Abstract
The Clostridium cellulovorans xynA gene encodes the cellulosomal endo-1,4-β-xylanase XynA, which consists of a family 11 glycoside hydrolase catalytic domain (CD), a dockerin domain, and a NodB domain. The recombinant acetyl xylan esterase (rNodB) encoded by the NodB domain exhibited broad substrate specificity and released acetate not only from acetylated xylan but also from other acetylated substrates. rNodB acted synergistically with the xylanase CD of XynA for hydrolysis of acetylated xylan. Immunological analyses revealed that XynA corresponds to a major xylanase in the cellulosomal fraction. These results indicate that XynA is a key enzymatic subunit for xylan degradation in C. cellulovorans.
Xylan, the major hemicellulose component in plant cell walls, has a backbone of β-1,4-linked xylopyranosyl residues and contains various substituted side groups, e.g., acetyl, l-arabinofuranosyl, and 4-o-methylglucuronyl residues (17). The enzymes involved in hydrolysis of the main chain of xylan are endoxylanase (1,4-β-d-xylan xylanohydrolase; EC 3.2.1.8), β-xylosidase (β-d-xyloside xylohydrolase; EC 3.2.1.37), and acetyl xylan esterase (EC 3.1.1.72) (17). On the basis of the amino acid sequences of catalytic domains (CDs), xylanases have been classified into two groups, families 10 and 11 of glycosyl hydrolases (P. M. Coutinho and B. Henrissat, http://afmb.cnrs-mrs.fr/∼pedro/CAZY/db.html). Clostridium cellulovorans ATCC 35296 (14) produces a large extracellular polysaccharolytic complex called the cellulosome, in which several cellulases are tightly bound to a scaffolding protein called CbpA (1). Our laboratory has characterized the genes necessary for the degradation of crystalline cellulose of this bacterium (1, 10, 15, 16). In this paper, we describe properties of the xynA gene that codes for a component of the C. cellulovorans cellulosome (7). To avoid confusion, we will call the complete product of the xynA gene XynA and the product of the acetyl xylan esterase domain NodB. The CD referred to is the product of the xylanase CD.
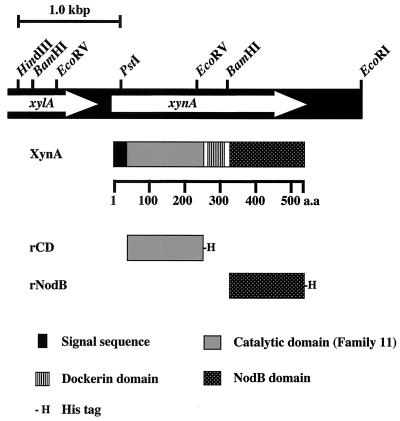
Nucleotide sequence of the xynA gene.
A previously constructed C. cellulovorans genomic library (15) was screened for xylanase activity by overlaying with soft agar containing birchwood xylan. Three positive clones were isolated, and they had an 8.7-kb EcoRI insert (pX13) in common. The coding region for xylanase was located on a 3.7-kb fragment between the HindIII and EcoRI sites (Fig. 1). The xynA gene consists of 1,563 nucleotides encoding a protein of 520 amino acids with a predicted molecular weight of 57,038. The assigned ATG initiation codon was preceded by a potential ribosome-binding sequence (GAAAGG) that was homologous to the consensus Shine-Dalgarno sequence (3). The xynA gene was located downstream of a hypothetical open reading frame (xylA) homologous to the C. acetobutylicum β-xylosidase (accession no. NC_001988.2) (11).
FIG. 1.
Restriction enzyme map of the HindIII-to-EcoRI fragment encoding xynA (A) and modular structure of XynA and its derivatives (B). The white arrows indicate the coding sequences for XynA and XylA polypeptides. a.a., amino acids.
Amino acid sequences and domains of XynA.
The N-terminal sequence of XynA exhibited a typical signal peptide (18). Comparison of the deduced amino acid sequence of XynA with sequences registered in protein databases such as SWSS-PROT revealed that mature XynA consists of three distinct functional domains, i.e., a CD of family 11 glycosyl hydrolases, a dockerin domain, and a nodulation protein domain (NodB) classified as a family 4 carbohydrate esterase (Fig. 1). The family 11 domain (CD) of XynA, spanning amino acids 29 to 232, exhibited extensive sequence homology with enzymes classified in family 11 of glycosyl hydrolases, such as C. thermocellum F1 XynA (67.7% identity) (4) and C. stercorarium XynA (54.9% identity) (13). A dockerin domain lies downstream of the family 11 CD (residues 249 to 306). The dockerin containing a 22-amino-acid repeat is highly conserved in enzymatic cellulosomal subunits of C. cellulovorans (1). The C-terminal domain, extending from residue 321 to residue 520, is homologous with NodB, and NodB-like domains are conserved in several xylanases, e.g., 27.8% identity with the NodB protein from Rhizobium leguminosarum (12).
Purification and characterization of rXynA.
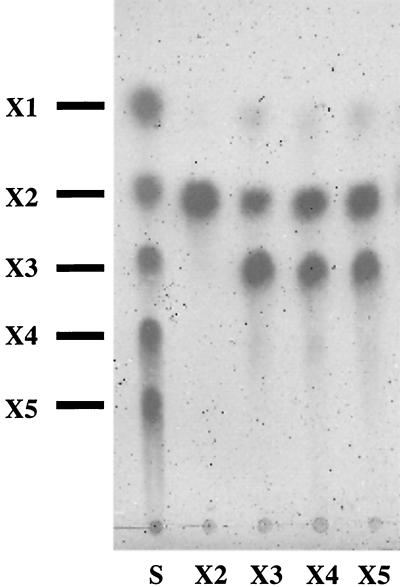
To characterize the properties of XynA, we designed a fusion protein with an S-protein tag for the N terminus and a six-histidine tag for the C terminus to isolate full-length XynA. The two primers containing artificial EcoRI or XhoI sites (underlined) were used to amplify full-length xynA (5′-CGAATTCGGCAACAAAAACGATCACC-3′ and 5′-CCGCTCGAGGAATGCACCATTTAACATTGT-3′). The PCR product was inserted into pET29b (Novagen) to generate pEXYNA29. When a culture of Escherichia coli BL21(DE3) (Novagen) harboring pEXYNA29 had reached an optical density at 600 nm of 0.5 at 30°C in Luria-Bertani medium supplemented with kanamycin (50 μg/ml), isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM and the cells were further cultivated at 30°C for 4 h. The cells were collected, suspended in buffer 1 (50 mM phosphate, 300 mM NaCl, 10 mM imidazole, pH 8.0) and disrupted by sonication. The cell extracts were applied to an Ni-nitrilotriacetic acid agarose column (Qiagen). Recombinant XynA (rXynA) was eluted by buffer 1 with 250 mM imidazole and applied to an S-protein agarose column (Novagen). The proteins were treated with the S-Tag thrombin purification kit (Novagen) to eliminate the S-protein tag sequence in accordance with the manufacturer's instructions. The eluted proteins were concentrated to 1.5 to 2.0 mg/ml by ultrafiltration (Ultra free biomax-30; Millipore). Protein concentrations were determined with a bicinchoninic acid protein assay kit (Pierce) with bovine serum albumin as the standard. As a result, rXynA was purified 347-fold from E. coli BL21 harboring pEXYNA29. Xylanase activity was measured in the presence of 0.2% (wt/vol) birchwood xylan (Sigma) at 37°C in 50 mM phosphate buffer (pH 7.0) or in Britton and Robinson's universal buffer (50 mM phosphoric acid, 50 mM boric acid, 50 mM acetic acid [pH adjusted to 2 to 11 with NaOH]) for 10 min. The reducing sugar released was measured by the Somogyi-Nelson method (19) after the reaction was stopped and the reaction mixture was stored on ice. One unit of activity was defined as the amount of enzyme that released 1 μmol of xylose per ml of sample per min. The glycosidase activities were determined by measuring the absorbance of liberated p-nitrophenol from p-nitrophenyl-β-d-xylopyranoside (Sigma), p-nitrophenyl-β-d-cellobioside (Sigma), and p-nitrophenyl-β-d-glucopyranoside (Sigma) at 410 nm. Assay mixtures containing each substrate at 1 mM in 50 mM phosphate buffer (pH 7.0) were incubated for 30 min at 37°C, and the reactions were stopped by addition of Na2CO3 (8). One unit of activity toward p-nitrophenyl derivatives was defined as the amount of enzyme liberating 1 μmol of p-nitrophenol per min. rXynA had a high specific activity with birchwood xylan (825 U/mg of protein), while no activity was observed with p-nitrophenyl-β-d-cellobioside, p-nitrophenyl-β-d-xylopyranoside, p-nitrophenyl-β-D-glucopyranoside, and carboxy methylcellulose. Figure 2 shows the pattern of several xylooligosaccharides hydrolyzed by rXynA and analyzed by thin-layer chromatography (7). The products of rXynA were mainly xylobiose and xylotriose. XynA was not active on xylobiose and less active with xylotriose than on xylooligosaccharides. The pH for optimum rXynA activity was 5.0, and the enzyme was stable over a pH range of 2.0 to 7.0 when incubated at 30°C for 12 h with no substrate. The temperature for maximum activity was found to be 60°C at pH 5.0. These enzymatic properties and the narrow substrate specificity of XynA are also very similar to those of C. thermocellum XynA (2, 4).
FIG. 2.
Thin-layer chromatography of hydrolysis products from xylooligosaccharides. Each xylooligosaccharide (xylobiose to xylopentaose, 3 mg of each; Megazyme) was incubated with purified enzyme (1 U) for 16 h, and the hydrolysates were analyzed by thin-layer chromatography (7). S, authentic oligosaccharides; X1, xylose; X2, xylobiose; X3, xylotriose; X4, xylotetraose; X5, xylopentaose.
Role of the NodB domain of XynA in xylan degradation.
To confirm whether the NodB domain of C. cellulovorans XynA is able to release acetyl groups from acetylated xylan, recombinant CD (rCD) containing the catalytic xylanase domain alone and rNodB containing the NodB domain alone were constructed. Two primers containing artificial EcoRI or XhoI sites (underlined) (5′-CGAATTCGGTTGCTCTCACATTTGAT-3′ for the sense primer of pEXNOD29 and 5′-CCGCTCGAGAAGTAATTTTTCTGGGGTAGGTTG-3′ for the antisense primer of pEXCD29) and primers used for full-length xynA were used to amplify its truncated derivatives by PCR. The amplified fragments were also inserted into pET29b to generate pEXCD29 and pEXNOD29, respectively. The rCD and rNodB proteins were also purified from E. coli BL21(DE3) harboring pEXCD29 or pEXNOD29 by the same purification steps as described for rXynA. Acetylated xylan was prepared from birchwood xylan by the method of Johnson et al. (6). Deacetylase activity was determined by measuring the amount of p-nitrophenol liberated at 410 nm after 10 min of incubation at 37°C in 50 mM phosphate buffer (pH 7.0) in the presence of 0.1 mM p-nitrophenyl acetate (Sigma). The reaction was also terminated by addition of Na2CO3. When acetyl xylan, N,N′-diacetylchitobiose (Sigma), galactose pentaacetate (Sigma), and cellulose acetate (Sigma) were used as substrates, the acetate released was measured with an acetic acid assay kit (Biopharm) after 10 min of incubation at 37°C in 50 mM phosphate buffer (pH 7.0) (6). One unit of deacetylase activity is defined as the amount of enzyme liberating 1 μmol of p-nitrophenol per min for p-nitrophenyl acetate or 1 μmol of acetic acid per min for acetylated substrates. As a result, rXynA and rNodB could release acetyl groups from acetylated xylan while the rCD did not show deacetylase activity, suggesting that the CD in XynA was not related to the activity of acetyl xylan esterase (Table 1). The xylanase activity of rXynA was twice as high as that of rCD for acetylated xylan; however, its activity for oat spelt xylan was not affected, suggesting that the NodB domain contributes synergistically to the efficient hydrolysis of acetylated xylan. In addition, rNodB and rXynA exhibited deacetylase activity against other acetylated substrates, such as 4-nitrophenyl acetate and chitobiose, suggesting that the deacetylase activity of rXynA has broad substrate specificity (Table 2). The NodB domain in Cellulomonas fimi XylD was also known to release acetate form acetylated xylan but not catalyze the deacetylation of chitobiose and 4-nitrophenyl acetate (9); however, our observations indicate that in C. cellulovorans, XynA is able to deacetylate the residues of chitooligosaccharides. In nitrogen-fixing bacteria such as Rhizobium meliloti, the NodB protein also deacetylates the nonreducing N-acetylglucosamine residues of a range of chitooligosaccharides (5). It is interesting with respect to the evolution of soil bacteria that the NodB action mode of C. cellulovorans XynA is similar to that of Rhizobium NodB. The optimum pH and temperature for the deacetylase activity of rXynA with 4-nitrophenyl acetate were in good agreement with those of the xylanase activity; i.e., the optimum pH and temperature were 6.0 and 50°C, respectively. The esterase activity was stable over a pH range of 3 to 7 when incubated with no substrate at 30°C for 12 h in Britton and Robinson's universal buffer (pH 2 to 9).
TABLE 1.
Comparison of xylanase and deacetylase activities for each domain of XynA
| Domain | Xylanasea
|
Deacetylasea
|
||
|---|---|---|---|---|
| Oat spelt xylan | Acetylated xylan | Oat spelt xylan | Acetylated xylan | |
| rXynA | 155.3 ± 0.1 | 309.3 ± 0.2 | NDb | 121.5 ± 0.9 |
| rCD | 155.6 ± 0.2 | 146.1 ± 0.3 | ND | ND |
| rNodB | ND | ND | ND | 103.1 ± 0.2 |
Activities are in units per milligram of protein. The reaction mixtures contained 0.2% (wt/vol) substrate, 50 mM phosphate buffer (pH 6.0), and 50 μg of protein. The incubation was carried out at 37°C for 10 min. Each value is the mean of three determinations ± the standard deviation.
ND, not detected.
TABLE 2.
Substrate specificity of deacetylase activity of XynA
| Substrate | Relative deacetylase rate (%)a
|
||
|---|---|---|---|
| rXynA | rCD | rNodB | |
| Acetylated xylan | 100 | NDb | 103 |
| Birchwood xylan | 49 | ND | 50 |
| N,N′-Diacetylchitobiose | 36 | ND | 69 |
| Galactose pentaacetate | 5.3 | 0.04 | 6.6 |
| Cellulose acetate | 0.6 | 0.02 | 0.6 |
| 4-Nitrophenyl acetatec | 100 | ND | 173 |
Deacetylase rates are relative to the amount of acetyl group liberated per milligram of protein when rXynA was incubated with acetyl xylan, which was set at 100%. The reaction mixtures contained 0.2% (wt/vol) substrate, except for 4-nitrophenol acetate (100 μM), 50 mM phosphate buffer (pH 6.0), and 50 μg of protein. The incubation was carried out at 37°C for 15 h. The measurements were done by three independent determinations.
ND, not detected.
The rate of 4-nitrophenyl acetate hydrolysis is also expressed relative to the amount of p-nitrophenol group liberated per milligram of protein by rXynA, which was set at 100%.
Identification of XynA in the C. cellulovorans cellulosome.
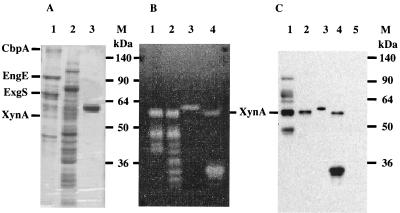
We performed immunoblot analyses with anti-XynA for cellulosomal and noncellulosomal fractions prepared from xylan-grown cultures (7). This antiserum reacted with proteins with molecular masses of 110, 75, 65, and 48 kDa, which corresponded to several cellulosomal subunits, e.g., EngE (15), ExgS (10), and EngB (1) (Fig. 3C). We believe that the antiserum was able to recognize several cellulosomal subunits through their dockerin domains, since these cross-reactive signals coincided with the migration of EngE, ExgS, and EngB on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Fig. 3A). One major immunoreactive band of 57 kDa corresponding to XynA was observed in the cellulosomal and noncellulosomal fractions (Fig. 3C). The 57-kDa immunoreactive protein also showed xylanase activity on zymogram analysis (Fig. 3B). We have reported that the N-terminal sequence of the 57-kDa immunoreactive protein is ATKTITXNETGNF (7). This sequence result was in good agreement with the deduced amino acid sequence of XynA. Therefore, these profiles strongly indicate that the xynA gene is a key component of the C. cellulovorans cellulosome and that it contributes significantly to xylan and plant cell wall degradation.
FIG. 3.
Identification of XynA in C. cellulovorans and expression of rXynA in E. coli. Gels were stained with Coomassie brilliant blue (A) or stained for xylanase activity (B). XynA proteins in immunoblot analyses were detected with a polyclonal rabbit antiserum raised against purified rXynA (C). Lanes: 1, cellulosomal fraction of C. cellulovorans; 2, noncellulosomal fraction of C. cellulovorans; 3, purified rXynA; 4, whole-cell proteins of E. coli XL1-Blue (Stratagene) harboring pX13; 5, whole-cell proteins of E. coli XL1-Blue; M, protein molecular mass standards. The proteins corresponding to the major components specified in the C. cellulovorans cellulosome (7) are indicated on the left of panel A.
Nucleotide sequence accession number.
The nucleotide sequence reported here has been submitted to the GenBank database and assigned accession no. AF435978.
Acknowledgments
This research was supported in part by grant DE-DDF03-92ER20069 from the U.S. Department of Energy.
REFERENCES
- 1.Doi, R. H., and Y. Tamaru. 2001. The Clostridium cellulovorans cellulosome: an enzyme complex with plant cell wall degrading activity. Chem. Rec. 1:24-32. [DOI] [PubMed] [Google Scholar]
- 2.Fernandes, A. C., C. M. G. A. Fontes, H. J. Gilbert, G. P. Hazlewood, T. H. Fernandes, and L. M. A. Ferreira. 1999. Homologous xylanases from Clostridium thermocellum: evidence for bi-functional activity, synergism between xylanase catalytic modules and the presence of xylan-binding domains in enzyme complexes. Biochem. J. 342:105-110. [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein, M. A., and R. H. Doi. 1995. Prokaryotic promoters in biotechnology. Biotechnol. Annu. Rev. 1:105-128. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi, H., M. Takehara, T. Hattori, T. Kimura, S. Karita, K. Sakka, and K. Ohmiya. 1999. Nucleotide sequences of two contiguous and highly homologous xylanase genes, xynA and xynB, and characterization of XynA from Clostridium thermocellum. Appl. Microbiol. Biotechnol. 51:348-357. [DOI] [PubMed] [Google Scholar]
- 5.John, M., H. Rohrig, J. Schmidt, U. Wieneke, and J. Schell. 1993. Rhizobium NodB protein involved in nodulation signal syntheses is a chitooligosaccharide deacetylase. Proc. Natl. Acad. Sci. USA 90:625-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson, K. G., J. D. Fontana, and C. R. Mackenzie. 1988. Measurement of acetylxylan esterase in Streptomyces. Methods Enzymol. 160:551-560. [Google Scholar]
- 7.Kosugi, A., K. Murashima, and R. H. Doi. 2001. Characterization of xylanolytic enzymes in Clostridium cellulovorans: expression of xylanase activity dependent on growth substrates. J. Bacteriol. 183:7037-7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lachke, A. H. 1988. 1,4-β-d-xylan xylohydrolase of Sclerotium rolfsii. Methods Enzymol. 160:679-684. [Google Scholar]
- 9.Laurie, J. I., J. H. Clarke, A. Ciruela, C. B. Faulds, G. Williamson, H. J. Gilbert, J. E. Rixon, J. Millward-Sadler, and G. P. Hazlewood. 1997. The NodB domain of a multidomain xylanase from Cellulomonas fimi deacetylates acetylxylan. FEMS Lett. 148:261-264. [Google Scholar]
- 10.Liu, C.-C., and R. H. Doi. 1998. Properties of exgS, a gene for a major subunit of the Clostridium cellulovorans cellulosomes. Gene 211:39-47. [DOI] [PubMed] [Google Scholar]
- 11.Nöling, J., G. Breton, M. V. Omelchenko, K. S. Makarova, Q. Zeng, R. Gibson, H. M. Lee, J. Dubois, D. Qiu, J. Hitti, Y. I. Wolf, R. L. Tatusov, F. Sabathe, L. Doucette-Stamm, P. Soucaille, M. J. Daly, G. N. Bennett, E. V. Koonin, and D. R. Smith. 2001. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J. Bacteriol. 183:4823-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossen, L., A. W. Johnston, and J. A. Downie. 1984. DNA sequence of Rhizobium leguminosarum nodulation genes nodA, -B and -C required for root hair curling. Nucleic Acids Res. 12:9497-9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakka, K., Y. Kojima, T. Kondo, S. Karita, K. Ohmiya, and K. Shimada. 1993. Nucleotide sequence of the Clostridium stercorarium xynA gene encoding xylanase A: identification of catalytic and cellulose binding domains. Biosci. Biotechnol. Biochem. 57:273-277. [DOI] [PubMed] [Google Scholar]
- 14.Sleat, R., R. A. Mah, and R. Robinson. 1984. Isolation and characterization of an anaerobic, cellulolytic bacterium, Clostridium cellulovorans sp. nov. Appl. Environ. Microbiol. 48:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamaru, Y., and R. H. Doi. 1999. Three surface layer homology domains at the N terminus of the Clostridium cellulovorans major cellulosomal subunit EngE. J. Bacteriol. 181:3270-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamaru, Y., and R. H. Doi. 2001. Pectate lyase A, an enzymatic subunit of Clostridium cellulovorans cellulosome. Proc. Natl. Acad. Sci. USA 98:4125-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomson, J. A. 1993. Molecular biology of xylan degradation. FEMS Microbiol. Rev. 104:65-82. [DOI] [PubMed] [Google Scholar]
- 18.Von Heijne, G. 1985. Signal sequences. The limits of variation. J. Mol. Biol. 184:99-105. [DOI] [PubMed] [Google Scholar]
- 19.Wood, W. A., and K. M. Bhat. 1988. Methods for measuring cellulose activities. Methods Enzymol. 160:87-112. [Google Scholar]