Abstract
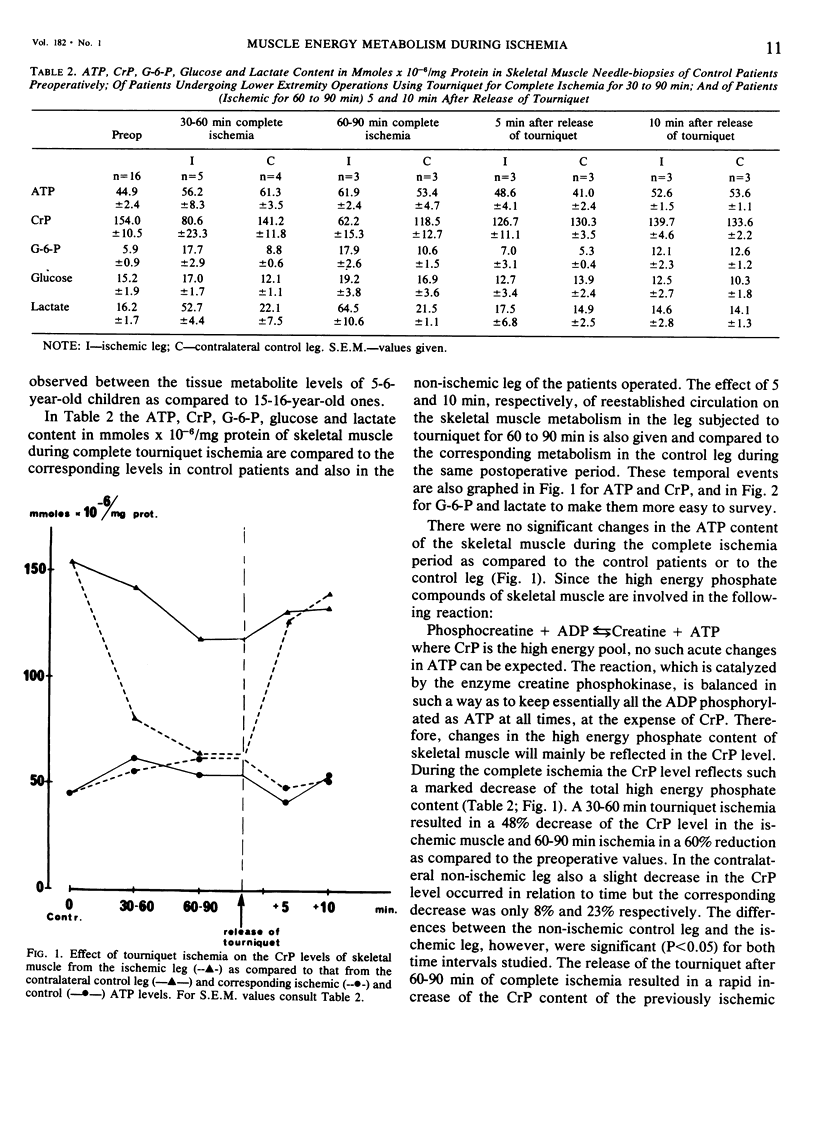
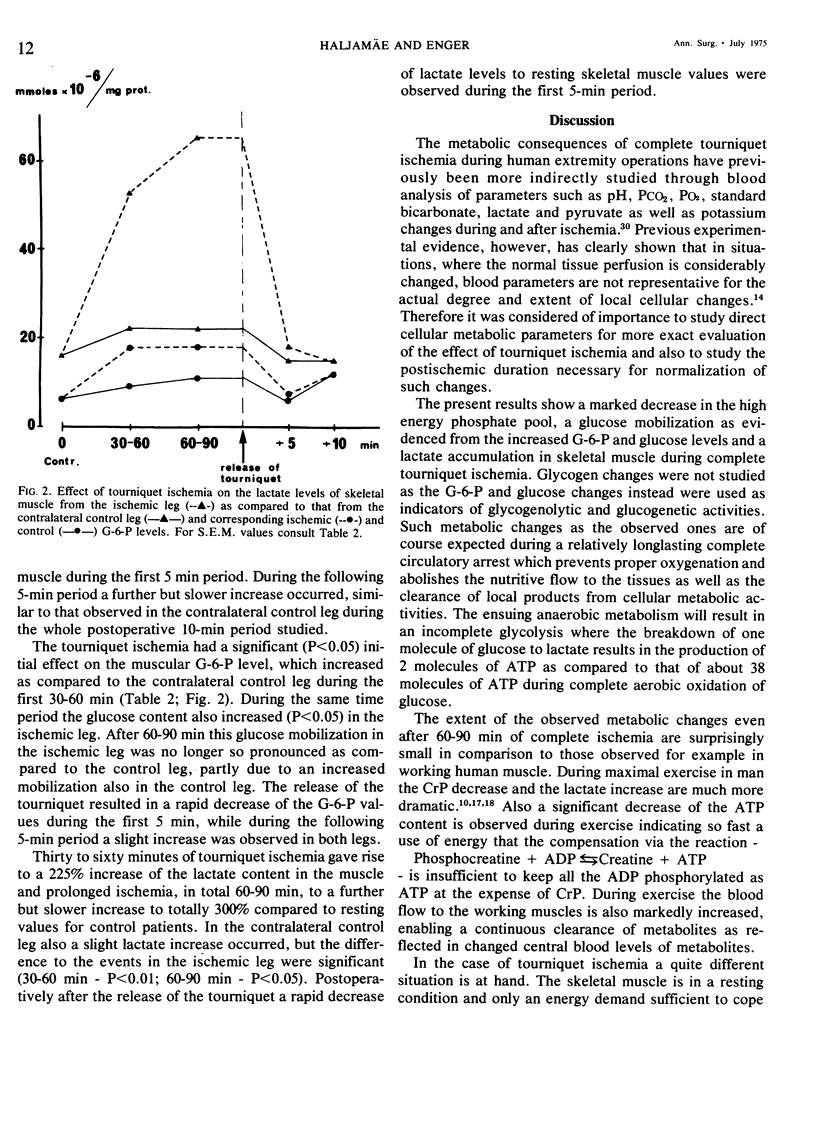
The extent of cellular metabolic deterioration and its reversibility was studied on human skeletal muscle needle biopsies during operations in bloodless field. The tissue levels of high energy phosphates and glycolytic metabolites were analyzed after various times of tourniquet ischemia and compared to contralateral control extremity levels. In the ischemic extremity the phosphocreatine (CrP) levels decreased by 40% within 30-60 min and after 60-90 min a 60% reduction was found. No significant ATP changes occurred. Lactate levels increased by 225% after 30-60 min and by 300% after 60-90 min. The glucose and G-6-P levels increased slightly and indicated glycogenolysis. The rate of the metabolic changes decreased with ischemia time. In the control leg no significant metabolic changes could be seen. After the release of the tourniquet there was a rapid restoration of the phosphagen content and clearance of lactate in the ischemic leg. Near control levels of these substances were seen already after 5 min. The present results show that clinical tourniquet ischemia of up to 90 min duration produces less pronounced metabolic alterations than those seen in working muscle.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruner J. M. Time, pressure, and temperature factors in the safe use of the tourniquet. Hand. 1970 Mar;2(1):39–42. doi: 10.1016/0072-968x(70)90033-1. [DOI] [PubMed] [Google Scholar]
- CALKINS E., TAYLOR I. M., HASTINGS A. B. Potassium exchange in the isolated diaphragm; effect of anoxia and cold. Am J Physiol. 1954 May;177(2):211–218. doi: 10.1152/ajplegacy.1954.177.2.211. [DOI] [PubMed] [Google Scholar]
- Clarke R. S. The hyperglycaemic response to different types of surgery and anaesthesia. Br J Anaesth. 1970 Jan;42(1):45–53. doi: 10.1093/bja/42.1.45. [DOI] [PubMed] [Google Scholar]
- Hagberg S., Haljamäe H., Röckert H. Shock reactions in skeletal muscle. IV. The effect of hypothermic treatment on cellular electrolyte responses to haemorrhagic shock. Acta Chir Scand. 1970;136(1):23–28. [PubMed] [Google Scholar]
- Haljamäe H. "Hidden" cellular electrolyte responses to hemorrhagic shock and their significance. Rev Surg. 1970 Sep-Oct;27(5):315–324. [PubMed] [Google Scholar]
- Haljamäe H. Effects of hemorrhagic shock and treatment with hypothermia on the potassium content and transport of single mammalian skeletal muscle cells. Acta Physiol Scand. 1970 Feb;78(2):189–200. doi: 10.1111/j.1748-1716.1970.tb04655.x. [DOI] [PubMed] [Google Scholar]
- Hilton S. M. Local chemical factors involved in vascular control. Angiologica. 1971;8(3-5):174–186. doi: 10.1159/000157893. [DOI] [PubMed] [Google Scholar]
- Karlsson J., Diamant B., Saltin B. Muscle metabolites during submaximal and maximal exercise in man. Scand J Clin Lab Invest. 1970 Dec;26(4):385–394. doi: 10.3109/00365517009046250. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Massion W. H., Blümel G. Irreversibility in shock: role of vasoactive kinins. Anesth Analg. 1971 Nov-Dec;50(6):970–976. doi: 10.1213/00000539-197150060-00014. [DOI] [PubMed] [Google Scholar]
- PALETTA F. X., SHEHADI S. I., MUDD J. G., COOPER T. Hypothermia and tourniquet ischemia. Plast Reconstr Surg Transplant Bull. 1962 May;29:531–538. doi: 10.1097/00006534-196205000-00001. [DOI] [PubMed] [Google Scholar]
- REICH T., DIEROLF B. M., REYNOLDS B. M. PLASMA CATHEPSIN-LIKE ACID PROTEINASE ACTIVITY DURING HEMORRHAGIC SHOCK. J Surg Res. 1965 Mar;5:116–119. doi: 10.1016/s0022-4804(65)80004-x. [DOI] [PubMed] [Google Scholar]
- Solonen K. A., Hjelt L. Morphological changes in striated muscle during ischaemia. A clinical and histological study in man. Acta Orthop Scand. 1968;39(1):13–19. doi: 10.3109/17453676808989435. [DOI] [PubMed] [Google Scholar]
- Solonen K. A., Tarkkanen L., Närvänen S., Gordin R. Metabolic changes in the upper limb during tourniquet ischaemia. A clinical study. Acta Orthop Scand. 1968;39(1):20–32. doi: 10.3109/17453676808989436. [DOI] [PubMed] [Google Scholar]
- Stock W., Bohn H. J., Isselhard W. Metabolic changes in rat sceletal muscle after acute arterial occlusion. Vasc Surg. 1971 Nov-Dec;5(5):249–255. doi: 10.1177/153857447100500508. [DOI] [PubMed] [Google Scholar]
- Tarhan S., Fulton R. E., Moffitt E. A. Body metabolism during general anesthesia without superimposed surgical stress. Anesth Analg. 1971 Nov-Dec;50(6):915–923. doi: 10.1213/00000539-197150060-00003. [DOI] [PubMed] [Google Scholar]
- Wilgis E. F. Observations on the effects of tourniquet ischemia. J Bone Joint Surg Am. 1971 Oct;53(7):1343–1346. [PubMed] [Google Scholar]


