for 42 to 48 h (Enterococcus spp.). Typical Acinetobacter spp. colonies were subcultured onto tryptone soya agar (Oxoid Ltd.), and identification was verified by colony hybridization with a genus-specific 16S rRNA-targeted alkaline phosphatase-labeled oligonucleotide probe (15). Typical Enterococcus colonies on Slanetz-Bartley medium agar plates were subcultured on tryptone soya agar plates, and identification was verified by genus-specific PCR detection (18) by using Ready-To-Go PCR beads (Amersham Pharmacia Biotech Inc., Piscataway, N.J.). Overnight broth cultures of isolates were stored in 15% glycerol and kept at −80°C. A total of 990 Acinetobacter and 244 Enterococcus isolates were obtained in this study.
Use of antimicrobials on integrated farms.
On the integrated fish farms, the chickens, pigs, and ducks were fed animal feed containing growth promoters. Additionally, the animals received antimicrobials in the drinking water prophylactically and for treatment of diseases. Only prophylactic and therapeutic treatments were recorded during the sampling period as information concerning the type of growth promoters used in the animal feed was not available. None of the fish in the ponds were given any antimicrobial treatment or given any traditional fish feed. The fish on the control farms were fed rice bran and other agricultural waste products.
Antimicrobial susceptibility testing.
Antimicrobial resistance was determined by the disk diffusion method on ISO Sensitest agar (Oxoid Ltd.). Six antimicrobials were selected for each of the two indicator organisms in order to have representatives of different classes of antimicrobials. Breakpoint values were selected to separate resistant and sensitive indicator bacteria based on the distributions of inhibition zone diameters, previous results obtained with environmental Acinetobacter spp. (15), and recommendations of the NCCLS (27). The concentrations of antimicrobials in the discs and the inhibition zone diameters for resistant isolates are indicated below in parentheses.
Acinetobacter spp. were tested for resistance to chloramphenicol (concentration in discs, 30 μg; inhibition zone diameters for resistant isolates, <17 mm), ciprofloxacin (5 μg; <23 mm), erythromycin (15 μg; <14 mm), oxytetracycline (30 μg; <17 mm), sulfamethoxazole (25 μg; <13 mm), and trimethoprim (5 μg; <14 mm). Due to limitations in the study, Acinetobacter spp. from integrated duck-fish and integrated pig-fish farms were tested only for resistance to chloramphenicol, oxytetracycline, and sulfamethoxazole. Enterococcus spp. were tested for resistance to chloramphenicol (30 μg; <12 mm), ciprofloxacin (5 μg; <13 mm), erythromycin (15 μg; <20 mm), gentamicin (200 μg; <13 mm), oxytetracycline (30 μg; <11 mm), and streptomycin (25 μg; <9 mm). All discs were purchased from Oxoid Ltd.
Statistical methods.
The structure of the resistance measurements was hierarchical and longitudinal. The hierarchical structure was sampling site nested within farm nested within type of farm, and the longitudinal structure was measurements over time within each sampling site. Various numbers of measurements were taken over time for the different farm type-farm-sampling site combinations. Due to this complex structure, data were analyzed within a generalized linear mixed model framework with a binomial family and logistic transformation (logit link). The results were analyzed in three steps. First, the results from farm B1 were analyzed with all factors included as fixed effects. Second, the effect of measurements over time within sampling site and the effect of sampling site within farm were tested by analysis of deviance (variance analysis of binomial data), with all factors as fixed effects. Both analyses were performed by using S-PLUS, version 6.0 (Insightful Corp., Seattle, Wash.). Finally, the effect of farm type was analyzed as a fixed effect, with farm as a random effect. Farm was chosen as a random effect to take into account the clustering at the farm level. Furthermore, the actual level of antimicrobial resistance on each farm was of minor importance compared to differences between farm types. The third analysis was performed by using the glimmix macro (39) and SAS, version 8.00 (SAS Institute Inc., Cary, N.C.). Fisher's exact test (S-PLUS, version 6.0) was used to test for differences in resistance between samples with the level at 0 or 100%. All tests for significance were performed at a 5% level.
RESULTS
Information about the farms studied, including the type of farm, the animals produced, and the use of antimicrobials for prophylactic and therapeutic purposes, in addition to the growth promoters (content unknown) in the animal feed, is shown in Table 1. Antimicrobials belonging to several different classes were used on all of the integrated fish farms.
TABLE 1.
Information about integrated and control fish farms studied
| Farm | Animals
|
No. of stocked fish | Antimicrobialsa | |
|---|---|---|---|---|
| Type | No. | |||
| Integrated farms | ||||
| B1 | Broilers | 5,000 | 60,000 | Amoxicillin, enrofloxacin, norfloxacin, tylosin |
| B2 | Broilers | 2,800 | 50,000 | Ampicillin, amoxicillin, enrofloxacin, erythromycin, neomycin, norfloxacin, sulfadiazine, trimethoprim |
| L1 | Layers | 2,000 | 70,000 | Enrofloxacin, sulfadimidine, tylosin |
| L2 | Layers | 1,000 | 55,000 | Chlorpheniamine, chlortetracycline, erythromycin, neomycin, oxytetracycline |
| P1 | Pigs | 30 | 20,000 | Amoxicillin, chlortetracycline, enrofloxacin, sulfadimethoxine |
| P2 | Pigs | 20 | 32,000 | Amoxicillin, chloramphenicol, chlortetracycline, norfloxacin, sulfadimethoxine |
| D1 | Ducks | 3,200 | 32,000 | Amoxicillin, chloramphenicol, chlortetracycline, oxytetracycline, sulfadimethoxine |
| D2 | Ducks | 3,000 | 40,000 | Chloramphenicol, chlortetracycline, oxytetracycline, sulfadimethoxine |
| Control farms | ||||
| C1 | NAb | 10,000 | NA | |
| C2 | NA | 30,000 | NA | |
| C3 | NA | 50,000 | NA | |
| C4 | NA | 10,000 | NA | |
Antimicrobial agents used prophylactically and for therapeutic treatment of the livestock during the sampling period. In addition, antimicrobials were introduced as growth promoters in feed.
NA, not applicable.
Integrated farm B1.
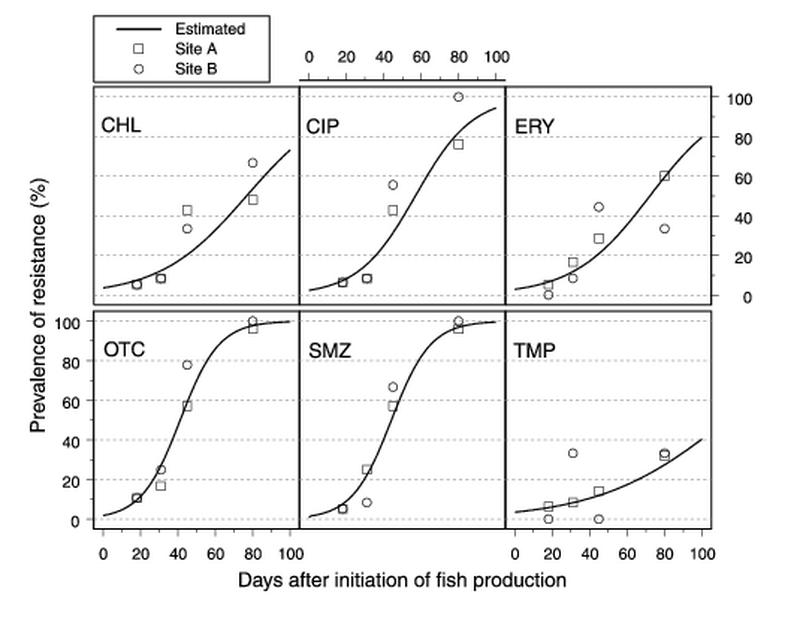
At the integrated broiler-fish farm which began a new fish production cycle 2 weeks prior to the first sampling date (farm B1), samples were taken on days 18, 31, 45, and 80 after fish production was initiated. A total of 106 Acinetobacter strains were isolated from water-sediment samples from farm B1, and equal numbers of isolates were obtained at the four sampling times. A fitted model of the temporal development of antimicrobial resistance was developed by using the parameters in Table 2. The corresponding time-response curves are shown in Fig. 2. The intercept was interpreted to be the estimated initial level of resistance, and the increase in odds per day was interpreted to be the factor that the odds of finding a resistant isolate was multiplied by per day (Table 2). The odds was the ratio of the probability of finding a resistant isolate to the probability of finding a sensitive isolate. The intercept varied between 0.9% (sulfamethoxazole) and 4.6% (trimethoprim), and the increase in the odds per day varied between 1.03 (trimethoprim) and 1.11 (sulfamethoxazole) (Table 2). For all antimicrobials, no significant difference in the increase in odds per day was found between sampling sites A and B, suggesting that there was little difference in resistance between bacteria isolated directly below an animal cage and bacteria isolated at a distance from the cage. The levels of resistance were more than 80% for ciprofloxacin and 100% for oxytetracycline and sulfamethoxazole at the end of the study period. Time-response relationships, such as the measurements obtained on farm B1, may be modeled in many ways. In this case the functional relationship was unknown, and the choice of the time-response function used was therefore in some ways arbitrary. Since the estimated odds were generally located in the full response area (0 to 100%), different time-response functions resulted in very similar responses (4, 9). A logistic link was therefore chosen in the model fitting.
TABLE 2.
Estimated parameters determined by logistic regression of temporal development of resistance in Acinetobacter spp. on integrated farm B1a
| Antimicrobial | Intercept (%) (95% confidence interval)b | Increase in odds per day (95% confidence interval)c |
|---|---|---|
| Chloramphenicol | 3.7 (0.98-11) | 1.04 (1.02-1.07) |
| Ciprofloxacin | 2.4 (0.62-7.1) | 1.07 (1.04-1.10) |
| Erythromycin | 2.9 (0.75-9.0) | 1.05 (1.03-1.07) |
| Oxytetracycline | 1.9 (0.37-6.6) | 1.10 (1.07-1.15) |
| Sulfamethoxazole | 0.9 (0.12-3.7) | 1.11 (1.08-1.17) |
| Trimethoprim | 4.6 (1.0-15) | 1.03 (1.01-1.05) |
See Fig. 2.
The intercept is an estimate of the background (or zero time) level of resistance.
The increase in odds per day is the factor that the odds of finding a resistant isolate is multiplied by per day.
FIG. 2.
Estimated time-response curves for the levels of antimicrobial resistance for Acinetobacter spp. on integrated farm B1. Abbreviations: CHL, chloramphenicol; CIP, ciprofloxacin; ERY, erythromycin; OTC, oxytetracycline; SMZ, sulfamethoxazole; TMP, trimethoprim.
No significant increase or decrease in the level of resistance over time (slope parameter not significantly different from 0) or at different sampling sites for any of the antimicrobials tested was found among the Enterococcus spp. isolated from water-sediment samples from farm B1. Accordingly, the resistance data for these isolates were regarded as replicate samples and included in the data presented for Enterococcus spp. Furthermore, the levels of resistance did not vary over time for the two indicator organisms isolated from manure samples from farm B1. Data on resistance levels in manure samples are presented in Tables 3 and 4.
TABLE 3.
Percentages of antimicrobial-resistant Acinetobacter isolates obtained from integrated and control fish farms
| Sample type | Farm type | No. of Acinetobacter isolates | % of Acinetobacter isolates resistant toa:
|
|||||
|---|---|---|---|---|---|---|---|---|
| Chloramphenicol | Ciprofloxacin | Erythromycin | Oxytetracycline | Sulfamethoxazole | Trimethoprim | |||
| Water-sediment | Control fish | 114 | 47 B | 3 A | 7 A | 20 A | 28 A | 17 A |
| Broiler-fish | 56 | 52 B | 33 B | 34 A | 38 AB | 25 A | 35 A | |
| Layer-fish | 208 | 9 A | 1 A | 20 A | 44 AB | 14 A | 23 A | |
| Duck-fish | 195 | 35 AB | NDb | ND | 45 AB | 36 A | ND | |
| Pig-fish | 186 | 23 AB | ND | ND | 66 B | 53 A | ND | |
| Manure | Broiler-fish | 12 | 42 A | 58 A | 42 A | 83 A∗ | 100 B∗ | 42 A |
| Layer-fish | 46 | 35 A∗ | 41 A∗ | 26 A | 91 A∗ | 83 B∗ | 13 A | |
| Duck-fish | 28 | 39 A | ND | ND | 86 A∗ | 50 A | ND | |
| Pig-fish | 39 | 54 A∗ | ND | ND | 97 A∗ | 97 B∗ | ND | |
For each antimicrobial, a statistical analysis was performed separately for water-sediment samples and manure samples, and different letters after the values indicate significant differences in levels of resistance between farm types. In addition, water-sediment and manure samples from similar farm types were compared; significantly higher levels of resistance in manure samples are indicated by asterisks.
ND, not determined.
TABLE 4.
Percentages of antimicrobial-resistant Enterococcus isolates obtained from integrated and control fish farms
| Sample type | Farm type | No. of Enterococcus isolates | % of Enterococcus isolates resistant toa:
|
|||||
|---|---|---|---|---|---|---|---|---|
| Chloramphenicol | Ciprofloxacin | Erythromycin | Gentamicin | Oxytetracycline | Streptomycin | |||
| Water-sediment | Control fish | 42 | 2 A | 0 A | 21 A | 0 A | 14 A | 2 A |
| Broiler-fish | 89 | 6 A | 7 AB | 97 B | 5 A | 67 B | 71 B | |
| Layer-fish | 40 | 5 A | 18 B | 60 AB | 0 A | 33 AB | 33 AB | |
| Manure | Broiler-fish | 40 | 0 A | 3 A | 100 B | 20 B∗ | 72 A | 85 B |
| Layer-fish | 33 | 12 B | 12 A | 76 A | 0 A | 52 A | 39 A | |
For each antimicrobial, a statistical analysis was performed separately for water-sediment samples and manure samples, and different letters after the values indicate significant differences in levels of resistance between farm types. In addition, water-sediment and manure samples from similar farm types were compared; a significantly higher level of resistance in manure samples is indicated by an asterisk.
The remaining integrated and control fish farms, all of which had been in operation for at least 3 months prior to sampling, were sampled between two and four times. Analysis of the deviance of the data obtained for Acinetobacter spp. and Enterococcus spp. isolated from manure and water-sediment samples showed no significant increase or decrease over time in the level of antimicrobial resistance for any antimicrobial tested during the sampling period (results not shown). Thus, the samples collected over time were considered replicate samples in the subsequent data analyses, reducing the data structure so that it was purely hierarchical (replicates within sites within farms within type). Furthermore, no significant differences in the antimicrobial resistance of bacterial isolates collected from pond sites A and B were found, reducing the complexity further to replicates within farms within types (results not shown).
Acinetobacter spp.
The mean levels of antimicrobial-resistant Acinetobacter spp. for the four types of integrated fish farms and the control farms are presented in Table 3. Due to the significant increases over time in the levels of resistance to all antimicrobials for isolates from farm B1 (Fig. 2), isolates obtained from water-sediment samples from this farm were not included in Table 3. Among Acinetobacter spp. isolated from water-sediment samples the level of resistance at the integrated farms was generally the same as or higher than the level of resistance at the control fish farms. The significant differences included differences in resistance to ciprofloxacin; the level of resistance to this antimicrobial at the broiler-fish farm (33%) was higher than the level of resistance at the control farms (2.6%) (P < 0.001). Also, resistance to oxytetracycline was higher among isolates from the pig-fish farms (66%) than among isolates from the control farms (20%) (P = 0.0252). The exceptions included resistance to chloramphenicol; the level of resistance to this antimicrobial was significantly higher at the control farms (47%) than at the integrated layer-fish farms (9.1%) (P = 0.0180).
The levels of antimicrobial resistance among Acinetobacter spp. from different manure samples were similar. One exception was resistance to sulfamethoxazole; the isolates from duck manure had a significantly lower level of resistance to this antimicrobial (50%) than isolates from other sources. More than 80% of the isolates from animal manure were resistant to oxytetracycline, regardless of the animal source (Table 3). Water-sediment and manure samples were compared within the same farm types to examine whether manure was a likely source of the antimicrobial-resistant bacteria found in the water-sediment samples. In 10 of 18 comparisons the level of resistance was significantly higher among isolates derived from manure samples; in particular, resistance to oxytetracycline was significantly higher (Table 3). In addition, the level of resistance to sulfamethoxazole was significantly higher for isolates from all manure samples except those from duck-fish farms.
Enterococcus spp.
The mean levels of antimicrobial resistance for Enterococcus spp. from the two types of integrated poultry-fish farms and the control farms are presented in Table 4. Although no significant temporal variations in resistance were found, the levels of resistance to most antimicrobials were higher among Enterococcus spp. isolated from the water-sediment samples from the integrated farms than among Enterococcus spp. isolated from the control fish farms. The differences were significant for resistance to erythromycin, oxytetracycline, and streptomycin among isolates from integrated broiler-fish farms and for ciprofloxacin resistance among isolates from integrated layer-fish farms.
Among isolates derived from manure samples, significantly higher levels of resistance to erythromycin and streptomycin were found for isolates from broiler-fish farms and significantly higher levels of resistance to chloramphenicol were found for isolates from layer-fish farms. Few differences between the levels of resistance were found for isolates derived from water-sediment and manure samples within the same farm type. Only the level of gentamicin resistance for isolates from manure samples from broiler-fish farms was significantly higher than the level of resistance for isolates from the corresponding water-sediment samples (P = 0.0302).
DISCUSSION
Significant temporal increases in resistance to all six antimicrobials included in this study were found for Acinetobacter spp. in water-sediment samples from farm B1. Sediment was removed from farm B1 immediately prior to initiation of the study, and therefore farm B1 constituted a pristine environment. The remaining integrated farms, which had all started fish production at least 3 months prior to sampling, did not show temporal increases in antimicrobial resistance, but generally higher resistance levels were found for the two indicator organisms when these levels were compared to levels found on the control farms.
The results obtained for integrated broiler-fish farm B1 showed that there was significant development of resistance during the first 2 months after fish production was initiated (Fig. 2). Significant increases in the levels of resistant Acinetobacter spp. were observed for all six antimicrobials studied, and the levels of resistance reached 100% for oxytetracycline and sulfamethoxazole (Fig. 2 and Table 2). The increases in the levels of antimicrobial resistance among Acinetobacter spp. on farm B1 could have been caused by several factors. The levels of resistance for Acinetobacter spp. from manure samples were higher than the levels of resistance for isolates from water-sediment samples (Table 3), suggesting that selection for resistant Acinetobacter spp. occurred in the gut of the chicken. On integrated broiler-fish farm B1 amoxicillin, enrofloxacin, norfloxacin, and tylosin were administered to the broilers during the 2-month sampling period (Table 1). Thus, the increases in resistance to ciprofloxacin, erythromycin, and oxytetracycline for isolates from water-sediment samples (Fig. 2) may have been associated with the use of these antimicrobials and the subsequent excretion of resistant bacteria. Upon release into the fish ponds, the resistant bacteria could have acted as donors of genes encoding antimicrobial resistance, or their presence could have been favored due to selection pressure exerted by the presence of antimicrobials or antimicrobial residues. Incorporation of manure into the sediment was believed to be greater directly under the animal cages, thereby creating higher selective pressure. However, no differences in levels of resistance were found when the susceptibilities of bacteria from different sites of the fish pond (site A and site B) were compared (Fig. 2). The manure could have been distributed evenly in the fish pond (e.g., by the bottom-feeding habits and activities of some of the fish in the polyculture system, such as the common carp), thereby eliminating site-specific selective pressure. Excessive chicken feed containing antimicrobial growth promoters and possibly antimicrobial-resistant bacteria could also have entered the pond environment. Preliminary studies showed that Acinetobacter spp. and Enterococcus spp. could not be isolated from commercial chicken feed (data not shown). Whether the increased levels of antimicrobial-resistant bacteria in the ponds were the result of introduction of resistant bacteria, selective pressure favoring growth of resistant isolates, and/or spread of resistance genes among the indicator populations was not determined. Genotyping of the indicator organisms and measurement of the concentrations of antimicrobials in the sediment would be needed to elucidate this. However, such analyses were beyond the scope of this study.
In contrast to Acinetobacter spp., Enterococcus spp. isolated from water-sediment samples from farm B1 showed no significant changes in the levels of antimicrobial resistance during the sampling period. Although Enterococcus spp. can survive in the aquatic environment (33), they do not appear to be widely distributed in this environment, unlike Acinetobacter spp. Accordingly, Enterococcus spp. with high levels of resistance present in chicken manure would be expected to make up a high proportion of the total number of Enterococcus spp. in a fish pond environment. This could explain why the levels of antimicrobial-resistant Enterococcus spp. in water-sediment samples did not increase during the 2-month sampling period. This hypothesis was supported by the results (Table 4) which showed little difference in the levels of resistance between Enterococcus spp. derived from manure and Enterococcus spp. derived from water-sediment samples from the same farm types.
The results in Tables 3 and 4 were obtained from different integrated farms and control fish farms which had commenced fish production at least 3 months prior to the sampling time. The levels of resistance among Acinetobacter spp. and Enterococcus spp. obtained from water-sediment samples from integrated farms were generally higher than the levels of resistance among isolates from control farms. Thus, the results obtained were an indication of the long-term effects on the development of antimicrobial resistance in integrated fish farms. No significant temporal increases or decreases in the levels of antimicrobial resistance among the indicator organisms from the integrated farms were seen. Population dynamics between resistant and sensitive bacteria in combination with variable selective pressures may have stabilized the levels of resistance, which fluctuated around a mean value. The mean resistance levels recorded for Acinetobacter spp. on farms that had produced fish for at least 3 months (Table 3) were lower than the levels obtained for the last sample obtained from newly started farm B1 (Fig. 2). This indicates that the impact of integrated fish farming on the development of antimicrobial resistance among Acinetobacter spp. was greatest at the beginning of a fish production cycle. Additional studies of longer duration on the development of antimicrobial resistance in newly established ponds are required to elucidate this phenomenon. Despite slightly lower levels of antimicrobial resistance for isolates from integrated layer-fish farms, this study did not reveal any significant differences in resistance levels among the different types of integrated farms.
The two types of indicator organisms were recovered by culture on agar media. No antimicrobials were added to select for resistant isolates. This approach may have underestimated the number of resistant bacteria in the populations since resistant bacteria may show reduced fitness and consequently reduced growth when there is no antimicrobial selective pressure. However, resistant bacteria have been reported to undergo mutational adaptations to recover general fitness (12). The relatively high levels of antimicrobial resistance found in this study indicate that resistant isolates were recovered effectively (Fig. 2 and Tables 3 and 4). The increased levels of antimicrobial-resistant bacteria in the ponds could be of concern since at harvest time the pond water is discharged into nearby streams or rivers. Selection of resistant bacteria and dissemination of such bacteria in natural habitats should be avoided to maintain a balance in the indigenous microbial populations in favor of susceptible organisms. It has been shown that the diversity of the microbial community in an aquatic environment is reduced when the community is exposed to water from fish farms with a recent history of antimicrobial treatment (14). It is not known whether the presence of antimicrobial-resistant bacteria in the pond environments of integrated farming systems and the possible presence of such bacteria in the fish gut represent a potential risk to humans through consumption of the fish and related products. Any assessment of possible human risk must take into account the impact of other food sources (e.g., consumption of poultry and pork products).
In conclusion, significant temporal increases in levels of antimicrobial resistance were found among Acinetobacter spp. isolated from water-sediment samples from a newly started integrated broiler-fish farm. The levels of resistance in indicator organisms suggested that there were long-term effects when integrated and control farms were compared. The input of animal manure on the integrated farms is likely to have been associated with the higher levels of resistance, either because of a high level of antimicrobial-resistant bacteria or because of antimicrobial residues in the manure.
Acknowledgments
This work was supported by Danida grant 90942 from the Danish Council for Development Research to Andreas Petersen.
The owners and workers on the study fish farms in Suphanburi and Nakhon Pathom, Thailand, are thanked for their cooperation and for the information provided about farming practices. The Aquatic Animal Health Research Institute (AAHRI), Bangkok, Thailand, provided logistics and laboratory facilities for this study. All staff members at AAHRI are thanked for their assistance.
REFERENCES
- 1.Aarestrup, F. M., F. Bager, N. E. Jensen, M. Madsen, A. Meyling, and H. C. Wegener. 1998. Resistance to antimicrobial agents used for animal therapy in pathogenic-, zoonotic- and indicator bacteria isolated from different food animals in Denmark: a baseline study for the Danish Integrated Antimicrobial Resistance Monitoring Programme (DANMAP). APMIS 106:745-770. [DOI] [PubMed] [Google Scholar]
- 2.Aarestrup, F. M., F. Bager, N. E. Jensen, M. Madsen, A. Meyling, and H. C. Wegener. 1998. Surveillance of antimicrobial resistance in bacteria isolated from food animals to antimicrobial growth promoters and related therapeutic agents in Denmark. APMIS 106:606-622. [DOI] [PubMed] [Google Scholar]
- 3.Alderman, D. J., and T. S. Hastings. 1998. Antibiotic use in aquaculture: development of antibiotic resistance—potential for consumer health risks. Int. J. Food Sci. Technol. 33:139-155. [Google Scholar]
- 4.Andersen, J. S. 1998. Statistical analysis of biotests. Ph.D. thesis. Technical University of Denmark, Lyngby, Denmark.
- 5.Bager, F., M. Madsen, J. Christensen, and F. M. Aarestrup. 1997. Avoparcin used as a growth promoter is associated with the occurrence of vancomycin-resistant Enterococcus faecium in Danish poultry and pig farms. Prev. Vet. Med. 31:95-112. [DOI] [PubMed] [Google Scholar]
- 6.Baumann, P. 1968. Isolation of Acinetobacter from soil and water. J. Bacteriol. 96:39-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Björklund, H., J. Bondestam, and G. Bylund. 1990. Residues of oxytetracycline in wild fish and sediments from fish farms. Aquaculture 86:359-367. [Google Scholar]
- 8.Cai, Y., P. Suyanandana, P. Saman, and Y. Benno. 1999. Classification and characterization of lactic acid bacteria isolated from the intestines of common carp and freshwater prawns. J. Gen. Appl. Microbiol. 45:177-184. [DOI] [PubMed] [Google Scholar]
- 9.Cox, D. R., and E. J. Snell. 1989. The analysis of binary data. Chapman & Hall, London, United Kingdom.
- 10.Coyne, R., M. Hiney, B. O'Connor, J. Kerry, D. Cazabon, and P. Smith. 1994. Concentration and persistence of oxytetracycline in sediments under a marine salmon farm. Aquaculture 123:31-42. [Google Scholar]
- 11.DePaola, A., J. T. Peeler, and G. E. Rodrick. 1995. Effect of oxytetracycline-medicated feed on antibiotic resistance of gram-negative bacteria in catfish ponds. Appl. Environ. Microbiol. 61:2335-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillespie, S. H. 2001. Antibiotic resistance in the absence of selective pressure. Int. J. Antimicrob. Agents 17:171-176. [DOI] [PubMed] [Google Scholar]
- 13.Guardabassi, L., A. Dalsgaard, and J. E. Olsen. 1999. Phenotypic characterisation and antibiotic resistance of Acinetobacter spp. isolated from aquatic sources. J. Appl. Microbiol. 87:659-667. [DOI] [PubMed] [Google Scholar]
- 14.Guardabassi, L., A. Dalsgaard, M. Raffatellu, and J. E. Olsen. 2000. Increase in the prevalence of oxolinic acid resistant Acinetobacter spp. observed in a stream receiving the effluent from a freshwater trout farm following the treatment with oxolinic acid-medicated feed. Aquaculture 188:205-218. [Google Scholar]
- 15.Guardabassi, L., A. Petersen, J. E. Olsen, and A. Dalsgaard. 1998. Antibiotic resistance in Acinetobacter spp. isolated from sewers receiving waste effluent from a hospital and a pharmaceutical plant. Appl. Environ. Microbiol. 64:3499-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inglis, V., S. Z. Abdullah, S. L. Angka, S. Chinabut, B. R. Chowdhury, E. M. Leano, I. H. MacRae, A. Sasongko, T. Somsiri, and A. V. Yambot. 1997. Survey of resistance to antibacterial agents used in aquaculture in five South East Asian countries, p. 331-337. In T. W. Flegel and I. H. MacRae (ed.), Diseases in Asian aquaculture III. Fish Health Section, Asian Fisheries Society, Manila, The Philippines.
- 17.Jacobsen, P., and L. Berglind. 1988. Persistence of oxytetracycline in sediments from fish farms. Aquaculture 70:365-370. [Google Scholar]
- 18.Ke, D., F. J. Picard, F. Martineau, C. Menard, P. H. Roy, M. Ouellette, and M. G. Bergeron. 1999. Development of a PCR assay for rapid detection of enterococci. J. Clin. Microbiol. 37:3497-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn, I., A. Iversen, L. G. Burman, B. Olsson-Liljequist, A. Franklin, M. Finn, F. Aarestrup, A. M. Seyfarth, A. R. Blanch, H. Taylor, J. Caplin, M. A. Moreno, L. Dominguez, and R. Mollby. 2000. Epidemiology and ecology of enterococci, with special reference to antibiotic resistant strains, in animals, humans and the environment. Example of an ongoing project within the European research programme. Int. J. Antimicrob. Agents 14:337-342. [DOI] [PubMed] [Google Scholar]
- 20.Laukova, A., and P. Juris. 1997. Distribution and characterization of Enterococcus species in municipal sewages. Microbios 89:73-80. [PubMed] [Google Scholar]
- 21.Little, D. C., and P. Edwards. 1999. Alternative strategies for livestock-fish integration with emphasis on Asia. Ambio 28:118-124. [Google Scholar]
- 22.Lunestad, B. T. 1992. Fate and effects of antibacterial agents in aquatic environments, p. 152-161. In C. Michel and D. J. Alderman (ed.), Chemotherapy in aquaculture: from theory to reality. Office international des epizooties, Paris, France.
- 23.Manero, A., and A. R. Blanch. 1999. Identification of Enterococcus spp. with a biochemical key. Appl. Environ. Microbiol. 65:4425-4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McPhearson, R. M., A. DePaola, S. R. Zywno, M. L. Motes, Jr., and A. M. Guarino. 1991. Antibiotic resistance in Gram-negative bacteria from cultured catfish and aquaculture ponds. Aquaculture 99:203-211. [Google Scholar]
- 25.Morrison, D., N. Woodford, and B. Cookson. 1997. Enterococci as emerging pathogens of humans. Soc. Appl. Bacteriol. Symp. Ser. 26:89S-99S. [PubMed] [Google Scholar]
- 26.Murray, B. E. 1998. Diversity among multidrug-resistant enterococci. Emerg. Infect. Dis. 4:37-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NCCLS. 2001. Performance standards for antimicrobial susceptibility testing: Eleventh informational supplement. NCCLS, Wayne, Pa.
- 28.Rice, E. W., J. W. Messer, C. H. Johnson, and D. J. Reasoner. 1995. Occurrence of high-level aminoglycoside resistance in environmental isolates of enterococci. Appl. Environ. Microbiol. 61:374-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samuelsen, O. B. 1989. Degradation of oxytetracycline in seawater at two different temperatures and light intensities, and the persistence of oxytetracycline in the sediments from a fish farm. Aquaculture 83:7-16. [Google Scholar]
- 30.Samuelsen, O. B., V. Torsvik, and A. Ervik. 1992. Long-range changes in oxytetracycline concentration and bacterial resistance towards oxytetracycline in a fish farm sediment after a medication. Sci. Total Environ. 114:25-36. [DOI] [PubMed] [Google Scholar]
- 31.Sandaa, R.-A., V. L. Torsvik, and J. Goksøyr. 1992. Transferable drug resistance in bacteria from fish-farm sediments. Can. J. Microbiol. 38:1061-1065. [Google Scholar]
- 32.Schmidt, A. S., M. S. Bruun, I. Dalsgaard, K. Pedersen, and J. L. Larsen. 2000. Occurrence of antimicrobial resistance in fish-pathogenic and environmental bacteria associated with four Danish rainbow trout farms. Appl. Environ. Microbiol. 66:4908-4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svec, P., and I. Sedlacek. 1999. Occurrence of Enterococcus spp. in waters. Folia Microbiol. 44:3-10. [DOI] [PubMed] [Google Scholar]
- 34.Toranzo, A. E., P. Combarro, M. L. Lemos, and J. L. Barja. 1984. Plasmid coding for drug resistance in bacteria isolated from cultured rainbow trout. Appl. Environ. Microbiol. 48:872-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Towner, K. J. 1996. Biology of Acinetobacter spp., p. 13-36. In E. Bergogne-Bérézin, M. L. Joly-Guillou, and K. J. Towner (ed.), Acinetobacter: microbiology, epidemiology, infections, management. CRC Press, Boca Raton, Fla.
- 36.Towner, K. J. 1997. Clinical importance and antibiotic resistance of Acinetobacter spp. J. Med. Microbiol. 46:721-746. [DOI] [PubMed] [Google Scholar]
- 37.Valdivia, E., I. Martin-Sanchez, R. Quirantes, M. Martinez-Bueno, A. Galvez, and M. Maqueda. 1996. Incidence of antibiotic resistance and sex pheromone response among enterococci isolated from clinical human samples and from municipal waste water. J. Appl. Bacteriol. 81:538-544. [DOI] [PubMed] [Google Scholar]
- 38.Wegener, H. C., F. M. Aarestrup, L. B. Jensen, A. M. Hammerum, and F. Bager. 1999. Use of antimicrobial growth promoters in food animals and Enterococcus faecium resistance to therapeutic antimicrobial drugs in Europe. Emerg. Infect. Dis. 5:329-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolfinger, R., and M. O'Connell. 1993. Generalised linear mixed models: a pseudo-likelihood approach. J. Stat. Comput. Sim. 48:233-243. [Google Scholar]