Abstract
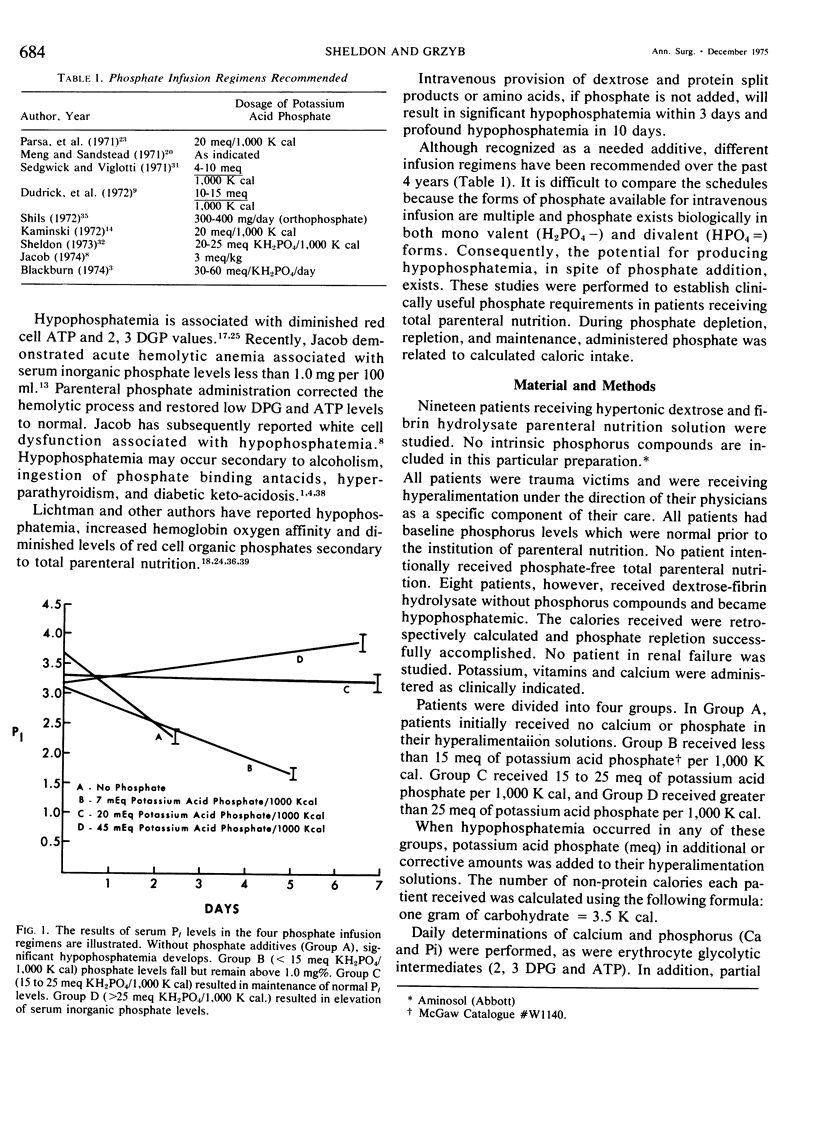
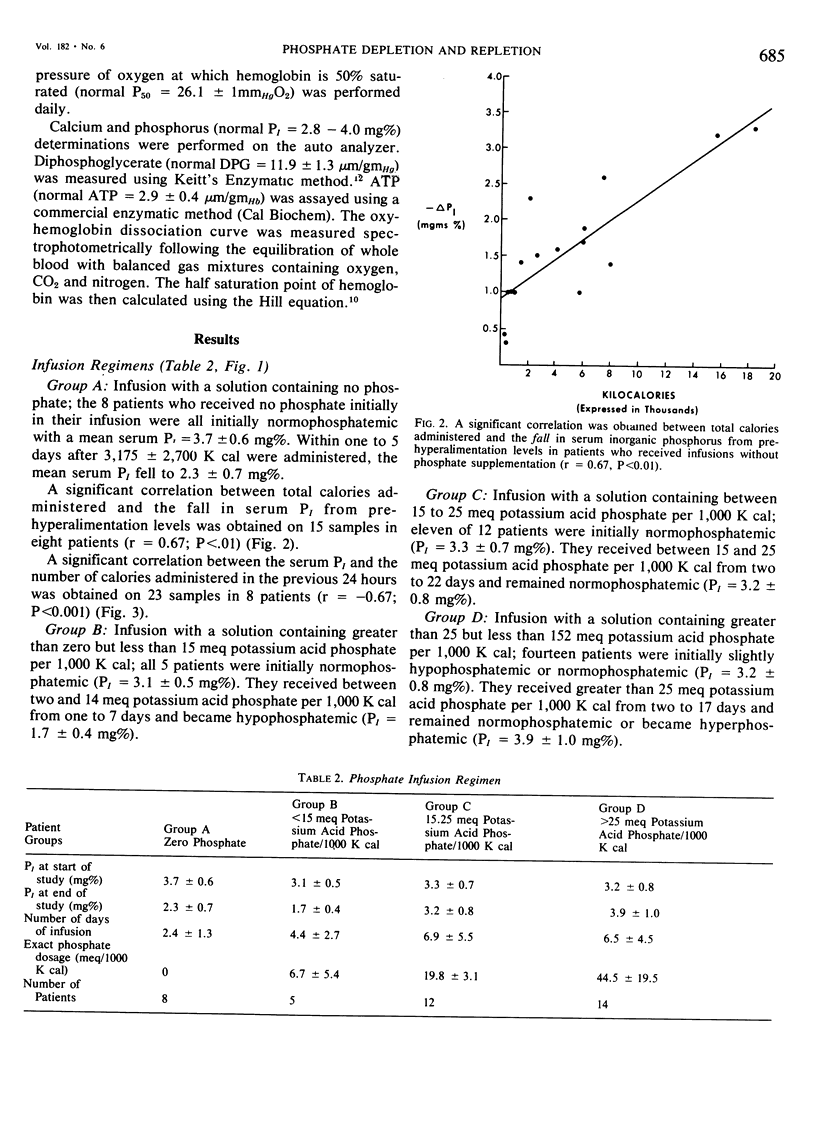
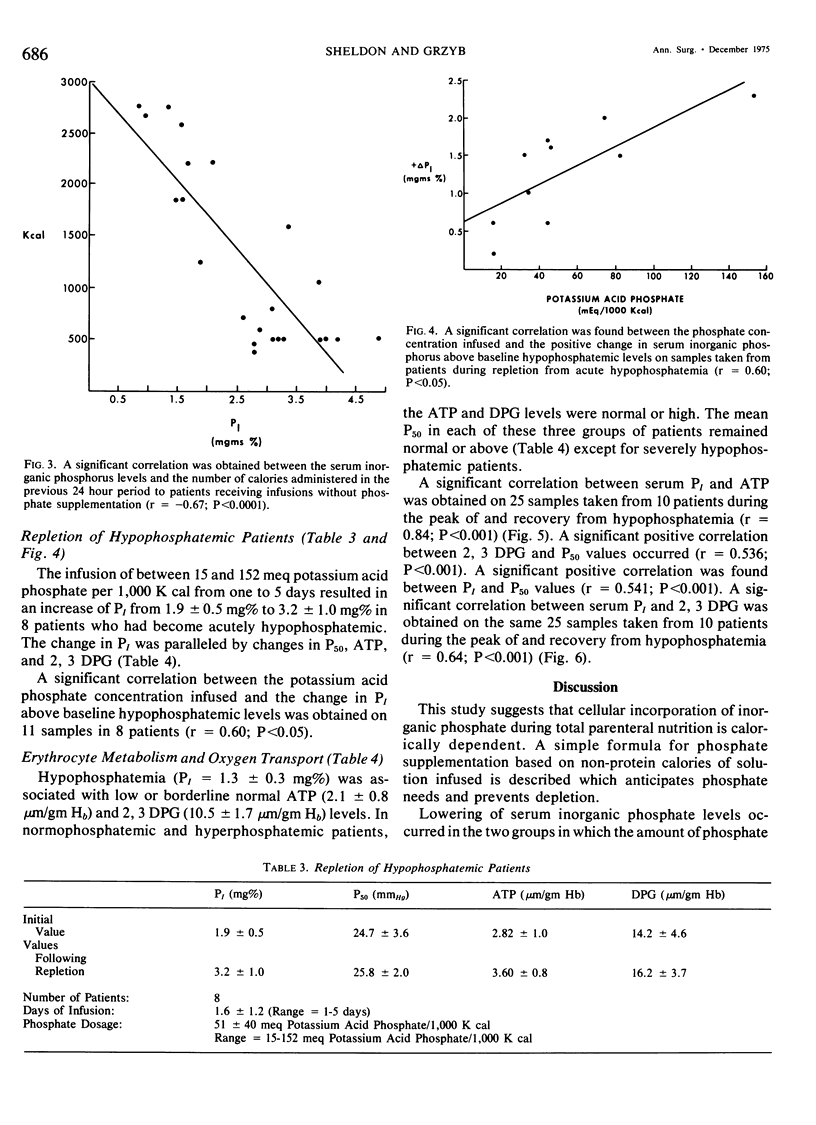
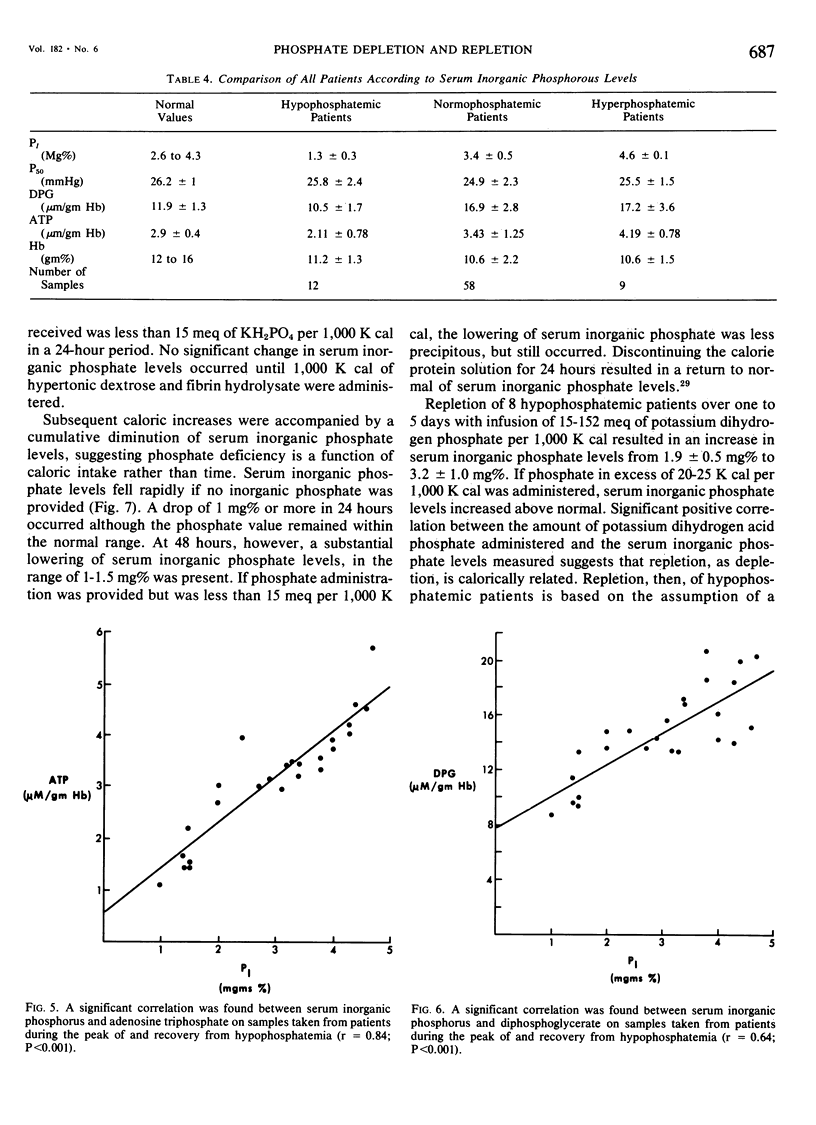
Phosphate depletion occurring during total parenteral nutrition has been frequently reported during the part 4 years. Hypophosphatemia may be associated with confusion, hyperventilation, and neuromuscular irritability, suggesting a total body phosphate deficiency. If inorganic phosphate levels fall below 1.0 mg %, diminished red cell glycolysis occurs with low erythrocyte levels of 2,3 diphosphoglycerate and adenosine triphosphate. Lowered red cell organic phosphates are associated with increased hemoglobin oxygen affinity. If severe hypophosphatemia occurs, hemolytic anemia, which is correctible by phosphate infusion, may result. In addition, leucocyte function is impaired by low levels of serum inorganic phosphate. While recognized as a needed additive, recommended phosphate supplements vary. Different infusion regimens have been suggested over the past 4 years, based primarily on assumed daily requirements. In the 19 trauma patients described who received hyperalimentation as part of their treatment, phosphate administration was calculated retrospectively and prospectively as a function of non-protein calories infused. Four different groups were studied. Group A received no phosphate additive and quickly became severely hypophosphatemic. Group B received from one to 15 meg of potassium acid phosphate per 1,000 K cal and developed a more gradual lowering of serum inorganic phosphate levels. Group C received 15 to 25 meg of potassium acid phosphate per 1,000 K cal and maintained normal phosphate levels throughout the course of treatment. Group D received greater than 25 meq of potassium acid phosphate per 1,000 K cal and gradually increased their serum inorganic phosphate levels. A significant positive correlation was found between serum inorganic phosphate levels, 2,3 diphosphoglycerate levels, adenosine triphosphate levels, and P50 of the oxy-hemoglobin dissociation curve. No patients developed hemolytic or neuromuscular syndromes which were attributable to hypophosphatemia. This study describes a simple method for the maintenance of adequate phosphate levels in patients whose dextrose-protein solutions may vary from day to day, by relating it to non-protein calories. Provision of 20 to 25 meq of potassium dihydrogen phosphate per 1,000 K cal will maintain normal serum levels of inorganic phosphate during total parenteral nutrition.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti K. G., Emerson P. M., Darley J. H., Hockaday T. D. 2,3-Diphosphoglycerate and tissue oxygenation in uncontrolled diabetes mellitus. Lancet. 1972 Aug 26;2(7774):391–395. doi: 10.1016/s0140-6736(72)91793-x. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biochem Biophys Res Commun. 1967 Jan 23;26(2):162–167. doi: 10.1016/0006-291x(67)90228-8. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biochem Biophys Res Commun. 1967 Jan 23;26(2):162–167. doi: 10.1016/0006-291x(67)90228-8. [DOI] [PubMed] [Google Scholar]
- Boelens P. A., Norwood W., Kjellstrand C., Brown D. M. Hypophosphatemia with muscle weakness due to antacids and hemodialysis. Am J Dis Child. 1970 Oct;120(4):350–353. doi: 10.1001/archpedi.1970.02100090124016. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Jandl J. H. Control of hemoglobin function within the red cell. N Engl J Med. 1970 Jun 18;282(25):1414–1421. doi: 10.1056/NEJM197006182822507. [DOI] [PubMed] [Google Scholar]
- Card R. T., Brain M. C. The "anemia" of childhood: evidence for a physiologic response to hyperphosphatemia. N Engl J Med. 1973 Feb 22;288(8):388–392. doi: 10.1056/NEJM197302222880803. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Yawata Y., VanSanten L., Gilberstadt S., Silvis S., Jacob H. S. Acquired phagocyte dysfunction. A complication of the hypophosphatemia of parenteral hyperalimentation. N Engl J Med. 1974 Jun 20;290(25):1403–1407. doi: 10.1056/NEJM197406202902504. [DOI] [PubMed] [Google Scholar]
- Jacob H. S., Amsden T. Acute hemolytic anemia with rigid red cells in hypophosphatemia. N Engl J Med. 1971 Dec 23;285(26):1446–1450. doi: 10.1056/NEJM197112232852602. [DOI] [PubMed] [Google Scholar]
- Keitt A. S. Reduced nicotinamide adenine dinucleotide-linked analysis of 2,3-diphosphoglyceric acid: spectrophotometric and fluorometric procedures. J Lab Clin Med. 1971 Mar;77(3):470–475. [PubMed] [Google Scholar]
- Lichtman M. A., Miller D. R., Cohen J., Waterhouse C. Reduced red cell glycolysis, 2, 3-diphosphoglycerate and adenosine triphosphate concentration, and increased hemoglobin-oxygen affinity caused by hypophosphatemia. Ann Intern Med. 1971 Apr;74(4):562–568. doi: 10.7326/0003-4819-74-4-562. [DOI] [PubMed] [Google Scholar]
- Lichtman M. A., Miller D. R. Erythrocyte glycolysis, 2,3-diphosphoglycerate and adenosine triphosphate concentration in uremic subjects: relationship to extracellular phosphate concentration. J Lab Clin Med. 1970 Aug;76(2):267–279. [PubMed] [Google Scholar]
- Lichtman M. A., Miller D. R., Freeman R. B. Erythrocyte adenosine triphosphate depletion during hypophosphatemia in a uremic subject. N Engl J Med. 1969 Jan 30;280(5):240–244. doi: 10.1056/NEJM196901302800504. [DOI] [PubMed] [Google Scholar]
- Lotz M., Zisman E., Bartter F. C. Evidence for a phosphorus-depletion syndrome in man. N Engl J Med. 1968 Feb 22;278(8):409–415. doi: 10.1056/NEJM196802222780802. [DOI] [PubMed] [Google Scholar]
- Oski F. A., Marshall B. E., Cohen P. J., Sugerman H. J., Miller L. D. The role of the left-shifted or right-shifted oxygen-hemoglobin equilibrium curve. Ann Intern Med. 1971 Jan;74(1):44–46. doi: 10.7326/0003-4819-74-1-44. [DOI] [PubMed] [Google Scholar]
- Rose I. A., Warms J. V. Control of glycolysis in the human red blood cell. J Biol Chem. 1966 Nov 10;241(21):4848–4854. [PubMed] [Google Scholar]
- Rose I. A., Warms J. V., O'Connell E. L. Role of inorganic phosphate in stimulating the glucose utilization of human red blood cells. Biochem Biophys Res Commun. 1964 Feb 18;15(1):33–37. doi: 10.1016/0006-291x(64)90098-1. [DOI] [PubMed] [Google Scholar]
- Ruberg R. L., Allen T. R., Goodman M. J., Long J. M., Dudrick S. J. Hypophosphatemia with hypophosphaturia in hyperalimentation. Surg Forum. 1971;22:87–88. [PubMed] [Google Scholar]
- Rudman D., Millikan W. J., Richardson T. J., Bixler T. J., 2nd, Stackhouse J., McGarrity W. C. Elemental balances during intravenous hyperalimentation of underweight adult subjects. J Clin Invest. 1975 Jan;55(1):94–104. doi: 10.1172/JCI107922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. A., Jr, Abel R. M., Abbott W. M., Hopkins C. C., Chesney T. M., Colley R., Phillips K., Fischer J. E. Catheter complications in total parenteral nutrition. A prospective study of 200 consecutive patients. N Engl J Med. 1974 Apr 4;290(14):757–761. doi: 10.1056/NEJM197404042901401. [DOI] [PubMed] [Google Scholar]
- Sedgwick C. E., Viglotti J. Hyperalimentation. Surg Clin North Am. 1971 Jun;51(3):681–686. doi: 10.1016/s0039-6109(16)39444-0. [DOI] [PubMed] [Google Scholar]
- Sheldon G. F. Defective hemoglobin function: a complication of hyperalimentation. J Trauma. 1973 Nov;13(11):971–979. [PubMed] [Google Scholar]
- Sheldon G. F., Fuchs R., Jelinek C. The role of inorganic phosphate in recovery from transfusion. Surg Forum. 1974;25(0):430–431. [PubMed] [Google Scholar]
- Sheldon G. F. Hyperphosphatemia, hypophosphatemia, and the oxy-hemoglobin dissociation curve. J Surg Res. 1973 Apr;14(4):367–372. doi: 10.1016/0022-4804(73)90040-1. [DOI] [PubMed] [Google Scholar]
- Shils M. E. Guidelines for total parenteral nutrition. JAMA. 1972 Jun 26;220(13):1721–1729. [PubMed] [Google Scholar]
- Silvis S. E., Paragas P. D., Jr Paresthesias, weakness, seizures, and hypophosphatemia in patients receiving hyperalimentation. Gastroenterology. 1972 Apr;62(4):513–520. [PubMed] [Google Scholar]
- Territo M. C., Tanaka K. R. Hypophosphatemia in chronic alcoholism. Arch Intern Med. 1974 Sep;134(3):445–447. [PubMed] [Google Scholar]
- Torrance J., Jacobs P., Restrepo A., Eschbach J., Lenfant C., Finch C. A. Intraerythrocytic adaptation to anemia. N Engl J Med. 1970 Jul 23;283(4):165–169. doi: 10.1056/NEJM197007232830402. [DOI] [PubMed] [Google Scholar]
- Travis S. F., Sugerman H. J., Ruberg R. L., Dudrick S. J., Delivoria-Papadopoulos M., Miller L. D., Oski F. A. Alterations of red-cell glycolytic intermediates and oxygen transport as a consequence of hypophosphatemia in patients receiving intravenous hyperalimentation. N Engl J Med. 1971 Sep 30;285(14):763–768. doi: 10.1056/NEJM197109302851402. [DOI] [PubMed] [Google Scholar]


