Abstract
A new molecular approach for the detection and identification of Listeria spp. and Listeria monocytogenes in food is presented here. The method is based on the PCR amplification of a fragment of the iap gene from the five species belonging to the genus and on the analysis of the PCR products obtained by denaturing gradient gel electrophoresis (DGGE). The protocol was first optimized by using strains from international collections. Based on the differences present in the sequences amplified, it was possible to obtain species-specific DGGE migration that allowed fast and easy identification of L. monocytogenes, L. innocua, L. welshimeri, L. seeligeri, and L. ivanovii. Moreover, for L. monocytogenes serotypes, partial differentiation was possible. The optimized protocol was used for identification of Listeria strains traditionally isolated from food and for direct detection and identification of Listeria members in food after an overnight enrichment. Identification of 48 food isolates and direct detection of Listeria spp. in 73 food samples show the potential of the method that can be used as a fast screening test to investigate the presence of Listeria spp. and L. monocytogenes in food.
The genus Listeria comprises a group of ubiquitous, gram-positive, nonsporulating bacteria. Previous results of DNA-DNA hybridization experiments unambiguously demonstrated that the species formerly called “Listeria monocytogenes” in fact contained five genomic groups deserving the species rank: L. monocytogenes, L. ivanovii, L. innocua, L. welshimeri, and L. seeligeri (48, 49). This subdivision has proven its usefulness for practical and epidemiological purposes, especially when problems caused by food contamination resulted in human listeriosis (29). Only the hemolytic species of Listeria, L. monocytogenes, L. ivanovii, and L. seeligeri, are associated with human pathogenicity. L. monocytogenes is the only species of the genus Listeria that has been involved in known food-borne outbreaks of listeriosis. L. ivanovii has been described to be involved in human pathology only rarely, and L. seeligeri has been reported only once to be the cause of meningitis in a nonimmunocompromised adult (35).
Several large outbreaks of listeriosis have been associated with contaminated commercial foodstuffs, such as vegetables, milk, and meat products, on which these bacteria can multiply even at low temperatures (52). Usually, the presence of any Listeria species in food is an indicator of poor hygiene (40).
Significant efforts have been dedicated to the development of enrichment media and protocols for L. monocytogenes and Listeria sp. isolation (1, 10, 11, 15, 24, 47). Ideal enrichment media would facilitate recovery of injured Listeria cells and enrichment of Listeria spp. and L. monocytogenes over competing microflora. In traditional culture-based assays, it becomes very difficult to detect L. monocytogenes at any level when it is greatly outnumbered by other Listeria spp., such as L. innocua, which appears to be present together with L. monocytogenes (10, 51). Consequently, detection of Listeria spp. is often used as an indication for the presence of L. monocytogenes (22).
Species-specific identification with biochemical standard methods, which include sugar fermentation or the CAMP test (53), is laborious and time-consuming and can require 1 to 2 weeks for species identification (28). Moreover, differentiation between species or strains within the same species is not always reached. For these reasons, little is known about the occurrence and distribution of Listeria species other than L. monocytogenes.
The first studies of isolation and detection of L. monocytogenes in food that exploited molecular methods date back to the 1990s (42, 50, 59). From then, protocols have been continuously proposed and only in the last few years a vast number of papers have been published (25, 27, 41, 44, 45). Only a few studies have considered the identification of the other members of the genus Listeria. Repetitive element sequence-based PCR (30), multiplex PCR (2, 6, 23, 32), rRNA gene restriction patterns (29), sequence analysis of the 16S-23S rRNA internal transcribed spacer loci (13, 18), temperature gradient gel electrophoresis (37), two-dimensional protein mapping (17), and colony-blot assay with anti-p60 antibodies (60) are some of the methods proposed to identify Listeria species. Often the protocols developed are able to detect only the genus Listeria, thus lacking the ability to identify different species of Listeria simultaneously. Moreover, the protocols described need a previous isolation of Listeria strains using traditional methods.
Currently, new methods are developed to directly characterize the microorganisms without the need for isolation (20). This approach eliminates the necessity for strain isolation, thereby negating the potential biases inherent to microbial enrichment. Studies that have employed such direct analysis have repeatedly demonstrated a tremendous variance between cultivated and naturally occurring species, thereby dramatically altering the perception of the true microbial diversity present in various habitats (26).
In this paper a direct identification in food samples of Listeria spp. by a molecular method is described. PCR coupled with denaturing gradient gel electrophoresis (DGGE) allowed a direct identification of Listeria spp. in food, based on specific migration patterns. Moreover, L. monocytogenes serotypes could be partially distinguished on the basis of PCR product mobility in the denaturing gel. The method described could represent a fast and interesting tool to study the ecology of the members of the genus Listeria, monitoring their spread in food and environmental samples and for epidemiological purposes as well.
(Partial results of this work were presented at the 101st General Meeting of the American Society for Microbiology, Orlando, Fla., 21 to 25 May 2001.)
MATERIALS AND METHODS
Bacterial strains.
The strains used in this study are listed in Table 1. Listeria spp. strains came from international collections or were isolated from patients with listeriosis. Non-Listeria strains came from the collection of the Dipartimento di Scienze degli Alimenti, Udine, Italy. Moreover, 48 strains of Listeria spp. previously isolated from food samples and identified by traditional methods were used. All the strains were cultured on brain heart infusion (BHI; Oxoid, Milan, Italy) broth and incubated at 30 or 37°C overnight before being subjected to analysis by molecular methods.
TABLE 1.
Strains used in this study
| Strain | Serotype | Strain no. | Sourcea |
|---|---|---|---|
| International collection strains | |||
| L. monocytogenes | 1/2a | NCTC 7979 | NCTC |
| 1/2b | NCTC 10887 | NCTC | |
| 1/2c | NCTC 9862 | NCTC | |
| 3a | NCTC 5105 | NCTC | |
| 3b | CIP 78.35 | CIP | |
| 4b | NCTC 10527 | NCTC | |
| ATCC 7644 | ATCC | ||
| L. ivanovii | DSMZ 20750 | DSMZ | |
| L. innocua | DSMZ 20649 | DSMZ | |
| L. welshimeri | DSMZ 20650 | DSMZ | |
| L. seeligeri | DSMZ 20751 | DSMZ | |
| Human isolate strains | |||
| L. monocytogenes | 1/2a | 26 | Azienda Ospedaliera Policlinico di Modena |
| 1/2b | 12 | Azienda Ospedaliera Policlinico di Modena | |
| 1/2b | 15 | Azienda Ospedaliera Policlinico di Modena | |
| 1/2c | 14 | Azienda Ospedaliera Policlinico di Modena | |
| 4b | 1 | Azienda Ospedaliera Policlinico di Modena | |
| 4b | 2 | Azienda Ospedaliera Policlinico di Modena | |
| 4b | 3 | Azienda Ospedaliera Policlinico di Modena | |
| 4b | 5 | Azienda Ospedaliera Policlinico di Modena | |
| 4b | 6 | Azienda Ospedaliera Policlinico di Modena | |
| 4b | 7 | Azienda Ospedaliera Policlinico di Modena | |
| 4b | 8 | Azienda Ospedaliera Policlinico di Modena | |
| 4b | 10 | Azienda Ospedaliera Policlinico di Modena | |
| 4b | 11 | Azienda Ospedaliera Policlinico di Modena | |
| 4b | 13 | Azienda Ospedaliera Policlinico di Modena | |
| 4b | 17 | Azienda Ospedaliera Policlinico di Modena | |
| 4b | 19 | Azienda Ospedaliera Policlinico di Modena | |
| 4b | 20 | Azienda Ospedaliera Policlinico di Modena | |
| 4b | 21 | Azienda Ospedaliera Policlinico di Modena | |
| 4b | 23 | Azienda Ospedaliera Policlinico di Modena | |
| 4b | 25 | Azienda Ospedaliera Policlinico di Modena | |
| Non-Listeria spp. strains | |||
| Aeromonas hydrophila | Dipartimento di Scienze degli Alimenti | ||
| Campylobacter jejuni | Dipartimento di Scienze degli Alimenti | ||
| Escherichia coli | Dipartimento di Scienze degli Alimenti | ||
| Escherichia coli O157:H7 | Dipartimento di Scienze degli Alimenti | ||
| Proteus vulgaris | Dipartimento di Scienze degli Alimenti | ||
| Proteus mirabilis | Dipartimento di Scienze degli Alimenti | ||
| Salmonella enterica serovar Typhimurium | Dipartimento di Scienze degli Alimenti | ||
| Salmonella enterica serovar Enteritidis | Dipartimento di Scienze degli Alimenti | ||
| Citrobacter freundii | Dipartimento di Scienze degli Alimenti | ||
| Shigella flexneri | Dipartimento di Scienze degli Alimenti | ||
| Yersinia enterocolitica | Dipartimento di Scienze degli Alimenti | ||
| Bacillus cereus | Dipartimento di Scienze degli Alimenti | ||
| Bacillus subtilis | Dipartimento di Scienze degli Alimenti | ||
| Enterococcus faecalis | Dipartimento di Scienze degli Alimenti | ||
| Lactobacillus plantarum | Dipartimento di Scienze degli Alimenti | ||
| Lactobacillus casei | Dipartimento di Scienze degli Alimenti | ||
| Staphylococcus aureus | Dipartimento di Scienze degli Alimenti | ||
| Staphylococcus carnosus | Dipartimento di Scienze degli Alimenti | ||
| Staphylococcus xylosus | Dipartimento di Scienze degli Alimenti |
NCTC, National Collection of Type Cultures; CIP, Collection Institut Pasteur; ATCC, American Type Culture Collection; DSMZ, Deutsche Sammlung von Mikroorganismen and Zellkulturen.
Traditional isolation and identification.
Forty-eight Listeria strains were isolated from food according to the method of the U.S. Department of Agriculture, Food Safety and Inspection Service (9), and identified using morphological, cultural, and biochemical criteria. In particular, Gram stain, catalase test, mobility, β-hemolysis, CAMP test, and production of acids from rhamnose and xylose were used, as described in Bergey's manual (53), to identify the species belonging to the genus.
DNA extraction.
One milliliter of an overnight culture was subjected to centrifugation at 14,000 × g at 4°C for 10 min, and the pellet was washed in 500 μl of lysozyme buffer solution (25% [wt/vol] sucrose, 0.1 g of lysozyme/ml). After a second centrifugation at 14,000 × g at 4°C for 10 min, the supernatant was discarded and the pellet was resuspended in 50 μl of lysozyme (50 mg/ml). A 30-min incubation at 37°C was performed, followed by a bead-beater treatment to break up the cells. In particular, the cell suspension was mixed with 300 μl of breaking buffer (2% Triton X-100, 1% sodium dodecyl sulfate, 100 mM NaCl, 10 mM Tris, 1 mM EDTA, pH 8) and transferred to a 1.5-ml screw-cap tube containing 0.3 g of glass beads. After the addition of 300 μl of phenol-chloroform-isoamyl alcohol (25:24:1) (pH 6.7) (Sigma, Milan, Italy), the tubes were subjected to three 30-s treatments, with a 15-s break in between them, at maximum speed in the Mini-Bead Beater 8 (Biospec Products, Inc., Bartlesville, Okla.). Three hundred microliters of 10 mM Tris-5 mM EDTA (pH 8) was added and a centrifugation at 12,000 × g at 4°C for 10 min was performed. The aqueous phase was collected, and the DNA was precipitated by the addition of 1 ml of ice-cold absolute ethanol and centrifugation at 14,000 × g at 4°C for 10 min. DNA pellets were dried under vacuum at room temperature, resuspended in 50 μl of RNase- and DNase-free sterile water, and treated with 1 μl of DNase-free RNase (500 μg/ml; Roche Diagnostics, Milan, Italy). After a 1-h incubation at 37°C, the tubes were stored at −20°C.
PCR amplification.
PCR was performed in a final volume of 50 μl containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 3.5 mM MgCl2, 0.2 mM (each) deoxynucleoside triphosphates, 1.25 IU of Taq polymerase (Applied Biosystems, Milan, Italy), and 0.2 μM (each) primers targeting the iap gene encoding the invasion-associated protein p60 in all Listeria spp. (7). Primers were named List-univ. 1 (5′-ATGTCATGGAATAA-3′) and List-univ. 2 (5′-GCTTTTCCAAGGTGTTTTT-3′) (L. Cocolin, M. Manzano, C. Cantoni, and G. Comi, Abstr. 101st Gen. Meet. Am. Soc. Microbiol. 2001, abstr. P-88, p. 575, 2001). A GC clamp was added to primer List-univ. 1 (5′-GCC AGC GGC CCG GCG CGG GCC CGG CGG CGG GGG CCG CGG C-3′) to improve its sensitivity in the detection of DNA sequence differences by DGGE analysis as previously described (55). After DNA addition (≈50 ng), the samples were subjected to amplification in a MiniCycler (Genenco, Florence, Italy) using the following program: 95°C for 5 min; 35 cycles of 95°C for 1 min, 36°C for 2 min, and 72°C for 3 min; and, finally, 72°C for 7 min. Five microliters of the product was analyzed by standard agarose gel electrophoresis before DGGE analysis.
DGGE analysis.
The Dcode Universal Mutation Detection system (Bio-Rad, Hercules, Calif.) was used for the sequence-specific separation of the PCR products. Electrophoresis was performed in a 0.8-mm-thick polyacrylamide gel (8% [wt/vol] acrylamide-bisacrylamide [37.5:1]) containing a 20 to 40% urea-formamide denaturing gradient (100% corresponds to 7 M urea and 40% [wt/vol] formamide), increasing in the direction of the electrophoretic run. The gels were subjected to a constant voltage of 130 V for 4 h at 60°C. After electrophoresis, they were stained for 10 min in a SYBR Green solution (Molecular Probes, Eugene, Oreg.) and analyzed under UV illumination. Pictures of the gels were digitized by using a charge-coupled device camera (Polaroid, St. Louis, Mo.), and images were analyzed using the Gel Pro Analyzer 3.0 (Immagini e Computer, Milan, Italy) for the identification of the bands present in the gel.
Direct analysis of food samples by PCR-DGGE.
Seventy-three food samples were collected from local laboratories and represented beef, pork, and poultry meats. Twenty-five grams of sample was added to 225 ml of BHI broth in a sterile bag and homogenized in a stomacher machine (P.B.I., Milan, Italy) for 1.5 min. After an overnight enrichment step at 37°C, 1 ml was collected and centrifuged at 14,000 × g at 4°C for 10 min. The pellet obtained was then subjected to DNA extraction, PCR amplification, and DGGE analysis as described above. One aliquot of the overnight enrichment was also streaked onto Oxford agar (Oxoid), and plates were incubated at 37°C for 48 h. After incubation, suspected Listeria sp. colonies were purified on BHI agar and, after propagation in BHI broth, were subjected to molecular identification by PCR-DGGE. The direct identification of Listeria spp. was also confirmed by direct sequencing of the bands migrating at the same level with appropriate controls loaded on the gel. In particular, bands of interest were punched out of the gel by use of sterile tips, resuspended in sterile water, and after an overnight incubation at 4°C, reamplified using the GC-clamped List-univ. 1 primer. A DGGE run was performed to check for the presence of a single band migrating at the same position with respect to the band isolated from the food sample, and after amplification using the List-univ. 1 primer lacking the GC tail, the PCR product was purified using a QiaQuick PCR purification kit (Qiagen, Milan, Italy) and sequenced by a commercial facility (MWG Biotech, Edelsberg, Germany). Moreover, the presence of L. monocytogenes was confirmed by specific amplification using the primers Mar 1 and Mar 2 (38) and restriction enzyme analysis (REA) as previously described (36).
RESULTS
Use of the iap gene as a target for specific PCR amplification of Listeria spp.
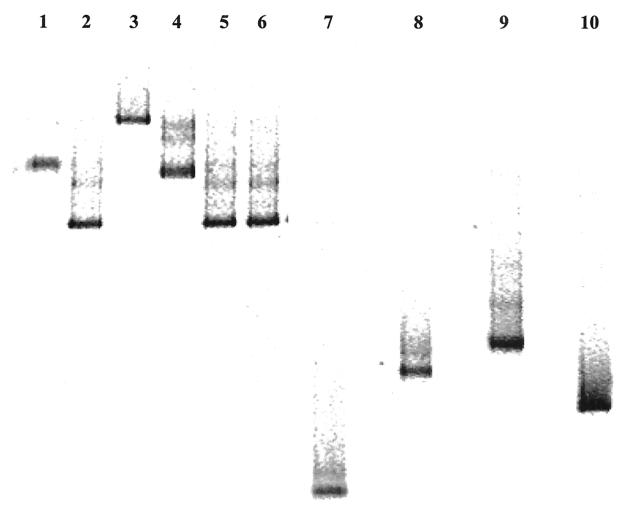
The set of primers developed was designed based on the partial alignments of the iap genes from the Listeria spp. considered in the study. The accession numbers of the reference sequences, primer sequences, positions of primer annealing sites, and expected PCR product sizes are reported in Table 2. The primers chosen are able to prime the amplification of all Listeria spp. tested, due to their high or complete homology to the sequence of the iap genes considered. More specifically, the forward primer was completely homologous to the reference sequence of all five species considered. In contrast, the reverse primer was identical to the L. monocytogenes sequence only, while a mismatch in one nucleotide position for each of the other four species was observed. The primers are able to amplify a region that contains significant differences in the sequence between the species of Listeria studied. L. monocytogenes and L. innocua are characterized by the presence of different deletions in the sequence compared to L. ivanovii, L. seeligeri, and L. welshimeri. Because of these deletions in the sequence for L. monocytogenes and L. innocua, PCR amplification gives amplicons with different molecular sizes as follows: L. ivanovii, L. seeligeri, and L. welshimeri are characterized by an amplicon of between 600 and 610 bp, whereas L. monocytogenes and L. innocua give PCR products of 457 and 472 bp, respectively. The expected sizes of the products, determined by using molecular biology software (14), were confirmed by agarose gel electrophoresis of the PCR amplicons. As shown in Fig. 1, L. ivanovii, L. seeligeri, and L. welshimeri (Fig. 1, lanes 3, 4, and 5) have a higher molecular weight band than L. monocytogenes and L. innocua (Fig. 1, lanes 6 to 12). No differences in the size of the PCR products were noticed when different serotypes of L. monocytogenes were amplified (Fig. 1, lanes 7 to 12). The primers designed for this study were proven to be highly specific for Listeria spp. When DNA from non-Listeria strains, as reported in Table 1, was used in the PCR, no PCR amplicon, of any size, was obtained, underlining the feasibility of the use of the primers directly on DNA extracted from microbial mixtures containing Listeria spp. for their specific detection by PCR amplification.
TABLE 2.
Sequence and position of annealing of the primers and PCR product sizes for the different Listeria spp. tested in this study
| Listeria sp. | Accession no. of reference sequence | 5′-3′ Sequence (nucleotide position) of the forward primer annealing sitea | 5′-3′ Sequence (nucleotide position) of the reverse primer annealing sitea,b | PCR product size (bp)a |
|---|---|---|---|---|
| L. ivanovii | M80350 | ATGTCATGGAATAA (661-674) | AAAAACATCTTGGAAAAGC (1250-1268) | 610 |
| L. seeligeri | M80353 | ATGTCATGGAATAA (667-680) | AAAAACACCTTGGTAAAGC (1247-1265) | 601 |
| L. welshimeri | M80354 | ATGTCATGGAATAA (661-674) | AAAAACATCTTGGAAAAGC (1250-1268) | 610 |
| L. innocua | M80349 | ATGTCATGGAATAA (670-683) | AAAAACATCTTGGAAAAGC (1121-1139) | 472 |
| L. monocytogenes | M80351 | ATGTCATGGAATAA (676-689) | AAAAACACCTTGGAAAAGC (1112-1130) | 457 |
Determined by using the molecular biology software Amplify (14).
Nucleotides in bold represent positions with a mismatch with the primer used in the study.
FIG. 1.
Agarose gel electrophoresis of the products obtained after amplification of the iap gene from different Listeria species. Lane 1, 100-bp ladder (Sigma); lane 2, negative control; lane 3, L. ivanovii DSMZ 20750; lane 4, L. seeligeri DSMZ 20751; lane 5, L. welshimeri DSMZ 20650; lane 6, L. innocua DSMZ 20649; lane 7, L. monocytogenes 1/2a NCTC 7979; lane 8, L. monocytogenes 1/2b NCTC 10887; lane 9, L. monocytogenes 1/2c NCTC 9862; lane 10, L. monocytogenes 3a NCTC 5105; lane 11, L. monocytogenes 3b CIP 78.35; lane 12, L. monocytogenes 4b NCTC 10527. The numbers on the left indicate the molecular size of the DNA bands in base pairs.
DGGE differentiation of iap-derived PCR products within Listeria spp.
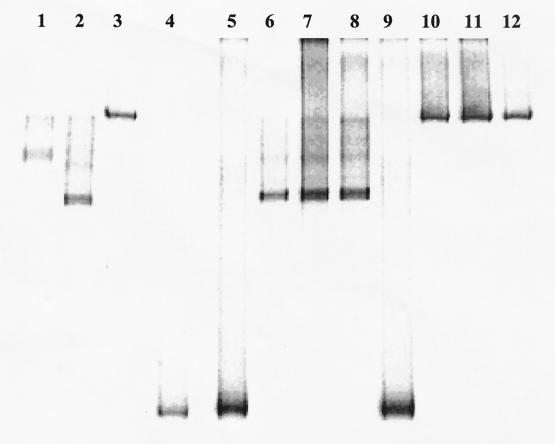
The DGGE profiles of the iap-derived PCR products for the different Listeria spp. considered in this study are shown in Fig. 2. Species-specific migrations were obtained for all the species tested. L. monocytogenes, L. innocua, L. ivanovii, L. seeligeri, and L. welshimeri all showed a different profile, making the identification simple and immediate. Moreover, for L. monocytogenes, differentiation between serotypes was possible. The PCR products obtained from serotypes 1/2a and 3a (Fig. 2, lanes 1 and 4) showed the same mobility in the gel, but it was different from that of serotype 1/2c (Fig. 2, lane 3) as well as those of 1/2b, 3b, and 4b (Fig. 2, lanes 2, 5, and 6). In addition, PCR-DGGE-obtained differentiation of L. monocytogenes from humans with listeriosis (Table 1) agreed with the previous serotyping (data not shown).
FIG. 2.
DGGE profiles of Listeria species obtained from international collections. Lane 1, L. monocytogenes 1/2a NCTC 7979; lane 2, L. monocytogenes 1/2b NCTC 10887; lane 3, L. monocytogenes 1/2c NCTC 9862; lane 4, L. monocytogenes 3a NCTC 5105; lane 5, L. monocytogenes 3b CIP 78.35; lane 6, L. monocytogenes 4b NCTC 10527; lane 7, L. innocua DSMZ 20649; lane 8, L. welshimeri DSMZ 20650; lane 9, L. seeligeri DSMZ 20751; lane 10, L. ivanovii DSMZ 20750.
Identification of Listeria strains isolated from food by traditional methods.
The results obtained from the biochemical and molecular identification of 48 Listeria strains isolated from food by traditional methods are reported in Table 3, and an example of DGGE strain identification is shown in Fig. 3. Only strains belonging to the species L. monocytogenes and L. innocua were identified, whereas no L. ivanovii, L. seeligeri, and L. welshimeri were found. Almost all of the strain identifications that were obtained by use of traditional methods were in agreement with the DGGE patterns. Among the 48 strains isolated, 9 were identified as L. monocytogenes by traditional methods but had the DGGE profile of L. innocua. Moreover, thanks to the capability of the DGGE protocol to differentiate L. monocytogenes serotypes, 20 strains of L. monocytogenes were grouped as follows: 12 serotype 1/2c strains, 5 serogroup a strains, and 3 serogroup b strains.
TABLE 3.
Comparison of the results obtained from the biochemical and PCR-DGGE identification on the 48 Listeria strains isolated from food samples using traditional methods (9)a
| Strain no. | Biochemical identification | PCR-DGGE identification |
|---|---|---|
| 1 | L. innocua | L. innocua |
| 4 | L. monocytogenes | L. monocytogenes serotype 1/2c |
| 5 | L. innocua | L. innocua |
| 6 | L. monocytogenes | L. monocytogenes serogroup a |
| 7 | L. monocytogenes | L. monocytogenes serogroup b |
| 9 | L. monocytogenes | L. monocytogenes serotype 1/2c |
| 11 | L. innocua | L. innocua |
| 13 | L. monocytogenes | L. monocytogenes serogroup a |
| 15 | L. innocua | L. innocua |
| 16 | L. innocua | L. innocua |
| 21 | L. innocua | L. innocua |
| 24 | L. monocytogenes | L. monocytogenes serogroup a |
| 26 | L. monocytogenes | L. monocytogenes serogroup a |
| 30 | L. innocua | L. innocua |
| 35 | L. innocua | L. innocua |
| 37 | L. monocytogenes | L. monocytogenes serotype 1/2c |
| 38 | L. monocytogenes | L. monocytogenes serotype 1/2c |
| 43 | L. monocytogenes | L. innocua |
| 45 | L. innocua | L. innocua |
| 46 | L. innocua | L. innocua |
| 47 | L. monocytogenes | L. innocua |
| 48 | L. monocytogenes | L. monocytogenes serotype 1/2c |
| 49 | L. monocytogenes | L. innocua |
| 50 | L. innocua | L. innocua |
| 51 | L. innocua | L. innocua |
| 52 | L. innocua | L. innocua |
| 53 | L. innocua | L. innocua |
| 54 | L. monocytogenes | L. innocua |
| 57 | L. monocytogenes | L. innocua |
| 60 | L. innocua | L. innocua |
| 64 | L. monocytogenes | L. innocua |
| 66 | L. monocytogenes | L. innocua |
| 68 | L. monocytogenes | L. innocua |
| 69 | L. innocua | L. innocua |
| 70 | L. monocytogenes | L. innocua |
| 71 | L. innocua | L. innocua |
| 79 | L. innocua | L. innocua |
| 81 | L. innocua | L. innocua |
| 83 | L. monocytogenes | L. monocytogenes serogroup b |
| 86 | L. monocytogenes | L. monocytogenes serotype 1/2c |
| 87 | L. monocytogenes | L. monocytogenes serotype 1/2c |
| 89 | L. monocytogenes | L. monocytogenes serotype 1/2c |
| 91 | L. monocytogenes | L. monocytogenes serotype 1/2c |
| 92 | L. monocytogenes | L. monocytogenes serogroup a |
| 94 | L. monocytogenes | L. monocytogenes serotype 1/2c |
| 96 | L. monocytogenes | L. monocytogenes serotype 1/2c |
| 98 | L. monocytogenes | L. monocytogenes serogroup b |
| 99 | L. monocytogenes | L. monocytogenes serotype 1/2c |
Strains reported in boldface were identified differently by biochemical and molecular methods.
FIG. 3.
DGGE patterns of Listeria strains isolated from food. Lanes 1 to 3, L. monocytogenes serotypes 1/2a NCTC 7979, 1/2b NCTC 10887, and 1/2c NCTC 9862, respectively; lane 4, L. innocua DSMZ 20649; lanes 5 to 12, strains isolated from food.
Identification of Listeria spp. in food samples.
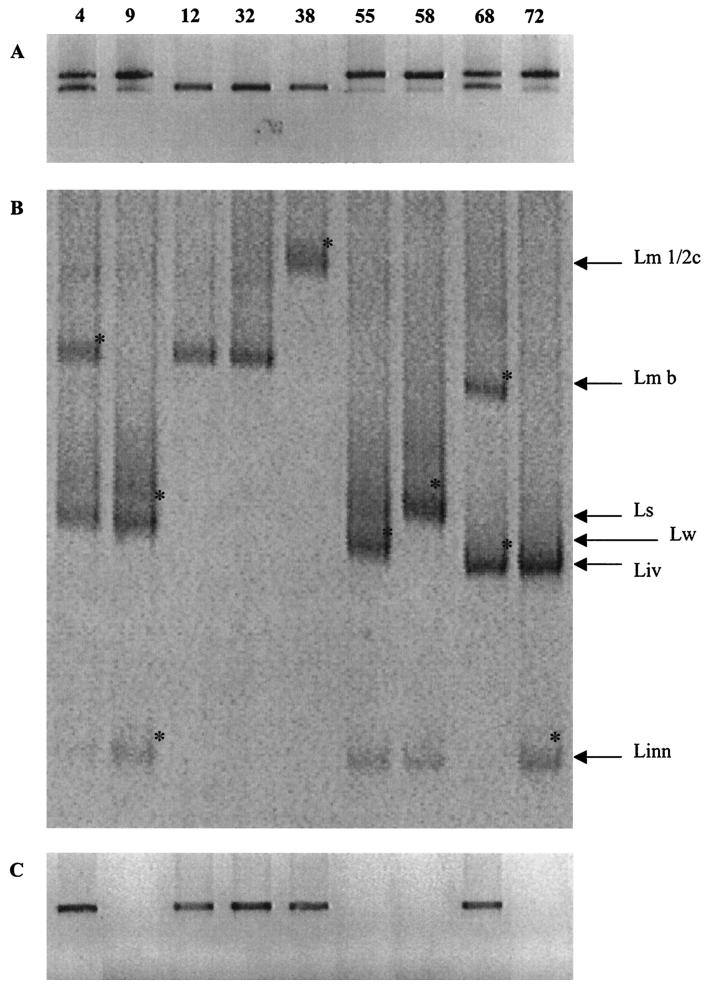
Seventy-three food samples of different origins were subjected to PCR-DGGE detection and isolation of Listeria spp. Of the 73 samples, 24 were positive for Listeria, whereas 49 showed no Listeria spp. The PCR products obtained by amplification of the DNA extracted directly from food, using the primers List-univ. 1 and List-univ. 2, were subjected to DGGE analysis, and suspected colonies grown on Oxford agar were isolated and identified by PCR-DGGE. Moreover, the samples containing L. monocytogenes were also subjected to PCR with specific primers for L. monocytogenes, Mar 1 and Mar 2, to validate the results obtained from the DGGE gels. The PCR product obtained was cut with the HindIII restriction endonuclease to confirm the differentiation of serotypes obtained by DGGE. Table 4 reports the profiling of Listeria sp. populations present in the positive food samples. Sixteen foods contained single populations of Listeria, or a single serogroup of L. monocytogenes, whereas eight samples showed mixed populations. Figure 4 shows the PCR products obtained directly from food samples by use of List-univ. 1 and List-univ. 2 primers, their relative DGGE profiles, and the results of the specific Mar 1 and Mar 2 L. monocytogenes amplification for nine food samples. The presence of different species in food samples was already determined by gel electrophoresis of the PCR products obtained from the DNA extracted from food samples, using primers List-univ. 1 and List-univ. 2. As shown in Fig. 4A, samples 4, 9, 55, 58, 68, and 72 presented a doublet, indicating populations belonging to the L. monocytogenes-L. innocua group and to the L. ivanovii-L. seeligeri-L. welshimeri group. Samples 3, 4, and 5 gave a single band that could correspond to mixed L. monocytogenes and L. innocua, to different L. monocytogenes serotypes, or to single populations of L. monocytogenes or L. innocua. In Fig. 4B, the profiles obtained by DGGE analysis of the amplicons shown in Fig. 4A are reported. Only samples 32 and 38 presented a single population of L. monocytogenes or mixed L. monocytogenes strains belonging to the same serogroup, whereas the other samples contained two to three different species of Listeria. Sample 4, in particular, showed the simultaneous presence of L. monocytogenes serogroup b, L. welshimeri, and L. innocua (faint DGGE band). To confirm the identification obtained by comparing electrophoretic mobilities, bands indicated in Fig. 4B were excised and sequenced. All of them showed high similarities (>99%) to the species previously identified by analyzing the electrophoretic pattern.
TABLE 4.
Results of the detection of Listeria spp. by the PCR-DGGE method and specific amplification and serogrouping of L. monocytogenes by the PCR-REA protocol directly in food samples and comparison with the identification by PCR-DGGE of the strains isolated on Oxford agar after overnight enrichmenta
| Sample no. | Species identified by direct PCR-DGGE | L. monocytogenes direct specific amplification | L. monocytogenes direct REA serogrouping | Species isolated from Oxford agar and identified by PCR-DGGE |
|---|---|---|---|---|
| 2 | L. monocytogenes group b | + | b | L. monocytogenes group b |
| 4 | L. monocytogenes group b, L. welshimeri, and L. innocua | + | b | L. monocytogenes group b and L. innocua |
| 5 | L. monocytogenes group a | + | a or 1/2c | L. monocytogenes group a |
| 8 | L. monocytogenes group a and L. monocytogenes group b | + | a or 1/2c plus b | L. monocytogenes group a and L. monocytogenes group b |
| 9 | L. innocua and L. welshimeri | − | NAb | L. innocua and L. welshimeri |
| 11 | L. monocytogenes group a and L. monocytogenes group 1/2c | + | a or 1/2c | L. monocytogenes group a and L. monocytogenes group 1/2c |
| 12 | L. monocytogenes group b | + | b | L. monocytogenes group b |
| 22 | L. innocua | − | NA | L. innocua |
| 28 | L. innocua | − | NA | L. innocua |
| 32 | L. monocytogenes group b | + | b | L. monocytogenes group b |
| 38 | L. monocytogenes group a | + | a or 1/2c | L. monocytogenes group a |
| 39 | L. monocytogenes group 1/2c | + | a or 1/2c | L. monocytogenes group 1/2c |
| 46 | L. innocua | − | NA | L. innocua |
| 51 | L. innocua | − | NA | L. innocua |
| 52 | L. welshimeri | − | NA | L. welshimeri |
| 55 | L. innocua and L. welshimeri | − | NA | L. innocua and L. welshimeri |
| 58 | L. innocua and L. seeligeri | − | NA | L. innocua |
| 61 | L. monocytogenes group 1/2c | + | a or 1/2c | L. monocytogenes group 1/2c |
| 64 | L. monocytogenes group a | + | a or 1/2c | L. monocytogenes group a |
| 65 | L. monocytogenes group b | + | b | L. monocytogenes group b |
| 66 | L. innocua | − | NA | L. innocua |
| 67 | L. innocua | − | NA | L. innocua |
| 68 | L. monocytogenes group b and L. ivanovii | + | b | L. monocytogenes group b and L. ivanovii |
| 72 | L. monocytogenes group b and L. ivanovii | + | b | L. monocytogenes group b and L. ivanovii |
Samples in bold are reported in Fig. 5.
NA, not applicable.
FIG. 4.
Results obtained from the direct amplification and characterization of Listeria spp. in food. Lane labels indicate the number of the food sample shown in the gel and also reported in Table 4. (A) List-univ. 1 and List-univ. 2 PCR amplification. (B) DGGE profiles of food samples. (C) Specific L. monocytogenes PCR using primers Mar 1 and Mar 2 (38). The DGGE bands indicated by an asterisk were excised, reamplified, sequenced, and identified by sequence analysis. Abbreviations: Lm 1/2c, L. monocytogenes serotype 1/2c; Lm b, L. monocytogenes serogroup b; Linn, L. innocua; Liv, L. ivanovii; Ls, L. seeligeri; Lw, L. welshimeri.
The DNA extracted directly from food samples was also subjected to PCR using primers Mar 1 and Mar 2, and the results obtained for the samples shown in Fig. 4A are reported in Fig. 4C. As shown, only samples 4, 12, 32, 38, and 68, which contained L. monocytogenes, gave the specific amplicon, confirming the DGGE differentiation and identification.
The identification of Listeria spp. by molecular methods directly in food samples was last compared with the characterization of the strains isolated from Oxford agar. Up to five suspected Listeria sp. colonies per sample were isolated and subjected to DNA extraction, PCR amplification, and DGGE analysis. The results obtained are reported in Table 4. For almost all of the samples, the results obtained were in agreement with the identification of the isolates and, in fact, the species isolated and identified were represented in the DGGE gels. Only for sample 4, containing three different populations, L. welshimeri was present in the DGGE gel, but no isolates belonged to this species.
DISCUSSION
The detection and identification of Listeria spp. have attracted the attention of many authors. This specific interest is related to the presence of L. monocytogenes, one of the most important food-borne pathogens, in the genus. It is often found in various uncooked foods, such as meat, cheese, and vegetables. It is widely diffused in the environment and this fact can cause the contamination of food during production and distribution. However, L. monocytogenes has been the main representative of the genus to be studied.
The advances in biotechnology over the past decades have resulted in the development of many methods for the detection of pathogenic microorganisms such as L. monocytogenes in food (56). Molecular methods have been applied with certain success to the classification and typing of Listeria. Multilocus enzyme electrophoresis (4, 46), REA (5, 43, 49), and ribotyping (19, 57) have been developed. The use of faster procedures like PCR and immunological or bacteriophage lysis techniques which might allow a more rapid monitoring of all Listeria species is limited because they detect only the genus Listeria or only L. monocytogenes (8, 12, 16, 31, 34), thus lacking the ability to simultaneously characterize all the species of Listeria. Moreover, the methods developed so far require pure cultures isolated by traditional methods.
In this study, the development of a PCR-DGGE method to directly identify the members of the genus Listeria in food samples is described. This approach exploited the potential of PCR to amplify, with specific primers, variable regions within the iap gene as well as the discriminatory power of DGGE to differentiate DNA molecules on the basis of differences in their sequence (33).
The iap gene has been demonstrated to be a reliable PCR target for differentiation of Listeria spp. (6, 21, 39, 58). Bubert et al. (7) characterized the iap gene from different Listeria spp. and demonstrated the presence of conserved regions at the 5′ and 3′ ends and a species-specific internal region.
Here, the information available on the iap gene was used to design primers that specifically amplify Listeria spp. and that could be used for specific identification using the DGGE method. Two primers were identified for the region of the sequences that were conserved among different Listeria spp. The regions amplified contained a high degree of heterogeneity among the sequences considered, allowing their differentiation by DGGE. In addition, just by PCR amplification, it was possible to differentiate two groups of species on the basis of the molecular weight of the amplicon obtained (Fig. 1). The primers developed showed high specificity toward Listeria spp., since no positive amplification was obtained by using DNA extracted from different non-Listeria organisms.
The PCR products produced from control strains obtained from international collections were used to optimize the experimental conditions (denaturant gradients, temperature, voltage, and length of the electrophoretic run) of the DGGE. Denaturants from 20 to 40% showed the best differentiation power, allowing the identification, on the basis of specific migration, of the five species considered in the study (Fig. 2). Moreover, L. monocytogenes strains were characterized by different electrophoretic patterns depending on the serotype. Profiles of serotypes 1/2a and 3a were different from those of serotype 1/2c and serotypes 1/2b, 3b, and 4b. The results obtained by analyzing different serotypes of L. monocytogenes isolated from humans with listeriosis by PCR-DGGE confirmed the discriminatory power of the method.
The PCR-DGGE protocol described was then first used to identify 48 strains that were previously isolated from food samples and identified by traditional methods. All the strains tested showed DGGE patterns matching with those of control strains, allowing a rapid identification at the species level. Moreover, for L. monocytogenes it was also possible to differentiate serogroups. Nine strains identified as L. monocytogenes by traditional methods showed DGGE profiles of L. innocua. The explanation of the different identification must be related to the subjective analysis of the biochemical tests used. Hemolysis is the only marker that can be used to differentiate L. monocytogenes from L. innocua (3) and its misinterpretation could lead to mistakes in the identification of these two species as described in this paper.
Due to the specificity of the primers developed, the protocol was then applied to the direct detection of Listeria spp. in food samples. After an overnight enrichment step to increase the number of target cells and to avoid the amplification of dead cells, DNA was extracted from the enriched broth and subjected to PCR and DGGE. Enrichments were also streaked onto Oxford agar, and after incubation, suspected colonies were isolated and identified to confirm the results obtained by direct analysis. The identification of the isolated strains agreed with the detection of Listeria spp. directly in the food samples. Only for sample 4, L. welshimeri was detected in the DGGE gels, but no colony belonging to this species was isolated, thus underscoring the biases of traditional methods. Of the 73 food samples tested, 24 gave PCR products indicating the presence of Listeria species, while 49 did not. After DGGE analysis it was possible to identify single populations of Listeria spp. or single L. monocytogenes serogroups in 16 samples and mixed Listeria spp. in 8 samples. The results of the direct identification from food samples, on the basis of comigration with control strains, were also confirmed by direct sequencing of different bands (indicated in Fig. 4A).
DNA extracted directly from food samples was also amplified by use of specific primers for L. monocytogenes, Mar 1 and Mar 2 (38), and the positive samples were subjected to restriction analysis as described previously (36) to confirm the results of the identification of L. monocytogenes by direct PCR-DGGE protocol. As described in Table 4, no differences in the results were obtained, underscoring the reliability and accuracy of the PCR-DGGE method.
The protocol developed in this paper allows for fast and easy identification of all the species belonging to the genus Listeria. DGGE analysis of Listeria sp. PCR products results in species-specific migration patterns. For identification, reference strains must be included in the PCR-DGGE analysis. Comigration in the gel of a PCR product obtained from an unknown sample with a control amplicon gives the identification. Further confirmation can be obtained by direct sequencing of the DGGE band under consideration. The method can be used for rapid identification of traditionally isolated strains or it can be applied directly in food samples to detect Listeria spp., avoiding time-consuming classical isolation and identification. The results reported in this study prove the tremendous impact of the protocol. Even if a pre-enrichment step is necessary, in 18 h it is possible to determine the presence of any Listeria spp. in the sample. The method described is also able to identify a single cell of Listeria present before enrichment (data not shown), becoming an important tool especially for the detection of L. monocytogenes in food samples, since no L. monocytogenes cell can be present in ready-to-eat or cooked foods (54). Moreover, the possibility for quickly screening food samples to assess the presence of L. monocytogenes represents a big benefit for both plant surveillance and safety and spoilage matters.
The protocol described here makes possible the study of Listeria sp. ecology in food samples. Its application allows for reliable monitoring of all Listeria species and it can be exploited for a better understanding of the occurrence and distribution of Listeria in the environment. Lastly, since the method allows for distinguishing L. monocytogenes serotypes directly in food, it may potentially be exploited for epidemiological purposes, too.
REFERENCES
- 1.Asperger, H., H. Heistinger, M. Wagner, A. Lehner, and E. Brandl. 1999. A contribution of Listeria enrichment methodology-growth of Listeria monocytogenes under varying conditions concerning enrichment broth composition, cheese matrices and competing microbial flora. Food Microbiol. 16:419-431. [Google Scholar]
- 2.Bansal, N. S., F. H. Y. McDonell, A. Smith, G. Arnold, and G. F. Ibrahim. 1996. Multiplex PCR assay for the routine detection of Listeria in food. Int. J. Food Microbiol. 33:293-300. [DOI] [PubMed] [Google Scholar]
- 3.Beumer, R. R., M. C. T. Giffel, M. T. C. Kok, and F. M. Rombouts. 1996. Confirmation and identification of Listeria spp. Lett. Appl. Microbiol. 22:448-452. [DOI] [PubMed] [Google Scholar]
- 4.Bibb, W. F., B. G. Schwartz, B. G. Gellin, B. D. Plikaytis, and R. E. Weaver. 1989. Analysis of Listeria monocytogenes by multilocus enzyme electrophoresis and application of the method to epidemiologic investigations. Int. J. Food Microbiol. 8:233-239. [DOI] [PubMed] [Google Scholar]
- 5.Brosch, R., C. Buchrieser, and J. Rocourt. 1991. Subtyping of Listeria monocytogenes serovar 4b by use of low-frequency-cleavage restriction endonucleases and pulsed-field gel electrophoresis. Res. Microbiol. 142:667-675. [DOI] [PubMed] [Google Scholar]
- 6.Bubert, A., I. Hein, M. Rauch, A. Lehner, B. Yoon, W. Goebel, and M. Wagner. 1999. Detection and differentiation of Listeria spp. by a single reaction based on multiplex PCR. Appl. Environ. Microbiol. 65:4688-4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bubert, A., S. Kohler, and W. Goebel. 1992. The homologous and heterologous regions within the iap gene allow genus-specific and species-specific identification of Listeria spp. by polymerase chain reaction. Appl. Environ. Microbiol. 58:2625-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bubert, A., P. Schubert, S. Kohler, R. Frank, and W. Goebel. 1994. Synthetic peptides derived from the Listeria monocytogenes p60 protein as antigens for the generation of polyclonal antibodies specific for secreted cell-free L. monocytogenes p60 proteins. Appl. Environ. Microbiol. 60:3120-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carnevale, R. A., and P. Johnston. 1989. Method for the isolation and identification of Listeria monocytogenes from meat and poultry products. Laboratory communication no. 57. U.S. Department of Agriculture, Washington, D.C.
- 10.Curiale, M. S., and C. Lewus. 1994. Detection of Listeria monocytogenes in samples containing Listeria innocua. J. Food Prot. 57:1048-1051. [DOI] [PubMed] [Google Scholar]
- 11.Curtis, G. D. W., and W. H. Lee. 1995. Culture media and methods for the isolation of Listeria monocytogenes. Int. J. Food Microbiol. 26:1-13. [DOI] [PubMed] [Google Scholar]
- 12.Deneer, H., and I. Boychuk. 1991. Species-specific identification of Listeria monocytogenes by DNA amplification. Appl. Environ. Microbiol. 57:606-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drebot, M., S. Neal, W. Schlech, and K. Rozee. 1996. Differentiation of Listeria isolates by PCR amplicon profiling and sequence analysis of 16S-23S rRNA internal transcribed spacer loci. J. Appl. Bacteriol. 80:174-178. [DOI] [PubMed] [Google Scholar]
- 14.Engels, B. 1992. Amplify, for analyzing PCR experiments. University of Wisconsin, Madison.
- 15.Flanders, K. J., and C. W. Donnelly. 1994. Injury, resuscitation and detection of Listeria spp. from frozen environments. Food Microbiol. 11:473-480. [Google Scholar]
- 16.Furrer, B., U. Candrian, C. Hoefelein, and J. Luethy. 1991. Detection and identification of Listeria monocytogenes in cooked sausage products and in milk by in vitro amplification of haemolysin gene fragments. J. Appl. Bacteriol. 70:372-379. [DOI] [PubMed] [Google Scholar]
- 17.Gormon, T., and L. Phanthanh. 1995. Identification and classification of Listeria by two-dimensional protein mapping. Res. Microbiol. 146:143-154. [DOI] [PubMed] [Google Scholar]
- 18.Graham, T. A., E. J. Golsteyn Thomas, J. E. Thomas, and V. P. J. Gannon. 1997. Inter- and intraspecies comparison of the 16S-23S rRNA operon intergenic spacer regions of six Listeria spp. Int. J. Syst. Bacteriol. 47:863-869. [DOI] [PubMed] [Google Scholar]
- 19.Graves, L. L., B. Swaminathan, M. W. Reeves, and J. Wenger. 1991. Ribosomal fingerprinting of L. monocytogenes using a digoxigenin-labeled DNA probe. Eur. J. Epidemiol. 7:77-82. [DOI] [PubMed] [Google Scholar]
- 20.Head, I. M., J. R. Saunders, and R. W. Pickup. 1998. Microbial evolution, diversity, and ecology: a decade of ribosomal RNA analysis of uncultivated microorganisms. Microb. Ecol. 35:1-21. [DOI] [PubMed] [Google Scholar]
- 21.Hein, I., D. Klein, A. Lehner, A. Bubert, E. Brandl, and M. Wagner. 2001. Detection and quantification of the iap gene of Listeria monocytogenes and Listeria innocua by a new real-time quantitative PCR assay. Res. Microbiol. 152:37-46. [DOI] [PubMed] [Google Scholar]
- 22.Heisick, J. E., L. I. Rosas-Marty, and S. R. Tatini. 1995. Enumeration of viable Listeria species and Listeria monocytogenes in foods. J. Food Prot. 58:733-736. [DOI] [PubMed] [Google Scholar]
- 23.Herman, L. M. F., H. F. M. Deridder, and G. M. M. Vlaemynck. 1995. A multiplex PCR method for the identification of Listeria spp. and Listeria monocytogenes in dairy samples. J. Food Prot. 58:867-872. [DOI] [PubMed] [Google Scholar]
- 24.Hitchins, A. D., and R. E. Duvall. 2000. Feasibility of a defined microflora challenge method for evaluating the efficacy of foodborne Listeria monocytogenes selective enrichments. J. Food Prot. 63:1064-1070. [DOI] [PubMed] [Google Scholar]
- 25.Hudson, J. A., R. J. Lake, M. G. Savill, P. Scholes, and R. E. McCormick. 2001. Rapid detection of Listeria monocytogenes in ham samples using immunomagnetic separation followed by polymerase chain reaction. J. Appl. Microbiol. 90:614-621. [DOI] [PubMed] [Google Scholar]
- 26.Hugenholtz, P., B. M. Goebel, and N. R. Pace. 1998. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J. Bacteriol. 180:4765-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingianni, A., M. Floris, P. Palomba, A. Madeddu, M. Quartuccio, and R. Pompei. 2001. Rapid detection of Listeria monocytogenes in foods, by a combination of PCR and DNA probe. Mol. Cell. Probes 15:275-280. [DOI] [PubMed] [Google Scholar]
- 28.International Dairy Federation. 1995. Milk and milk products—detection of Listeria monocytogenes. IDF standard 143A:1995. International Dairy Federation, Brussels, Belgium.
- 29.Jacquet, C., S. Aubert, N. Elsolh, and J. Rocourt. 1992. Use of rRNA gene restriction patterns for the identification of Listeria species. Syst. Appl. Microbiol. 15:42-46. [Google Scholar]
- 30.Jersek, B., E. Tcherneva, N. Rijpens, and L. Herman. 1996. Repetitive element sequence-based PCR for species and strain discrimination in the genus Listeria. Lett. Appl. Microbiol. 23:55-60. [DOI] [PubMed] [Google Scholar]
- 31.Johnson, W. M., S. D. Tyler, E. P. Ewan, F. E. Ashton, G. Wang, and K. R. Rozee. 1992. Detection of genes coding for listeriolysin and Listeria monocytogenes antigen A (lmaA) in Listeria spp. by the polymerase chain reaction. Microb. Pathog. 12:79-86. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence, L. M., and A. Gilmour. 1994. Incidence of Listeria spp. and Listeria monocytogenes in a poultry processing environment and in poultry products and their rapid confirmation by multiplex PCR. Appl. Environ. Microbiol. 60:4600-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lerman, L. S., S. G. Fischer, I. Hurley, K. Silverstein, and N. Lumelsky. 1984. Sequence-determined DNA separation. Annu. Rev. Biophys. Bioeng. 13:399-423. [DOI] [PubMed] [Google Scholar]
- 34.Loessner, M. J., C. E. D. Rees, G. S. A. B. Stewart, and S. Scherer. 1996. Construction of luciferase reporter bacteriophage A511::luxAB for rapid and sensitive detection of viable Listeria cells. Appl. Environ. Microbiol. 62:1133-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovett, J., and R. Twedt. 1988. Listeria. Outstanding symposia in food science and technology. Food Technol. 8:188-191. [Google Scholar]
- 36.Manzano, M., L. Cocolin, C. Cantoni, and G. Comi. 1998. A rapid method for the identification and partial serotyping of Listeria monocytogenes in food by PCR and restriction enzyme analysis. Int. J. Food Microbiol. 42:207-212. [DOI] [PubMed] [Google Scholar]
- 37.Manzano, M., L. Cocolin, C. Cantoni, and G. Comi. 2000. Temperature gradient gel electrophoresis of the amplified product of a small 16S rRNA gene fragment for the identification of Listeria species isolated from food. J. Food Prot. 63:659-661. [DOI] [PubMed] [Google Scholar]
- 38.Manzano, M., L. Cocolin, P. Ferroni, C. Cantoni, and G. Comi. 1997. A simple and fast PCR protocol to detect Listeria monocytogenes from meat. J. Sci. Food Agric. 74:25-30. [Google Scholar]
- 39.Manzano, M., L. Cocolin, P. Ferroni, V. Gasparini, D. Narduzzi, C. Cantoni, and G. Comi. 1996. Identification of Listeria species by a semi-nested polymerase chain reaction. Res. Microbiol. 147:637-640. [DOI] [PubMed] [Google Scholar]
- 40.McLauchlin, J. 1997. The identification of Listeria spp. Int. J. Food Microbiol. 38:77-81. [DOI] [PubMed] [Google Scholar]
- 41.Medeiros, D., and J. M. Farber. 2001. A single space polymerase chain reaction for combined gene detection and epidemiological typing of Listeria monocytogenes. Food Microbiol. 18:375-386. [Google Scholar]
- 42.Niederhauser, C., U. Candrian, C. Hofelein, M. Jermini, H. P. Buhler, and J. Luthy. 1992. Use of polymerase chain reaction for detection of Listeria monocytogenes in food. Appl. Environ. Microbiol. 58:1564-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nocera, D., E. Bannerman, J. Rocort, J. Jaton-Ogay, and J. Bille. 1990. Characterization by DNA restriction endonuclease analysis of Listeria monocytogenes strains related to the Swiss epidemic of listeriosis. J. Clin. Microbiol. 28:2259-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nogva, H. K., K. Rudi, K. Naterstad, A. Holck, and D. Lillehaug. 2000. Application of 5′-nuclease PCR for quantitative detection of Listeria monocytogenes in pure cultures, water, skim milk, and unpasteurized whole milk. Appl. Environ. Microbiol. 66:4266-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norton, D. M., M. A. McCamey, K. L. Gall, J. M. Scarlett, K. J. Boor, and M. Wiedmann. 2001. Molecular studies on the ecology of Listeria monocytogenes in the smoked fish processing industry. Appl. Environ. Microbiol. 67:198-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piffaretti, J. C., H. Kressbuch, M. Aeschbacher, J. Bille, E. Bannerman, J. M. Musser, R. K. Salander, and J. Rocourt. 1989. Genetic characterization of clones of the bacterium Listeria monocytogenes causing epidemic diseases. Proc. Natl. Acad. Sci. USA 86:3818-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pritchard, T. J., and C. W. Donnelly. 1998. Combined secondary enrichment of primary enrichment broths increases Listeria detection. J. Food Prot. 62:532-535. [DOI] [PubMed] [Google Scholar]
- 48.Rocourt, J., J. M. Alonso, and H. P. R. Seeliger. 1983. Virulence comparée des cinq groupes génomiques de Listeria monocytogenes (sensu lato). Ann. Microbiol. (Inst. Pasteur) 134A:359-364. [PubMed] [Google Scholar]
- 49.Rocourt, J., F. Grimont, P. A. D. Grimont, and H. P. R. Seeliger. 1982. DNA relatedness among serovars of Listeria monocytogenes sensu lato. Curr. Microbiol. 7:383-388. [Google Scholar]
- 50.Rossen, L., K. Holmstrom, J. E. Olsen, and O. F. Rasmussen. 1991. A rapid polymerase chain reaction (PCR)-based assay for the identification of Listeria monocytogenes in food samples. Int. J. Food Microbiol. 14:145-151. [DOI] [PubMed] [Google Scholar]
- 51.Ryser, E. T., S. M. Arimi, M. Bunduki, and C. W. Connelly. 1996. Recovery of different Listeria ribotypes from naturally contaminated, raw refrigerated meat and poultry products with two primary enrichment media. Appl. Environ. Microbiol. 62:1781-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schuchat, A., B. Swaminathan, and C. V. Broome. 1991. Epidemiology of human listeriosis. Clin. Microbiol. Rev. 4:169-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seeliger, H. P. R., and D. Jones. 1986. Genus Listeria Pirie 1940, 383AL, p. 1235-1245. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. Williams & Wilkins, Baltimore, Md.
- 54.Shank, F. R., E. L. Elliot, I. K. Wachsmuth, and M. E. Losikoff. 1996. U.S. position on Listeria monocytogenes in foods. Food Control 7:229-234. [Google Scholar]
- 55.Sheffield, V. C., D. R. Cox, L. S. Lerman, and R. M. Myers. 1989. Attachment of a 40-base pair G+C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc. Natl. Acad. Sci. USA 86:232-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swaminathan, B., and P. Feng. 1994. Rapid detection of foodborne pathogenic bacteria. Annu. Rev. Microbiol. 48:401-425. [DOI] [PubMed] [Google Scholar]
- 57.Vogt, R. L., C. Donelly, B. Gellin, W. Bibb, and B. Swaminathan. 1990. Linking environmental and human strains of Listeria monocytogenes with isoenzyme and ribosomal RNA typing. Eur. J. Epidemiol. 6:229-230. [DOI] [PubMed] [Google Scholar]
- 58.Wagner, M., A. Lehner, D. Klein, and A. Bubert. 2000. Single-strand conformation polymorphisms in the hly gene and polymerase chain reaction analysis of a repeat region in the iap gene to identify and type Listeria monocytogenes. J. Food Prot. 63:332-336. [DOI] [PubMed] [Google Scholar]
- 59.Wang, R. F., W. W. Cao, and M. G. Johnson. 1992. 16S rRNA-based probes and polymerase chain reaction method to detect Listeria monocytogenes cells added to foods. Appl. Environ. Microbiol. 58:2827-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wieckowska-Szakiel, M., A. Bubert, M. Rozalski, U. Krajewska, W. Rudnicka, and B. Rozalska. 2002. Colony-blot assay with anti-p60 antibodies as a method for quick identification of Listeria in food. Int. J. Food Microbiol. 72:63-71. [DOI] [PubMed] [Google Scholar]