Abstract
Cork taint is a musty or moldy off-odor in wine mainly caused by 2,4,6-trichloroanisole (2,4,6-TCA). We examined the role of 14 fungal strains isolated from cork samples in the production of 2,4,6-TCA by O methylation of 2,4,6-trichlorophenol (2,4,6-TCP). The fungal strains isolated belong to the genera Penicillium (four isolates); Trichoderma (two isolates); and Acremonium, Chrysonilia, Cladosporium, Fusarium, Mortierella, Mucor, Paecilomyces, and Verticillium (one isolate each). Eleven of these strains could produce 2,4,6-TCA when they were grown directly on cork in the presence of 2,4,6-TCP. The highest levels of bioconversion were carried out by the Trichoderma and Fusarium strains. One strain of Trichoderma longibrachiatum could also efficiently produce 2,4,6-TCA in liquid medium. However, no detectable levels of 2,4,6-TCA production by this strain could be detected on cork when putative precursors other than 2,4,6-TCP, including several anisoles, dichlorophenols, trichlorophenols, or other highly chlorinated compounds, were tested. Time course expression studies with liquid cultures showed that the formation of 2,4,6-TCA was not affected by a high concentration of glucose (2% or 111 mM) or by ammonium salts at concentrations up to 60 mM. In T. longibrachiatum the O methylation of 2,4,6-TCP was catalyzed by a mycelium-associated S-adenosyl-l-methionine (SAM)-dependent methyltransferase that was strongly induced by 2,4,6-TCP. The reaction was inhibited by S-adenosyl-l-homocysteine, an inhibitor of SAM-dependent methylation, suggesting that SAM is the natural methyl donor. These findings increase our understanding of the mechanism underlying the origin of 2,4,6-TCA on cork, which is poorly understood despite its great economic importance for the wine industry, and they could also help us improve our knowledge about the biodegradation and detoxification processes associated with chlorinated phenols.
Cork, a material of vegetable origin, is the bark of the cork oak (Quercus suber), whose main component is suberin, a complex aromatic biopolymer structurally related to lignin and, accordingly, highly resistant to degradation. The main application of cork is in the manufacture of stoppers for wine bottles, especially for high-quality wines. Cork is probably the best material that can effectively and safely close a bottle while allowing proper maturation of the wine (2). Nevertheless, some wines (2 to 7%) suffer from a defect, commonly known as cork taint, that is attributed to the cork stopper (5, 31). This off-odor problem is usually perceived as a moldy, musty, and/or earthy aroma that can mask the natural wine aroma and lessen its quality. Several chemical compounds, including anisoles, guaiacol, geosmine, 2-methylisoborneol, pyrazines, and several aliphatic compounds, such as 1-octen-3-one or 1-octen-3-ol, have been related to this problem (2). Of these, anisoles, especially 2,4,6-trichloroanisole (2,4,6-TCA) and to a lesser extent 2,3,4,6-tetrachloroanisole (2,3,4,6-TeCA) and pentachloroanisole (PCA), are responsible for at least 80% of the cases of cork taint reported (5). Although the worldwide cost of spoilage of wine due to this problem exceeds $10 billion per year (5), very few studies of the biosynthesis of 2,4,6-TCA have been conducted (30, 31). The nontoxic anisoles are thought to arise by O methylation of the highly toxic chlorophenol precursors, as part of a normal detoxification reaction mediated by different microbial species. In fact, the environmental importance of O methylation of chlorophenols as a normal detoxification event has been suggested by several authors (1, 6, 24). The formation of anisoles and thioanisoles by methylation of halogenated phenols and thiophenols has been reported in Rhodococcus, Acinetobacter, and Pseudomonas strains (1, 24). This reaction is not confined to prokaryotes. Thus, O methylation of pentachlorophenol (PCP) by Trichoderma virgatum (6), production of 2,3,4,6-TeCA by several fungi (13), and S methylation of several phenolic compounds by the yeast Saccharomycopsis lipolytica (10) and by the protozoans Euglena gracilis (8) and Tetrahymena thermophila (9) have been reported. Also, the ability of some fungal strains isolated from food-packaging materials (33, 35) and of several fungi and actinomycetes isolated from drinking water (25) to methylate 2,4,6-trichlorophenol (2,4,6-TCP) to produce 2,4,6-TCA has also been documented.
In some Rhodococcus and Acinetobacter strains, an S-adenosyl-l-methionine (SAM)-dependent methyltransferase has been implicated in the production of anisoles (24). However, chloromethane is the methyl donor for the biosynthesis of anisole in the wood-rotting fungus Phellinus pomaceus (14, 22).
Moreover, other authors have suggested other hypothetical mechanisms to explain the formation of 2,4,6-TCA. Maujean and coworkers (21) postulated that this compound originates in the hypochlorite wash of cork, a process that today has fallen into disuse. This treatment could yield chlorophenols that should be methylated by microorganisms present in cork. Other authors have suggested that 2,4,6-TCA may originate from the catabolism of higher chlorinated compounds, such as PCP, tetrachlorophenol (TeCP), hexachlorocyclohexane, or hexachlorobenzene (32). Putative biosynthesis of 2,4,6-TCA by chlorination from phenol or anisol has also been described (23).
The cork ecosystem is a very complex microbial ecosystem, and as a consequence, the microbiota associated with cork during the various steps of the manufacturing process is difficult to establish (19, 31). Until recently, no exhaustive studies had been done to unambiguously identify the microorganisms responsible for the production of 2,4,6-TCA and the biochemical pathways or mechanisms leading to its formation. This is especially true for filamentous fungi, which traditionally have been blamed for cork taint, even though there is no clear correlation between growth of any particular fungal strain and the appearance of anisoles on cork.
The main objectives of this work were to establish a clear relationship between fungal strains growing on cork and the biosynthesis of 2,4,6-TCA and to increase our knowledge about this process.
MATERIALS AND METHODS
Chemicals.
All the dichlorophenols used, 2,3,6-TCP, 2,4,5-TCP, 2,4,6-TCA, 2,3,4,6-TeCP, 2,3,4,6-TeCA, hexachlorocyclohexane, hexaclorobenzene, anisole, and 1,3,5-trichlorobenzene were obtained from Aldrich-Chemie (Steinheim, Germany). 2,4,6-TCP and 2,3,4,6-TeCP were obtained from Fluka Chemie AG (Buchs, Switzerland). PCA was obtained from Supelco (Bellefonte, Pa.). Phenol and l-[U-14C]2,4,6-TCP (10.2 mCi/mmol) were obtained from Sigma Chemical Co. (St. Louis, Mo.).
Microorganisms.
All the fungi were isolated from cork samples kindly provided by the cork factory of Sanvicork S.A. (San Vicente de Alcántara, Badajoz, Spain) and by IPROCOR Institute (Mérida, Badajoz, Spain). The strains isolated have been deposited in the Spanish Type Culture Collection (CECT) under the following strain numbers: Acremonium strictum CECT 20419, Chrysonilia sitophila CECT 20424, Cladosporium oxysporum CECT 20421, Fusarium oxysporum CECT 20420, Mortierella alpina CECT 20430, Mucor plumbeus CECT 20422, Paecilomyces viridis CECT 20427, Penicillium citreonigrum CECT 20426, Penicillium decumbens CECT 20418, Penicillium chrysogenum CECT 20429, Penicillium purpurogenum CECT 20425, Trichoderma longibrachiatum CECT 20431, Trichoderma viride CECT 20423, and Verticillium psalliotae CECT 20428.
Isolation and conservation of filamentous fungi.
Fungi were isolated from different samples obtained at different points during the manufacture of cork stoppers. For a typical agglomerated cork stopper, the manufacturing process begins with the removal, once every 9 years, of the bark of mature cork trees, followed by storage of the planks in the country or the factory to allow them to dry or stabilize (sample A; raw cork). The stabilized corkwood is submerged in a water bath at 80 to 100°C for up to 60 min (sample B; boiled cork). The boiled planks are stacked and allowed to season in a storeroom under conditions that favor fungal growth (sample C; mold growth room). The best planks are used to manufacture natural cork stoppers. The remaining planks are stacked in the factory, usually exposed to the environment (sample D; stacked planks), and are used to manufacture agglomerated cork stoppers. This material is cleaned and pulverized to a granulated powder consisting of particles that are 2 to 4 mm in diameter (sample E; granulated cork). The granulated material is mixed with chemical agglutinating compounds, such as latex, polyurethane, and glue, to produce stoppers (sample F; glued agglomerated cork stoppers). Finally, the stoppers are polished with an abrasive stone and treated with SO2 to prevent microbial growth during storage or transport (sample G; final cork stoppers, SO2 treated).
Microorganisms were isolated from 1-g cork samples (cut into small fragments with a scalpel) in 50 ml of saline solution, followed by mixing in an orbital incubator (New Brunswick Scientific, Edison, N.J.) at 25°C and 220 rpm for 2 h. Dilutions were prepared and plated on Rose Bengal agar (RB agar) (Microkit Laboratories, Madrid, Spain) plates (Rose Bengal strongly restricts the growth of invasive fungi) supplemented with chloramphenicol (250 μg ml−1) to prevent bacterial contamination. Bacteria were isolated on plate count agar plates (Sigma) containing cycloheximide (500 μg ml−1) to inhibit fungal growth. After 3 to 7 days of incubation at 25 or 30°C, individual colonies were subcultured under the same conditions. Strains were maintained on plate count agar, RB agar, or malt extract agar (MEA) (Sigma) plates at 4°C or, when possible, as spore suspensions in 40% glycerol at −20°C (spore suspensions were obtained for Cladosporium, Chrysonilia, Paecilomyces, Penicillium, and Trichoderma strains).
The fungal and bacterial populations were quantified by using samples taken at several stages during the manufacture of cork stoppers and plating 10-fold serial dilutions on RB agar or plate count agar plates containing the appropriate antibiotics.
Identification of filamentous fungi.
Initially, we isolated 25 putative different fungal isolates (designated F1 to F25) from cork samples on the basis of their macroscopic morphological characteristics. All of these isolates were identified morphologically at the genus level by using the traditional method based on both macroscopic cultural traits and microscopic structures, as described for each group (11, 12, 15, 16, 26, 27, 28). All the media used were provided by Sigma.
In order to identify organisms at the species level, the isolates belonging to the genus Penicillium were grown for 7 days in Czapeck yeast extract agar at 4, 25, and 37°C, in MEA at 4 and 25°C, and in 25% glycerol nitrate agar at 25°C by using the methods described by Pitt (26, 27).
The isolates belonging to the genus Trichoderma were identified by the method described by Gams and Bissett (11). These isolates were grown on corn meal agar, MEA, potato dextrose agar, and oatmeal agar for 7 days at 25°C.
Morphological identification of the isolates belonging to the genera Acremonium (16, 28), Chrysonilia (28), Cladosporium (16), Fusarium (16, 28), Mortierella (12), Mucor (28), and Paecilomyces (16) was carried out by using the traditional method described for each group. Briefly, we checked the ability of an organism to grow on MEA, oatmeal agar, potato carrot agar, and potato dextrose agar at 25, 30, and 37°C for 7 to 10 days. Color was tested by growing organisms at 26°C for 10 days in the dark and then for 2 days in daylight.
Molecular methods were used to confirm the identities of some problematic strains. For the Trichoderma strains we analyzed the restriction patterns of PCR-amplified ribosomal DNA (rDNA) products, including the spacers ITS1 and ITS2 and 5.8S rDNA (17, 20), that were amplified by using primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (34). Fungal DNA was isolated as described by Lee and Taylor (18). Each PCR was performed by using a 50-μl (total volume) mixture containing 1× PCR amplification buffer (Gibco BRL, Bethesda, Md.), 1 mM MgCl2, each primer (Gibco BRL) at a concentration of 1 μM, each deoxynucleoside triphosphate (Boehringer, Mannheim, Germany) at a concentration of 50 μM, and 1.2 U of Taq DNA polymerase (Gibco BRL). The reaction mixtures were subjected to an initial denaturation step of 95°C for 3 min; then the sample was denatured at 94°C for 1 min, annealed at 52°C for 40 s, and extended at 72°C for 1 min, and this cycle was repeated 35 times, followed by a final extension step of 10 min at 72°C. PCR-amplified rDNA products were digested overnight at 37°C with restriction endonuclease CfoI, HinfI, or ScrfI (Boehringer). DNA restriction fragments were separated on a 2% agarose gel in 0.5× Tris-borate-EDTA buffer. The restriction patterns were compared to those obtained for previously identified type strains deposited in the CECT. The reference strains used were Trichoderma harzianum CECT 2413 and CECT 2927, T. longibrachiatum CECT 2606 and CECT 20106, and T. viride CECT 2944 and CECT 20101.
Identification of P. chrysogenum and P. citreonigrum was also confirmed by analyzing the restriction length polymorphism of the rDNA as described above. The strains used as reference strains were P. citreonigrum CECT 20438 and CECT 20440 and P. chrysogenum CECT 2306 and CECT 20231.
Because the single isolate of Verticillium did not exhibit clear morphological structures and cultural traits (28) that allowed us to assign it to any species, this organism was identified by partial sequencing of its rDNA. The primers used were NL-1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL-4 (5′-GGTCCGTGTTTCAAGACGG-3′). The PCR conditions were the same as those described above, except that the extension step consisted of 72°C for 2 min.
Analysis of 2,4,6-TCA biosynthesis from 2,4,6-TCP by fungi growing on cork.
We placed 20 g of pieces of granulated cork that were 2 to 4 mm in diameter (supplied by Sanvicork S.A.) in a 500-ml flask containing 20 ml of distilled water, 20 mM ammonium acetate, and 20 μg of 2,4,6-TCP and inoculated the preparation with 5 × 105 CFU of a fungal strain. Duplicate flasks in two independent experiments were incubated statically for 28 days at 22°C in the dark. After incubation, 0.5 g of cork was removed from each flask, and the microorganisms were recovered and quantified on RB agar plates. This procedure allowed us to confirm the absence of contaminating organisms and to check the ability of each strain to grow on cork under the conditions of the assay. The rest of the culture was extracted with 200 ml of ethanol for 48 h at 22 to 25°C with agitation (220 rpm), and the solution was filtered through filter paper to remove any solid debris. After this, 100 ml of water was added and mixed, and the resulting mixture was extracted twice with 5 ml of pentane. We added 1 μg of lindane (Sigma) as an internal standard, and the organic phase was evaporated under a vacuum in an N2 atmosphere at room temperature (22 to 25°C). The concentrated sample was analyzed by gas chromatography-mass spectrometry (GC-MS) at 70 eV by using a 5973A mass spectrometer (Hewlett-Packard, Wilmington, Del.) fitted with a Hewlett-Packard 6890 gas chromatograph. The sample was injected into an Hp-5 MS column (length, 30 m; inside diameter, 0.25 mm; film thickness, 0.25 μm; Hewlett-Packard). The operating temperature was 40°C for 2 min, and then the temperature was raised to 250°C at a rate of 7°C min−1 and finally maintained at 250°C for 6 min. Quantification and identification of the extracted 2,4,6-TCA were carried out by peak area determination and comparison of the retention time with that of a commercial standard obtained from Aldrich.
Analysis of other putative precursors for formation of 2,4,6-TCA by T. longibrachiatum.
In order to determine if T. longibrachiatum could produce 2,4,6-TCA from precursors other than 2,4,6-TCP or by mechanisms different than O methylation, we prepared cultures of this organism on cork as described above. In all cases 20-μg portions of different putative precursors were added alone or in combination with 40 μg of sodium hypochlorite as a chlorinating agent (this amount of sodium hypochlorite did not have a negative effect on growth of this organism). The cultures were grown and 2,4,6-TCA production was determined as described above.
Bioconversion of 2,4,6-TCP to 2,4,6-TCA in liquid cultures.
Fungi were routinely cultured in 500-ml Erlenmeyer flasks containing 100 ml of malt extract broth (MEB) supplemented with ammonium acetate (12 mM) containing 2,4,6-TCP (10 μg/ml). Media were supplemented with ammonium acetate (25, 50, or 60 mM), glucose (111 mM), or ammonium acetate (50 mM) plus glucose (111 mM) to check for the existence of putative general regulatory mechanisms due to either the carbon or nitrogen source. The flasks were inoculated with 106 CFU and incubated at 28°C with agitation at 200 rpm in an orbital incubator (Sanyo Gallemkamp, Loughborough, United Kingdom). Samples (5 ml) were removed at various times, and the mycelium was separated from the medium by filtration through a filter or glass fiber. Each filtrate was acidified by adding 50 μl of 10 N HCl and then extracted twice with 5 ml of diethyl ether. The organic phase was evaporated, and the residue was dissolved in methanol and analyzed by high-performance liquid chromatography (HPLC) (1100 Series; Hewlett-Packard) by using a Zorbax SB-C8 column (4.5 by 150 mm; Agilent Technologies, Madrid, Spain) with methanol-water (75:25) as the mobile phase at a flow rate of 1 ml/min. Eluted peaks were detected at 230 nm. Quantification of 2,4,6-TCA and 2,4,6-TCP was performed by using 1,3,5-trichlorobenzene and 2,3,4,6-TeCA as internal standards. The area under each peak was referred to calibration curves obtained with authentic products. Microbial growth was estimated by determining mycelial dry weight.
Mineralization of 2,4,6-TCP.
Triplicate T. longibrachiatum cultures were grown in MEB alone or MEB supplemented with glucose (111 mM) or ammonium acetate (50 mM) in 250-ml serum bottles. We added 105 cpm of l-[U-14C]2,4,6-TCP to every bottle after 3 days of incubation in order to prevent putative inhibition of growth by the substrate. The inoculated bottles (equipped with two-air-exit screw caps) were each sealed with a Teflon ribbon. The cultures were flushed weekly with sterile humidified CO2-free air for 1 h, and the 14CO2 was trapped by using 4 ml of a 2-phenylaethylamine-ethanol-water solution (2:1:1); the efficiency of trapping was more than 96%. After this 4 ml of liquid scintillation cocktail (Ready-safe; Beckman Coulter) was added to each sample, and the radioactivity was determined with a liquid scintillation analyzer (LKB 1219 Rackbeta; Wallac). The counting efficiency was more than 80%, as determined by using an external standard.
Preparation of fungal cell extracts.
Following incubation in liquid medium, mycelia were harvested by filtration as described above, washed with a sterile saline solution to remove any trace of phenolic compounds, dried between folds of filter paper, and kept at −20°C until they were used. The frozen mycelium was thawed slowly in an ice bath, resuspended (2 ml g of mycelium−1) in 100 mM phosphate buffer (pH 7.5) containing 1 mM MgCl2, 100 mM NaCl, 1 mM dithiothreitol, and 1 mM phenylmethylsulfonyl fluoride as a protease inhibitor, and disrupted by sonication (150-W MSE ultrasonic disintegrator) by using 10 15-s bursts. Cell debris was removed by centrifugation at 10,000 × g for 20 min at 4°C. The supernatant was desalted by passage through a PD-10 column (Amersham Pharmacia Biotech, Uppsala, Sweden) to remove any trace of phenolic compounds, and the eluate, in 100 mM phosphate buffer (pH 7.5), was used as the crude enzyme preparation.
Assay of enzyme activity.
The reaction mixture used for the assay of SAM-dependent methyltransferase activity contained cell extract (about 2 mg of protein in 250 μl), 625 nmol of S-adenosyl-l-methionine p-toluene sulfonate (Sigma), 500 nmol of 2,4,6-TCP, MgCl2 (1 mM), and the appropriate buffer in a total volume of 0.5 ml. After incubation at 28°C for 3 h, the reaction was stopped by addition of 20 μl of 6 N HCl. Proteins were removed by centrifugation at 15,000 × g for 20 min at room temperature. Phenolic compounds were extracted twice with 500 μl of diethyl ether. The organic phase was evaporated under reduced pressure, and the residue was dissolved in 100 μl (total volume) of methanol. The 2,4,6-TCP and 2,4,6-TCA were analyzed by HPLC as described above. Specific activities were expressed in picomoles of product formed per minute per milligram of protein. Total soluble protein contents were determined by the method of Bradford (4) by using the Bio-Rad protein reagent (Bio-Rad, Hercules, Calif.) and bovine serum albumin as the standard.
Induction of the methyltransferase activity.
In order to assess putative induction of methyltransferase activity by 2,4,6-TCP, we performed a typical resting cell experiment. Mycelia were grown as described above in MEB for 48 h, harvested by filtration through 3MM Chr paper (Whatman International Ltd., Maidstone, England) under sterile conditions, washed with 2 volumes of a sterile saline solution, and finally suspended in 0.1 volume of 100 mM phosphate buffer (pH 7.5) containing 1 mM MgCl2 and 100 mM NaCl. After this, 2,4,6-TCP was added to a final concentration of 10 μg/μl, and the preparation was incubated under the same conditions. Samples were taken at several times, cell extracts were obtained, and methyltransferase activity was determined as described above. Experiments with negative noninduced controls were performed in parallel.
RESULTS
Evolution of the microbial population during the manufacture of an agglomerated cork stoppers.
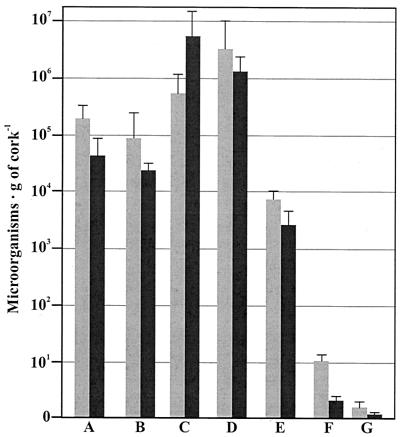
The changes in the fungal and bacterial population during the manufacture of agglomerated cork stoppers is summarized in Fig. 1. The numbers of CFU in the initial microbial population were rather high, 3.01 × 105 ± 2.57 × 105 bacterial CFU per g of cork and 5.68 × 104 ± 3.77 × 104 fungal CFU per g of cork. These levels were reduced only slightly when the cork samples were submerged in a hot water bath; after this the numbers of microbes were 9.02 × 104 ± 4.88 × 104 bacterial CFU per g of cork and 2.73 × 104 ± 1.33 × 104 fungal CFU per g of cork. The size of the microbial population increased significantly and reached the maximum levels, 7.12 × 105 ± 3.84 × 105 bacterial CFU per g of cork and 6.20 × 106 ± 4.71 × 106 fungal CFU per g of cork, following stacking of cork slabs in the molding room, where the temperature and humidity conditions favored rapid increases in the numbers of microorganisms. The size of the microbial population was rather stable or decreased slightly during storage at the factory under ambient environmental conditions (sample D). However, the size of the population drastically decreased during the next stages (samples E and F), probably as a consequence of the combination of mechanical and chemical treatments, reaching its lowest level in the final product (sample G), in which less than 4 microorganisms per g of cork were detected.
FIG. 1.
Evolution of bacterial populations (gray bars) and fungal populations (solid bars) during the manufacture of agglomerated cork stoppers. The samples correspond to several stages of the manufacturing process described in Materials and Methods, as follows: raw cork (bars A), boiled cork (bars B), mold growth room stage (bars C), stacked planks exposed to the ambient environmental conditions (bars D), granulated cork (bars E), glued agglomerated cork stoppers (bars F), and final cork stoppers (SO2 treated) (bars G). The results are the means of four independent experiments.
Composition of the population of filamentous fungi growing on cork.
Although initially we recovered 25 different putative fungal isolates, identification of these isolates let us to conclude that these strains represented only 14 species belonging to the following 10 genera: Acremonium, Chrysonilia, Cladosporium, Fusarium, Mortierella, Mucor, Paecilomyces, Penicillium, Trichoderma, and Verticillium (Table 1). All of the species were cosmopolitan species which are usually isolated from soil, plant debris, and air, especially during seasons in which the humidity is elevated. The isolates included four species of the genus Penicillium (P. chrysogenum, P. citreonigrum, P. decumbens, and P. purpurogenum) and two species of Trichoderma (T. viride and T. longibrachiatum), which were identified by comparison of the restriction patterns of PCR-amplified rDNA products with the restriction patterns of well-characterized type strains deposited in the CECT (data not shown).
TABLE 1.
Relative amounts of the filamentous fungi isolated from samples at each stage during the production of agglomerated cork stoppers
| Fungus | % ina:
|
||||||
|---|---|---|---|---|---|---|---|
| Sample A | Sample B | Sample C | Sample D | Sample E | Sample F | Sample G | |
| Acremonium strictum | 3.0 (0.2) | NDb | 0.8 (0.1) | 0.8 (0.2) | ND | ND | ND |
| Chrysonilia sitophila | 6.6 (0.4) | 2.8 (0.3) | 57.4 (3.9) | 6.1 (0.3) | 2.2 (0.2) | 1.3 (0.3) | ND |
| Cladosporium oxysporum | 10.4 (1.2) | 6.3 (0.4) | 6.4 (0.3) | 15.0 (1.0) | 20.4 (1.7) | 38.5 (2.8) | 34.2 (4.6) |
| Fusarium oxysporum | 2.1 (0.1) | ND | ND | 1.6 (0.2) | ND | ND | ND |
| Mortierella alpina | 1.8 (0.2) | ND | ND | ND | ND | ND | ND |
| Mucor plumbeus | 2.4 (0.2) | ND | ND | ND | ND | ND | ND |
| Paecilomyces viridis | 5.2 (0.1) | 8.4 (0.7) | 2.5 (0.2) | 6.0 (0.7) | ND | ND | ND |
| Penicillium chrysogenum | 12.9 (0.5) | 9.2 (0.7) | 3.4 (0.1) | 10.5 (0.8) | ND | 8.4 (0.5) | ND |
| Penicillium citreonigrum | 10.2 (0.2) | 15.2 (0.9) | 3.0 (0.1) | 20.8 (1.2) | 38.0 (4.1) | 38.6 (3.2) | 57.4 (7.8) |
| Penicillium decumbens | 14.8 (0.7) | 2.8 (0.1) | 4.6 (0.2) | 9.9 (1.0) | ND | 3.0 (0.2) | ND |
| Penicillium purpurogenum | 4.0 (0.4) | 12.1 (0.5) | 1.0 (0.1) | 17.9 (2.0) | 22.4 (3.3) | ND | ND |
| Trichoderma longibrachiatum | 13.2 (1.0) | 20.3 (1.6) | 11.3 (0.6) | 5.1 (0.4) | 10.2 (1.2) | 7.2 (0.8) | 8.4 (1.3) |
| Trichoderma viride | 10.3 (0.9) | 18.5 (1.4) | 9.6 (0.6) | 3.1 (0.4) | 6.8 (0.7) | 3.0 (0.5) | ND |
| Verticillium psalliotae | 3.1 (0.4) | 4.4 (0.1) | ND | 3.2 (0.4) | ND | ND | ND |
Sample A, raw cork; Sample B, boiled cork; Sample C, mold growth room; Sample D, stacked planks; Sample E, granulated cork; Sample F, glued agglomerated cork stoppers; Sample G, final cork stoppers (SO2 treated). The values are the averages for duplicate estimates obtained in three independent experiments. The values in parentheses are standard errors.
ND, not detected.
The rest of the species isolated (only a single species of each genus) were identified by using morphological characteristics as A. strictum, C. sitophila, C. oxysporum, F. oxysporum, M. alpina, M. plumbeus, P. viridis, and Verticillium sp.
The traits of all of the isolates except the Verticillium isolate clearly resembled the typical macroscopic and microscopic traits reported for the species. The Verticillium species was identified as V. psalliotae by partial sequencing of its 28S rDNA (accession number AF500907). The sequence data showed 100% identity with a partial sequence of the 28S rDNA of V. psalliotae NRRL 26999 (accession number AF049178) and 99% identity with the 28S rDNA sequence of V. psalliotae NRRL 26542 (accession number U17421). Slightly lower levels of homology (97%) were obtained with the strains Verticillium lecanii ATCC 46578 and ATCC 58909.
Table 1 shows the relative frequencies at which the different strains were detected in cork samples from each stage of the manufacturing process. Only three strains (C. oxysporum, P. citreonigrum, and T. longibrachiatum) were detected in all manufacturing steps. C. sitophila was detected at all stages except the final product. In contrast, M. alpina and M. plumbeus were isolated only from the initial sample (raw cork), while the rest of the strains were detected in at least three different samples, mainly samples from the initial stages of the manufacturing process.
O methylation of 2,4,6-TCP and formation of 2,4,6-TCA by filamentous fungi growing on cork.
Of the 14 fungal species isolated (Table 2), 11 could significantly increase the 2,4,6-TCA content of the cork when the starting material (granulated cork) was inoculated and supplemented with the putative precursor 2,4,6-TCP. Noninoculated cork samples or inoculated samples lacking 2,4,6-TCP showed little or no increase in the low levels of endogenous 2,4,6-TCA. These results suggest that conversion of 2,4,6-TCP to 2,4,6-TCA is a biological process mediated by the action of some microorganisms, such as the filamentous fungi discussed above. In addition, our findings confirm a previous hypothesis that 2,4,6-TCA might arise directly from the highly toxic precursor 2,4,6-TCP by a methylation reaction as part of a normal detoxification process. This experiment mimicked the conditions existing when the cork is maturing in the mold growth room.
TABLE 2.
Abilities of several filamentous fungi to produce 2,4,6-TCA in the absence or presence of 2,4,6-TCP when they are growing directly on cork
| Fungus added | 2,4,6-TCA production (ng g of cork−1)a
|
% Bioconversion of 2,4,6-TCP to 2,4,6-TCA | Growth on corkb | |
|---|---|---|---|---|
| Without 2,4,6-TCP | With 2,4,6-TCP | |||
| None (noninoculated cork sample) | 9.9 | 10.1 | 0 | ND |
| Acremonium strictum | 11.0 | 153.4 | 14.24 | + |
| Chrysonilia sitophila | 18.0 | 128.2 | 11.02 | + |
| Cladosporium oxysporum | 12.4 | 155.5 | 14.31 | + |
| Fusarium oxysporum | 6.0 | 292.5 | 28.65 | + |
| Mortierella alpina | 5.5 | 6.6 | 0.11 | − |
| Mucor plumbeus | 6.3 | 6.6 | 0.03 | − |
| Paecilomyces viridis | 9.9 | 88.7 | 7.88 | + |
| Penicillium chrysogenum | 9.7 | 86.5 | 7.68 | + |
| Penicillium citreonigrum | 11.0 | 143.8 | 13.28 | + |
| Penicillium decumbens | 9.5 | 10.6 | 0.11 | − |
| Penicillium purpurogenum | 6.4 | 116.6 | 11.02 | + |
| Trichoderma longibrachiatum | 20.5 | 396.1 | 37.56 | + |
| Trichoderma viride | 17.2 | 278.9 | 26.17 | + |
| Verticillium psalliotae | 6.5 | 75.5 | 6.9 | + |
The values are the averages for duplicate estimates obtained in two independent experiments. The variations were always less than 10%.
ND, not detected; +, number of CFU per gram of cork after incubation was more than 5.5 × 105 CFU; −, number of CFU per gram of cork after incubation was less than 4.5 × 105 CFU.
We grouped the fungi analyzed into four classes based on the final 2,4,6-TCA yield. The strong producers (both Trichoderma strains and the Fusarium strain) could transform more than 25% of the precursor into 2,4,6-TCA. The moderate producers could transform 10 to 25% of the 2,4,6-TCP and included two Penicillium strains, A. strictum, C. sitophila, and C. oxysporum. The low producers, which reproducibly biotransformed 5 to 10% of the precursor, included P. viridis, P. chrysogenum, and V. psalliotae. Finally, the strains of M. alpina, M. plumbeus, and P. decumbens were considered nonproducers (less than 1% bioconversion), since only trace amounts of 2,4,6-TCA (differences less than 0.15%) were detected in their cultures and these amounts could be attributed to normal variation in the measurements. Of these three organisms, only P. decumbens showed significant ability to grow on cork, whereas M. alpina and M. plumbeus were unable to grow under the conditions used. Since the latter two organisms were also unable to produce 2,4,6-TCA in liquid cultures (data not shown), the absence of this compound in the inoculated flasks probably was due to the inability of the fungi to biosynthesize it rather than to their inability to grow on granulated cork. Accordingly, these two filamentous fungi could be occasional contaminants present on cork planks rather than saprophytic microorganisms on cork. By contrast, the rest of the strains tested could overgrow cork in our assays and, therefore, should be considered part of its normal microbiota.
Analysis of the production of 2,4,6-TCA from different putative precursors by T. longibrachiatum growing on cork.
The rates of production of 2,4,6-TCA by T. longibrachiatum from putative precursors other than 2,4,6-TCP are shown in Table 3. It is clear that 2,4,6-TCP could act as an efficient precursor since a 34.72% rate of bioconversion was detected. In contrast, incubation with all the other putative precursors tested did not yield a significant amount of 2,4,6-TCA (all the bioconversion values obtained were less than 2.5%). Several dichlorophenols (alone or in combination with sodium hypochlorite as a chlorinating agent) failed to support 2,4,6-TCA biosynthesis (the highest bioconversion value obtained was 0.83%). Also, highly chlorinated compounds, such as hexachlorocyclohexane, hexachlorobenzene, PCP, PCA, 2,3,4,6-TeCA, and 2,3,4,6-TeCP, did not yield significant amounts of 2,4,6-TCA; their bioconversion rates were always lower than 0.15%, and occasionally the 2,4,6-TCA levels detected were less than the endogenous content of the cork.
TABLE 3.
Analysis of the production of 2,4,6-TCA from different putative precursors by T. longibrachiatum
| Precursor | Sodium hypochlorite | T. longibrachiatum | 2,4,6-TCA production (ng g of cork−1)a | % Bioconversion of precursor to 2,4,6-TCAb |
|---|---|---|---|---|
| None | − | − | 8.6c | |
| None | − | + | 9.6 | |
| None | + | + | 13.2 | |
| 2,3-DCPd | − | + | 8.0 | 0 |
| 2,3-DCP | + | + | 9.6 | 0.10 |
| 2,3-DCP | + | − | 8.0 | 0 |
| 2,4-DCP | − | + | 4.5 | 0 |
| 2,4-DCP | + | + | 8.9 | 0.03 |
| 2,4-DCP | + | − | 4.7 | 0 |
| 2,5-DCP | − | + | 7.2 | 0 |
| 2,5-DCP | + | + | 9.7 | 0.11 |
| 2,5-DCP | + | − | 15.5 | 0.83 |
| 2,6-DCP | − | + | 6.2 | 0 |
| 2,6-DCP | + | + | 3.1 | 0 |
| 2,6-DCP | + | − | 3.2 | 0 |
| 3,4-DCP | − | + | 8.3 | 0 |
| 3,4-DCP | + | + | 9.1 | 0.05 |
| 3,4-DCP | + | − | 6.8 | 0 |
| Hexachlorocyclohexane | − | + | 7.0 | 0 |
| Hexachlorobenzene | − | + | 7.0 | 0 |
| PCP | − | + | 9.7 | 0.11 |
| PCA | − | + | 7.3 | 0 |
| 2,3,4,6-TeCP | − | + | 9.9 | 0.13 |
| 2,3,4,6-TeCA | − | + | 4.6 | 0 |
| 2,3,6-TCP | − | + | 1.5 | 0 |
| 2,4,5-TCP | − | + | 7.5 | 0 |
| 2,4,6-TCP | − | + | 355.8 | 34.72 |
| Anisole | − | + | 8.8 | 0 |
| Anisole | + | + | 18.8 | 1.02 |
| Anisole | + | − | 15.5 | 0.69 |
| Phenol | − | + | 17.4 | 0.88 |
| Phenol | + | + | 25.2 | 1.66 |
| Phenol | + | − | 13.6 | 0.50 |
The values are averages for duplicate estimates obtained in two independent experiments.
The values were obtained by subtracting the endogenous 2,4,6-TCA content of cork from the average 2,4,6-TCA values detected.
Endogenous 2,4,6-TCA content of cork.
DCP, dichlorophenol.
We also examined whether anisole or phenol, alone or in the presence of sodium hypochlorite, could support 2,4,6-TCA production, but again the levels of bioconversion were less than 2% for all the combinations tested and should be attributed to normal variations in the method rather than to a very low capacity for biosynthesis.
In vivo bioconversion of 2,4,6-TCP to 2,4,6-TCA by T. longibrachiatum.
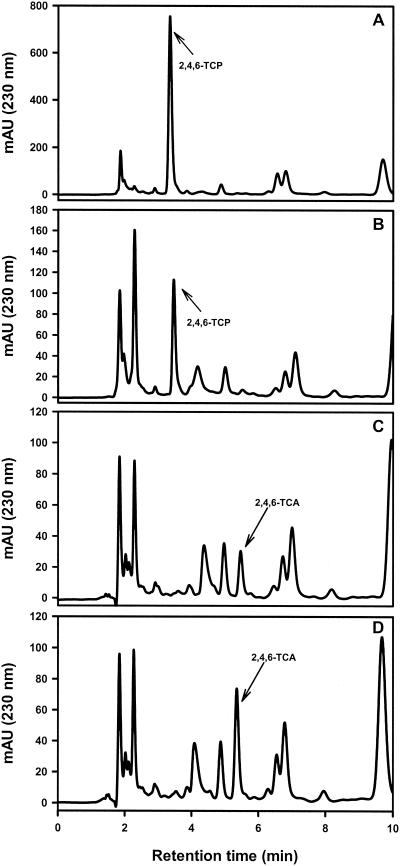
Since T. longibrachiatum produced the highest levels of 2,4,6-TCA from 2,4,6-TCP when it was grown on cork (Table 2), it was selected for further studies in order to investigate the kinetics and the mechanism of this bioconversion in a liquid culture. As shown in Fig. 2, a sample of the supernatant of a culture taken at zero time yielded a prominent peak of 2,4,6-TCP when it was added as a precursor (Fig. 2A). After 24 h, the size of this peak decreased drastically (Fig. 2B), and the peak was undetectable in the 72-h sample (Fig. 2C). At this time, a new peak with the retention time of 2,4,6-TCA was detected. In order to confirm the nature of this peak, we added a small amount of commercial 2,4,6-TCA to the 72-h supernatant. As shown in Fig. 2D, this resulted in an increase in the area of the new peak. Unambiguous characterization of the nature of this peak was performed by GC-MS.
FIG. 2.
HPLC analysis of 2,4,6-TCA and 2,4,6-TCP levels in culture supernatants of T. longibrachiatum. (A) Culture at zero time; (B) 24-h culture; (C) 72-h culture; and (D) 72-h culture containing exogenous pure 2,4,6-TCA. Peaks corresponding to 2,4,6-TCA and 2,4,6-TCP are indicated by arrows. mAU, milliunits of absorbance.
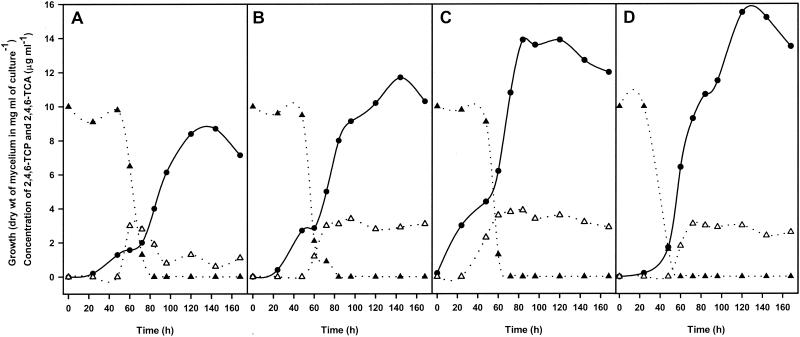
To assess the putative regulation of the biosynthesis of 2,4,6-TCA by general regulatory mechanisms, such as carbon catabolite repression by glucose or repression by ammonium, we grew parallel cultures in MEB (Fig. 3A), MEB supplemented with 111 mM glucose (Fig. 3B), MEB supplemented with 50 mM ammonium acetate (Fig. 3C), and MEB supplemented with 111 mM glucose plus 50 mM ammonium acetate (Fig. 3D). As deduced from the data obtained, the rates of formation of 2,4,6-TCA were very similar in all the cultures if the effect of mechanisms of repression by glucose or ammonium salts under the conditions tested was excluded. Similar results were obtained when the concentration of ammonium acetate was increased to 60 mM (data not shown). In contrast, the growth rate was clearly influenced by addition of glucose and/or ammonium. In fact, higher levels of growth were obtained when the medium was supplemented with glucose, ammonium, or both compounds (Fig. 3B, C, and D). Under all conditions 29 to 39% of the original 2,4,6-TCP was converted to 2,4,6-TCA, suggesting that the latter compound is not the only metabolic product of 2,4,6-TCP. Under all of the experimental conditions the highest levels of 2,4,6-TCA were detected after between 60 and 84 h of incubation. Longer incubations resulted in a slight decreases in the level of 2,4,6-TCA (Fig. 3A), suggesting that some degradation of this compound could have occurred. We cannot exclude the possibility that these decreases were due in part to evaporation of 2,4,6-TCA during opening of the flasks to take the samples. Binding of 2,4,6-TCA to the mycelium was ruled out because extraction with diethyl ether did not yield significant amounts of this compound (data not shown). Besides, the decreases in the 2,4,6-TCA levels (Fig. 3) coincided with some degree of mycelial lysis during the later stages of fermentation.
FIG. 3.
Time course of bioconversion of 2,4,6-TCP, production of 2,4,6-TCA, and growth curves for T. longibrachiatum cultures grown in MEB (A), in MEB supplemented with 111 mM glucose (B), in MEB supplemented with 50 mM ammonium acetate (C), and in MEB containing 111 mM glucose and 50 mM ammonium acetate (D). Growth (•) was estimated by determining mycelial dry weight. Concentrations of 2,4,6-TCP (▴) and 2,4,6-TCA (▵) in supernatants were determined by HPLC. Each data set is the average data for duplicate samples obtained in three independent experiments.
These studies showed that 61 to 71% of 2,4,6-TCP was not converted into 2,4,6-TCA, although 2,4,6-TCP completely disappeared from the culture by 60 to 84 h. Mineralization studies of radiolabeled 2,4,6-TCP under several different conditions were negative, indicating that 2,4,6-TCP was not degraded to CO2. Different attempts to identify putative intermediates of 2,4,6-TCP degradation in supernatants of cultures were performed as described previously (3), and all gave negative results.
O methylation of 2,4,6-TCP is catalyzed by an inducible SAM-dependent methyltransferase.
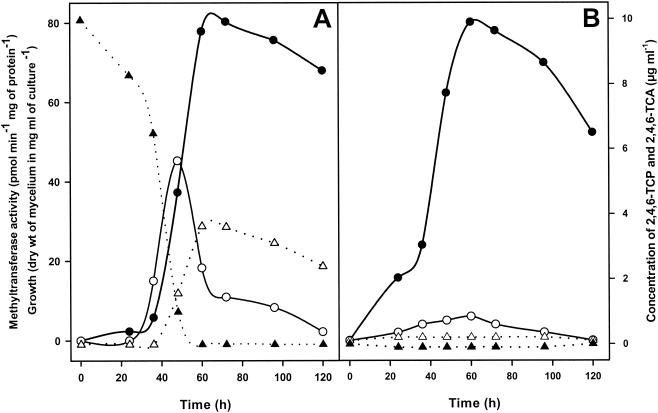
As expected from the experiments described above (Table 2), the production of 2,4,6-TCA in liquid cultures was dependent on addition of 2,4,6-TCP to the medium, which is also consistent with the absence of any traces of this compound in the absence of the precursor (Fig. 4B). The presence of 2,4,6-TCP inhibited the growth of T. longibrachiatum, since growth decreased significantly during the first 36 h after its addition (compare Fig. 4A and B). However, once 2,4,6-TCP was metabolized, the culture reached a biomass similar to that of a counterpart lacking 2,4,6-TCP.
FIG. 4.
Growth curves, levels of 2,4,6-TCP and 2,4,6-TCA, and cell-associated methyltransferase activity in liquid cultures of T. longibrachiatum grown in the presence (A) or in the absence (B) of 2,4,6-TCP (10 μg ml−1). Growth (•) was measured by determining mycelial dry weight. Concentrations of 2,4,6-TCP (▴) and 2,4,6-TCA (▵) in supernatants were determined by HPLC after extraction from culture medium. SAM-dependent methyltransferase activity (○) was estimated by using mycelia. Each data set is the average data for duplicate samples obtained in three independent experiments.
Conversion of 2,4,6-TCP to 2,4,6-TCA in liquid medium was preceded by the appearance of a mycelium-associated SAM-dependent enzymatic activity (Fig. 4A). The maximum levels of activity were detected after 48 h of culture, 12 h before the maximum yield of 2,4,6-TCA was obtained. The levels decreased rapidly after 48 h of culture, when 2,4,6-TCP had been completely removed from the medium, indicating that the activity is not a constitutive activity but is induced by the presence of 2,4,6-TCP. In support of this hypothesis, only residual levels of activity were detected in mycelia growing in the absence of 2,4,6-TCP (Fig. 4B). To confirm these data, we performed a typical resting-cell system experiment (data not shown), which showed that only residual levels of enzyme activity could be detected in the absence of 2,4,6-TCP as an inducer. In contrast, clear activity was detected 1 h after addition of 2,4,6-TCP to the culture, and the level continued to increase up to 8 h after induction and then decreased after incubation for longer times. This activity was significantly lower (87.1% inhibition) when 1 mM S-adenosyl-l-homocysteine, a typical inhibitor of SAM-dependent methylation, was added to the reaction mixture (data not shown), confirming the role of SAM as a natural methyl donor.
DISCUSSION
The microbiology of cork has been reviewed by several authors. Most previous studies have dealt with fungal populations, not only because it has been traditionally accepted that molds have a beneficial effect on cork maturation but also because molds have been identified as putative 2,4,6-TCA producers (19, 31). Of the 14 fungal strains which we isolated, 11 have previously been found on cork (19), which means that the fungal population characterized in this study should be considered representative. However, isolation of P. citreonigrum, M. alpina, and V. psalliotae is new. This result is not surprising, as we expected to find a great variety of fungi growing on cork and significant variation in the fungal populations depending on the origin of the cork.
Unexpectedly, we found that 11 of our 14 isolates could produce significant amounts of 2,4,6-TCA when they grew directly on cork containing sublethal concentrations (1 μg ml−1) of 2,4,6-TCP as a precursor. As cork taint can result from as little as 46.6 ng of 2,4,6-TCA per liter for white wines and 22 ng per liter for red wines (5), it is clear that almost all the fungal isolates could potentially be blamed for this unpleasant problem. Chloroanisoles that cause unpleasant aromas and tastes are not associated solely with wines. In fact, 2,3,4,6-TeCA causes musty taint in chickens (7, 13), and other chloroanisoles, including 2,4,6-TCA, also produce off-odor problems in potato chips (6), dried fruit (33, 35), coffee (31), and drinking water (25).
For a long time there has been controversy concerning the putative origin of 2,4,6-TCA on cork (31). For T. longibrachiatum the absence of significant levels of this compound in cultures that developed on cork in the presence of several putative precursors, including anisole, phenol, and several dichlorophenols, with and without sodium hypochlorite, argues against a putative origin due to chlorination and/or biomethylation of these compounds. In addition, 2,4,6-TCA was not detected after potential bioconversion or catabolism of highly chlorinated precursors, including hexachlorocyclohexane, hexachlorobenzene, PCP, PCA, 2,3,4,6-TeCP, and 2,3,4,6-TeCA. This is not an unexpected result if we bear in mind that the catabolism of some of these compounds, such as PCP, 2,4,5-TCP, and 2,4,6-TCP, proceeds under aerobic conditions mainly through an oxidative dehalogenating mechanism catalyzed by monooxygenase-type enzymes, yielding different quinones as intermediates (3, 36, 37, 38), although reductive dechlorination can also occur in the presence of oxygen (3). Besides, to our knowledge, 2,4,6-TCA has never been detected as an intermediate during degradation of these compounds, which obviously rules out a putative origin of 2,4,6-TCA through this mechanism. Finally, of all of the TCPs tested, only 2,4,6-TCP yielded a significant rate of biosynthesis. Nevertheless, we cannot exclude the possibility that 2,4,6-TCA could be synthesized from these or other precursors as result of microbial interactions between the microorganisms forming the complex populations found on cork.
Chlorophenols constitute a significant class of environmental pollutants, and significant efforts have been made to isolate and characterize microorganisms able to degrade these compounds. The toxicity of chlorophenols is mainly due to the nonspecific binding of these compounds to biological molecules which results in inactivation of the highly reactive hydroxyl group (6). 2,4,6-TCP is recognized as a major pollutant, and considerable work has been done to elucidate pathways leading to its degradation. Lignin-degrading microorganisms are ideal for removing this compound because the enzymes implicated in lignin degradation, including peroxidases, dioxygenases, and quinone reductases, are also effective for eliminating 2,4,6-TCP (3). O methylation is an alternative mechanism to detoxify phenols, since this reaction results in the biosynthesis of the corresponding anisoles (1, 22), which are not toxic at all. For instance, our fungal strains can normally grow in the presence of up to 1 mg of 2,4,6-TCA per ml, but their growth is completely inhibited by 10 to 40 μg of 2,4,6-TCP per ml, depending on the strain tested. Similar data were reported by Cserjesi and Johnson (6). Despite its importance as an alternative mechanism for detoxification of chlorophenols, very little is known about O methylation. A SAM-dependent methyltransferase, implicated in biomethylation of chlorophenols and thiophenols, has been detected in cell extracts of some gram-positive and gram-negative bacteria (24). Interestingly, a similar SAM-dependent activity was detected in the present study in T. longibrachiatum. It should be noted that chloromethane, which has been reported to be a putative methyl donor for biosynthesis of anisol in the wood-rooting fungus P. pomaceus (14, 22, 29), failed to support O methylation of 2,4,6-TCP in our study. It is also important to point out that this bacterial methyltransferase activity is constitutive (24). This property is environmentally significant, since it implies that previous exposure to toxic phenols is not required to carry out O methylation. By contrast, in T. longibrachiatum only residual levels of this activity can be detected in the absence of 2,4,6-TCP, but clear induction is observed when this compound is included in the culture medium. The induction mechanism observed for the fungal enzyme can be advantageous, since it probably results in a better and stronger response to 2,4,6-TCP in a shorter time.
Traditionally, the growth of filamentous fungi on cork has been considered beneficial. These microorganisms are supposed to contribute to the softening of cork, to the cleaning of the cork surface, and to filling of the internal lenticular channels (31), although extensive studies to confirm these ideas have not been performed. In agreement with this point of view, it has been postulated that some organisms, such as C. sitophila, which not only exhibit very low efficiency for 2,4,6-TCP biomethylation (0.03%) and a limited ability to degrade 2,4,6-TCP but also restrict the growth of other molds, could be exploited industrially to inoculate cork planks (30). However, under our experimental conditions, C. sitophila, as well as other isolates, showed moderate 2,4,6-TCP biomethylation ability (11.02%). We do not know the reasons for such a discrepancy, but it could be due to differences in the experimental approaches. In any case, these observations emphasize the importance of studying both the biochemical pathways and the regulatory processes controlling biodegradation of 2,4,6-TCP and biosynthesis of 2,4,6-TCA.
Previous studies indicated that 85.3% of fungi isolated from broiler house litter could metabolize 2,3,4,6-TeCP and that 58.6% of these strains could produce 2,3,4,6-TeCA (13). Also, several fungi isolated from food-packaging materials or drinking water could accomplish O methylation of 2,4,6-TCP to 2,4,6-TCA (25, 33, 35). These data and the data reported in the present study suggest that biomethylation of chlorophenols by filamentous fungi might be a more common phenomenon than previously thought. Little is known about regulation of this process, although Phanerochaete chrysosporium, a lignin-degrading basidiomycete, can degrade 2,4,6-TCP efficiently when it is grown under high-carbon-low-nitrogen conditions. Curiously, an increase in the concentration of nitrogen in the culture medium to 12 mM ammonium tartrate (high-carbon-high-nitrogen conditions) results in very slow O methylation of 2,4,6-TCP to 2,4,6-TCA as the sole product (3). These data suggest that better control of the environmental conditions could also help reduce the 2,4,6-TCA levels on cork. It is important to point out that no regulatory effects on 2,4,6-TCA biosynthesis and 2,4,6-TCP disappearance were noticed when T. longibrachiatum was grown in the presence of 2% glucose in the presence of up to 60 mM ammonium acetate (high-carbon-high-nitrogen conditions). The absence of regulation by these compounds under the conditions tested represents a significant difference if we compare the findings with the mechanism underlying 2,4,6-TCA biosynthesis and 2,4,6-TCP degradation by P. chrysosporium (3), and it suggests that in T. longibrachiatum 2,4,6-TCA biosynthesis is induced by the presence of a suitable substrate independent of the environmental conditions. This result also supports the putative role of O methylation of chlorophenols as a detoxification process. It is clear that in nature it would be dangerous for any microorganism to be exposed to a chlorophenol under environmental conditions that repress the O methylation reaction, which obviously would prevent detoxification of the compound to the corresponding anisole.
From our experiments we concluded that only a small percentage of 2,4,6-TCP is converted to 2,4,6-TCA by T. longibrachiatum, which clearly suggests that this product is not the only metabolite derived from 2,4,6-TCP. Mineralization studies indicated that 2,4,6-TCP is not fully degraded to CO2. Unfortunately, our efforts to identify intermediate products of 2,4,6-TCP degradation, such as those described for the basidiomycete P. chrysosporium and several gram-negative bacteria (3, 36, 37, 38), were unsuccessful. Thus, we cannot rule out the possibility that in T. longibrachiatum incomplete degradation of 2,4,6-TCP involves the mechanism proposed for these microorganisms. Our results may also mean that the levels of intermediates in the culture media were too low to be detected by our GC-MS analysis, perhaps because intracellular degradation prevented the accumulation of appreciable levels of intermediates in the media. It is also possible that 2,4,6-TCP is degraded to some compound that is not released into culture media but perhaps is incorporated into cellular material. Further studies should be carried out to clarify this point.
Future strategies to efficiently control the levels of chlorophenolic precursors in cork may include the development of modified fungal strains that have greater ability to degrade the chlorophenolic precursors while lacking the ability to biosynthesize 2,4,6-TCA under environmental conditions close to those found in nature. These strains could be inoculated into cork planks in order to overgrow the undesirable natural flora leading to synthesis of 2,4,6-TCA.
A number of issues remain to be elucidated. Purification of the methyltransferase, currently in progress, should be of interest to investigate the rates of O methylation when different precursors are used, since the enzymes apparently use a wide range of substrates (14, 24). On the other hand, biomethylation of chlorophenols could be a powerful alternative strategy for detoxification of these xenobiotic compounds from the environment.
Finally, it should be emphasized that in spite of the cork taint problem, cork is the best material for sealing wines and other beverages. A satisfactory solution to this problem not only should help the companies that manufacture cork and the wine industry but also should contribute to conservation of the threatened Mediterranean forests in which the cork oak is one of the most representative trees.
Acknowledgments
We gratefully acknowledge Miguel Elena Rosselló, Luis Javier Ávila, and Teresa Morientes of IPROCOR for their assistance with GC-MS assays, Leocadia Franco for technical assistance, and Richard Calderone and Pedro Fernández for helpful comments. Cork samples were kindly provided by Sanvicork S.A.
This work was supported by the European Community and the Ministerio de Educación y Cultura of Spain (grant 1FD97-1172) and also by the Junta de Extremadura (grant IPR00C004).
REFERENCES
- 1.Allard, A.-S., M. Remberger, and A. H. Neilson. 1987. Bacterial O methylation of halogen-substituted phenols. Appl. Environ. Microbiol. 53:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amon, J. M., J. M. Vandepeer, and R. F. Simpson. 1989. Compounds responsible for cork taint in wine. Wine Ind. J. 4:62-69. [Google Scholar]
- 3.Bhasker Reddy, G. V., M. D. Sollewijn Gelpke, and M. H. Gold. 1998. Degradation of 2,4,6-trichlorophenol by Phanerochaete chrysosporium: involvement of reductive dechlorination. J. Bacteriol. 180:5159-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Butzke, C. E., T. J. Evans, and S. E. Ebeler. 1999. Detection of cork taint in wine using automated solid-phase microextraction in combination with GC/MS-SIM. ACS Symp. Ser. 714:208-216. [Google Scholar]
- 6.Cserjesi, A. J., and E. L. Johnson. 1972. Methylation of pentachlorophenol by Trichoderma virgatum. Can. J. Microbiol. 18:45-49. [DOI] [PubMed] [Google Scholar]
- 7.Curtis, R. F., D. G. Land, N. M. Griffiths, M. G. Gee, D. Robinson, J. L. Peel, C. Dennis, and J. M. Gee. 1974. 2,3,4,6-Tetrachloroanisole: association with musty taint in chickens and microbiological formation. Nature 235:223-224. [DOI] [PubMed] [Google Scholar]
- 8.Drotar, A. M., and R. Fall. 1985. Methylation of xenobiotic thiols by Euglena gracilis: characterization of a cytoplasmic thiol methyltransferase. Plant Cell Physiol. 26:847-850. [Google Scholar]
- 9.Drotar, A. M., and R. Fall. 1986. Characterization of a xenobiotic methyltransferase and its role in detoxification in Tetrahymena thermophila. Pestic. Biochem. Physiol. 25:396-406. [Google Scholar]
- 10.Drotar, A. M., G. A. Burton, Jr., J. E. Tavernier, and R. Fall. 1987. Widespread occurrence of bacterial thiol methyltransferases and the biogenic emission of methylated sulfur gases. Appl. Environ. Microbiol. 53:1626-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gams, W., and J. Bissett. 1998. Morphology and identification of Trichoderma, p. 3-34. In C. P. Kubicek and G. E. Harman (ed.), Trichoderma and Gliocladium, vol. 1. Basic biology, taxonomy and genetics. Taylor & Francis Ltd., London, United Kingdom.
- 12.Gams, W., E. S. Hoekstra, and A. Aptroot. 1998. CBS course of mycology. Centraalbureau voor Schimmelcultures, Baarn, The Netherdlands.
- 13.Gee, J. M., and J. L. Peel. 1974. Metabolism of 2,3,4,6-tetrachlorophenol by microorganisms from broiler house litter. J. Gen. Microbiol. 85:237-243. [DOI] [PubMed] [Google Scholar]
- 14.Harper, D. B., J. T. G. Hamilton, J. T. Kennedy, and K. J. McNally. 1989. Chloromethane, a novel methyl donor for biosynthesis of esters and anisoles in Phellinus pomaceus. Appl. Environ. Microbiol. 55:1981-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawksworth, D. L., P. M. Kirk, B. B. Sutton, and D. N. Pegler. 1995. Dictionary of the fungi, 8th ed. CAB International, Wallingford, United Kingdom.
- 16.Hoog, G. S., and J. Guarro. 1995. Atlas of clinical fungi. Centraalbureau voor Schimmelcultures, Baarn, The Netherlands.
- 17.Kuhls, K., E. Lieckfeldt, G. J. Samuels, W. Meyer, C. P. Kubicek, and T. Börner. 1997. Revision of Trichoderma sect. Longibrachiatum including related teleomorphs based on analysis of ribosomal DNA internal transcribed spacer sequences. Mycologia 89:442-460. [Google Scholar]
- 18.Lee, S. L., and J. W. Taylor. 1990. Isolation of DNA from fungal mycelia and single spores, p. 282-286. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and, T. J. White (ed.), PCR protocols. A guide to methods and applications. Academic Press, San Diego, Calif.
- 19.Lee, T. H., and R. F. Simpson. 1993. Microbiology and chemistry of cork taints in wine, p. 353-372. In G. H. Fleet (ed.), Wine microbiology and bio/technology. G. Harwood Academic Publishers, Chur, Switzerland.
- 20.Lübeck, M., S. K. Poulsen, P. S. Lübeck, D. F. Jensen, and U. Thrane. 2000. Identification of Trichoderma strains from building materials by ITS 2 ribotyping, UP-PCR fingerprinting and UP-PCR cross hybridization. FEMS Microbiol. Lett. 185:129-134. [DOI] [PubMed] [Google Scholar]
- 21.Maujean, A., P. Millery, and H. Lemaresquier. 1985. Explications biochimiques et metaboliques de la confusion entre goût de bouchon et goût de moisi. Rev. Fr. Oenolog. 99:55-59. [Google Scholar]
- 22.McNally, K. J., and D. B. Harper. 1991. Methylation of phenol by chloromethane in the fungus Phellinus pomaceus. J. Gen. Microbiol. 137:1029-1032. [Google Scholar]
- 23.Neidleman, S. L., and J. Geigert. 1986. Biohalogenations: principles, basic roles and applications. Ellis Harwood, Chichester, United Kingdom.
- 24.Neilson, A. H., C. Lindgren, P.-A. Hynning, and M. Remberger. 1988. Methylation of halogenated phenols and thiophenols by cell extracts of gram-positive and gram-negative bacteria. Appl. Environ. Microbiol. 54:524-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nystrom, A., A. Grimvall, C. Krantzrulcker, R. Savenhed, and K. Akerstrand. 1992. Drinking-water off-flavor caused by 2,4,6-trichloroanisole. Water Sci. Technol. 25:241-249. [Google Scholar]
- 26.Pitt, J. I. 1973. An appraisal of identification methods for Penicillium species: novel taxonomic criteria based on temperature and water relations. Mycologia 65:1135-1157. [PubMed] [Google Scholar]
- 27.Pitt, J. I. 1985. A laboratory guide to common Penicillium species. CSIRO Food Research Laboratory, North Ryde, Australia.
- 28.Samson, R. A., and E. S. Hoekstra. 1988. Introduction to food-borne fungi, 3rd ed. Centraalbureau voor Schimmelcultures, Baarn, The Netherlands.
- 29.Saxena, D., S. Aouad, J. Attieh, and H. S. Saini. 1998. Biochemical characterization of chloromethane emission from the wood-rotting fungus Phellinus pomaceus. Appl. Environ. Microbiol. 64:2831-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva Pereira, C., A. Pires, M. J. Valle, L. Vilas Boas, J. J. Figueiredo Marques, and M. V. San Romao. 2000. Role of Chrysonilia sitophila in the quality of cork stoppers for sealing wine bottles. J. Ind. Microbiol. Biotechnol. 24:256-261. [Google Scholar]
- 31.Silva Pereira, C., J. J. Figueiredo Marques, and M. V. San Romao. 2000. Cork taint in wine: scientific knowledge and public perception—a critical review. Crit. Rev. Microbiol. 26:147-162. [DOI] [PubMed] [Google Scholar]
- 32.Tanner, H., C. Zanner, and G. Würdig. 1981. Zur analytischen Differenzierung von Muffon und Korkgeschmack in Wein. Schweiz. Z. Obst Weinbau 117:752-757. [Google Scholar]
- 33.Tindale, C. R., F. B. Whitfield, S. D. Levingston, and T. H. L. Nguyen. 1989. Fungi isolated from packaging materials: their role in the production of 2,4,6-trichloroanisole. J. Sci. Food Agric. 49:437-447. [Google Scholar]
- 34.White, T. J., T. Bruns, S. Lee, and J. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols. A guide to methods and applications. Academic Press, San Diego, Calif.
- 35.Whitfield, F. B., T. H. L. Nguyen, and C. R. Tindale. 1991. Effect of relative-humidity and incubation-time on the O-methylation of chlorophenols in fiberboard by Paecilomyces variotii. J. Sci. Food Agric. 55:19-26. [Google Scholar]
- 36.Wieser, M., B. Wagner, J. Eberspächer, and F. Lingens. 1997. Purification and characterization of 2,4,6-trichlorophenol-4-monooxygenase, a dehalogenating enzyme from Azotobacter sp. strain GP1. J. Bacteriol. 179:202-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xun, L. 1996. Purification and characterization of chlorophenol-4-monooxygenase from Burkholderia cepacia AC1100. J. Bacteriol. 178:2645-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xun, L., and C. S. Orser. 1991. Purification and properties of pentachlorophenol hydroxylase, a flavoprotein from Flavobacterium sp. strain ATCC 39723. J. Bacteriol. 173:4447-4453. [DOI] [PMC free article] [PubMed] [Google Scholar]