Abstract
Rhizobia are soil bacteria that are able to establish symbiotic associations with leguminous hosts. In iron-limited environments these bacteria can use iron present in heme or heme compounds (hemoglobin, leghemoglobin). Here we report the presence in Sinorhizobium meliloti of an iron-regulated outer membrane protein that is able to bind hemin but not hemoglobin. Protein assignment was done by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Tryptic peptides correlated with the mass measurements obtained accounted for 54% of the translated sequence of a putative heme receptor gene present in the chromosome of S. meliloti 1021. The results which we obtained suggest that this protein (designated ShmR for Sinorhizobium heme receptor) is involved in high-affinity heme-mediated iron transport.
Rhizobia are N2-fixing bacteria that are able to adapt to diverse nutrient availability conditions. These soil bacteria can exist as free-living organisms in natural environments, where they have to compete with other microbes to obtain nutrients. Rhizobia can also be found in legume nodules as endosymbionts, and this form completely depends on nutrients supplied by the host plant. Among the nutrients, iron has a particular trait. This metal is essential for many metabolic processes, including nitrogen fixation. However, intracellular iron is potentially toxic because it is able to catalyze the formation of oxygen free radicals. To cope with this conflict, acquisition of nutritional iron and maintenance of the intracellular iron concentration are monitored and regulated in bacteria by internal and external signal systems (8).
Like most other microbes, free-living rhizobia are able to acquire iron from their own ferri-siderophore, exogenous ferri-siderophores, and ferric citrate (3, 17). Although the ability to use heme iron sources has been thought for many years to be restricted to bacterial pathogens of animals, our group demonstrated that many soil bacteria, including rhizobia, can also acquire iron from heme, myoglobin, hemoglobin, and leghemoglobin (14).
Heme uptake systems in Rhizobium leguminosarum and Bradyrhizobium japonicum have recently been described genetically. Wexler et al. (22) found a chromosomal region in R. leguminosarum that contains a tonB homologue and hmuPSTUV operon genes that are very similar to transporter genes involved in iron acquisition in some pathogenic bacteria. A presumptive outer membrane heme receptor was not present in the genomic region studied and has not been found in this genus yet. Genome sequencing (7) revealed an equivalent region with almost the same gene arrangement in the Sinorhizobium meliloti 1021 chromosome. In B. japonicum, Nienabier et al. (13) identified an Hmu cluster that encodes a putative heme receptor, HmuR, and HmuTUV, a presumptive heme ABC transporter. These authors demonstrated by using growth assays that an hmuR mutant is unable to use heme compounds as sole iron sources.
In silico studies of the S. meliloti 1021 genome predicted the existence of two heme receptor proteins designated Smc 04205 and Smc 02726 (2). To our knowledge, expression of either of these receptors has not been reported previously. In this paper we describe biochemical methods used for detection and identification at the protein level of one of these presumptive receptors, Smc 02726 (designated ShmR here), and we provide evidence that it is able to bind hemin.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The parent strain of S. meliloti 242 is a streptomycin-resistant derivative of the field isolate S. meliloti 259 (5). Rhizobia were grown on defined M3 medium. M3 medium is a slightly modified version of minimal Vincent medium (20) and contains (per liter) 1.0 g of KH2PO4, 1.0 g of K2HPO4, 124 mg of MgSO4, 50 mg of CaCl2, 0.2 mg of biotin, 45 mg of l-methionine, 1.0 g of glutamate, and 1.0 g of d-mannitol (pH 7.2). Iron-rich M3 medium also contained 37 μM FeCl3. Iron-chelated media (M3E) were obtained by adding di-o-hydroxyphenylacetic acid (EDDHA). As indicated below, in some experiments growth media were supplemented with 16 μM hemin, 4 μM hemoglobin, 10 μM protoporphyrin IX (PPIX), 50 μg of kanamycin per ml, 50 μg of neomycin per ml, or 100 μg of streptomycin per ml. EDDHA, hemin, bovine hemoglobin, and PPIX were obtained from Sigma (St. Louis, Mo.).
Outer membrane protein extraction.
S. meliloti 242 was grown on 50 ml of iron-rich medium and on iron-chelated minimal medium to the early stationary growth phase. Cells were pelleted by centrifugation for 10 min at 10,000 × g, washed twice with 10 mM Tris-HCl (pH 7.5), and resuspended in 10 ml of the same buffer. Cells were disrupted by three passages through a French pressure cell at 25,000 lb/in2, and the suspension obtained was incubated for 90 min with a solution containing 0.4 mg of DNase per ml, 0.4 mg of RNase per ml, and 10 mg of lysozyme per ml and for an additional 60 min with 0.75% N-laurylsarcosine at room temperature. Cells debris was removed by centrifugation for 10 min at 10,000 × g, and the supernatant was centrifuged for 2 h at 60,000 × g. The pellet obtained was used for sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) or for affinity chromatography assays.
SDS-PAGE.
Pellets enriched with outer membrane proteins were resuspended in 500 μl of water and mixed with 500 μl of sample buffer (24). The preparations were heated for 10 min at 98°C and stored frozen at −20°C until they were analyzed by discontinuous slab gel electrophoresis with SDS (SDS-PAGE) (11). Resolving gels containing 10% acrylamide were used. Proteins were visualized by staining with Coomassie brilliant blue R-250 or by silver staining (23). The apparent molecular weights of the proteins detected were estimated by comparison with a broad-range protein standard obtained from Bio-Rad (Richmond, Calif.).
Hemin and hemoglobin affinity chromatography.
Hemin-binding proteins present in the outer membrane were purified by the method described by Mills and Payne (12), with minor modifications. Briefly, a pellet containing outer membrane proteins (obtained from ca. 1010 cells) was resuspended in 500 μl of 50 mM Tris-HCl (pH 8.0)-1 M NaCl and incubated for 1 h at 30°C with 250 μl of a washed hemin-agarose or hemoglobin-agarose suspension (Sigma). Nonadherent proteins were removed by incubation for 90 min at 30°C with a solution containing 10 mM Tris-HCl (pH 7.8), 150 mM NaCl, 10 mM EDTA, 1% (vol/vol) N-laurylsarcosine, 0.2% (wt/vol) sodium deoxycholate, and 0.1% (wt/vol) SDS. The suspension was centrifuged for 5 min at 5,000 × g, and the supernatant was discarded. The agarose beds were then washed three times with a high-salt solution containing 50 mM Tris-HCl (pH 8.0), 1 M NaCl, 10 mM EDTA, 0.75% (vol/vol) N-laurylsarcosine, 0.15% (wt/vol) sodium deoxycholate, and 0.075% (wt/vol) SDS and once with a solution containing 50 mM Tris-HCl (pH 8.0) and 1 M NaCl. Samples were centrifuged for 5 min at 5,000 × g between washes. Finally, washed beads were suspended in 400 μl of the electrophoresis sample buffer, heated for 10 min at 98°C, and centrifuged for 5 min at 5,000 × g before they were analyzed by SDS-PAGE.
Competition binding assay.
Outer membrane preparations were resuspended in 500 μl of 50 mM Tris-HCl (pH 8.0)-1 M NaCl and were incubated with 0.1 mM hemin or 0.1 mM PPIX for 1 h at 37°C with shaking prior to hemin affinity chromatography.
Mass spectrometric analysis and sample preparation.
Proteins of interest were obtained from an SDS-PAGE gel and stained as described above. Each protein band was manually excised from the gel with a scalpel on a clean bench and placed into a 1.5-ml Eppendorf tube. Destaining, washing, proteolytic digestion, and peptide extraction were done by using previously described protocols (9). Overnight digestion at 37°C with trypsin (Promega) was performed in 0.1 M NH4HCO3, while for digestion with endolysin C (Wako, Osaka, Japan), 0.1 M Tris-HCl (pH 9.25) was used.
A treated piece of gel containing the protein band was transferred to a microcentrifuge tube, and 0.63 μg of trypsin per μl or 0.25 μg of endolysin C per μl was added. Digestion was allowed to proceed overnight at 37°C. Samples containing peptides resulting from proteolytic cleavage were analyzed by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry with a Voyager-DE-PRO mass spectrometer (Perspective Biosystems, Framingham, Mass.). Aliquots (1 μl) of the sample were mixed with the same volume of a matrix-saturated solution of α-cyano-4-hydroxycinnamic acid (Sigma) in 50% acetonitride-0.2% trifluoroacetic acid. The mixture was put directly onto the laser target and allowed to crystallize at room temperature for at least 1 min before mass analysis. All spectra were acquired by operating the mass spectrometer in the reflector mode with an accelerating voltage of 20 kV, and the average was determined for 250 laser shots per spectrum. External calibration was done by using a peptide standard mixture spotted immediately next to the sample. The standard mixture included (final concentration and monoisotopic molecular mass for each peptide) angiotensin I (2 pmol/μl and 1,296.685 Da), human adrenocorticotropic hormone (ACTH) 1-17 clip (2 pmol/μl and 2,093.087 Da), ACTH 18-39 clip (1.5 pmol/μl and 2,465.199 Da), and ACTH 7-38 clip (3 pmol/μl and 3,658.932 Da). The molecular mass corresponds to the monoisotopic mass of each protein.
Some peptides were sequenced by performing postsource decay fragmentation analysis according to the manufacturer's instructions. The measured peptide masses for each protein digest were compared to theoretical masses for proteolytic digests from protein sequences available from the National Center for Biotechnology Information by using the Profound program (http://prowl.rockefeller.edu/cbi-bin/Profound). Theoretical digestion of protein sequences with trypsin or endolysin C was performed by using the Protein Prospector 3.4.1 MS-Digest program (http://www.prospector.ucsf.edu).
RESULTS
Binding of ShmR to heme-agarose.
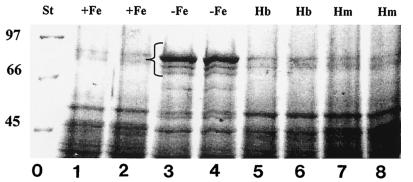
In a previous study we demonstrated that S. meliloti 242 grown on TY rich medium is able to express at least two iron-regulated outer membrane proteins (IROMPs), which have molecular masses of 82 and 91 kDa as estimated by SDS-PAGE (6). In this work we examined the ability of S. meliloti 242 to induce expression of specific proteins when hemin or hemoglobin was present in the growth medium. As shown in Fig. 1, 82- and 91-kDa proteins were present in outer membrane preparations obtained from cultures grown on iron-limited minimal medium. Under these conditions an additional iron-regulated protein, which had a molecular mass of about 80 kDa, was also detected. Addition of 16 μM hemin or 4 μM hemoglobin to iron-limited medium did not induce detectable expression of any of these IROMPs. Moreover, the protein profiles obtained for cultures grown on iron-rich M3 medium and cultures grown on M3E containing hemin or hemoglobin were almost the same.
FIG. 1.
SDS-PAGE analysis of S. meliloti 242 outer membrane proteins. S. meliloti 242 was grown in M3 medium containing 37 μM FeCl3 (lanes 1 and 2), 500 μM EDDHA (lanes 3 and 4), 500 μM EDDHA and 4 μM hemoglobin (lanes 5 and 6), or 500 μM EDDHA and 16 μM hemin (lanes 7 and 8). Outer membrane proteins were extracted as described in the text, electrophoresed in a 10% acrylamide gel, and stained with 0.1% Coomassie brilliant blue. The bracket indicates the position of IROMPs. The positions of molecular mass standards (in kilodaltons) (lane 0) are indicated on the left. St, standards; Hb, hemoglobin; Hm, hemin.
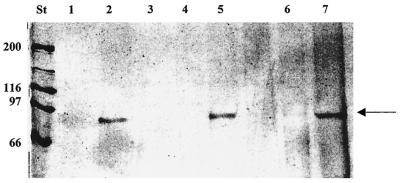
To detect the presence of outer membrane proteins able to bind heme or heme compounds, we utilized heme or hemoglobin immobilized on agarose beads, a strategy that has been successfully used to identify heme receptors in pathogenic bacteria (4, 12). In our experiments the 91-kDa protein was retained in heme-agarose affinity gels. This protein was detected in preparations obtained from cultures grown on M3E or M3E containing PPIX (Fig. 2, lanes 2 and 5). In contrast, the 91-kDa protein was not found on affinity gels when preparations were obtained from cultures grown on medium containing 37 μM FeCl3, 16 μM hemin, or 4 μM hemoglobin (lanes 1, 3, and 4).
FIG. 2.
Binding of the 91-kDa protein to hemin-agarose. Outer membrane proteins were isolated from bacteria grown in M3 medium containing 37 μM FeCl3 (lane 1), 500 μM EDDHA (lane 2), 500 μM EDDHA and 4 μM hemoglobin (lane 3), 500 μM EDDHA and 16 μM hemin (lane 4), or 16 μM PPIX (lane 5), and they were added to hemin-agarose resin. Lanes 6 and 7 contained the hemin-binding proteins from competition assays performed with hemin (lane 6) and PPIX (lane 7). Hemin-binding proteins were eluted as described in Materials and Methods, separated by SDS-PAGE, and silver stained. The arrow indicates the position of the 91-kDa hemin-binding outer membrane protein. The positions of molecular mass standards (in kilodaltons) (lane St) are indicated on the left.
Specificity conferred by the presence of iron in the heme molecule was tested by competitive assays. Preincubation of an outer membrane preparation with hemin but not PPIX inhibited protein binding to heme-agarose (Fig. 2, lanes 6 and 7). These results indicated that the 91-kDa protein recognized the presence of hemin in the target molecule.
Next, we examined the ability of any outer membrane protein to bind hemoglobin immobilized in agarose, but no such protein was detected by this method (data not shown).
MALDI-TOF mass spectrometry analysis.
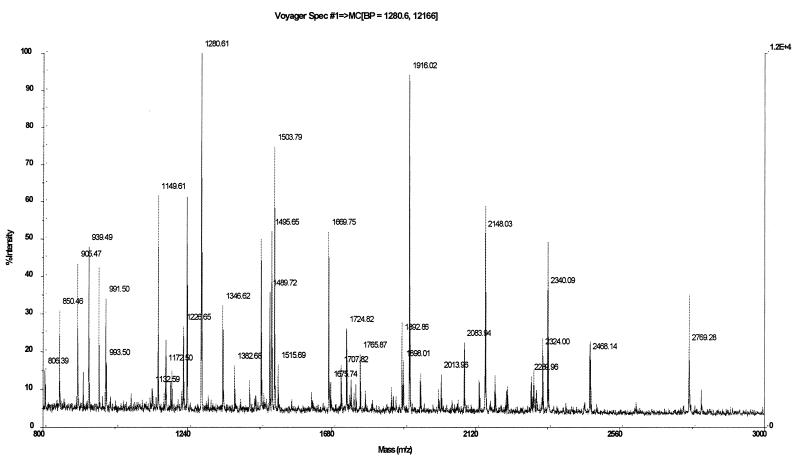
The mass spectrum shown in Fig. 3 was obtained by trypsin digestion of the 91-kDa protein band isolated from the Coomassie brilliant blue-stained SDS gel shown in Fig. 1, lane 3. A similar peptide map was obtained by spectrometry when the sample came from material retained by hemin-agarose and was analyzed on an SDS-PAGE gel like the one shown in Fig. 2, lane 2 (data not shown). Protein Smc 02726 from S. meliloti 1021 resulted in the highest score when a search was conducted by submitting the mass spectrometric peptide data to the National Center for Biotechnology Information database. Table 1 lists peptide masses obtained from spectra in Fig. 3 compared to theoretical peptide masses that differed by less than 0.1 Da, as determined by virtual tryptic digestion of the candidate proteins. An analysis of the primary structures of three peptides whose sequences were identical to those of three peptides having the same masses resulting from the theoretical digestion of protein Smc 02726 reinforced the protein identification results.
FIG. 3.
Mass spectrum obtained by MALDI-TOF mass spectrometry by using the 91-kDa outer membrane protein in a gel digested with trypsin.
TABLE 1.
Comparison of experimental peptide masses obtained from the spectra shown in Fig. 3 with theoretical peptide masses obtained from virtual tryptic digestion of the ShmR protein from S. meliloti 1021
| Peptide sequence | Theoretical mass (Da) | Exptl mass (Da) | Difference (Da) |
|---|---|---|---|
| G109-R114 | 586.33 | 586.33 | 0.00 |
| L55-R60 | 615.35 | 615.36 | 0.01 |
| T632-K637 | 662.38 | 662.38 | 0.00 |
| V737-F743a | 850.47 | 850.47 | 0.00 |
| A101-R108a | 905.48 | 905.48 | 0.00 |
| I706-R713a | 939.50 | 939.52 | 0.02 |
| D726-R733 | 970.43 | 970.42 | −0.01 |
| T351-R359 | 991.52 | 991.51 | −0.01 |
| I275-R283 | 993.54 | 993.52 | −0.02 |
| K705-R713 | 1,067.60 | 1,067.59 | −0.01 |
| T734-R742 | 1,079.62 | 1,079.69 | 0.07 |
| Q81-R90 | 1,131.56 | 1,131.56 | 0.00 |
| I275-R284 | 1,149.63 | 1,149.62 | −0.01 |
| I300-R309 | 1,172.52 | 1,172.51 | −0.01 |
| G714-R725 | 1,187.60 | 1,187.59 | −0.01 |
| S426-R436 | 1,191.60 | 1,191.60 | 0.00 |
| T734-F743 | 1,226.68 | 1,226.66 | −0.02 |
| F265-R274 | 1,237.61 | 1,237.60 | −0.01 |
| A334-R343 | 1,280.62 | 1,280.61 | −0.01 |
| T180-K191 | 1,302.64 | 1,302.57 | −0.07 |
| D726-K736 | 1,346.63 | 1,346.62 | −0.01 |
| V694-K705 | 1,382.73 | 1,382.69 | −0.04 |
| Y298-R309 | 1,463.68 | 1,463.67 | −0.01 |
| A101-R114 | 1,472.80 | 1,472.74 | −0.06 |
| F218-R230 | 1,489.74 | 1,489.74 | 0.00 |
| D726-K736 | 1,495.67 | 1,495.66 | −0.01 |
| I437-R451 | 1,503.81 | 1,503.79 | −0.02 |
| D285-R297 | 1,515.73 | 1,515.70 | −0.03 |
| F218-K231 | 1,617.84 | 1,617.81 | −0.03 |
| G543-R558 | 1,669.78 | 1,669.76 | −0.02 |
| I298-R311 | 1,734.80 | 1,734.78 | −0.02 |
| S91-R108 | 1,875.96 | 1,875.89 | −0.07 |
| T249-K264 | 1,892.90 | 1,892.87 | −0.03 |
| I115-R132 | 1,916.04 | 1,916.02 | −0.02 |
| S199-R217 | 1,949.88 | 1,949.86 | −0.02 |
| T605-K622 | 2,004.95 | 2,004.92 | −0.03 |
| D377-R395 | 2,083.97 | 2,083.94 | −0.03 |
| T180-R198 | 2,148.06 | 2,148.03 | −0.03 |
| D726-F743 | 2,178.09 | 2,178.07 | −0.02 |
| F265-R283 | 2,212.13 | 2,212.09 | −0.04 |
| V228-R333 | 2,288.99 | 2,288.96 | −0.03 |
| R694-R713 | 2,303.21 | 2,303.19 | −0.02 |
| A374-R395 | 2,340.12 | 2,340.10 | −0.02 |
| T351-R373 | 2,468.18 | 2,468.15 | −0.03 |
| V559-R583 | 2,769.31 | 2,769.28 | −0.03 |
Peptide sequenced.
As predicted from the DNA sequence, the hypothetical heme receptor protein Smc 02726 has a putative TonB box and a FRAP-NPNL motif reported to be involved in heme binding. These characteristics are consistent with the ability of the 91-kDa IROMP to bind heme.
Figure 4 shows the amino acid sequence, and the peptides identified by mass spectrometry are indicated. As Fig. 4 shows, coverage of 54% of the theoretical protein sequence was obtained. Only peptides whose measured masses differed by less than 0.1 mass unit from the expected values were considered.
FIG. 4.
Amino acid sequence of the putative iron transport protein from S. meliloti 1021 (ShmR). Boldface type indicates the experimental peptides identified by MALDI-TOF mass spectrometry. A probable TonB box (residues 149 to 180) and the FRAP-NPNL motif of heme receptors (residues 509 to 545) are underlined. Highly conserved residues present in the TonB box are indicated by a black background, whereas highly conserved residues of the FRAP-NPNL region are indicated by a grey background. The conserved histidine between the FRAP and NPNL motifs is probably replaced by an asparagine (residue 523) in ShmR.
DISCUSSION
In this study we identified in S. meliloti an IROMP (ShmR) able to bind hemin but not hemoglobin. This protein was identified as a translation product of an smc 02726 gene (predicted to be a hemin transport protein) by mass spectrometry. The 54% protein sequence coverage resulting from tryptic peptide mass measurements together with the identification of three peptides by sequencing surpass current stringent criteria for protein identification (10, 19).
By using competition assays we also found that the presence of a metal ion in the hemin molecule is required for receptor recognition. This result is in agreement with recent observations made with Porphyromonas gingivalis showing that iron, copper, and zinc metalloprotoporphyrins bind to the heme receptor HmuR more tightly than PPIX alone (16).
The ShmR protein could be detected only in outer membrane preparations from bacteria grown on M3E. When 16 μM hemin or 4 μM hemoglobin was included in the growth medium, ShmR could not be detected either by SDS-PAGE or by affinity chromatography. Moreover, any additional IROMPs could be detected under these conditions. The finding suggests that this heme receptor could be involved in high-affinity heme-mediated iron transport. When extracellular medium has a low free iron concentration but a high hemin concentration, iron transport must proceed by a different pathway. Qi and O'Brian (18) showed that hemin concentrations as low as 0.05 μM could satisfy heme auxotrophy in a hemH mutant of B. japonicum, indicating that the hemin is internalized and sustains growth in this mutant. These authors also demonstrated that heme can serve as a signaling molecule mediated by the Irr protein in B. japonicum. Although iron- and heme-dependent regulation in S. meliloti has not been studied in detail yet, our results suggest that the heme concentration may regulate heme receptor expression via a homologous mechanism.
Previously (14), it was demonstrated that S. meliloti 242 is able to grow in M3E supplemented with hemin, hemoglobin, or leghemoglobin. Nonetheless, in the present study we could not detect an outer membrane protein able to bind hemoglobin. This result indicates that ShmR could be involved in heme recognition but not in hemoglobin recognition. An analysis of the S. meliloti genome by Capela et al. (2) suggested two TonB-dependent receptors that could participate in heme transport: Smc 02726 (ShmR) and Smc 04205. Amino acid alignments constructed by Nienabier et al. (13) found that Smc 04205 is highly homologous to HmuR from B. japonicum, to HasR from Serratia marscecens, and to other hemin receptors present in different pathogenic bacteria. HasR is involved in heme transport mediated by the hemophore HasA. In S. marscecens HasA is able to bind extracellular heme, and HasR recognizes the heme-HasA complex. In a situation in which Smc 04205 would require the presence of another molecule to recognize heme-containing compounds, this presumptive membrane receptor would not be detected by affinity chromatography with immobilized heme or hemoglobin. Although HasA-like proteins have not been detected in rhizobia, such a mechanism would be an attractive mechanism to explain our observation.
The FRAP-NPNL conserved motif, which has been reported to be a common sequence in heme receptors, is present in both the ShmR and Smc 04205 proteins (13). However, the highly conserved histidine residue in the FRAP-NPNL region is present only in Smc 04205. As mutational studies have directly implicated the invariant histidine residue (His-461 in Yersinia enterocolitica) in hemin recognition and internalization (1, 21), the mechanism operating in the ShmR receptor may proceed in a different way.
It is interesting that this receptor was detected in a free-living form of rhizobia. Evaluation of the role of heme-mediated iron transport mechanisms during the symbiotic association with leguminous hosts remains an open area for research. Nonetheless, data presented here support the notion that “pathogens and symbionts depend on similar mechanisms for interacting with hosts” (15), although the organisms may respond to different regulatory pathways.
Acknowledgments
We are very grateful to Mark R. O'Brian for fruitful discussions concerning the manuscript and Gordana Bothe from GATC for providing unpublished S. meliloti sequences.
This work was supported in part by PEDECIBA, CONICYT-FCE, and IFS.
REFERENCES
- 1.Bracken, C. S., M. T. Baer, A. Abdur-Rashid, W. Helmes, and I. Stojiljkovic. 1999. Use of heme-protein complex by the Yersinia enterocolitica HemR receptor: histidine residues are essential for receptor function. J. Bacteriol. 181:6063-6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capela, D., F. Barloy-Hubler, J. Gouzy, G. Bothe, F. Ampe, J. Batut, P. Boistard, A. Becker, M. Boutry, E. Cadieu, S. Dréano, S. Gloux, T. Godrie, A. Goffeau, D. Kahn, E. Kiss, V. Lelaure, D. Masuy, T. Pohl, D. Portetelle, A. Pühler, B. Purnelle, U. Ramsperger, C. Renard, P. Thébault, M. Vandenbol, S. Weidner, and F. Galibert. 2001. Analysis of the chromosome sequence of the legume symbiont Sinorhizobium meliloti strain 1021. Proc. Natl. Acad. Sci. USA 98:9877-9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carson, K. C., A. R. Glenn, and M. J. Dilworth. 1994. Specificity of siderophore-mediated transport of iron in rhizobia. Arch. Microbiol. 161:333-339. [Google Scholar]
- 4.Dashper, S. G., A. Hendtlass, N. Slakeski, C. Jackson, K. J. Croos, L. Brownfield, R. Hamilton, I. Barr, and E. C. Reynolds. 2000. Characterization of a novel outer membrane hemin-binding protein of Porphyromonas gingivalis. J. Bacteriol. 182:6456-6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabiano, E., G. Gualtieri, C. Pritch, G. Polla, and A. Arias. 1994. Extent of high-affinity iron transport systems in field isolates of rhizobia. Plant Soil 164:177-185. [Google Scholar]
- 6.Fabiano, E., P. R. Gill, F. Noya, P. Bagnasco, L. De La Fuente, and A. Arias. 1995. Siderophore-mediated iron acquisition mutants in Rhizobium meliloti 242 and its effect on the nodulation kinetic of alfalfa nodules. Symbiosis 19:197-211. [Google Scholar]
- 7.Galibert, F., T. M. Finan, S. R. Long, A. Pühler, P. Abola, F. Ampe, F. Barloy-Hubler, M. J. Barnett, A. Becker, P. Boistard, G. Bothe, M. Boutry, L. Bowser, J. Buhrmester, E. Cadieu, D. Capela, P. Chain, A. Cowie, R. W. Davis, S. Dréano, N. A. Federspiel, R. F. Fisher, S. Gloux, T. Godrie, A. Goffeau, B. Golding, J. Gouzy, M. Gurjal, I. Hernandez-Lucas, A. Hong, L. Huizar, R. W. Hyman, T. Jones, D. Kahn, M. L. Kahn, S. Kalman, D. H. Keating, E. Kiss, C. Komp, V. Lelaure, D. Masuy, C. Palm, M. C. Peck, T. M Pohl, D. Portetelle, B. Purnelle, U. Ramsperger, R. Surzycki, P. Thébault, M. Vandenbol, F. J. Vorhülter, S. Weidner, D. H. Wells, K. Wong, Y. Kuo-Chen, and J. Batut. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668-672. [DOI] [PubMed] [Google Scholar]
- 8.Hantke, K. 2001. Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 4:172-177. [DOI] [PubMed] [Google Scholar]
- 9.Hellman, U. 2000. Sample preparation by SDS-PAGE and in-gel digestion, p. 43-54. In P. Jollès and H. Jörnvall (ed.), Proteomics in functional genomics. Protein structure analysis. Birkhäuser Verlag, Basel, Switzerland.
- 10.Jiménez, C. R., L. Huang, Y. Qiu, and A. L. Burlingame. 1998. Searching sequence database over the internet: protein identification using MS-Fit, p. 16.5.1-16.5.3. In J. E. Coligan, B. M. Dunn, H. L. Ploogh, D. W. Speicher, and P. T. Wingfield (ed.), Current protocols in protein science, vol. 1. John Wiley and Sons, Inc., New York, N.Y. [DOI] [PubMed]
- 11.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 12.Mills, M., and S. M. Payne. 1995. Genetics and regulation of heme iron transport in Shigella dysenteriae and detection of an analogous system in Escherichia coli O157:H7. J. Bacteriol. 177:3004-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nienabier, A., H. Hennecke, and H. Fischer. 2001. Discovery of a haem uptake system in the soil bacterium Bradyrhizobium japonicum. Mol. Microbiol. 41:787-800. [DOI] [PubMed] [Google Scholar]
- 14.Noya, F., A. Arias, and E. Fabiano. 1997. Heme compounds as iron sources for nonpathogenic Rhizobium bacteria. J. Bacteriol. 179:3076-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochman, H., and N. A. Moran. 2001. Genes lost and genes found: evolution of bacterial pathogenesis and symbiosis. Science 292:1096-1098. [DOI] [PubMed] [Google Scholar]
- 16.Olczak, T., D. W. Dixon, and C. A. Genco. 2001. Binding specificity of the Porphyromonas gingivalis heme and hemoglobin receptor HmuR, gingipain K, and gingipain R1 for heme, porphyrins, and metallophorphyrins. J. Bacteriol. 183:5599-5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plessner, O., T. Klapatcht, and M. L. Guerinot. 1993. Siderophore utilization by Bradyrhizobium japonicum. Appl. Environ. Microbiol. 59:1688-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi, Z., and M. O'Brian. 2002. Interaction between the bacterial iron response regulator and ferrochelatase mediates genetic control of heme biosynthesis. Mol. Cell 9:155-162. [DOI] [PubMed] [Google Scholar]
- 19.Rappsilber, S., and M. Mann. 2002. What does it mean to identify a protein in proteomics? Trends Biochem. Sci. 27:74-78. [DOI] [PubMed] [Google Scholar]
- 20.Vincent, J. M. 1970. A manual for the practical study of root-nodule bacteria. IBP handbook N15. Blackwell, Oxford, United Kingdom.
- 21.Wandersman, C., and I. Stojiljkovic. 2000. Bacterial heme sources. The role of heme, hemoprotein receptors and hemophores. Curr. Opin. Microbiol. 3:215-220. [DOI] [PubMed] [Google Scholar]
- 22.Wexler, M., K. H. Yeoman, J. B. Stevens, N. G. de Luca, and A. W. B., Johnston. 2001. The Rhizobium leguminosarum tonB gene is required for the uptake of siderophore and haem as sources of iron. Mol. Microbiol. 41:801-816. [DOI] [PubMed] [Google Scholar]
- 23.Wray, V. P., and R. Hancock. 1981. Silver staining of proteins in polyacrylamide gels. Anal. Biochem. 118:197-203. [DOI] [PubMed] [Google Scholar]
- 24.Wright, S. F., J. G. Foster, and O. L. Bennett. 1986. Production and use of monoclonal antibodies for identification of strains of Rhizobium trifolii. Appl. Environ. Microbiol. 52:119-123. [DOI] [PMC free article] [PubMed] [Google Scholar]